Photopolymer-Based Composite with Substance Release Capability Manufactured Additively with DLP Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Composites
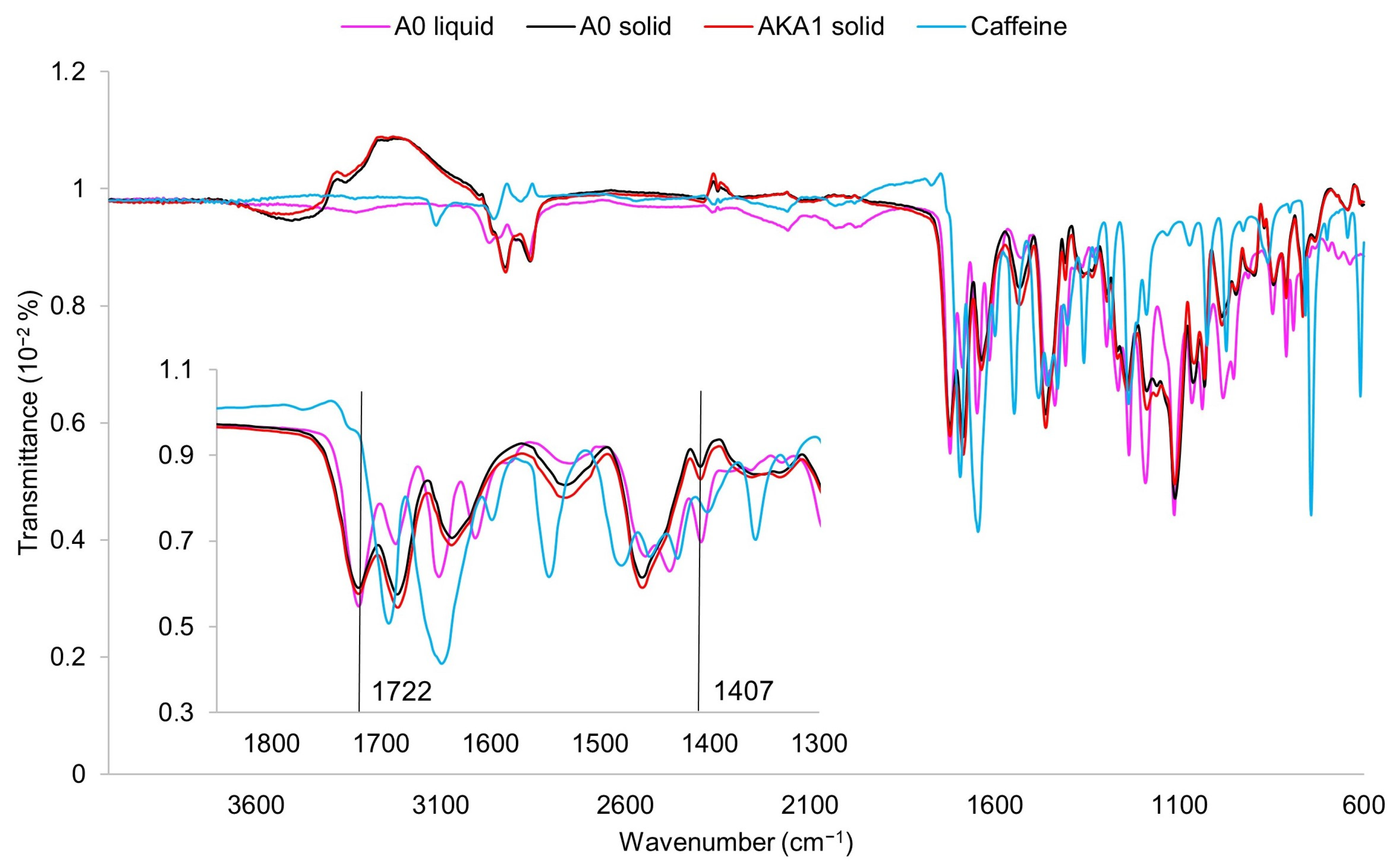
2.3. FTIR-ATR Measurement
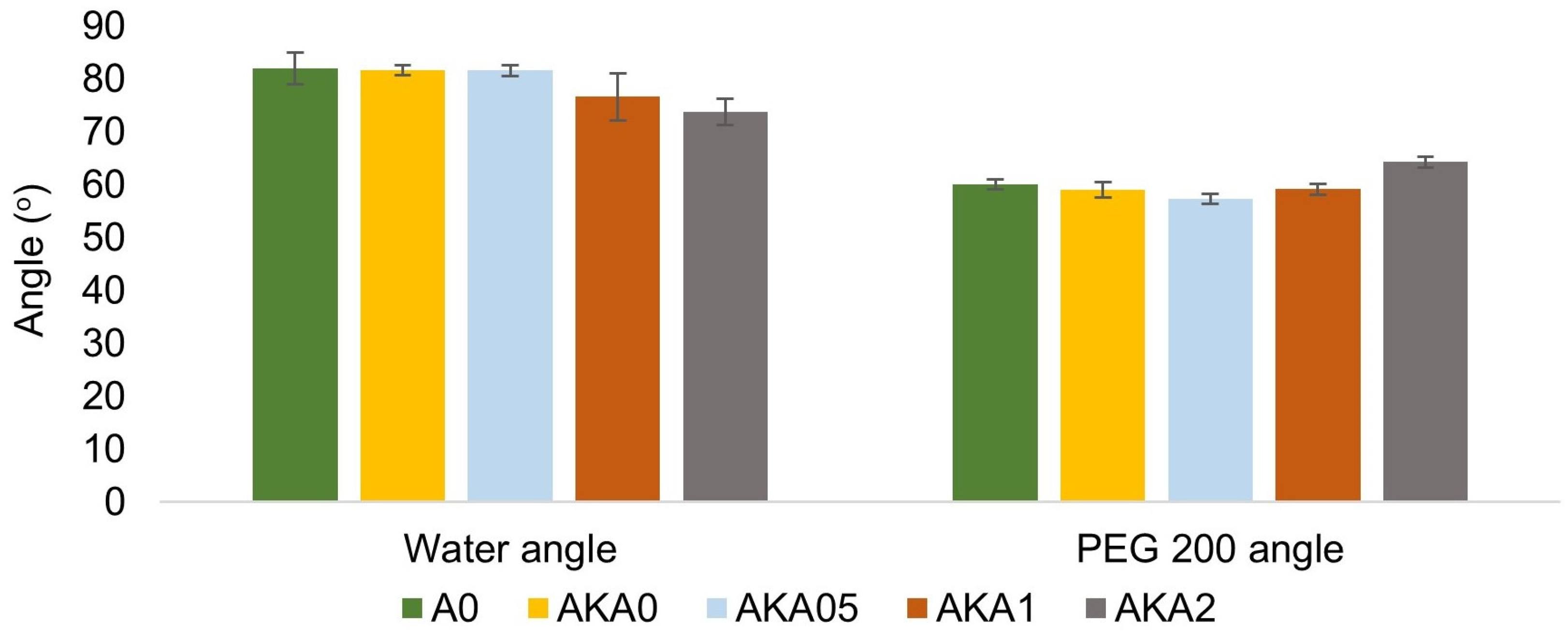
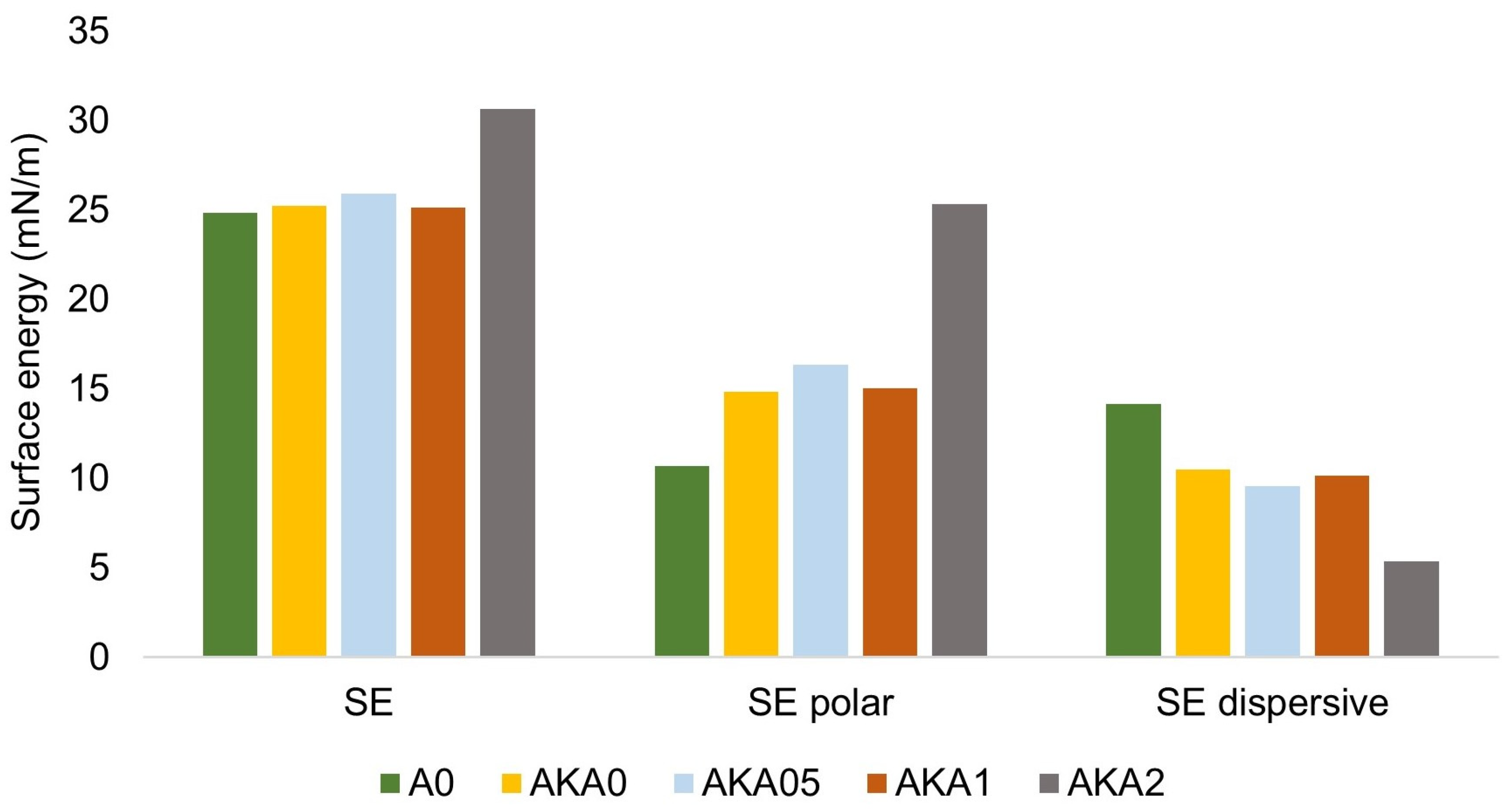
2.4. Wettability Measurement
2.5. Evaluation of Caffeine Release by UV-Vis
2.6. Tensile Testing
2.7. Nanoindentation
3. Results
3.1. Evaluation of the Structure and Composition of the Samples
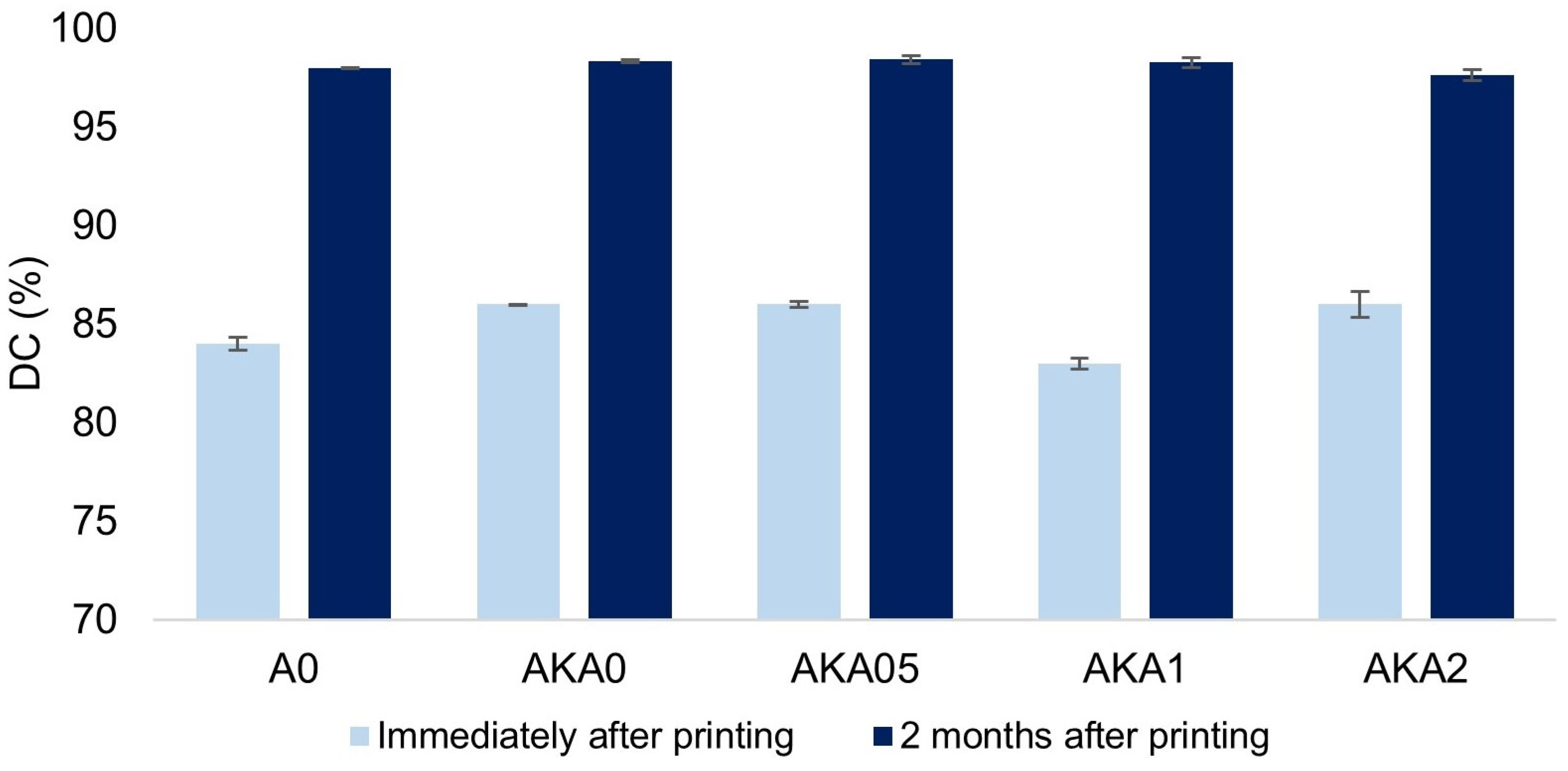
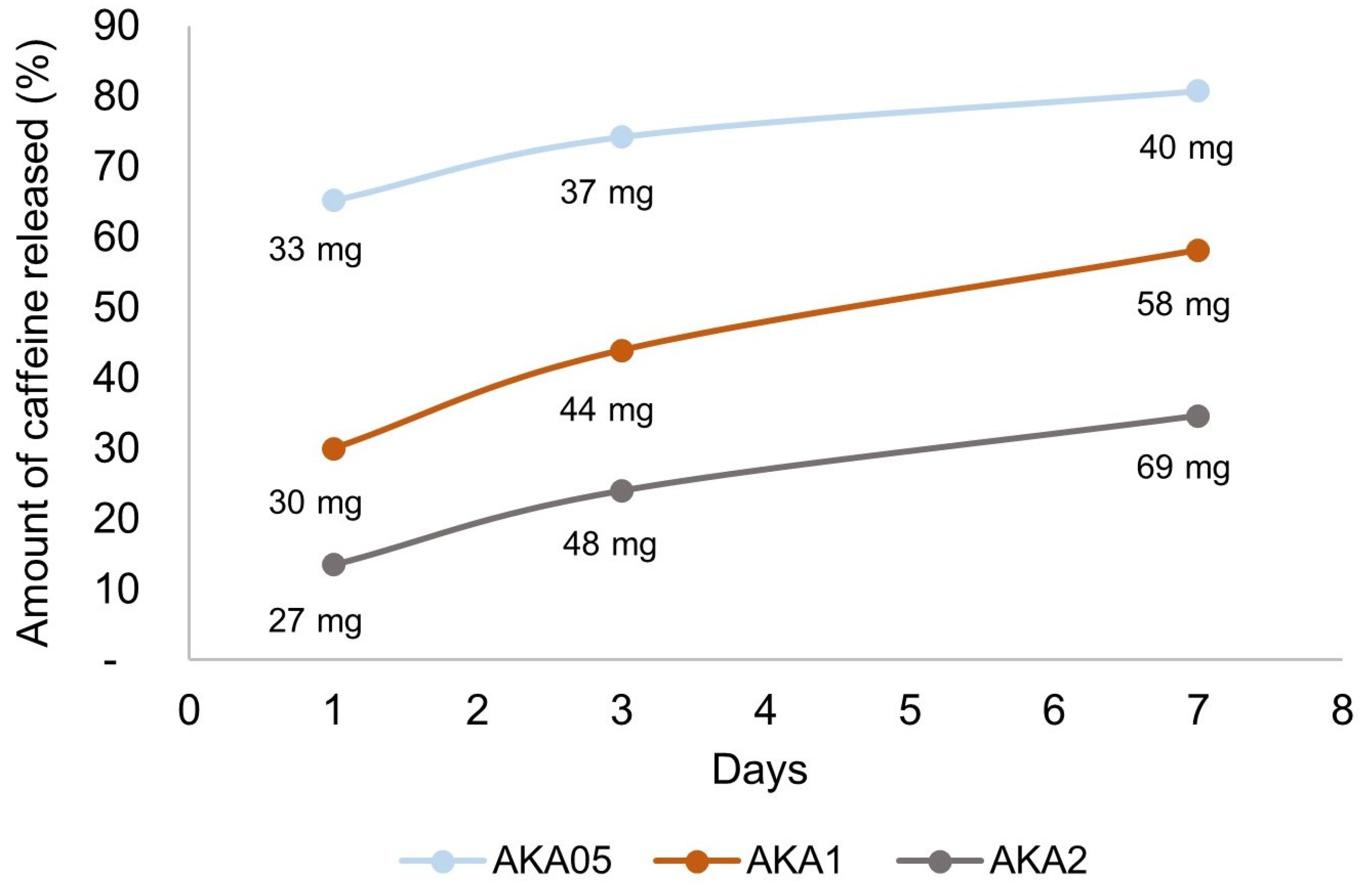
3.2. The Drug Release Process
3.3. Mechanical Properties of Composites
3.4. Advantages and Potential of the Developed System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomczak, D.; Wichniarek, R.; Kuczko, W.; Górski, F. Manufacturing of Thin-Walled, Complex Polymer Parts by DLP Printing—The Influence of Process Parameters on Crosslinking Density. Bull. Pol. Acad. Sci. Tech. Sci. 2023, 71, 145936. [Google Scholar] [CrossRef]
- Jiang, T.; Yan, B.; Jiang, M.; Xu, B.; Gao, S.; Xu, Y.; Yu, Y.; Ma, T.; Qin, T. Study of Forming Performance and Characterization of DLP 3D Printed Parts. Materials 2023, 16, 3847. [Google Scholar] [CrossRef] [PubMed]
- Barone, S.; Neri, P.; Paoli, A.; Razionale, A.V.; Tamburrino, F. Development of a DLP 3D Printer for Orthodontic Applications. Procedia Manuf. 2019, 38, 1017–1025. [Google Scholar] [CrossRef]
- Żukowska, M.; Górski, F.; Wichniarek, R.; Kuczko, W. Methodology of Low Cost Rapid Manufacturing of Anatomical Models with Material Imitation of Soft Tissues. Adv. Sci. Technol. Res. J. 2019, 13, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, G.; Medarević, D.; Adamov, I.; Pešić, N.; Kovačević, J.; Ibrić, S. Tailoring Atomoxetine Release Rate from DLP 3D-Printed Tablets Using Artificial Neural Networks: Influence of Tablet Thickness and Drug Loading. Molecules 2021, 26, 111. [Google Scholar] [CrossRef] [PubMed]
- Madzarevic, M.; Medarevic, D.; Vulovic, A.; Sustersic, T.; Djuris, J.; Filipovic, N.; Ibric, S. Optimization and Prediction of Ibuprofen Release from 3D DLP Printlets Using Artificial Neural Networks. Pharmaceutics 2019, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Abdella, S.; Youssef, S.H.; Afinjuomo, F.; Song, Y.; Fouladian, P.; Upton, R.; Garg, S. 3D Printing of Thermo-Sensitive Drugs. Pharmaceutics 2021, 13, 1524. [Google Scholar] [CrossRef]
- Adamov, I.; Medarević, D.; Ivković, B.; Ivković, A.; Ibrić, S. Digital Light Processing (DLP) 3D Printing Technique Applied in the Fabrication of Two-Layered Tablets: The Concept of a Combined Polypill. Arh. Farm. 2022, 72, 674–688. [Google Scholar] [CrossRef]
- Mau, R.; Reske, T.; Eickner, T.; Grabow, N.; Seitz, H. DLP 3D Printing of Dexamethasoneincorporated PEGDA-Based Photopolymers: Compressive Properties and Drug Release. Curr. Dir. Biomed. Eng. 2020, 6, 20203105. [Google Scholar] [CrossRef]
- Li, H.; Dai, J.; Wang, Z.; Zheng, H.; Li, W.; Wang, M.; Cheng, F. Digital Light Processing (DLP)-based (Bio)Printing Strategies for Tissue Modeling and Regeneration. Aggregate 2023, 4, 270. [Google Scholar] [CrossRef]
- Chen, C.H.; Shyu, V.B.H.; Chen, C.T. Dissolving Microneedle Patches for Transdermal Insulin Delivery in Diabetic Mice: Potential for Clinical Applications. Materials 2018, 11, 1625. [Google Scholar] [CrossRef]
- Ali, Z.; Türeyen, E.B.; Karpat, Y.; Çakmakci, M. Fabrication of Polymer Micro Needles for Transdermal Drug Delivery System Using DLP Based Projection Stereo-Lithography. Procedia CIRP 2016, 42, 87–90. [Google Scholar] [CrossRef]
- Huang, X.; Chang, Q.; Gao, J.; Lu, F. Sustained Release Microneedles: Materials and Applications in Facial Rejuvenation. Tissue Eng. Part B Rev. 2023, 29, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Arnett, P.; Bagde, A.; Azim, N.; Kouagou, E.; Singh, M.; Rajaraman, S. DLP 3D Printed “Intelligent” Microneedle Array (IμNA) for Stimuli Responsive Release of Drugs and Its in Vitro and Ex Vivo Characterization. J. Microelectromech. Syst. 2020, 29, 685–691. [Google Scholar] [CrossRef]
- Erkus, H.; Bedir, T.; Kaya, E.; Tinaz, G.B.; Gunduz, O.; Chifiriuc, M.C.; Ustundag, C.B. Innovative Transdermal Drug Delivery System Based on Amoxicillin-Loaded Gelatin Methacryloyl Microneedles Obtained by 3D Printing. Materialia 2023, 27, 101700. [Google Scholar] [CrossRef]
- Dardano, P.; Caliò, A.; Di Palma, V.; Bevilacqua, M.F.; Di Matteo, A.D.; Stefano, L. A Photolithographic Approach to Polymeric Microneedles Array Fabrication. Materials 2015, 8, 8661–8673. [Google Scholar] [CrossRef] [PubMed]
- Alafnan, A.; Seetharam, A.A.; Hussain, T.; Gupta, M.S.; Rizvi, S.M.D.; Moin, A.; Alamri, A.; Unnisa, A.; Awadelkareem, A.M.; Elkhalifa, A.E.O.; et al. Development and Characterization of PEGDA Microneedles for Localized Drug Delivery of Gemcitabine to Treat Inflammatory Breast Cancer. Materials 2022, 15, 7693. [Google Scholar] [CrossRef] [PubMed]
- Halz, M.; Kaszuba, M.; Gawel, D.; Jarosz, J.; Matykiewicz, P.; Bichowska, M. Influence of Caffeine Supplementation on Bench Press Performance—Review. Trends Sport Sci. 2021, 28, 69–82. [Google Scholar] [CrossRef]
- Chandran, R.; Mohd Tohit, E.R.; Stanslas, J.; Salim, N.; Tuan Mahmood, T.M. Investigation and Optimization of Hydrogel Microneedles for Transdermal Delivery of Caffeine. Tissue Eng. Part C Methods 2022, 28, 545–556. [Google Scholar] [CrossRef]
- Foudah, A.I.; Shakeel, F.; Salkini, M.A.; Alshehri, S.; Ghoneim, M.M.; Alam, P. A Green High-Performance Thin-Layer Chromatography Method for the Determination of Caffeine in Commercial Energy Drinks and Formulations. Materials 2022, 15, 2965. [Google Scholar] [CrossRef]
- Safety Data Sheet for Aqua Clear Resin by Phrozen. Available online: https://cdn.shopify.com/s/files/1/0436/6965/1618/files/SDS_Aqua_Clear.pdf?v=1686222900 (accessed on 31 October 2023).
- Tomczak, D.; Wichniarek, R.; Kuczko, W. Caffeine–Acrylic Resin DLP-Manufactured Composite as a Modern Biomaterial. Designs 2023, 7, 49. [Google Scholar] [CrossRef]
- ISO 527 Plastics; Determination of Tensile Properties. CEN: Brussels, Belgium, 2014.
- Li, X.; Wang, D.; Zhao, L.; Hou, X.; Liu, L.; Feng, B.; Li, M.; Zheng, P.; Zhao, X.; Wei, S. UV LED Curable Epoxy Soybean-Oil-Based Waterborne PUA Resin for Wood Coatings. Prog. Org. Coat. 2021, 151, 105942. [Google Scholar] [CrossRef]
- Luo, Q.; Wen, X.; Xu, R.; Liu, Z.; Xiang, H.; Li, Z.; Liu, X. Preparation and Properties of Novel Modified Waterborne Polyurethane Acrylate. Coatings 2022, 12, 1135. [Google Scholar] [CrossRef]
- Ugur, M.H.; Kılıç, H.; Berkem, M.L.; Güngör, A. Synthesis by UV-Curing and Characterisation of Polyurethane Acrylate-Lithium Salts-Based Polymer Electrolytes in Lithium Batteries. Chem. Pap. 2014, 68, 1561–1572. [Google Scholar] [CrossRef]
- Nezu, T.; Nagano-Takebe, F.; Endo, K. Designing an Antibacterial Acrylic Resin Using the Cosolvent Method —Effect of Ethanol on the Optical and Mechanical Properties of a Cold-Cure Acrylic Resin. Dent. Mater. J. 2017, 36, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Yamada, B. Free-Radical Addition Polymerization (Fundamental). In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Olowe, M.; Parupelli, S.K.; Desai, S. A Review of 3D-Printing of Microneedles. Pharmaceutics 2022, 14, 2693. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, M.; Fu, J.; Sun, Y.; Lu, C.; Quan, G.; Pan, X.; Wu, C. Recent Advances in Microneedles-Mediated Transdermal Delivery of Protein and Peptide Drugs. Acta Pharm. Sin. B 2021, 11, 2326–2343. [Google Scholar] [CrossRef]
| Amount of Resin (g) | Amount of Ethanol (mL) | Amount of Caffeine (mg) | |
|---|---|---|---|
| A0 | 50 | - | - |
| AKA0 | 50 | 10 | - |
| AKA05 | 50 | 10 | 50 |
| AKA1 | 50 | 10 | 100 |
| AKA2 | 50 | 10 | 200 |
| E (MPa) | Rm (MPa) | A (%) | |
|---|---|---|---|
| A0 AKA0 AKA05 | 778.0 ± 59 | 20.4 ± 0.3 | 18.9 ± 0.7 |
| 361.0 ± 7.8 | 8.5 ± 0.2 | 11.2 ± 1.9 | |
| 343.5 ± 5.9 | 8.2 ± 0.3 | 9.2 ± 1.8 | |
| AKA1 AKA2 | 346.3 ± 7.4 | 8.3 ± 0.5 | 9.2 ± 2.8 |
| 356.5 ± 12 | 8.6 ± 0.2 | 9.2 ± 2.3 |
| E (MPa) | Rm (MPa) | A (%) | |
|---|---|---|---|
| A0 AKA0 AKA05 | 742.2 ± 43 | 16.6 ± 0.6 | 18.6 ± 0.7 |
| 435.0 ± 20 | 10.4 ± 0.5 | 12.5 ± 4.7 | |
| 372.0 ± 25 | 9.1 ± 0.4 | 10.9 ± 2.6 | |
| AKA1 AKA2 | 367.5 ± 3.5 | 9.8 ± 0.7 | 12.0 ± 2.0 |
| 364.7 ± 14 | 8.9 ± 1.2 | 9.2 ± 0.4 |
| ER (GPa) | nH (MPa) | |
|---|---|---|
| A0 | 4.4 ± 0.2 | 141 ± 14 |
| AKA0 | 3.7 ± 0.2 | 109 ± 11 |
| AKA05 | 5.1 ± 0.2 | 142 ± 10 |
| AKA1 | 5.3 ± 0.4 | 159 ± 21 |
| AKA2 | 3.6 ± 0.1 | 94 ± 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, D.; Borysiak, S.; Kuczko, W.; Nowicka, A.B.; Osmałek, T.; Strzemiecka, B.; Wichniarek, R. Photopolymer-Based Composite with Substance Release Capability Manufactured Additively with DLP Method. Materials 2024, 17, 322. https://doi.org/10.3390/ma17020322
Tomczak D, Borysiak S, Kuczko W, Nowicka AB, Osmałek T, Strzemiecka B, Wichniarek R. Photopolymer-Based Composite with Substance Release Capability Manufactured Additively with DLP Method. Materials. 2024; 17(2):322. https://doi.org/10.3390/ma17020322
Chicago/Turabian StyleTomczak, Dorota, Sławomir Borysiak, Wiesław Kuczko, Ariadna B. Nowicka, Tomasz Osmałek, Beata Strzemiecka, and Radosław Wichniarek. 2024. "Photopolymer-Based Composite with Substance Release Capability Manufactured Additively with DLP Method" Materials 17, no. 2: 322. https://doi.org/10.3390/ma17020322






