Gamma-Camera Direct Imaging of the Plasma and On/Intra Cellular Distribution of the 99mTc-DPD-Fe3O4 Dual-Modality Contrast Agent in Peripheral Human Blood
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Radiolabeling of Fe3O4-DPD with 99mTc Radionuclide
2.3. Healthy Donors and Peripheral Whole Blood Collection
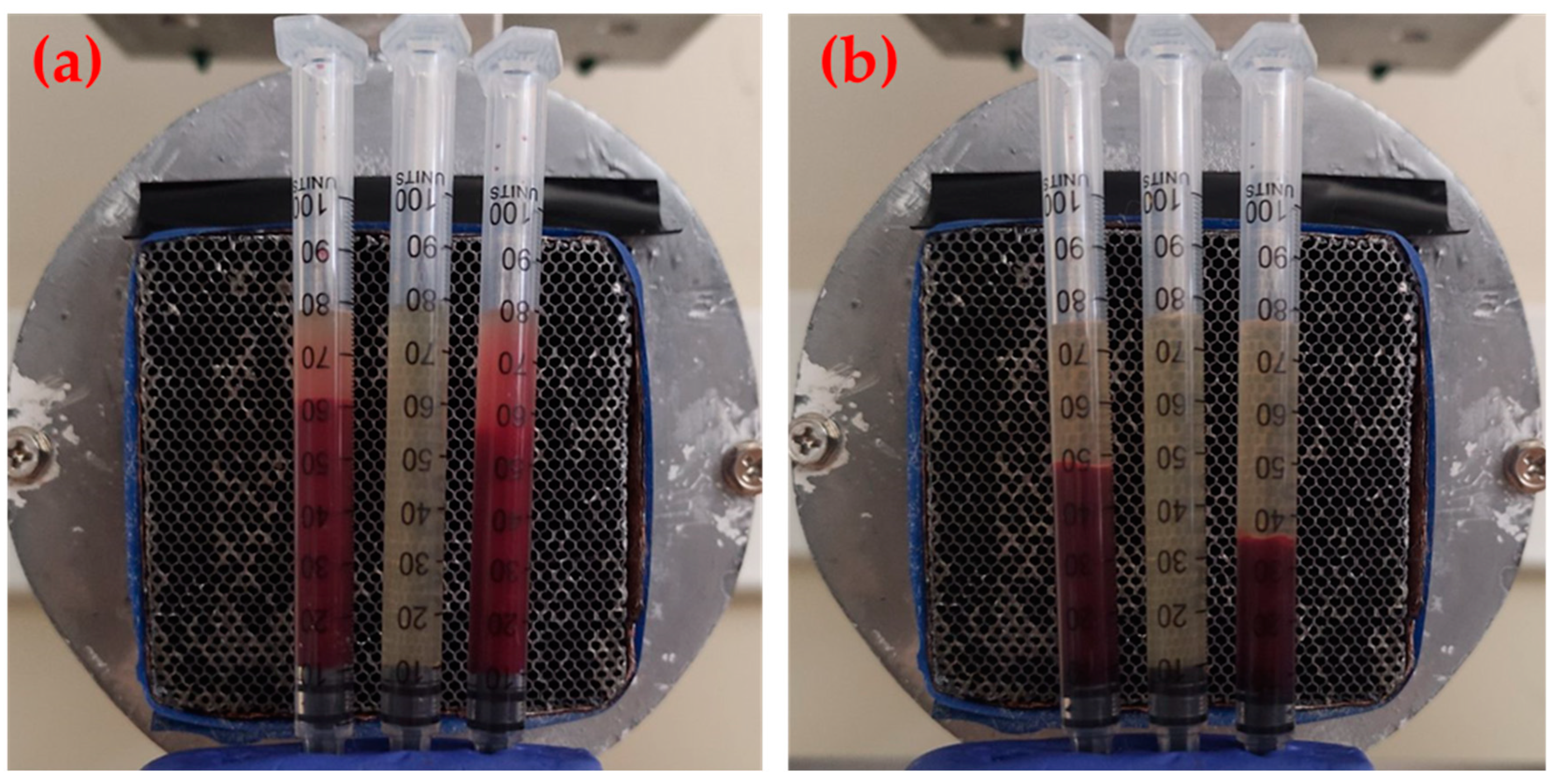
2.4. Preparation of Samples
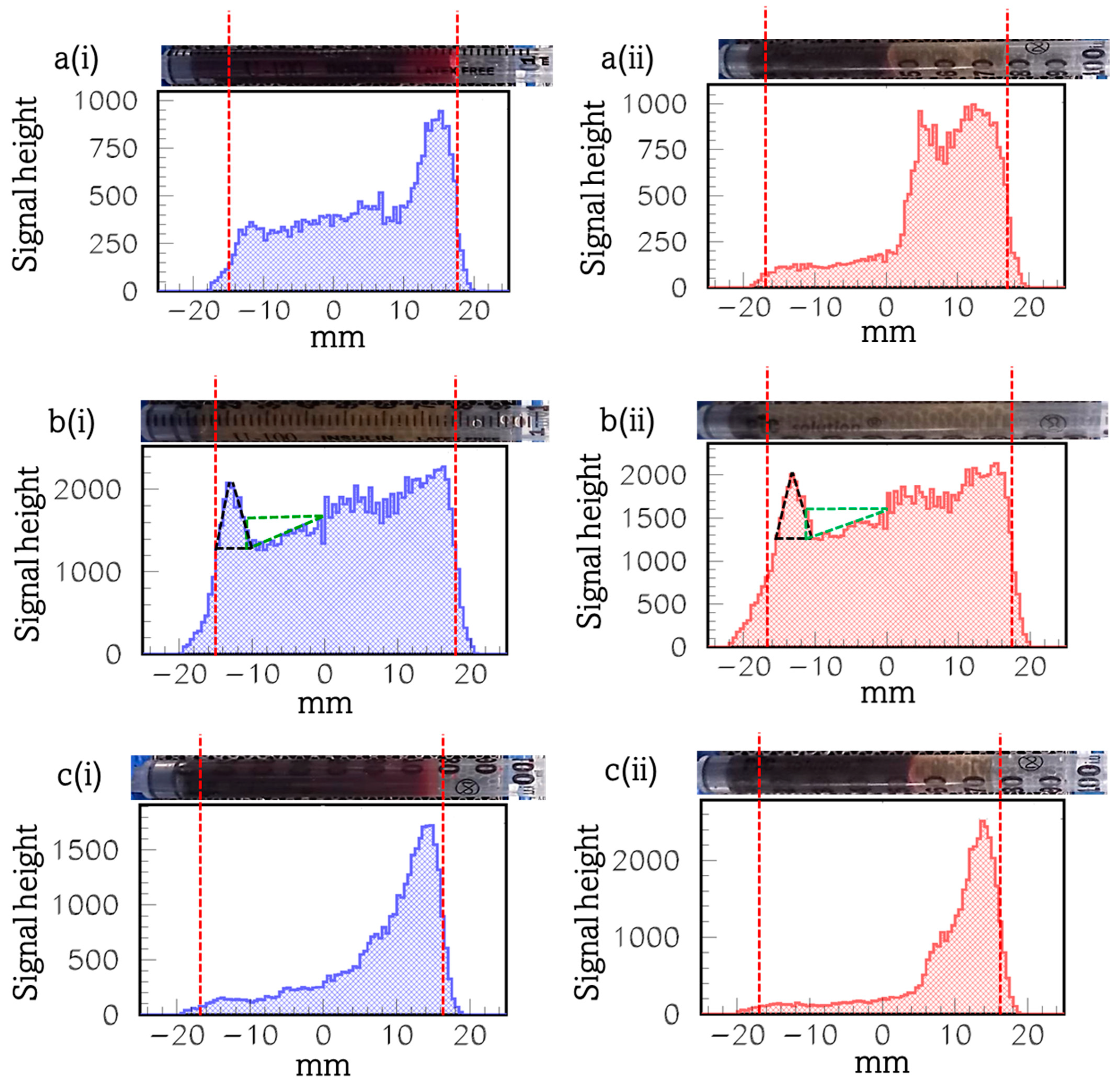
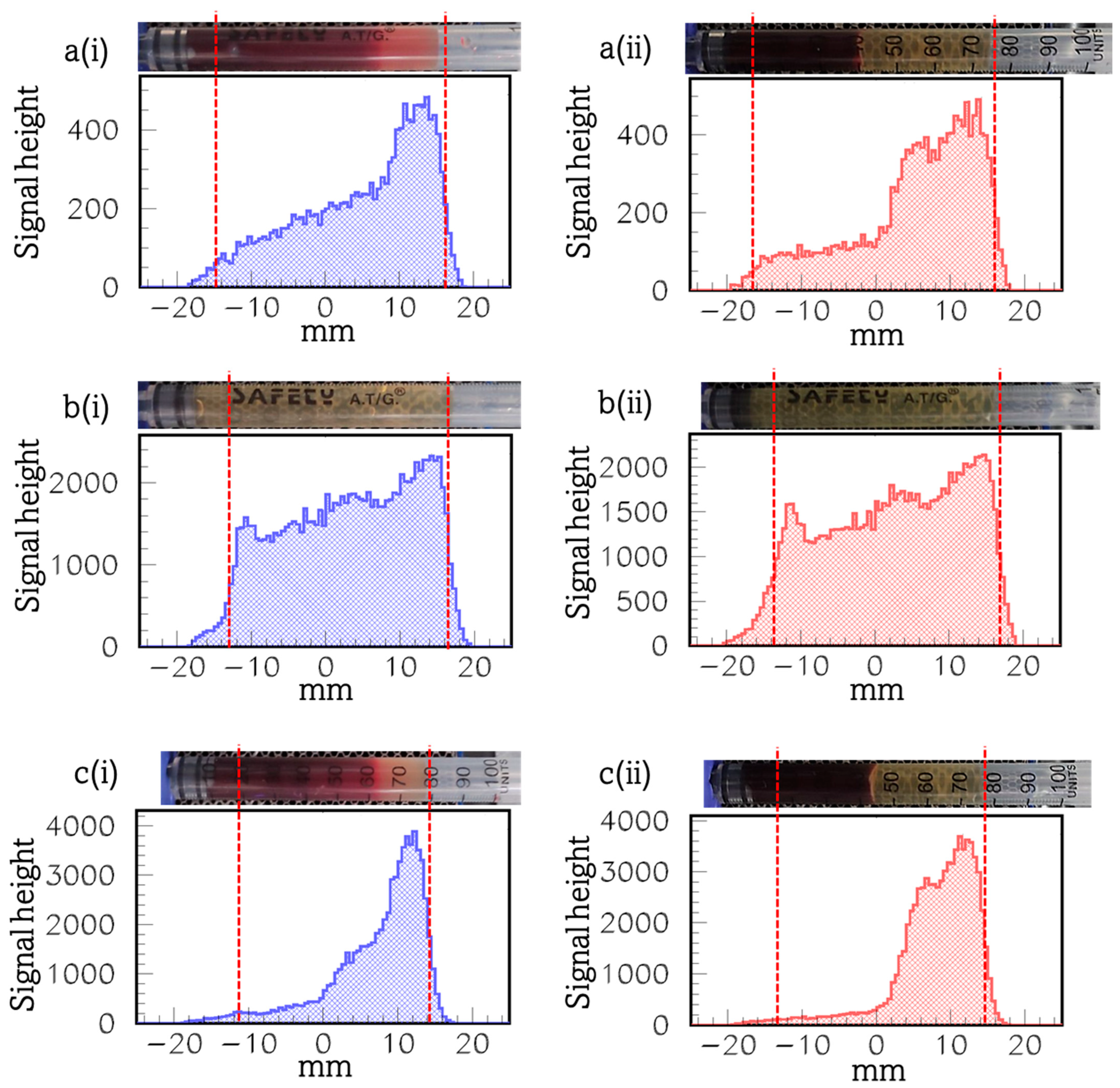
2.5. Gamma-Camera Imaging of Samples
2.6. Statistical Analysis
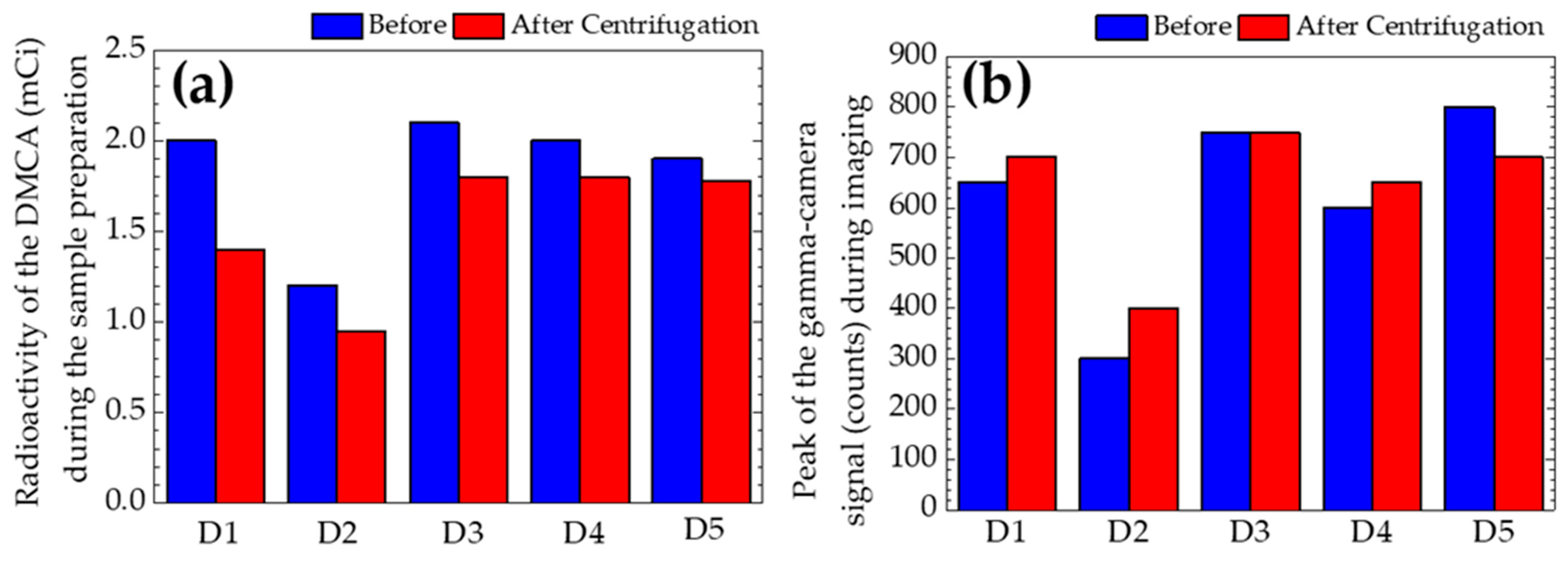
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.H.; Choi, Y.; Kim, S.; Cheon, J. Recent advances in magnetic nanoparticle-based multi-modal imaging. Chem. Soc. Rev. 2015, 44, 4501–4516. [Google Scholar] [CrossRef] [PubMed]
- Burke, B.P.; Cawthorne, C.; Archibald, S.J. Multimodal nanoparticle imaging agents: Design and applications. Phil. Trans. R. Soc. A 2017, 375, 20170261. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Zhang, Y.; Sun, J.; Cai, W. Molecular imaging and therapy of cancer with radiolabeled nanoparticles. Nano Today 2009, 4, 399–413. [Google Scholar] [CrossRef]
- Thomas, R.; Park, I.-K.; Jeong, Y.Y. Magnetic Iron Oxide Nanoparticles for Multimodal Imaging and Therapy of Cancer. Int. J. Mol. Sci. 2013, 14, 15910–15930. [Google Scholar] [CrossRef]
- Xing, Y.; Zhao, J.; Conti, P.S.; Chen, K. Radiolabeled Nanoparticles for Multimodality Tumor Imaging. Theranostics 2014, 4, 290–306. [Google Scholar] [CrossRef]
- Ai, F.; Ferreira, C.A.; Chen, F.; Cai, W. Engineering of radiolabeled iron oxide nanoparticles for dual modality imaging. WIREs Nanomed. Nanobiotechnol. 2016, 8, 619–630. [Google Scholar] [CrossRef]
- Pellico, J.; Ruiz-Cabello, J.; Herranz, F. Radiolabeled Iron Oxide Nanomaterials for Multimodal Nuclear Imaging and Positive Contrast Magnetic Resonance Imaging (MRI): A Review. ACS Appl. Nano Mater. 2023, 6, 20523–20538. [Google Scholar] [CrossRef]
- De Barros, A.L.B.; Tsourkas, A.; Saboury, B.; Cardoso, V.N.; Alavi, A. Emerging role of radiolabeled nanoparticles as an effective diagnostic technique. EJNMMI Res. 2012, 2, 39. [Google Scholar] [CrossRef]
- Goel, M.; Mackeyev, Y.; Krishnan, S. Radiolabeled nanomaterial for cancer diagnostics and therapeutics: Principles and concepts. Cancer Nano. 2023, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Braeken, Y.; Cheruku, S.; Ethirajan, A.; Maes, W. Conjugated Polymer Nanoparticles for Bioimaging. Materials 2017, 10, 1420. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.C.; Daniel-da-Silva, A.L.; Tavares, D.S.; Calatayud, M.P.; Goya, G.F.; Trindade, T. Fluorescent Magnetic Bioprobes by Surface Modification of Magnetite Nanoparticles. Materials 2013, 6, 3213–3225. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Miao, Q.; Liang, G. Quantum Dots as Multifunctional Materials for Tumor Imaging and Therapy. Materials 2013, 6, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.M.; Thorat, N.D.; Shete, P.B.; Bedge, P.A.; Gavde, S.; Joshi, M.G.; Tofail, S.A.M.; Bohara, R.A. Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticles. Biochem. Biophys. Rep. 2018, 13, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Hanot, C.C.; Choi, Y.S.; Anani, T.B.; Soundarrajan, D.; David, A.E. Effects of Iron-Oxide Nanoparticle Surface Chemistry on Uptake Kinetics and Cytotoxicity in CHO-K1 Cells. Int. J. Mol. Sci. 2016, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Ling-Ling, J.; Yun, Z.; Gang, L. Toxicity of superparamagnetic iron oxide nanoparticles: Research strategies and implications for nanomedicine. Chin. Phys. B 2013, 22, 127503. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, C.; Wu, L.; Bai, X.; Zhai, S. Cytotoxicity-Related Bioeffects Induced by Nanoparticles: The Role of Surface Chemistry. Front. Bioeng. Biotechnol. 2019, 7, 414. [Google Scholar] [CrossRef]
- Patil, U.S.; Adireddy, S.; Jaiswal, A.; Mandava, S.; Lee, B.R.; Chrisey, D.B. In Vitro/In Vivo Toxicity Evaluation and Quantification of Iron Oxide Nanoparticles. Int. J. Mol. Sci. 2015, 16, 24417–24450. [Google Scholar] [CrossRef]
- Liao, S.H.; Liu, C.H.; Bastakoti, B.P.; Suzuki, N.; Chang, Y.; Yamauchi, Y.; Lin, F.H.; Wu, K.C.-W. Functionalized magnetic iron oxide/alginate core-shell nanoparticles for targeting hyperthermia. Int. J. Nanomed. 2015, 10, 3315–3328. [Google Scholar] [CrossRef]
- Cótica, L.F.; Freitas, V.F.; Dias, G.S.; Santos, I.A.; Vendrame, S.C.; Khalil, N.M.; Mainardes, R.M.; Staruch, M.; Jain, M. Simple and facile approach to synthesize magnetite nanoparticles and assessment of their effects on blood cells. J. Magn. Magn. Mater. 2012, 324, 559–563. [Google Scholar] [CrossRef]
- Sivalingam, S.; Santhanakrishnan, M.; Parthasarathy, V. Synthesis, Characterization and In-Vitro Toxicity Assessment of Superparamagnetic Iron Oxide Nanoparticles for Biomedical Applications. Nano. Biomed. Eng. 2022, 14, 201–207. [Google Scholar] [CrossRef]
- Karageorgou, M.A.; Bouziotis, P.; Vranješ-Djurić, S.; Stamopoulos, D. Hemocompatibility of gallium-68 labeled iron oxide nanoparticles coated with 2,3-dicarboxypropane1,1-diphosphonic acid. Mater. Sci. Eng. C 2020, 115, 111121. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Sethu, S.; Vadukumpully, S.; Zhong, S.; Lim, C.T.; Hande, M.P.; Valiyaveettil, S. Investigations on the structural damage in human erythrocytes exposed to silver, gold, and platinum nanoparticles. Adv. Funct. Mater. 2010, 20, 1233–1242. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, X.; Zhang, G.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.Y. Interaction of mesoporous silica nanoparticles with human red blood cell membranes: Size and surface effects. ACS Nano 2011, 5, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Woo, H.J.; Kim, Y.H.; Lee, H.J.; Park, K.H.; Park, S.; Youn, B. Optimizing hemocompatibility of surfactant-coated silver nanoparticles in human erythrocytes. J. Nanosci. Nanotechnol. 2012, 12, 6168–6175. [Google Scholar] [CrossRef]
- Chen, L.Q.; Fang, L.; Ling, J.; Ding, C.Z.; Kang, B.; Huang, C.Z. Nanotoxicity of silver nanoparticles to red blood cells: Size dependent adsorption, uptake, and hemolytic activity. Chem. Res. Toxicol. 2015, 28, 501–509. [Google Scholar] [CrossRef]
- He, Z.; Liu, J.; Du, L. The unexpected effect of PEGylated gold nanoparticles on the primary function of erythrocytes. Nanoscale 2014, 6, 9017–9024. [Google Scholar] [CrossRef]
- Ran, Q.; Xiang, Y.; Liu, Y.; Xiang, L.; Li, F.; Deng, X.; Xiao, Y.; Chen, L.; Chen, L.; Li, Z. Eryptosis indices as a novel predictive parameter for biocompatibility of Fe3O4 magnetic nanoparticles on erythrocytes. Sci. Rep. 2015, 5, 16209. [Google Scholar] [CrossRef]
- Liu, T.; Bai, R.; Zhou, H.; Wang, R.; Liu, J.; Zhao, Y.; Chen, C. The effect of size and surface ligands of iron oxide nanoparticles on blood compatibility. RSC Adv. 2020, 10, 7559–7569. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.O.; Bañobre-López, M.; Gallo, J.; Tavares, P.B.; Silva, A.M.T.; Lima, R.; Gomes, H.T. Haemocompatibility of iron oxide nanoparticles synthesized for theranostic applications: A high-sensitivity microfluidic tool. J. Nanopart. Res. 2016, 18, 194. [Google Scholar] [CrossRef]
- Dai, Q.; Wilhelm, S.; Ding, D.; Syed, A.M.; Sindhwani, S.; Zhang, Y.; Chen, Y.Y.; MacMillan, P.; Chan, W.C.W. Quantifying the ligand-coated nanoparticle delivery to cancer cells in solid tumors. ACS Nano 2018, 12, 8423–8435. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yang, Y.; Xu, P.; Xu, D.; Liu, Y.; Castillo, R.; Yan, R.; Ren, J.; Zhou, G.; Liu, C.; et al. Real-time quantification of cell internalization kinetics by functionalized bioluminescent nanoprobes. Adv. Mater. 2019, 31, 1902469. [Google Scholar] [CrossRef] [PubMed]
- Braun, G.B.; Friman, T.; Pang, H.-B.; Pallaoro, A.; Hurtado de Mendoza, T.; Willmore, A.-M.A.; Kotamraju, V.R.; Mann, A.P.; She, Z.G.; Sugahara, K.N.; et al. Etchable plasmonic nanoparticle probes to image and quantify cellular internalization. Nat. Mater. 2014, 13, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Karageorgou, M.A.; Rapsomanikis, A.N.; Mirković, M.; Vranješ-Ðurić, S.; Stiliaris, E.; Bouziotis, P.; Stamopoulos, D. 99mTc-Labeled Iron Oxide Nanoparticles as Dual-Modality Contrast Agent: A Preliminary Study from Synthesis to Magnetic Resonance and Gamma-Camera Imaging in Mice Models. Nanomaterials 2022, 12, 2728. [Google Scholar] [CrossRef]
- Karageorgou, M.A.; Vranješ-Ðurić, S.; Radović, M.; Lyberopoulou, A.; Antić, B.; Rouchota, M.; Gazouli, M.; Loudos, G.; Xanthopoulos, S.; Sideratou, Z.; et al. Gallium-68 Labeled Iron Oxide Nanoparticles Coated with 2,3-Dicarboxypropane-1,1-diphosphonic Acid as a Potential PET/MR Imaging Agent: A Proof-of-Concept Study. Contrast Media Mol. Imaging 2017, 2017, 6951240. [Google Scholar] [CrossRef]
- Spanoudaki, V.; Giokaris, N.D.; Karabarbounis, A.; Loudos, G.K.; Maintas, D.; Papanicolas, C.N.; Paschalis, P.; Stiliaris, E. Design and development of a position-sensitive γ-camera for SPECT imaging based on PCI electronics. Nucl. Instrum. Methods Phys. Res. A 2004, 527, 151–156. [Google Scholar] [CrossRef]
- Thanasas, D.; Georgiou, E.; Giokaris, N.; Karabarbounis, A.; Maintas, D.; Papanicolas, C.N.; Polychronopoulou, A.; Stiliaris, E. A correction method of the spatial distortion in planar images from γ-Camera systems. JINST 2009, 4, P06012. [Google Scholar] [CrossRef]
| Donors | Sex | Age (Years) |
|---|---|---|
| Donor 1 | Male | 50 |
| Donor 2 | Female | 60 |
| Donor 3 | Female | 27 |
| Donor 4 | Male | 54 |
| Donor 5 | Female | 32 |
| Donors | Whole Blood Indices | Physiological Range | Results |
|---|---|---|---|
| Donor 1 | Red blood cells (RBCs) | 4.50–5.70 M/μL | 5.14 |
| Hemoglobin (HGB) | 11–17 g/dL | 16.00 | |
| Hematocrit (HCT) | 35–55% | 44.90 | |
| Mean corpuscular volume (MCV) | 76–97 fL | 87.40 | |
| Mean corpuscular hemoglobin concentration (MCHC) | 26–37 g/dL | 35.70 | |
| Mean corpuscular hemoglobin (MCH) | 26–36 pg | 31.20 | |
| Red cell distribution width (RDW-CV) | 11.00–16.00% | 13.80 | |
| White blood cells (WBCs) | 4.00–11.00 K/μL | 6.60 | |
| Platelets (PLTs) | 150–400 K/μL | 237 | |
| Mean platelet volume (MPV) | 6.00–12.00 fL | 8.70 | |
| Plateletcrit (PCT) | 0.17–2.82% | 2.06 | |
| Donor 2 | Red blood cells (RBCs) | 4.50–5.70 M/μL | 4.52 |
| Hemoglobin (HGB) | 11–17 g/dL | 13.90 | |
| Hematocrit (HCT) | 35–55% | 42.80 | |
| Mean corpuscular volume (MCV) | 76–97 fL | 94.60 | |
| Mean corpuscular hemoglobin concentration (MCHC) | 26–37 g/dL | 32.50 | |
| Mean corpuscular hemoglobin (MCH) | 26–36 pg | 30.80 | |
| Red cell distribution width (RDW-CV) | 11.00–16.00% | 12.40 | |
| White blood cells (WBCs) | 4.00–11.00 K/μL | 6.04 | |
| Platelets (PLTs) | 150–400 K/μL | 317 | |
| Mean platelet volume (MPV) | 6.00–12.00 fL | 7.80 | |
| Plateletcrit (PCT) | 0.17–2.82% | 2.01 | |
| Donor 3 | Red blood cells (RBCs) | 4.50–5.70 M/μL | 4.90 |
| Hemoglobin (HGB) | 11–17 g/dL | 13.20 | |
| Hematocrit (HCT) | 35–55% | 41.30 | |
| Mean corpuscular volume (MCV) | 76–97 fL | 83.80 | |
| Mean corpuscular hemoglobin concentration (MCHC) | 26–37 g/dL | 32.00 | |
| Mean corpuscular hemoglobin (MCH) | 26–36 pg | 26.80 | |
| Red cell distribution width (RDW-CV) | 11.00–16.00% | 13.10 | |
| White blood cells (WBCs) | 4.00–11.00 K/μL | 5.40 | |
| Platelets (PLTs) | 150–400 K/μL | 229 | |
| Mean platelet volume (MPV) | 6.00–12.00 fL | 8.60 | |
| Plateletcrit (PCT) | 0.17–2.82% | 0.42 | |
| Donor 4 | Red blood cells (RBCs) | 4.50–5.70 M/μL | 4.59 |
| Hemoglobin (HGB) | 11–17 g/dL | 14.30 | |
| Hematocrit (HCT) | 35–55% | 43.00 | |
| Mean corpuscular volume (MCV) | 76–97 fL | 93.00 | |
| Mean corpuscular hemoglobin concentration (MCHC) | 26–37 g/dL | 33.40 | |
| Mean corpuscular hemoglobin (MCH) | 26–36 pg | 31.10 | |
| Red cell distribution width (RDW-CV) | 11.00–16.00% | 13.90 | |
| White blood cells (WBCs) | 4.00–11.00 K/μL | 7.73 | |
| Platelets (PLTs) | 150–400 K/μL | 187 | |
| Mean platelet volume (MPV) | 6.00–12.00 fL | 13.70 | |
| Plateletcrit (PCT) | 0.17–2.82% | 0.26 | |
| Donor 5 | Red blood cells (RBCs) | 4.50–5.70 M/μL | 4.78 |
| Hemoglobin (HGB) | 11–17 g/dL | 12.8 | |
| Hematocrit (HCT) | 35–55% | 39.9 | |
| Mean corpuscular volume (MCV) | 76–97 fL | 83.50 | |
| Mean corpuscular hemoglobin concentration (MCHC) | 26–37 g/dL | 32.08 | |
| Mean corpuscular hemoglobin (MCH) | 26–36 pg | 26.78 | |
| Red cell distribution width (RDW-CV) | 11.00–16.00% | 14.20 | |
| White blood cells (WBCs) | 4.00–11.00 K/μL | 8.20 | |
| Platelets (PLTs) | 150–400 K/μL | 269 | |
| Mean platelet volume (MPV) | 6.00–12.00 fL | 7.50 | |
| Plateletcrit (PCT) | 0.17–2.82% | 0.21 |
| Donors | Radioactivity of the DMCA (mCi) (MV ± SD) | Peak of the Signal (MV ± SD) |
|---|---|---|
| Donor 1 | 1.70 ± 0.42 | 675 ± 35 |
| Donor 2 | 1.08 ± 0.18 | 350 ± 71 |
| Donor 3 | 1.95 ± 0.21 | 750 ± 0 |
| Donor 4 | 1.90 ± 0.14 | 625 ± 35 |
| Donor 5 | 1.84 ± 0.08 | 750 ± 71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karageorgou, M.-A.; Apostolopoulou, A.; Tomazinaki, M.-E.; Stanković, D.; Stiliaris, E.; Bouziotis, P.; Stamopoulos, D. Gamma-Camera Direct Imaging of the Plasma and On/Intra Cellular Distribution of the 99mTc-DPD-Fe3O4 Dual-Modality Contrast Agent in Peripheral Human Blood. Materials 2024, 17, 335. https://doi.org/10.3390/ma17020335
Karageorgou M-A, Apostolopoulou A, Tomazinaki M-E, Stanković D, Stiliaris E, Bouziotis P, Stamopoulos D. Gamma-Camera Direct Imaging of the Plasma and On/Intra Cellular Distribution of the 99mTc-DPD-Fe3O4 Dual-Modality Contrast Agent in Peripheral Human Blood. Materials. 2024; 17(2):335. https://doi.org/10.3390/ma17020335
Chicago/Turabian StyleKarageorgou, Maria-Argyro, Adamantia Apostolopoulou, Mina-Ermioni Tomazinaki, Dragana Stanković, Efstathios Stiliaris, Penelope Bouziotis, and Dimosthenis Stamopoulos. 2024. "Gamma-Camera Direct Imaging of the Plasma and On/Intra Cellular Distribution of the 99mTc-DPD-Fe3O4 Dual-Modality Contrast Agent in Peripheral Human Blood" Materials 17, no. 2: 335. https://doi.org/10.3390/ma17020335
APA StyleKarageorgou, M.-A., Apostolopoulou, A., Tomazinaki, M.-E., Stanković, D., Stiliaris, E., Bouziotis, P., & Stamopoulos, D. (2024). Gamma-Camera Direct Imaging of the Plasma and On/Intra Cellular Distribution of the 99mTc-DPD-Fe3O4 Dual-Modality Contrast Agent in Peripheral Human Blood. Materials, 17(2), 335. https://doi.org/10.3390/ma17020335







