Possibility of High Ionic Conductivity and High Fracture Toughness in All-Dislocation-Ceramics
Abstract
:1. Introduction
2. High Ionic Conductivity without Dendrite Formation
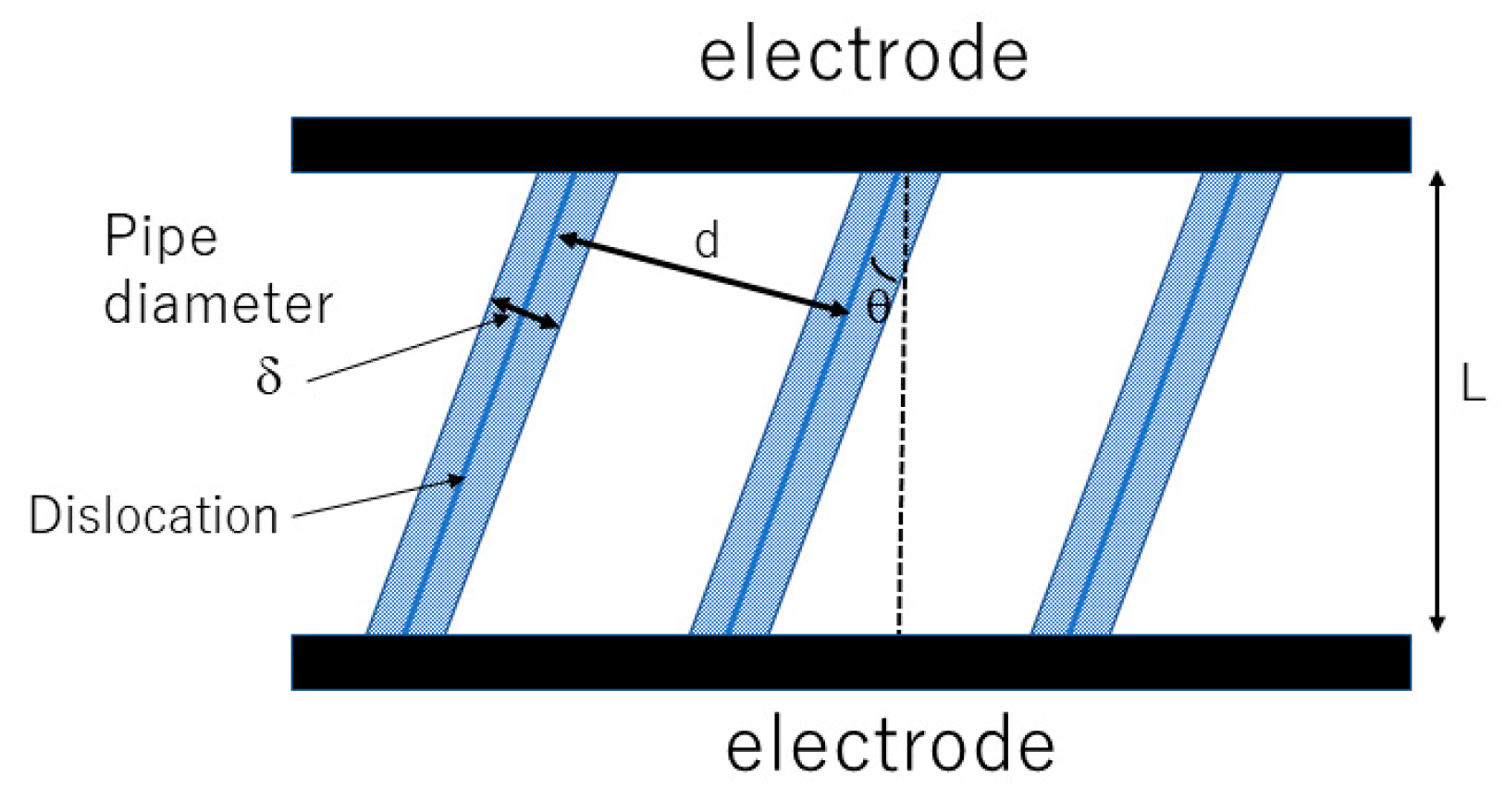
2.1. High Ionic Conductivity in All-Dislocation-Ceramics
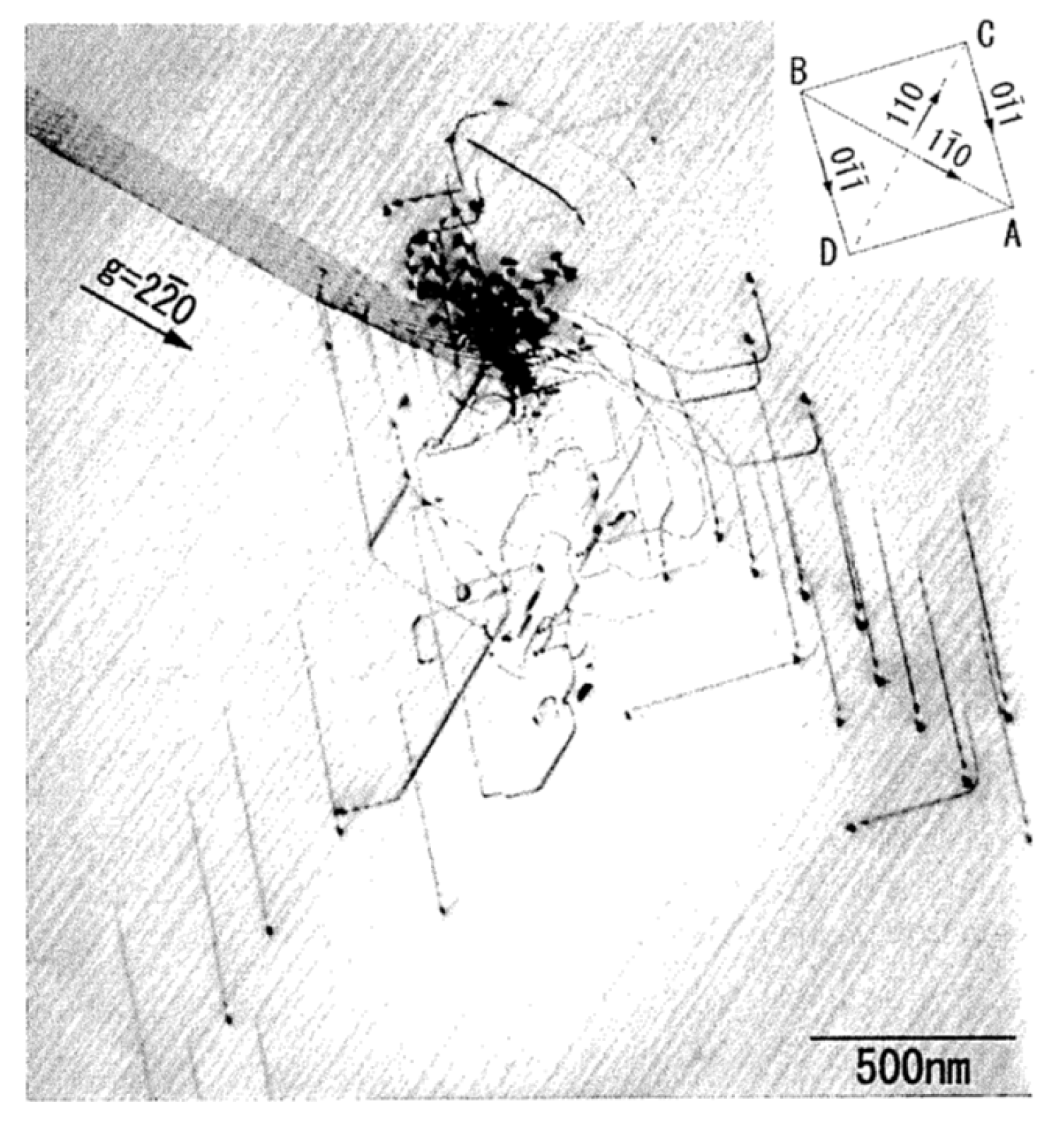
2.2. Theoretical Upper Limit of Dislocation Density in Ceramics
3. Mechanism of Toughening by Pre-Existing Dislocations
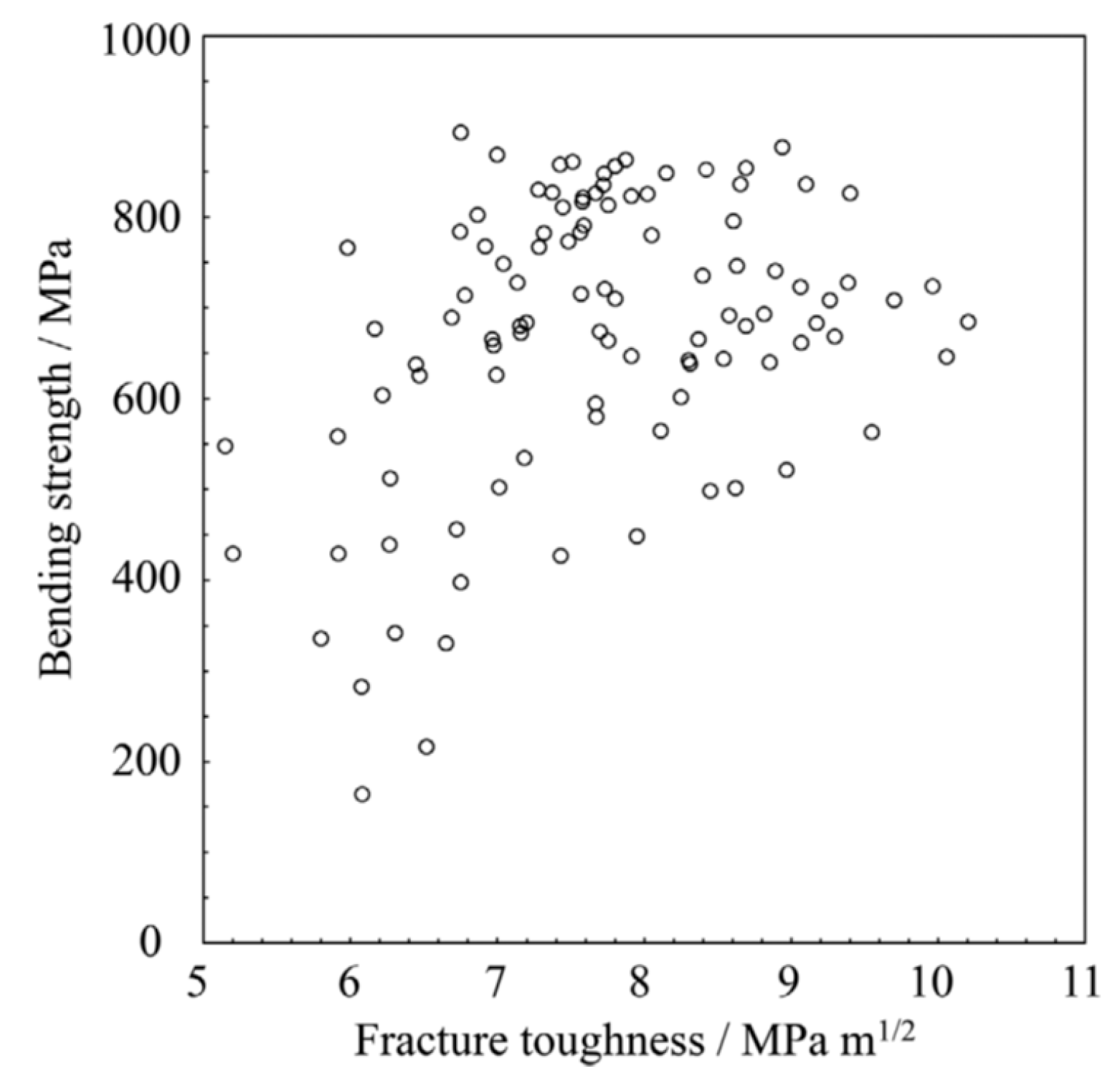
3.1. Fracture Toughness
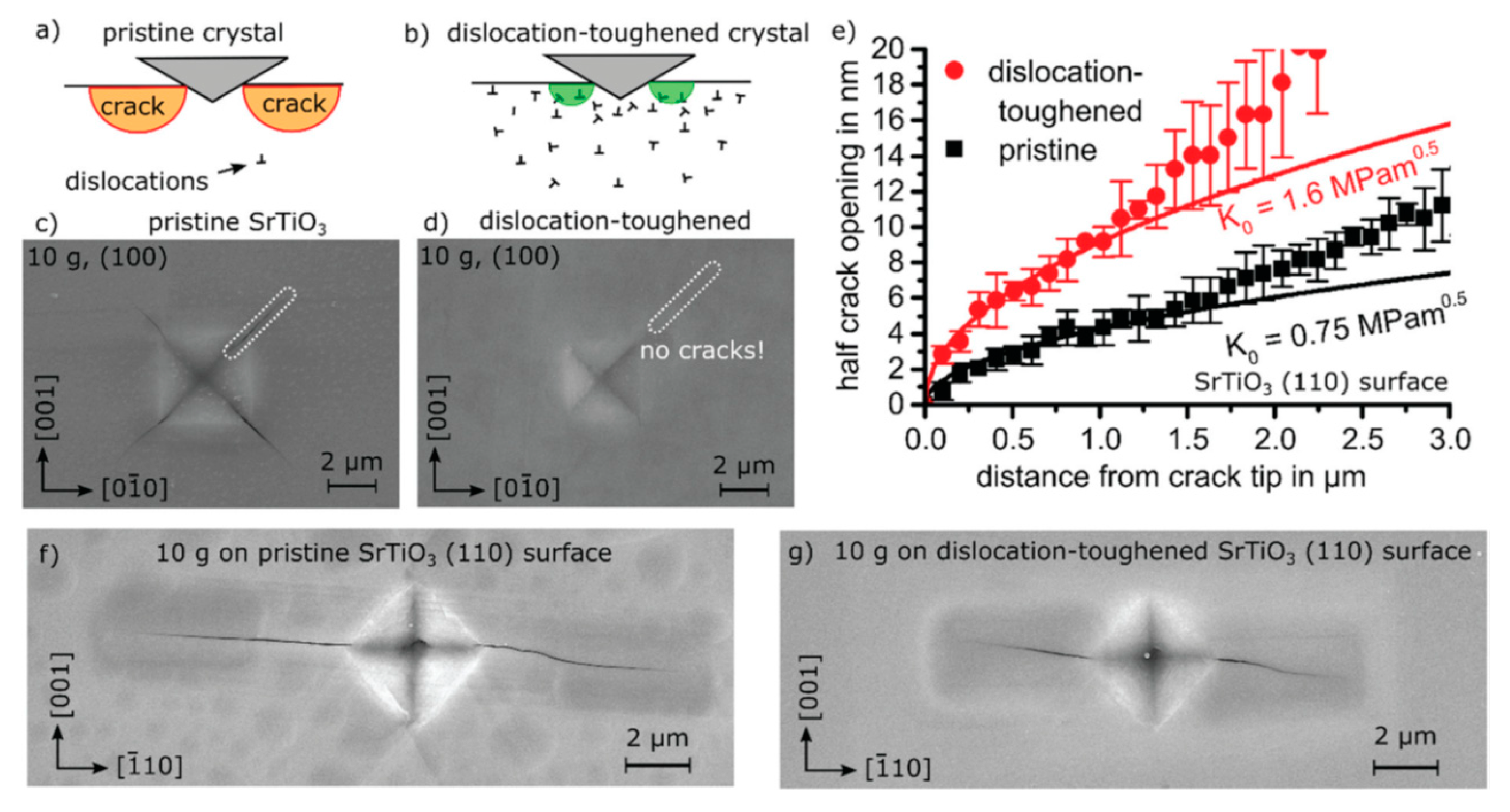
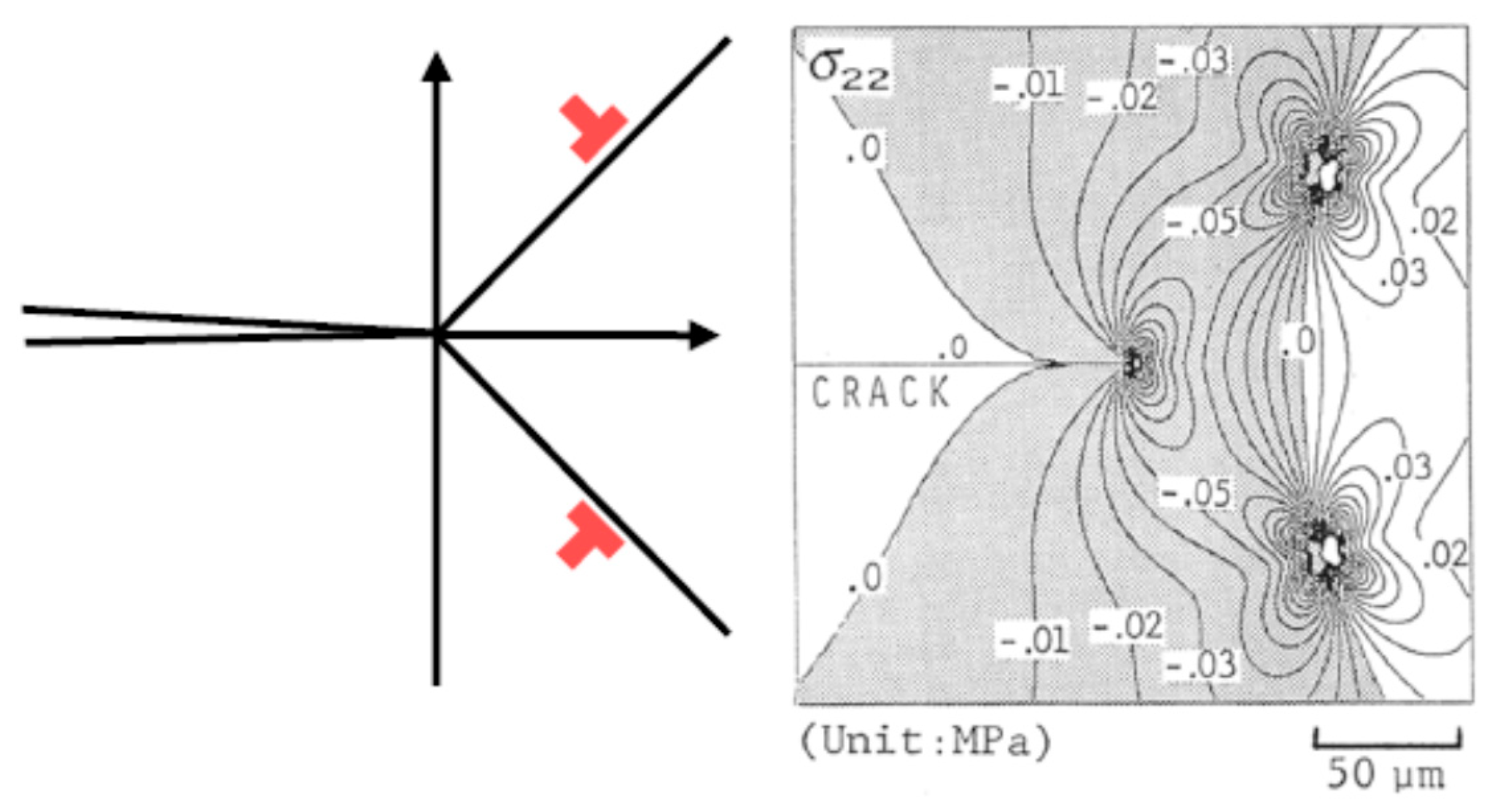
3.2. Toughening Mechanism by Pre-Existing Dislocations
4. Conclusions and Unsolved Problems
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| crack length (m) | |
| amplitude of reduction in ionic conductivity by crossing dislocations (S/m) | |
| magnitude of the Burgers vector (m) | |
| dimensionless factor which depends on specimen geometry | |
| distance between neighboring dislocations (m) | |
| characteristic diameter of pre-existing microcracks (m) | |
| critical diameter of a pre-existing microcrack for fracture (m) | |
| diameter of a pre-existing penny-shaped (circular) microcrack (m) | |
| Young’s modulus (Pa) | |
| applied electric field (V/m) | |
| shear modulus (Pa) | |
| number concentration of pre-existing microcracks with diameter between and ( | |
| hardness of a specimen measured by the Vickers indentation test | |
| ionic current density on a dislocation (A/) | |
| ionic current density on the other area (A/) | |
| stress intensity factor caused by dislocations and takes negative value for shielding dislocations () | |
| crack-tip toughness () | |
| stress intensity factor () | |
| fracture toughness () | |
| half-diagonal of the indentation (m) | |
| shape factor of the Weibull distribution | |
| number concentration of pre-existing microcracks ( | |
| density of dislocations () | |
| upper limit of the dislocation density () | |
| number of pre-existing microcracks in a specimen | |
| probability of fracture | |
| distance from a crack tip (m) | |
| strength ratio | |
| cross section of a dislocation pipe () | |
| half-opening of a crack (m) | |
| volume of a specimen () | |
| numerical factor defined in Equation (9) | |
| surface energy of a solid specimen (J/) | |
| diameter of a dislocation pipe (m) | |
| critical diameter of a dislocation pipe (m) | |
| diameter of a dislocation core (m) | |
| angle of parallel straight dislocations relative to the electrode (rad) | |
| angle defined in Figure 5 (rad) | |
| mean ionic conductivity (S/m) | |
| ionic conductivity in the bulk (S/m) | |
| ionic conductivity along a dislocation (S/m) | |
| ionic conductivity across a dislocation (S/m) | |
| Poisson’s ratio | |
| distance from a crack tip along the center line of the crack (m) | |
| applied compressive stress (Pa) | |
| magnitude of the applied stress on a specimen (Pa) | |
| compressive strength (Pa) | |
| tensile strength (Pa) | |
| stress component defined in Figure 5 (Pa) | |
| stress component defined in Figure 5 (Pa) | |
| stress component defined in Figure 5 (Pa) | |
| numerical factor used in Equation (24) |
References
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Sunandana, C.S. Introduction to Solid State Ionics Phenomenology and Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Julien, C.; Nazri, G.-A. Solid State Batteries: Materials Design and Optimization; Springer Sci. Business Media: New York, NY, USA, 1994. [Google Scholar]
- Kotobuki, M.; Song, S.; Chen, C.; Lu, L. Ceramic Electrolytes for All-Solid-State Li Batteries; World Scientific: Singapore, 2018. [Google Scholar]
- Randau, S.; Weber, D.A.; Kötz, O.; Koerver, R.; Braun, P.; Weber, A.; Ivers-Tiffee, E.; Adermann, T.; Kulisch, J.; Zeier, W.G.; et al. Benchmarking the performance of all-solid-state lithium batteries. Nat. Energy 2020, 5, 259–270. [Google Scholar] [CrossRef]
- Kataoka, K.; Nagata, H.; Akimoto, J. Lithium-ion conducting oxide single crystal as solid electrolyte for advanced lithium battery application. Sci. Rep. 2018, 8, 9965. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, N. Ionic conductivity and ion transport mechanisms of solid-state lithium-ion battery electrolytes: A review. Energy Sci. Eng. 2022, 10, 1643–1671. [Google Scholar] [CrossRef]
- Southall, J.P.; Hubbard, H.V.S.A.; Johnston, S.F.; Rogers, V.; Davies, G.R.; McIntyre, J.E.; Ward, I.M. Ionic conductivity and viscosity correlations in liquid electrolytes for incorporation into PVDF gel electrolytes. Solid State Ionics 1996, 85, 51–60. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Sun, H.; Mei, L.; Liang, J.; Zhao, Z.; Lee, C.; Fei, H.; Ding, M.; Lau, J.; Li, M.; Wang, C.; et al. Three-dimensional holey-graphene/niobia composite architectures for ultrahigh-rate energy storage. Science 2017, 356, 599–604. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef]
- Yuan, C.; Lu, W.; Xu, J. Unlocking the electrochemical-mechanical coupling behaviors of dendrite growth and crack propagation in all-solid-state batteries. Adv. Energy Mater. 2021, 11, 2101807. [Google Scholar] [CrossRef]
- Athanasiou, C.E.; Jin, M.Y.; Ramirez, C.; Padture, N.P.; Sheldon, B.W. High-toughness inorganic solid electrolytes via the use of reduced graphene oxide. Matter 2020, 3, 212–229. [Google Scholar] [CrossRef]
- Yan, G.; Malzbender, J.; Fu, S.; Gross, J.P.; Yu, S.; Eichel, R.-A.; Schwaiger, R. Fracture behavior of solid electrolyte LATP material based on micro-pillar splitting method. J. Eur. Ceram. Soc. 2021, 41, 5240–5247. [Google Scholar] [CrossRef]
- Ke, X.; Wang, Y.; Ren, G.; Yuan, C. Towards rational mechanical design of inorganic solid electrolytes for all-solid-state lithium ion batteries. Energy Stor. Mater. 2020, 26, 313–324. [Google Scholar] [CrossRef]
- Yasui, K.; Hamamoto, K. Influence of dislocations on ionic conductivity and dendrite formation in solid electrolytes. Phys. Scr. 2023, 98, 045811. [Google Scholar] [CrossRef]
- Yasui, K.; Hamamoto, K. Theoretical upper limit of dislocation density in slightly-ductile single-crystal ceramics. J. Phys. Condens. Matter 2023, 35, 455701. [Google Scholar] [CrossRef] [PubMed]
- Porz, L.; Klomp, A.J.; Fang, X.; Li, N.; Yildirim, C.; Detlefs, C.; Bruder, E.; Höfling, M.; Rheinheimer, W.; Patterson, E.A.; et al. Dislocation-toughened ceramics. Mater. Horiz. 2021, 8, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Preuβ, O.; Bruder, E.; Lu, W.; Zhuo, F.; Minnert, C.; Zhang, J.; Rödel, J.; Fang, X. Dislocation toughening in single-crystal KNbO3. J. Am. Ceram. Soc. 2023, 106, 4371–4381. [Google Scholar] [CrossRef]
- Salem, M.N.; Ding, K.; Rödel, J.; Fang, X. Thermally enhanced dislocation density improves both hardness and fracture toughness in single-crystal SrTiO3. J. Am. Ceram. Soc. 2023, 106, 1344–1355. [Google Scholar] [CrossRef]
- Tang, X.; Lagerlöf, K.P.D.; Heuer, A.H. Determination of pipe diffusion coefficients in undoped and magnesia-doped sapphire (a-Al2O3): A study based on annihilation of dislocation dipoles. J. Am. Ceram. Soc. 2003, 86, 560–565. [Google Scholar] [CrossRef]
- Otsuka, K.; Matsunaga, K.; Nakamura, A.; Ii, S.; Kuwabara, A.; Yamamoto, T.; Ikuhara, Y. Effects of dislocations on the oxygen ionic conduction in yttria stabilized zirconia. Mater. Trans. 2004, 45, 2042–2047. [Google Scholar] [CrossRef]
- Armstrong, M.D.; Lan, K.-W.; Guo, Y.; Perry, N.H. Dislocation-mediated conductivity in oxides: Progress, challenges, and opportunities. ACS Nano 2021, 15, 9211–9221. [Google Scholar] [CrossRef] [PubMed]
- Legros, M.; Dehm, G.; Arzt, E.; Balk, T.J. Observation of giant diffusivity along dislocation cores. Science 2008, 319, 1646–1649. [Google Scholar] [CrossRef] [PubMed]
- Börgers, J.M.; Kler, J.; Ran, K.; Larenz, E.; Weirich, T.E.; Dittmann, R.; De Souza, R.A. Faster diffusion of oxygen along dislocations in (La,Sr)MnO3+d is a space-charge phenomenon. Adv. Funct. Mater. 2021, 31, 2105647. [Google Scholar] [CrossRef]
- Sakaguchi, I.; Yurimoto, H.; Sueno, S. Impurities dislocation diffusion in single-crystal MgO. Mater. Sci. Eng. 1992, B13, L1–L4. [Google Scholar] [CrossRef]
- Heitjans, P.; Indris, S. Diffusion and ionic conduction in nanocrystalline ceramics. J. Phys. Condens. Matter 2003, 15, R1257–R1289. [Google Scholar] [CrossRef]
- Rabier, J.; Puls, M.P. Atomistic model calculations of pipe-diffusion mechanisms in MgO. Philos. Mag. 1985, 52, 461–473. [Google Scholar] [CrossRef]
- Miller, K.M.; Ingle, K.W.; Crocker, A.G. A computer simulation study of pipe diffusion in body centred cubic metals. Acta Metall. 1981, 29, 1599–1606. [Google Scholar] [CrossRef]
- Pun, G.P.P.; Mishin, Y. A molecular dynamics study of self-diffusion in the cores of screw and edge dislocations in aluminum. Acta Mater. 2009, 57, 5531–5542. [Google Scholar] [CrossRef]
- Luther, L.C. Diffusion along dislocations. J. Chem. Phys. 1965, 43, 2213–2218. [Google Scholar] [CrossRef]
- Porz, L.; Frömling, T.; Nakamura, A.; Li, N.; Maruyama, R.; Matsunaga, K.; Gao, P.; Simons, H.; Dietz, C.; Rohnke, M.; et al. Conceptual framework for dislocation-modified conductivity in oxide ceramics deconvoluting mesoscopic structure, core, and space charge exemplified for SrTiO3. ACS Nano 2021, 15, 9355–9367. [Google Scholar] [CrossRef]
- Marrocchelli, D.; Sun, L.; Yildiz, B. Dislocations in SrTiO3: Easy to reduce but not so fast for oxygen transport. J. Am. Chem. Soc. 2015, 137, 4735–4748. [Google Scholar] [CrossRef] [PubMed]
- Adepalli, K.K.; Yang, J.; Maier, J.; Tuller, H.L.; Yildiz, B. Tunable oxygen diffusion and electronic conduction in SrTiO3 by dislocation-induced space charge fields. Adv. Funct. Mater. 2017, 27, 1700243. [Google Scholar] [CrossRef]
- Garbrecht, M.; Saha, B.; Schroeder, J.L.; Hultman, L.; Sands, T.D. Dislocation-pipe diffusion in nitride superlattices observed in direct atomic resolution. Sci. Rep. 2017, 7, 46092. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.J.; Tarafdar, S.; Middya, T.R. Effective medium theory for ionic conductivity in polycrystalline solid electrolytes. Solid State Ion. 1997, 95, 283–288. [Google Scholar] [CrossRef]
- Ikuhara, Y. Nanowire design by dislocation technology. Prog. Mater. Sci. 2009, 54, 770–791. [Google Scholar] [CrossRef]
- Muhammad, Q.K.; Porz, L.; Nakamura, A.; Matsunaga, K.; Rohnke, M.; Janek, J.; Rödel, J.; Frömling, T. Donor and acceptor-like self-doping by mechanically induced dislocations in bulk TiO2. Nano Energy 2021, 85, 105944. [Google Scholar] [CrossRef]
- Muhammad, Q.K.; Bishara, H.; Porz, L.; Dietz, C.; Ghidelli, M.; Dehm, G.; Frömling, T. Dislocation-mediated electronic conductivity in rutile. Mater. Today Nano 2022, 17, 100171. [Google Scholar] [CrossRef]
- Li, Y.; Fang, X.; Tochigi, E.; Oshima, Y.; Hoshino, S.; Tanaka, T.; Oguri, H.; Ogata, S.; Ikuhara, Y.; Matsunaga, K.; et al. Shedding new light on the dislocation-mediated plasticity in wurtzite ZnO single crystals by photoindentation. J. Mater. Sci. Technol. 2023, 156, 206–216. [Google Scholar] [CrossRef]
- Ikuhara, Y. Oxide ceramics with high density dislocations and their properties. Mater. Trans. 2009, 50, 1626–1632. [Google Scholar] [CrossRef]
- Ren, P.; Höfling, M.; Koruza, J.; Lauterbach, S.; Jiang, X.; Frömling, T.; Khatua, D.K.; Dietz, C.; Porz, L.; Ranjan, R.; et al. High temperature creep-mediated functionality in polycrystalline barium titanate. J. Am. Ceram. Soc. 2020, 103, 1891–1902. [Google Scholar] [CrossRef]
- Höfling, M.; Zhou, X.; Riemer, L.M.; Bruder, E.; Liu, B.; Zhou, L.; Groszewicz, P.B.; Zhuo, F.; Xu, B.-X.; Durst, K.; et al. Control of polarization in bulk ferroelectrics by mechanical dislocation imprint. Science 2021, 372, 861–964. [Google Scholar] [CrossRef] [PubMed]
- Johanning, M.; Porz, L.; Dong, J.; Nakamura, A.; Li, J.-F.; Rödel, J. Influence of dislocations on thermal conductivity of strontium titanate. Appl. Phys. Lett. 2020, 117, 021902. [Google Scholar] [CrossRef]
- Kissel, M.; Porz, L.; Frömling, T.; Nakamura, A.; Rödel, J.; Alexe, M. Enhanced photoconductivity at dislocations in SrTiO3. Adv. Mater. 2022, 34, 2203032. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.; Pelc, D.; Anderson, Z.W.; Klein, A.; Spieker, R.J.; Yue, L.; Das, B.; Ramberger, J.; Lukas, M.; Liu, Y.; et al. superconductivity and ferroelectric quantum criticality in plastically deformed strontium titanate. Nat. Mater. 2022, 21, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Kuwabara, A.; Nakamura, A.; Yamamoto, T.; Matsunaga, K.; Ikuhara, Y. Dislocation-enhanced ionic conductivity of yttria-stabilized zirconia. Appl. Phys. Lett. 2003, 82, 877–879. [Google Scholar] [CrossRef]
- Nakamura, A.; Matsunaga, K.; Tohma, J.; Yamamoto, T.; Ikuhara, Y. Conducting nanowires in insulating ceramics. Nat. Mater. 2003, 2, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Porz, L.; Knez, D.; Scherer, M.; Ganschow, S.; Kothleitner, G.; Rettenwander, D. Dislocations in ceramic electrolytes for solid-state Li Batteries. Sci. Rep. 2021, 11, 8949. [Google Scholar] [CrossRef]
- Jin, L.; Guo, X.; Jia, C.L. TEM study of <110>-type 35.26° dislocations specially induced by polishing of SrTiO3 single crystals. Ultramicroscopy 2013, 134, 77–85. [Google Scholar] [CrossRef]
- Okafor, C.; Ding, K.; Zhou, X.; Durst, K.; Rödel, J.; Fang, X. Mechanical tailoring of dislocation densities in SrTiO3 at room temperature. J. Am. Ceram. Soc. 2022, 105, 2399–2402. [Google Scholar] [CrossRef]
- Szot, K.; Rodenbücher, C.; Bihlmayer, G.; Speier, W.; Ishikawa, R.; Shibata, N.; Ikuhara, Y. Influence of Dislocations in Transition Metal Oxides on Selected Physical and Chemical Properties. Crystals 2018, 8, 241. [Google Scholar] [CrossRef]
- Amelinckx, S.; Delavignette, P. Dislocations in layer structures. In Direct Observation of Imperfections in Crystals; Newkirk, J.B., Wernick, J.H., Eds.; Intersci. Pub. (John Wiley & Sons): New York, NY, USA, 1962; pp. 295–356. [Google Scholar]
- Kasemchainan, J.; Zekoll, S.; Jolly, D.S.; Ning, Z.; Hartley, G.O.; Marrow, J.; Bruce, P.G. Critical stripping current leads to dendrite formation on plating in lithium anode solid electrolyte cells. Nat. Mater. 2019, 18, 1105–1111. [Google Scholar] [CrossRef]
- Li, G.; Monroe, C.W. Dendrite nucleation in lithium-conductive ceramics. Phys. Chem. Chem. Phys. 2019, 21, 20354–20359. [Google Scholar] [CrossRef]
- Wu, B.; Wang, S.; Lochala, J.; Desrochers, D.; Liu, B.; Zhang, W.; Yang, J.; Xiao, J. The role of the solid electrolyte interphase layer in preventing Li dendrite growth in solid-state batteries. Energy Environ. Sci. 2018, 11, 1803–1810. [Google Scholar] [CrossRef]
- Porz, L.; Swamy, T.; Sheldon, B.W.; Rettenwander, D.; Frömling, T.; Thaman, H.L.; Berendts, S.; Uecker, R.; Carter, W.C.; Chiang, Y.-M. Mechanism of lithium metal penetration through inorganic solid electrolytes. Adv. Energy Mater. 2017, 7, 1701003. [Google Scholar] [CrossRef]
- Shishvan, S.S.; Fleck, N.A.; McMeeking, R.M.; Deshpande, V.S. Growth rate of lithium filaments in ceramic electrolytes. Acta Mater. 2020, 196, 444–455. [Google Scholar] [CrossRef]
- Shishvan, S.S.; Fleck, N.A.; McMeeking, R.M.; Deshpande, V.S. Dendrite as climb dislocations in ceramic electrolytes: Initiation of growth. J. Power Sources 2020, 456, 227989. [Google Scholar] [CrossRef]
- Cheng, E.J.; Sharafi, A.; Sakamoto, J. Intergranular Li metal propagation through polycrystalline Li6.25Al0.25La3Zr2O12 ceramic electrolyte. Electrochim. Acta 2017, 223, 85–91. [Google Scholar] [CrossRef]
- Maier, J.; Prill, S.; Reichert, B. Space charge effects in polycrystalline, micropolycrystalline and thin film samples: Application to AgCl and AgBr. Solid State Ion. 1988, 28–30, 1465–1469. [Google Scholar] [CrossRef]
- Tuller, H.L. Ionic conduction in nanocrystalline materials. Solid State Ion. 2000, 131, 143–157. [Google Scholar] [CrossRef]
- Bellino, M.G.; Lamas, D.G.; de Reca, N.E.W. Enhanced ionic conductivity in nanostructured, heavily doped ceria ceramics. Adv. Funct. Mater. 2006, 16, 107–113. [Google Scholar] [CrossRef]
- Anderson, T.L. Fracture Mechanics, 4th ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Shih, C.J.; Meyers, M.A.; Nesterenko, V.F.; Chen, S.J. Damage evolution in dynamic deformation of silicon carbide. Acta Mater. 2000, 48, 2399–2420. [Google Scholar] [CrossRef]
- Dai, J.; Su, H.; Wang, Z.; Xu, J.; Fu, Y.; Chen, J. Damage formation mechanisms of sintered silicon carbide during single-diamond grinding. Ceram. Int. 2021, 47, 28419–28428. [Google Scholar] [CrossRef]
- Yasui, K. Critical roles of impurities and imperfections in various phases of materials. Materials 2023, 16, 1612. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Nix, W.D. Imperfections in Crystalline Solids; Cambridge Univ. Press: Cambridge, UK, 2016. [Google Scholar]
- Bailey, J.E.; Hirsch, P.B. The dislocation distribution, flow stress, and stored energy in cold-worked polycrystalline silver. Philos. Mag. 1960, 5, 485–497. [Google Scholar] [CrossRef]
- François, D.; Pineau, A.; Zaoui, A. Mechanical Behaviour of Materials, Volume II: Viscoplasticity, Damage, Fracture and Contact Mechanics; Springer-Science+Business Media: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Cai, M. Practical estimates of tensile strength and Hoek-Brown strength parameter mi of brittle rocks. Rock Mech. Rock Eng. 2010, 43, 167–184. [Google Scholar] [CrossRef]
- Sheorey, P.R. Empirical Rock Failure Criteria; A. A. Balkema: Cape Town, South Africa, 1997. [Google Scholar]
- Lee, S.; Kim, S.; Hwang, B.; Lee, B.S.; Lee, C.G. Effect of carbide distribution on the fracture toughness in the transition temperature region of an SA 508 steel. Acta Mater. 2002, 50, 4755–4762. [Google Scholar] [CrossRef]
- Tanguy, B.; Besson, J.; Pineau, A. Comment on “Effect of carbide distribution on the fracture toughness in the transition temperature region of an SA 508 steel”. Scr. Mater. 2003, 49, 191–197. [Google Scholar] [CrossRef]
- Pineau, A. Development of the local approach to fracture over the past 25 years: Theory and applications. Int. J. Fract. 2006, 138, 139–166. [Google Scholar] [CrossRef]
- Anderson, P.M.; Hirth, J.P.; Lothe, J. Theory of Dislocations, 3rd ed.; Cambridge Univ. Press: Cambridge, UK, 2017. [Google Scholar]
- Munz, D.; Fett, T. Ceramics: Mechanical Properties, Failure Behaviour, Materials Selection; Springer: Berlin, Germany, 1999. [Google Scholar]
- Chiang, Y.-M.; Birnie, D.P., III; Kingery, W.D. Physical Ceramics; John Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Reichel, F.; Jeurgens, L.P.H.; Mittemeijer, E.J. The thermodynamic stability of amorphous oxide overgrowths on metals. Acta Mater. 2008, 56, 659–674. [Google Scholar] [CrossRef]
- Canepa, P.; Dawson, J.A.; Gautam, G.S.; Statham, J.M.; Parker, S.C.; Islam, M.S. Particle morphology and lithium segregation to surfaces of the Li7La3Zr2O12 solid electrolyte. Chem. Mater. 2018, 30, 3019–3027. [Google Scholar] [CrossRef]
- Barsoum, M.W. Fundamentals of Ceramics, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Pelleg, J. Mechanical Properties of Materials; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Tada, H.; Paris, P.C.; Irwin, G.R. The Stress Analysis of Cracks Handbook, 3rd ed.; ASME Press: New York, NY, USA, 2000. [Google Scholar]
- Murakami, Y. (Ed.) Stress Intensity Factors Handbook, Volume 1 and 2; Pergamon Press: Oxford, UK, 1987. [Google Scholar]
- Niu, H.; Niu, S.; Oganov, A.R. Simple and accurate model of fracture toughness of solids. J. Appl. Phys. 2019, 125, 065105. [Google Scholar] [CrossRef]
- Jackman, S.D.; Cutler, R.A. Effect of microcracking on ionic conductivity in LATP. J. Power Sources 2012, 218, 65–72. [Google Scholar] [CrossRef]
- Nonemacher, J.F.; Arinicheva, Y.; Yan, G.; Finsterbusch, M.; Krüger, M.; Malzbender, J. Fracture toughness of single grains and polycrystalline Li7La3Zr2O12 electrolyte material based on a pillar splitting method. J. Eur. Ceram. Soc. 2020, 40, 3057–3064. [Google Scholar] [CrossRef]
- Ghosh, S.; Tarafder, M.; Sivaprasad, S.; Tarafder, S. Experimental and numerical study of ball indentation for evaluation of mechanical properties and fracture toughness of structural steel. Trans. Indian Inst. Met. 2010, 63, 617–622. [Google Scholar] [CrossRef]
- Courtney, T.H. Mechanical Behavior of Materials, 2nd ed.; Waveland Press: Long Grove, IL, USA, 2005. [Google Scholar]
- Cho, D.-H.; Kim, Y.-W.; Kim, W. Strength and fracture toughness of in situ-toughened silicon carbide. J. Mater. Sci. 1997, 32, 4777–4782. [Google Scholar] [CrossRef]
- Furushima, R.; Nakashima, Y.; Maruyama, Y.; Hirao, K.; Ohji, T.; Fukushima, M. Artificial intelligence-based determination of fracture toughness and bending strength of silicon nitride ceramics. J. Am. Ceram. Soc. 2023, 106, 4944–4954. [Google Scholar] [CrossRef]
- Rice, R.W. Mechanical Properties of Ceramics and Composites; Marcel Dekker: New York, NY, USA, 2000. [Google Scholar]
- Inoue, R.; Yang, J.M.; Kakisawa, H.; Kagawa, Y. Mode I fracture toughness of short carbon fiber-dispersed SiC matrix composite fabricated by melt infiltration process. Ceram. Int. 2013, 39, 8341–8346. [Google Scholar] [CrossRef]
- Du, J.; Zhang, H.; Geng, Y.; Ming, W.; He, W.; Ma, J.; Cao, Y.; Li, X.; Liu, K. A review on machining of carbon fiber reinforced ceramic matrix composites. Ceram. Int. 2019, 45, 18155–18166. [Google Scholar] [CrossRef]
- Seidel, J.; Rödel, J. Measurement of crack tip toughness in alumina as a function of grain size. J. Am. Ceram. Soc. 1997, 80, 433–438. [Google Scholar] [CrossRef]
- Knowles, K.M. The plane strain Young’s modulus in cubic materials. J. Elast. 2017, 128, 147–173. [Google Scholar] [CrossRef]
- Niihara, K.; Morena, R.; Hasselman, D.P.H. Evaluation of KIC of brittle solids by the indentation method with low crack-to-indent ratios. J. Mater. Sci. Lett. 1982, 1, 13–16. [Google Scholar] [CrossRef]
- Niihara, K. A fracture mechanics analysis of indentation-induced Palmqvist crack in ceramics. J. Mater. Sci. Lett. 1983, 2, 221–223. [Google Scholar] [CrossRef]
- Patterson, E.A.; Major, M.; Donner, W.; Durst, K.; Webber, K.G.; Rödel, J. Temperature-dependent deformation and dislocation density in SrTiO3 (001) single crystals. J. Am. Ceram. Soc. 2016, 99, 3411–3420. [Google Scholar] [CrossRef]
- Higashida, K.; Tanaka, M.; Sadamatsu, S. Effect of crack-tip shielding by dislocations on fracture toughness—In relation to hydrogen embrittlement. ISIJ Int. 2022, 62, 2074–2080. [Google Scholar] [CrossRef]
- Xu, Y.B.; Ha, K.F.; Wang, Z.G.; Wang, X.H.; Li, J. Dislocation distribution near the crack tip of I and II modes in bulk aluminum single crystal. J. Appl. Phys. 1991, 69, 203–206. [Google Scholar] [CrossRef]
- Narita, N.; Higashida, K.; Torii, T.; Miyaki, S. Crack-tip shielding by dislocations and fracture toughness in NaCl crystals. Mater. Trans. 1989, 30, 895–907. [Google Scholar] [CrossRef]
- Ohr, S.M. Antishielding dislocations at a crack tip. Scr. Metall. 1987, 21, 1681–1684. [Google Scholar] [CrossRef]
- Hirsch, P.B. Crack-tip plasticity and quasi-brittle fracture of single crystals. In Plastic Deformation of Ceramics; Bradt, R.C., Brookes, C.A., Routbort, J.L., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 1–19. [Google Scholar]
- Ohr, S.M. An electron microscope study of crack tip deformation and its impact on the dislocation theory of fracture. Mater. Sci. Eng. 1985, 72, 1–35. [Google Scholar] [CrossRef]
- Bitzek, E.; Gumbsch, P. Mechanisms of dislocation multiplication at crack tips. Acta Mater. 2013, 61, 1394–1403. [Google Scholar] [CrossRef]
- Higashida, K.; Narita, N.; Tanaka, M.; Morikawa, T.; Miura, Y.; Onodera, R. Crack tip dislocations in silicon characterized by high-voltage electron microscopy. Philos. Mag. 2002, 82, 3263–3273. [Google Scholar] [CrossRef]
- Friedel, J. Dislocations; Pergamon Press: Oxford, UK, 1964. [Google Scholar]
- Brechet, Y.; Louchet, F. A physical approach to the toughness problem: From thermodynamics to kinetics—I. The homogeneous case. Acta Metall. Mater. 1993, 41, 783–791. [Google Scholar] [CrossRef]
- Narita, N.; Higashida, K.; Kitano, S. Dislocation distribution around a crack tip and the fracture toughness in NaCl crystals. Scr. Metall. 1987, 21, 1273–1278. [Google Scholar] [CrossRef]
- Mishin, Y. Atomistic modeling of the ɣ and ɣ′-phases of the Ni-Al system. Acta Mater. 2004, 52, 1451–1467. [Google Scholar] [CrossRef]
- Bitzek, E.; Gumbsch, P. Atomistic study of drag, surface and inertial effect on edge dislocations in face-centered cubic metals. Mater. Sci. Eng. A 2004, 387–389, 11–15. [Google Scholar] [CrossRef]
- Bitzek, E.; Gumbsch, P. Dynamic aspects of dislocation motion: Atomistic simulations. Mater. Sci. Eng. A 2005, 400–401, 40–44. [Google Scholar] [CrossRef]
- Sakamoto, M. High-velocity dislocations: Effective mass, effective line tension and multiplication. Philos. Mag. A 1991, 63, 1241–1248. [Google Scholar] [CrossRef]
- Frank, F.C. On the equations of motion of crystal dislocations. Proc. Phys. Soc. A 1949, 62, 131–134. [Google Scholar] [CrossRef]
- Schoeck, G. The formation of dislocation rings on a crack front. Philos. Mag. A 1996, 74, 419–430. [Google Scholar] [CrossRef]
- Hirsch, P.B.; Roberts, S.G.; Samuels, J. The brittle-ductile transition in silicon. II. Interpretation. Proc. R. Soc. Lond. A 1989, 421, 25–53. [Google Scholar] [CrossRef]
- Michot, G. Interaction of a dislocation with a crack tip: From stimulated emission to avalanche generation. Acta Mater. 2011, 59, 3864–3871. [Google Scholar] [CrossRef]
- de Oliveira, M.A.L.; Michot, G. Three dimensional analysis of the interaction between a crack and a dislocation loop. Acta Mater. 1998, 46, 1371–1383. [Google Scholar] [CrossRef]
- Hirsch, P.B.; Roberts, S.G. The brittle-ductile transition in silicon. Philos. Mag. A 1991, 64, 55–80. [Google Scholar] [CrossRef]
- Scandian, C.; Azzouzi, H.; Maloufi, N.; Michot, G.; George, A. Dislocation nucleation and multiplication at crack tips in silicon. Phys. Stat. Sol. A 1999, 171, 67–82. [Google Scholar] [CrossRef]
- Langenecker, B. Ultrasonic treatment of specimens in the electron microscope. Rev. Sci. Instrum. 1966, 37, 103–106. [Google Scholar] [CrossRef]
- Westmacott, K.H.; Langenecker, B. Dislocation structure in ultrasonically irradiated aluminum. Phys. Rev. Lett. 1965, 14, 221–222. [Google Scholar] [CrossRef]
- Yasui, K.; Hamamoto, K. Importance of dislocations in ultrasound-assisted sintering of silver nanoparticles. J. Appl. Phys. 2021, 130, 194901. [Google Scholar] [CrossRef]
- Tsuji, K.; Fan, Z.; Bang, S.H.; Dursun, S.; Trolier-McKinstry, S.; Randall, C.A. Cold sintering of the ceramic potassium sodium niobate, (K0.5Na0.5)NbO3, and influences on piezoelectric properties. J. Eur. Ceram. Soc. 2022, 42, 105–111. [Google Scholar] [CrossRef]
- Nur, K.; Zubair, M.; Gibson, J.S.K.-L.; Sandlöbes-Haut, S.; Mayer, J.; Bram, M.; Guillon, O. Mechanical properties of cold sintered ZnO investigated by nanoindentation and micro-pillar testing. J. Eur. Ceram. Soc. 2022, 42, 512–524. [Google Scholar] [CrossRef]
- Pezzotti, G.; Kleebe, H.-J. Quantitative characterization of dislocation density in a-SiC crystals after high-pressure sintering in Si3N4 matrix. J. Mater. Sci. Lett. 1996, 15, 500–504. [Google Scholar] [CrossRef]
- Yasui, K.; Hamamoto, K. Comparison between cold sintering and dry pressing of CaCO3 at room temperature by numerical simulations. AIP Adv. 2022, 12, 45304. [Google Scholar] [CrossRef]
- Bouville, F.; Studart, A.R. Geologically-inspired strong bulk ceramics made with water at room temperature. Nat. Commun. 2017, 8, 14655. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasui, K.; Hamamoto, K. Possibility of High Ionic Conductivity and High Fracture Toughness in All-Dislocation-Ceramics. Materials 2024, 17, 428. https://doi.org/10.3390/ma17020428
Yasui K, Hamamoto K. Possibility of High Ionic Conductivity and High Fracture Toughness in All-Dislocation-Ceramics. Materials. 2024; 17(2):428. https://doi.org/10.3390/ma17020428
Chicago/Turabian StyleYasui, Kyuichi, and Koichi Hamamoto. 2024. "Possibility of High Ionic Conductivity and High Fracture Toughness in All-Dislocation-Ceramics" Materials 17, no. 2: 428. https://doi.org/10.3390/ma17020428




