Study on the Performance Mechanism of Polyformaldehyde Glycol Ether Polymer for Crude Oil Recovery Enhancement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. PGEP Prepared
2.3. Surface Tension Test Methods
2.4. Interfacial Tension Test Method
2.5. Oil Washing Efficiency Test Method
2.6. Drainage Efficiency Test Method
3. Result and Disscusion
3.1. PGEP Infrared Spectral Characterization and Analysis
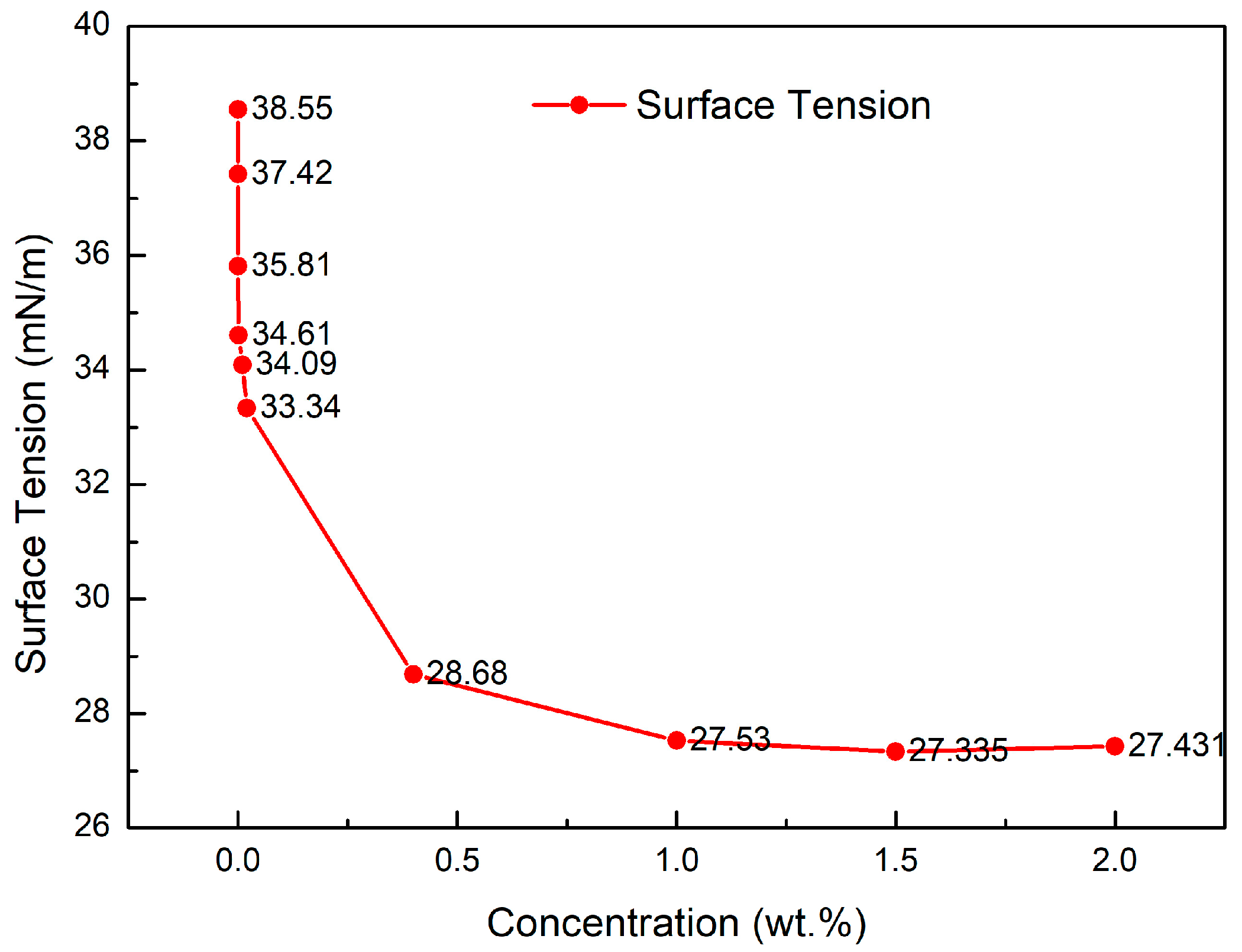
3.2. Study on PGEP Surface Tension Performance
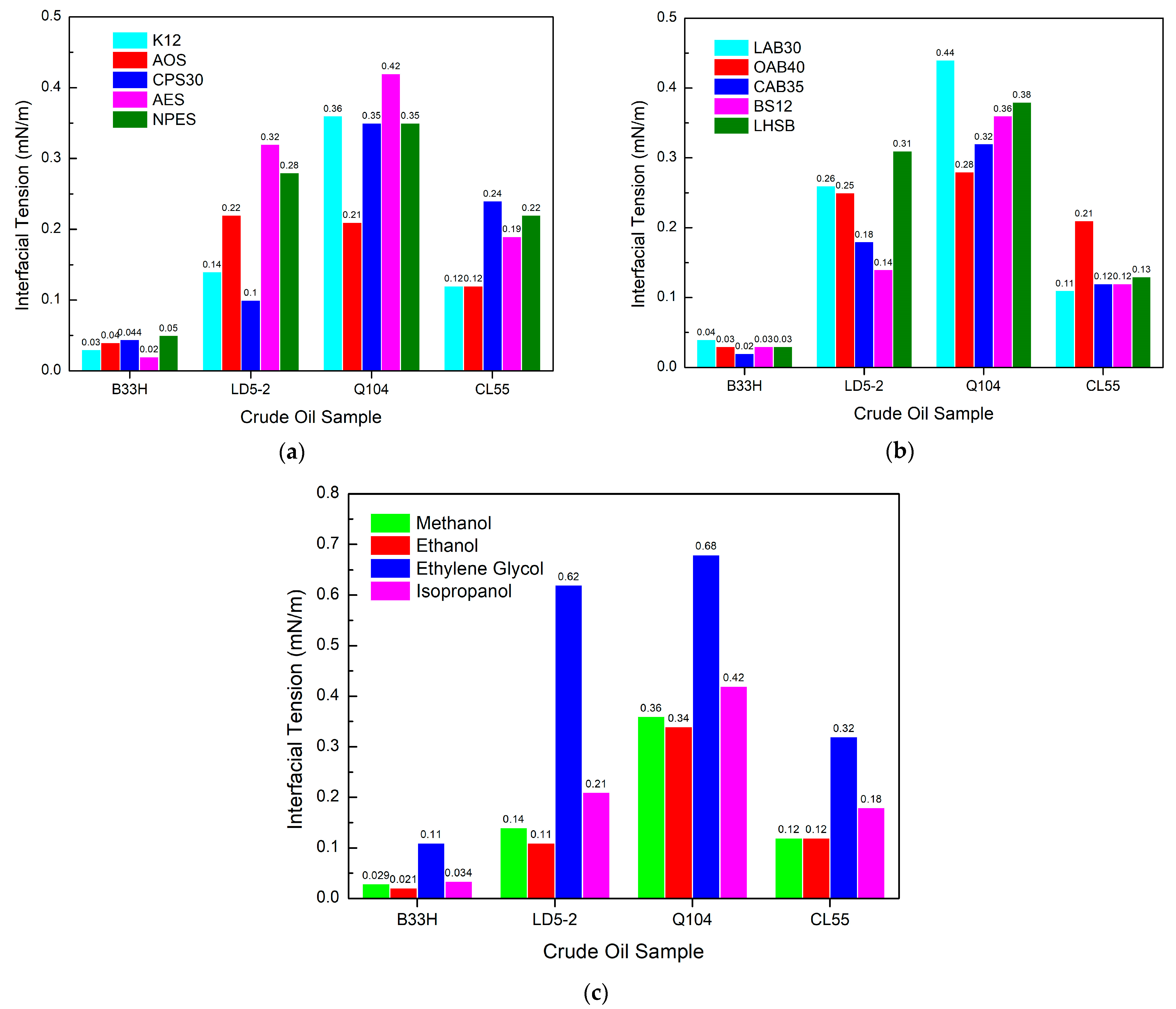
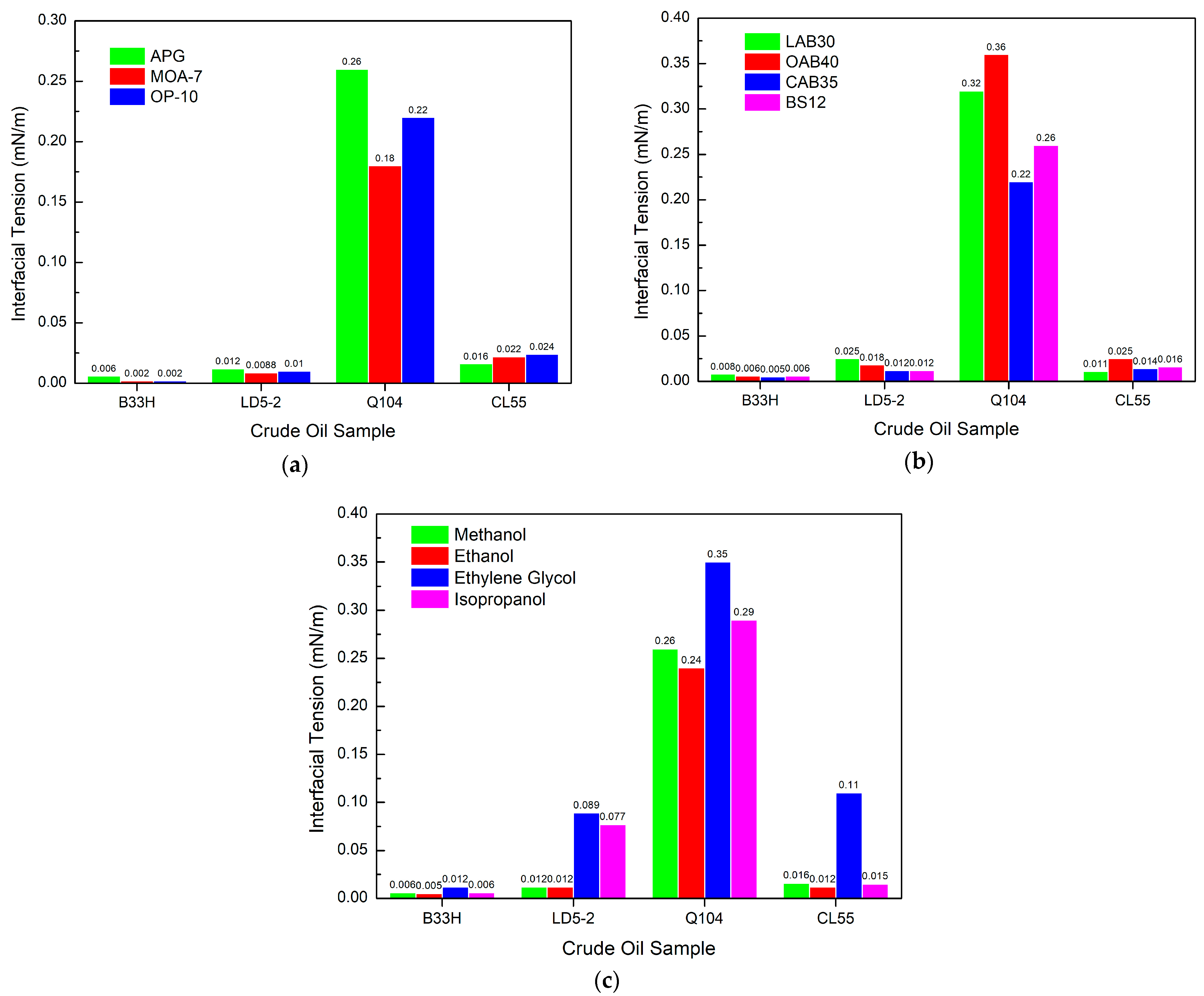
3.3. Study on the Interfacial Tension Performance of PGEP after Compounding with Different Surfactants
3.3.1. Effect of Different Components in Cationic Systems on Interfacial Tension
3.3.2. Effect of Different Components in Anionic Systems on Interfacial Tension
3.3.3. Effect of Different Components in Nonionic Systems on Interfacial Tension
Effect of Different Nonionic Surfactants on Interfacial Tension
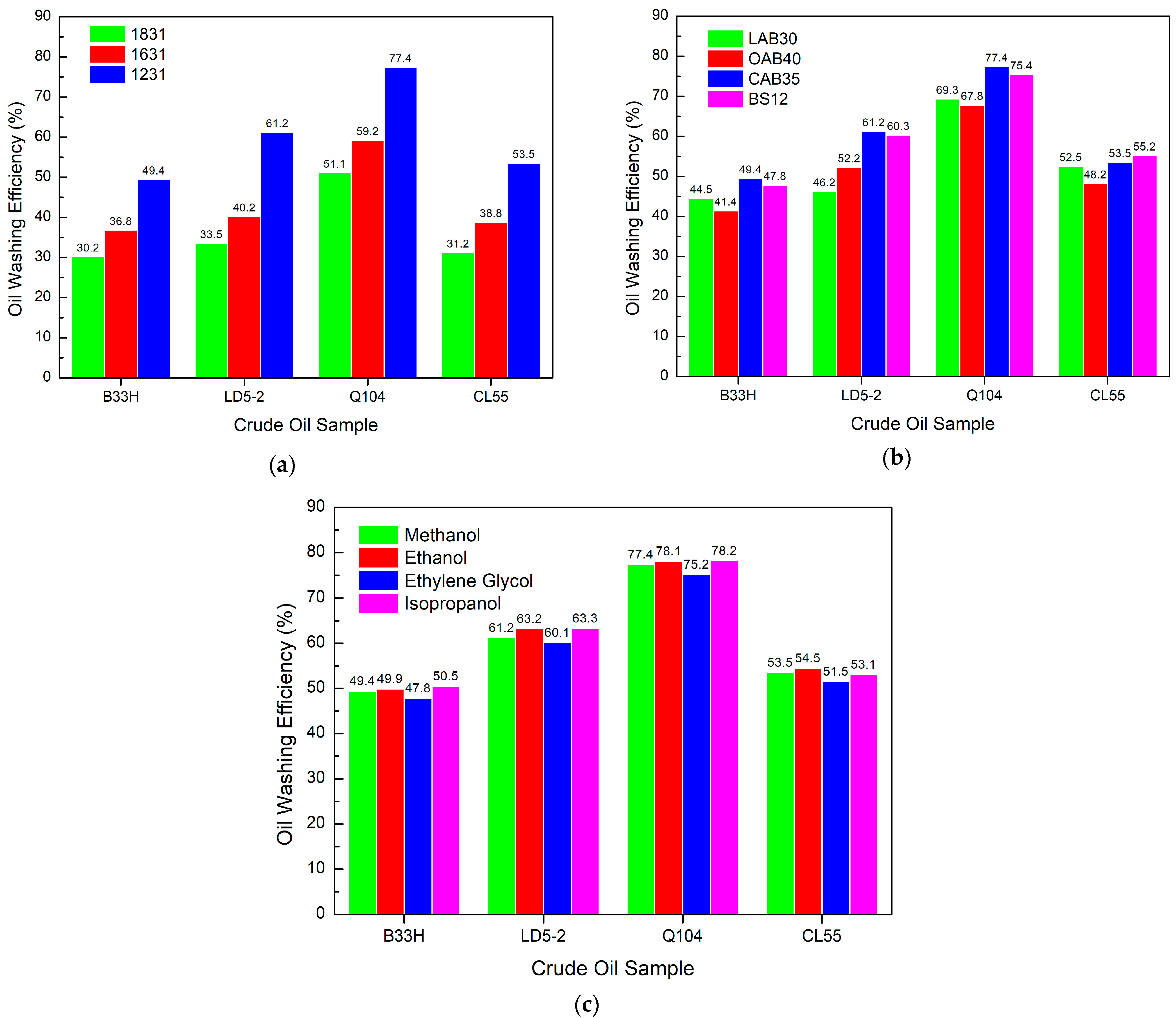
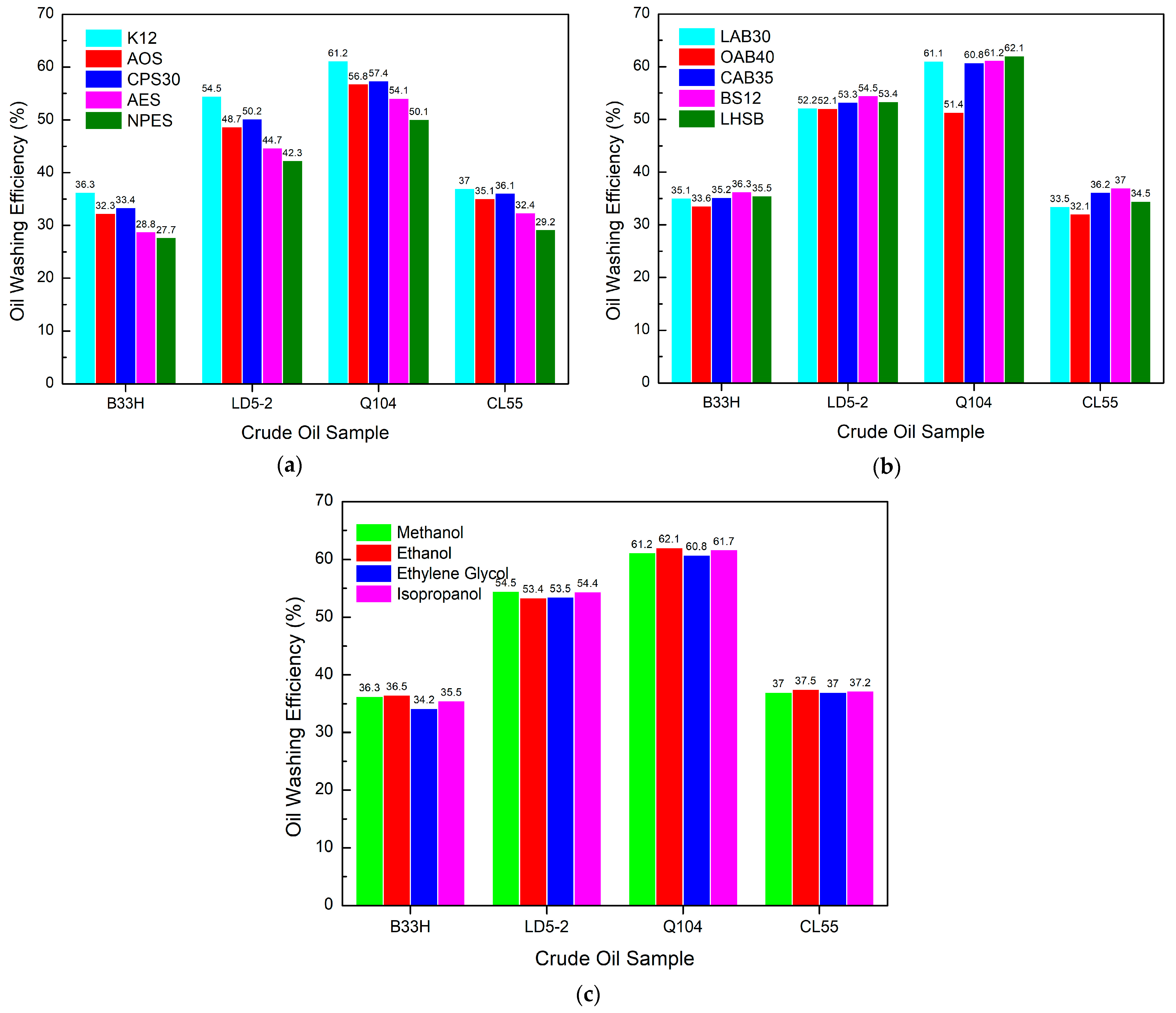
3.4. Study on the Performance of PGEP Compounded with Different Surfactants on Oil Washing Efficiency
3.4.1. Effect of Different Components in Cationic Systems on Oil Washing Efficiency
3.4.2. Effect of Different Components in Anionic Systems on Oil Washing Efficiency
3.4.3. Effect of Different Components in a Nonionic System on Oil Washing Efficiency
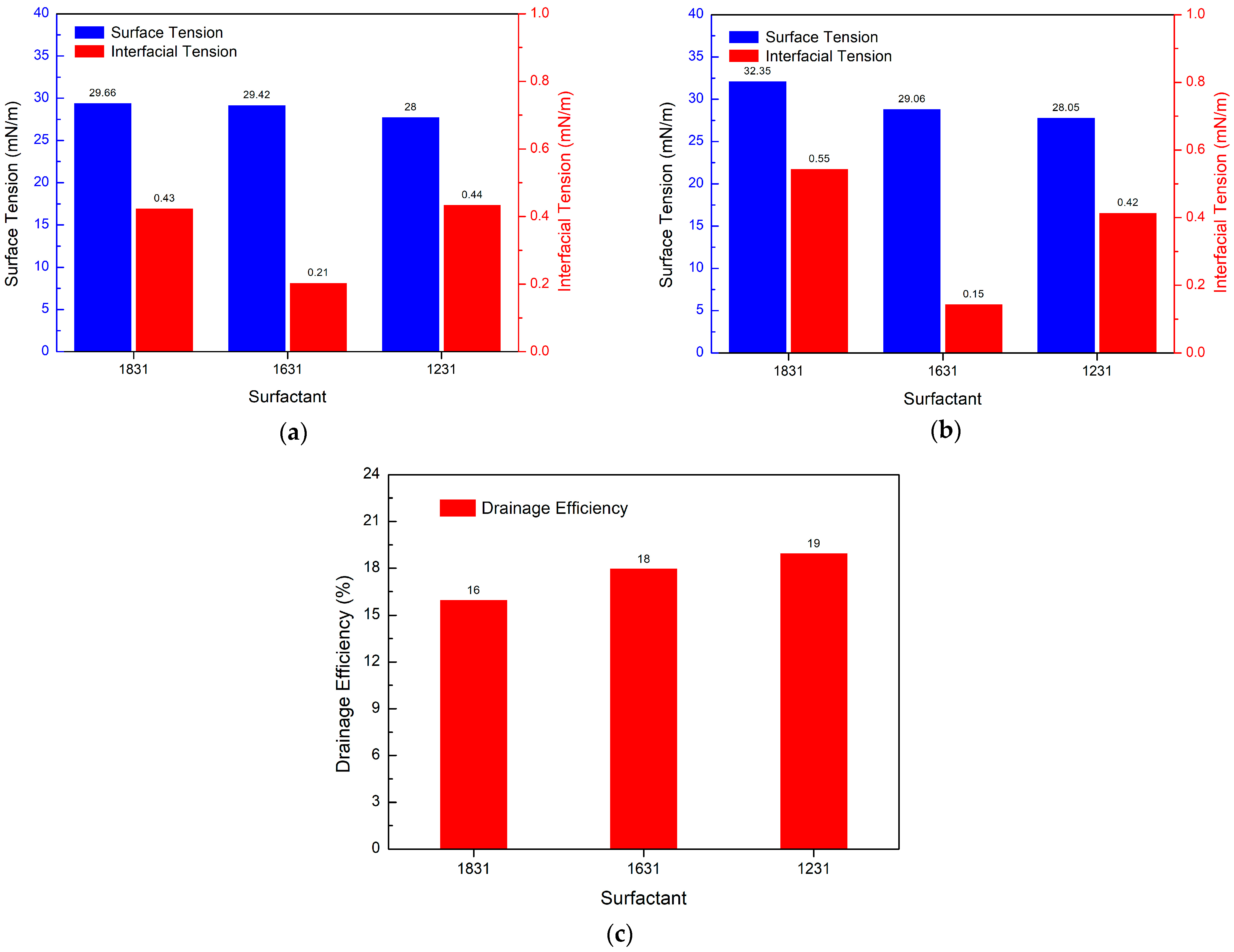
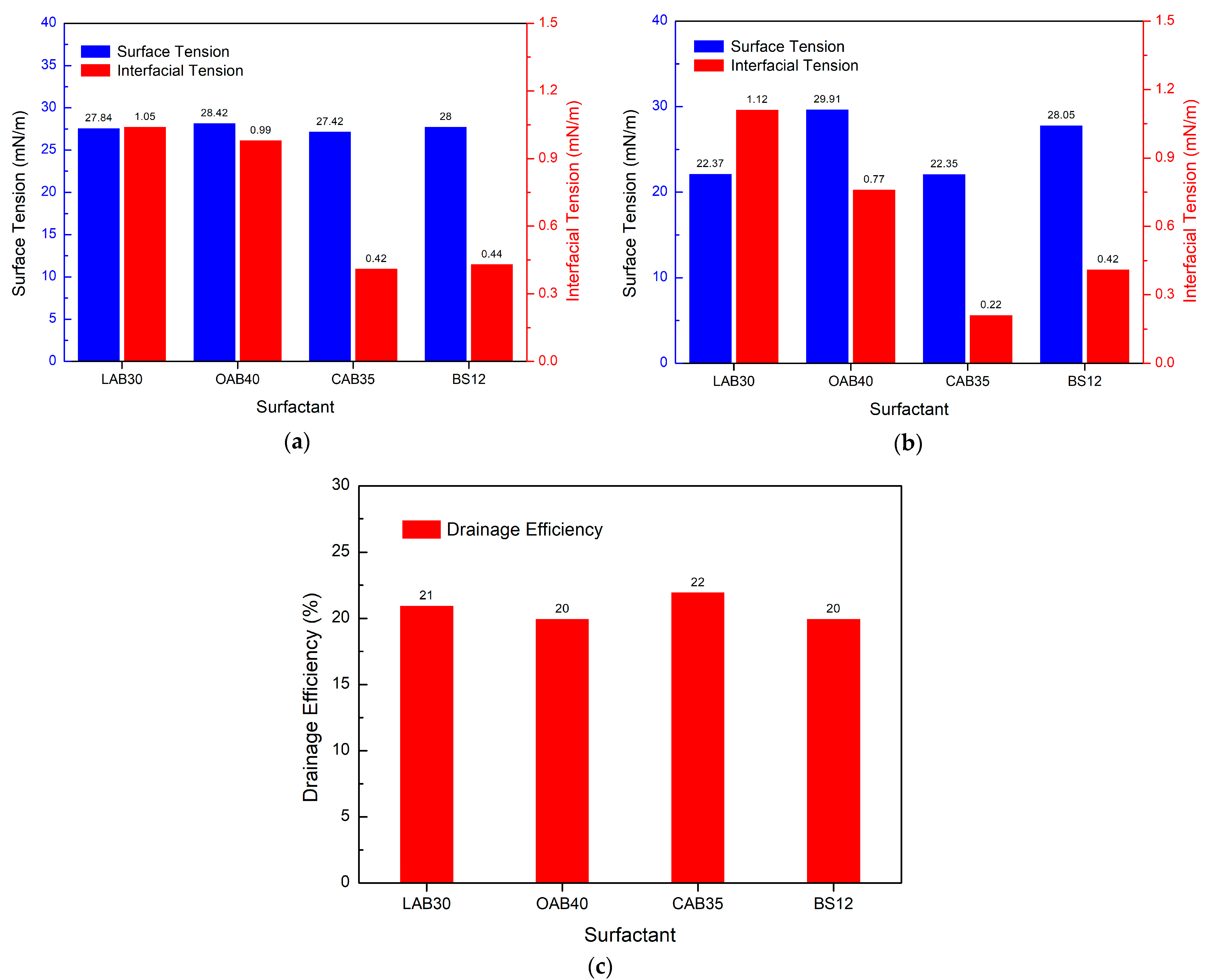
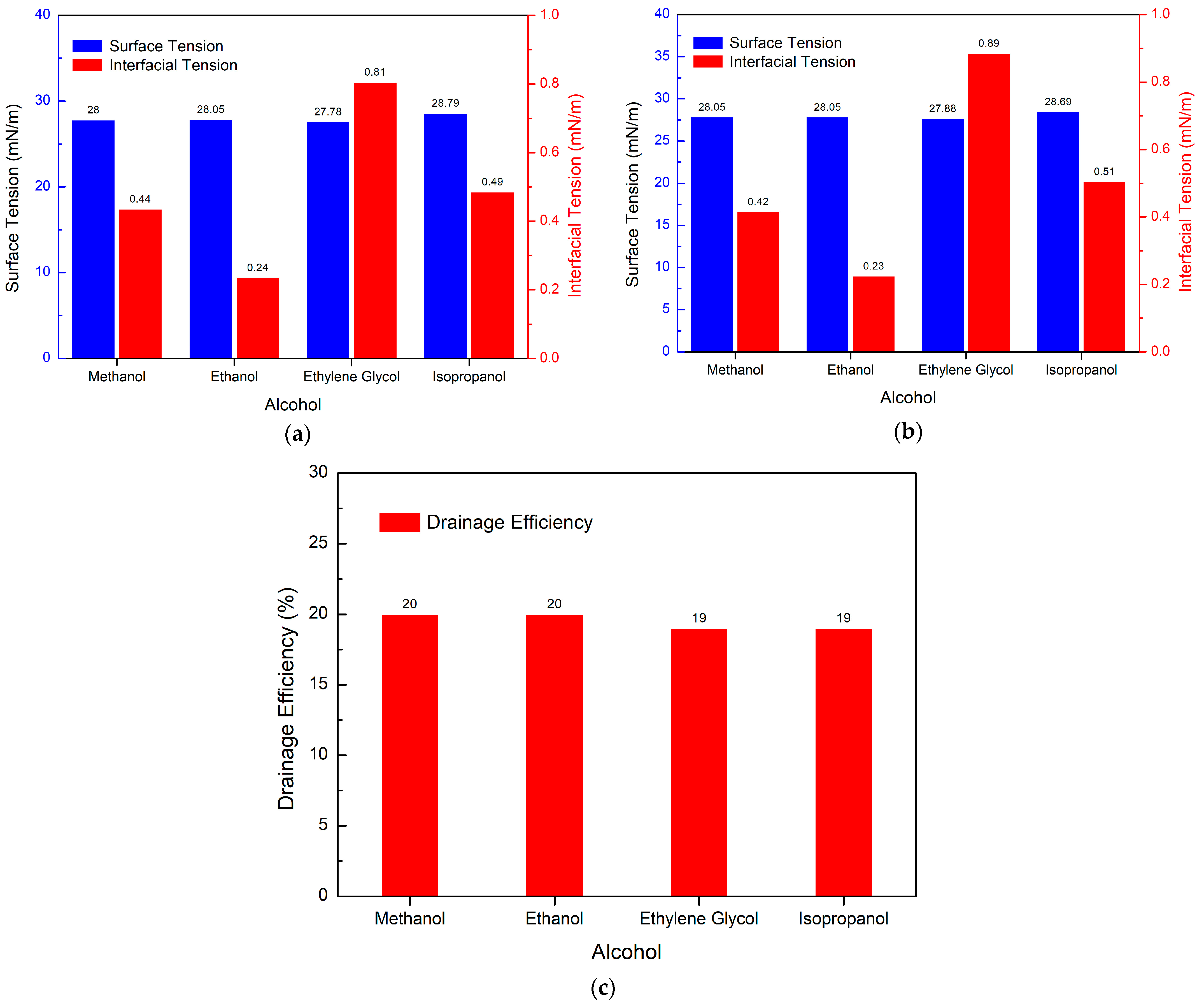
3.5. Study on the Performance of PGEP Compounded with Different Surfactants on Fracturing Fluid Flowback
3.5.1. Effect of Different Cationic Surfactants on Drainage Efficiency
3.5.2. Effect of Different Amphoteric Surfactants on Drainage Efficiency
3.5.3. Effect of Different Alcohols on Drainage Efficiency
4. Conclusions
- The interfacial tension performance of PGEP after compounding with different surfactants can reach as low as 0.00034 mN/m. It can meet the technical requirements of interfacial tension (interfacial tension ≤ 5 × 10−3 mN/m) in the oilfield.
- The oil washing rate performance of PGEP after compounding with different surfactants is best up to 78.2%. It can meet the technical requirements of oil washing efficiency (Oil washing efficiency ≥ 40%) in an oilfield. Alcohols have little effect on the oil washing efficiency of the system.
- The surface tension of PGEP after compounding with different surfactants reaches as low as 27.42 mN/m, and the interfacial tension reaches as low as 0.21 mN/m. After high temperature (150 °C), the surface tension of PGEP after compounding with different surfactants reaches 22.35 mN/m, and the interfacial tension reaches 0.15 mN/m. The drainage efficiency of PGEP after compounding with different surfactants reaches 22%. It can meet the technical requirements of surface/interfacial tension and drainage efficiency (surface tension ≤ 30 mN/m, interfacial tension ≤ 3 mN/m; after high temperature (150 °C), surface tension ≤ 32 mN/m, interfacial tension ≤ 5 mN/m; drainage efficiency ≥ 15%) in the oilfield. The surface interfacial tension of the system remains constant after the concentration exceeds 0.2% and decreases with lower concentrations. The drainage efficiency increases with increasing concentrations in the range below 0.6%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afolabi, R.O.; Oluyemi, G.F.; Officer, S.; Ugwu, J.O. Hydrophobically associating polymers for enhanced oil recovery—Part A: A review on the effects of some key reservoir conditions. J. Pet. Sci. Eng. 2019, 180, 681–698. [Google Scholar] [CrossRef]
- Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymers for enhanced oil recovery: A paradigm for structure–property relationship in aqueous solution. Prog. Polym. Sci. 2011, 36, 1558–1628. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Kandile, N.G.; El-Ghazawy, R.A.; El-Din, M.R.N.; El-Sharaky, E.A. Solution properties of hydrophobically modified polyacrylamides and their potential use for polymer flooding application. Egypt J. Pet. 2016, 25, 433–444. [Google Scholar] [CrossRef]
- Levitt, D.; Pope, G.A. Selection and screening of polymers for enhanced-oil recovery. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20–23 April 2008. SPE-113845-MS. [Google Scholar]
- Raffa, P.; Broekhuis, A.A.; Picchioni, F. Polymeric surfactants for enhanced oil recovery: A review. J. Pet. Sci. Eng. 2016, 145, 723–733. [Google Scholar] [CrossRef]
- Taylor, K.C.; Nasr-El-Din, H.A. Water-soluble hydrophobically associating polymers for improved oil recovery: A literature review. J. Pet. Sci. Eng. 1998, 19, 265–280. [Google Scholar] [CrossRef]
- Kumar, A.; Mandal, A. Core-scale modelling and numerical simulation of zwitterionic surfactant flooding: Designing of chemical slug for enhanced oil recovery. J. Pet. Sci. Eng. 2020, 192, 107333. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Chen, S.; Mbia, E.; Wu, K. The second critical capillary number for chemical flooding in low permeability reservoirs: Experimental and numerical investigations. Chem. Eng. Sci. 2019, 196, 202–213. [Google Scholar] [CrossRef]
- Li, Z.; Wu, H.; Yang, M.; Jiang, J.; Xu, D.; Feng, H.; Lu, Y.; Kang, W.; Bai, B.; Hou, J. Spontaneous emulsification via once bottom-up cycle for the crude oil in low-permeability reservoirs. Energy Fuels 2018, 32, 3119–3126. [Google Scholar] [CrossRef]
- Yang, H.; Shao, S.; Zhu, T.; Chen, C.; Liu, S.; Zhou, B.; Hou, X.; Zhang, Y.; Kang, W. Shear resistance performance of low elastic polymer microspheres used for conformance control treatment. J. Ind. Eng. Chem. 2019, 79, 295–306. [Google Scholar] [CrossRef]
- Kumar, S.; Mandal, A. Studies on interfacial behavior and wettability change phenomena by ionic and nonionic surfactants in presence of alkalis and salt for enhanced oil recovery. Appl. Surf. Sci. 2016, 372, 42–51. [Google Scholar] [CrossRef]
- Sheng, J.J. Enhanced oil recovery in shale reservoirs by gas injection. J. Nat. Gas Sci. Eng. 2015, 22, 252–259. [Google Scholar] [CrossRef]
- Khandoozi, S.; Sharifi, A.; Riazi, M. Enhanced oil recovery using surfactants. Chem. Methods 2022, 95–139. [Google Scholar] [CrossRef]
- Alfarge, D.; Wei, M.; Bai, B. Ior methods in unconventional reservoirs of north america: Comprehensive review. In Proceedings of the SPE Western Regional Meeting, Bakersfield, CA, USA, 23–27 April 2017; SPE-185640-MS. [Google Scholar]
- Blagojević, S.N.; Blagojević, S.M.; Pejić, N.D. Performance and Efficiency of Anionic Dishwashing Liquids with Amphoteric and Nonionic Surfactants. J. Surfactants Deterg. 2016, 19, 363–372. [Google Scholar] [CrossRef]
- Ishak, S.A. Ecotoxicological behaviour of poorly water soluble fatty alcohol ethoxylates in freshwater environment. J. Oil Palm Res. 2020, 32, 665–673. [Google Scholar] [CrossRef]
- Lechuga, M.; Fernández-Serrano, M.; Jurado, E.; Núñez-Olea, J.; Ríos, F. Acute toxicity of anionic and non-ionic surfactants to aquatic organisms. Ecotoxicol. Environ. Saf. 2016, 125, 1–8. [Google Scholar] [CrossRef]
- Fainerman, V.B.; Möbius, D.; Miller, R. Surfactants: Chemistry, Interfacial Properties, Applications; Elsevier Science: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Mitrinova, Z.; Tcholakova, S.; Denkov, N. Control of surfactant solution rheology using medium-chain cosurfactants. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 173–184. [Google Scholar] [CrossRef]
- Vu, T.; Reynolds, G.; Hutton, H.D.; Kasting, G.B.; Koenig, P. Rheology Control Using Nonionic Cosurfactants and pH Titration in an Amino Acid-Derived Surfactant Composition. Langmuir 2021, 37, 12327–12334. [Google Scholar] [CrossRef]
- Fangwei, H.; Yue, Z.; Mei, L.; Fuhong, H.; Yingying, P.; Liang, M. Wetting behavior during impacting bituminous coal surface for dust suppression droplets of fatty alcohol polyoxyethylene ether. Environ. Sci. Pollut. Res. 2023, 30, 51816–51829. [Google Scholar]
- Munkajohnpong, P.; Kesornpun, C.; Buttranon, S.; Jaroensuk, J.; Weeranoppanant, N.; Chaiyen, P. Fatty alcohol production: An opportunity of bioprocess. Biofuels Bioprod. Biorefining 2020, 14, 986–1009. [Google Scholar] [CrossRef]
- Park, S.; Lee, E.S.; Sulaiman, W.R.W. Adsorption behaviors of surfactants for chemical flooding in enhanced oil recovery. J. Ind. Eng. Chem. 2015, 21, 1239–1245. [Google Scholar] [CrossRef]
- Daghlian Sofla, S.J.; Sharifi, M.; Hemmati Sarapardeh, A. Toward mechanistic understanding of natural surfactant flooding in enhanced oil recovery processes: The role of salinity, surfactant concentration and rock type. J. Mol. Liq. 2016, 222, 632–639. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Mohammadi, A.H.; Manshad, A.K. Chemical enhanced oil recovery by different scenarios of slug injection into carbonate/sandstone composite oil reservoirs using an anionic surfactant derived from rapeseed oil. Energy Fuels 2021, 35, 1248–1258. [Google Scholar] [CrossRef]
- Sagir, M.; Mushtaq, M.; Tahir, M.S.; Tahir, M.B.; Shaik, A.R. Surfactants for Enhanced Oil Recovery Applications; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Pan, F.; Zhang, Z.; Zhang, X.; Davarpanah, A. Impact of anionic and cationic surfactants interfacial tension on the oil recovery enhancement. Powder Technol. 2020, 373, 93–98. [Google Scholar] [CrossRef]
- Atta, D.Y.; Negash, B.M.; Yekeen, N.; Habte, A.D. A state-of-the-art review on the application of natural surfactants in enhanced oil recovery. J. Mol. Liq. 2020, 321, 114888. [Google Scholar] [CrossRef]
- Yekeen, N.; Malik, A.A.; Idris, A.K.; Reepei, N.I.; Ganie, K. Foaming properties, wettability alteration and interfacial tension reduction by saponin extracted from soapnut (sapindus mukorossi) at room and reservoir conditions. J. Pet. Sci. Eng. 2020, 195, 107591. [Google Scholar] [CrossRef]
- Yarveicy, H.; Haghtalab, A. Effect of amphoteric surfactant on phase behavior of hydrocarbon-electrolyte-water system-an application in enhanced oil recovery. J. Dispers. Sci. Technol. 2018, 39, 522–530. [Google Scholar] [CrossRef]
- Dawson, M.; Nguyen, D.; Champion, N.; Li, H. Designing an optimized surfactant flood in the Bakken. In Proceedings of the SPE/CSUR Unconventional Resources Conference, Calgary, AB, Canada, 20–22 October 2015. SPE-175937-MS. [Google Scholar]
- Nguyen, D.; Wang, D.; Oladapo, A.; Zhang, J.; Sickorez, J.; Butler, R.; Mueller, B. Evaluation of surfactants for oil recovery potential in shale reservoirs. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. SPE-169085-MS. [Google Scholar]
- Shuler, P.; Tang, H.; Zayne, L.; Tang, Y. Chemical processes for improved oil recovery from Bakken shale. In Proceedings of the Canadian Unconventional Resources Conference, Society of Petroleum Engineers, Calgary, AB, Canada, 15–17 November 2011. [Google Scholar]
- Wang, D.; Butler, R.; Liu, H.; Ahmed, S. Flow-rate behavior and imbibition in shale. SPE Reserv. Eval. Eng. 2011, 14, 485–492. [Google Scholar] [CrossRef]
- Wang, D.; Butler, R.; Zhang, J.; Seright, R. Wettability survey in Bakken shale with surfactant-formulation imbibition. SPE Reserv. Eval. Eng. 2012, 15, 695–705. [Google Scholar] [CrossRef]
- Gong, L.; Liao, G.; Luan, H.; Chen, Q.; Nie, X.; Liu, D.; Feng, Y. Oil solubilization in sodium dodecylbenzenesulfonate micelles: New insights into surfactant enhanced oil recovery. J. Colloid Interface Sci. 2020, 569, 219–228. [Google Scholar] [CrossRef]
- Yun, W.; Chang, S.; Cogswell, D.A.; Eichmann, S.L.; Gizzatov, A.; Thomas, G.; Al-Hazza, N.; Abdel-Fattah, A.; Wang, W. Toward reservoir-on-a-chip: Rapid performance evaluation of enhanced oil recovery surfactants for carbonate reservoirs using a calcite-coated micromodel. Sci. Rep. 2020, 10, 782. [Google Scholar] [CrossRef]
- Deljooei, M.; Zargar, G.; Nooripoor, V.; Takassi, M.A.; Esfandiarian, A. Novel green surfactant made from L-aspartic acid as enhancer of oil production from sandstone reservoirs: Wettability, IFT, microfluidic, and core flooding assessments. J. Mol. Liq. 2021, 323, 115037. [Google Scholar] [CrossRef]
- Niu, J.; Wang, A.; He, Y.; Yin, H.; Xu, Q.; Li, C.; Xiao, L. Catalytic Reaction between Fatty Alcohol Polyoxyethylene Ether and Sodium Hydroxide to Sodium Fatty Alcohol Polyoxyethylene Ether Acetate on ZnO and SnO2 Catalysts. React. Kinet. Mech. Catal. 2023. [Google Scholar] [CrossRef]
- Takaoka, N.; Horinaka, J.; Takigawa, T. Crossover Behavior on Temperature Dependence of Volume of Poly (Ethylene Oxide)-Poly (Propylene Oxide)-Poly (Ethylene Oxide)–Based Hydrogels. Colloid Polym. Sci. 2019, 297, 1177–1182. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Lu, W.; Li, T.; Ma, F.; Yao, D.; Li, Q. Study on the Performance Mechanism of Polyformaldehyde Glycol Ether Polymer for Crude Oil Recovery Enhancement. Materials 2024, 17, 437. https://doi.org/10.3390/ma17020437
Jiang S, Lu W, Li T, Ma F, Yao D, Li Q. Study on the Performance Mechanism of Polyformaldehyde Glycol Ether Polymer for Crude Oil Recovery Enhancement. Materials. 2024; 17(2):437. https://doi.org/10.3390/ma17020437
Chicago/Turabian StyleJiang, Shaohui, Wenxue Lu, Tao Li, Fujun Ma, Dahu Yao, and Qingsong Li. 2024. "Study on the Performance Mechanism of Polyformaldehyde Glycol Ether Polymer for Crude Oil Recovery Enhancement" Materials 17, no. 2: 437. https://doi.org/10.3390/ma17020437
APA StyleJiang, S., Lu, W., Li, T., Ma, F., Yao, D., & Li, Q. (2024). Study on the Performance Mechanism of Polyformaldehyde Glycol Ether Polymer for Crude Oil Recovery Enhancement. Materials, 17(2), 437. https://doi.org/10.3390/ma17020437



