The Influence of Different Aggregates on the Physico-Mechanical Performance of Alkali-Activated Geopolymer Composites Produced Using Romanian Fly Ash
Abstract
:1. Introduction
SiO3²⁻ + 2αAlO²⁻ + βCa²⁺ + 2γNa⁺ + 2δFe³⁺ + εHTiO³− + (1 + α)H2O + 2(β + γ + 3δ)
2. Materials and Methods
2.1. Preparation of the Geopolymer Binder
2.2. Preparation of the Geopolymer Composite Samples
2.3. Physico-Mechanical Analysis of the Alkali-Activated Geopolymer Samples
2.4. Optical, SEM and EDS Analysis of Samples
3. Results and Discussions
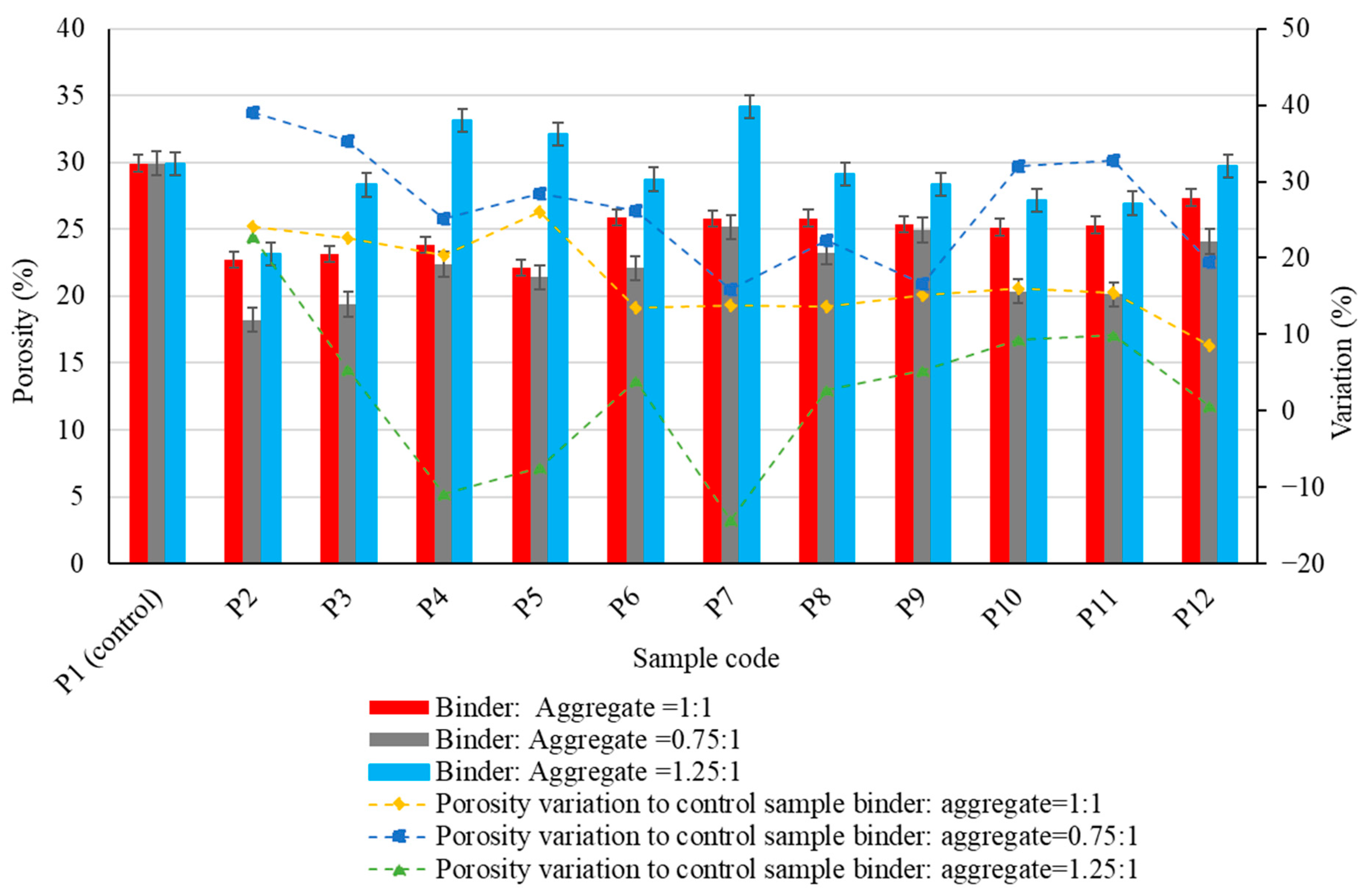
3.1. Physico-Mechanical Properties of the Samples
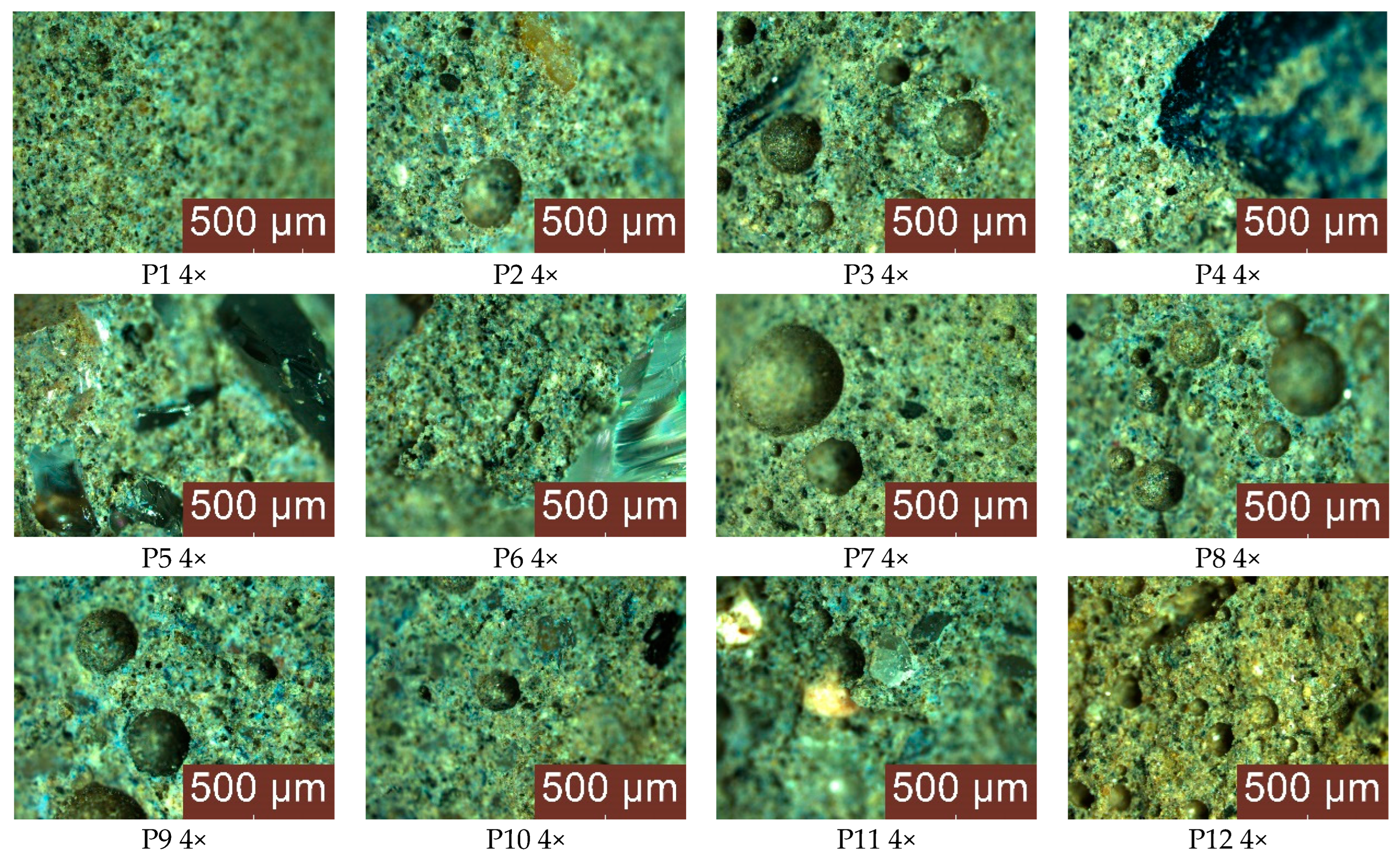
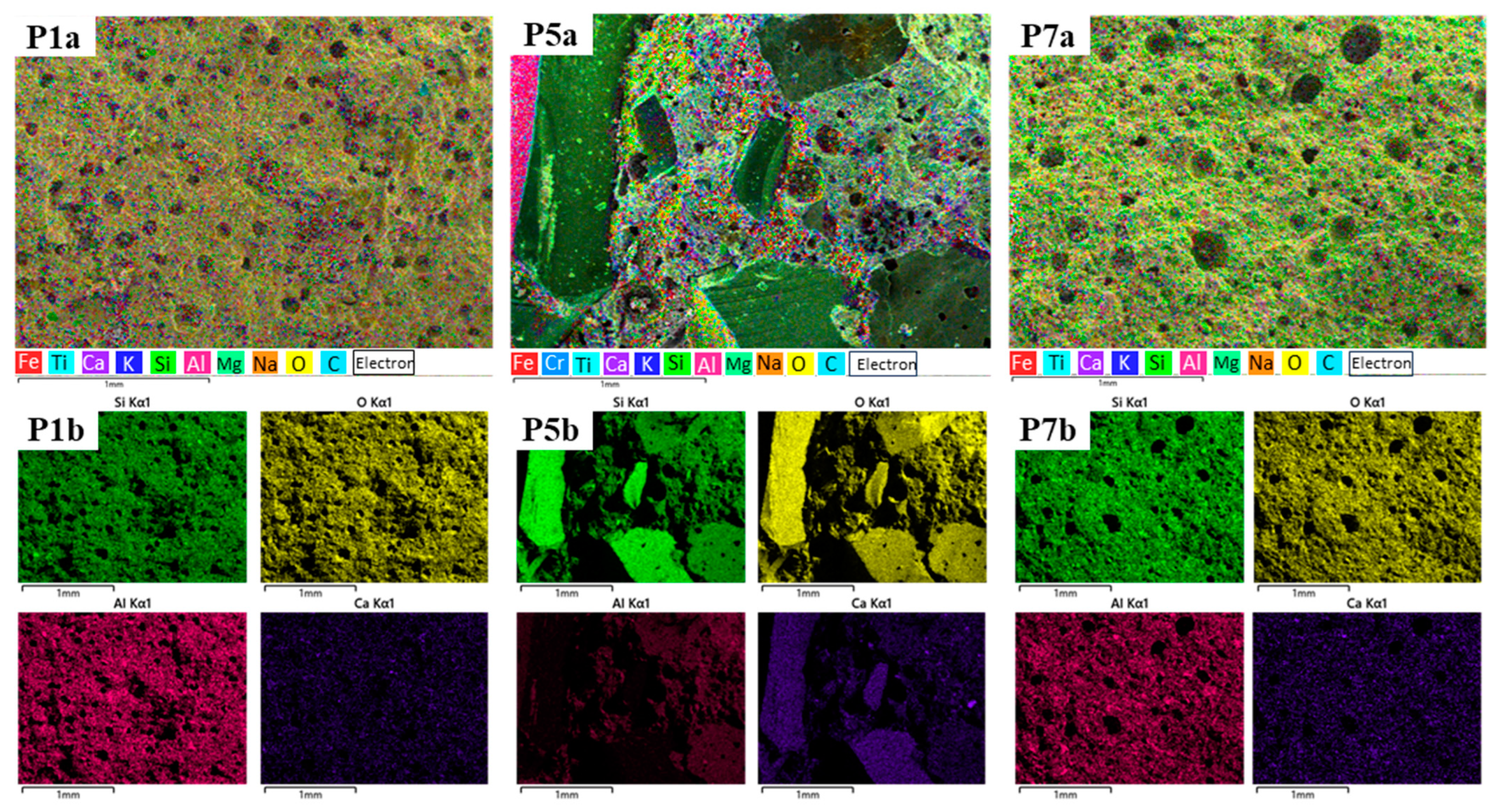
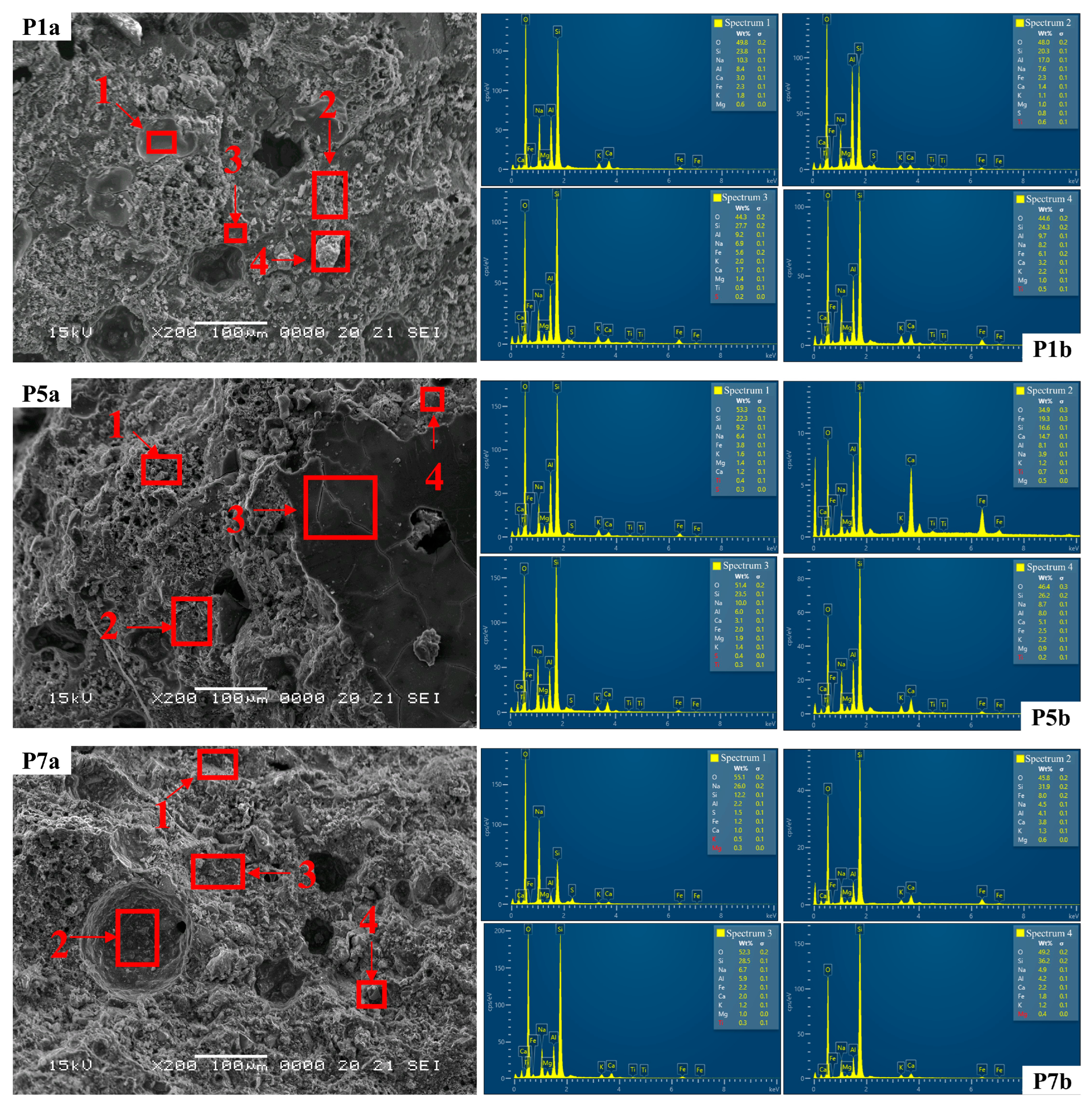
3.2. Optical, SEM and EDS Analysis of Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cembureau. Available online: https://www.cembureau.eu/library/reports/2050-carbon-neutrality-roadmap/ (accessed on 21 February 2023).
- Aitcin, P.C. Cements of yesterday and today: Concrete of tomorrow. Cem. Concr. Res. 2000, 30, 1349–1359. [Google Scholar] [CrossRef]
- Sandu, A.V. Obtaining and Characterization of New Materials. Materials 2021, 14, 6606. [Google Scholar] [CrossRef] [PubMed]
- Jamaludin, L.; Razak, R.A.; Abdullah, M.M.; Vizureanu, P.; Bras, A.; Imjai, T.; Sandu, A.V.; Abd Rahim, S.Z.; Yong, H.C. The Suitability of Photocatalyst Precursor Materials in Geopolymer Coating Applications: A Review. Coatings 2022, 12, 1348. [Google Scholar] [CrossRef]
- Warid Wazien, A.Z.; Mustafa, M.; Abdullah, A.B.; Razak, R.A.; Rozainy, M.M.A.Z.R.; Faheem, M.; Tahir, M.; Faris, M.A.; Hamzah, H.N. Review on Potential of Geopolymer for Concrete Repair and Rehabilitation. MATEC Web Conf. 2016, 78, 01065. [Google Scholar] [CrossRef]
- Lloyd, N.; Rangan, B. Geopolymer Concrete with Fly Ash. In Second International Conference on Sustainable Construction Materials and Technologies; Zachar, J., Claisse, P., Naik, T., Ganjian, G., Eds.; UWM Center for By-Products Utilization: Ancona, Italy, 2010; Volume 3, pp. 1493–1504. [Google Scholar]
- Stengel, T.; Reger, J.; Heinz, D. Life cycle assessment of geopolymer concrete—What is the environmental benefit? In Proceedings of the Concrete Solutions 09: Proceedings of the 24th Biennial Conference of the Concrete Institute of Australia, Sydney, Australia, 17–19 September 2009. [Google Scholar]
- Weil, M.; Dombrowski, K.; Buchwald, A. Life-cycle analysis of geopolymers. In Geopolymers: Structures, Processing, Properties and Industrial Applications; Provis, J.L., Van Deventer, J.S.J., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 194–210. [Google Scholar]
- Al Bakri Abdullah, A.M.; Kamarudin, H.; Binhussain, M.; Nizar, K.; Mastura, W.I.W. Mechanism and Chemical Reaction of Fly Ash Geopolymer Cement—A Review. Asian J. Sci. Res. 2011, 1, 247–253. [Google Scholar]
- Amran, M.; Fediuk, R.; Murali, G.; Avudaiappan, S.; Ozbakkaloglu, T.; Vatin, N.; Karelina, M.; Klyuev, S.; Gholampour, A. Fly Ash-Based Eco-Efficient Concretes: A Comprehensive Review of the Short-Term Properties. Materials 2021, 14, 4264. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, A. What are geopolymers? Current State of Research and Technology, The Opportunities They Offer, and Their Significance for the Precast Industry. Concr. Precast. Plant Technol. (Betonw. Fert.-Tech.) 2006, 72, 42–49. [Google Scholar]
- Tian, L.; He, D.; Zhao, J.; Wang, H. Durability of geopolymers and geopolymer concretes: A review. Rev. Adv. Mater. Sci. 2021, 60, 1–14. [Google Scholar]
- Fernandez-Jimenez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Panagiotopoulou, C.; Kontori, E.; Perraki, T.; Kakali, G. Dissolution of aluminosilicate minerals and by-products in alkaline media. J. Mater. Sci. 2007, 42, 2967–2973. [Google Scholar] [CrossRef]
- Adewuyi, Y.G. Recent Advances in Fly-Ash-Based Geopolymers: Potential on the Utilization for Sustainable Environmental Remediation. ACS Omega 2021, 6, 15532–15542. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.; Khereby, M.A.; Ghorab, H.Y.; Elkhoshkhany, N. Preparation of Geo-polymer Concrete Using Egyptian Kaolin Clay and the Study of Its Environmental Effects and Economic Cost. Clean Technol. Environ. Policy 2020, 22, 669–687. [Google Scholar] [CrossRef]
- Albidah, A.; Alghannam, M.; Abbas, H.; Almusallam, T.; Al-Salloum, Y. Charac-teristics of Metakaolin-Based Geopolymer Concrete for Different Mix Design Parameters. J. Mater. Res. Technol. 2021, 10, 84–98. [Google Scholar] [CrossRef]
- Ionescu, B.A.; Lăzărescu, A.-V.; Hegyi, A. The Possibility of Using Slag for the Production of Geopolymer Materials and Its Influence on Mechanical Performances—A Review. Proceedings 2020, 63, 30. [Google Scholar]
- Xu, H.; van Deventer, J. The effect of alkali metals on the formation of geopolymeric gels from alkali-feldspats. Colloids Surf. A Physicochem. Eng. Asp. 2013, 216, 27–44. [Google Scholar] [CrossRef]
- Weng, L.; Sagoe-Crentsil, K. Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: Part I—Low Si/Al ratio systems. J. Mater. Sci. 2007, 42, 2997–3006. [Google Scholar] [CrossRef]
- Andini, S.; Cioffi, R.; Colangelo, F.; Grieco, T.; Montagnaro, F.; Santoro, L. Coal fly ash as raw material for the manufacture of geopolymer-based product. J. Waste Manag. 2008, 28, 416–423. [Google Scholar] [CrossRef]
- Duxon, P.; Fernande-Jimenez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Moreno, N.; Querol, X.; Andrés, J.M.; Stanton, K.; Towler, M.; Jurcovicova, M.; Jones, R. Physico-chemical characteristics of European pulverized coal combustion fly ashes. Fuel 2005, 84, 1351–1563. [Google Scholar] [CrossRef]
- Chen-Tan, N.W.; Van Riessen, A.; Ly, C.V.; Southam, D. Determining the reactivity of a fly ash for production of geopolymer. J. Am. Ceram. Soc. 2009, 92, 881–887. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K.; Rangan, V.B. Early Age Properties of Low-calcium Fly Ash Geopolymer Concrete Suitable for Ambient Curing. Procedia Eng. 2015, 125, 601–607. [Google Scholar] [CrossRef]
- Swanepoel, J.C.; Strydom, C.A. Utilisation of fly ash in a geopolymeric material. Appl. Geochem. 2002, 17, 114–148. [Google Scholar] [CrossRef]
- Goretta, K.C.; Gutierrez-Mora, F.; Singh, D.; Routbort, J.L.; Lukey, G.C.; van Deventer, J.S.J. Erosion of geopolymers made from industrial waste. J. Mater. Sci. 2007, 42, 3066–3072. [Google Scholar] [CrossRef]
- Puertas, F.; Martinez-Ramirez, S.; Alonso, S.; Vazquez, T. Alkali activated fly ash/slag cements: Strength behavior and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Farhana, Z.; Kamarudin, H.; Rahmat, A.; Al Bakri, A.M. The Relationship between Water Absorption and Porosity for Geopolymer Paste. Mater. Sci. Forum 2014, 803, 166–172. [Google Scholar] [CrossRef]
- Aly, M.; Hashmi, M.S.; Olabi, A.G.; Messeiry, M. Effect of colloidal nano-silica on the mechanical and physical behavior of waste-glass cement mortar. Mater. Des. 2012, 33, 127135. [Google Scholar] [CrossRef]
- Khater, M.H. Effect of nano-silica on microstructure formation of low-cost geopolymer binder. Nanocomposites 2016, 2, 84–97. [Google Scholar] [CrossRef]
- Khater, M.H. Physicomechanical properties of nano-silica effect on geopolymer composites. J. Build. Mater. Struct. 2016, 3, 1–14. [Google Scholar] [CrossRef]
- Assaedi, H.; Shaikh, F.U.; Low, I.M. Effect of nanoclay on durability and mechanical properties of flax fabric reinforced geopolymer composites. J. Asian Ceram. Soc. 2017, 5, 62–70. [Google Scholar] [CrossRef]
- Adak, D.; Sarkar, M.; Mandal, S. Effect of nano-silica on strength and durability of fly ash based geopolymer mortar. Construct. Build. Mater. 2014, 70, 453–459. [Google Scholar] [CrossRef]
- Shaikh, F.U.; Supit, S.W.; Sarker, P.K. A study on the effect of nano silica on compressive strength of high volume fly ash mortars and concretes. Mater. Des. 2014, 60, 433–442. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Wang, R.; Chen, F.; Jia, X.; Cong, P. Effects of Reactive MgO on the Reaction Process of Geopolymer. Materials 2019, 12, 526. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhu, X.; Long, F. Alkali-activated fly ash-based geopolymers with zeolite or bentonite as additives. Cem. Concr. Compos. 2009, 31, 762–768. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Atis, C.D.; Görür, E.B.; Karahan, O.; Bilim, C.; Ilkentapar, S.; Luga, E. Very high strength (120 MPa) Class F fly ash geopolymer mortar activated at different NaOH amount, heat curing temperature and heat curing duration. Constr Build. Mater. 2015, 96, 673–678. [Google Scholar] [CrossRef]
- Al Bakri, M.M.; Mohammed, H.; Kamarudin, H.; Niza, K.; Zarina, Y. Review of Fly Ash-Based Geopolymer Concrete Without Portland Cement. J. Eng. Technol. 2011, 3, 1–4. [Google Scholar]
- Hardjito, D.; Rangan, B.V. Development and Properties of Low-Calcium Fly Ash-Based Geopolymer Concrete; Technical Report GC1; Civil Engineering Faculty, Technical University: Perth, Australia, 2005. [Google Scholar]
- Al Bakri Mustafa, A.M.; Kamarudin, H.; Binhussain, M.; Niza, I.K. The effect of curing temperature on physical and chemical properties of geopolymers. Phys. Procedia 2011, 22, 286–291. [Google Scholar]
- Al Bakri Mustafa, A.M.; Kamarudin, H.; Bnhussain, M.; Nizar, I.K.; Rafiza, A.R.; Zarina, Y. The processing, characterization, and properties of fly ash based geopolymer concrete. Rev. Adv. Mater. Sci. 2012, 30, 90–97. [Google Scholar]
- Chindaprasirt, P.; Chareerat, T.; Sirivivatnano, V. Workability and strength of coarse high calcium fly ash geopolymer. Cem. Conc. Comp. 2007, 29, 224–229. [Google Scholar] [CrossRef]
- Morsy, M.S.; Alsaye, S.H.; Al-Salloum, Y.; Almusallam, T. Effect of sodium silicate to sodium hydroxide ratios on strength and microstructure of fly ash geopolymer binder. Arab. J. Sci. Eng. 2014, 39, 4333–4339. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, N.; Izquierdo, M.; Font, O.; Moreno, T.; Diez, S.; Vasquez, K.; et al. Environmental, physical and structural characterisation of geopolymer matrixes synthesised from coal (co-)combustion fly ashes. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef]
- Hardjito, D.; Rangan, B.V. Development and Properties of Low-Calcium Fly Ash-Based Geopolymer Concrete; Technical Report GC2; Civil Engineering Faculty, Technical University: Perth, Australia, 2005. [Google Scholar]
- Provis, J.L.; Yong, C.Z.; Duxson, P.; van Deventer, J. Correlating mechanical and thermal properties of sodium silicate-fly ash geopolymers. Colloids Surf. A Physicochem. Eng. 2009, 336, 57–63. [Google Scholar] [CrossRef]
- Sumajouw, D.; Hardjito, D.; Wallah, S.; Rangan, B. Fly ash-based geopolymer concrete: Study of slender reinforced columns. J. Mater. Sci. 2007, 42, 3124–3130. [Google Scholar] [CrossRef]
- Vora, P.; Dave, U. Parametric Studies on Compressive Strength of Geopolymer Concrete. Procedia Eng. 2013, 51, 210–219. [Google Scholar] [CrossRef]
- Sindhunata; van Deventer, J.S.J.; Lukey, G.C.; Xu, H. Effect of Curing Temperature and Silicate Concentration on Fly-Ash-Based Geopolymerization. Ind. Eng. Chem. Res. 2006, 45, 3559–3569. [Google Scholar] [CrossRef]
- Raijiwala, D.B.; Patil, H.S. Geopolymer concrete: A green concrete. In Proceedings of the 2nd International Conference on Chemical, Biological and Environmental Engineering (ICBEE 2010), Cairo, Egypt, 2–4 November 2010. [Google Scholar]
- Alonso, S.; Palomo, A. Alkaline activation of metakaolin and calcium hydroxide mixtures: Influence of temperature, activator concentration and solids ratio. Mater. Lett. 2001, 47, 55–62. [Google Scholar] [CrossRef]
- Memon, F.; Nuruddin, M.F.; Khan, S.H.; Shafiq, N.R. Effect of sodium hydroxide concentration on fresh properties and compressive strength of self-compacting geopolymer concrete. J. Eng. Sci. Technol. 2013, 8, 44–56. [Google Scholar]
- Barbosa, V.; Mackenzie, K.; Thaumaturgo, C. Synthesis and characterisation of sodium polysialate inorganic polymer based on alumina and silica. In Proceedings of the Geopolymer’99 International Conference, Saint-Quentin, Geopolymer Institute, Saint-Quentin, France, 30 June–2 July 1999. [Google Scholar]
- Luhar, S.; Dave, U. Investigations on mechanical properties of fly ash and slag based geopolymer concrete. Ind. Concr. J. 2016, 34–41. [Google Scholar]
- Ma, Y.; Hu, J.; Ye, G. The effect of activating solution on the mechanical strength, reaction rate, mineralogy, and microstructure of alkali-activated fly ash. J. Mater. Sci. 2012, 47, 4568–4578. [Google Scholar] [CrossRef]
- Xie, J.; Yin, J.; Chen, J.; Xu, J. Study on the geopolymer based on fly ash and slag. Energy Environ. 2009, 3, 578–581. [Google Scholar]
- van Jaarsveld, J.G.S.; van Deventer, J.S.J.; Lukey, G.C. The effect of composition and temperature on the properties of fly ash-and kaolinite-based geopolymers. J. Chem. Eng. 2002, 89, 63–73. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Chareerat, T.; Hatanaka, S.; Cao, T. High-strength geopolymer using fine high-calcium fly ash. Mater. Civ. Eng. 2010, 23, 264–270. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Garcia-Lodeiro, I.; Palomo, A. Durability of alkali-activated fly ash cementitious materials. J. Mater. Sci. 2007, 42, 3055–3065. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T.; Sinsiri, T. Workability and compressive strength of geopolymer mortar from fly ash containing diatomite. Eng. J. 2011, 38, 11–26. [Google Scholar]
- Kong, D.; Sanjayan, J. Effect of elevated temperatures on geopolymer paste, mortar and concrete. Cem. Concr. Res. 2010, 40, 334–339. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H. Self-solidification/stabilization of heavy metal wastes of class C fly ash-based geopolymers. J. Mater. Civ. Eng. 2012, 25, 491–496. [Google Scholar] [CrossRef]
- Rashad, A.; Zeedan, S. The effect of activator concentration on the residual strength of alkali-activated fly ash pastes subjected to thermal load. Constr. Build. Mater. 2011, 25, 3098–3107. [Google Scholar] [CrossRef]
- Sukmak, P.; Horpibulsuk, S.; Shen, S. Strength development in clay-fly ash geopolymer. Contr. Build. Mater. 2013, 40, 566–574. [Google Scholar] [CrossRef]
- Taebuanhuad, S.; Rattanasak, U.; Jenjirapanya, S. Strength behavior of fly ash geopolymer with microwave pre-radiation curing. J. Ind. Technol. 2012, 8, 1–8. [Google Scholar]
- Lăzărescu, A.V.; Szilagyi, H.; Baeră, C.; Ioani, A. Parameters Affecting the Mechanical Properties of Fly Ash-Based Geopolymer Binders–Experimental Results. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012035. [Google Scholar] [CrossRef]
- Hardjito, D.; Rangan, B.V. Development and Properties of Low-Calcium Fly Ash-Based Geopolymer Concrete; Technical Report GC3; Civil Engineering Faculty, Technical University: Perth, Australia, 2006. [Google Scholar]
- Omar, O.M.; Heniegal, A.M.; Abd Elhameed, G.D.; Mohamadien, H.A. Effect of Local Steel Slag as a Coarse Aggregate on Properties of Fly Ash Based-Geopolymer Concrete. Int. J. Civ. Environ. 2015, 3, 1452–1460. [Google Scholar]
- Perera, D.S.; Uchida, O.; Vance, E.R.; Finnie, K.S. Influence of curing schedule on the integrity of geopolymers. J. Mater. Sci. 2007, 42, 3099–3106. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. Microstructural characterisation of geopolymers synthesised from kaolinite/stilbite mixtures using XRD, MAS-NMR, SEM/EDX, TEM/EDX, and HREM. Cem. Concr. Res. 2002, 32, 1705–1716. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Kim, A.G. Fly ash characterization by SEM–EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Kumari, N.; Mohan, C. Basics of clay minerals and their characteristic properties. Clay Clay Miner. 2021, 24, 1–29. [Google Scholar]
- Velde, B. Electron microprobe analysis of clay minerals. Clay Miner. 1984, 19, 243–247. [Google Scholar] [CrossRef]
- Gomes, S.; Francois, M. Characterisation of mullite in silicoaluminous fly ash by XRD, TEM, and 29Si MAS NMR. Cem. Concr. Res. 2020, 30, 175–181. [Google Scholar] [CrossRef]
- Derbe, T.; Temesgen, S.; Bitew, M. A Short Review on Synthesis, Characterization, and Applications of Zeolites. Hindawi Adv. Mater. Sci. Eng. 2021, 2021, 6637898. [Google Scholar] [CrossRef]
- Chen, X.; Wang, G.; Dong, Q.; Zhao, X.; Wang, Y. Microscopic characterizations of pervious concrete using recycled Steel Slag Aggregate. J. Clean. Prod. 2020, 254, 120149. [Google Scholar] [CrossRef]
- Lyu, K.; She, W.; Miao, C.; Chang, H.; Gu, Y. Quantitative characterization of pore morphology in hardened cement paste via SEM-BSE image analysis. Constr. Build. Mater. 2019, 202, 589–602. [Google Scholar] [CrossRef]
- Galiano, L.Y.; Pereira, F.C.; Izquierdo, M. Contributions to the study of porosity in fly ash-based geopolymers. Relationship between degree of reaction, porosity and compressive strength. Mater. Construcción. 2016, 66, 324. [Google Scholar]
- Hudson, B.P. Modification to the fine aggregate angularity test investigation into the way we measure fine aggregate angularity. In Proceedings of the 7th Annual Symposium, International Center for Aggregate Research (ICAR) Symposium, Austin, TX, USA, 19–21 April 1999. [Google Scholar]
- Sreenivasulu, C.; Jawahar, J.G.; Reddy, M.V.S.; Kumar, D.P. Effect of fine aggregate blending on short-term mechanical properties of geopolymer concrete. Asian J. Civ. Eng. (BHRC) 2016, 17, 537–550. [Google Scholar]
- Nuaklong, P.; Sata, V.; Chindaprasirt, P. Influence of recycled aggregate on fly ash geopolymer concrete properties. J. Clean. Prod. 2016, 112, 2300–2307. [Google Scholar] [CrossRef]
- Jahromi, S.G. Estimation of resistance to moisture destruction in asphalt mixtures. Constr. Build. Mater. 2009, 23, 2324–2331. [Google Scholar] [CrossRef]
- Bagampadde, U.; Isacsson, U.; Kiggundu, B.M. Impact of bitumen and aggregate composition on stripping in bituminous mixtures. Mater. Struct. 2006, 39, 303–315. [Google Scholar] [CrossRef]
- Lăzărescu, A.-V.; Ionescu, B.A.; Hegyi, A.; Florean, C. Analysis Regarding the Mechanical Properties of Alkali-Activated Fly Ash-Based Geopolymer Concrete Containing Spent Garnet as Replacement for Sand Aggregates. EJMSE 2023, 8, 11–21. [Google Scholar] [CrossRef]
- SR EN 196-1:2016; Methods of Testing Cement—Part 1: Determination of Strength. ASRO: Bucharest, Romania, 2016. (In Romanian)
- SR EN 1097-3:2002; Tests for Mechanical and Physical Properties of Aggregates Determination of Loose Bulk Density and Voids. ASRO: Bucharest, Romania, 2003. (In Romanian)
- Lloyd, R.R.; Provis, J.L.; Smeaton, K.J.; Van Deventer, J.S.J. Spatial distribution of pores in fly ash-based inorganic polymer gels visualised by Wood’s metal intrusion. Micropor. Mesopor. Mater. 2009, 126, 32–39. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, Y.; Huajun, Z. Potential application of geopolymers as protection coatings for marine concrete. II. Microstructure and anticorrosion mechanism. Appl. Clay. Sci. 2010, 49, 7–12. [Google Scholar] [CrossRef]
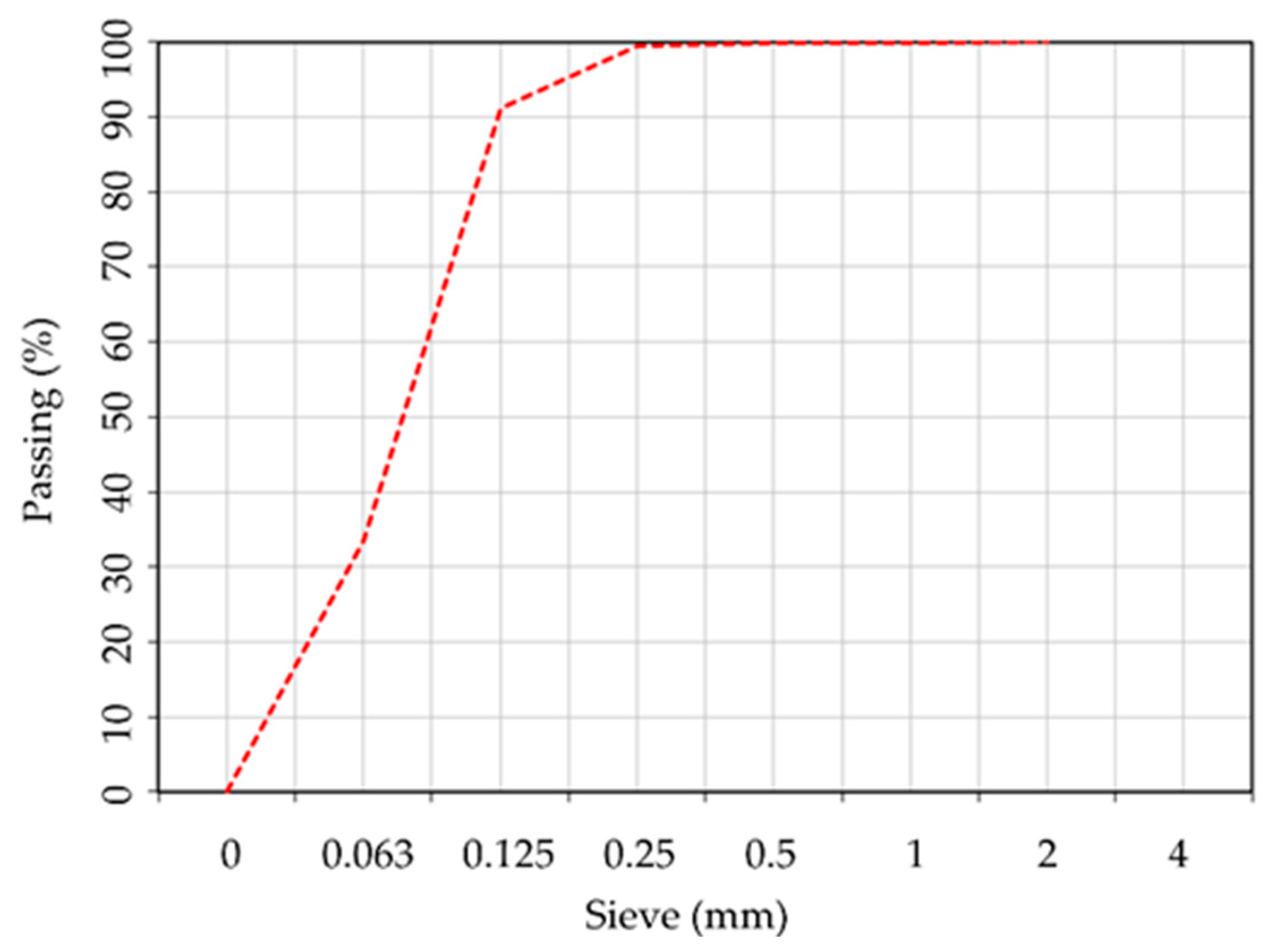

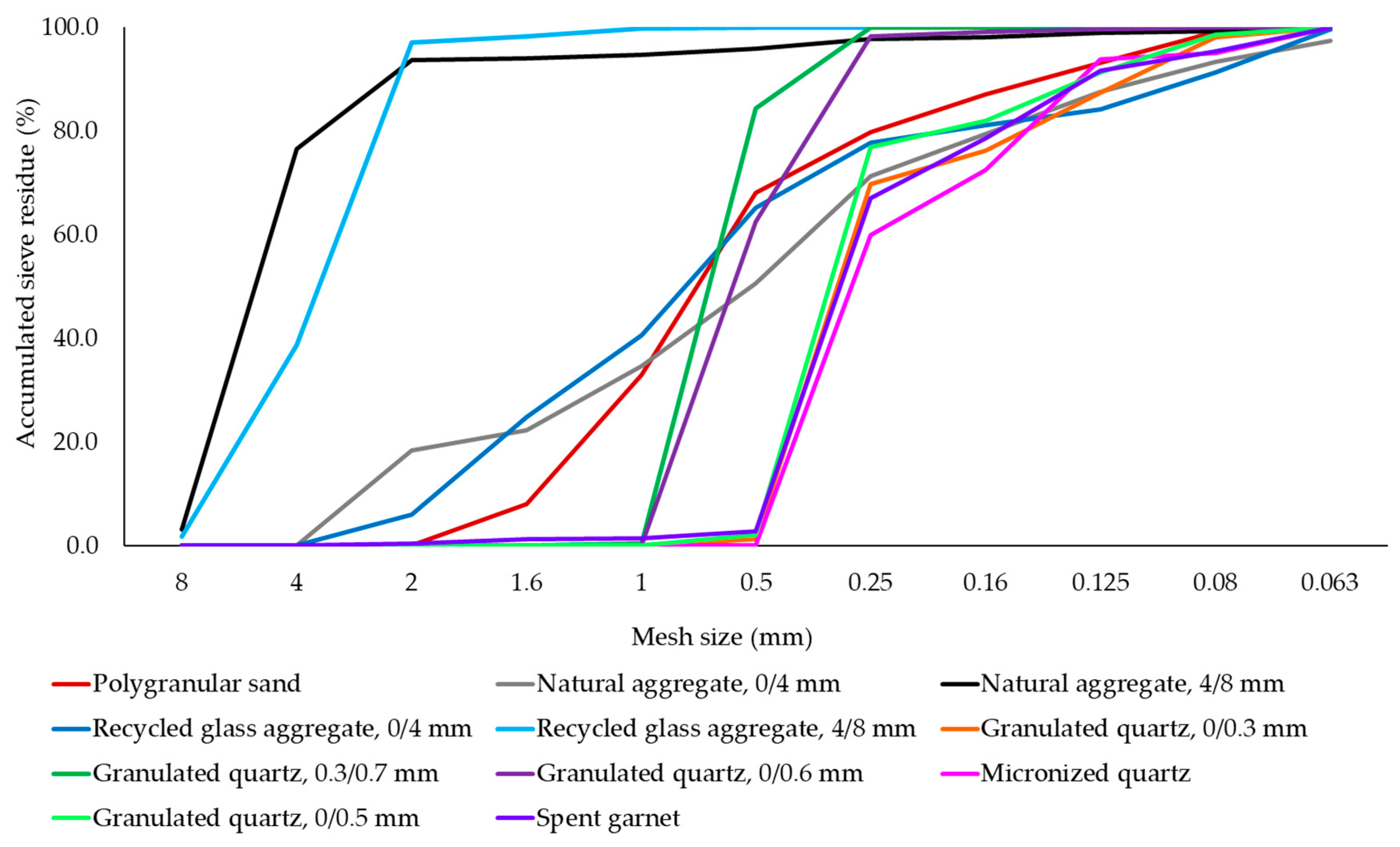


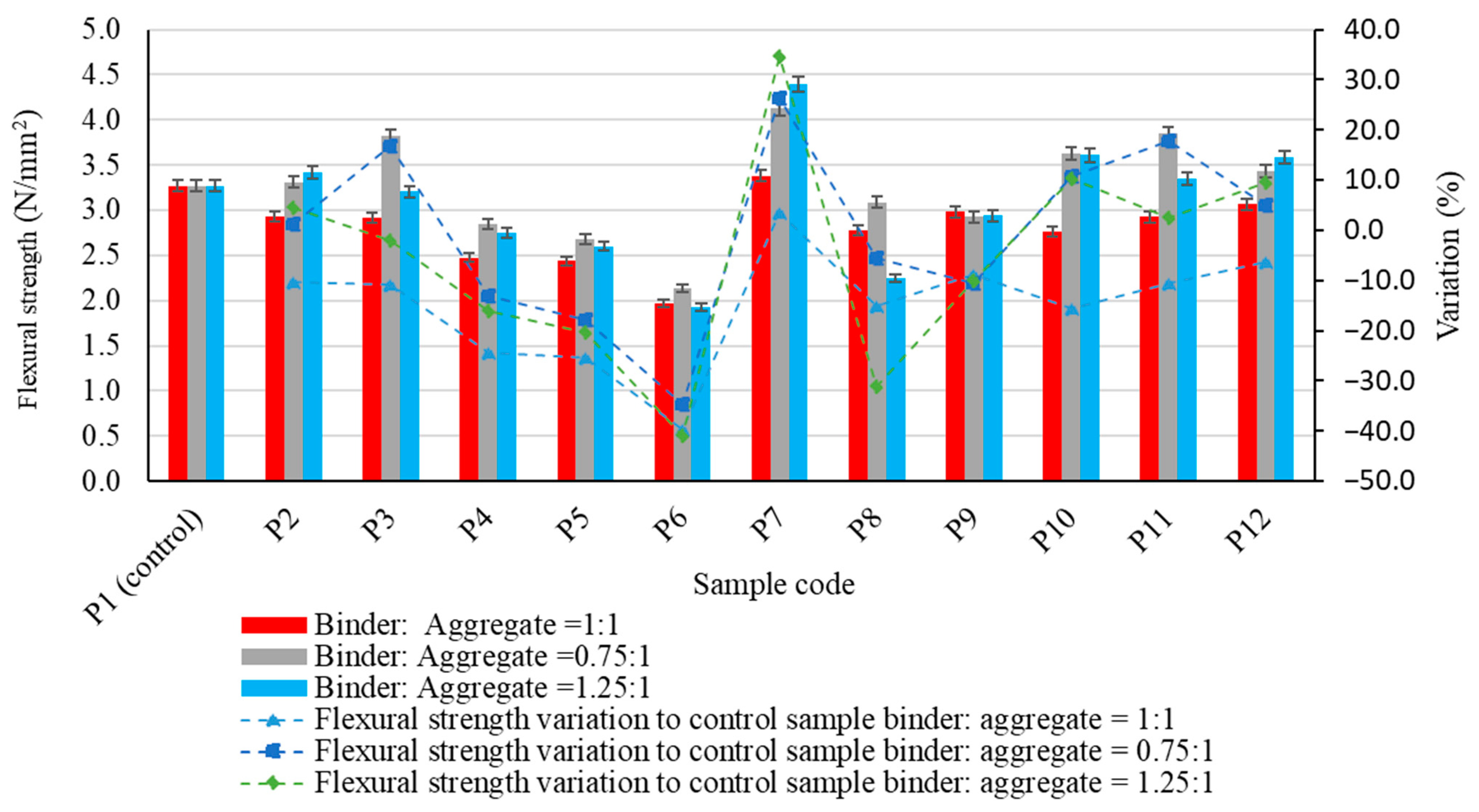
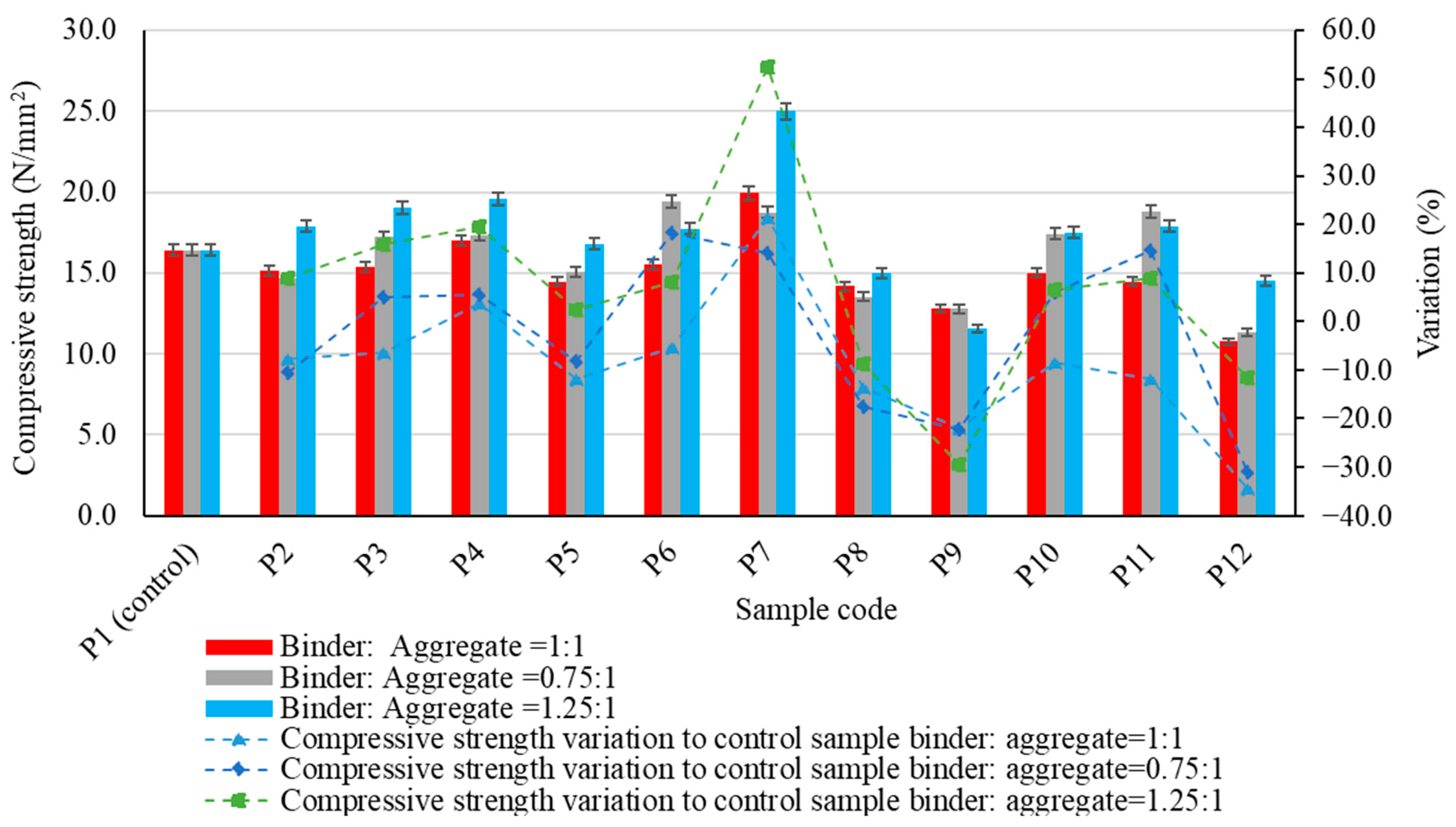


| Oxide composition (%) | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | P2O₅ |
| 46.94 | 23.83 | 10.08 | 10.72 | 2.63 | 0.45 | 0.62 | 1.65 | 0.25 | |
| TiO2 | Cr2O3 | Mn2O3 | ZnO | SrO | CO2 | P.C. | SiO2+Al2O3 | ||
| 0.92 | 0.02 | 0.06 | 0.02 | 0.03 | - | 2.11 | 70.77 | ||
| R0.045 (%) | 31.40 | ||||||||
| Aggregate Type | Polygranular Sand | Natural Aggregates 0/4 mm | Natural Aggregates 4/8 mm | Glass Aggregate Reciclată 0/4 mm | Glass Aggregate Reciclată 4/8 mm | Micronized Quartz | Granulated Quartz 0/0.3 mm | Granulated Quartz 0/0.5 mm | Granulated Quartz 0/0.6 mm | Granulated Quartz 0.3/0.7 mm | Spent Garnet |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bulk apparent density (kg/m3) | 1660 | 1580 | 1530 | 1350 | 1430 | 1030 | 1430 | 1440 | 1450 | 1420 | 2690 |
| Mixture | Spectra | O % | S %i | Na % | Al % | Ca % | Fe % | K % | Mg % |
|---|---|---|---|---|---|---|---|---|---|
| P1a | S1 | 49.8 | 23.8 | 10.3 | 8.4 | 3 | 2.3 | 1.8 | 0.6 |
| S2 | 48 | 20.3 | 7.6 | 17 | 1.4 | 2.3 | 1.1 | 1 | |
| S3 | 44.3 | 27.7 | 6.9 | 9.2 | 1.7 | 5.6 | 2 | 1.4 | |
| S4 | 44.6 | 24.3 | 8.2 | 9.7 | 3.2 | 6.1 | 2.2 | 1 | |
| P5a | S1 | 53.3 | 22.3 | 6.4 | 9.2 | 1.2 | 3.8 | 1.6 | 1.4 |
| S2 | 34.9 | 16.6 | 3.9 | 8.1 | 14.7 | 19.3 | 1.2 | 0.5 | |
| S3 | 51.4 | 23.5 | 10 | 6 | 3.1 | 2 | 1.4 | 1.9 | |
| S4 | 46.4 | 26.2 | 8.7 | 8 | 5.1 | 2.5 | 2.2 | 0.9 | |
| P7a | S1 | 55.1 | 12.2 | 26 | 2.2 | 1 | 1.2 | 0.5 | 0.3 |
| S2 | 45.8 | 31.9 | 4.5 | 4.1 | 3.8 | 8 | 1.3 | 0.6 | |
| S3 | 52.3 | 28.5 | 6.7 | 5.9 | 2 | 2.2 | 1.2 | 1 | |
| S4 | 49.2 | 36.2 | 4.9 | 4.2 | 2.2 | 1.8 | 1.2 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lăzărescu, A.-V.; Hegyi, A.; Csapai, A.; Popa, F. The Influence of Different Aggregates on the Physico-Mechanical Performance of Alkali-Activated Geopolymer Composites Produced Using Romanian Fly Ash. Materials 2024, 17, 485. https://doi.org/10.3390/ma17020485
Lăzărescu A-V, Hegyi A, Csapai A, Popa F. The Influence of Different Aggregates on the Physico-Mechanical Performance of Alkali-Activated Geopolymer Composites Produced Using Romanian Fly Ash. Materials. 2024; 17(2):485. https://doi.org/10.3390/ma17020485
Chicago/Turabian StyleLăzărescu, Adrian-Victor, Andreea Hegyi, Alexandra Csapai, and Florin Popa. 2024. "The Influence of Different Aggregates on the Physico-Mechanical Performance of Alkali-Activated Geopolymer Composites Produced Using Romanian Fly Ash" Materials 17, no. 2: 485. https://doi.org/10.3390/ma17020485





