Mapping Uncharted Lead-Free Halide Perovskites and Related Low-Dimensional Structures
Abstract
:1. Introduction: Synthetic Lead-Free Halide Perovskites and Related Low-Dimensional Structures
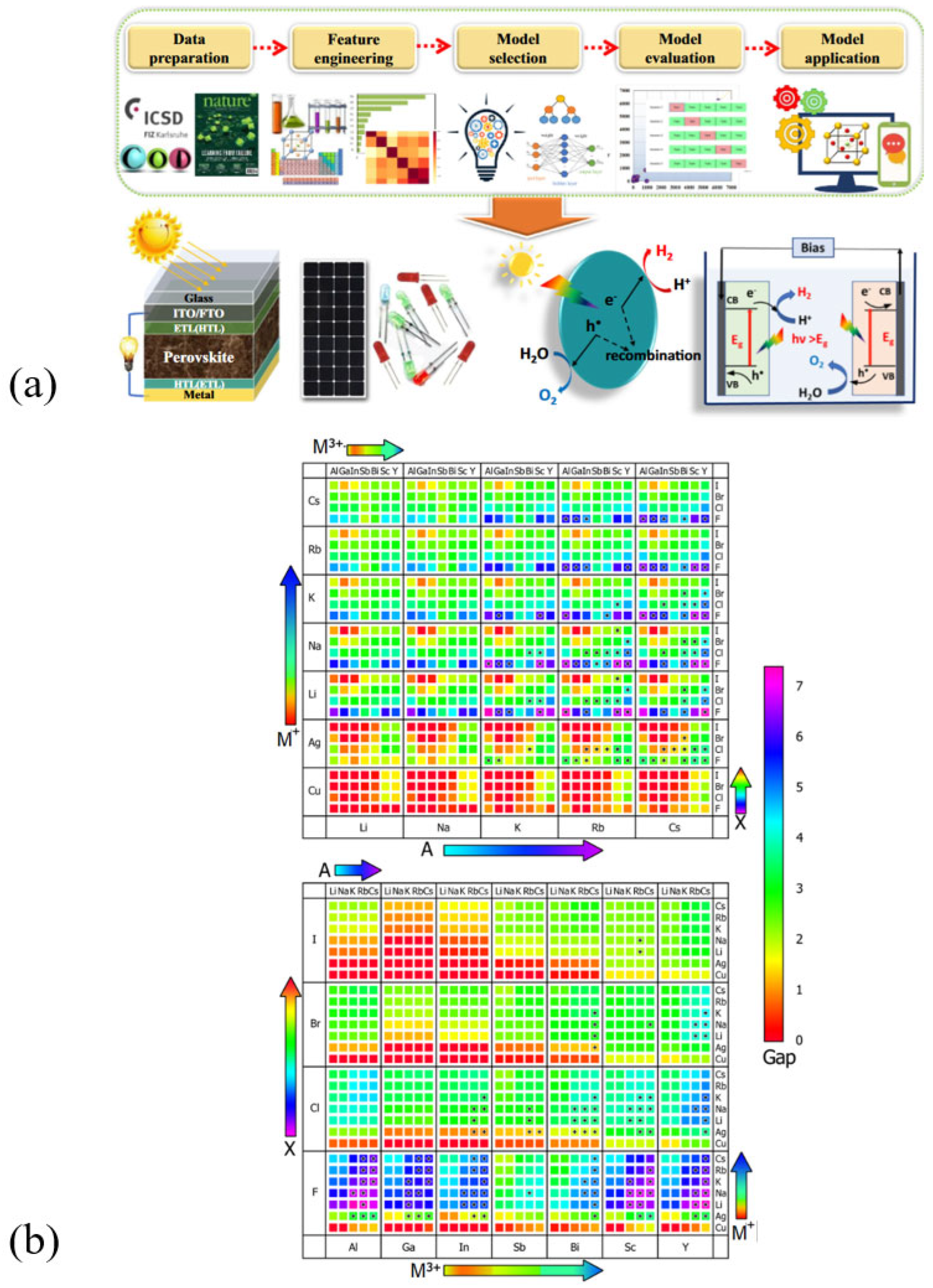
2. Extract of Roadmaps on Inorganic Lead-Free Halide Double Perovskites: Versatility, Properties, Development, and Challenges
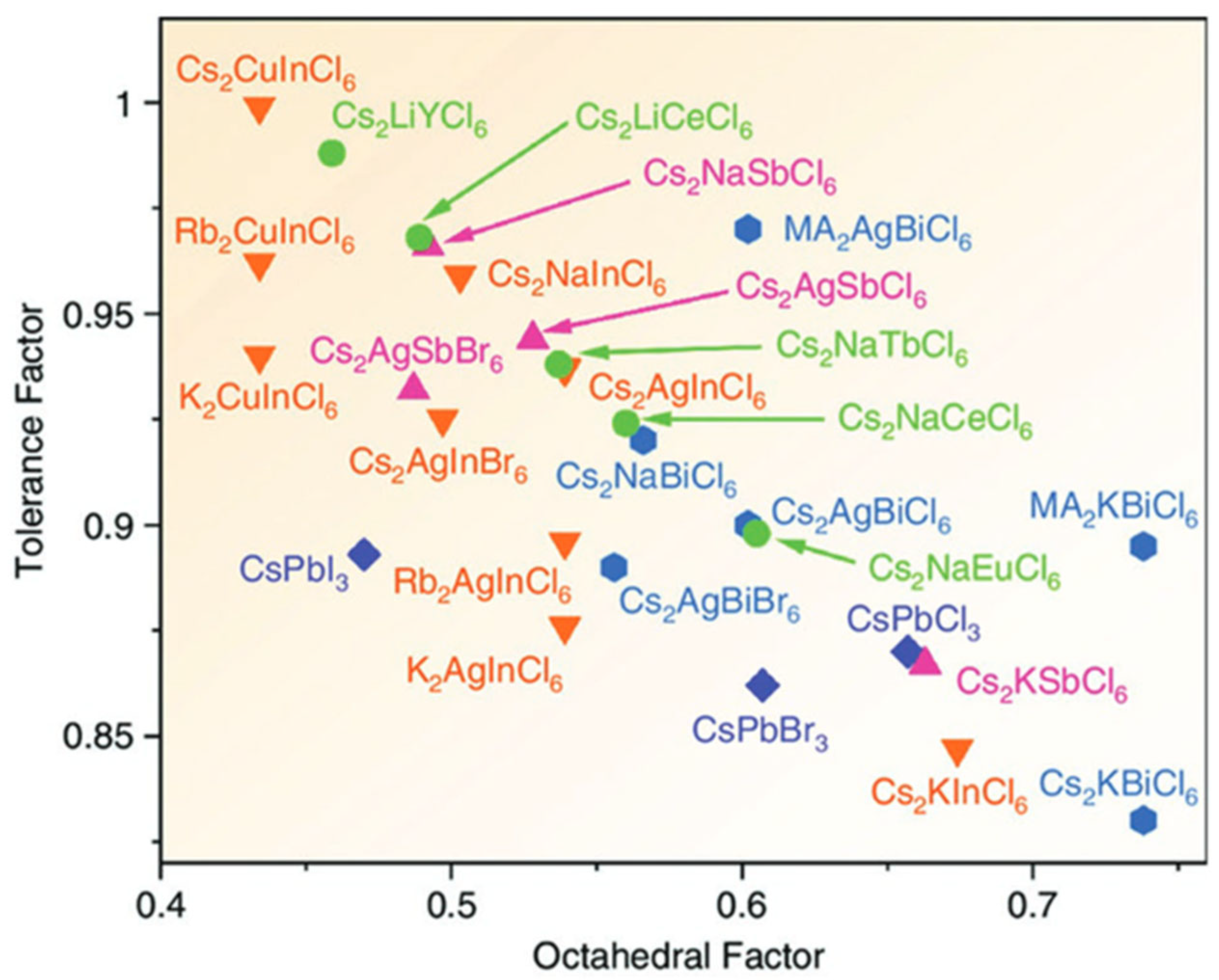
2.1. Mapping the Stability of Halide Double Perovskites
- -
- Goldschmidt tolerance factor:
- -
- Octahedral factor:
- -
- Tolerance factor by Bartel et al. [34]:
2.2. Electronic and Optical Characteristics and Application of Halide DPs
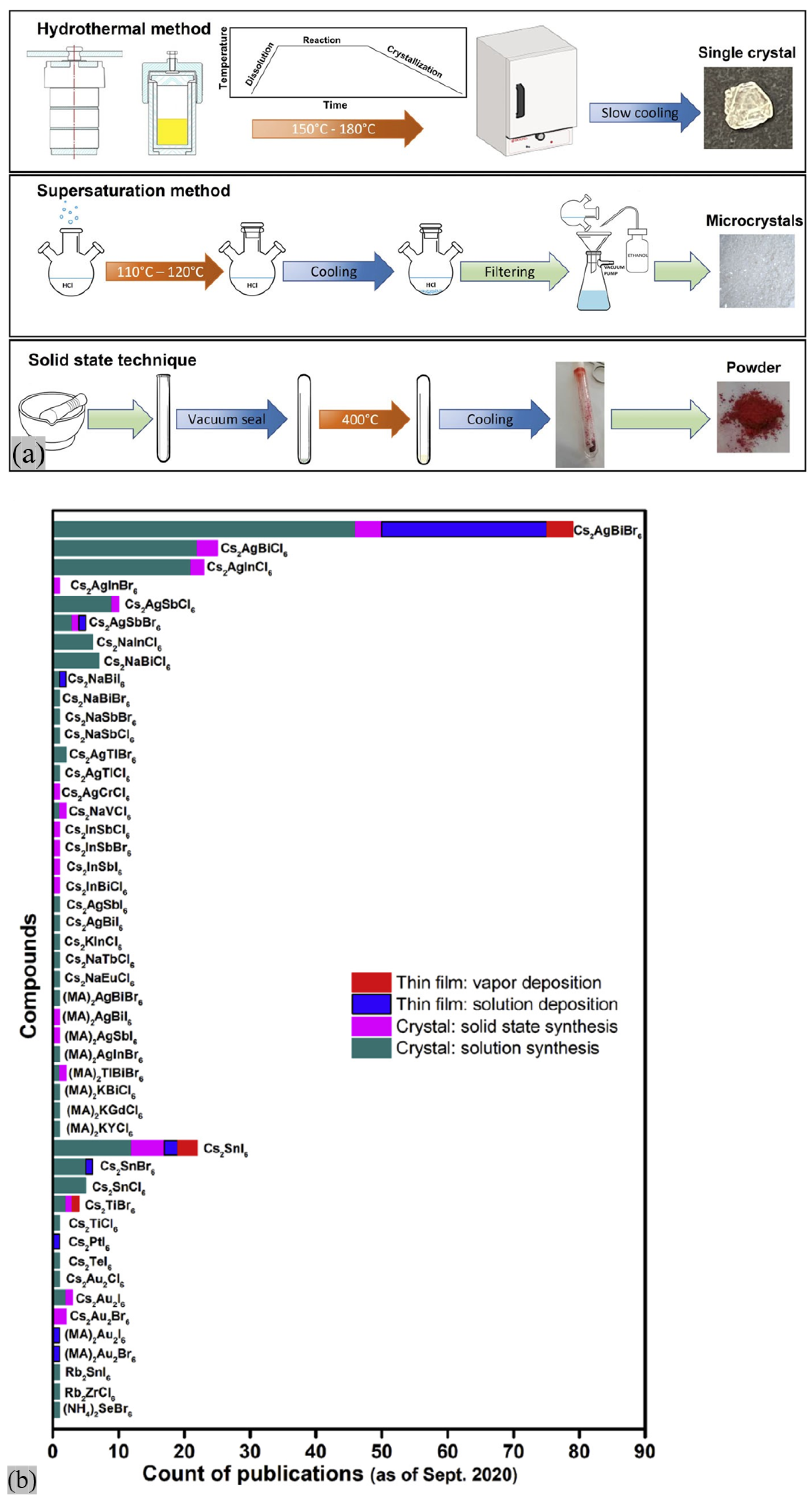
2.3. Manifesto of DPs: Synthetic Techniques and Technological Challenges
3. Low-Dimensional Lead-Free Halide Perovskite Derivatives
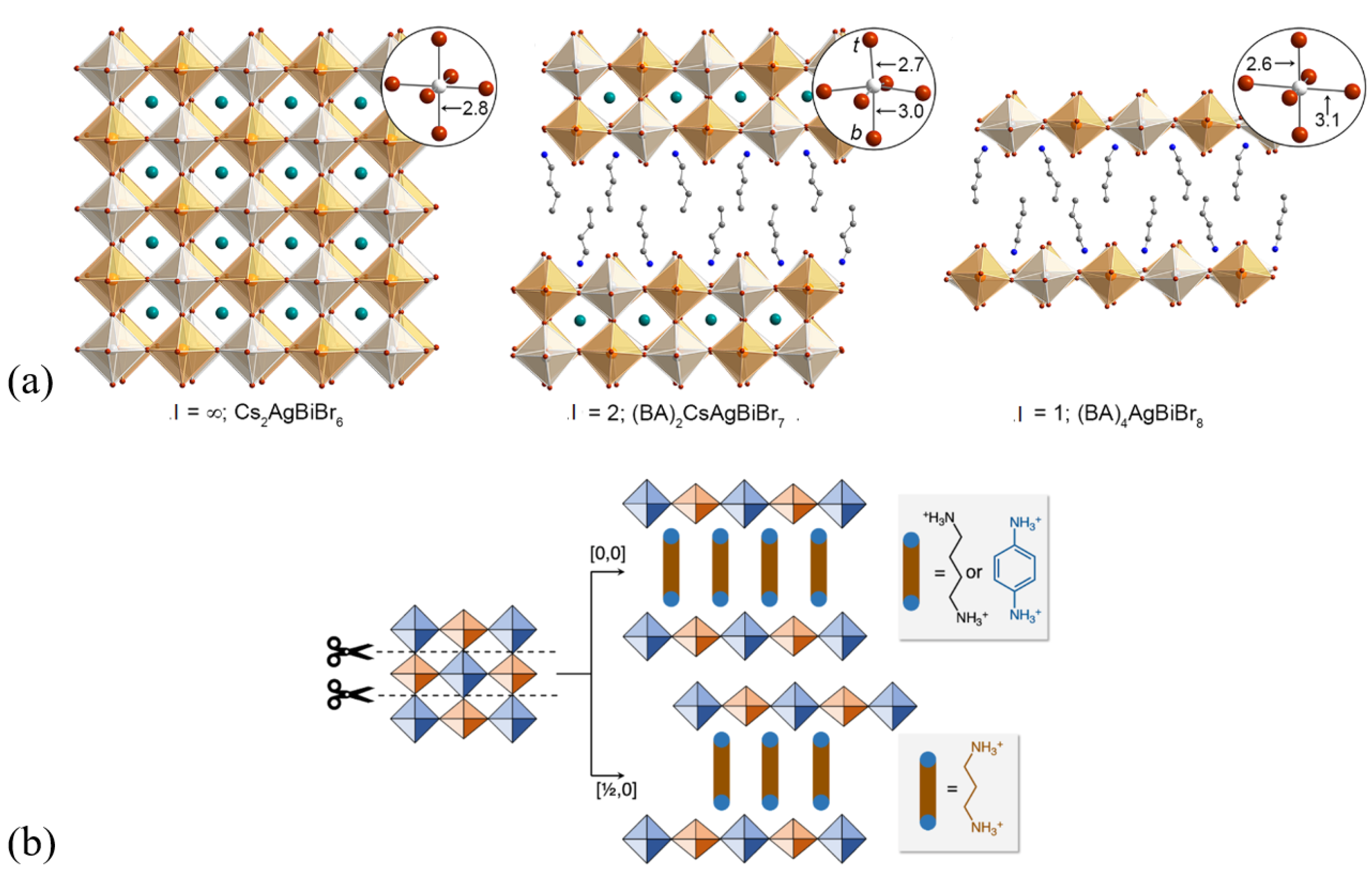
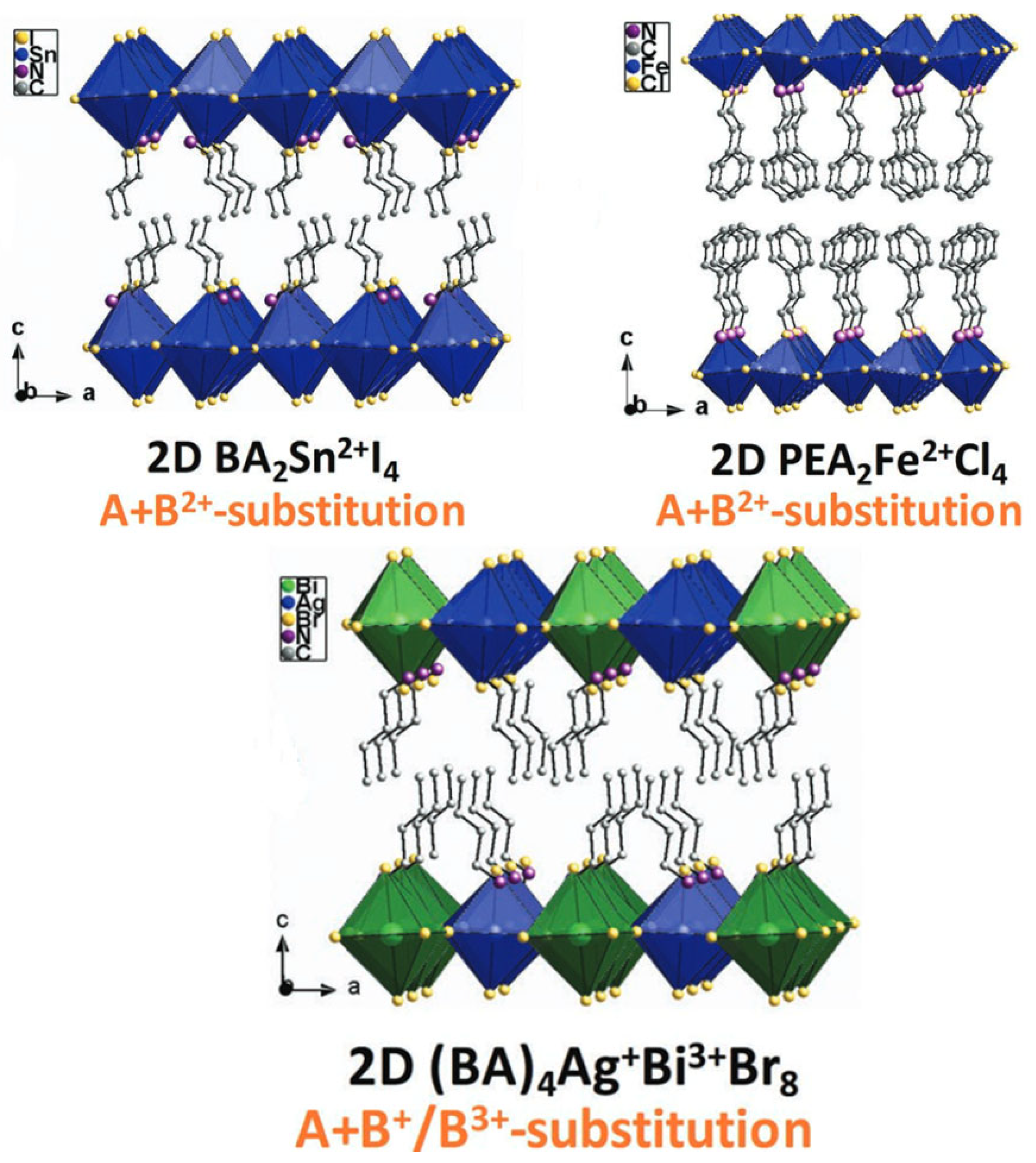
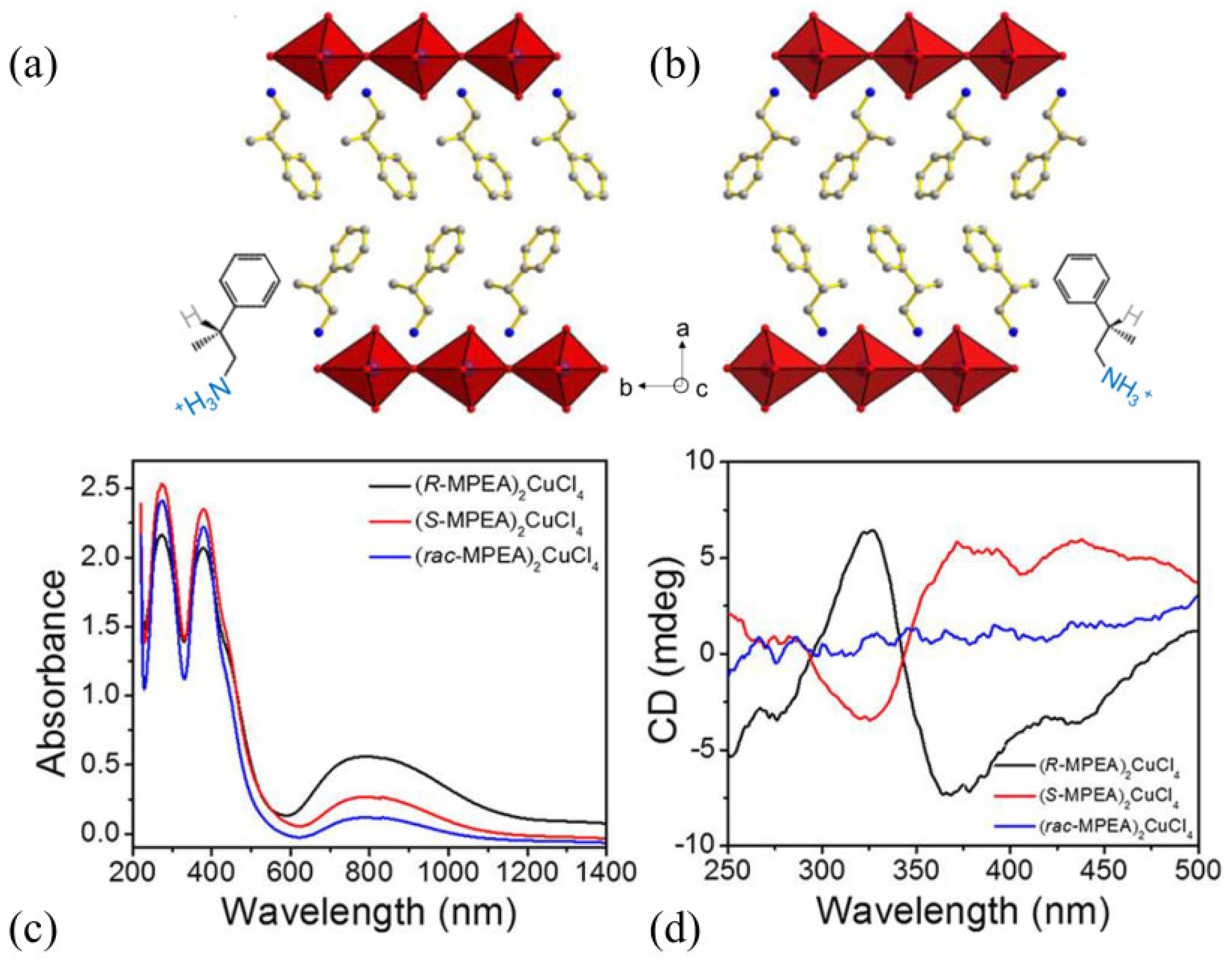
3.1. Hybrid Organic–Inorganic 2D Layered Halide-Perovskite-Related Structures
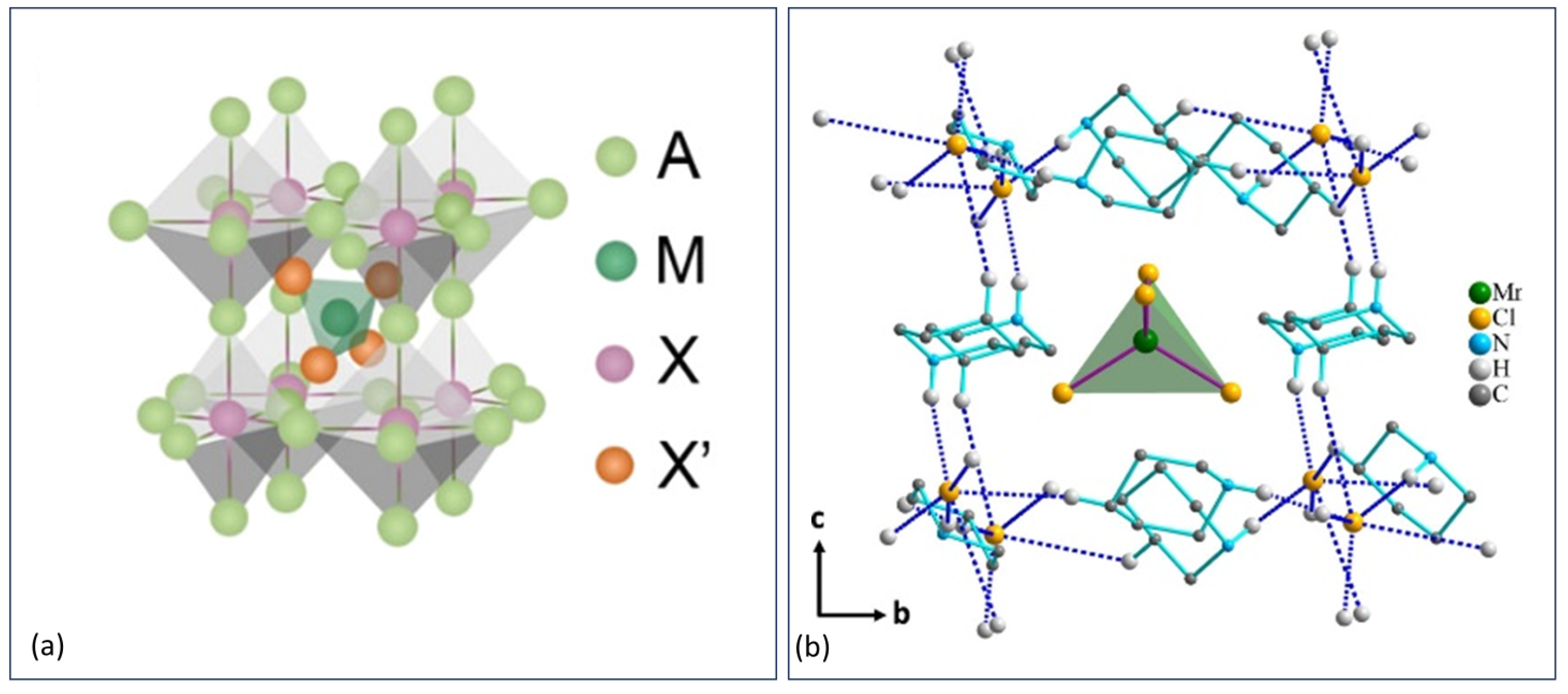
3.2. Electronically Zero-Dimensional and Perovskite-Inspired Crystal Structures
3.2.1. 0D Anti-Perovskites
3.2.2. Perovskite-Inspired Structures
Definition of Different Crystals
Properties
Applications
Synthesis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stranks, S.D.; Snaith, H.J. Metal-Halide Perovskites for Photovoltaic and Light-Emitting Devices. Nat. Nanotechnol. 2015, 10, 391–402. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed]
- Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 8 November 2023).
- Jiang, P.; Acharya, D.; Volonakis, G.; Zacharias, M.; Kepenekian, M.; Pedesseau, L.; Katan, C.; Even, J. Pb-Free Halide Perovskites for Solar Cells, Light-Emitting Diodes, and Photocatalysts. APL Mater. 2022, 10, 060902. [Google Scholar] [CrossRef]
- Argillander, J.; Alarcón, A.; Bao, C.; Kuang, C.; Lima, G.; Gao, F.; Xavier, G.B. Quantum Random Number Generation Based on a Perovskite Light Emitting Diode. Commun. Phys. 2023, 6, 157. [Google Scholar] [CrossRef]
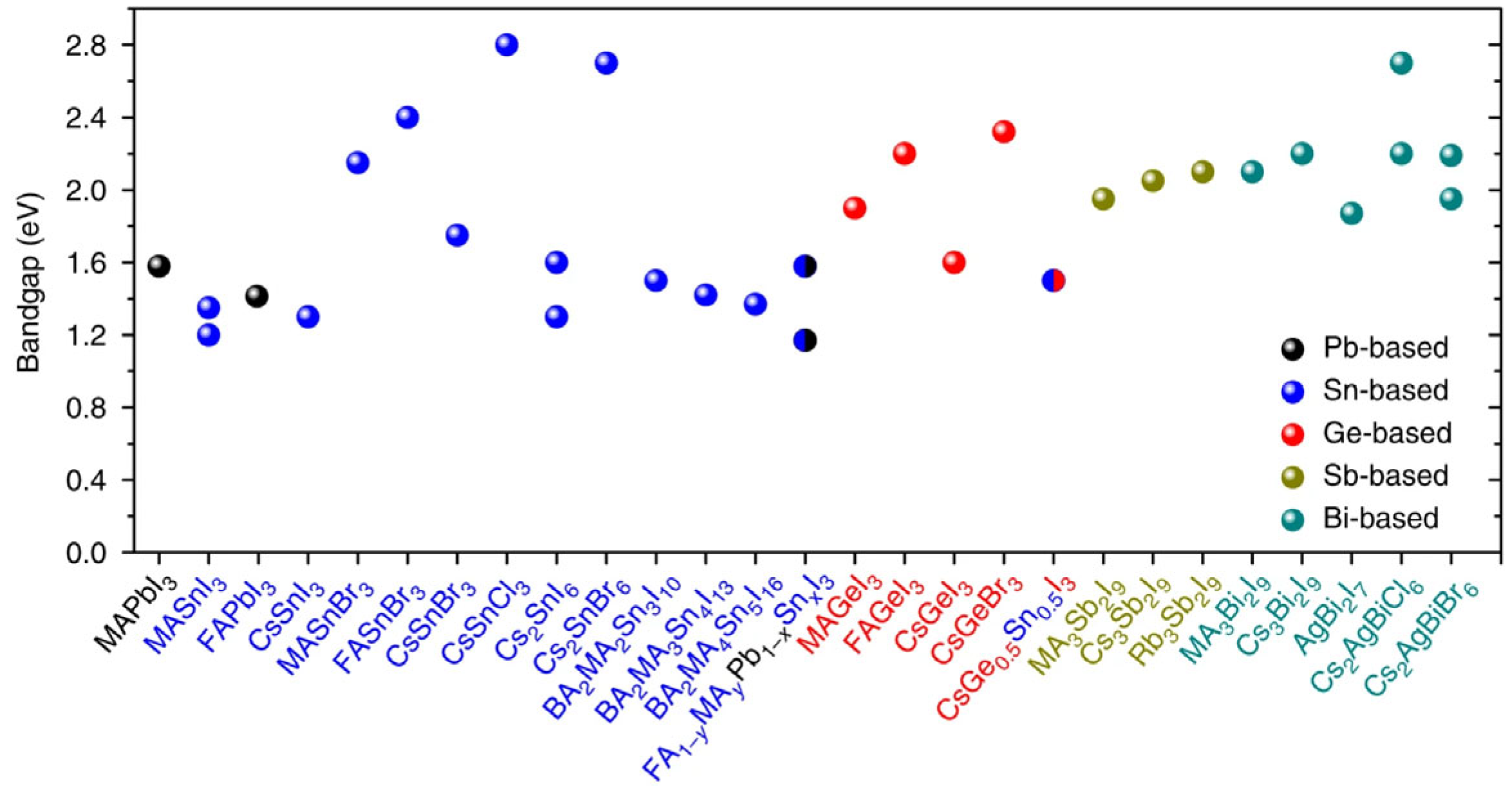
- Xiao, Z.; Song, Z.; Yan, Y. From Lead Halide Perovskites to Lead-Free Metal Halide Perovskites and Perovskite Derivatives. Adv. Mater. 2019, 31, 1803792. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wang, C.; Li, J.; Su, Z.; Xing, G.; Gao, X.; Chen, S. In Situ and Operando Characterization Techniques in Stability Study of Perovskite-Based Devices. Nanomaterials 2023, 13, 1983. [Google Scholar] [CrossRef] [PubMed]
- Schileo, G.; Grancini, G. Lead or No Lead? Availability, Toxicity, Sustainability and Environmental Impact of Lead-Free Perovskite Solar Cells. J. Mater. Chem. C 2021, 9, 67–76. [Google Scholar] [CrossRef]
- Sofia, S.E.; Wang, H.; Bruno, A.; Cruz-Campa, J.L.; Buonassisi, T.; Peters, I.M. Roadmap for Cost-Effective, Commercially-Viable Perovskite Silicon Tandems for the Current and Future PV Market. Sustain. Energy Fuels 2020, 4, 852–862. [Google Scholar] [CrossRef]
- Vidal, R.; Alberola-Borràs, J.; Sánchez-Pantoja, N.; Mora-Seró, I. Comparison of Perovskite Solar Cells with Other Photovoltaics Technologies from the Point of View of Life Cycle Assessment. Adv. Energy Sustain. Res. 2021, 2, 2000088. [Google Scholar] [CrossRef]
- Li, J.; Cao, H.-L.; Jiao, W.-B.; Wang, Q.; Wei, M.; Cantone, I.; Lü, J.; Abate, A. Biological Impact of Lead from Halide Perovskites Reveals the Risk of Introducing a Safe Threshold. Nat. Commun. 2020, 11, 310. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public. Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, X.; Deng, Y.; Li, B.; Yuan, C. Life Cycle Assessment of Titania Perovskite Solar Cell Technology for Sustainable Design and Manufacturing. ChemSusChem 2015, 8, 3882–3891. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cruse, K.; Abdelsamie, M.; Ceder, G.; Sutter-Fella, C.M. Synthetic Approaches for Thin-Film Halide Double Perovskites. Matter 2021, 4, 1801–1831. [Google Scholar] [CrossRef]
- Abate, A. Perovskite Solar Cells Go Lead Free. Joule 2017, 1, 659–664. [Google Scholar] [CrossRef]
- Ke, W.; Kanatzidis, M.G. Prospects for Low-Toxicity Lead-Free Perovskite Solar Cells. Nat. Commun. 2019, 10, 965. [Google Scholar] [CrossRef] [PubMed]
- Ben Bechir, M.; Dhaou, M.H.; Altrifi, S.M. Photoluminescence, Raman and Photosensitive Dielectric Properties of Lead-Free Antimony-Based Cs3Sb2Br9 Single Crystals for Energy Storage Devices. Mater. Res. Bull. 2023, 167, 112381. [Google Scholar] [CrossRef]
- Zhao, X.-G.; Yang, D.; Ren, J.-C.; Sun, Y.; Xiao, Z.; Zhang, L. Rational Design of Halide Double Perovskites for Optoelectronic Applications. Joule 2018, 2, 1662–1673. [Google Scholar] [CrossRef]
- Meng, W.; Wang, X.; Xiao, Z.; Wang, J.; Mitzi, D.B.; Yan, Y. Parity-Forbidden Transitions and Their Impact on the Optical Absorption Properties of Lead-Free Metal Halide Perovskites and Double Perovskites. J. Phys. Chem. Lett. 2017, 8, 2999–3007. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Bao, J.; Puttisong, Y.; Moro, F.; Kobera, L.; Shimono, S.; Wang, L.; Ji, F.; Cuartero, M.; Kawaguchi, S.; et al. Magnetizing Lead-Free Halide Double Perovskites. Sci. Adv. 2020, 6, eabb5381. [Google Scholar] [CrossRef]
- Ye, H.-Y.; Tang, Y.-Y.; Li, P.-F.; Liao, W.-Q.; Gao, J.-X.; Hua, X.-N.; Cai, H.; Shi, P.-P.; You, Y.-M.; Xiong, R.-G. Metal-Free Three-Dimensional Perovskite Ferroelectrics. Science 2018, 361, 151–155. [Google Scholar] [CrossRef]
- Ning, W.; Gao, F. Structural and Functional Diversity in Lead-Free Halide Perovskite Materials. Adv. Mater. 2019, 31, 1900326. [Google Scholar] [CrossRef] [PubMed]
- Morana, M.; Malavasi, L. Pressure Effects on Lead-Free Metal Halide Perovskites: A Route to Design Optimized Materials for Photovoltaics. Sol. RRL 2021, 5, 2100550. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Manna, L. What Defines a Halide Perovskite? ACS Energy Lett. 2020, 5, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, Y.; Dong, H.; Li, J.; Zhu, X.; Xu, J.; Pan, F.; Yuan, F.; Dai, J.; Jiao, B.; et al. Highly Efficient and Stable Perovskite Solar Cells Enabled by Low-Dimensional Perovskitoids. Sci. Adv. 2022, 8, eabk2722. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández, I.; Valli, D.; Wang, C.; Samanta, S.; Okamoto, T.; Huang, Y.; Sun, K.; Liu, Y.; Chirvony, V.S.; Patra, A.; et al. Lead-Free Halide Perovskite Materials and Optoelectronic Devices: Progress and Prospective. Adv. Funct. Mater. 2023, 2307896. [Google Scholar] [CrossRef]
- Bhaumik, S.; Ray, S.; Batabyal, S.K. Recent Advances of Lead-Free Metal Halide Perovskite Single Crystals and Nanocrystals: Synthesis, Crystal Structure, Optical Properties, and Their Diverse Applications. Mater. Today Chem. 2020, 18, 100363. [Google Scholar] [CrossRef]
- Ji, G.; Xiao, Z. Jahn−Teller Distortion-Stabilized Halide Double Perovskites with Unusual Rock-Salt-Type Ordering of Divalent B-Site Cations. Chem. Mater. 2022, 34, 8207–8212. [Google Scholar] [CrossRef]
- Ji, G.; Han, C.; Hu, S.; Fu, P.; Chen, X.; Guo, J.; Tang, J.; Xiao, Z. B-Site Columnar-Ordered Halide Double Perovskites: Theoretical Design and Experimental Verification. J. Am. Chem. Soc. 2021, 143, 10275–10281. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, J.; Chen, J.; Tan, Z.; Lei, H. Lead-Free Halide Double Perovskite for High-Performance Photodetectors: Progress and Perspective. Materials 2023, 16, 4490. [Google Scholar] [CrossRef]
- Hutter, E.M.; Gélvez-Rueda, M.C.; Bartesaghi, D.; Grozema, F.C.; Savenije, T.J. Band-Like Charge Transport in Cs2AgBiBr6 and Mixed Antimony–Bismuth Cs2AgBi1–xSbxBr6 Halide Double Perovskites. ACS Omega 2018, 3, 11655–11662. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Wang, F.; Gao, F.; Boschloo, G. Challenges and Progress in Lead-Free Halide Double Perovskite Solar Cells. Sol. RRL 2023, 7, 2201112. [Google Scholar] [CrossRef]
- Bartel, C.J.; Sutton, C.; Goldsmith, B.R.; Ouyang, R.; Musgrave, C.B.; Ghiringhelli, L.M.; Scheffler, M. New Tolerance Factor to Predict the Stability of Perovskite Oxides and Halides. Sci. Adv. 2019, 5, eaav0693. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, X.; Zeng, H. Lead-Free Halide Double Perovskites: Structure, Luminescence, and Applications. Small Struct. 2021, 2, 2000071. [Google Scholar] [CrossRef]
- Xiao, Z.; Du, K.; Meng, W.; Mitzi, D.B.; Yan, Y. Chemical Origin of the Stability Difference between Copper(I)- and Silver(I)-Based Halide Double Perovskites. Angew. Chem. 2017, 129, 12275–12279. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A Materials Genome Approach to Accelerating Materials Innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Zhang, T.; Cai, Z.; Chen, S. Chemical Trends in the Thermodynamic Stability and Band Gaps of 980 Halide Double Perovskites: A High-Throughput First-Principles Study. ACS Appl. Mater. Interfaces 2020, 12, 20680–20690. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.-Q.; Zhang, J. A Machine Learning Model for Screening Thermodynamic Stable Lead-Free Halide Double Perovskites. Comput. Mater. Sci. 2022, 204, 111172. [Google Scholar] [CrossRef]
- Tao, Q.; Xu, P.; Li, M.; Lu, W. Machine Learning for Perovskite Materials Design and Discovery. npj Comput. Mater. 2021, 7, 23. [Google Scholar] [CrossRef]
- Lu, S.; Zhou, Q.; Ouyang, Y.; Guo, Y.; Li, Q.; Wang, J. Accelerated Discovery of Stable Lead-Free Hybrid Organic-Inorganic Perovskites via Machine Learning. Nat. Commun. 2018, 9, 3405. [Google Scholar] [CrossRef]
- Muscarella, L.A.; Hutter, E.M. Halide Double-Perovskite Semiconductors beyond Photovoltaics. ACS Energy Lett. 2022, 7, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Tailor, N.K.; Listorti, A.; Colella, S.; Satapathi, S. Lead-Free Halide Double Perovskites: Fundamentals, Challenges, and Photovoltaics Applications. Adv. Mater. Technol. 2023, 8, 2200442. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, L.; Teo, S.; Guo, Z.; Xu, Z.; Zhao, S.; Ma, T. Design of a Novel and Highly Stable Lead-Free Cs2NaBiI6 Double Perovskite for Photovoltaic Application. Sustain. Energy Fuels 2018, 2, 2419–2428. [Google Scholar] [CrossRef]
- Grandhi, G.K.; Toikkonen, S.; Al-Anesi, B.; Pecunia, V.; Vivo, P. Perovskite-Inspired Cu2AgBiI6 for Mesoscopic Indoor Photovoltaics under Realistic Low-Light Intensity Conditions. Sustain. Energy Fuels 2023, 7, 66–73. [Google Scholar] [CrossRef]
- Sansom, H.C.; Longo, G.; Wright, A.D.; Buizza, L.R.V.; Mahesh, S.; Wenger, B.; Zanella, M.; Abdi-Jalebi, M.; Pitcher, M.J.; Dyer, M.S.; et al. Highly Absorbing Lead-Free Semiconductor Cu2AgBiI6 for Photovoltaic Applications from the Quaternary CuI–AgI–BiI3 Phase Space. J. Am. Chem. Soc. 2021, 143, 3983–3992. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, P.; Wei, S.-H. Band Structure Engineering of Cs2AgBiBr6 Perovskite through Order–Disordered Transition: A First-Principle Study. J. Phys. Chem. Lett. 2018, 9, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Zhang, T.; Wu, Y.; Chen, S. Halide Double-Perovskite Light-Emitting Centers Embedded in Lattice-Matched and Coherent Crystalline Matrix. Adv. Funct. Mater. 2020, 30, 2000653. [Google Scholar] [CrossRef]
- Liu, A.; Zhu, H.; Reo, Y.; Kim, M.-G.; Chu, H.Y.; Lim, J.H.; Kim, H.-J.; Ning, W.; Bai, S.; Noh, Y.-Y. Modulation of Vacancy-Ordered Double Perovskite Cs2SnI6 for Air-Stable Thin-Film Transistors. Cell Rep. Phys. Sci. 2022, 3, 100812. [Google Scholar] [CrossRef]
- Kaltzoglou, A.; Antoniadou, M.; Perganti, D.; Siranidi, E.; Raptis, V.; Trohidou, K.; Psycharis, V.; Kontos, A.G.; Falaras, P. Mixed-Halide Cs2SnI3Br3 Perovskite as Low Resistance Hole-Transporting Material in Dye-Sensitized Solar Cells. Electrochim. Acta 2015, 184, 466–474. [Google Scholar] [CrossRef]
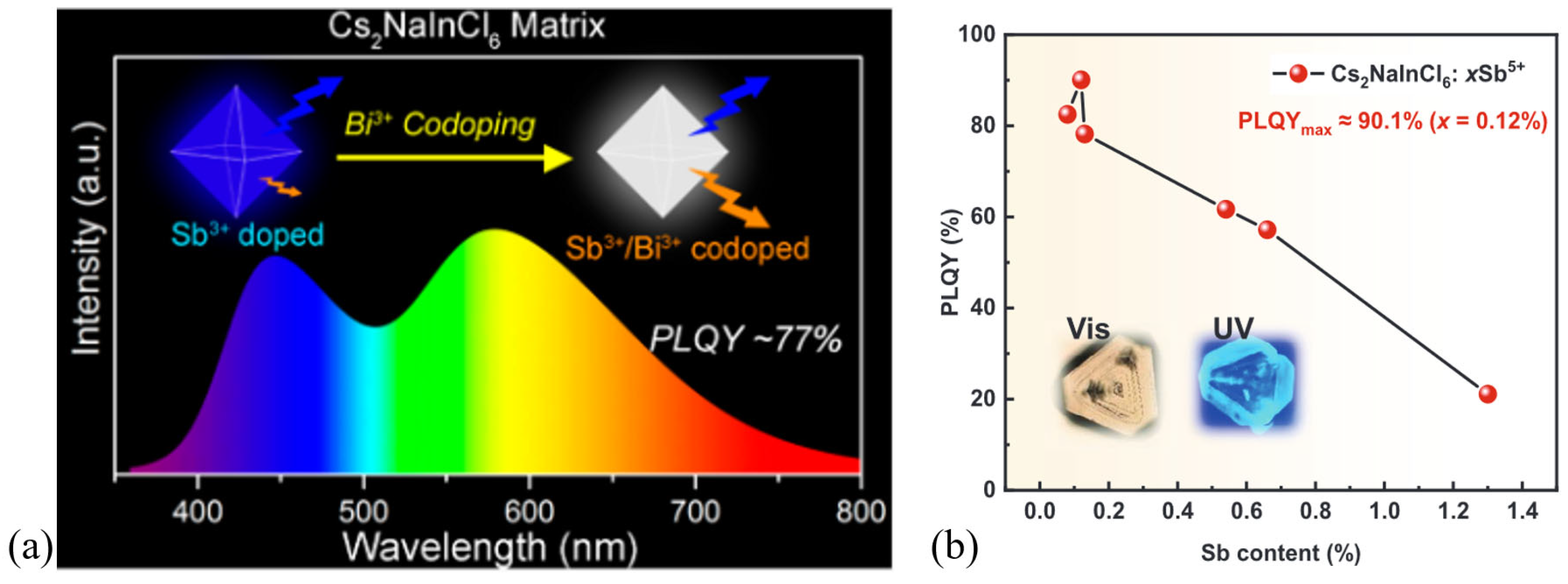
- Zhou, B.; Liu, Z.; Fang, S.; Zhong, H.; Tian, B.; Wang, Y.; Li, H.; Hu, H.; Shi, Y. Efficient White Photoluminescence from Self-Trapped Excitons in Sb3+/Bi3+-Codoped Cs2NaInCl6 Double Perovskites with Tunable Dual-Emission. ACS Energy Lett. 2021, 6, 3343–3351. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, X.; Zeng, X.; Yuan, X.; Wang, Y.; Song, Y.; Chen, H.; Zhang, C.; Wang, Y.; Wan, L.; et al. High-Efficient Blue Emission and Bandgap Engineering from Jahn–Teller Distorted Halide Double Perovskites. Adv. Opt. Mater. 2023, 2301576. [Google Scholar] [CrossRef]
- Noculak, A.; Morad, V.; McCall, K.M.; Yakunin, S.; Shynkarenko, Y.; Wörle, M.; Kovalenko, M.V. Bright Blue and Green Luminescence of Sb(III) in Double Perovskite Cs2MInCl6 (M = Na, K) Matrices. Chem. Mater. 2020, 32, 5118–5124. [Google Scholar] [CrossRef]
- Zhao, F.; Song, Z.; Zhao, J.; Liu, Q. Double Perovskite Cs2AgInCl6:Cr3+: Broadband and near-Infrared Luminescent Materials. Inorg. Chem. Front. 2019, 6, 3621–3628. [Google Scholar] [CrossRef]
- Manna, D.; Das, T.K.; Yella, A. Tunable and Stable White Light Emission in Bi3+-Alloyed Cs2AgInCl6 Double Perovskite Nanocrystals. Chem. Mater. 2019, 31, 10063–10070. [Google Scholar] [CrossRef]
- Li, H.; Tian, L.; Shi, Z.; Li, Y.; Li, C.; Feng, J.; Zhang, H. Double Perovskite Cs2 NaInCl6 Nanocrystals with Intense Dual-Emission via Self-Trapped Exciton-to-Tb3+ Dopant Energy Transfer. J. Mater. Chem. C 2022, 10, 10609–10615. [Google Scholar] [CrossRef]
- Li, S.; Hu, Q.; Luo, J.; Jin, T.; Liu, J.; Li, J.; Tan, Z.; Han, Y.; Zheng, Z.; Zhai, T.; et al. Self-Trapped Exciton to Dopant Energy Transfer in Rare Earth Doped Lead-Free Double Perovskite. Adv. Opt. Mater. 2019, 7, 1901098. [Google Scholar] [CrossRef]
- Hu, M.; Luo, J.; Li, S.; Liu, J.; Li, J.; Tan, Z.; Niu, G.; Wang, Z.; Tang, J. Broadband Emission of Double Perovskite Cs2Na0.4Ag0.6In0.995Bi0.005Cl6:Mn2+ for Single-Phosphor White-Light-Emitting Diodes. Opt. Lett. 2019, 44, 4757–4760. [Google Scholar] [CrossRef]
- Gray, M.B.; Majher, J.D.; Strom, T.A.; Woodward, P.M. Broadband White Emission in Cs2AgIn1–xBixCl6 Phosphors. Inorg. Chem. 2019, 58, 13403–13410. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Fan, T.; Wu, X.; Lü, J.; Fan, W.; Deng, T.; He, H. Highly Efficient Double Perovskite (Bi,Gd)-Codoped Cs2Ag0.4Na0.6InCl6 Phosphors for Warm White LEDs. Ceram. Int. 2022, 48, 29991–29996. [Google Scholar] [CrossRef]
- Li, S.; Shi, Z.; Zhang, F.; Wang, L.; Ma, Z.; Wu, D.; Yang, D.; Chen, X.; Tian, Y.; Zhang, Y.; et al. Ultrastable Lead-Free Double Perovskite Warm-White Light-Emitting Devices with a Lifetime above 1000 Hours. ACS Appl. Mater. Interfaces 2020, 12, 46330–46339. [Google Scholar] [CrossRef]
- Zeng, Z.; Sun, M.; Zhang, S.; Zhang, H.; Shi, X.; Ye, S.; Huang, B.; Du, Y.; Yan, C.-H. Rare-Earth-Based Perovskite Cs2AgScCl6:Bi for Strong Full Visible Spectrum Emission. Adv. Funct. Mater. 2022, 32, 2204780. [Google Scholar] [CrossRef]
- Facile Melting-Crystallization Synthesis of Cs2NaxAg1–xInCl6: Bi Double Perovskites for White Light-Emitting Diodes | Inorganic Chemistry. Available online: https://pubs.acs.org/doi/full/10.1021/acs.inorgchem.1c03996 (accessed on 8 January 2024).
- Guedel, H.U.; Snellgrove, T.R. Jahn-Teller Effect in the 4T2g State of Chromium(III) in Dicesium Sodium Indium(III) Hexachloride. Inorg. Chem. 1978, 17, 1617–1620. [Google Scholar] [CrossRef]
- Reber, C.; Gudel, H.U. Near-infrared luminescence spectroscopy of Al2O3: V3+ and YP3O9: V3+. Chem. Phys. Lett. 1989, 154, 7. [Google Scholar] [CrossRef]
- Arfin, H.; Nag, A. Origin of Luminescence in Sb3+- and Bi3+-Doped Cs2SnCl6 Perovskites: Excited State Relaxation and Spin–Orbit Coupling. J. Phys. Chem. Lett. 2021, 12, 10002–10008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, X.; Li, D.; Yuan, Y.; Xue, X.; Li, Q.; Xu, J.; Wang, H.; Hu, F.; Zhang, X. Investigation on Lead-Free Mn-Doped Cs2NaInCl6 Double Perovskite Phosphors and Their Optical Properties. Opt. Mater. 2021, 122, 111802. [Google Scholar] [CrossRef]
- Li, X.; Shen, X.; Lu, M.; Wu, J.; Zhong, Y.; Wu, Z.; Yu, W.W.; Gao, Y.; Hu, J.; Zhu, J.; et al. Wide-Coverage and Efficient NIR Emission from Single-Component Nanophosphors through Shaping Multiple Metal-Halide Packages. Angew. Chem. Int. Ed. 2023, 62, e202217832. [Google Scholar] [CrossRef] [PubMed]
- Boutinaud, P. ERRATUM: Revisiting Duffy’s Model for Sb3+ and Bi3+ in Double Halide Perovskites: Emergence of a Descriptor for Machine Learning. Opt. Mater. X 2021, 12, 100109. [Google Scholar] [CrossRef]
- Ji, F.; Huang, Y.; Wang, F.; Kobera, L.; Xie, F.; Klarbring, J.; Abbrent, S.; Brus, J.; Yin, C.; Simak, S.I.; et al. Near-Infrared Light-Responsive Cu-Doped Cs2AgBiBr6. Adv. Funct. Mater. 2020, 30, 2005521. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Z.; Wu, C.; Zhang, Y.; Li, X.; Yu, W.; Yao, G.; Liu, S.; Shi, J.; Liu, K.; et al. Extending Absorption of Cs2AgBiBr6 to Near-Infrared Region (≈1350 nm) with Intermediate Band. Adv. Funct. Mater. 2022, 32, 2109891. [Google Scholar] [CrossRef]
- Mopoung, K.; Dávid, A.; Liu, X.; Fahlman, M.; Buyanova, I.A.; Chen, W.M.; Puttisong, Y. Spin Centers in Vanadium-Doped Cs2NaInCl6 Halide Double Perovskites. ACS Mater. Lett. 2024, 6, 566–571. [Google Scholar] [CrossRef]
- Dahl, J.C.; Osowiecki, W.T.; Cai, Y.; Swabeck, J.K.; Bekenstein, Y.; Asta, M.; Chan, E.M.; Alivisatos, A.P. Probing the Stability and Band Gaps of Cs2AgInCl6 and Cs2AgSbCl6 Lead-Free Double Perovskite Nanocrystals. Chem. Mater. 2019, 31, 3134–3143. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, H.; Wang, J.; Yuan, Y.; Hills-Kimball, K.; Cai, T.; Wang, P.; Tang, A.; Chen, O. Synthesis of Lead-Free Cs2AgBiX6 (X = Cl, Br, I) Double Perovskite Nanoplatelets and Their Application in CO2 Photocatalytic Reduction. Nano Lett. 2021, 21, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Zdražil, L.; Kalytchuk, S.; Langer, M.; Ahmad, R.; Pospíšil, J.; Zmeškal, O.; Altomare, M.; Osvet, A.; Zbořil, R.; Schmuki, P.; et al. Transparent and Low-Loss Luminescent Solar Concentrators Based on Self-Trapped Exciton Emission in Lead-Free Double Perovskite Nanocrystals. ACS Appl. Energy Mater. 2021, 4, 6445–6453. [Google Scholar] [CrossRef]
- Wu, D.; Tao, Y.; Huang, Y.; Huo, B.; Zhao, X.; Yang, J.; Jiang, X.; Huang, Q.; Dong, F.; Tang, X. High Visible-Light Photocatalytic Performance of Stable Lead-Free Cs2AgBiBr6 Double Perovskite Nanocrystals. J. Catal. 2021, 397, 27–35. [Google Scholar] [CrossRef]
- Cong, M.; Zhang, Q.; Yang, B.; Chen, J.; Xiao, J.; Zheng, D.; Zheng, T.; Zhang, R.; Qing, G.; Zhang, C.; et al. Bright Triplet Self-Trapped Excitons to Dopant Energy Transfer in Halide Double-Perovskite Nanocrystals. Nano Lett. 2021, 21, 8671–8678. [Google Scholar] [CrossRef]
- Ahmad, R.; Nutan, G.V.; Singh, D.; Gupta, G.; Soni, U.; Sapra, S.; Srivastava, R. Colloidal Lead-Free Cs2AgBiBr6 Double Perovskite Nanocrystals: Synthesis, Uniform Thin-Film Fabrication, and Application in Solution-Processed Solar Cells. Nano Res. 2021, 14, 1126–1134. [Google Scholar] [CrossRef]
- Zhou, W.; Li, C.; Wu, T.; Liu, R.; Ding, Z.; Zhang, R.; Yu, Y.; Han, P.; Lu, R. Bright Green-Emitting All-Inorganic Terbium Halide Double Perovskite Nanocrystals for Low-Dose X-Ray Imaging. J. Phys. Chem. Lett. 2023, 14, 8577–8583. [Google Scholar] [CrossRef]
- Locardi, F.; Cirignano, M.; Baranov, D.; Dang, Z.; Prato, M.; Drago, F.; Ferretti, M.; Pinchetti, V.; Fanciulli, M.; Brovelli, S.; et al. Colloidal Synthesis of Double Perovskite Cs2AgInCl6 and Mn-Doped Cs2AgInCl6 Nanocrystals. J. Am. Chem. Soc. 2018, 140, 12989–12995. [Google Scholar] [CrossRef]
- Yang, B.; Mao, X.; Hong, F.; Meng, W.; Tang, Y.; Xia, X.; Yang, S.; Deng, W.; Han, K. Lead-Free Direct Band Gap Double-Perovskite Nanocrystals with Bright Dual-Color Emission. J. Am. Chem. Soc. 2018, 140, 17001–17006. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, Y.; Zhao, J.; Liu, Q.; Xia, Z. Design Optimization of Lead-Free Perovskite Cs2AgInCl6:Bi Nanocrystals with 11.4% Photoluminescence Quantum Yield. Chem. Mater. 2019, 31, 3333–3339. [Google Scholar] [CrossRef]
- Ahmad, R.; Zdražil, L.; Kalytchuk, S.; Naldoni, A.; Mohammadi, E.; Schmuki, P.; Zboril, R.; Kment, Š. Robust Dual Cationic Ligand for Stable and Efficient Warm-White Light Emission in Lead-Free Double Perovskite Nanocrystals. Appl. Mater. Today 2022, 26, 101288. [Google Scholar] [CrossRef]
- Li, L.; Shao, H.; Wu, X.; Chen, W.; Zhu, J.; Dong, B.; Xu, L.; Xu, W.; Hu, J.; Zhou, M.; et al. Aluminum-Doped Lead-Free Double Perovskite Cs2AgBiCl6 Nanocrystals with Ultrahigh Stability towards White Light Emitting Diodes. Mater. Res. Bull. 2022, 147, 111645. [Google Scholar] [CrossRef]
- Han, P.; Mao, X.; Yang, S.; Zhang, F.; Yang, B.; Wei, D.; Deng, W.; Han, K. Lead-Free Sodium–Indium Double Perovskite Nanocrystals through Doping Silver Cations for Bright Yellow Emission. Angew. Chem. 2019, 131, 17391–17395. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Li, B.; Yang, L.; Li, Q.; Jiang, H.; Xu, D. Tunable Dual-Emission in Monodispersed Sb3+/Mn2+ Codoped Cs2NaInCl6 Perovskite Nanocrystals through an Energy Transfer Process. Small 2020, 16, 2002547. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Zdražil, L.; Kalytchuk, S.; Naldoni, A.; Rogach, A.L.; Schmuki, P.; Zboril, R.; Kment, Š. Uncovering the Role of Trioctylphosphine on Colloidal and Emission Stability of Sb-Alloyed Cs2NaInCl6 Double Perovskite Nanocrystals. ACS Appl. Mater. Interfaces 2021, 13, 47845–47859. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, R.; Mao, X.; Zheng, D.; Liu, S.; Liu, F.; Han, K.; Yang, B. Boosting the Self-Trapped Exciton Emission in Cs2NaYCl6 Double Perovskite Single Crystals and Nanocrystals. J. Phys. Chem. Lett. 2022, 13, 8613–8619. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, B.; Chen, J.; Wei, D.; Zheng, D.; Kong, Q.; Deng, W.; Han, K. Efficient Thermally Activated Delayed Fluorescence from All-Inorganic Cesium Zirconium Halide Perovskite Nanocrystals. Angew. Chem. 2020, 132, 22109–22113. [Google Scholar] [CrossRef]
- Liu, S.; Yang, B.; Chen, J.; Zheng, D.; Tang, Z.; Deng, W.; Han, K. Colloidal Synthesis and Tunable Multicolor Emission of Vacancy-Ordered Cs2HfCl6 Perovskite Nanocrystals. Laser Photonics Rev. 2022, 16, 2100439. [Google Scholar] [CrossRef]
- Luo, C.; Han, P.; Hou, J.; Li, J.; Sun, F.; Li, C.; Liu, J.; Yang, B. Elucidating the Role of Antimony Dopant in Optical Properties of Brightly Luminescent Zero-Dimensional Organic–Inorganic Metal Halides. J. Phys. Chem. C 2023, 127, 10720–10729. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Cai, T.; Yang, H.; Zhao, J.; Yin, T.; Hao, C.; Chen, M.; Shi, W.; Li, X.; et al. Two-Dimensional Cs2AgInxBi1-xCl6 Alloyed Double Perovskite Nanoplatelets for Solution-Processed Light-Emitting Diodes. Adv. Mater. 2023, 35, 2211235. [Google Scholar] [CrossRef]
- Jin, S.; Yuan, H.; Pang, T.; Zhang, M.; Li, J.; Zheng, Y.; Wu, T.; Zhang, R.; Wang, Z.; Chen, D. Highly Bright and Stable Lead-Free Double Perovskite White Light-Emitting Diodes. Adv. Mater. 2023, 2308487. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Yang, B.; Chen, J.; Zheng, D.; Tang, Z.; Wang, X.; Zhao, Y.; Lu, R.; Han, K. Efficient Luminescent Halide Quadruple-Perovskite Nanocrystals via Trap-Engineering for Highly Sensitive Photodetectors. Adv. Mater. 2021, 33, 2007215. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wu, X.; Zhang, S.; Li, Z.; Gao, D.; Chen, X.; Xiao, S.; Chueh, C.-C.; Jen, A.K.-Y.; Zhu, Z. Efficient and Stable Cs2AgBiBr6 Double Perovskite Solar Cells through In-Situ Surface Modulation. Chem. Eng. J. 2022, 446, 137144. [Google Scholar] [CrossRef]
- Marongiu, D.; Lai, S.; Liu, F.; Simbula, A.; Quochi, F.; Saba, M.; Mura, A.; Bongiovanni, G. Halide Double-Perovskites: High Efficient Light Emission and Beyond. APL Energy 2023, 1, 021501. [Google Scholar] [CrossRef]
- Hu, Q.; Deng, Z.; Hu, M.; Zhao, A.; Zhang, Y.; Tan, Z.; Niu, G.; Wu, H.; Tang, J. X-Ray Scintillation in Lead-Free Double Perovskite Crystals. Sci. China Chem. 2018, 61, 1581–1586. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Bakr, O.M.; Mohammed, O.F. Metal Halide Perovskites for X-Ray Imaging Scintillators and Detectors. ACS Energy Lett. 2021, 6, 739–768. [Google Scholar] [CrossRef]
- He, X.; Deng, Y.; Ouyang, D.; Zhang, N.; Wang, J.; Murthy, A.A.; Spanopoulos, I.; Islam, S.M.; Tu, Q.; Xing, G.; et al. Recent Development of Halide Perovskite Materials and Devices for Ionizing Radiation Detection. Chem. Rev. 2023, 123, 1207–1261. [Google Scholar] [CrossRef]
- Cheng, X.; Qian, W.; Wang, J.; Yu, C.; He, J.; Li, H.; Xu, Q.; Chen, D.; Li, N.; Lu, J. Environmentally Robust Memristor Enabled by Lead-Free Double Perovskite for High-Performance Information Storage. Small 2019, 15, 1905731. [Google Scholar] [CrossRef]
- Ji, F.; Klarbring, J.; Zhang, B.; Wang, F.; Wang, L.; Miao, X.; Ning, W.; Zhang, M.; Cai, X.; Bakhit, B.; et al. Remarkable Thermochromism in the Double Perovskite Cs2NaFeCl6. Adv. Opt. Mater. 2023, 2301102. [Google Scholar] [CrossRef]
- Ning, W.; Zhao, X.; Klarbring, J.; Bai, S.; Ji, F.; Wang, F.; Simak, S.I.; Tao, Y.; Ren, X.; Zhang, L.; et al. Thermochromic Lead-Free Halide Double Perovskites. Adv. Funct. Mater. 2019, 29, 1807375. [Google Scholar] [CrossRef]
- García-Espejo, G.; Rodríguez-Padrón, D.; Luque, R.; Camacho, L.; de Miguel, G. Mechanochemical Synthesis of Three Double Perovskites: Cs2AgBiBr6, (CH3NH3)2TlBiBr6 and Cs2AgSbBr6. Nanoscale 2019, 11, 16650–16657. [Google Scholar] [CrossRef]
- Giovilli, G.; Albini, B.; Grisci, V.; Bonomi, S.; Moroni, M.; Mosconi, E.; Kaiser, W.; De Angelis, F.; Galinetto, P.; Malavasi, L. Band Gap Tuning through Cation and Halide Alloying in Mechanochemically Synthesized Cs3(Sb1−xBix)2Br9 and Cs3Sb2(I1−xBrx)9 Solid Solutions. J. Mater. Chem. C 2023, 11, 10282–10291. [Google Scholar] [CrossRef]
- Knochenmuss, R.; Reber, C.; Rajasekharan, M.V.; Güdel, H.U. Broadband Near-infrared Luminescence of Cr+3 in the Elpasolite Lattices Cs2NaInCl6, Cs2NaYCl6, and Cs2NaYBr6. J. Chem. Phys. 1986, 85, 4280–4289. [Google Scholar] [CrossRef]
- Cao, X.; Kang, L.; Guo, S.; Zhang, M.; Lin, Z.; Gao, J. Cs2NaVCl6: A Pb-Free Halide Double Perovskite with Strong Visible and Near-Infrared Light Absorption. ACS Appl. Mater. Interfaces 2019, 11, 38648–38653. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, H.; Aldamasy, M.H.; Frasca, C.; Abate, A.; Zhao, K.; Hu, Y. Advances in the Synthesis of Halide Perovskite Single Crystals for Optoelectronic Applications. Chem. Mater. 2023, 35, 2683–2712. [Google Scholar] [CrossRef]
- Deng, Y.-H.; Yang, Z.-Q.; Ma, R.-M. Growth of Centimeter-Scale Perovskite Single-Crystalline Thin Film via Surface Engineering. Nano Converg. 2020, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Vidyasagar, C.C.; Muñoz Flores, B.M.; Jiménez Pérez, V.M. Recent Advances in Synthesis and Properties of Hybrid Halide Perovskites for Photovoltaics. Nano-Micro Lett. 2018, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, K.; Wu, X.; Zhu, M.; Zhang, H.; Zhang, K.; Wang, Y.; Loh, K.P.; Shi, Y.; Xu, Q.-H. In Situ Synthesis of Lead-Free Halide Perovskite Cs2AgBiBr6 Supported on Nitrogen-Doped Carbon for Efficient Hydrogen Evolution in Aqueous HBr Solution. ACS Appl. Mater. Interfaces 2021, 13, 10037–10046. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Matei, L.; Jung, H.J.; McCall, K.M.; Chen, M.; Stoumpos, C.C.; Liu, Z.; Peters, J.A.; Chung, D.Y.; Wessels, B.W.; et al. High Spectral Resolution of Gamma-Rays at Room Temperature by Perovskite CsPbBr3 Single Crystals. Nat. Commun. 2018, 9, 1609. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Ran, C.; Xi, J.; Jiao, B.; Zhang, W.; Wu, M.; Hou, X.; Wu, Z. High-Quality Cs2AgBiBr6 Double Perovskite Film for Lead-Free Inverted Planar Heterojunction Solar Cells with 2.2% Efficiency. ChemPhysChem 2018, 19, 1696–1700. [Google Scholar] [CrossRef]
- Usman, M.; Yan, Q. Recent Advancements in Crystalline Pb-Free Halide Double Perovskites. Crystals 2020, 10, 62. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Y.; Chen, B.; Kuang, D.; Su, C. Synthesis and Photocatalytic Application of Stable Lead-Free Cs2 AgBiBr6 Perovskite Nanocrystals. Small 2018, 14, 1703762. [Google Scholar] [CrossRef] [PubMed]
- Creutz, S.E.; Crites, E.N.; De Siena, M.C.; Gamelin, D.R. Colloidal Nanocrystals of Lead-Free Double-Perovskite (Elpasolite) Semiconductors: Synthesis and Anion Exchange To Access New Materials. Nano Lett. 2018, 18, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
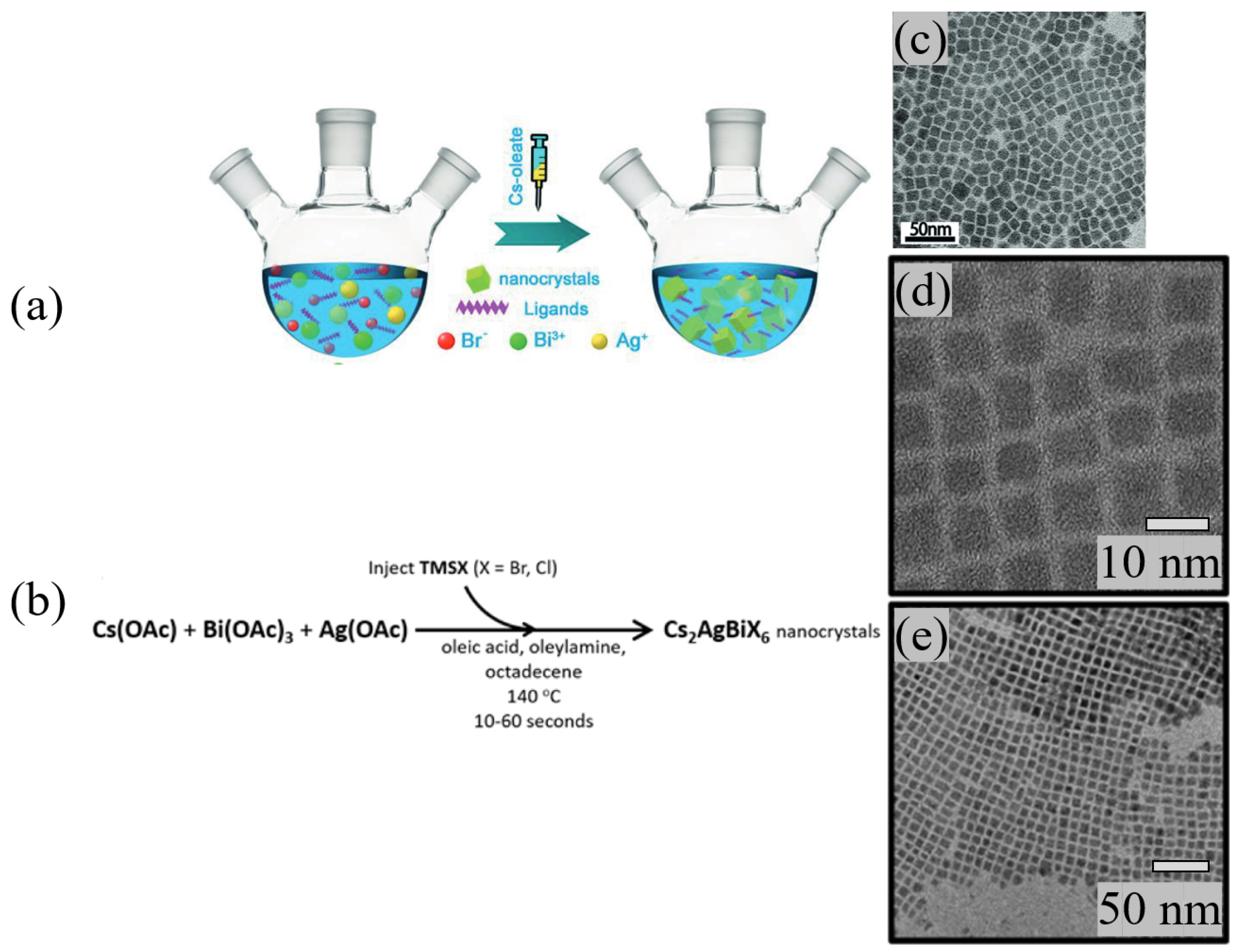
- Bekenstein, Y.; Dahl, J.C.; Huang, J.; Osowiecki, W.T.; Swabeck, J.K.; Chan, E.M.; Yang, P.; Alivisatos, A.P. The Making and Breaking of Lead-Free Double Perovskite Nanocrystals of Cesium Silver–Bismuth Halide Compositions. Nano Lett. 2018, 18, 3502–3508. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Qi, S.; Liu, G.; Lou, Y.; Chen, J.; Zhao, Y. Lead-Free Silver-Antimony Halide Double Perovskite Quantum Dots with Superior Blue Photoluminescence. Chem. Commun. 2019, 55, 14741–14744. [Google Scholar] [CrossRef]
- Mahor, Y.; Mir, W.J.; Nag, A. Synthesis and Near-Infrared Emission of Yb-Doped Cs2AgInCl6 Double Perovskite Microcrystals and Nanocrystals. J. Phys. Chem. C 2019, 123, 15787–15793. [Google Scholar] [CrossRef]
- Lee, W.; Choi, D.; Kim, S. Colloidal Synthesis of Shape-Controlled Cs2NaBiX6 (X = Cl, Br) Double Perovskite Nanocrystals: Discrete Optical Transition by Non-Bonding Characters and Energy Transfer to Mn Dopants. Chem. Mater. 2020, 32, 6864–6874. [Google Scholar] [CrossRef]
- Tang, H.; Xu, Y.; Hu, X.; Hu, Q.; Chen, T.; Jiang, W.; Wang, L.; Jiang, W. Facile One-Pot Preparation and Luminescence Mechanism of Stable Bi3+-Doped Cs2AgInCl6 Double Perovskite Nanocrystals. J. Lumin. 2022, 252, 119246. [Google Scholar] [CrossRef]
- Yang, J.-N.; Song, Y.; Yao, J.-S.; Wang, K.-H.; Wang, J.-J.; Zhu, B.-S.; Yao, M.-M.; Rahman, S.U.; Lan, Y.-F.; Fan, F.-J.; et al. Potassium Bromide Surface Passivation on CsPbI3-xBrx Nanocrystals for Efficient and Stable Pure Red Perovskite Light-Emitting Diodes. J. Am. Chem. Soc. 2020, 142, 2956–2967. [Google Scholar] [CrossRef]
- Ugur, E.; Aydin, E.; De Bastiani, M.; Harrison, G.T.; Yildirim, B.K.; Teale, S.; Chen, B.; Liu, J.; Wang, M.; Seitkhan, A.; et al. Front-Contact Passivation through 2D/3D Perovskite Heterojunctions Enables Efficient Bifacial Perovskite/Silicon Tandem Solar Cells. Matter 2023, 6, 2919–2934. [Google Scholar] [CrossRef]
- Garai, R.; Gupta, R.K.; Iyer, P.K. Trap State Passivation for Stabilizing Perovskite Solar Cells via Multifunctional Molecules. Acc. Mater. Res. 2023, 4, 560–565. [Google Scholar] [CrossRef]
- Aktas, E.; Rajamanickam, N.; Pascual, J.; Hu, S.; Aldamasy, M.H.; Di Girolamo, D.; Li, W.; Nasti, G.; Martínez-Ferrero, E.; Wakamiya, A.; et al. Challenges and Strategies toward Long-Term Stability of Lead-Free Tin-Based Perovskite Solar Cells. Commun. Mater. 2022, 3, 104. [Google Scholar] [CrossRef]
- Hsu, S.-N.; Zhao, W.; Gao, Y.; Akriti; Segovia, M.; Xu, X.; Boudouris, B.W.; Dou, L. Thermoelectric Performance of Lead-Free Two-Dimensional Halide Perovskites Featuring Conjugated Ligands. Nano Lett. 2021, 21, 7839–7844. [Google Scholar] [CrossRef] [PubMed]
- Akriti; Shi, E.; Dou, L. A Leap towards High-Performance 2D Perovskite Photodetectors. Trends Chem. 2019, 1, 365–367. [Google Scholar] [CrossRef]
- Xue, J.; Wang, Z.; Comstock, A.; Wang, Z.; Sung, H.H.Y.; Williams, I.D.; Sun, D.; Liu, J.; Lu, H. Chemical Control of Magnetic Ordering in Hybrid Fe–Cl Layered Double Perovskites. Chem. Mater. 2022, 34, 2813–2823. [Google Scholar] [CrossRef]
- Chao, I.-H.; Yang, Y.-T.; Yu, M.-H.; Chen, C.-H.; Liao, C.-H.; Lin, B.-H.; Ni, I.-C.; Chen, W.-C.; Ho-Baillie, A.W.Y.; Chueh, C.-C. Performance Enhancement of Lead-Free 2D Tin Halide Perovskite Transistors by Surface Passivation and Its Impact on Non-Volatile Photomemory Characteristics. Small 2023, 19, 2207734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shao, M.; Wang, C.; Wen, W.; Shi, W.; Qin, M.; Huang, H.; Wei, X.; Guo, Y.; Liu, Y. Photoinduced Nonvolatile Memory Transistor Based on Lead-Free Perovskite Incorporating Fused Π-Conjugated Organic Ligands. Adv. Mater. 2024, 36, 2307326. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Liu, X.-F.; Li, X.-Y.; Zhang, Y.; Shao, X.; Yang, D.; Zhang, H.-L. Two-Dimensional Perovskite Chiral Ferromagnets. Chem. Mater. 2020, 32, 8914–8920. [Google Scholar] [CrossRef]
- Milić, J.V. Multifunctional Layered Hybrid Perovskites. J. Mater. Chem. C 2021, 9, 11428–11443. [Google Scholar] [CrossRef]
- Connor, B.A.; Leppert, L.; Smith, M.D.; Neaton, J.B.; Karunadasa, H.I. Layered Halide Double Perovskites: Dimensional Reduction of Cs2 AgBiBr6. J. Am. Chem. Soc. 2018, 140, 5235–5240. [Google Scholar] [CrossRef]
- Mao, L.; Stoumpos, C.C.; Kanatzidis, M.G. Two-Dimensional Hybrid Halide Perovskites: Principles and Promises. J. Am. Chem. Soc. 2019, 141, 1171–1190. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zuo, C.; Niu, X.; Ding, L.; Ding, J.; Hao, F. Recent Promise of Lead-Free Halide Perovskites in Optoelectronic Applications. Chem. Eng. J. 2023, 451, 138926. [Google Scholar] [CrossRef]
- McClure, E.T.; McCormick, A.P.; Woodward, P.M. Four Lead-Free Layered Double Perovskites with the n = 1 Ruddlesden–Popper Structure. Inorg. Chem. 2020, 59, 6010–6017. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, P.; Zuo, J.L.; Li, X.; Binwal, D.C.; Wyckoff, K.E.; Mao, L.; Kautzsch, L.; Wu, G.; Wilson, S.D.; Kanatzidis, M.G.; et al. Hybrid Layered Double Perovskite Halides of Transition Metals. J. Am. Chem. Soc. 2022, 144, 6661–6666. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Z.; Li, G.; Aldamasy, M.H.; Li, M.; Abate, A. Dimensional Tuning in Lead-Free Tin Halide Perovskite for Solar Cells. Adv. Energy Mater. 2023, 13, 2204233. [Google Scholar] [CrossRef]
- Sun, P.-P.; Zhang, X.; Yuan, S.; Chi, W.; Cai, G. High-Performance Photoabsorber Based on 2D Perovskites with Alternating Cations in the Interlayer Space: GA(MA)3M3I10. Sol. RRL 2023, 7, 2300441. [Google Scholar] [CrossRef]
- Li, Z.; Kavanagh, S.R.; Napari, M.; Palgrave, R.G.; Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Davies, D.W.; Laitinen, M.; Julin, J.; Isaacs, M.A.; et al. Bandgap Lowering in Mixed Alloys of Cs2Ag(SbxBi1−x)Br6 Double Perovskite Thin Films. J. Mater. Chem. A 2020, 8, 21780–21788. [Google Scholar] [CrossRef]
- Jana, M.K.; Janke, S.M.; Dirkes, D.J.; Dovletgeldi, S.; Liu, C.; Qin, X.; Gundogdu, K.; You, W.; Blum, V.; Mitzi, D.B. Direct-Bandgap 2D Silver–Bismuth Iodide Double Perovskite: The Structure-Directing Influence of an Oligothiophene Spacer Cation. J. Am. Chem. Soc. 2019, 141, 7955–7964. [Google Scholar] [CrossRef]
- Coccia, C.; Moroni, M.; Malavasi, L. Chiral Metal Halide Perovskites: Focus on Lead-Free Materials and Structure-Property Correlations. Molecules 2023, 28, 6166. [Google Scholar] [CrossRef]
- Li, Q.; Cao, D.; Liu, X.; Zhou, X.; Chen, X.; Shu, H. Hierarchical Computational Screening of Layered Lead-Free Metal Halide Perovskites for Optoelectronic Applications. J. Mater. Chem. A 2021, 9, 6476–6486. [Google Scholar] [CrossRef]
- Chen, M.; Shan, Z.; Dong, X.; Liu (Frank), S.; Xu, Z. Discovering Layered Lead-Free Perovskite Solar Absorbers via Cation Transmutation. Nanoscale Horiz. 2023, 8, 483–488. [Google Scholar] [CrossRef]
- Panda, D.P.; Swain, D.; Rohj, R.K.; Sarma, D.D.; Sundaresan, A. Elucidating Structure–Property Correlation in Perovskitoid and Antiperovskite Piperidinium Manganese Chloride. Inorg. Chem. 2023, 62, 3202–3211. [Google Scholar] [CrossRef]
- Zhao, Y.; Daemen, L.L. Superionic Conductivity in Lithium-Rich Anti-Perovskites. J. Am. Chem. Soc. 2012, 134, 15042–15047. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Tang, K.; Lin, Y.; Ren, Y.; Tian, W.; Chen, H.; Lin, T.; Qiu, L.; Pan, X.; Wang, W. Light-Emitting Diodes with Manganese Halide Tetrahedron Embedded in Anti-Perovskites. ACS Energy Lett. 2021, 6, 1901–1911. [Google Scholar] [CrossRef]
- Ju, M.-G.; Dai, J.; Ma, L.; Zhou, Y.; Zeng, X.C. Zero-Dimensional Organic–Inorganic Perovskite Variant: Transition between Molecular and Solid Crystal. J. Am. Chem. Soc. 2018, 140, 10456–10463. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.M.S.; Ganose, A.M.; Pieters, L.; Winnie Leung, W.W.; Wade, J.; Zhang, L.; Scanlon, D.O.; Palgrave, R.G. Anion Distribution, Structural Distortion, and Symmetry-Driven Optical Band Gap Bowing in Mixed Halide Cs2SnX6 Vacancy Ordered Double Perovskites. Chem. Mater. 2019, 31, 9430–9444. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, S.; Wei, Q.; Cao, S.; Zhao, J.; Zou, B.; Zeng, R. Stoichiometry-Controlled Phase Engineering of Cesium Bismuth Halides and Reversible Structure Switch. Adv. Opt. Mater. 2022, 10, 2101406. [Google Scholar] [CrossRef]
- Dave, K.; Huang, W.-T.; Liu, R.-S. All Inorganic Lead-Free Zero-Dimensional Metal Halide Luminescent Materials and Applications. Crystals 2023, 13, 499. [Google Scholar] [CrossRef]
- Peng, H.; Tian, Y.; Yu, Z.; Wang, X.; Ke, B.; Zhao, Y.; Dong, T.; Wang, J.; Zou, B. (C16H28N)2SbCl5: A New Lead-Free Zero-Dimensional Metal-Halide Hybrid with Bright Orange Emission. Sci. China Mater. 2022, 65, 1594–1600. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Shi, H.; Tian, Y.; Pak, C.; Shatruk, M.; Zhou, Y.; Djurovich, P.; Du, M.-H.; Ma, B. A Zero-Dimensional Organic Seesaw-Shaped Tin Bromide with Highly Efficient Strongly Stokes-Shifted Deep-Red Emission. Angew. Chem. Int. Ed. 2018, 57, 1021–1024. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, L.-J.; Lee, S.; Lin, H.; Ma, B. Recent Advances in Luminescent Zero-Dimensional Organic Metal Halide Hybrids. Adv. Opt. Mater. 2021, 9, 2001766. [Google Scholar] [CrossRef]
- Xiao, H.; Dang, P.; Yun, X.; Li, G.; Wei, Y.; Wei, Y.; Xiao, X.; Zhao, Y.; Molokeev, M.S.; Cheng, Z.; et al. Solvatochromic Photoluminescent Effects in All-Inorganic Manganese(II)-Based Perovskites by Highly Selective Solvent-Induced Crystal-to-Crystal Phase Transformations. Angew. Chem. Int. Ed. 2021, 60, 3699–3707. [Google Scholar] [CrossRef]
- Tang, H.; Yuan, K.J.; Zheng, P.F.; Xiao, T.Q.; Zhang, H.W.; Zhao, X.C.; Zhou, W.; Wang, S.Y.; Liu, W.F. Synthesis, Crystal Structure and Optical Properties of the Quasi-0D Lead-Free Organic-Inorganic Hybrid Crystal (C6H14N)3Bi2I9·H2O. J. Solid State Chem. 2023, 323, 124011. [Google Scholar] [CrossRef]
- Chu, Z.; Chu, X.; Zhao, Y.; Ye, Q.; Jiang, J.; Zhang, X.; You, J. Emerging Low-Dimensional Crystal Structure of Metal Halide Perovskite Optoelectronic Materials and Devices. Small Struct. 2021, 2, 2000133. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Wang, W.-Q.; Zhang, B.-L.; Wang, Y.-J.; Ren, M.-P.; Jing, Z.; Yue, C.-Y. Zero-Dimensional Organic–Inorganic Hybrid Zinc Halide with Broadband Yellow Light Emission. CrystEngComm 2023, 25, 444–449. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, S.; Teng, Q.; Li, C.; Zhuang, B.; Zhang, R.; Huang, F.; Chen, D.; Yuan, F. Opportunity of Lead-Free Metal Halide Perovskites for Electroluminescence. Innov. Mater. 2023, 1, 100015–100088. [Google Scholar] [CrossRef]
- Sun, S.; Lu, M.; Gao, X.; Shi, Z.; Bai, X.; Yu, W.W.; Zhang, Y. 0D Perovskites: Unique Properties, Synthesis, and Their Applications. Adv. Sci. 2021, 8, 2102689. [Google Scholar] [CrossRef] [PubMed]
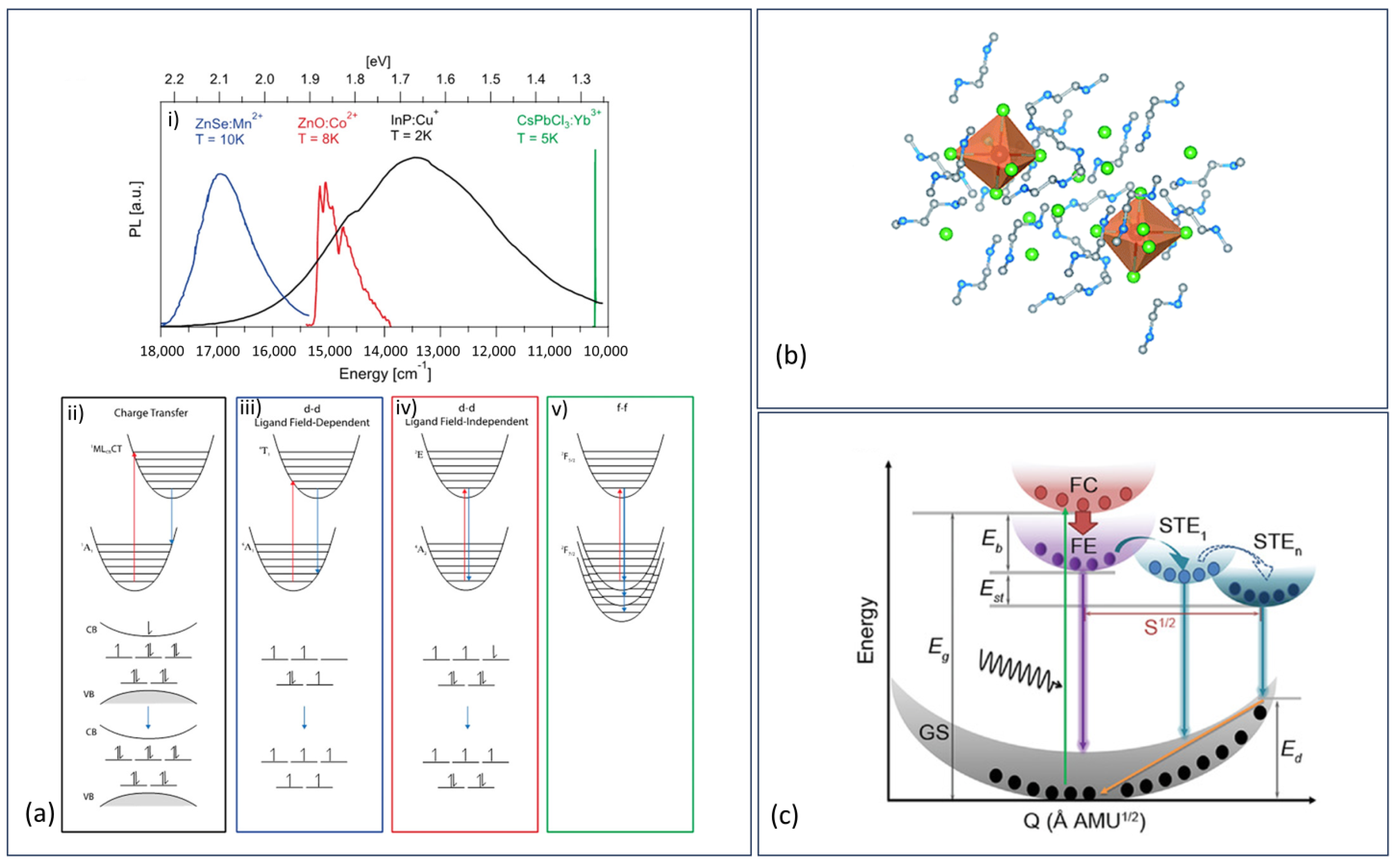
- Li, S.; Luo, J.; Liu, J.; Tang, J. Self-Trapped Excitons in All-Inorganic Halide Perovskites: Fundamentals, Status, and Potential Applications. J. Phys. Chem. Lett. 2019, 10, 1999–2007. [Google Scholar] [CrossRef]
- Zhao, J.-Q.; Shi, H.-S.; Zeng, L.-R.; Ge, H.; Hou, Y.-H.; Wu, X.-M.; Yue, C.-Y.; Lei, X.-W. Highly Emissive Zero-Dimensional Antimony Halide for Anti-Counterfeiting and Confidential Information Encryption-Decryption. Chem. Eng. J. 2022, 431, 134336. [Google Scholar] [CrossRef]
- Williams, R.T.; Song, K.S. The Self-Trapped Exciton. J. Phys. Chem. Solids 1990, 51, 679–716. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Tian, Y.; Yuan, Z.; Clark, R.; Chen, B.; van de Burgt, L.J.; Wang, J.C.; Zhou, Y.; Hanson, K.; et al. Luminescent Zero-Dimensional Organic Metal Halide Hybrids with near-Unity Quantum Efficiency. Chem. Sci. 2018, 9, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.G.; Datta, D. Ligand Field Splitting in Homoleptic Tetrahedral D10 Transition Metal Complexes. Spectrochemical Series. Comput. Theor. Chem. 2018, 1130, 77–82. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Dixon, G.; Dou, F.Y.; Gallagher, S.; Gibbs, S.; Ladd, D.M.; Marino, E.; Ondry, J.C.; Shanahan, J.P.; Vasileiadou, E.S.; et al. Design Rules for Obtaining Narrow Luminescence from Semiconductors Made in Solution. Chem. Rev. 2023, 123, 7890–7952. [Google Scholar] [CrossRef] [PubMed]
- Kitzmann, W.R.; Moll, J.; Heinze, K. Spin-Flip Luminescence. Photochem. Photobiol. Sci. 2022, 21, 1309–1331. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.-M.; Yang, Q.-L.; Xing, X.-X.; Li, J.-P.; Meng, F.-L.; Zhang, X.; Xiao, P.-C.; Yue, C.-Y.; Lei, X.-W. Enhancement of the Photoluminescence Efficiency of Hybrid Manganese Halides through Rational Structural Design. Chem. Commun. 2021, 57, 6907–6910. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhao, X.; Song, B.; Luo, J.; Tang, J. Light Emission of Self-Trapped Excitons in Inorganic Metal Halides for Optoelectronic Applications. Adv. Mater. 2022, 34, 2201008. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, L.; Yang, L.; Duan, J.; Du, H.; Ji, G.; Liu, N.; Zhao, X.; Chen, C.; Xu, L.; et al. Spectra Stable Deep-Blue Light-Emitting Diodes Based on Cryolite-like Cerium(III) Halides with Nanosecond d-f Emission. Sci. Adv. 2022, 8, eabq2148. [Google Scholar] [CrossRef]
- Lian, L.; Zhang, T.; Ding, H.; Zhang, P.; Zhang, X.; Zhao, Y.-B.; Gao, J.; Zhang, D.; Zhao, Y.S.; Zhang, J. Highly Luminescent Zero-Dimensional Organic Copper Halide with Low-Loss Optical Waveguides and Highly Polarized Emission. ACS Mater. Lett. 2022, 4, 1446–1452. [Google Scholar] [CrossRef]
- Luo, H.; Bu, K.; Yin, Y.; Wang, D.; Shi, C.; Guo, S.; Fu, T.; Liang, J.; Liu, B.; Zhang, D.; et al. Anomalous Charge Transfer from Organic Ligands to Metal Halides in Zero-Dimensional [(C6H5)4P]2SbCl5 Enabled by Pressure-Induced Lone Pair-π Interaction. Angew. Chem. Int. Ed. 2023, 62, e202304494. [Google Scholar] [CrossRef]
- Ran, Q.; Zhang, Y.; Yang, J.; He, R.; Zhou, L.; Hu, S. White-Light Defect Emission and Enhanced Photoluminescence Efficiency in a 0D Indium-Based Metal Halide. J. Mater. Chem. C 2022, 10, 1999–2007. [Google Scholar] [CrossRef]
- Zhang, Y.; Tirani, F.F.; Pattison, P.; Schenk-Joß, K.; Xiao, Z.; Nazeeruddin, M.K.; Gao, P. Zero-Dimensional Hybrid Iodobismuthate Derivatives: From Structure Study to Photovoltaic Application. Dalton Trans. 2020, 49, 5815–5822. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Tian, W.; Chen, H.; Tang, K.; Lin, T.; Zhong, G.; Qiu, L.; Pan, X.; Wang, W. Synthesis of 0D Manganese-Based Organic–Inorganic Hybrid Perovskite and Its Application in Lead-Free Red Light-Emitting Diode. Adv. Funct. Mater. 2021, 31, 2100855. [Google Scholar] [CrossRef]
- Xu, L.-J.; Sun, C.-Z.; Xiao, H.; Wu, Y.; Chen, Z.-N. Green-Light-Emitting Diodes Based on Tetrabromide Manganese(II) Complex through Solution Process. Adv. Mater. 2017, 29, 1605739. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Tao, P.; Gao, L.; She, P.; Liu, S.; Li, X.; Li, F.; Wang, H.; Zhao, Q.; Miao, Y.; et al. Designing Highly Efficient Phosphorescent Neutral Tetrahedral Manganese(II) Complexes for Organic Light-Emitting Diodes. Adv. Opt. Mater. 2019, 7, 1801160. [Google Scholar] [CrossRef]
- Li, J.-L.; Sang, Y.-F.; Xu, L.-J.; Lu, H.-Y.; Wang, J.-Y.; Chen, Z.-N. Highly Efficient Light-Emitting Diodes Based on an Organic Antimony(III) Halide Hybrid. Angew. Chem. 2022, 134, e202113450. [Google Scholar] [CrossRef]
- Liu, H.; Shonde, T.B.; Gonzalez, F.; Olasupo, O.J.; Lee, S.; Luong, D.; Lin, X.; Vellore Winfred, J.S.R.; Lochner, E.; Fatima, I.; et al. Efficient Red Light Emitting Diodes Based on a Zero-Dimensional Organic Antimony Halide Hybrid. Adv. Mater. 2023, 35, 2209417. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhu, L.; Xue, C.; Liu, P.; Du, X.; Wen, K.; Zhang, H.; Xu, L.; Xiang, C.; Lin, C.; et al. Efficient and Bright Warm-White Electroluminescence from Lead-Free Metal Halides. Nat. Commun. 2021, 12, 1421. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, F.; Zhu, C.; Li, J.; Lv, X.; Xing, G.; Wei, Q.; Wang, G.; Dai, J.; Dong, H.; et al. Near-Unity Blue Luminance from Lead-Free Copper Halides for Light-Emitting Diodes. Nano Energy 2022, 91, 106664. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Q.; Duan, J.; Xie, W.; Ji, G.; Li, S.; Chen, C.; Li, J.; Yang, L.; Tan, Z.; et al. Exploration of Nontoxic Cs3CeBr6 for Violet Light-Emitting Diodes. ACS Energy Lett. 2021, 6, 4245–4254. [Google Scholar] [CrossRef]
- Liu, S.; Fang, X.; Lu, B.; Yan, D. Wide Range Zero-Thermal-Quenching Ultralong Phosphorescence from Zero-Dimensional Metal Halide Hybrids. Nat. Commun. 2020, 11, 4649. [Google Scholar] [CrossRef]
- Cai, W.; Kuang, C.; Liu, T.; Shang, Y.; Zhang, J.; Qin, J.; Gao, F. Multicolor Light Emission in Manganese-Based Metal Halide Composites. Appl. Phys. Rev. 2022, 9, 041409. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Hu, S.; Xu, J.; Zhang, Z.; Qi, X.; Lu, X.; Jin, J.; Huang, X.-Y.; Xu, Q.; Deng, Z.; et al. Bright Green Emitter of Mn-Doped C4H12N2ZnX4 (X = Cl, Br) for X-Ray Radiography and WLEDs. Chem. Eng. J. 2023, 468, 143818. [Google Scholar] [CrossRef]
- Li, M.; Zhou, J.; Molokeev, M.S.; Jiang, X.; Lin, Z.; Zhao, J.; Xia, Z. Lead-Free Hybrid Metal Halides with a Green-Emissive [MnBr4] Unit as a Selective Turn-On Fluorescent Sensor for Acetone. Inorg. Chem. 2019, 58, 13464–13470. [Google Scholar] [CrossRef]
- Shen, W.; Sui, S.; Yuan, W.; Wang, A.; Tao, Y.; Chen, S.; Deng, Z. Precise, Sensitive, and Reversible Thermochromic Luminescent Sensing Facilitated via Bright High-Temperature Luminescent PEAMnBrxI3−x (x = 0/1/2/3). J. Mater. Chem. C 2021, 9, 2729–2737. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Y.; Yuan, F.; Zhao, C.; Dong, H.; Jiao, B.; Wu, Z. Vacuum Dual-Source Thermal-Deposited Lead-Free Cs3Cu2I5 Films with High Photoluminescence Quantum Yield for Deep-Blue Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2020, 12, 52967–52975. [Google Scholar] [CrossRef] [PubMed]
- Roccanova, R.; Yangui, A.; Nhalil, H.; Shi, H.; Du, M.-H.; Saparov, B. Near-Unity Photoluminescence Quantum Yield in Blue-Emitting Cs3Cu2Br5–xIx (0 ≤ x ≤ 5). ACS Appl. Electron. Mater. 2019, 1, 269–274. [Google Scholar] [CrossRef]
- Peng, H.; Tian, Y.; Wang, X.; Huang, T.; Yu, Z.; Zhao, Y.; Dong, T.; Wang, J.; Zou, B. Pure White Emission with 91.9% Photoluminescence Quantum Yield of [(C3H7)4N]2Cu2I4 out of Polaronic States and Ultra-High Color Rendering Index. ACS Appl. Mater. Interfaces 2022, 14, 12395–12403. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, X.; Tian, Y.; Zou, B.; Yang, F.; Huang, T.; Peng, C.; Yao, S.; Yu, Z.; Yao, Q.; et al. Highly Efficient Cool-White Photoluminescence of (Gua)3Cu2I5 Single Crystals: Formation and Optical Properties. ACS Appl. Mater. Interfaces 2021, 13, 13443–13451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, S.; He, M.; Zheng, W.; Wan, Q.; Liu, M.; Liao, X.; Zhan, W.; Yuan, C.; Liu, J.; et al. Stable Lead-Free Tin Halide Perovskite with Operational Stability >1200 h by Suppressing Tin (II) Oxidation. Angew. Chem. Int. Ed. 2022, 61, e202205463. [Google Scholar] [CrossRef] [PubMed]
- Morad, V.; Shynkarenko, Y.; Yakunin, S.; Brumberg, A.; Schaller, R.D.; Kovalenko, M.V. Disphenoidal Zero-Dimensional Lead, Tin, and Germanium Halides: Highly Emissive Singlet and Triplet Self-Trapped Excitons and X-Ray Scintillation. J. Am. Chem. Soc. 2019, 141, 9764–9768. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wei, Y.; Chen, Y.; Liu, C.; Jiang, F.; Liu, Y.; Hong, M. Blue-Emitting 0D Cs3ZnX5 (X = Cl, Br) Perovskite Nanocrystals Based on Self-Trapped Excitons. J. Lumin. 2022, 249, 119048. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, T.; Chen, J.; Qin, H.; Wu, J.; Zhang, Q.; Zheng, J.; Li, X.; Sun, Y.-Y.; He, Y.; et al. Zero-Dimensional Organic–Inorganic Hybrid Zinc Halides for Multiple Applications in Anti-Counterfeiting, X-Ray Imaging and White LEDs. Adv. Opt. Mater. 2023, 13, 2301864. [Google Scholar] [CrossRef]
- He, S.; Hao, S.; Fan, L.; Liu, K.; Cai, C.; Wolverton, C.; Zhao, J.; Liu, Q. Efficient Solar Spectrum-Like White-Light Emission in Zinc-Based Zero-Dimensional Hybrid Metal Halides. Adv. Opt. Mater. 2023, 11, 2300218. [Google Scholar] [CrossRef]
- Kong, Q.; Yang, B.; Chen, J.; Zhang, R.; Liu, S.; Zheng, D.; Zhang, H.; Liu, Q.; Wang, Y.; Han, K. Phase Engineering of Cesium Manganese Bromides Nanocrystals with Color-Tunable Emission. Angew. Chem. Int. Ed. 2021, 60, 19653–19659. [Google Scholar] [CrossRef] [PubMed]
- Worku, M.; Tian, Y.; Zhou, C.; Lee, S.; Meisner, Q.; Zhou, Y.; Ma, B. Sunlike White-Light-Emitting Diodes Based on Zero-Dimensional Organic Metal Halide Hybrids. ACS Appl. Mater. Interfaces 2018, 10, 30051–30057. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, Z.; Tao, X. (1-C5H14N2Br)2MnBr4: A Lead-Free Zero-Dimensional Organic-Metal Halide with Intense Green Photoluminescence. Front. Chem. 2020, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Liang, D.; Qian, Q.; Mo, Q.; Zhao, S.; Cai, W.; Chen, J.; Zang, Z. Near-Unity Quantum Yield in Zero-Dimensional Lead-Free Manganese-Based Halides for Flexible X-Ray Imaging with High Spatial Resolution. eScience 2023, 3, 100089. [Google Scholar] [CrossRef]
- Yang, H.; Cai, T.; Liu, E.; Hills-Kimball, K.; Gao, J.; Chen, O. Synthesis and Transformation of Zero-Dimensional Cs3BiX6 (X = Cl, Br) Perovskite-Analogue Nanocrystals. Nano Res. 2020, 13, 282–291. [Google Scholar] [CrossRef]
- Yang, B.; Chen, J.; Hong, F.; Mao, X.; Zheng, K.; Yang, S.; Li, Y.; Pullerits, T.; Deng, W.; Han, K. Lead-Free, Air-Stable All-Inorganic Cesium Bismuth Halide Perovskite Nanocrystals. Angew. Chem. Int. Ed. 2017, 56, 12471–12475. [Google Scholar] [CrossRef]
- Chen, D.; Dai, F.; Hao, S.; Zhou, G.; Liu, Q.; Wolverton, C.; Zhao, J.; Xia, Z. Crystal Structure and Luminescence Properties of Lead-Free Metal Halides (C6H5CH2NH3)3MBr6 (M = Bi and Sb). J. Mater. Chem. C 2020, 8, 7322–7329. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, D.; Shi, Z.; Qin, C.; Cui, M.; Ma, Z.; Wang, L.; Wang, M.; Ji, X.; Chen, X.; et al. Stable Zero-Dimensional Cesium Indium Bromide Hollow Nanocrystals Emitting Blue Light from Self-Trapped Excitons. Nano Today 2021, 38, 101153. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, J.-F.; Huang, Z.-G.; Wei, J.-H.; Wang, X.-D.; Li, W.-G.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. A Highly Red-Emissive Lead-Free Indium-Based Perovskite Single Crystal for Sensitive Water Detection. Angew. Chem. Int. Ed. 2019, 58, 5277–5281. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, C.; Wang, Y.; Sun, M.; Zhao, G. Efficient White Light Emission of 0D Lead-Free Indium-Based Halide Perovskite and the Intermediate State Promotion Mechanism of the Nonadiabatic Transition to Self-Trapped Exciton via Antimony(III) Cation Doping. J. Phys. Chem. C 2023, 127, 11616–11622. [Google Scholar] [CrossRef]
- Shao, H.; Wu, X.; Zhu, J.; Xu, W.; Xu, L.; Dong, B.; Hu, J.; Dong, B.; Bai, X.; Cui, H.; et al. Mn2+ Ions Doped Lead-Free Zero-Dimensional K3SbCl6 Perovskite Nanocrystals towards White Light Emitting Diodes. Chem. Eng. J. 2021, 413, 127415. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Liang, P.; Zhou, T.; Wang, L.; Xie, R.-J. Dual-Band Luminescent Lead-Free Antimony Chloride Halides with Near-Unity Photoluminescence Quantum Efficiency. Chem. Mater. 2019, 31, 9363–9371. [Google Scholar] [CrossRef]
- He, Q.; Zhou, C.; Xu, L.; Lee, S.; Lin, X.; Neu, J.; Worku, M.; Chaaban, M.; Ma, B. Highly Stable Organic Antimony Halide Crystals for X-Ray Scintillation. ACS Mater. Lett. 2020, 2, 633–638. [Google Scholar] [CrossRef]
- Peng, Y.-C.; Zhou, S.-H.; Jin, J.-C.; Zhuang, T.-H.; Gong, L.-K.; Lin, H.-W.; Wang, Z.-P.; Du, K.-Z.; Huang, X.-Y. [PPh3H]2[SbCl5]: A Zero-Dimensional Hybrid Metal Halide with a Supramolecular Framework and Stable Dual-Band Emission. J. Phys. Chem. C 2022, 126, 17381–17389. [Google Scholar] [CrossRef]
- Lv, L.; Yang, H.; Cheng, X.; Lin, Y.; Chang, X.; Cheng, T.; Xie, Y.; Han, Y.; Li, J.; Yin, J.; et al. Yellow Phosphor Based on Zero-Dimensional Antimony Halide for White Light-Emitting Diodes. J. Mater. Chem. C 2023, 11, 13335–13342. [Google Scholar] [CrossRef]
- Chen, D.; Hao, S.; Zhou, G.; Deng, C.; Liu, Q.; Ma, S.; Wolverton, C.; Zhao, J.; Xia, Z. Lead-Free Broadband Orange-Emitting Zero-Dimensional Hybrid (PMA)3InBr6 with Direct Band Gap. Inorg. Chem. 2019, 58, 15602–15609. [Google Scholar] [CrossRef]
- Ben-Akacha, A.; Zhou, C.; Chaaban, M.; Beery, D.; Lee, S.; Worku, M.; Lin, X.; Westphal, R.; Ma, B. Mechanochemical Synthesis of Zero Dimensional Organic-Inorganic Metal Halide Hybrids. ChemPhotoChem 2021, 5, 326–329. [Google Scholar] [CrossRef]
- Wei, J.-H.; Liao, J.-F.; Zhou, L.; Luo, J.-B.; Wang, X.-D.; Kuang, D.-B. Indium-Antimony-Halide Single Crystals for High-Efficiency White-Light Emission and Anti-Counterfeiting. Sci. Adv. 2021, 7, eabg3989. [Google Scholar] [CrossRef] [PubMed]
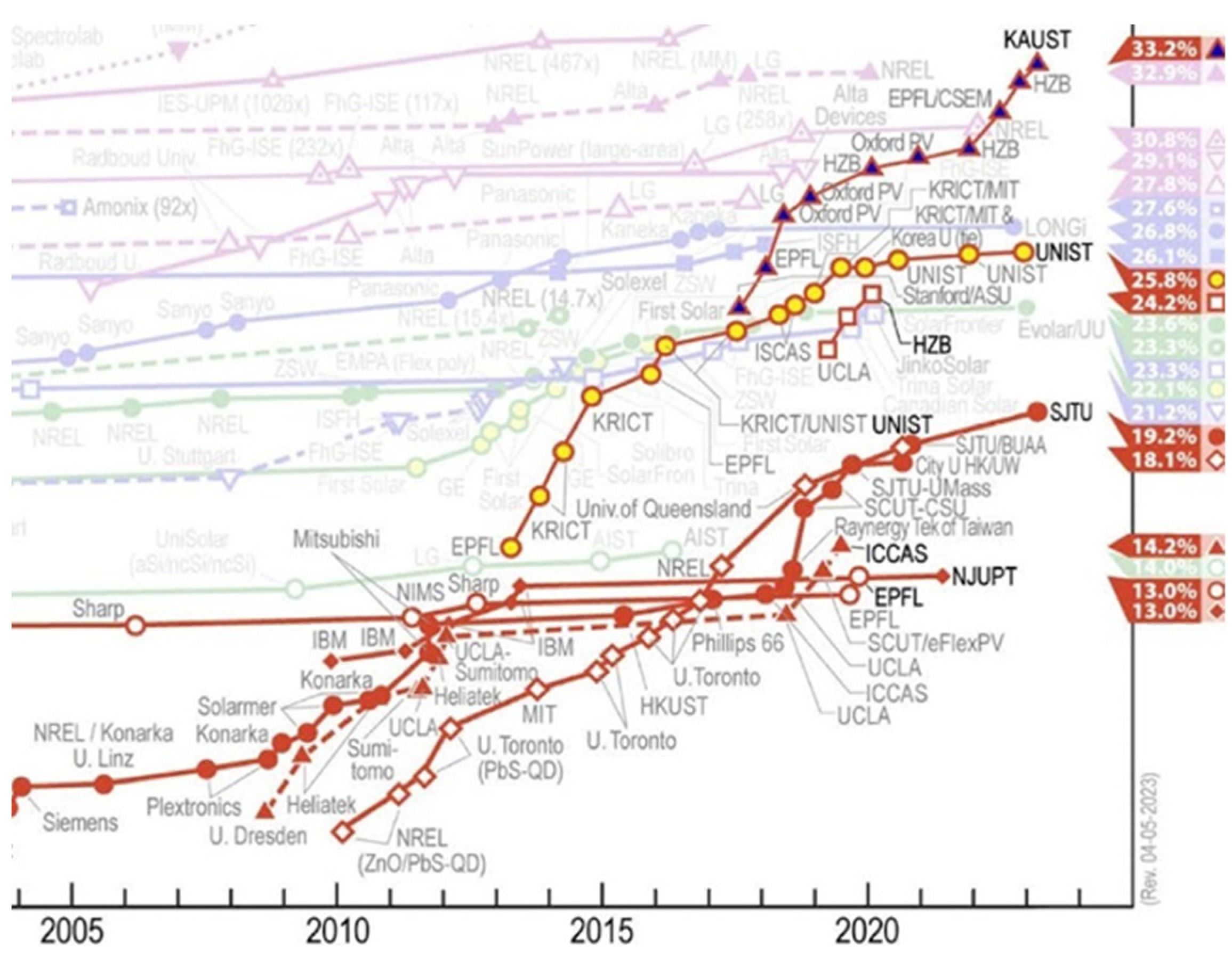
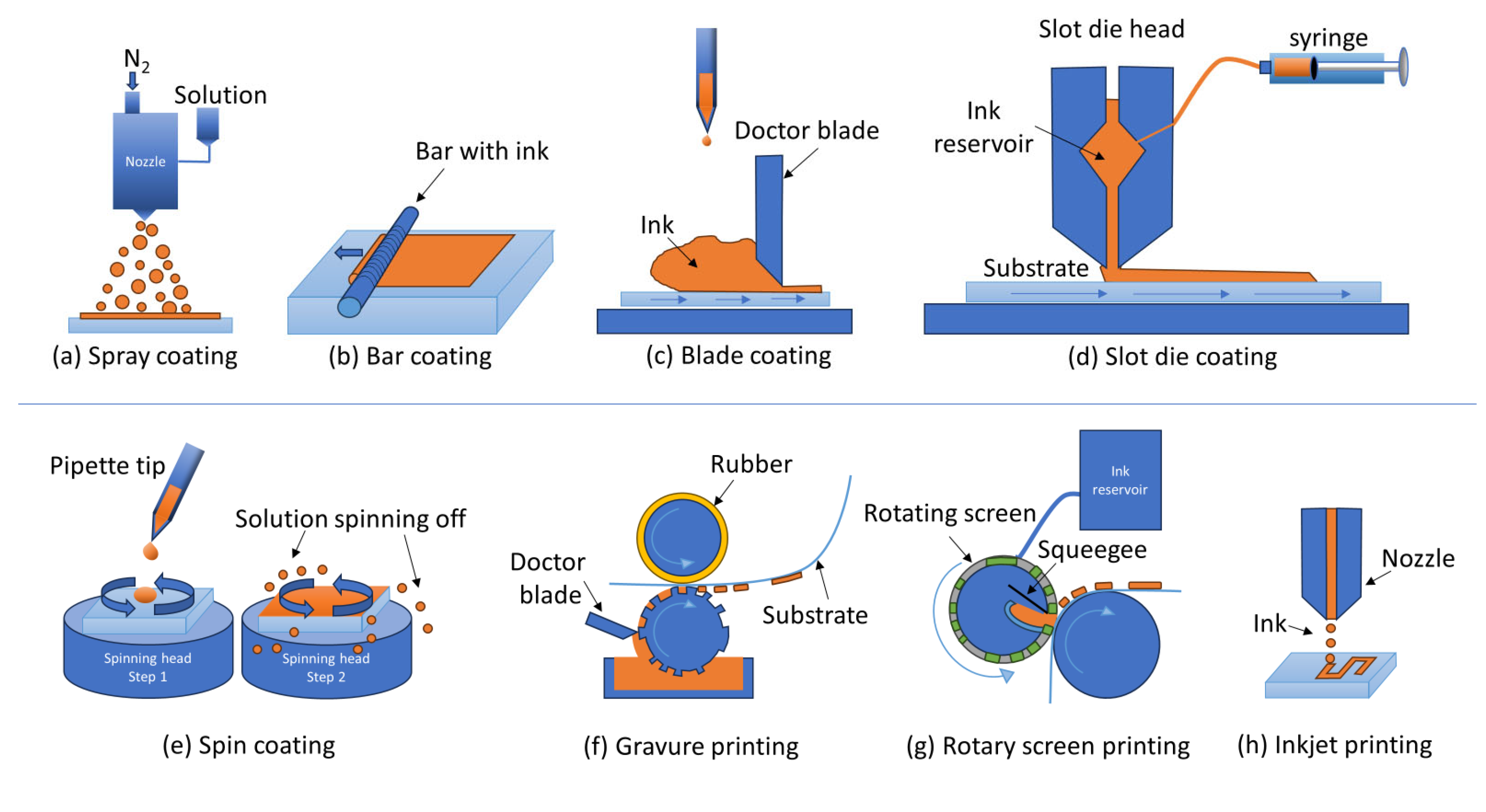
- Park, N.-G.; Zhu, K. Scalable Fabrication and Coating Methods for Perovskite Solar Cells and Solar Modules. Nat. Rev. Mater. 2020, 5, 333–350. [Google Scholar] [CrossRef]
- Faure, B.; Salazar-Alvarez, G.; Ahniyaz, A.; Villaluenga, I.; Berriozabal, G.; De Miguel, Y.R.; Bergström, L. Dispersion and Surface Functionalization of Oxide Nanoparticles for Transparent Photocatalytic and UV-Protecting Coatings and Sunscreens. Sci. Technol. Adv. Mater. 2013, 14, 023001. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, L.; Stergiopoulos, T.; Zachariadis, A.; Gravalidis, C.; Laskarakis, A.; Logothetidis, S. Perovskite Solar Cells from Small Scale Spin Coating Process towards Roll-to-Roll Printing: Optical and Morphological Studies. Mater. Today Proc. 2017, 4 Pt B, 5082–5089. [Google Scholar] [CrossRef]
- Li, F.; Bao, C.; Gao, H.; Zhu, W.; Yu, T.; Yang, J.; Fu, G.; Zhou, X.; Zou, Z. A Facile Spray-Assisted Fabrication of Homogenous Flat CH3NH3PbI3 Films for High Performance Mesostructure Perovskite Solar Cells. Mater. Lett. 2015, 157, 38–41. [Google Scholar] [CrossRef]
- Richards, D.; Burkitt, D.; Patidar, R.; Beynon, D.; Watson, T. Predicting a Process Window for the Roll-to-Roll Deposition of Solvent-Engineered SnO2 in Perovskite Solar Cells. Mater. Adv. 2022, 3, 8588–8596. [Google Scholar] [CrossRef]
- Razza, S.; Castro-Hermosa, S.; Di Carlo, A.; Brown, T.M. Research Update: Large-Area Deposition, Coating, Printing, and Processing Techniques for the Upscaling of Perovskite Solar Cell Technology. APL Mater. 2016, 4, 091508. [Google Scholar] [CrossRef]
- Peng, X.; Yuan, J.; Shen, S.; Gao, M.; Chesman, A.S.R.; Yin, H.; Cheng, J.; Zhang, Q.; Angmo, D. Perovskite and Organic Solar Cells Fabricated by Inkjet Printing: Progress and Prospects. Adv. Funct. Mater. 2017, 27, 1703704. [Google Scholar] [CrossRef]
- Sliz, R.; Czajkowski, J.; Fabritius, T. Taming the Coffee Ring Effect: Enhanced Thermal Control as a Method for Thin-Film Nanopatterning. Langmuir 2020, 36, 9562–9570. [Google Scholar] [CrossRef]
- Sumaiya, S.; Kardel, K.; El-Shahat, A. Organic Solar Cell by Inkjet Printing—An Overview. Technologies 2017, 5, 53. [Google Scholar] [CrossRef]
- Parida, B.; Singh, A.; Kalathil Soopy, A.K.; Sangaraju, S.; Sundaray, M.; Mishra, S.; Liu, S.; Najar, A. Recent Developments in Upscalable Printing Techniques for Perovskite Solar Cells. Adv. Sci. 2022, 9, 2200308. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.W.; Jang, J.; Kim, U.; Lee, Y.; Ji, S.-G.; Noh, E.; Hong, S.; Choi, M.; Seok, S.I. Efficient Perovskite Solar Mini-Modules Fabricated via Bar-Coating Using 2-Methoxyethanol-Based Formamidinium Lead Tri-Iodide Precursor Solution. Joule 2021, 5, 2420–2436. [Google Scholar] [CrossRef]
- Søndergaard, R.R.; Hösel, M.; Krebs, F.C. Roll-to-Roll Fabrication of Large Area Functional Organic Materials. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 16–34. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Shi, C.; Qin, J.; Fu, Y.; Song, Y.; Li, Y.; Ling, Z. Roll-To-Roll Fabricating MXene Membranes with Ordered Interlayer Distances for Molecule and Ion Separation. Adv. Mater. Interfaces 2023, 10, 2300301. [Google Scholar] [CrossRef]
- Bae, S.-R.; Heo, D.Y.; Kim, S.Y. Recent Progress of Perovskite Devices Fabricated Using Thermal Evaporation Method: Perspective and Outlook. Mater. Today Adv. 2022, 14, 100232. [Google Scholar] [CrossRef]



| Body Organ/System | Clinical Symptoms of Pb Poisoning |
|---|---|
| Eyes | Blindness of parts of visual field Hallucinations |
| Ears | Hearing loss |
| Mouth | Unusual taste Slurred speech Blue line along the gum |
| Kidney | Structural damage and failure Changes in the excretory function |
| Liver | Jaundice Lead-induced oxidative stress Decreased liver function Microvesicular and macrovesicular steatosis Hemosiderosis and cholestasis |
| Skin | Pallor and/or lividity |
| Central nervous system | Insomnia Loss of appetite Decreased libido Irritability Cognitive deficits Memory loss Headache Personality changes Delirium Coma Encephalopathy |
| Reproductive organs | Sperm dysfunctions Pregnancy complications Preterm birth |
| Abdomen/Stomach | Pain Nausea Diarrhea Constipation |
| Blood | Anaemia |
| General | Malaise Fatigue Weight loss |
| Neuro-muscular | Tremor Pain Delayed reaction times Loss of coordination Convulsions Foot or ankle drop Seizers Weakness |
| Bones | Mineralizing bones and teeth Decreased bone density |
| A2M(I)M(III)X6 | Optoelectronic Properties | Synthesized Compounds | (Potential) Application |
|---|---|---|---|
| Type I: s2 + s2 | direct bandgap suitable bandgap values strong light absorption high electronic dimensionality expected defect tolerance | (MA)2TlBiBr6 | (solar cell) (light-emission device) |
| Type II: s0 + s2 | indirect bandgap large bandgap values reduced electronic dimensionality long carrier lifetime not good carrier transport | Cs2AgBiCl6 Cs2AgBiBr6 (MA)2AgBiBr6 (MA)2AgbiI6 Cs2AgSbCl6 (MA)2AgSbCl6 (MA)2KBiCl6 Cs2NaBiCl6 | solar cell X-ray detector photocatalysis (X-ray imaging) |
| Type III: s0 + s0 | direct bandgap dipole-forbidden transition large bandgap values reduced electronic dimensionality | Cs2AgInCl6 (MA)2KGdCl6 (MA)2KYCl6 Cs2NaGaF6 | photodetector laser light-emission device |
| Vacancy-ordered | direct bandgap strong light absorption existence of deep mid-gap defects not good carrier transport | Cs2SnI6 Cs2PdBr6 Cs2Ti(Br/I)6 Cs2TeI6 | solar cell light-emission device (X-ray imaging) |
| Device Structure | PLQY [%] | CIE (x, y) | CCT [K] | CRI | Ref. |
|---|---|---|---|---|---|
| UV LED/Cs2AgIn0.7Bi0.3Cl6 NCs/PMMA | 4 | (0.36, 0.35) | 4443 | 91 | [55] |
| UV LED/Cs2NaInCl6:2.5%Sb, 45%Tb, 3%Mn NCs | 74 | (0.41, 0.39) | 3371 | 89.2 | [56] |
| UV LED/Cs2(Na, Ag)InCl6:7.09%Ho3+ | 57.09 | (0.39, 0.46) | N/A | 75.4 | [57] |
| UV LED/Cs2Na0.4Ag0.6In0.995Bi0.005Cl6:Mn2+ | 31.8 | (0.3784, 0.4216) | 4323.4 | 82.6 | [58] |
| UV LED/Cs2AgIn1−xBixCl6 | 39 | (0.417, 0.391) | 3119 | 85 | [59] |
| UV LED/Cs2Ag0.4Na0.6InCl6:1%Bi, 1%/BaMgAl10O17:Eu2+ | 98.6 | (0.4, 0.38) | 4430 | 95.7 | [60] |
| UV LED/Cs2Ag0.4Na0.6InCl6:Bi, Gd/BaMgAl10O17:Eu2+ | 87.57 | (0.3464, 0.3224) | 4818 | 93.9 | [60] |
| UV LED/Cs2Ag0.7Na0.3InCl6:Bi | 87.2 | (0.38, 0.44) | 4347 | 87.8 | [61] |
| UV LED/Cs2AgScCl6:0.05Bi | 60 | (0.366, 0.367) | 4100 | 96 | [62] |
| UV LED/Cs2Na0.4Ag0.6InCl6:Bi | 73.3 | (0.461, 0.443) | 2930–6957 | 84.8–97.1 | [63] |
| Luminescence | |||
|---|---|---|---|
| Composition | PL Peak [nm] | PLQY (%) | Ref. |
| Cs2AgInCl6:Mn | 620 | 16.4 | [80] |
| Cs2AgBiCl6:In | 570 | 36.6 | [81] |
| Cs2AgInCl6:Bi | 580 | 11.4 | [82] |
| Cs2AgInCl6:Na/Bi | 600 | 40 | [83] |
| Cs2AgBiCl6:Al | 630 | 17.2 | [84] |
| Cs2NaInCl6:Ag | 535 | 31.1 | [85] |
| Cs2NaInCl6:Sb/Mn | 455/622 | 24 | [86] |
| Cs2NaInCl6:Sb | 448 | 50 | [87] |
| Cs2KInCl6:Sb | 515 | 95 | [77] |
| Cs2KInCl6:Sb/Mn | 510/630 | 87 | [77] |
| Cs2NaYCl6:Sb | 461 | 51.8 | [88] |
| Cs2NaTbCl6:Sb | Multiple peaks (green emission) | 24 | [79] |
| Cs2ZrCl6 | 446 | 60.37 | [89] |
| Cs2HfCl6 | 628 | 40.71 | [90] |
| MA4InCl7:Sb | 620 | 84 | [91] |
| Light-emitting diodes | |||
| EL (1) peak [nm] | Efficinecy | ||
| Cs2AgInCl6:Bi | 557 | 58 cd/m2 (luminance); 0.01% (EQE (2)) | [92] |
| Cs2AgInCl6:Na/Bi/Tb | 610 | 2793 cd/m2 (luminance); 0.76% (EQE (2)) | [93] |
| Photocatalysis | |||
| Composition | Reaction | Efficiency | Ref. |
| Cs2AgBiX6 (X = Cl, Br, I) | CO2 photoreduction | 0.035% (EQE (2)) | [74] |
| Cs2AgBiBr6 | NO removal | 97% (removal rate) | [76] |
| Luminescent solar concentrators | |||
| Cs2AgInCl6:Na/Bi | 21.2% (internal OQE (3)) | [75] | |
| Solar cells | |||
| Cs2AgBiBr6 | 0.46% (PCE (4)) | [78] | |
| Scintillator | |||
| Cs2NaTbCl6:Sb | 140 nGyair/s (detection limit) | [79] | |
| Photodetector | |||
| Cs4Cd0.75Mn0.25Bi2Cl12 | 0.98 × 104 A W−1 (responsivity); 3 × 106% (EQE (2)) | [94] | |
| Material | Metal | Device Structure | EQE (%) | Emission Wavelength [nm] | Luminescence [cd m−2] | PLQY (%) | Comment | Ref. |
|---|---|---|---|---|---|---|---|---|
| (ABI)4MnBr6 | Mn | ITO/PEDOT:PSS/poly TPD/(ABI)4MnBr6/TPBi/LiF/Al | 9.8 | 629 | 4700 | 80 | PEO 1 wt% additive | [174] |
| [PPh4]2[MnBr4] | Mn | ITO/PEDOT:PSS/TCTA:26DCZPPY (1:2):(Ph4P)2[MnBr4]/BmPyPb LiF/Al | 9.6 | 518 | 2339 | Active layer mixed with hole transport | [175] | |
| DBFDPO-MnBr2 | Mn | ITO/MoO3/TAPC/TCTA (50 wt%) DBFDPO-MnBr2/TmPyPB/LiF/Al | 10.5 | 552 | 81.4 | Active layer mixed with hole transport | [176] | |
| (MePPh3)2SbCl5 | Sb | ITO/PEDOT:PSS/Poly-TP/TAPC:2,6-DCZPPY:(MePPh3)2SbCl5 (6:3:1)/TPBi/LiF/A | 3.1 | 593 | 3500 | 99.4 | Active layer mixed with hole and electron transport | [177] |
| TPPcarzSbBr4 | Sb | ITO/PEDOT:PSS/PVK/TPPcarzSbBr4/ZnO/LiF/Al | 5.12 | 653 nm | 5957 cd m−2 | 93.8% | [178] | |
| Cs3Cu2I5:CsCu2I3 | Cu | ITO/PEDOT:PSS, /Cs3Cu2I5:CsCu2I3/TmPyPB, /LiF/Al | 3.1 | 565 | 1570 | 30 | Mixture of 0D and 1D | [179] |
| TEA2Cu2Br4 | Cu | ITO/PEDOT:PSS/TEA2Cu2Br4/TPBi/LiF/Al | 0.11 | 463 | 85 | 94.73 | [Cu2Br4] units | [180] |
| Cs3CeBr6 | Ce | (ITO)/ZnO/Al2O3/Cs3CeBr6/TCTA/TAPC/HAT-CN/Al | 0.42 | 391/421 | 91 | [181] |
| Formula | PLQY (%) | Form | Valency | Em λ [nm] | Isolated Polyhedral | FWHM/ Stokes Shift [nm] | Method/ Application/ Emission | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cs3Cu2I5 | 58 | film | I | 440 | [Cu2I3]− | 81/155 | Thermal evaporation/LED/STE | [187] |
| Cs3Cu2I5 | 98.71 | SC | I | 443–456 | [Cu2I5]3− | 99/135 | Annealed together/ /STE | [188] |
| [(C3H7)4N]2Cu2I4 | 91.9 | SC | I | 483, 637 | [CuI2]− | Solvent evaporation/ /STE | [189] | |
| (Gua)3Cu2I5 | 96 | SC | I | 481 | [Cu2I5]3− | 125/156 | Heated solution/WLED/STE | [190] |
| Cs4SnBr6 with SnF2 | 62.8 | powder | II | 540 | [SnBr6]4− | Ball milling/WLED/STE | [191] | |
| (C4N2H14Br)4SnBr6 | 95 | SC | II | 570 | [SnBr6]4− | 105/215 | Antisolvent diffusion/phosphor/STE | [163] |
| (C4N2H14I)4SnI6 | 75 | SC | II | 620 | [SnBr6]4− | 118/210 | Antisolvent diffusion/phosphor/ | [163] |
| (Bmpip)2SnBr4 | 75 | SC | 666 | [SnBr4]2− | 69/326 | LTC/X-ray scintillator/ | [192] | |
| Cs3ZnBr5 | 7.89 | NC | II | 468 | [ZnBr4]2− | 76/193 | Hot injection/SSL, display X-ray scintillator/STE | [193] |
| (ABI)2ZnCl4 | 24.28 | SC | II | 395 | [ZnCl4]2− | 92 | Solvent evaporation/Anti-Counterfeiting, X-ray scintillator, WLED/ | [194] |
| (PMA)2ZnCl4 | 37.2 | crystal | II | 413, 440 | [ZnX4]2− | LTC */WLED | [195] | |
| Cs3MnBr5 | 48 (1.29 with 2H2O) | NCs | II | 520 | [MnBr4]2− | 43 | Hot injection/anti-counterfeiting/d-d transition, and possible STE | [196] |
| (C9NH20)2MnBr4 | 81.08 | Crystal | II | 528 | [MnBr4]2− | 64 | LTC/sensor/d–d transition | [185] |
| (Ph4P)2MnBr4 | 98 | Crystal | II | 512 | [MnBr4]2− | 48/52 | Diffusion, ball milling/WLED/d–d transition | [197] |
| (1-mPQBr)2MnBr4 | 60.70 | SC | II | 520 | [MnBr4]2− | 43 | Solvent evaporation/ /d–d transition | [198] |
| (TBA)2MnBr4 | 93.76 | SC | II | 512 | [MnBr4]2− | 38.7/ | Solvent evaporation/X-ray scintillator/d–d transition | [199] |
| Cs3BiCl6 | NCs | III | 391 | [BiCl6]3− | 60/59 | Hot injection/ /STE | [200] | |
| Cs3Bi2Br9with oleic acid | 4.5 | NCs | III | 460 | [BiBr6]3− | 45 | antisolvent injection | [201] |
| (PMA)3BiBr6 | <1% | Crystal | III | 405/510 | [BiBr6]3− | 153/160 | LTC/ /STEs | [202] |
| Cs3InBr6 | 22.3 | NCs, lower PLQY as SC | III | 450 | [InBr6]3− | /75 | Hot injection/ /singlet and triplet STE/lighting, displays | [203] |
| Cs2InBr5(H2O) | 33 | SC | III | 695 | [InBr5(H2O)]2− | /340 | LTC/Water sensor/STE | [204] |
| (DETA)InBr6 | 1.40 (24.12 Sb3+doping) | SC | III | 400, 500-700 | [InBr6]3− | 134/200 | LTC/ /STE | [205] |
| K3SbCl6 Mn2+-doped | 22.3 | NCs | III | 440 | [SbCl6]3− | 102/120 | Hot injection/WLED/STE and defect | [206] |
| (C9NH20)2SbCl5 | 98 | SC | II | 590 | [SbCl5]2− | 119/210 | Antisolvent diffusion/phosphor/ | [163] |
| TEBA2SbCl5 | 98 | SC or powder | III | 590 | [SbCl5]2− | 140/250 | antisolvent diffusion or injection/WLED/STE | [207] |
| (PPN)2SbCl5 | 98.1 | SC | III | 635 | [SbCl5]2− | 142/225 | Antisolvent injection/X-ray scintillator | [208] |
| (PPh3H]2SbCl5 | 74.50 | SC | III | 653 | [SbCl5]2− | /283 | Autoclave/ /singlet, triplet emission | [209] |
| (NII)2SbCl5 | 88.9 | SC | III | 610 | [SbCl5]2− | 118/248 | Antisolvent diffusion/SSL/STE | [210] |
| (Bmpip)2GeBr4 | 1 | SC | II | 670 | [GeBr4]2− | /330 | Antisolvent injection/SSL, X-ray scintillator/singlet, triplet exciton emission, lone pairs | [192] |
| (PMA)3InBr6 | 35 | Crystal | III | 610 | [InBr6]3− | LTC/WLED/STEs | [211] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dávid, A.; Morát, J.; Chen, M.; Gao, F.; Fahlman, M.; Liu, X. Mapping Uncharted Lead-Free Halide Perovskites and Related Low-Dimensional Structures. Materials 2024, 17, 491. https://doi.org/10.3390/ma17020491
Dávid A, Morát J, Chen M, Gao F, Fahlman M, Liu X. Mapping Uncharted Lead-Free Halide Perovskites and Related Low-Dimensional Structures. Materials. 2024; 17(2):491. https://doi.org/10.3390/ma17020491
Chicago/Turabian StyleDávid, Anna, Julia Morát, Mengyun Chen, Feng Gao, Mats Fahlman, and Xianjie Liu. 2024. "Mapping Uncharted Lead-Free Halide Perovskites and Related Low-Dimensional Structures" Materials 17, no. 2: 491. https://doi.org/10.3390/ma17020491
APA StyleDávid, A., Morát, J., Chen, M., Gao, F., Fahlman, M., & Liu, X. (2024). Mapping Uncharted Lead-Free Halide Perovskites and Related Low-Dimensional Structures. Materials, 17(2), 491. https://doi.org/10.3390/ma17020491






