The Influence of Manganese Slag on the Properties of Ultra-High-Performance Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of the Specimens
2.3. Measuring Methods
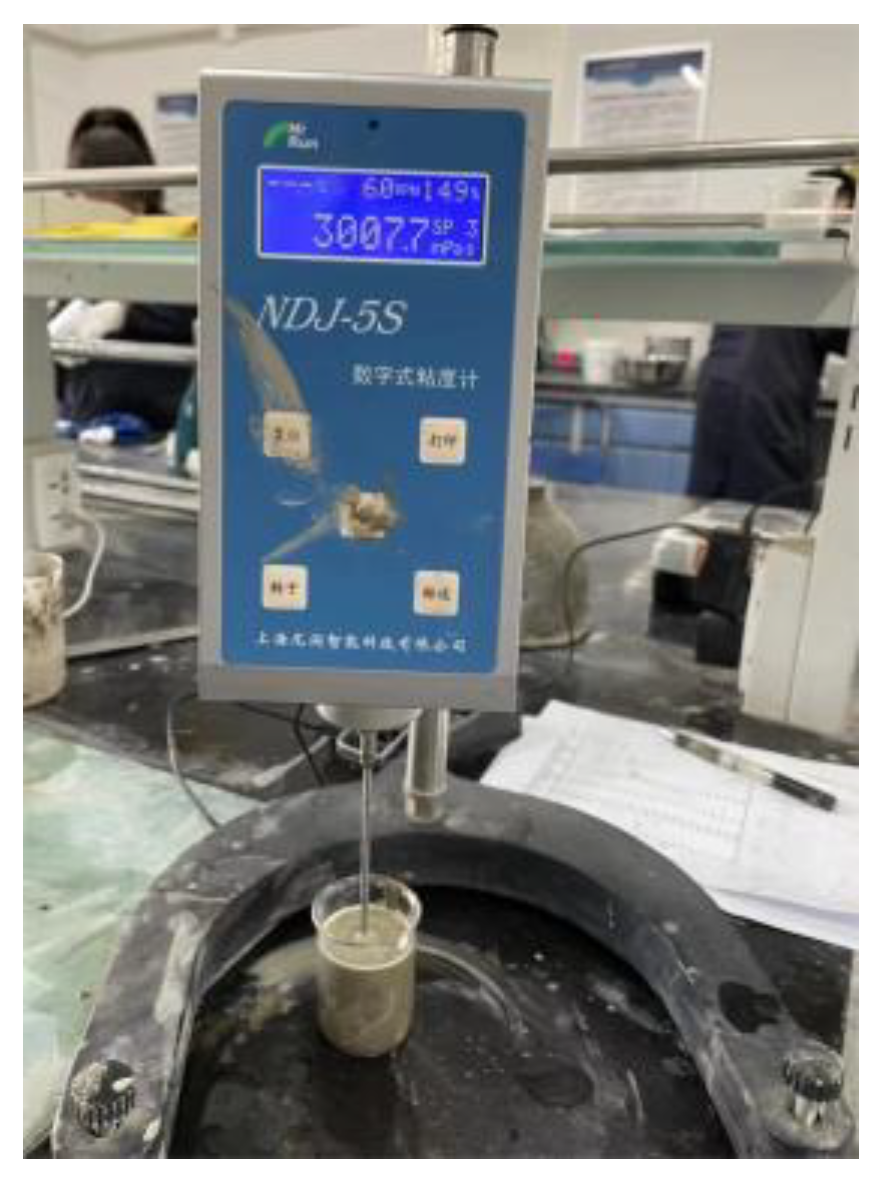
2.3.1. The Measurement of Rheological Parameters
2.3.2. The Initial Setting Time
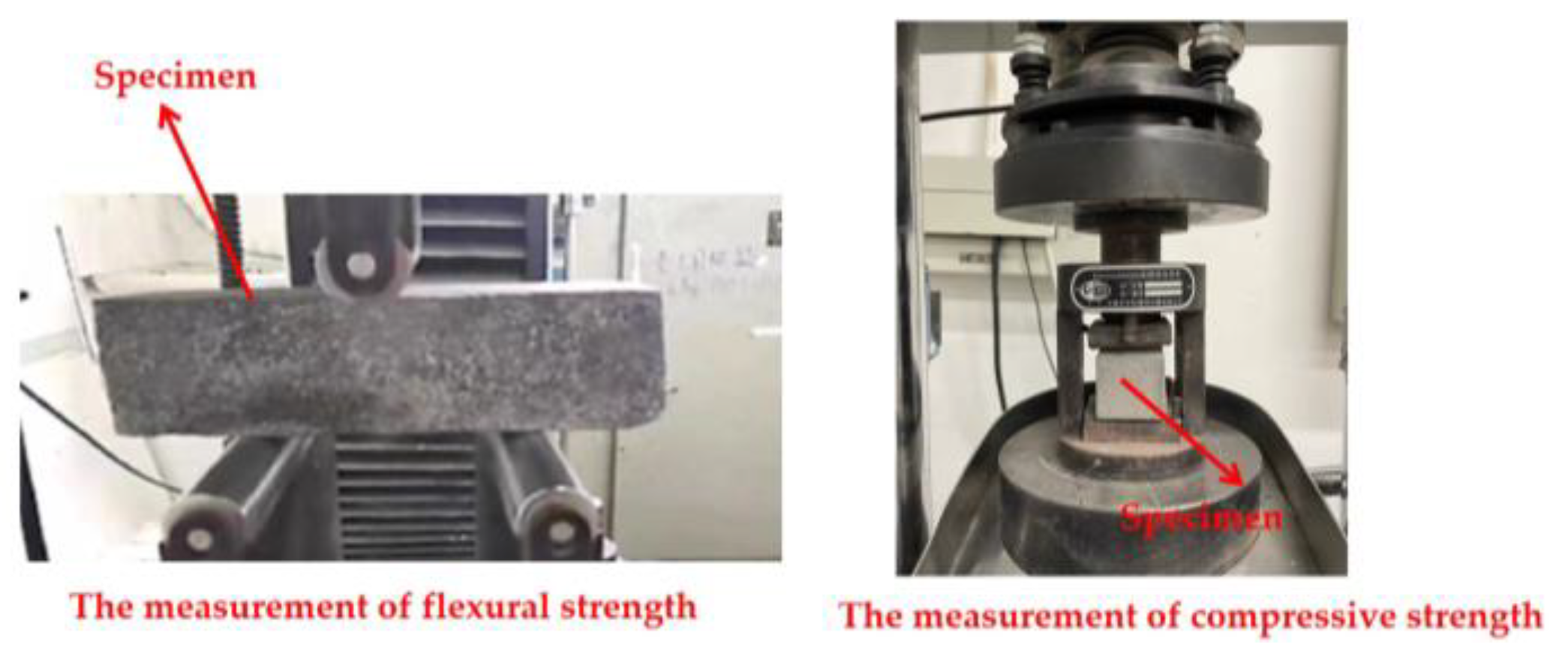
2.3.3. The Mechanical Strengths
3. Results and Discussion
3.1. The Rheological Parameters of UHPC
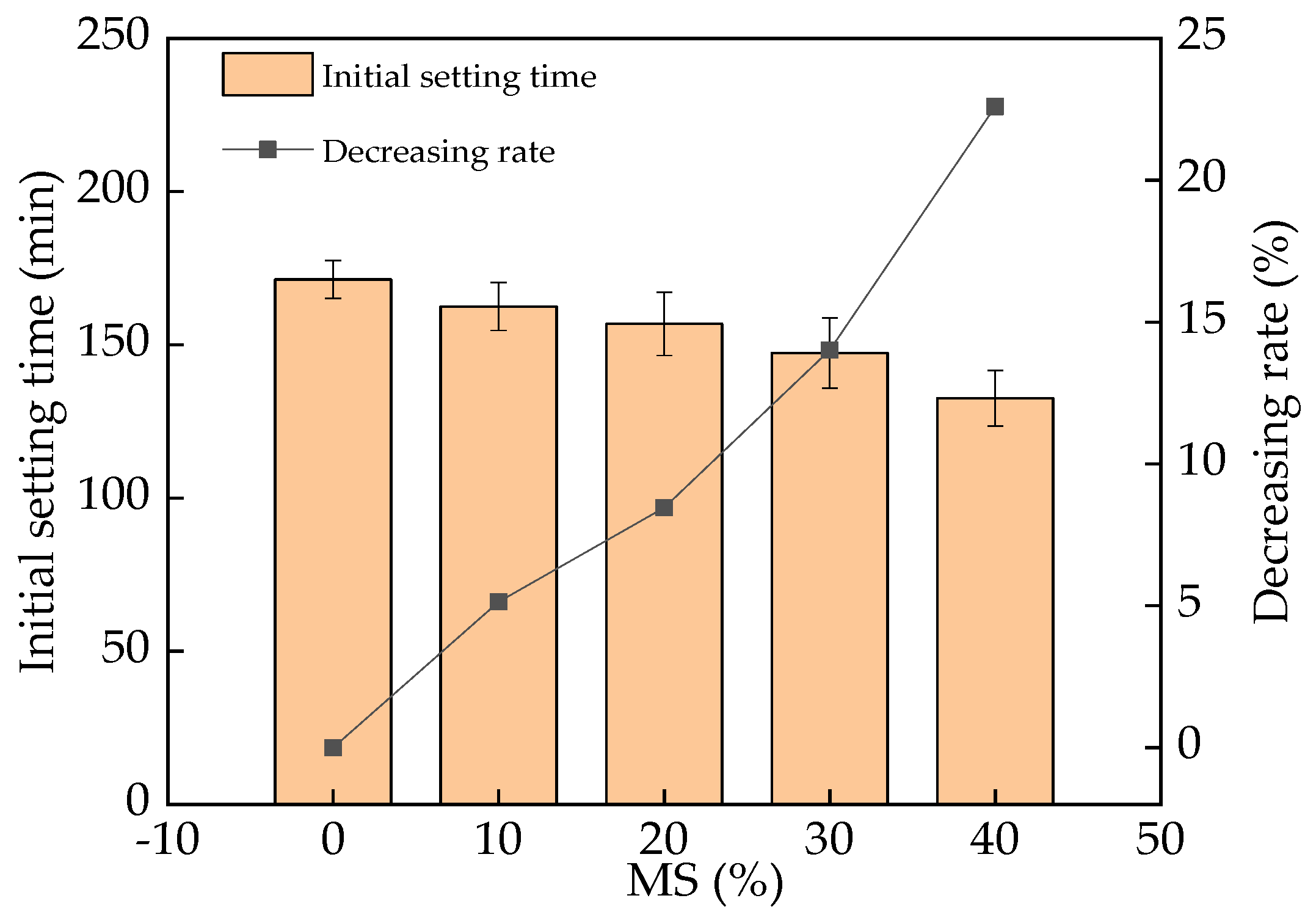
3.2. The Initial Setting Time of UHPC
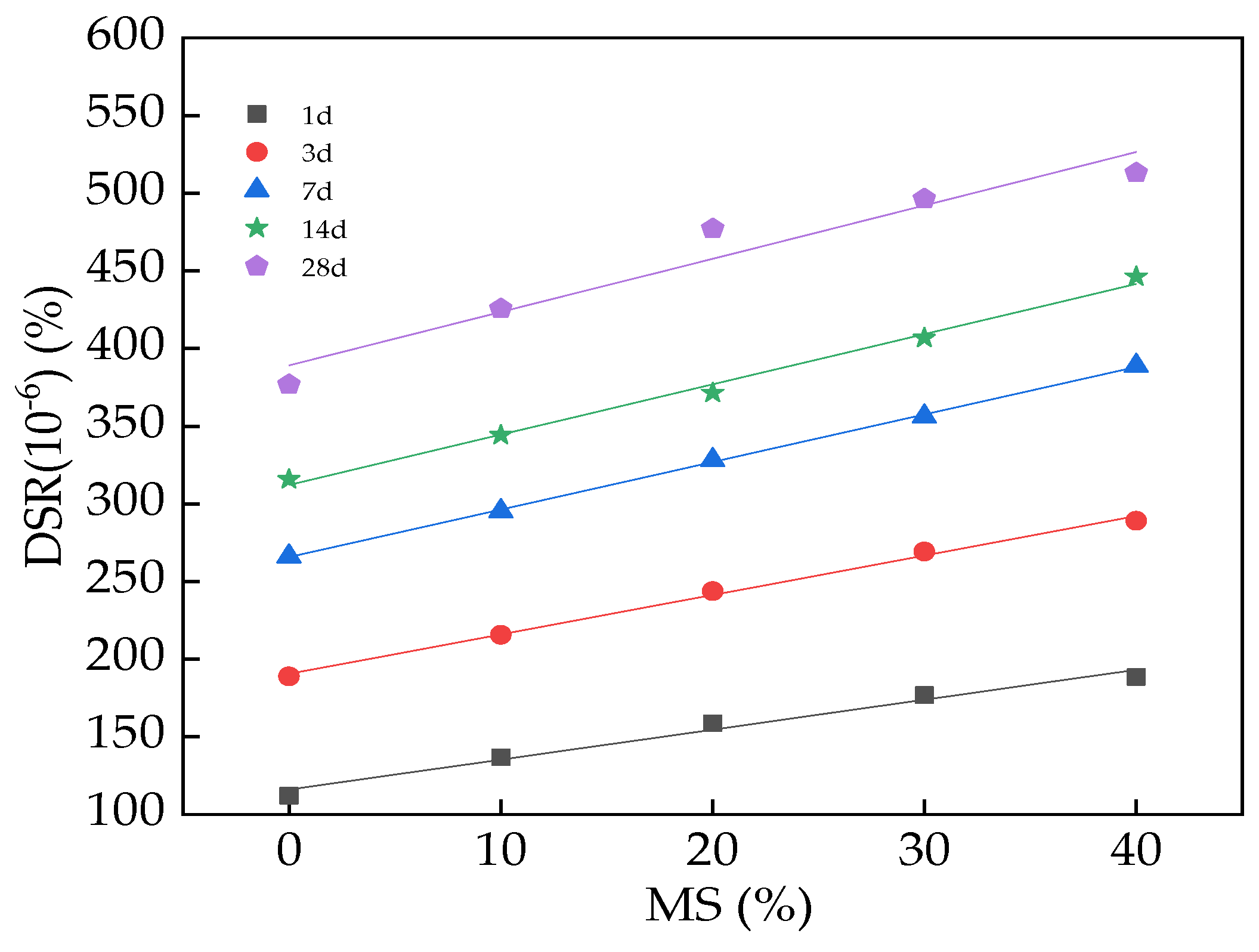
3.3. The DSR of UHPC
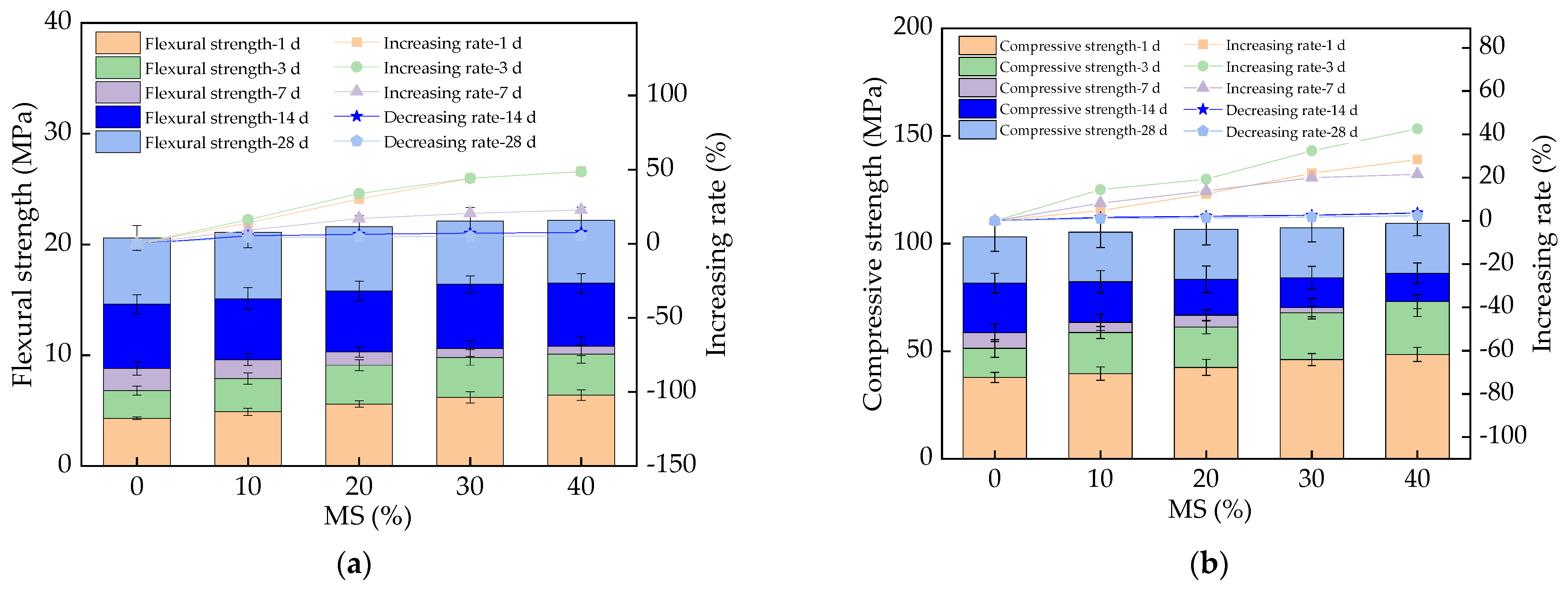
3.4. The Mechanical Strength of UHPC
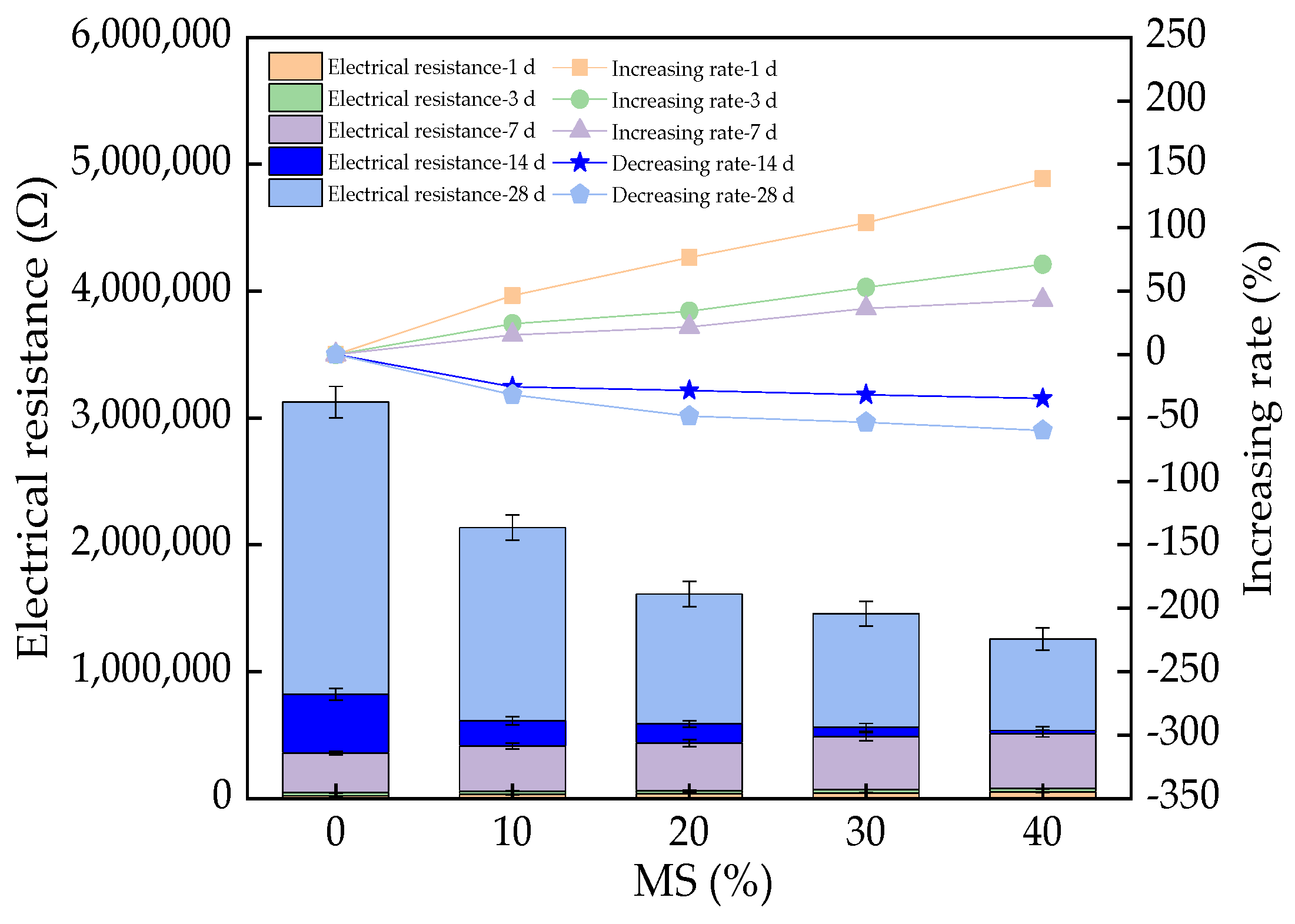
3.5. The Electrical Resistance of UHPC
3.6. The AC Impedance Spectrum of UHPC
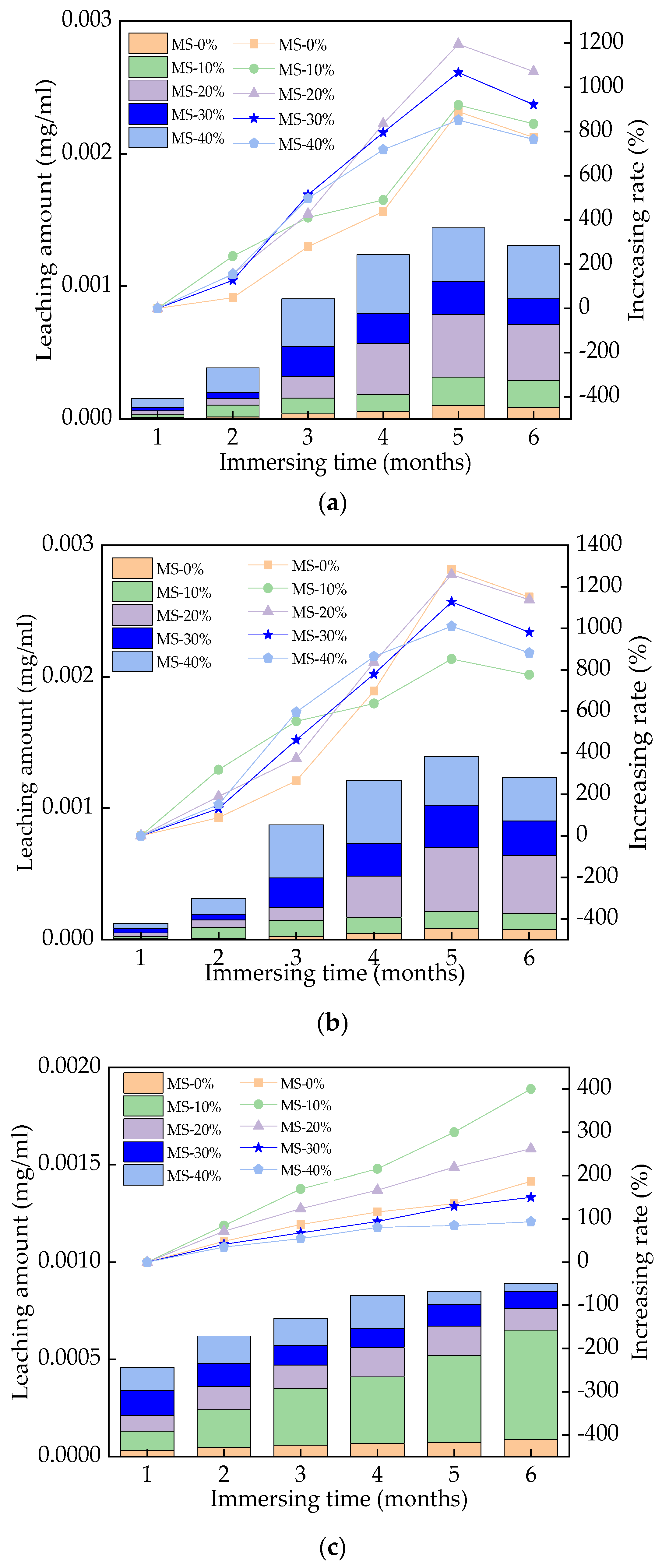
3.7. The Leached Toxic Substances
3.8. The SEM-EDS of UHPC
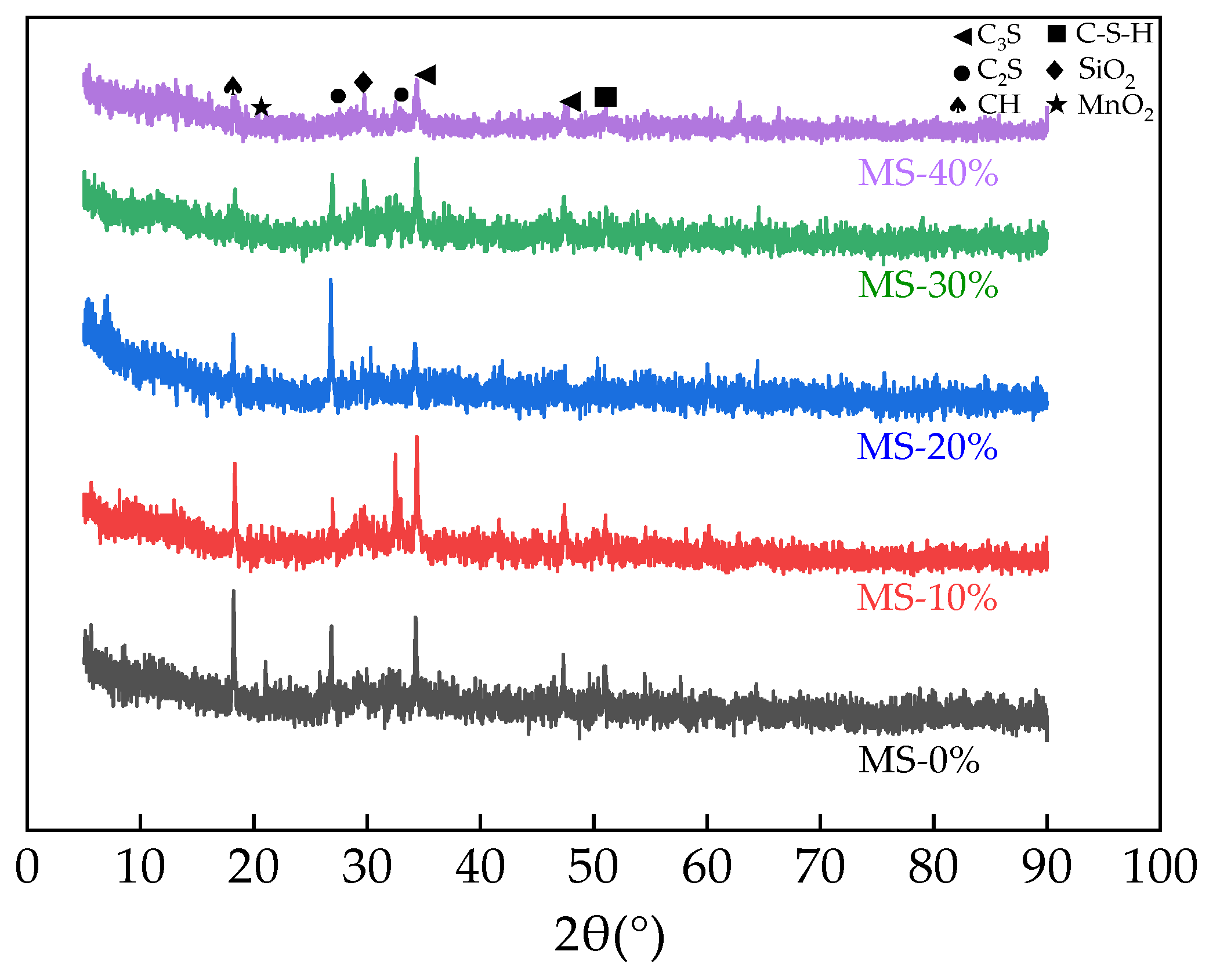
3.9. The XRD of UHPC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kudyba, A.; Akhtar, S.; Johansen, I.; Safarian, J. Aluminothermic Reduction of Manganese Oxide from Selected MnO-Containing Slags. Materials 2021, 14, 356. [Google Scholar] [CrossRef] [PubMed]
- El-Faramawy, H.; Mattar, T.; Eissa, M.; El-Fawakhry, K.; Ahmed, A.M. Demanganisation of high manganese pig iron to produce high manganese stag. Ironmak. Steelmak. 2004, 31, 23–30. [Google Scholar] [CrossRef]
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Functionalization of graphite oxide with magnetic chitosan for the preparation of a nanocomposite dye adsorbent. Langmuir 2013, 29, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, M.; Karmakar, M.; Dutta, A.; Mondal, H.; Roy, J.S.D.; Chattopadhyay, P.K. Microstructural analyses of loaded and/or unloaded semisynthetic porous material for understanding of superadsorption and optimization by response surface methodology. J. Environ. Chem. Eng. 2018, 6, 289–310. [Google Scholar] [CrossRef]
- Nishad, P.A.; Bhaskarapillai, A. Antimony, a pollutant of emerging concern: A review on industrial sources and remediation technologies. Chemosphere 2021, 277, 130252. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Lakouraj, M.M.; Kasirian, N. Development of effective nanobiosorbent based on poly m-phenylenediamine grafted dextrin for removal of Pb (II) and methylene blue from water. Carbohydr. Polym. 2018, 201, 539–548. [Google Scholar] [CrossRef]
- Toro, N.; Rodríguez, F.; Rojas, A.; Robles, P.; Ghorbani, Y. Leaching manganese nodules with iron-reducing agents-a critical review. Miner. Eng. 2021, 163, 106748. [Google Scholar] [CrossRef]
- Luther, G.W.; Thibault de Chanvalon, A.; Oldham, V.E.; Estes, E.R.; Tebo, B.M.; Madison, A.S. Reduction of manganese oxides: Thermodynamic, kinetic and mechanistic considerations for one- versus twoelectron transfer steps. Aquat. Geochem. 2018, 24, 257–277. [Google Scholar] [CrossRef]
- Janis, D.; Carsten, J.; Mathias, K. The first step of the oxidation of elemental sulfur: Crystal structure of the homopolyatomic sulfur radical cation [S8]. Angew. Chem. Int. Ed. 2017, 56, 8281–8284. [Google Scholar]
- Gupta, S.; Sireesha, S.; Sreedhar, I.; Patel, C.M.; Anitha, K.L. Latest trends in heavy metal removal from wastewater by biochar based sorbents. J. Water Process Eng. 2020, 38, 101561. [Google Scholar] [CrossRef]
- Syukor, A.R.A.; Sulaiman, S.; Siddique, M.N.I.; Zularisam, A.W.; Said, M.I.M. Integration of phytogreen for heavy metal removal from wastewater. J. Clean. Prod. 2016, 112, 3124–3131. [Google Scholar] [CrossRef]
- Maleki, A.; Hajizadeh, Z.; Sharifi, V.; Emdadi, Z. A green, porous and eco-friendly magnetic geopolymer adsorbent for heavy metals removal from aqueous solutions. J. Clean. Prod. 2019, 215, 1233–1245. [Google Scholar] [CrossRef]
- Oulguidoum, A.; Bouiahya, K.; Bouyarmane, H.; Talbaoui, A.; Nunzi, J.M.; Laghzizil, A. Mesoporous nanocrystalline sulfonated hydroxyapatites enhance heavy metal removal and antimicrobial activity. Sep. Purif. Technol. 2021, 255, 117777. [Google Scholar] [CrossRef]
- Benavent, V.; Steins, P.; Sobrados, I.; Sanz, J.; Lambertin, D.; Frizon, F.; Rossignol, S.; Poulesquen, A. Impact of aluminum on the structure of geopolymers from the early stages to consolidated material. Cem. Concr. Res. 2016, 90, 27–35. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Guibal, E. A novel algal-based sorbent for heavy metal removal. Chem. Eng. J. 2018, 332, 582–595. [Google Scholar] [CrossRef]
- Esfahani, A.R.; Zhang, Z.; Sip, Y.Y.L.; Zhai, L.; Sadmani, A.H.M.A. Removal of heavy metals from water using electrospun polyelectrolyte complex fiber mats. J. Water Process Eng. 2020, 37, 101438. [Google Scholar] [CrossRef]
- Esrafili, L.; Firuzabadi, F.D.; Morsali, A.; Hu, M.-L. Reuse of predesigned dualfunctional metal organic frameworks (DF-MOFs) after heavy metal removal. J. Hazard. Mater. 2021, 403, 123696. [Google Scholar] [CrossRef]
- Epold, I.; Trapido, M.; Dulova, N. Degradation of levofloxacin in aqueous solutions by Fenton, ferrous ion-activated persulfate and combined Fenton/persulfate systems. Chem. Eng. 2015, 279, 452–462. [Google Scholar] [CrossRef]
- Coetsee, T.; Nell, J.; Pistorius, P.C. Phase Characterization of High Basicity Manganese Slags. Metall. Mater. Trans. B-Process Metall. Mater. Process. Sci. 2017, 48, 1463–1485. [Google Scholar] [CrossRef]
- Rai, A.; Prabakar, J.; Raju, C.B.; Morchalle, R.K. Metallurgical slag as a component in blended cement. Constr. Build. Mater. 2002, 16, 489–494. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Brice, D.G.; Kilcullen, A.R.; Hamdan, S.; Deventer, J.S. Influence of fly ash on the water and chloride permeability of alkali-activated slag mortars and concretes. Constr. Build. Mater. 2013, 48, 1187–1201. [Google Scholar] [CrossRef]
- Sumanjali, P.; Satyanarayana, P.V.V. A comparative study of slope stability analysis of Manganese slag stabilized soil to an ordinary soil. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1025, 012009. [Google Scholar]
- Maurilio, G.P.; Micael, R.C.; Danielle, S.V.; Marcelo, S.P. Manganese mining waste as a novel supplementary material in Portland cement. Mater. Lett. 2022, 309, 131459. [Google Scholar]
- Luo, W.J.; Li, B.; Yang, G.; Xu, M.X. Utilisation of electrolytic manganese residue as a sulphate activator in producing concrete blocks with high-volume fly ash. J. Clean. Prod. 2023, 434, 139813. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, C.; Gao, Z.; Wang, F. A review on fracture properties of steel fiber reinforced concrete. J. Build. Eng. 2023, 67, 105975. [Google Scholar] [CrossRef]
- Rahat, U.; Jawad, A.; Yuan, Q.; Nikolai, I.V. Ultra-High-Performance Concrete (UHPC): A State-of-the-Art Review. Materials 2022, 15, 4131. [Google Scholar]
- Sun, M.; Visintin, P.; Bennett, T. The effect of specimen size on autogenous and total shrinkage of ultra-high performance concrete (UHPC). Constr. Build. Mater. 2022, 327, 126952. [Google Scholar] [CrossRef]
- Yalçınkaya, Ç.; Yazıcı, H. Effects of ambient temperature and relative humidity on early-age shrinkage of UHPC with high-volume mineral admixtures. Constr. Build. Mater. 2017, 144, 252–259. [Google Scholar] [CrossRef]
- Mohebbi, A.; Graybeal, B.; Haber, Z. Time-dependent properties of ultrahigh-performance concrete: Compressive creep and shrinkage. J. Mater. Civ. Eng. 2022, 34, 04022096. [Google Scholar] [CrossRef]
- Yoo, D.; Yoon, Y.; Min, K. Shrinkage and cracking of restrained ultra-high-performance fiber-reinforced concrete slabs at early age. Constr. Build. Mater. 2014, 73, 357–365. [Google Scholar] [CrossRef]
- Abdullah, W.A.; AbdulKadir, M.R.; Muhammad, M.A. Effect of high temperature on mechanical properties of rubberized concrete using recycled tire rubber as fine aggregate replacement. Eng. Technol. 2018, 36, 906–913. [Google Scholar] [CrossRef]
- Bušić, R.; Miličević, I.; Dokšanović, T. Durability Performance and Thermal Resistance of Structural Self-Compacting Concrete Improved with Waste Rubber and Silica Fume. Buildings 2023, 13, 1331. [Google Scholar] [CrossRef]
- Hajj, O.; Douzane, O.; Freitas, D.; Promis, G.; Laidoudi, B.; Bordet, F.; Langlet, T. Physical and Mechanical Properties of Rapeseed Straw Concrete. Materials 2022, 15, 8611. [Google Scholar] [CrossRef] [PubMed]
- Ashour, T.; Morsy, M.; Korjenic, A.; Fischer, H.; Khalil, M.; Sesto, E.; Orabi, M.; Yehia, I. Engineering Parameters of Rice Straw Concrete with Granulated Blast Furnace Slag. Energies 2021, 14, 343. [Google Scholar] [CrossRef]
- Athira, G.; Bahurudeen, A. Rheological properties of cement paste blended with sugarcane bagasse ash and rice straw ash. Constr. Build. Mater. 2022, 332, 127377. [Google Scholar] [CrossRef]
- Luo, W.J.; Liu, S.; Hu, Y.; Pang, C. Sustainable reuse of excavated soil and recycled concrete aggregate in manufacturing concrete blocks. Constr. Build. Mater. 2022, 342, 127917. [Google Scholar] [CrossRef]
- Khayat, K.H.; Meng, W.; Vallurupalli, K. Rheological properties of ultra-high-performance concrete-An overview. Cem. Concr. Res. 2019, 124, 105828. [Google Scholar] [CrossRef]
- Choi, M.S.; Lee, J.S.; Ryu, K.S. Estimation of rheological properties of UHPC using mini slump test. Constr. Build. Mater. 2016, 106, 632–639. [Google Scholar] [CrossRef]
- Sadrmomtazi, A.; Tajasosi, S.; Tahmouresi, B. Effect of materials proportion on rheology and mechanical strength and microstructure of ultra-high performance concrete (UHPC). Constr. Build. Mater. 2018, 187, 1103–1112. [Google Scholar] [CrossRef]
- Dils, J.; Boel, V.; De Schutter, G. Influence of cement type and mixing pressure on air content, rheology and mechanical properties of UHPC. Constr. Build. Mater. 2013, 41, 455–463. [Google Scholar] [CrossRef]
- Abdulkareem, O.M.; Fraj, A.B.; Bouasker, M. Mixture design and early age investigations of more sustainable UHPC. Constr. Build. Mater. 2018, 163, 235–246. [Google Scholar] [CrossRef]
- Norhasri, M.S.M.; Hamidah, M.S.; Fadzil, A.M. Inclusion of nano metaclayed as additive in ultra high performance concrete (UHPC). Constr. Build. Mater. 2019, 201, 590–598. [Google Scholar] [CrossRef]
- Soliman, A.M.; Nehdi, M.L. Effect of partially hydrated cementitious materials and superabsorbent polymer on early-age shrinkage of UHPC. Constr. Build. Mater. 2013, 41, 270–275. [Google Scholar] [CrossRef]
- Yang, C.; Lv, X.X.; Tian, X.K.; Wang, Y.X.; Komarneni, S. An investigation on the use of electrolytic manganese residue as filler in sulfur concrete. Constr. Build. Mater. 2014, 73, 305–310. [Google Scholar] [CrossRef]
- Akbar, N.A.; Aziz, H.A.; Adlan, M.N. Potential of high quality limestone as adsorbent for iron and manganese removal in groundwater. J. Teknol. Sci. Eng. 2016, 78, 77–82. [Google Scholar] [CrossRef]
- Adegoloye, G.; Beaucour, A.L.; Ortola, S.; Noumowé, A. Concretes made of EAF slag and AOD slag aggregates from stainless steel process: Mechanical properties and durability. Constr. Build. Mater. 2015, 76, 313–321. [Google Scholar] [CrossRef]
- Chousidis, N.; Ioannou, I.; Batis, G. Utilization of Electrolytic Manganese Dioxide (E.M.D.) waste in concrete exposed to salt crystallization. Constr. Build. Mater. 2018, 158, 708–718. [Google Scholar] [CrossRef]
- Nath, S.K.; Randhawa, N.S.; Kumar, S. A review on characteristics of silico-manganese slag and its utilization into construction materials. Resour. Conserv. Recycl. 2022, 176, 105946. [Google Scholar] [CrossRef]
- Simao, L.; Fernandes, E.; Hotza, D.; Ribeiro, M.J.; Montedo, O.R.K.; Raupp-Pereira, F. Controlling efflorescence in geopolymers: A new approach. Case Stud. Constr. Mater. 2021, 15, e00740. [Google Scholar] [CrossRef]
- Zhou, Y. Reusing electrolytic manganese residue as an activator: The effect of calcination on its mineralogy and activity. Constr. Build. Mater. 2021, 294, 123533. [Google Scholar] [CrossRef]
- Padilla-Encinas, P.; Palomo, A.; Blanco-Varela, M.T.A. Fernandez-Jimenez, Calcium sulfoaluminate clinker hydration at different alkali concentrations. Cem. Concr. Res. 2020, 138, 106251. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Schmitt; Ben Haha, D.M. Effect of raw mix design and of clinkering process on the formation and mineralogical composition of (ternesite) belite calcium sulphoaluminate ferrite clinker. Cem. Concr. Res. 2014, 59, 87–95. [Google Scholar] [CrossRef]
- Pan, L.J. Effect of Manganese Slag on the Mechanical Properties and Durability of Concrete. Bull. Chin. Ceram. Soc. 2017, 36, 2508–2513. [Google Scholar]
- Dong, S.; Han, B.; Ou, J.; Li, Z.; Han, L.; Yu, X. Electrically conductive behaviors and mechanisms of short-cut super-fine stainless wire reinforced reactive powder concrete. Cem. Concr. Compos. 2016, 72, 48–65. [Google Scholar] [CrossRef]
- Cao, J.; Chung, D. Electric polarization and depolarization in cement-based materials, studied by apparent electrical resistance measurement. Cem. Concr. Res. 2004, 34, 481–485. [Google Scholar] [CrossRef]
- Han, B.; Zhang, L.; Ou, J. Influence of water content on conductivity and piezoresistivity of cement-based material with both carbon fiber and carbon black. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2010, 25, 147–151. [Google Scholar] [CrossRef]
- Shimoda, K.; Hinoki, T.; Kohyama, A. Effect of carbon nanofibers (CNFs) content on thermal and mechanical properties of CNFs/SiC nanocomposites. Compos. Sci. Technol. 2010, 70, 387–392. [Google Scholar] [CrossRef]
- Lindroos, M.; Andersson, T.; Laukkanen, A.; Suarez, L.; Kajberg, J.; Jonsen, P.; Terva, J.; Kallio, M. Micromechanical and multi-scale modeling of manganese containing slag comminution in the design of energy efficient secondary raw material beneficiation processes. Miner. Eng. 2021, 170, 106995. [Google Scholar] [CrossRef]
- Baisanov, A.; Maishina, Z.; Isagulov, A.; Smagulova, N.; Yudakova, V. Experimental melting of high-silicon ferromanganese with the use of ferromanganesian ore and manganese slag. Metalurgija 2021, 60, 89–92. [Google Scholar]










| Types | Particle Size/μm | ||||||
|---|---|---|---|---|---|---|---|
| 0.3 | 0.6 | 1 | 4 | 8 | 64 | 360 | |
| OPC | 0.12 | 0.37 | 3.09 | 14.77 | 29.14 | 92.61 | 100 |
| SF | 32.04 | 59.01 | 82.99 | 99.8 | 99.9 | 100 | 100 |
| BFP | 0.03 | 0.11 | 3.4 | 19.3 | 35.1 | 98.3 | 100 |
| QS | 0 | 0 | 0 | 0 | 0.036 | 23.95 | 100 |
| MS | 0.05 | 0.32 | 1.33 | 8.72 | 23.52 | 98.43 | 100 |
| Types | SiO2 | Al2O3 | FexOy | MgO | CaO | SO3 | K2O | MnO2 | Loss on Ignition |
|---|---|---|---|---|---|---|---|---|---|
| OPC | 20.2 | 5.7 | 3.8 | 1.8 | 62.3 | 2.9 | - | - | 3.0 |
| SF | 90.1 | 0.3 | 0.6 | 0.3 | 0.45 | 0.2 | 7.4 | - | - |
| BFP | 33.8 | 14.7 | 0.6 | 9.9 | 37.1 | 0.2 | 3.4 | - | - |
| QS | 98.4 | - | 1.4 | - | - | - | - | - | - |
| MS | 34.22 | 7.32 | 12.03 | 1.91 | 13.92 | 0.08 | 30.52 | - |
| Types | Water | OPC | MS | SF | BFP | QS | WR |
|---|---|---|---|---|---|---|---|
| MS-1 | 240.2 | 839.9 | 0 | 910 | 111.1 | 961.5 | 16.0 |
| MS-2 | 240.2 | 839.9 | 91 | 819 | 111.1 | 961.5 | 16.0 |
| MS-3 | 240.2 | 839.9 | 182 | 728 | 111.1 | 961.5 | 16.0 |
| MS-4 | 240.2 | 839.9 | 273 | 637 | 111.1 | 961.5 | 16.0 |
| MS-5 | 240.2 | 839.9 | 364 | 546 | 111.1 | 961.5 | 16.0 |
| Equation | Types | a | b | R2 |
|---|---|---|---|---|
| DSR = a + bM | DSR(10−6)(%)-1d | 116 | 1.93 | 0.97 |
| DSR(10−6)(%)-3d | 190.52 | 2.54 | 0.99 | |
| DSR(10−6)(%)-7d | 265.7 | 3.06 | 0.99 | |
| DSR(10−6)(%)-14d | 312.2 | 3.24 | 0.99 | |
| DSR(10−6)(%)-28d | 389.14 | 3.43 | 0.92 |
| Equation | Types | A | B | C | R2 |
|---|---|---|---|---|---|
| MS-0%-3 days | 0.0030 | −123.33 | 1.16 × 106 | 1.00 | |
| MS-10%-3 days | 0.0021 | −75.22 | 3.08 × 105 | 1.00 | |
| MS-20%-3 days | 3.83 × 10−4 | 0.51 | −5.79 × 105 | 1.00 | |
| MS-30%-3 days | 8.19 × 10−4 | −68.29 | 1.29 × 106 | 1.00 | |
| MS-40%-3 days | 4.84 × 10−4 | −40.41 | 6.89 × 105 | 1.00 | |
| MS-0%-14 days | 3.70 × 10−6 | −2.88 | 5.85 × 105 | 1.00 | |
| MS-10%-14 days | 1.29 × 10−5 | −9.95 | 1.98 × 106 | 1.00 | |
| MS-20%-14 days | 8.31 × 10−6 | −5.18 | 8.19 × 105 | 1.00 | |
| MS-30%-14 days | 1.55 × 10−5 | −8.34 | 1.15 × 106 | 1.00 | |
| MS-40%-14 days | 2.23 × 10−5 | −8.77 | 8.75 × 105 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Yu, J.; Wang, H. The Influence of Manganese Slag on the Properties of Ultra-High-Performance Concrete. Materials 2024, 17, 497. https://doi.org/10.3390/ma17020497
Xu W, Yu J, Wang H. The Influence of Manganese Slag on the Properties of Ultra-High-Performance Concrete. Materials. 2024; 17(2):497. https://doi.org/10.3390/ma17020497
Chicago/Turabian StyleXu, Wenyu, Jia Yu, and Hui Wang. 2024. "The Influence of Manganese Slag on the Properties of Ultra-High-Performance Concrete" Materials 17, no. 2: 497. https://doi.org/10.3390/ma17020497




