Effect of Tempering Temperature on the Aqueous Corrosion Resistance of 9Cr Series Heat-Resistant Steel
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Material Preparation
2.2. Microstructure Analysis
2.3. Mechanical Testing
2.4. Corrosion Testing
3. Results and Discussion
3.1. Microstructure Observation
3.1.1. Microstructure Observation before Tempering
3.1.2. Microstructure after Tempering
3.2. Hardness
3.3. Electrochemical Aqueous Corrosion Performance
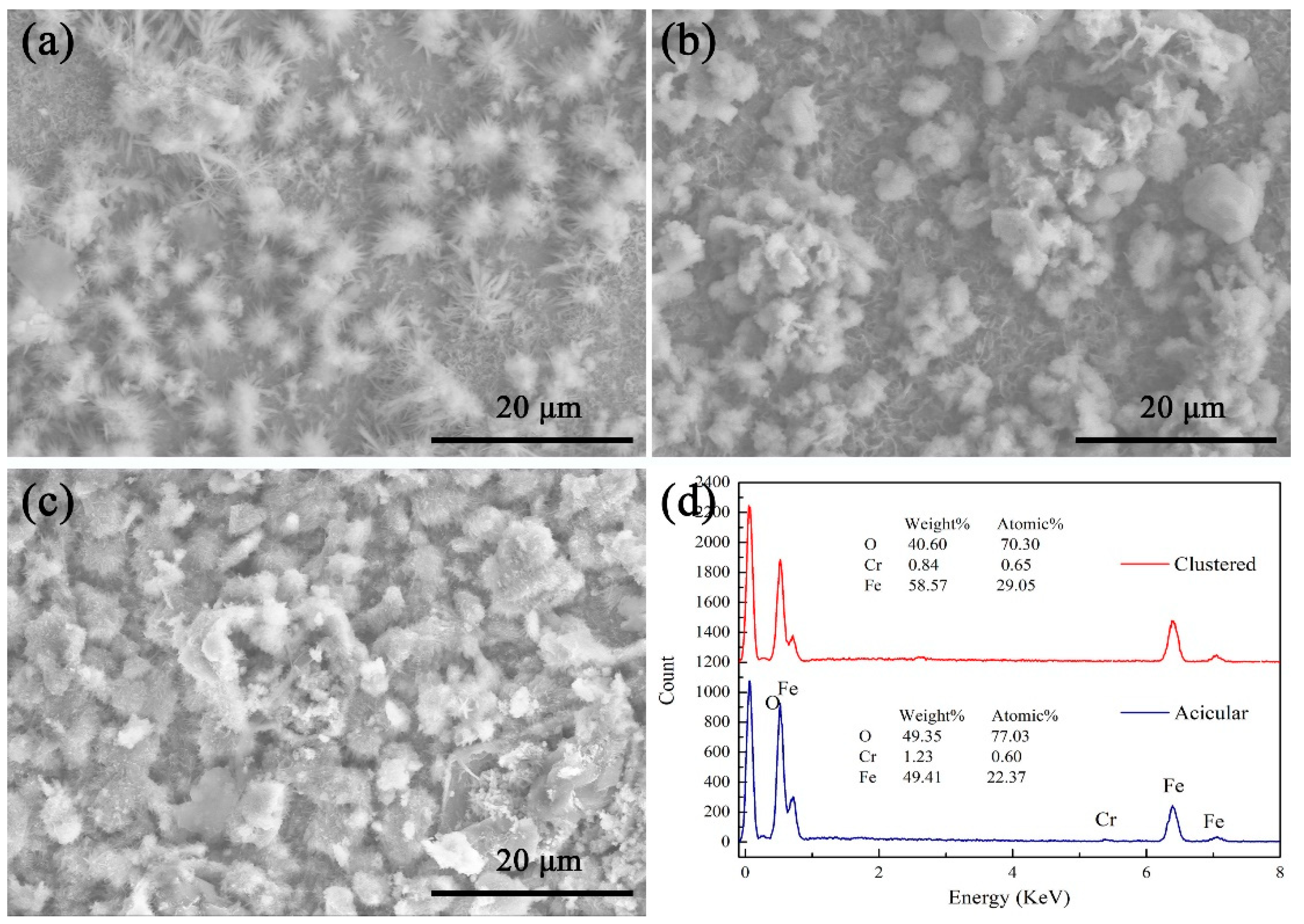
3.4. Aqueous Corrosion Behavior in Neutral Salt Spray Environment
3.5. Discussion
- Influence law
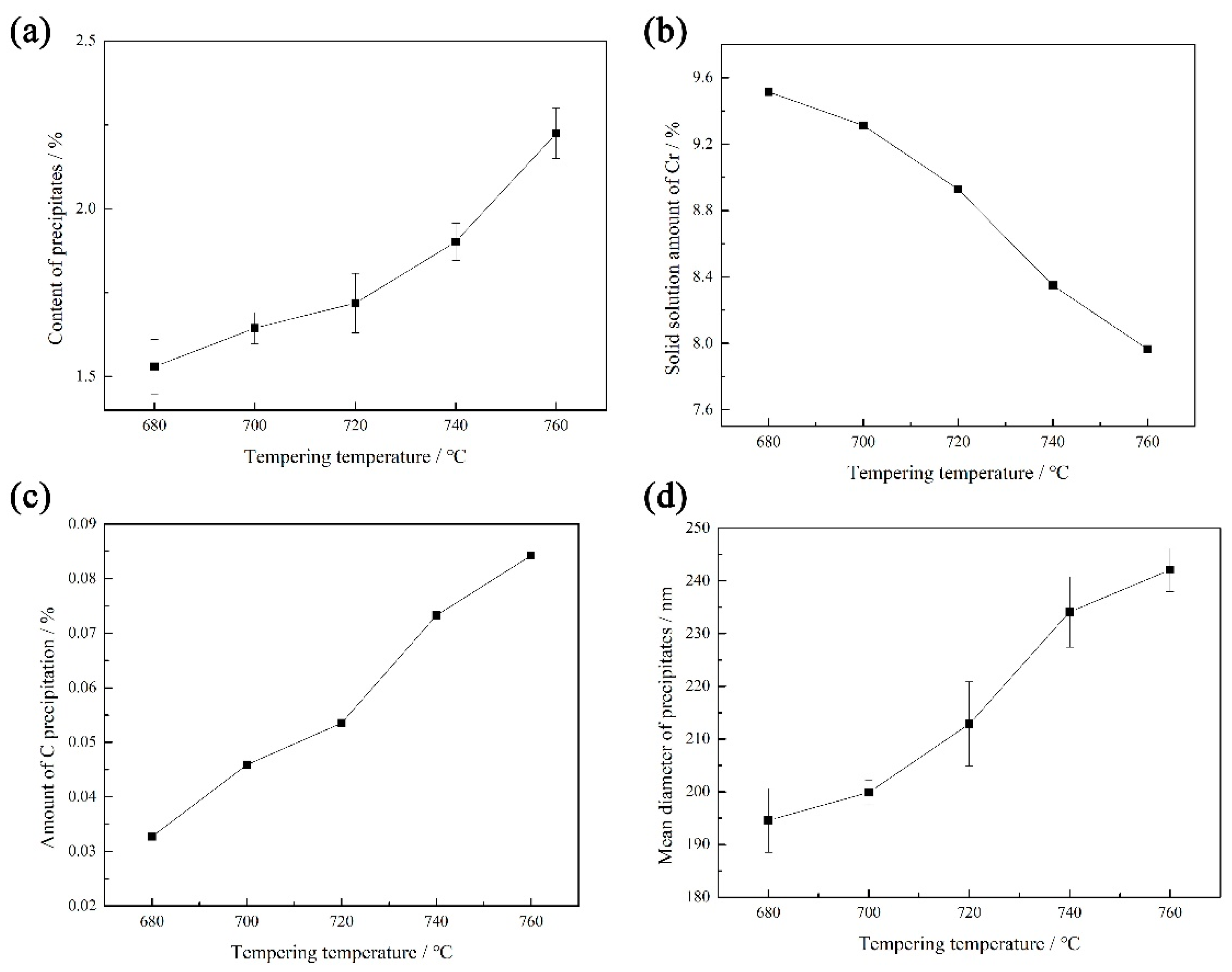
- Cr elements and carbides
- Dislocation density
4. Conclusions
- The microstructure of the heat-resistant steel after tempering was composed of lath-tempered martensite and fine precipitates. With the tempering temperature increasing from 680 °C to 760 °C, the average size and amount of the precipitates increased slowly at first and then significantly.
- The aqueous corrosion resistance of the heat-resistant steel decreased with the decrease in the Cr content in the solid solution and increased with the decrease in the dislocation density. With the increase in the tempering temperature, the solid solution Cr content and the dislocation density continued to decrease, which is the main reason for the increase and then decrease in corrosion resistance. The aqueous corrosion resistance of the heat-resistant steel tempered at 720 °C was the best. The tempering temperature of 720 °C provides a certain reference value for the industrial production of 9Cr heat-resistant steel.
- The transformation of surface oxide morphology from acicular to cluster correlates with improved stability and reduced susceptibility to localized corrosion at 720 °C.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, G.; Was, G.S. Improved creep behavior of ferritic-martensitic alloy T91 by subgrain boundary density enhancement. Metall. Mater. Trans. A 2008, 39, 150–164. [Google Scholar] [CrossRef]
- Yan, P.; Liu, Z.D.; Bao, H.S.; Weng, Y.Q.; Liu, W. Effect of normalizing temperature on the strength of 9Cr-3W-3Co martensitic heat resistant steel. Mater. Sci. Eng. A 2014, 597, 148–156. [Google Scholar] [CrossRef]
- Gibbons, T.J.M.S. Technology, Superalloys in modern power generation applications. Mater. Sci. Technol. 2009, 25, 129–135. [Google Scholar] [CrossRef]
- Hagarová, M.; Baranová, G.; Jablonský, G.; Buľko, B.; Vojtko, M.; Komanický, V.; Vorobiov, S.; Bednarčík, J. Influence of flowing water vapor containing environment on high-temperature behavior of 9Cr creep-resistant steels. J. Mater. Res. Technol. 2023, 23, 3840–3855. [Google Scholar] [CrossRef]
- Chen, H.; He, Z.; Lu, L. Correlation of surface features with corrosion behaviors of interstitial free steel processed by temper rolling. J. Mater. Sci. Technol. 2020, 36, 37–44. [Google Scholar] [CrossRef]
- Feng, H.; Jiang, Z.; Li, H.; Lu, P.; Zhang, S.; Zhu, H.; Zhang, B.; Zhang, T.; Xu, D.; Chen, Z. Influence of nitrogen on corrosion behaviour of high nitrogen martensitic stainless steels manufactured by pressurized metallurgy. Corros. Sci. 2018, 144, 288–300. [Google Scholar] [CrossRef]
- Fetni, S.; Toumi, A.; Mkaouar, I.; Boubahri, C.; Briki, J. Microstructure evolution and corrosion behaviour of an ASTM A213 T91 tube after long term creep exposure. Eng. Fail. Anal. 2017, 79, 575–591. [Google Scholar] [CrossRef]
- Ye, Q.F.; Feng, K.; Li, Z.G.; Lu, F.G.; Li, R.F.; Huang, J.; Wu, Y.X. Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl. Surf. Sci. 2017, 396, 1420–1426. [Google Scholar] [CrossRef]
- Okoro, S.C.; Montgomery, M.; Frandsen, F.J.; Pantleon, K. Influence of preoxidation on high temperature corrosion of a Ni-based alloy under conditions relevant to biomass firing. Surf. Coat Technol. 2017, 319, 76–87. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, L.; Gao, Q.; Liu, C.; Ma, Z.; Li, H.; Liu, Y.; Wang, H. Development of weld filler material to match the advanced martensitic heat resistance steel G115 and tailoring the performance by tempering temperature. J. Mater. Res. Technol. 2022, 21, 2515–2531. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, X.; Kang, Z.; Ye, F.; Chen, Y.; Zhou, D.; Zhang, X.; Gong, J. Creep lifetime prediction of 9% Cr martensitic heat-resistant steel based on ensemble learning method. J. Mater. Res. Technol. 2022, 21, 4745–4760. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, C.; Xiong, X.; Zheng, Z.; Zhu, M. Intergranular corrosion susceptibility of a novel Super304H stainless steel. Eng. Fail. Anal. 2012, 24, 26–32. [Google Scholar] [CrossRef]
- Zhou, Q.; Zheng, Z.; Gao, Y.J. Abnormal selective dissolution by the partial recrystallization in a plastically deformed austenitic stainless steel. Corros. Sci. 2021, 188, 109548. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, R.; Zhan, X.; Liu, J.; Wang, W.; Wang, Y.; Tang, W. Austenite Grain Growth and Its Effect on the Mechanical Properties of Super304H Heat-Resistant Steel. J. Mater. Eng. Perform. 2022, 32, 2228–2236. [Google Scholar] [CrossRef]
- Montoya-Rangel, M.; Garza-Montes-De-Oca, N.F.; Gaona-Tiburcio, C.; Almeraya-Calderón, F. Corrosion mechanism of advanced high strength dual-phase steels by electrochemical noise analysis in chloride solutions. Mater. Today Commun. 2023, 35, 105663. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Kang, Z.; Zhang, T.; Jiang, Y.; Zhang, X.; Gong, J. Characterization of the cyclic softening and remaining creep behaviour of P92 steel weldment. J. Mater. Res. Technol. 2021, 15, 1446–1456. [Google Scholar] [CrossRef]
- Han, K.; Ding, H.; Fan, X.; Li, W.; Lv, Y.; Feng, Y. Study of the creep cavitation behavior of P91 steel under different stress states and its effect on high-temperature creep properties. J. Mater. Res. Technol. 2022, 20, 47–59. [Google Scholar] [CrossRef]
- Yin, H.-F.; Yang, G.; Zhao, J.-Q.; Bao, H.-S.J.M.C. Mo-rich Laves phase in a 9.5 Cr-1.5 MoCoVNbNB heat-resistant steel during long-term aging at 620 °C. Mater. Charact. 2021, 182, 111588. [Google Scholar] [CrossRef]
- Ren, J.; Li, C.; Han, Y.; Li, E.; Gao, C.; Qiu, C. Effect of initial martensite and tempered carbide on mechanical properties of 3Cr2MnNiMo mold steel. Mater. Sci. Eng. A 2021, 812, 141080. [Google Scholar] [CrossRef]
- Guillou, S.; Cabet, C.; Desgranges, C.; Marchetti, L.; Wouters, Y. Influence of hydrogen and water vapour on the kinetics of chromium oxide growth at high temperature. Oxid. Met. 2011, 76, 193–214. [Google Scholar] [CrossRef]
- Yang, X.; Xu, Y.; Lu, J.; Guo, Z.; Dang, Y.; Huang, J.; Li, W. Subsurface microstructural degeneration of Super304H boiler tube under the synergy of fireside corrosion and creep stress. Corros. Sci. 2022, 200, 110250. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, G.; Wang, M.; Guo, Q.; Wang, Z.; Wu, Y.; Xu, H.; Chen, D. Corrosion behavior of heat-resistant steel T91 in high-temperature supercritical carbon dioxide with impurity O2, SO2 or H2S. Fluids 2023, 198, 105936. [Google Scholar] [CrossRef]
- Staszuk, M.J.V. Investigations of CrN+ Cr2O3/TiO2 coatings obtained in a PVD/ALD hybrid method on austenitic 316L steel substrate. Vacuum 2023, 207, 111653. [Google Scholar] [CrossRef]
- Guo, T.; Chen, Y.; Shao, H.; Zhao, Q.; Liang, Z. Molecular dynamics, thermodynamics and experimental studies on the corrosion mechanism of T92 and TP347H steels in high-pressure CO2 and H2O at 600 °C. Appl. Surf. Sci. 2023, 621, 156879. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Sun, X.; Lu, Z. Effects of silicon additions on the oxide scale formation of an alumina-forming austenitic alloy. Corros. Sci. 2012, 65, 317–321. [Google Scholar] [CrossRef]
- Li, J.; Zhang, P.; He, T.; Cheng, L.; Wang, L.; Li, H. Effect of carbides on high-temperature aging embrittlement in 12%Cr martensitic heat-resistant steel. J. Mater. Res. Technol. 2019, 8, 5833–5846. [Google Scholar] [CrossRef]
- Gorunov, A.J.S.; Technology, C. Investigation of M7C3, M23C6 and M3C carbides synthesized on austenitic stainless steel and carbon fibers using laser metal deposition. Surf. Coat. Technol. 2020, 401, 126294. [Google Scholar] [CrossRef]
- Jiang, C.C.; Dong, Z.; Song, X.L.; Jia, J.; Xiang, Z.D. Long-term creep rupture strength prediction for a new grade of 9Cr martensitic creep resistant steel (G115)—An application of a new tensile creep rupture model. J. Mater. Res. Technol. 2020, 9, 5542–5548. [Google Scholar] [CrossRef]
- Sobolev, A.; Mirzoev, A. Structure and stability of (Cr, Fe)7C3 ternary carbides in solid and liquid state. J. Alloys Compd. 2019, 804, 566–572. [Google Scholar] [CrossRef]
- Wu, H.; Wang, S.; Zhao, Q.; Liang, Z. High-temperature corrosion data and mechanisms for T122, Super304H and HR3C after 15 years in 1000MW ultra-supercritical power plant. Mater. High Temp. 2023, 40, 88–98. [Google Scholar] [CrossRef]
- Kasl, J.; Mikmekova, S.; Jandova, D. SEM, TEM and SLEEM (scanning low energy electron microscopy) of CB2 steel after creep testing. IOP Conf. Ser. Mater. Sci. Eng. 2014, 55, 012008. [Google Scholar] [CrossRef]
- Jandova, D.; Kasl, J.; Chvostova, E. Microstructure of CB2 Steel before and after Long-term Creep Tests. Mater. Sci. Forum 2014, 782, 311–318. [Google Scholar] [CrossRef]
- Dou, P.; Kimura, A.; Kasada, R.; Okuda, T.; Inoue, M.; Ukai, S.; Ohnuki, S.; Fujisawa, T.; Abe, F. TEM and HRTEM study of oxide particles in an Al-alloyed high-Cr oxide dispersion strengthened steel with Zr addition. J. Nucl. Mater. 2014, 444, 441–453. [Google Scholar] [CrossRef]
- Yang, S.; Che, Z.; Liu, W.; Liu, Z.; Cheng, X.; Liu, C.; Li, X. Influence mechanism of heat treatment on corrosion resistance of Te-containing 15–5PH stainless steel. Corros. Sci. 2023, 225, 111610. [Google Scholar] [CrossRef]
- Song, C.; Zhang, Z.; Wu, W.; Wang, H.; Sun, Z.; Yang, Y.; He, W.; Xu, J.; Xia, Y.; Yin, W.; et al. Effect of Si on the dislocation state within martensite of ultra-high strength hot-rolled medium Mn steel with good ductility. Mater. Sci. Eng. A 2023, 869, 144825. [Google Scholar] [CrossRef]
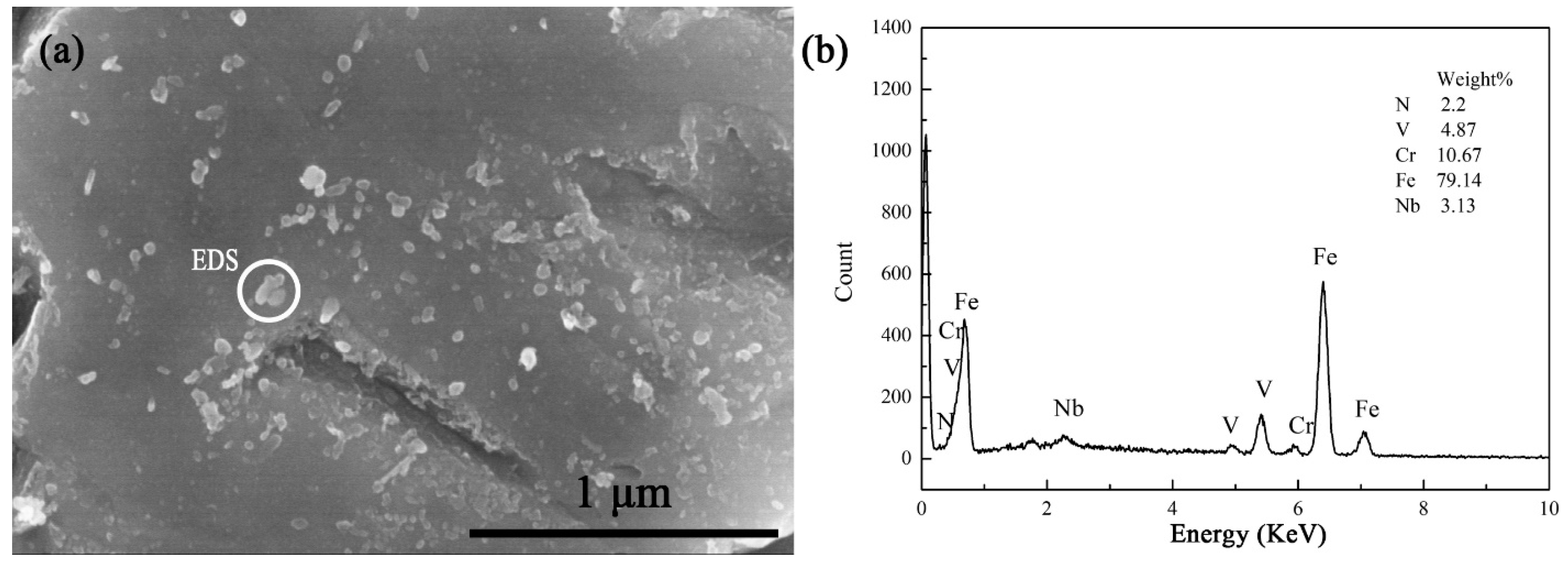
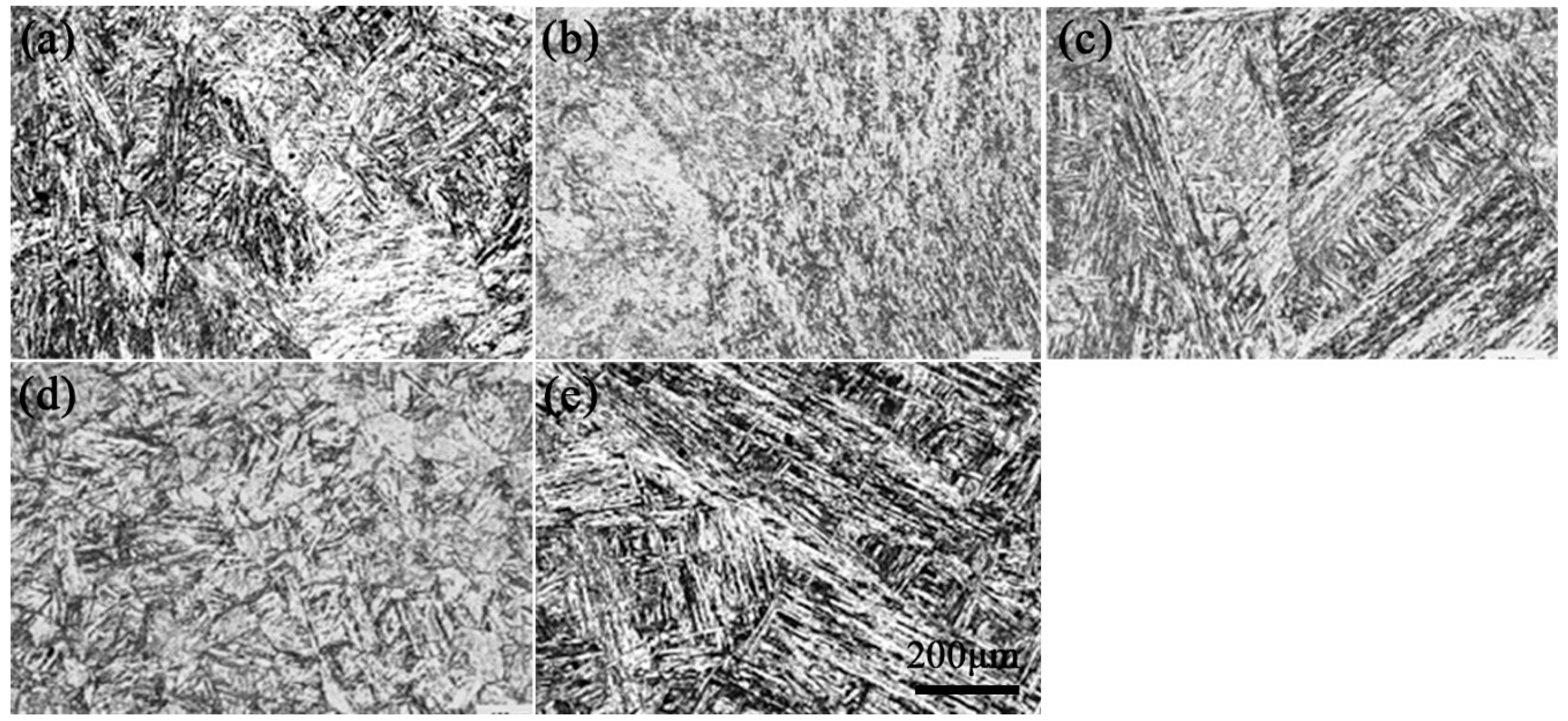


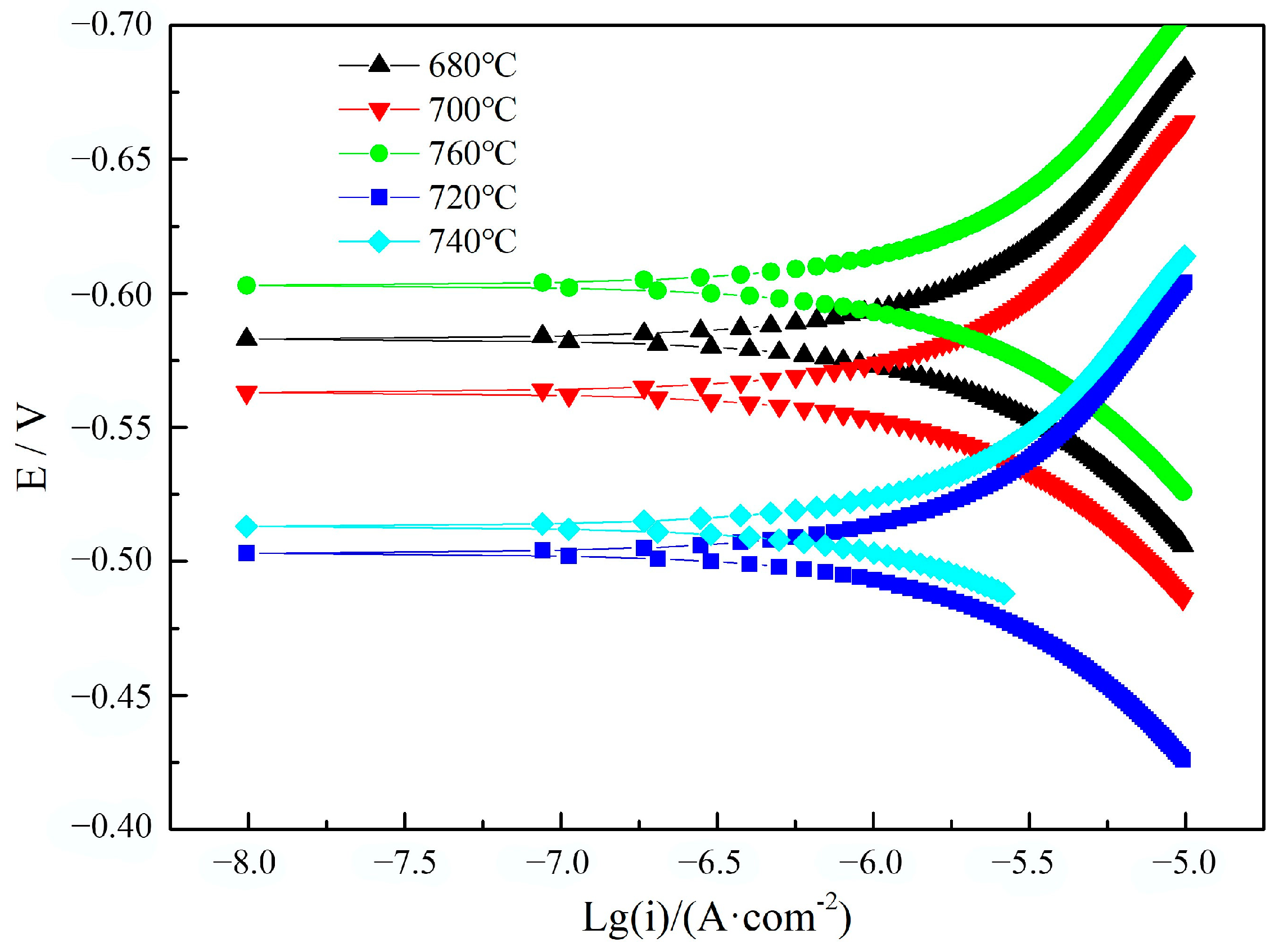
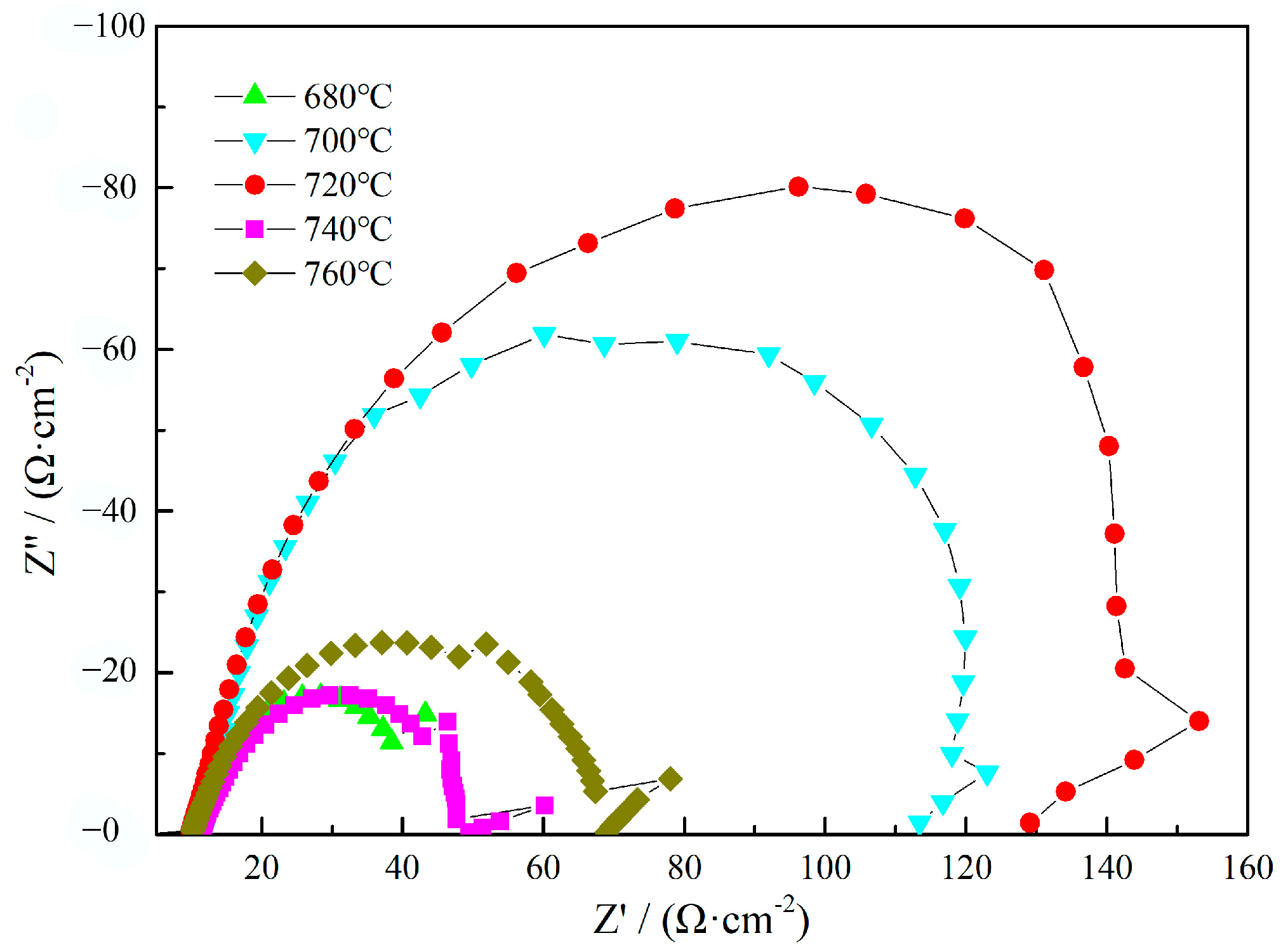

| Tempering Temperature/°C | 680 | 700 | 720 | 740 | 760 |
|---|---|---|---|---|---|
| Corrosion Current Density/(μA·cm−2) | 0.692 | 0.653 | 0.612 | 0.619 | 0.704 |
| Tempering Temperature/ °C | Solution Resistance Rs/(Ω·cm2) | Charge Transfer Resistance Rt/(Ω·cm2) | Film Resistance Rf/(Ω·cm2) | Polarization Resistance Rp/(Ω·cm2) |
|---|---|---|---|---|
| 680 | 8.68 | 22.87 | 73.51 | 107.61 |
| 700 | 8.74 | 15.55 | 87.82 | 113.84 |
| 720 | 9.73 | 31.89 | 124.9 | 138.87 |
| 740 | 9.88 | 19.04 | 75.83 | 115.82 |
| 760 | 9.95 | 15.43 | 89.43 | 114.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Bai, H. Effect of Tempering Temperature on the Aqueous Corrosion Resistance of 9Cr Series Heat-Resistant Steel. Materials 2024, 17, 4960. https://doi.org/10.3390/ma17204960
Li H, Bai H. Effect of Tempering Temperature on the Aqueous Corrosion Resistance of 9Cr Series Heat-Resistant Steel. Materials. 2024; 17(20):4960. https://doi.org/10.3390/ma17204960
Chicago/Turabian StyleLi, Hui, and Hao Bai. 2024. "Effect of Tempering Temperature on the Aqueous Corrosion Resistance of 9Cr Series Heat-Resistant Steel" Materials 17, no. 20: 4960. https://doi.org/10.3390/ma17204960





