Corrosion Behavior of 700L Automotive Beam Steel in Marine Atmospheric Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Atmospheric Exposure Tests
2.3. Corrosion Product Analysis
2.4. Electrochemical Measurements
3. Results and Discussion
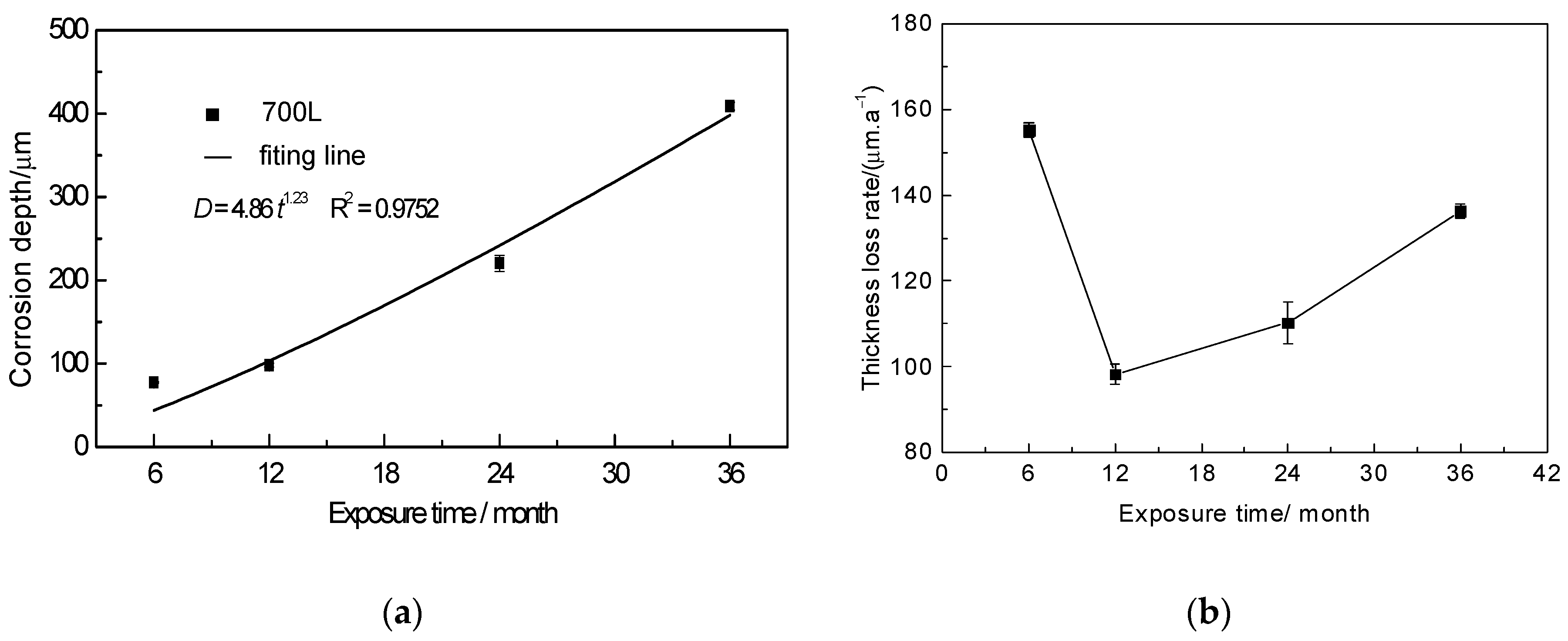
3.1. Corrosion Kinetics
3.2. Macroscopic Corrosion Product Morphology
3.3. Microscopic Corrosion Product Morphology
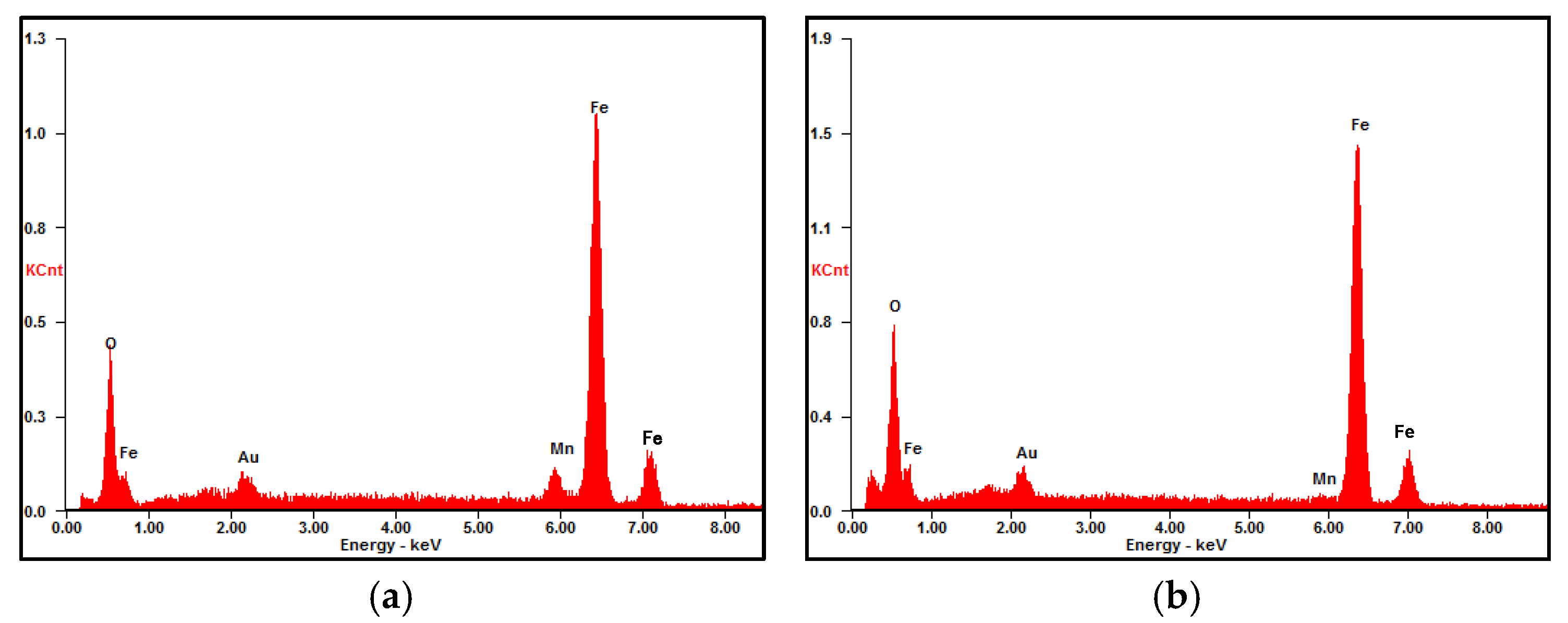
3.3.1. Surface Morphology
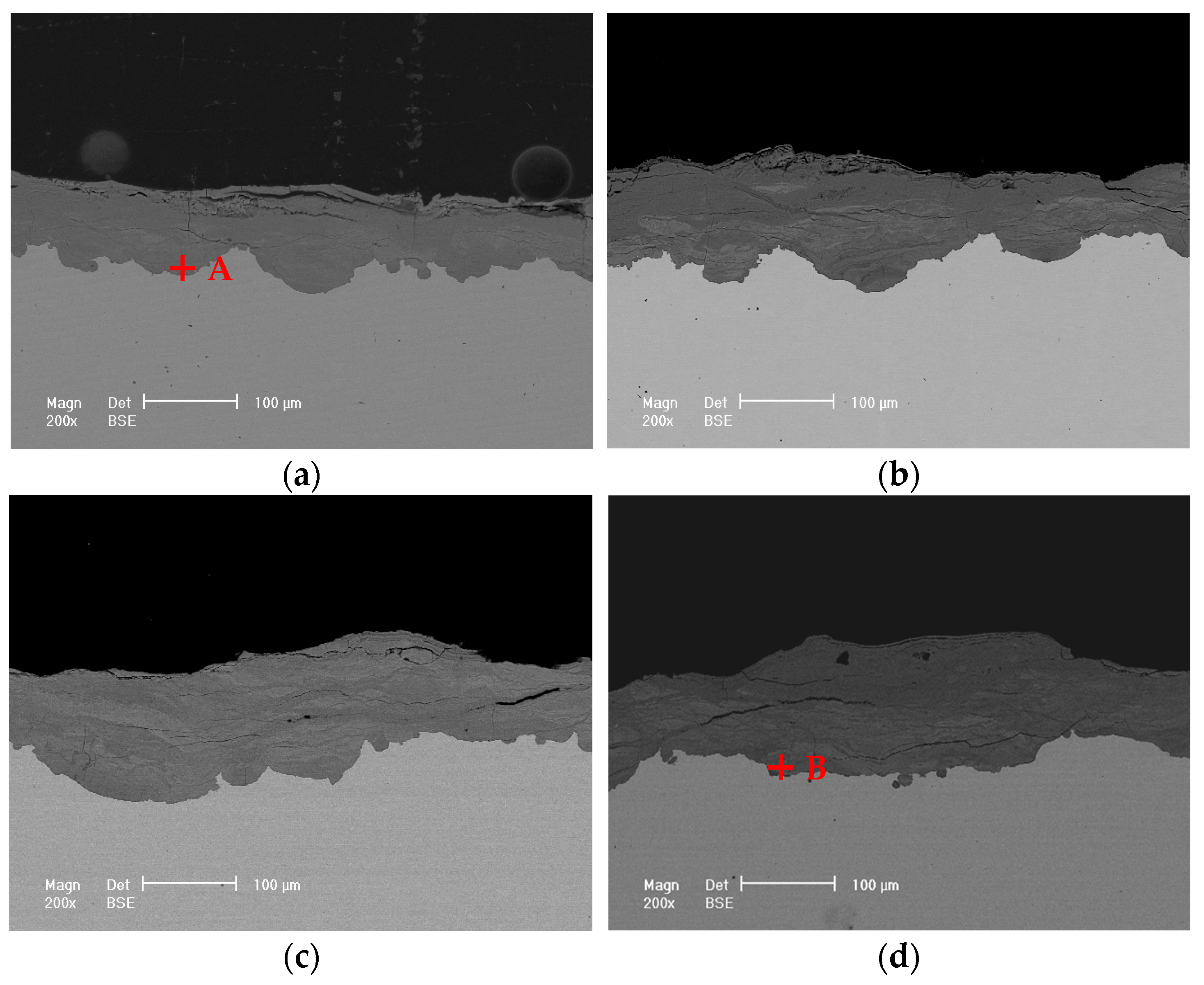
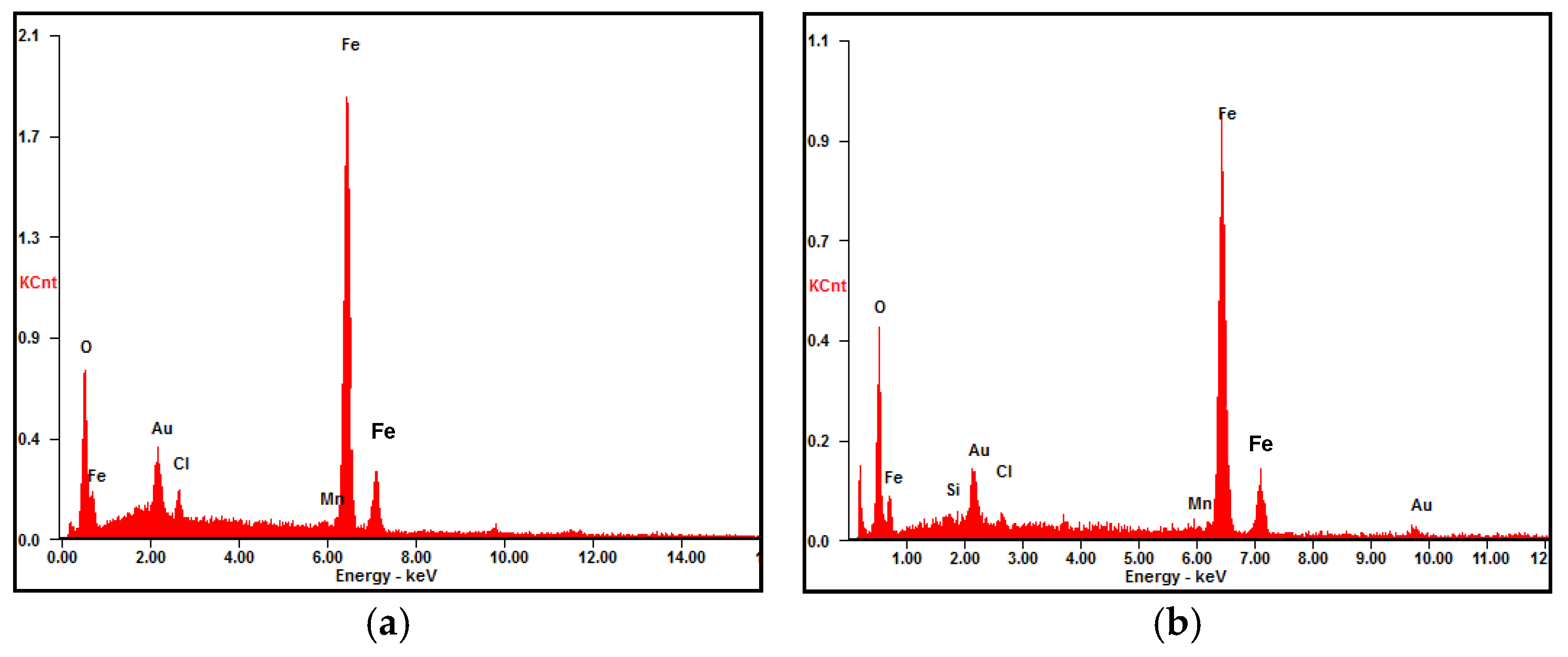
3.3.2. Cross-Sectional Morphology
3.4. Corrosion Morphology
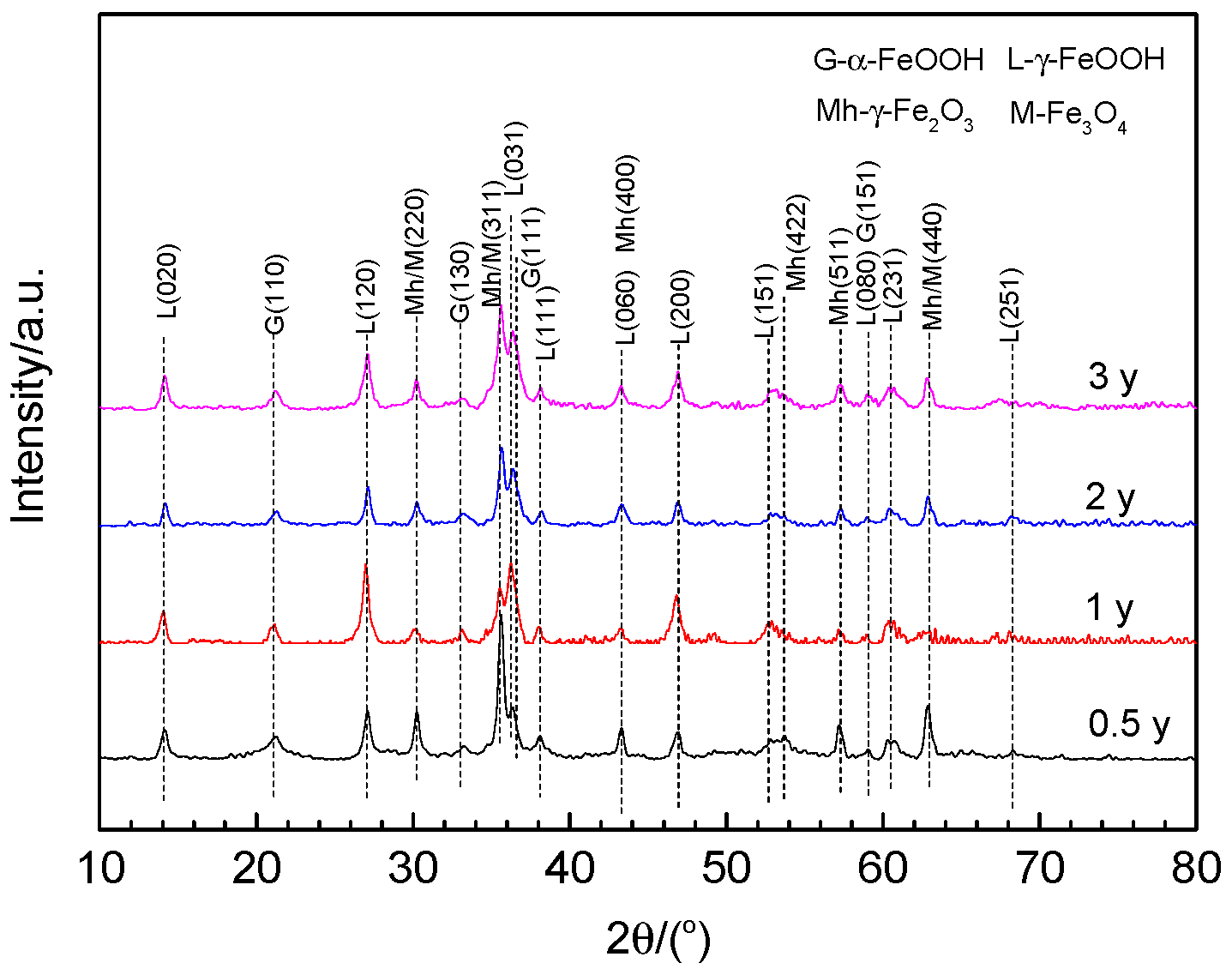
3.5. Phase Composition of the Rust Layer
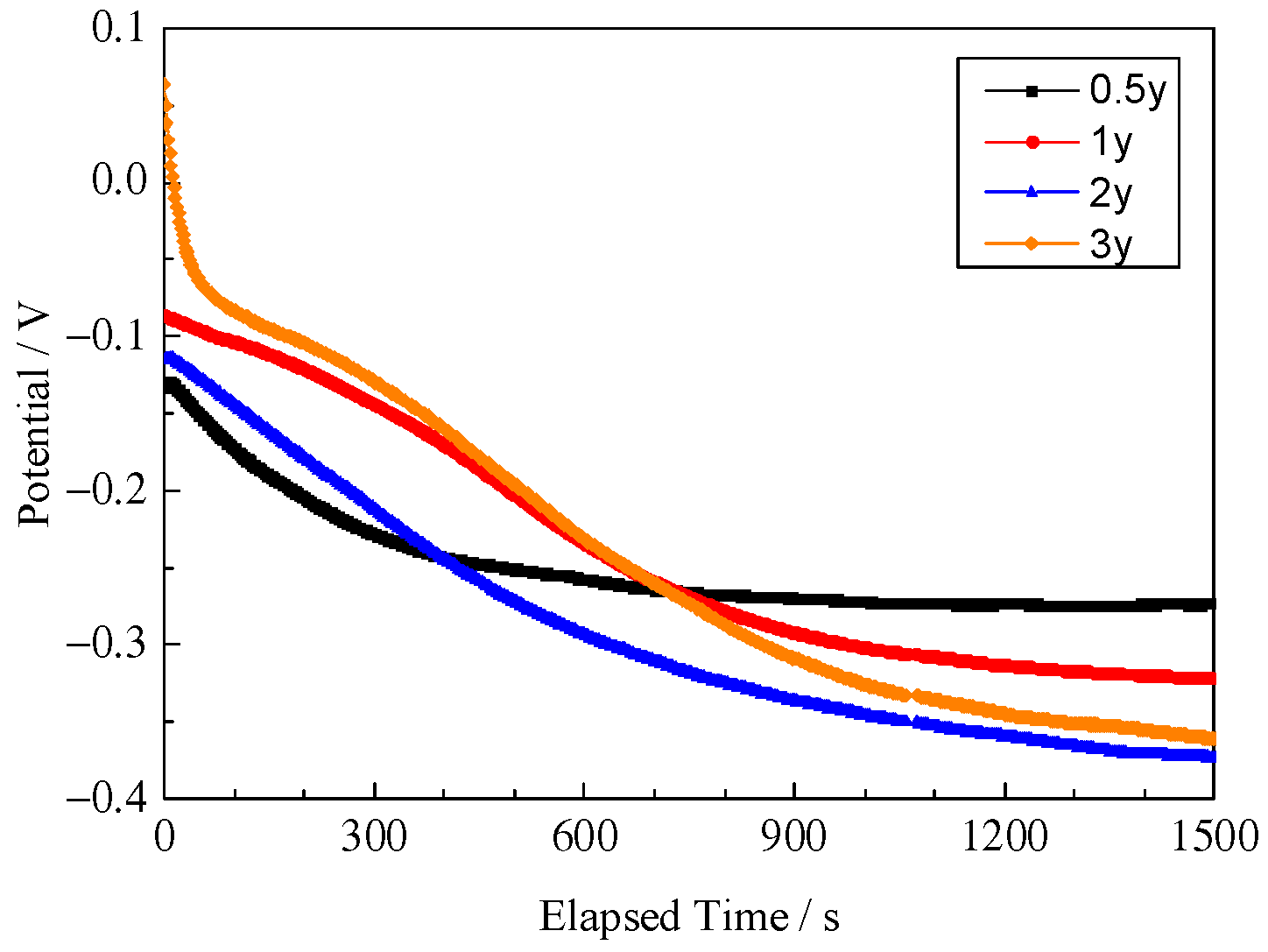
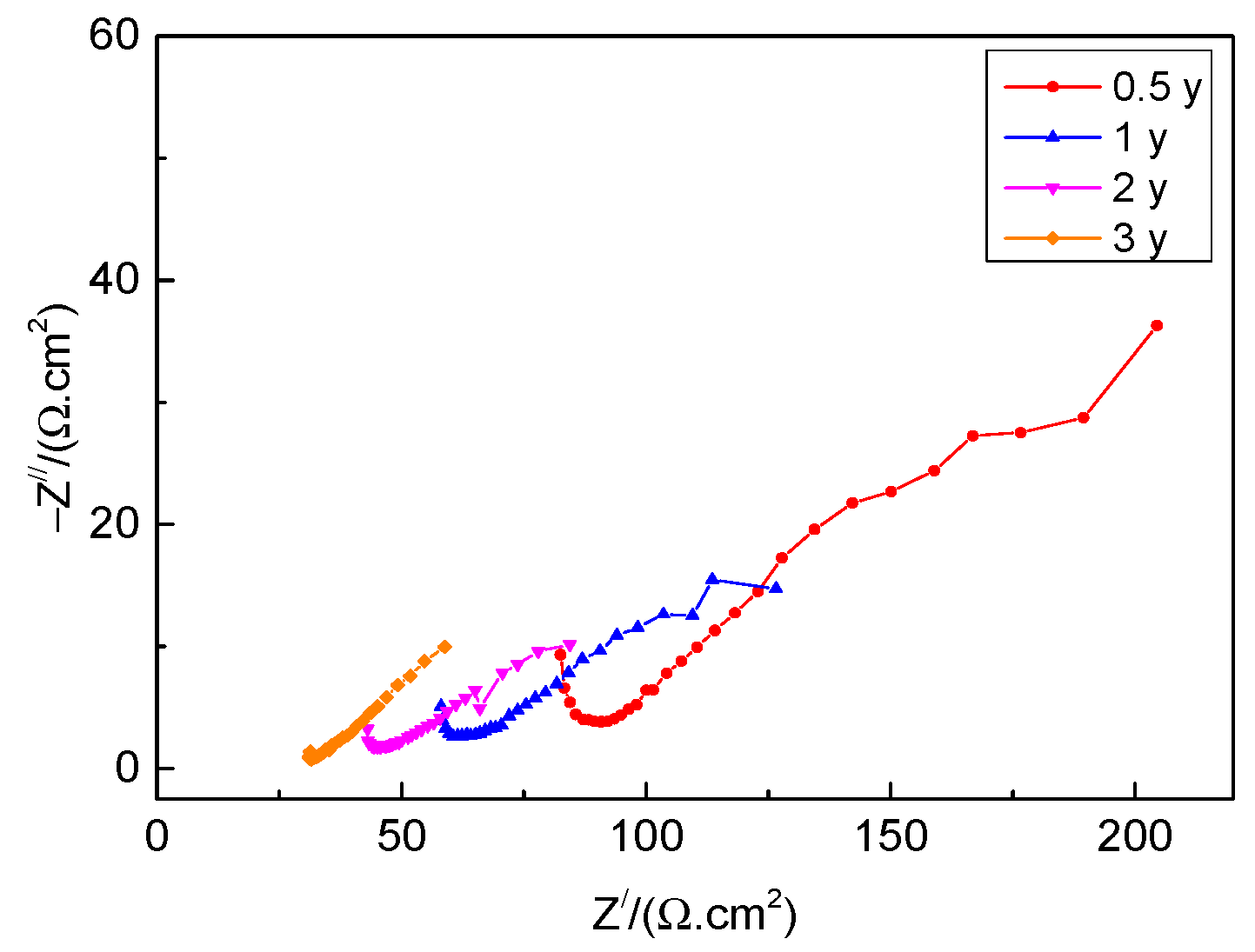
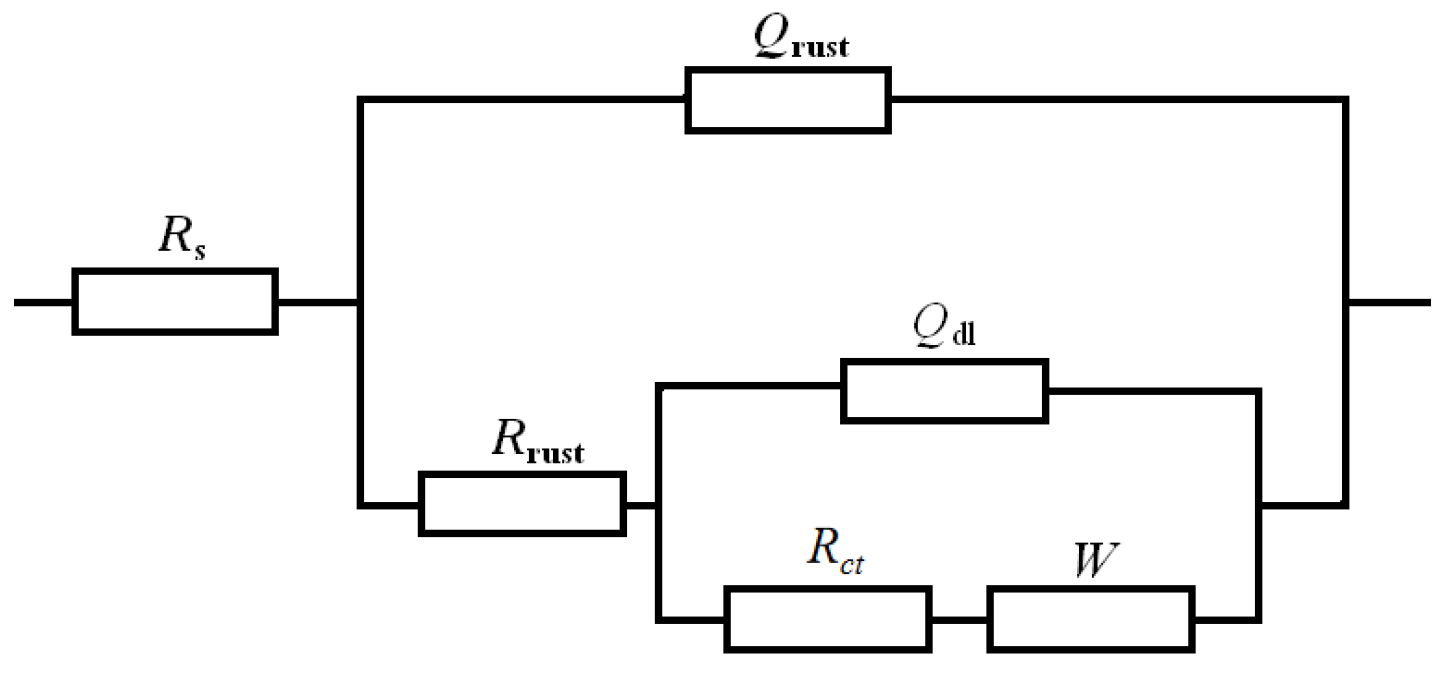
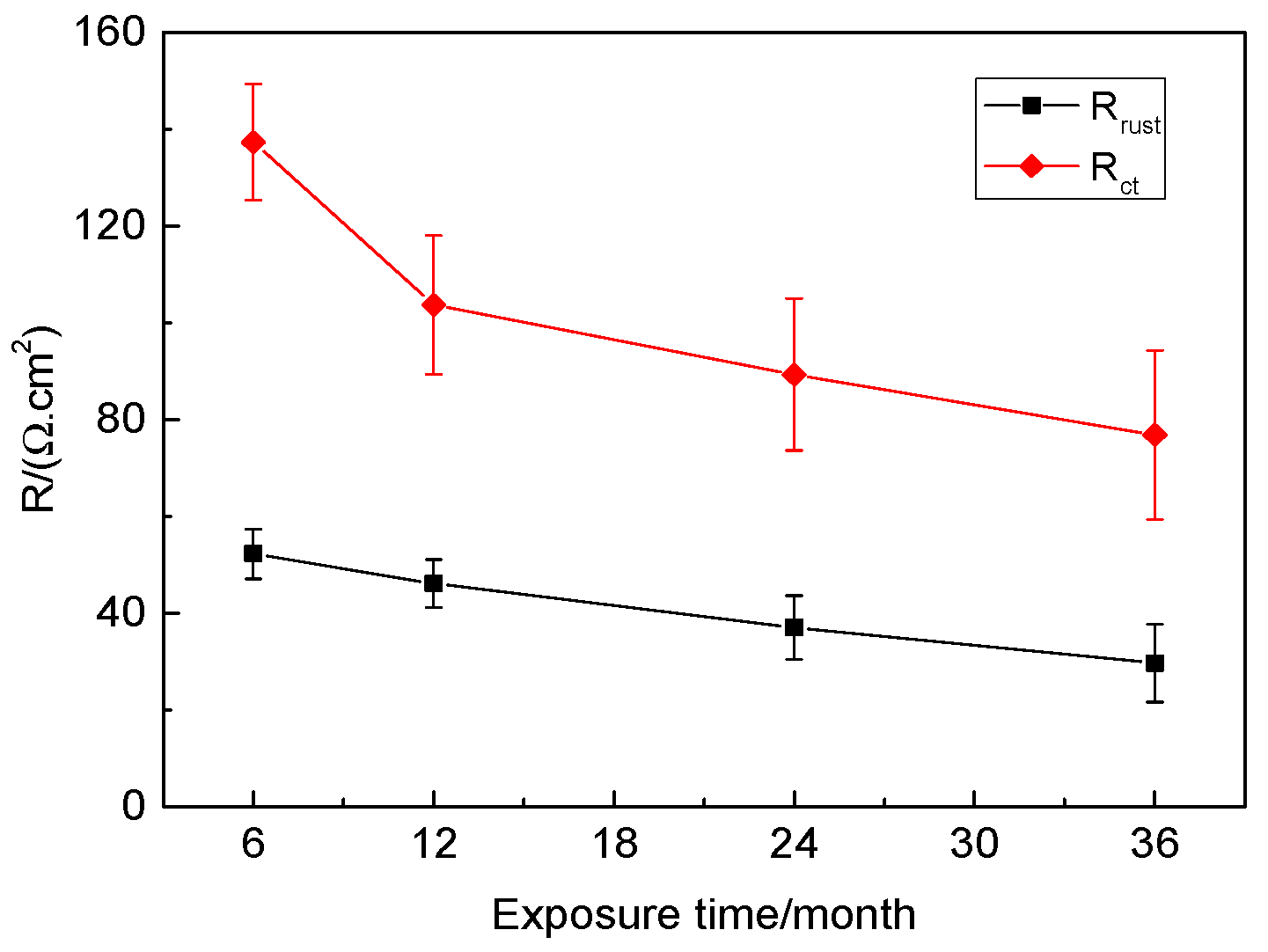
3.6. Electrochemical Corrosion Properties
4. Conclusions
- (1)
- The corrosion kinetics of 700L steel exposed to the marine atmosphere for 36 months follow the exponential function D = 4.85t1.23, and the corrosion process is accelerated with exposure time.
- (2)
- The rust phases on the 700L steel are mainly composited of γ-FeOOH, α-FeOOH, Fe3O4, and γ-Fe2O3, regardless of the exposure duration.
- (3)
- Lots of porosity, spalling, and cracks in the rust layer are present, which are the easy pathways for chloride ions and oxygen to reach the steel substrate, thereby accelerating the corrosion process.
- (4)
- Based on the electrochemical data from the OCP, polarization curves, and EIS, the corrosion resistance of rusted 700L steel decreases with exposure time, which is associated with the increase in porosity, cracking, and spalling.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, B.; Shi, X.G.; Dong, Y.; Xu, X.; Liu, R.D. Development and production of ultra-high strength steel for automotive heavy beam. Angang Technol. 2012, 10–13. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Tzeng, H.J.; Wei, L.I.; Wang, L.H.; Oung, J.C.; Shih, H.C. Corrosion resistance and mechanical properties of low-alloy steels under atmospheric conditions. Corros. Sci. 2005, 47, 1001–1021. [Google Scholar] [CrossRef]
- Giray, D.; Sonmez, M.S.; Yamanoglu, R.; Yavuz, H.I.; Muratal, O. Characterization of corrosion products formed in high-strength dual-phase steels under an accelerated corrosion test. Eng. Sci. Technol. Int. J. 2024, 57, 101796. [Google Scholar] [CrossRef]
- Yang, X.K.; Zhang, L.W.; Zhang, S.Y.; Zhou, K.; Li, M.; He, Q.Y.; Wang, J.C.; Wu, S.; Yang, H.M. Atmospheric corrosion behaviour and degradation of high-strength bolt in marine and industrial atmosphere environments. Int. J. Electrochem. Sci. 2021, 16, 151015. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, J.S.; Seong, H.G. Effects of Cu-alloying in minute quantities on the corrosion-induced mechanical degradation behaviors of medium C-based ultrahigh-strength steel in aqueous environments. J. Mater. Res. Technol. 2021, 15, 5682–5693. [Google Scholar] [CrossRef]
- Mokhtari, E.; Heidarpour, A.; Javidan, F. Mechanical performance of high strength steel under corrosion: A review study. J. Constr. Steel Res. 2024, 220, 108840. [Google Scholar] [CrossRef]
- Liu, Y.W.; Zhang, J.; Wei, Y.H.; Wang, Z.Y. Effect of different UV intensity on corrosion behavior of carbon steel exposed to simulated Nansha atmospheric environment. Mater. Chem. Phys. 2019, 237, 121855. [Google Scholar] [CrossRef]
- Pei, Z.B.; Cheng, X.Q.; Yang, X.J.; Li, Q.; Xi, C.H.; Zhang, D.W.; Li, X.G. Understanding environmental impacts on initial atmospheric corrosion based on corrosion monitoring sensors. J. Mater. Sci. Technol. 2021, 64, 214–221. [Google Scholar] [CrossRef]
- Liu, M.R.; Lu, X.; Yin, Q.; Pan, C.; Wang, C.; Wang, Z.Y. Effect of acidified aerosols on initial corrosion behavior of Q235 carbon steel. Acta Metall. Sin. (English Lett.) 2019, 32, 995–1006. [Google Scholar] [CrossRef]
- Morcillo, M.; Díaz, I.; Cano, H.; Chico, B.; de la Fuente, D. Atmospheric corrosion of weathering steels. Overview for engineers. Part I: Basic concepts. Constr. Build. Mater. 2019, 213, 723–737. [Google Scholar] [CrossRef]
- Liao, X.N.; Cao, F.H.; Zheng, L.Y.; Liu, W.J.; Chen, A.N.; Zhang, J.Q.; Cao, C.N. Corrosion behaviour of copper under chloride-containing thin electrolyte layer. Corros. Sci. 2011, 53, 3289–3298. [Google Scholar] [CrossRef]
- Liu, Y.W.; Zhao, H.T.; Wang, Z.Y.; Wei, Y.H.; Pan, C.; Lv, C.X. Corrosion behavior of low-carbon steel and weathering steel in a coastal zone of the Spratly Islands: A tropical marine atmosphere. Int. J. Electrochem. Sci. 2020, 15, 6464–6477. [Google Scholar] [CrossRef]
- Hou, W.; Liang, C. Eight-year atmospheric corrosion exposure of steels in China. Corrosion 1999, 55, 65–73. [Google Scholar] [CrossRef]
- To, D.; Shinohara, T.; Umezawa, O. Experimental investigation on the corrosivity of atmosphere through the atmospheric corrosion monitoring (ACM) sensors. ECS Trans. 2017, 75, 1–10. [Google Scholar] [CrossRef]
- Wang, L.B.; Wang, J.H.; Hu, W.B. Influence of Cl− on the initial corrosion of weathering steel in simulated marine-industrial atmosphere. Int. J. Electrochem. Sci. 2018, 13, 7356–7369. [Google Scholar] [CrossRef]
- Han, W.; Yu, G.C.; Wang, Z.Y.; Wang, J. Characterisation of initial atmospheric corrosion carbon steels by field exposure and laboratory simulation. Corros. Sci. 2007, 49, 2920–2935. [Google Scholar] [CrossRef]
- Townsend, H.E. Effects of alloying elements on the corrosion of steel in industrial atmospheres. Corrosion 2001, 57, 497–501. [Google Scholar] [CrossRef]
- ISO 9223:2012; Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Clasification, Determination and Estimation, 2nd ed. International Organization for Standardization: Geneva, Switzerland, 2012.
- Ma, Y.T.; Li, Y.; Wang, F.H. Corrosion of low carbon steel in atmospheric environments of different chloride content. Corros. Sci. 2009, 51, 997–1006. [Google Scholar] [CrossRef]
- de la Fuente, D.; Díaz, I.; Alcántara, J.; Chico, B.; Simancas, J.; Llorente, I.; García-Delgado, A.; Jiménez, J.A.; Adeva, P.; Morcillo, M. Corrosion mechanisms of mild steel in chloride-rich atmospheres. Mater. Corros. 2016, 67, 227–238. [Google Scholar] [CrossRef]
- Nishimura, T.; Katayama, H.; Noda, K.; Kodama, T. Electrochemical behavior of rust formed on carbon steel in a wet/dry environment containing chloride ions. Corrosion 2000, 56, 935–941. [Google Scholar] [CrossRef]
- Alcántara, J.; Chico, B.; Díaz, I.; de la Fuente, D.; Morcillo, M. Airborne chloride deposit and its effect on marine atmospheric corrosion of mild steel. Corros. Sci. 2015, 97, 74–88. [Google Scholar] [CrossRef]
- Alcántara, J.; de la Fuente, D.; Chico, B.; Simancas, J.; Díaz, I.; Morcillo, M. Marine atmospheric corrosion of carbon steel: A review. Materials 2017, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.B.; Wang, P.X.; Ma, H.C.; Cheng, X.Q.; Li, X.G. Essential role of Si in enhancing corrosion resistance of high strength low alloy steels in marine environment. J. Mater. Res. Technol. 2024, 30, 3328–3339. [Google Scholar] [CrossRef]
- Wu, W.; Hao, W.K.; Liu, Z.Y.; Li, X.G.; Du, C.W.; Liao, W.J. Corrosion behavior of E690 high-strength steel in alternating wet-dry marine environment with different pH values. J. Mater. Eng. Perform. 2015, 24, 4636–4646. [Google Scholar] [CrossRef]
- Du, C.C.; Wu, Z.F.; Qin, M.H.; Li, D.L.; Zhao, L.; Li, X.Y.; Wang, H.Z. Corrosion behavior and mechanism of 921 A high-strength low-alloy steel in harsh marine atmospheric environments. Int. J. Electrochem. Sci. 2024, 19, 10027. [Google Scholar] [CrossRef]
- Calderon-Hernandez, J.W.; da Fonseca, D.P.M.; Prada-Ramirez, O.M.; Hincapie-Ladino, D.; de Melo, H.G.; Padilha, A.F. Effect of Mo content on microstructure and corrosion behavior in HCl of ultra-high strength maraging steels. J. Mater. Res. Technol. 2024, 30, 3112–3121. [Google Scholar] [CrossRef]
- Liu, L.L.; Fei, R.S.; Sun, F.; Bi, H.Y.; Chang, E.; Li, M.C. Effect of cold rolling deformation on the pitting corrosion behavior of high-strength metastable austenitic stainless steel 14Cr10Mn in simulated coastal atmospheric environments. J. Mater. Res. Technol. 2024, 29, 1476–1486. [Google Scholar] [CrossRef]
- Liu, Y.W. Corrosion Behavior and Mechanism of Carbon Steel at Spratly Islands Marine Atmospheric Environment. Ph.D. Thesis, University of Science and Technology of China, Hefei, China, 2020. [Google Scholar]
- Xiao, K.; Yao, Q.; Wu, J.S.; Gao, J. Atmospheric Corrosion of Metallic Materials in Coastal Atmospheric Corrosion of Metallic Materials in Coastal Environment of Wenchang, Hainan; Chemical Industry Press: Beijing, China, 2022. [Google Scholar]
- Suzuki, I.; Hisamatsu, Y.; Masuk, N. Nature of atmospheric rust on iron. J. Electrochem. Soc. 1980, 127, 2210–2215. [Google Scholar] [CrossRef]
- Yang, X.J.; Yang, Y.; Sun, M.H.; Jia, J.H.; Cheng, X.Q.; Pei, Z.B.; Li, Q.; Xu, D.; Xiao, K.; Li, X.G. A new understanding of the effect of Cr on the corrosion resistance evolution of weathering steel based on big data technology. J. Mater. Sci. Technol. 2022, 104, 67–80. [Google Scholar] [CrossRef]
- Oh, S.J.; Cook, D.C.; Townsend, H.E. Atmospheric corrosion of different steels in marine, rural and industrial environments. Corros. Sci. 1999, 41, 1687–1702. [Google Scholar] [CrossRef]
- Corvo, F.; Minotas, J.; Delgado, J.; Arroyave, C. Changes in atmospheric corrosion rate caused by chloride ions depending on rain regime. Corros. Sci. 2005, 47, 883–892. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; de la Fuente, D.; Alcantara, J.; Wallinder, I.O.; Leygraf, C. On the mechanism of rust exfoliation in marine environments. J. Electrochem. Soc. 2017, 164, C8–C16. [Google Scholar] [CrossRef]
- Pino, E.; Alcántara, J.; Chico, B.; Díaz, I.; Simancas, J.; de la Fuente, D.; Morcillo, M. Atmospheric corrosion of mild steel in marine atmospheres. Corros. Prot. Mater. 2015, 34, 35–41. [Google Scholar]
- Kamimura, T.; Kashima, K.; Sugae, K.; Miyuki, H.; Kudo, T. The role of chloride ion on the atmospheric corrosion of steel and corrosion resistance of Sn-bearing steel. Corros. Sci. 2012, 62, 34–41. [Google Scholar] [CrossRef]
- Evans, U.R. Electrochemical mechanism of atmospheric rusting. Nature 1965, 206, 980–982. [Google Scholar] [CrossRef]
- Misawa, T.; Asami, K.; Hashimoto, K.; Shimodaira, S. The mechanism of atmospheric rusting and protective amorphous rust on low-alloy steel. Corros. Sci. 1974, 14, 279–289. [Google Scholar] [CrossRef]
- Wang, J.H.; Wei, F.I.; Chang, Y.S.; Shih, H.C. The corrosion mechanisms of carbon steel and weathering steel in SO2 polluted atmospheres. Mater. Chem. Phys. 1997, 47, 1–8. [Google Scholar] [CrossRef]
- Morcillo, M.; Díaz, I.; Cano, H.; Chico, B.; de la Fuente, D. Atmospheric corrosion of weathering steels. Overview for engineers. Part II: Testing, inspection, maintenance. Constr. Build. Mater. 2019, 222, 750–765. [Google Scholar] [CrossRef]




| C | Mn | S | P | Si | Als | Cu | Cr | Ni | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 0.072 | 1.68 | 0.0041 | 0.013 | 0.132 | 0.030 | 0.016 | 0.028 | 0.022 | Bal. |
| Average Temperature (°C/year) | Average Relative Humidity (%) | Average Sunshine Time (h/year) | Average Rain Time (h/year) | Average Cl− Ions Deposition (mg·m−2·d−1) |
|---|---|---|---|---|
| 24.6 | 86 | 2043 | 391.7 | 50.39 |
| Time/ Year | Rs/ (Ω·cm2) | Qrust/ (Ω−1·cm−2·s−n) | nrust | Rrust/ (Ω·cm2) | Qdl/ (Ω−1·cm−2·s−n) | ndl | Rct/ (Ω·cm2) | W/ (Ω−1·cm−2·s−0.5) |
|---|---|---|---|---|---|---|---|---|
| 0.5 | 6.43 × 10−5 | 1.344 × 10−9 | 0.9958 | 52.27 | 0.007753 | 0.09241 | 137.31 | 0.003124 |
| 1 | 9.999 × 10−4 | 1.563 × 10−9 | 1 | 46.12 | 0.01663 | 0.1126 | 103.7 | 0.004857 |
| 2 | 9.993 × 10−4 | 1.852 × 10−9 | 1 | 37.03 | 0.02868 | 0.1281 | 89.30 | 0.009986 |
| 3 | 0.0989 | 2.001 × 10−9 | 0.9901 | 29.66 | 0.05399 | 0.2024 | 76.83 | 0.01298 |
| Time/year | Icorr/(A·cm−2) | Ecorr/V | βc/(V·dec−1) | βa/(V·dec−1) | Rp/(Ω·cm2) |
|---|---|---|---|---|---|
| 0.5 | 4.340 × 10−5 | −0.460 | 0.103 | 0.144 | 604.0 |
| 1 | 1.166 × 10−4 | −0.502 | 0.142 | 0.314 | 364.6 |
| 2 | 2.346 × 10−4 | −0.568 | 0.138 | 0.436 | 194.3 |
| 3 | 2.863 × 10−4 | −0.551 | 0.112 | 0.368 | 130.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Liu, Y.; Wang, C.; Cao, G.; He, C.; Wang, Z. Corrosion Behavior of 700L Automotive Beam Steel in Marine Atmospheric Environment. Materials 2024, 17, 4964. https://doi.org/10.3390/ma17204964
He Y, Liu Y, Wang C, Cao G, He C, Wang Z. Corrosion Behavior of 700L Automotive Beam Steel in Marine Atmospheric Environment. Materials. 2024; 17(20):4964. https://doi.org/10.3390/ma17204964
Chicago/Turabian StyleHe, Younian, Yuwei Liu, Chuan Wang, Gongwang Cao, Chunlin He, and Zhenyao Wang. 2024. "Corrosion Behavior of 700L Automotive Beam Steel in Marine Atmospheric Environment" Materials 17, no. 20: 4964. https://doi.org/10.3390/ma17204964





