Development of Cost-Effective Sn-Free Al-Bi-Fe Alloys for Efficient Onboard Hydrogen Production through Al–Water Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
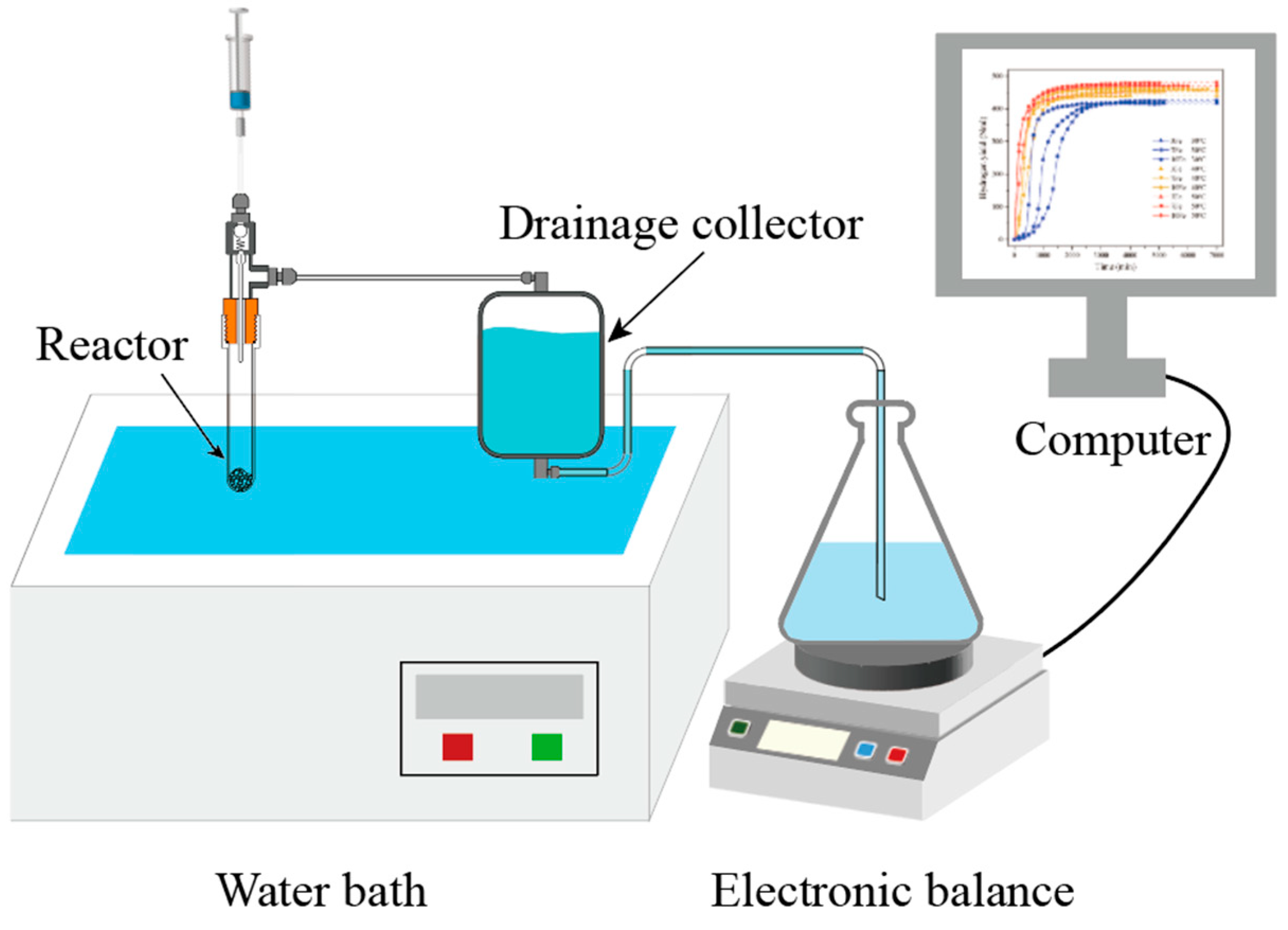
2.2. Hydrolysis Test
2.3. Characterization
3. Results and Discussion
3.1. Design of Powder Composition
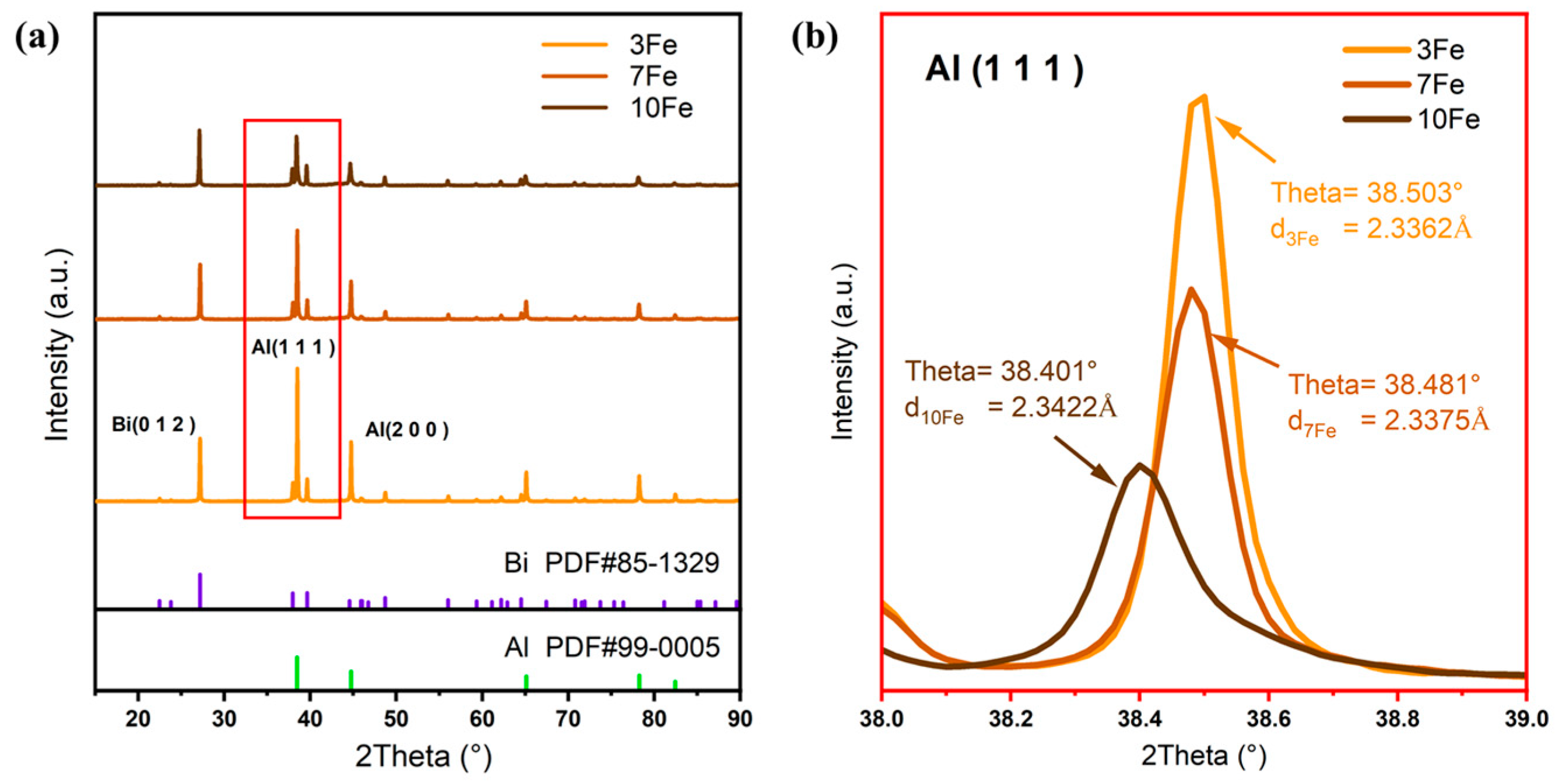
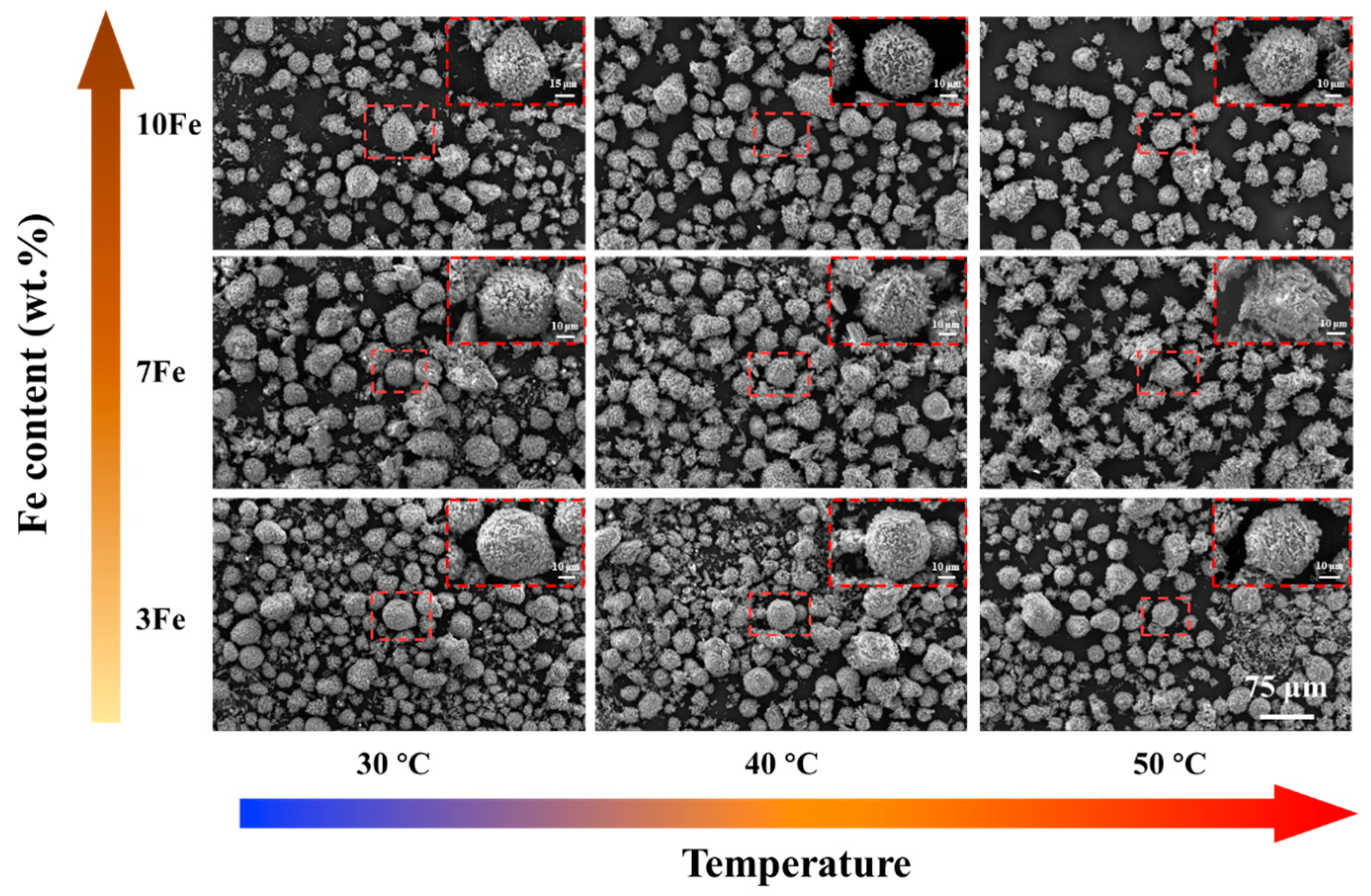
3.2. Microstructure Characterization of As-Atomized Powders
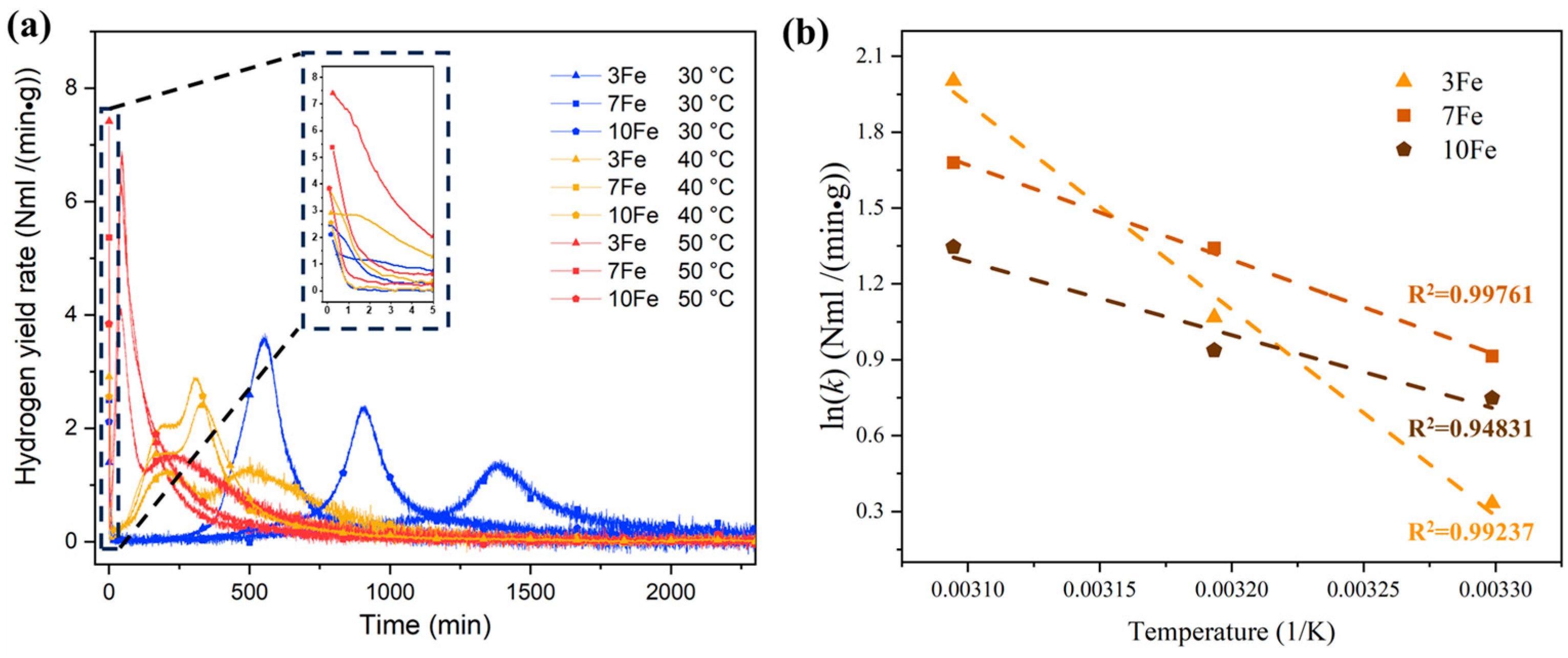
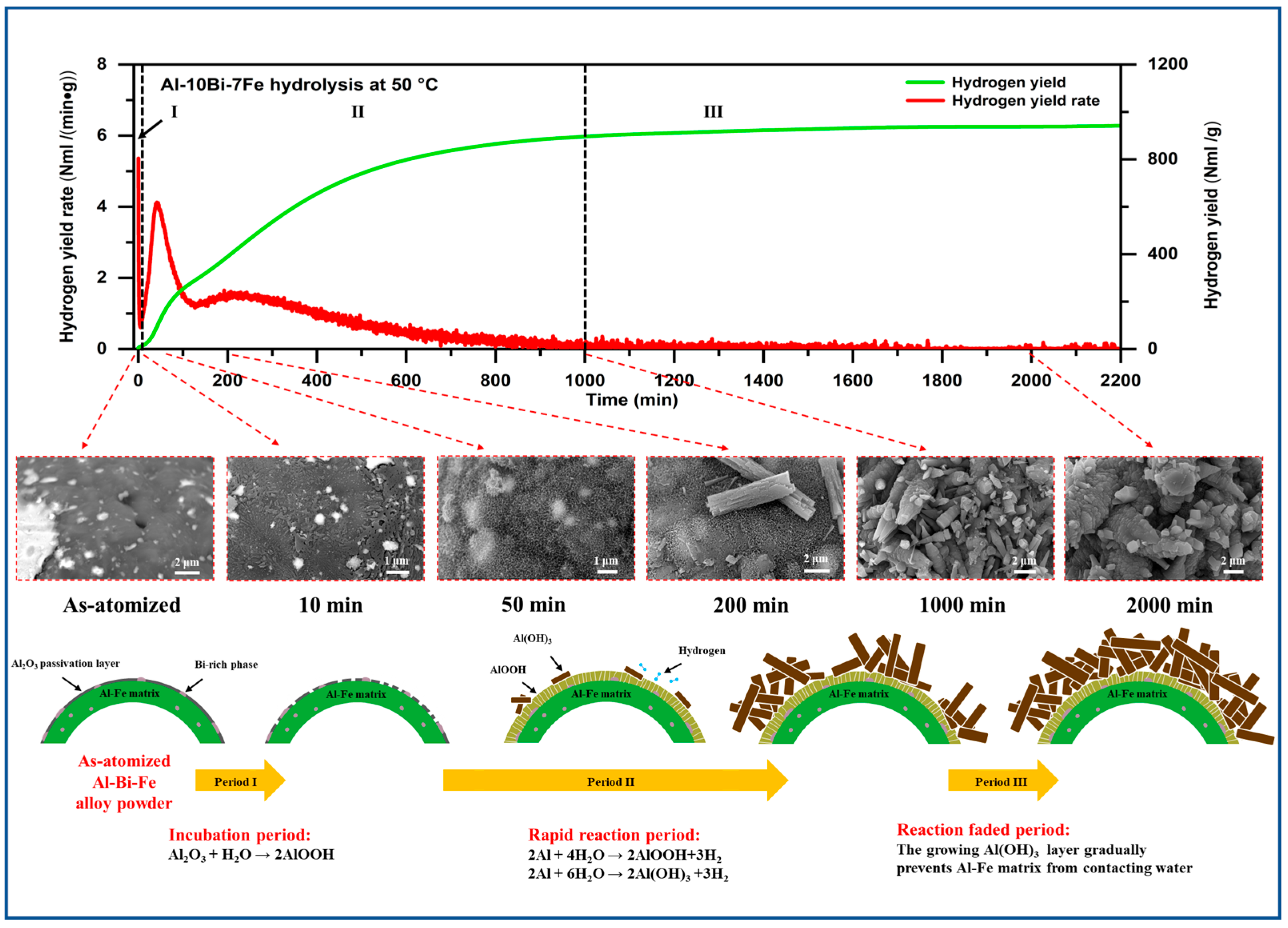
3.3. Hydrolysis Performance
3.4. Chemical Kinetics of Hydrolysis Reaction
3.5. Hydrolysis Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thampan, T.; Atwater, T.; Cook, C.; Novoa, J.; Sutorik, A.C. Hydrogen generation from aluminum hydride for wearable polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2016, 41, 9402–9409. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y.; Li, B.; Mo, T.; Li, Y.; Feng, S.-P.; Qu, J.; Chu, P.K. Development and application of fuel cells in the automobile industry. J. Energy Storage 2021, 42, 103124. [Google Scholar] [CrossRef]
- Pulsone, N.B.; Hart, D.P.; Siegel, A.M.; Edwards, J.R.; Railey, K.E. Aluminum-Water Energy System for Autonomous Undersea Vehicles. Linc. Lab. J. 2017, 22, 79–92. [Google Scholar]
- Wang, S.; Sun, L.-X.; Xu, F.; Jiao, C.-L.; Zhang, J.; Zhou, H.-Y.; Huang, F.-L. Hydrolysis reaction of ball-milled Mg-metal chlorides composite for hydrogen generation for fuel cells. Int. J. Hydrogen Energy 2012, 37, 6771–6775. [Google Scholar] [CrossRef]
- Qin, H.; Li, H.; Fu, Q.; Yu, R.; Zhao, Y.; Kang, Z.; Chen, X.; Wang, M. Process optimisation of controlled and continuous MgH2 hydrolysis to produce hydrogen. Sustain. Mater. Technol. 2022, 33, e00505. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, H.; Zhu, M. AlH3 as a hydrogen storage material: Recent advances, prospects and challenges. Rare Met. 2021, 40, 3337–3356. [Google Scholar] [CrossRef]
- Yu, K.M.K.; Tong, W.; West, A.; Cheung, K.; Li, T.; Smith, G.; Guo, Y.; Tsang, S.C.E. Non-syngas direct steam reforming of methanol to hydrogen and carbon dioxide at low temperature. Nat. Commun. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Netskina, O.; Tayban, E.; Rogov, V.; Ozerova, A.; Mukha, S.; Simagina, V.; Komova, O. Solid-state NaBH4 composites for hydrogen generation: Catalytic activity of nickel and cobalt catalysts. Int. J. Hydrogen Energy 2021, 46, 5459–5471. [Google Scholar] [CrossRef]
- Fan, M.Q.; Liu, S.; Sun, L.X.; Xu, F.; Wang, S.; Zhang, J.; Mei, D.S.; Huang, F.L.; Zhang, Q.M. Synergistic hydrogen generation from AlLi alloy and solid-state NaBH4 activated by CoCl2 in water for portable fuel cell. Int. J. Hydrogen Energy 2012, 37, 4571–4579. [Google Scholar] [CrossRef]
- Wang, H.; Leung, D.Y.; Leung, M.; Ni, M. A review on hydrogen production using aluminum and aluminum alloys. Renew. Sustain. Energy Rev. 2009, 13, 845–853. [Google Scholar] [CrossRef]
- Liparoti, S.; Sofia, D.; Romano, A.; Marra, F.; Pantani, R. Fused filament deposition of PLA: The role of interlayer adhesion in the mechanical performances. Polymers 2021, 13, 399. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chai, Y.; Yang, F.; Zhang, Q.; Liu, H.; Wang, N. Hydrogen generation by aluminum-water reaction in acidic and alkaline media and its reaction dynamics. Int. J. Energy Res. 2018, 42, 1594–1602. [Google Scholar] [CrossRef]
- He, T.; Wang, W.; Chen, D.; Yang, K. Effect of Ti on the microstructure and Al–water reactivity of Al-rich alloy. Int. J. Hydrogen Energy 2014, 39, 684–691. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, X.; Liu, J. Liquid metal activated aluminum-water reaction for direct hydrogen generation at room temperature. Renew. Sustain. Energy Rev. 2018, 92, 17–37. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.; Szpunar, J. Effect of addition of water-soluble salts on the hydrogen generation of aluminum in reaction with hot water. J. Alloys Compd. 2016, 679, 364–374. [Google Scholar] [CrossRef]
- Dupiano, P.; Stamatis, D.; Dreizin, E.L. Hydrogen production by reacting water with mechanically milled composite aluminum-metal oxide powders. Int. J. Hydrogen Energy 2011, 36, 4781–4791. [Google Scholar] [CrossRef]
- Su, M.; Wang, H.; Xu, H.; Chen, F.; Hu, H.; Gan, J. Enhanced hydrogen production properties of a novel aluminum-based composite for instant on-site hydrogen supply at low temperature. Int. J. Hydrogen Energy 2022, 47, 9969–9985. [Google Scholar] [CrossRef]
- Huang, X.-N.; Lv, C.-J.; Wang, Y.; Shen, H.-Y.; Chen, D.; Huang, Y.-X. Hydrogen generation from hydrolysis of aluminum/graphite composites with a core–shell structure. Int. J. Hydrogen Energy 2012, 37, 7457–7463. [Google Scholar] [CrossRef]
- Woodall, J.; Ziebarth, J.T.; Allen, C.R.; Sherman, D.M.; Jeon, J.; Choi, G. Recent results on splitting water with aluminum alloys. Mater. Innov. Emerg. Hydrog. Econ. 2008, 1, 119–127. [Google Scholar]
- Cuomo, J.; Woodall, J. Solid State Renewable Energy Supply. U.S. Patent 4358291, 9 November 1982. [Google Scholar]
- Choi, G.; Ziebarth, J.T.; Woodall, J.M.; Kramer, R.; Sherman, D.; Allen, C.R. Mechanism of hydrogen generation via water reaction with aluminum alloys. In Proceedings of the 2010 18th Biennial University/Government/Industry Micro/Nano Symposium, West Lafayette, IN, USA, 28 June–1 July 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 1–4. [Google Scholar]
- Ziebarth, J.T.; Woodall, J.M.; Kramer, R.A.; Choi, G. Liquid phase-enabled reaction of Al–Ga and Al–Ga–In–Sn alloys with water. Int. J. Hydrogen Energy 2011, 36, 5271–5279. [Google Scholar] [CrossRef]
- An, Q.; Jin, Z.; Li, N.; Wang, H.; Schmierer, J.; Wei, C.; Hu, H.; Gao, Q.; Woodall, J.M. Study on the liquid phase-derived activation mechanism in Al-rich alloy hydrolysis reaction for hydrogen production. Energy 2022, 247, 123489. [Google Scholar] [CrossRef]
- Wang, H.; Chang, Y.; Dong, S.; Lei, Z.; Zhu, Q.; Luo, P.; Xie, Z. Investigation on hydrogen production using multicomponent aluminum alloys at mild conditions and its mechanism. Int. J. Hydrogen Energy 2013, 38, 1236–1243. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, H.; Shi, J.; Wang, H.; Gao, X.; Gao, Q.; Sun, X. Unveiling the role of indium and tin in Al–Ga based alloys for on-demand hydrogen supply from simulation to validation. J. Power Sources 2023, 554, 232268. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Liu, H.; Yang, T.; Chen, X.; Yang, S.; Liu, X. A novel self-assembling Al-based composite powder with high hydrogen generation efficiency. Sci. Rep. 2015, 5, 17428. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Chen, X.; Yang, S.; Wang, C. Hydrogen generation from hydrolysis of activated Al-Bi, Al-Sn powders prepared by gas atomization method. Int. J. Hydrogen Energy 2017, 42, 10943–10951. [Google Scholar] [CrossRef]
- Chen, X.; Wang, C.; Liu, Y.; Shen, Y.; Zheng, Q.; Yang, S.; Lu, H.; Zou, H.; Lin, K.; Liu, H. Popcorn-like aluminum-based powders for instant low-temperature water vapor hydrogen generation. Mater. Today Energy 2020, 19, 100602. [Google Scholar] [CrossRef]
- Wang, C.; Lin, K.; Liu, Y.; Chen, X.; Zou, H.; Qiu, C.; Yang, S.; Liu, X. Design and fabrication of high activity retention Al-based composite powders for mild hydrogen generation. Materials 2019, 12, 3328. [Google Scholar] [CrossRef]
- Wang, C.; Yin, B.; Lin, K.; Wang, M.; Deng, R.; Guo, Y.; Zhang, J.; Yang, S.; Liu, X. Effect of Fe on the Hydrogen Production Properties of Al-Bi-Sn Composite Powders. Materials 2022, 15, 6702. [Google Scholar] [CrossRef]
- Xiao, F.; Guo, Y.; Li, J.; Yang, R. Hydrogen generation from hydrolysis of activated aluminum composites in tap water. Energy 2018, 157, 608–614. [Google Scholar] [CrossRef]
- Gai, W.-Z.; Liu, W.-H.; Deng, Z.-Y.; Zhou, J.-G. Reaction of Al powder with water for hydrogen generation under ambient condition. Int. J. Hydrogen Energy 2012, 37, 13132–13140. [Google Scholar] [CrossRef]
- Kahveci, O.; Kaya, M.F. Hydrogen production from Al–Cu alloy using electric vehicle’s waste DC motor coils. Int. J. Hydrogen Energy 2022, 47, 12179–12188. [Google Scholar] [CrossRef]
- Kaya, M.F.; Kahveci, O.; Erol, H.; Akkaya, A. Effect of low B addition on Al-Zn alloy’s hydrogen production performance. Int. J. Hydrogen Energy 2021, 46, 15192–15202. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Eckhoff, R.K. Kinetics study of hydration reaction between aluminum powder and water based on an improved multi-stage shrinking core model. Int. J. Hydrogen Energy 2021, 46, 33635–33655. [Google Scholar] [CrossRef]
- Bunker, B.C.; Nelson, G.C.; Zavadil, K.; Barbour, J.; Wall, F.; Sullivan, J.; Windisch, C.F.; Engelhardt, M.; Baer, D.R. Hydration of passive oxide films on aluminum. J. Phys. Chem. B 2002, 106, 4705–4713. [Google Scholar] [CrossRef]
- Rider, A.; Arnott, D. Boiling water and silane pre-treatment of aluminium alloys for durable adhesive bonding. Int. J. Adhes. Adhes. 2000, 20, 209–220. [Google Scholar] [CrossRef]
- Wang, C.; Huang, Y.; Wei, H.; Yu, F.; Wang, M.; Guo, Y.; Zhang, J.; Deng, R.; Yang, S.; Liu, X. A brief strategy for designing self-encapsulated Al-Si base phase change materials with high thermal energy storage performance. J. Energy Storage 2023, 62, 106957. [Google Scholar] [CrossRef]
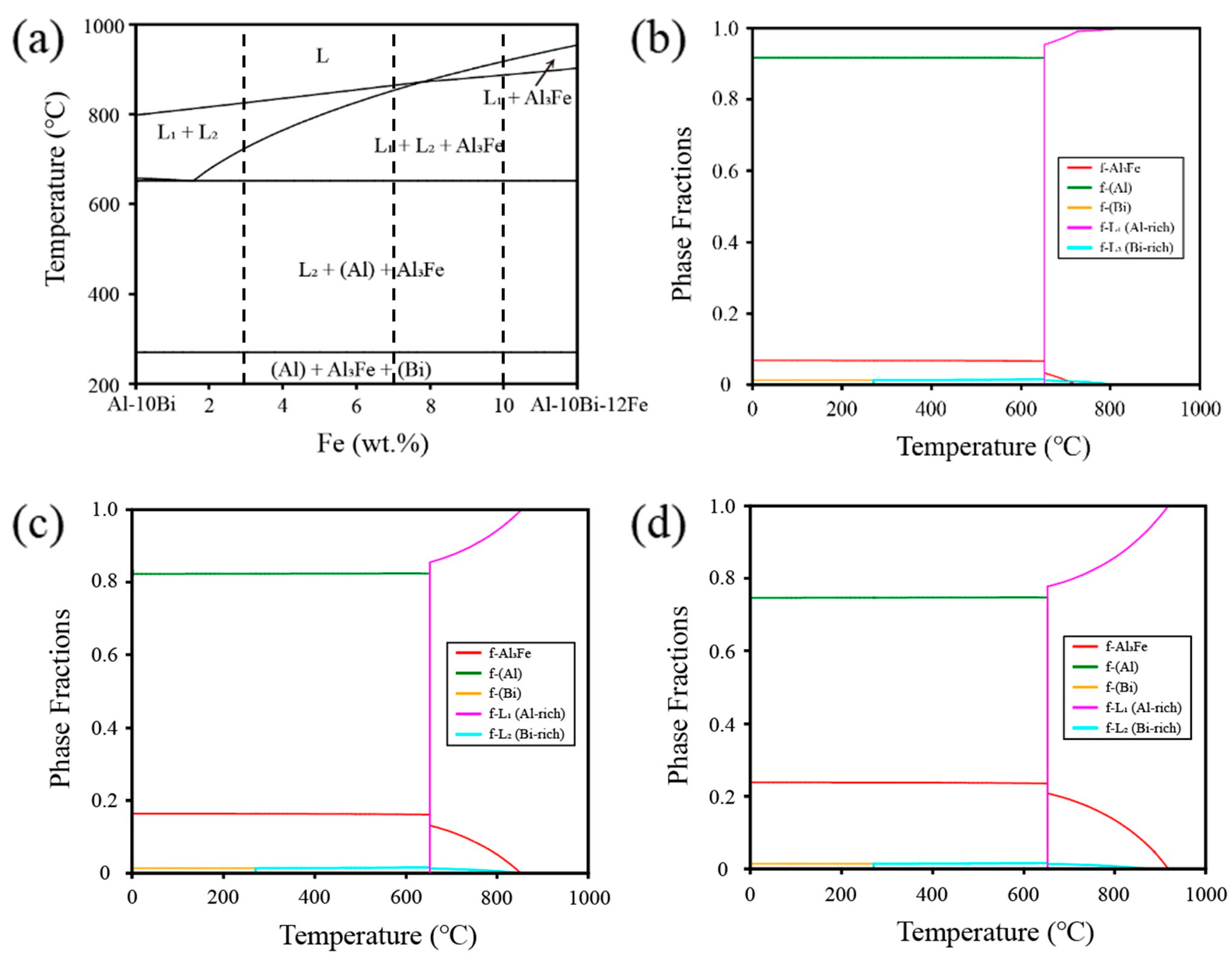




| Alloys (wt.%) | L1(Al-Rich) | L2(Bi-Rich) | (Al) | (Bi) | Al3Fe |
|---|---|---|---|---|---|
| Al-10Bi-3Fe | 0.982 | 0.006 | 0 | 0 | 0.012 |
| Al-10Bi-7Fe | 0.841 | 0.015 | 0 | 0 | 0.144 |
| Al-10Bi-10Fe | 0.791 | 0.017 | 0 | 0 | 0.192 |
| Alloys (wt.%) | Initial Hydrogen Yield Rate at 30 °C (NmL H2/(min·g)) | Initial Hydrogen Yield Rate at 40 °C (NmL H2/(min·g)) | Initial Hydrogen Yield Rate at 50 °C (NmL H2/(min·g)) | Activation Energy (kJ/mol) |
|---|---|---|---|---|
| Al-10Bi-3Fe | 1.396 | 2.910 | 7.412 | 67.888 |
| Al-10Bi-7Fe | 2.496 | 3.826 | 5.363 | 31.178 |
| Al-10Bi-10Fe | 2.114 | 2.551 | 3.842 | 24.224 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, R.; Wang, M.; Zhang, H.; Yao, R.; Zhen, K.; Liu, Y.; Liu, X.; Wang, C. Development of Cost-Effective Sn-Free Al-Bi-Fe Alloys for Efficient Onboard Hydrogen Production through Al–Water Reaction. Materials 2024, 17, 4973. https://doi.org/10.3390/ma17204973
Deng R, Wang M, Zhang H, Yao R, Zhen K, Liu Y, Liu X, Wang C. Development of Cost-Effective Sn-Free Al-Bi-Fe Alloys for Efficient Onboard Hydrogen Production through Al–Water Reaction. Materials. 2024; 17(20):4973. https://doi.org/10.3390/ma17204973
Chicago/Turabian StyleDeng, Rui, Mingshuai Wang, Hao Zhang, Ruijun Yao, Kai Zhen, Yifei Liu, Xingjun Liu, and Cuiping Wang. 2024. "Development of Cost-Effective Sn-Free Al-Bi-Fe Alloys for Efficient Onboard Hydrogen Production through Al–Water Reaction" Materials 17, no. 20: 4973. https://doi.org/10.3390/ma17204973





