The Effect of Inorganic Filler Content on the Properties of BPA-Free Bulk-Fill Dental Resin Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Dental Resin Matrix and Composites
2.3. The Mixing Degree
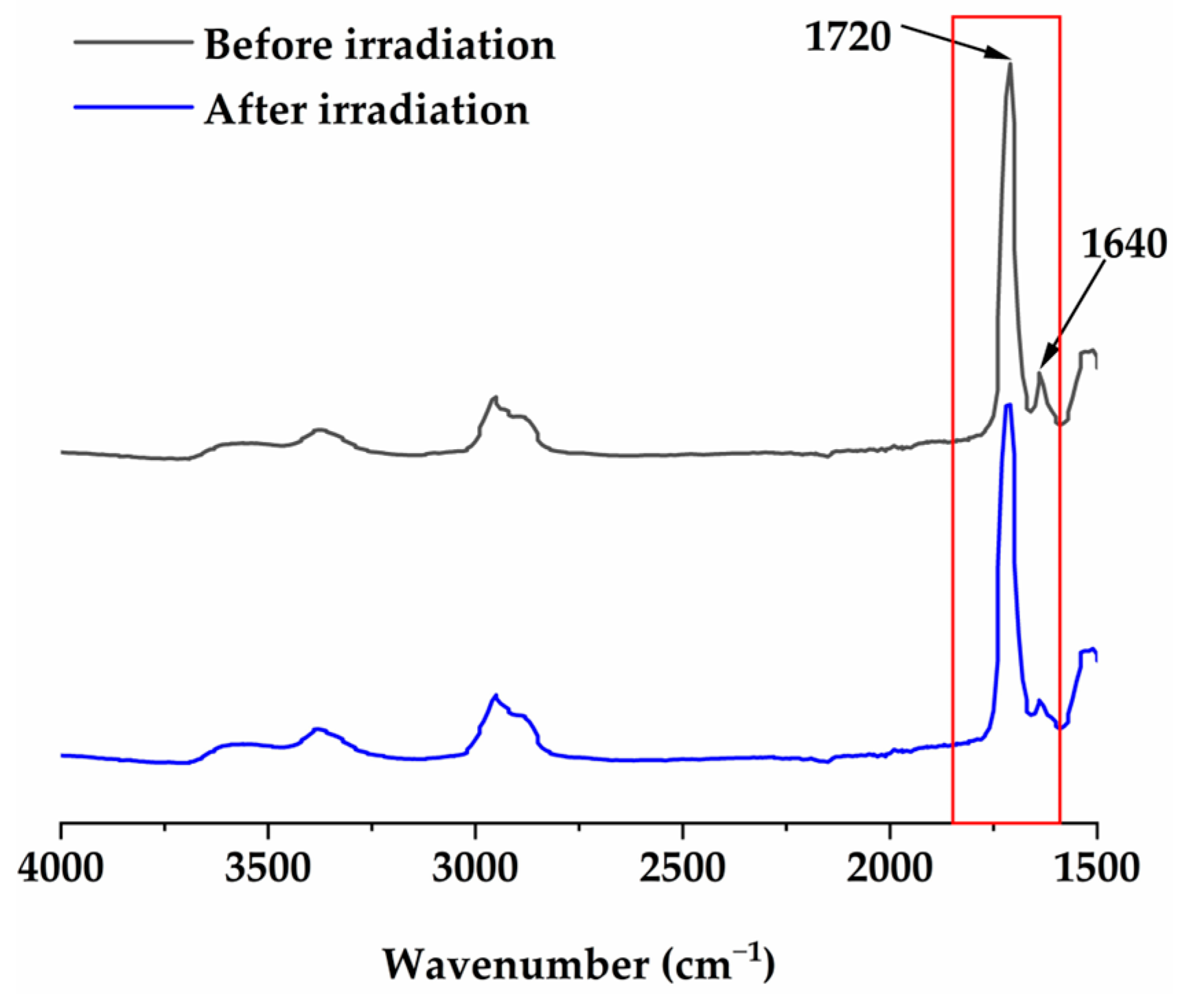
2.4. Double-Bond Conversion (DC)
2.5. Depth of Cure (DOC)
2.6. Vickers Microhardness (VHN)
2.7. Volumetric Shrinkage (VS)
2.8. Shrinkage Stress (SS)
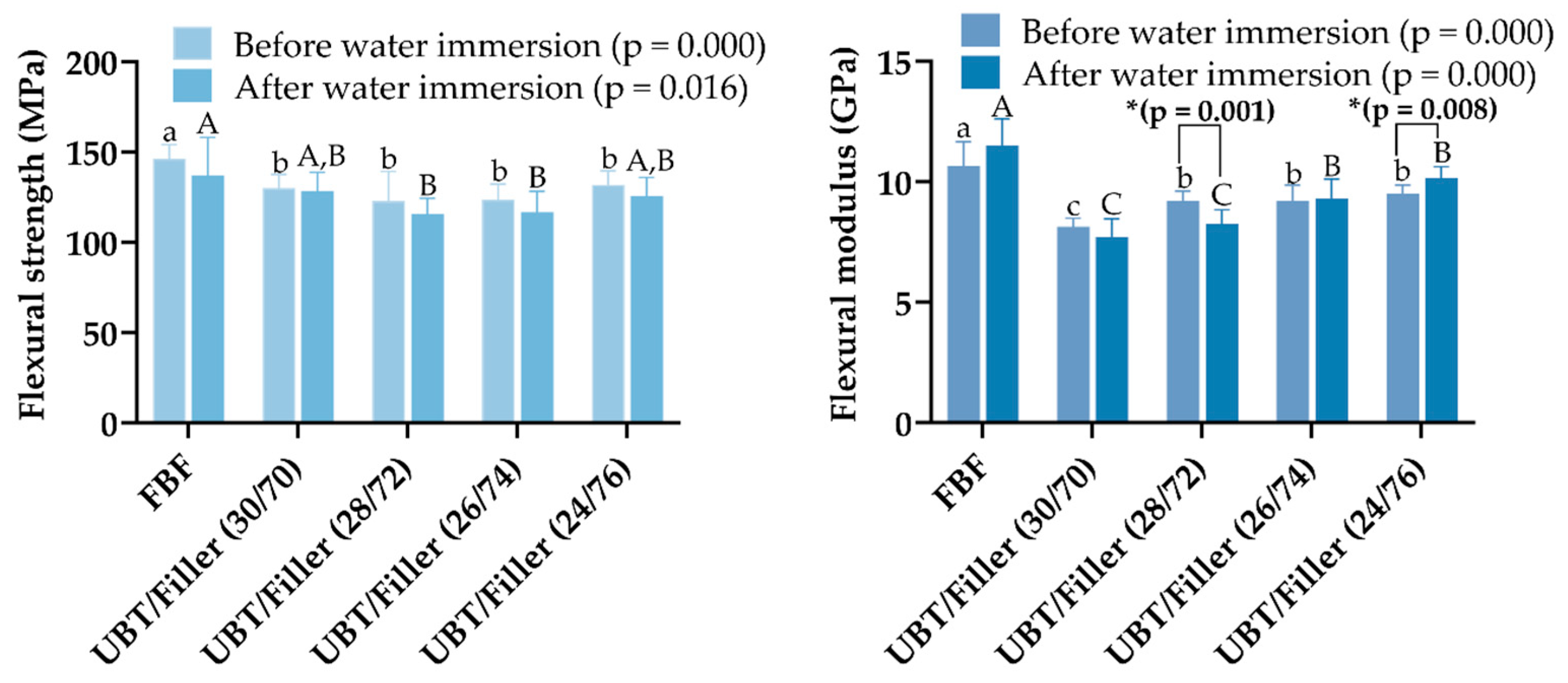
2.9. Flexural Strength (FS) and Flexural Modulus (FM)
2.10. Water Sorption (WS) and Solubility (SL)
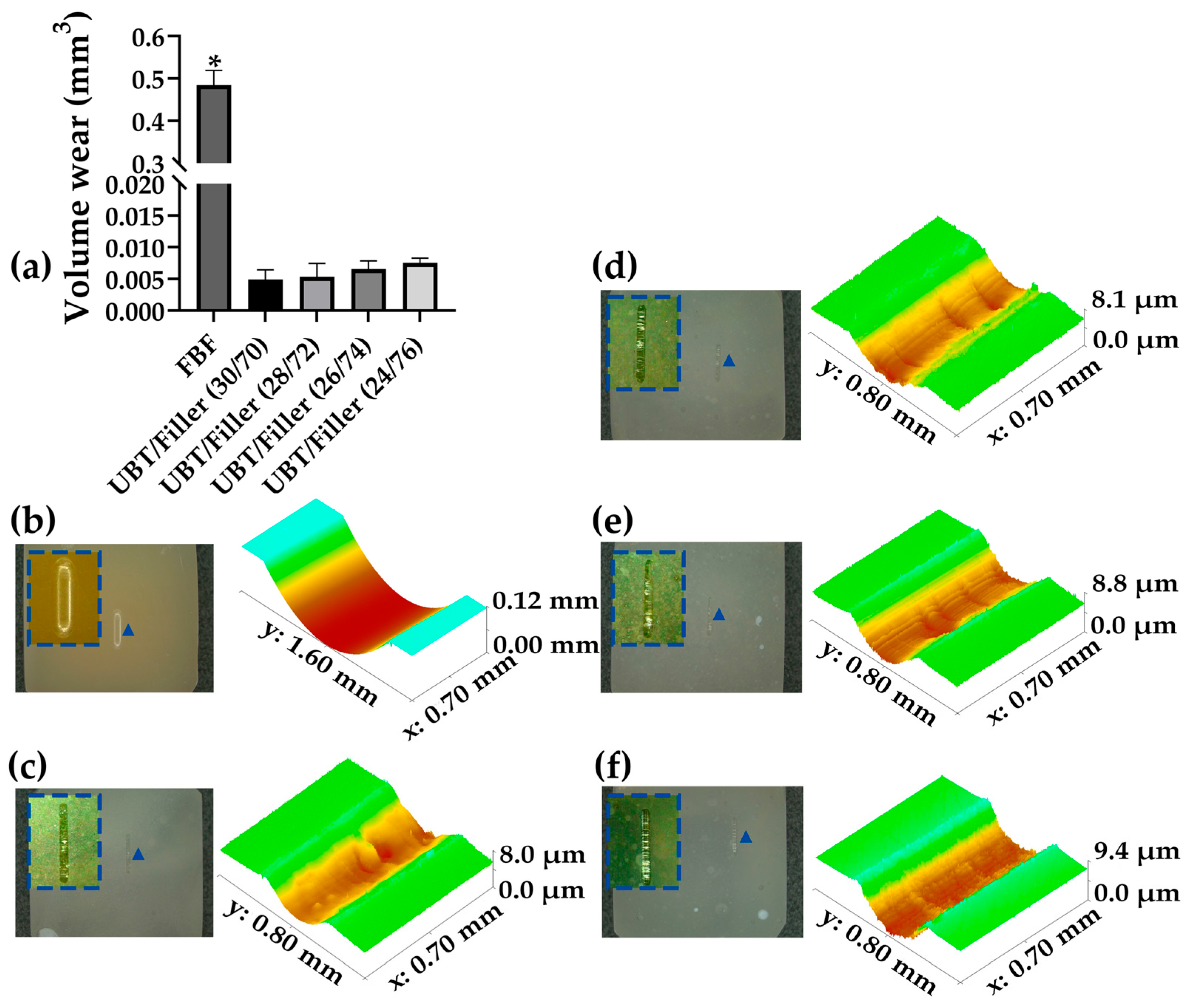
2.11. Wear Resistance
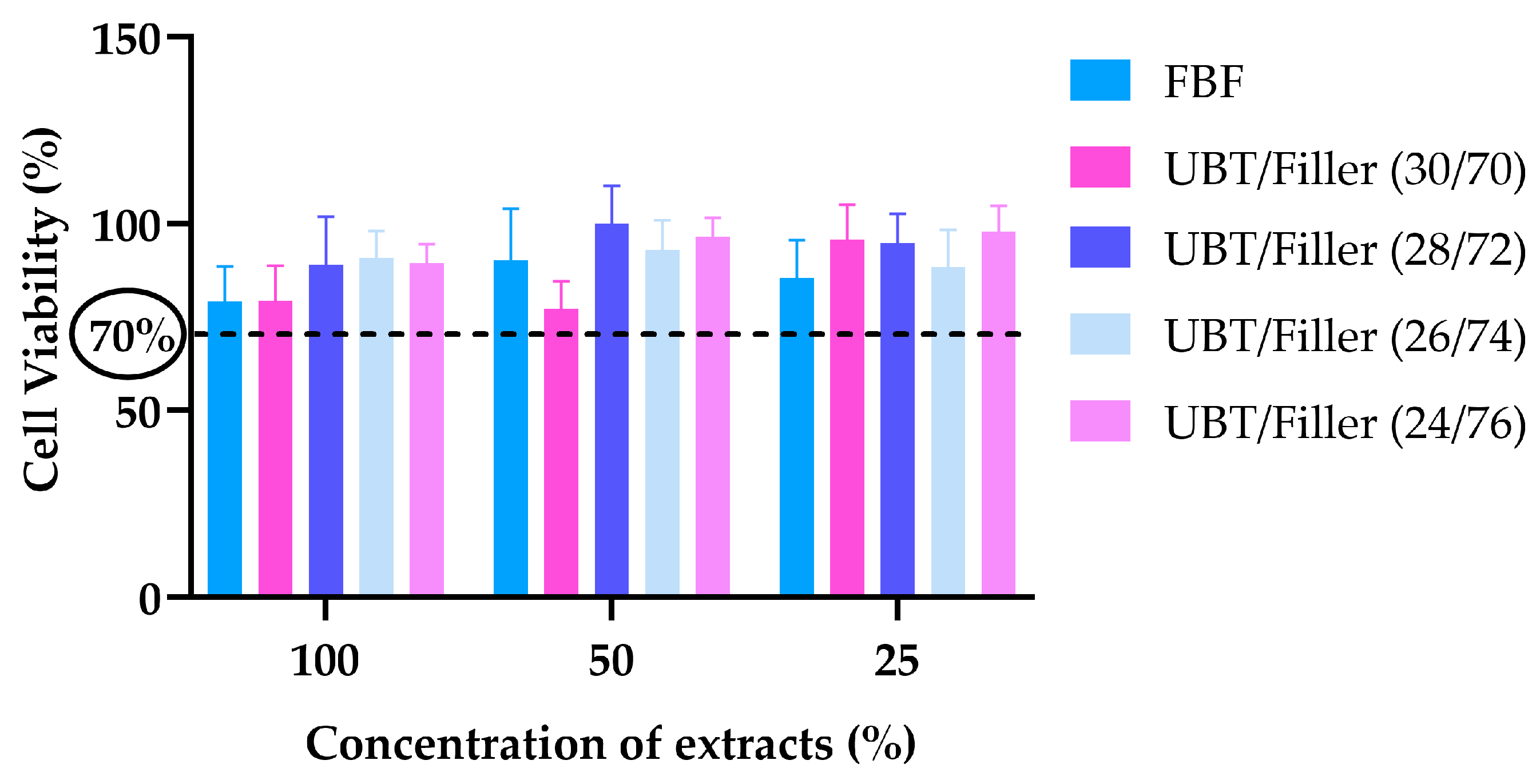
2.12. Measurement of Cytotoxicity
2.13. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- The increase in inorganic filler content did not significantly affect the DOC, DC, SL, and cytotoxicity, and it could notably reduce the WS of the system of Bis-EFMA-based BF-DRCs.
- The increase in inorganic filler content could significantly enhance the VHN and FM of Bis-EFMA-based BF-DRCs, aiding in the improvement of the mechanical properties of the system.
- The increase in inorganic filler content could significantly reduce the VS of Bis-EFMA-based BF-DRC, to a certain extent, which could help improve the polymeric shrinkage of the system.
- The UBT/Filler system with UBT/Filler (24/76) exhibited superior performance compared to commercial bulk-fill resin composite FBF in all aspects, except for flexural strength, flexural modulus, and polymerization shrinkage stress. While the flexural strength of UBT/Filler (24/76) was significantly lower than that of FBF, it still exceeded the minimum flexural strength requirement of 80 MPa, as stipulated by ISO 4049:2019(E), thereby meeting the mechanical performance criteria for posterior dental restorative materials.
- Bis-EFMA-based BF-DRCs can achieve good operability, exceptional mechanical strength, and reduced polymerization shrinkage through an increase in filler content within a certain range. However, considering the prospects for clinical application, their biocompatibility requires further experimental discussion. Additionally, this study has limitations, such as testing the in vitro properties, which may not fully replicate the complexities of the oral environment. Moreover, the introduction of a single filler system cannot adequately balance the modulus of the system and polymerization shrinkage stress.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miletic, V. Dental Composite Materials for Direct Restorations; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Cho, K.; Rajan, G.; Farrar, P.; Prentice, L.; Prusty, B.G. Dental resin composites: A review on materials to product realizations. Compos. Part B Eng. 2022, 230, 109495. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Nawrot, T.; Geebelen, B.; De Munck, J.; Snauwaert, J.; Yoshihara, K.; Scheers, H.; Godderis, L.; Hoet, P.; Van Meerbeek, B.; et al. How much do resin-based dental materials release? A meta-analytical approach. Dent. Mater. 2011, 27, 723–747. [Google Scholar] [CrossRef]
- De Nys, S.; Turkalj, M.; Duca, R.C.; Covaci, A.; Elskens, M.; Godderis, L.; Vanoirbeek, J.; Van Meerbeek, B.; Van Landuyt, K.L. Level of BPA contamination in resin composites determines BPA release. Dent. Mater. 2024, 40, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; Sarteur, N.; Buonvivere, M.; Vadini, M.; Šteffl, M.; D’Arcangelo, C. Meta-analytical analysis on components released from resin-based dental materials. Clin. Oral Investig. 2022, 26, 6015–6041. [Google Scholar] [CrossRef] [PubMed]
- Roussou, K.; Nikolaidis, A.K.; Ziouti, F.; Arhakis, A.; Arapostathis, K.; Koulaouzidou, E.A. Cytotoxic Evaluation and Determination of Organic and Inorganic Eluates from Restorative Materials. Molecules 2021, 26, 4912. [Google Scholar] [CrossRef] [PubMed]
- Snijder, C.A.; Heederik, D.; Pierik, F.H.; Hofman, A.; Jaddoe, V.W.; Koch, H.M.; Longnecker, M.P.; Burdorf, A. Fetal Growth and Prenatal Exposure to Bisphenol A: The Generation R Study. Environ. Health Perspect. 2013, 121, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Ejaredar, M.; Lee, Y.; Roberts, D.J.; Sauve, R.; Dewey, D. Bisphenol A exposure and children’s behavior: A systematic review. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 175–183. [Google Scholar] [CrossRef]
- González-Casanova, J.E.; Bermúdez, V.; Fuentes, N.J.C.; Angarita, L.C.; Caicedo, N.H.; Muñoz, J.R.; Rojas-Gómez, D.M. New Evidence on BPA’s Role in Adipose Tissue Development of Proinflammatory Processes and Its Relationship with Obesity. Int. J. Mol. Sci. 2023, 24, 8231. [Google Scholar] [CrossRef] [PubMed]
- Hessel, E.V.; Ezendam, J.; van Broekhuizen, F.A.; Hakkert, B.; DeWitt, J.; Granum, B.; Guzylack, L.; Lawrence, B.P.; Penninks, A.; Rooney, A.A.; et al. Assessment of recent developmental immunotoxicity studies with bisphenol A in the context of the 2015 EFSA t-TDI. Reprod. Toxicol. 2016, 65, 448–456. [Google Scholar] [CrossRef]
- Lopes-Rocha, L.; Hernandez, C.; Gonçalves, V.; Pinho, T.; Tiritan, M.E. Analytical Methods for Determination of BPA Released from Dental Resin Composites and Related Materials: A Systematic Review. Crit. Rev. Anal. Chem. 2024, 54, 653–668. [Google Scholar] [CrossRef]
- Attik, N.; Hallay, F.; Bois, L.; Brioude, A.; Grosgogeat, B.; Colon, P. Mesoporous silica fillers and resin composition effect on dental composites cytocompatibility. Dent. Mater. 2017, 33, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, D.; Liu, F.; He, J. Preparation and Characterization of Light-Cured Dental Resin without Methacrylate Monomers Derived from Bisphenol A. Adv. Polym. Technol. 2014, 33, 21417. [Google Scholar] [CrossRef]
- Luo, S.; Zhu, W.; Liu, F.; He, J. Preparation of a Bis-GMA-Free Dental Resin System with Synthesized Fluorinated Dimethacrylate Monomers. Int. J. Mol. Sci. 2016, 17, 2014. [Google Scholar] [CrossRef] [PubMed]
- Fugolin, A.P.; de Paula, A.B.; Dobson, A.; Huynh, V.; Consani, R.; Ferracane, J.L.; Pfeifer, C.S. Alternative monomer for BisGMA-free resin composites formulations. Dent. Mater. 2020, 36, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Khan, M.U.A.; Abdullah, A.M.; Razak, S.I.A. A Review on Current Trends of Polymers in Orthodontics: BPA-Free and Smart Materials. Polymers 2021, 13, 1409. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Z.; Jiang, H.; Duan, Y.; Li, J.; Liu, X.; Hong, L.; Zhao, C. Preparation and evaluation of novel bio-based Bis-GMA-free dental composites with low estrogenic activity. Dent. Mater. 2022, 38, 281–293. [Google Scholar] [CrossRef]
- He, J.; Kopperud, H.M. Preparation and characterization of Bis-GMA-free dental composites with dimethacrylate monomer derived from 9,9-Bis[4-(2-hydroxyethoxy)phenyl]fluorene. Dent. Mater. 2018, 34, 1003–1013. [Google Scholar] [CrossRef]
- Cidreira Boaro, L.C.; Pereira Lopes, D.; de Souza, A.S.C.; Lie Nakano, E.; Ayala Perez, M.D.; Pfeifer, C.S.; Gonçalves, F. Clinical performance and chemical-physical properties of bulk fill composites resin—A systematic review and meta-analysis. Dent. Mater. 2019, 35, 249–264. [Google Scholar] [CrossRef]
- Ludovichetti, F.S.; Lucchi, P.; Zambon, G.; Pezzato, L.; Bertolini, R.; Zerman, N.; Stellini, E.; Mazzoleni, S. Depth of Cure, Hardness, Roughness and Filler Dimension of Bulk-Fill Flowable, Conventional Flowable and High-Strength Universal Injectable Composites: An In Vitro Study. Nanomaterials 2022, 12, 1951. [Google Scholar] [CrossRef]
- Hatipoğlu, Ö.; Turumtay, E.A.; Saygın, A.G.; Hatipoğlu, F.P. Evaluation of Color Stability of Experimental Dental Composite Resins Prepared from Bis-EFMA, A Novel Monomer System. J. Photopolym. Sci. Technol. 2021, 34, 297–305. [Google Scholar] [CrossRef]
- Hatipoğlu, Ö.; Turumtay, E.A.; Saygın, A.G. Evaluation of monomer elution, microhardness, and roughness of experimental dental composite resins prepared from Bis-EFMA, a novel monomer system. Polym. Compos. 2022, 43, 584–592. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, X.; Liao, M.; Liu, F.; Wei, Q.; Shi, Z.; Mai, S.; He, J. Properties of Bis-GMA free bulk-filled resin composite based on high refractive index monomer Bis-EFMA. J. Mech. Behav. Biomed. Mater. 2022, 134, 105372. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, X.; Huang, X.; Liu, F.; He, J.; Mai, S. Low shrinkage bulk-filled dental resin composites with non-estrogenic dimethacrylate. Biomater. Sci. 2023, 11, 3669–3682. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, X.; Huang, X.; Liu, F.; He, J.; Mai, S. Performance of low shrinkage Bis-EFMA based bulk-fill dental resin composites. Dent. Mater. 2024, 40, 1378–1389. [Google Scholar] [CrossRef]
- ISO 4049-2019; Dentistry—Polymer-Based Restorative Materials. International Organization for Standardization: Geneva, Switzerland, 2019.
- Bucuta, S.; Ilie, N. Light transmittance and micro-mechanical properties of bulk fill vs. conventional resin based composites. Clin. Oral Investig. 2014, 18, 1991–2000. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Si, W.; Feng, H.; Tao, Y.; Ma, Z. Friction and wear behaviors of dental ceramics against natural tooth enamel. J. Eur. Ceram. Soc. 2012, 32, 2599–2606. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Palin, W.M.; Leprince, J.G.; Hadis, M.A. Shining a light on high volume photocurable materials. Dent. Mater. 2018, 34, 695–710. [Google Scholar] [CrossRef]
- Moore, B.K.; Platt, J.A.; Borges, G.; Chu, T.-M.G.; Katsilieri, I. Depth of Cure of Dental Resin Composites: ISO 4049 Depth and Microhardness of Types of Materials and Shades. Oper. Dent. 2008, 33, 408–412. [Google Scholar] [CrossRef]
- Paolone, G.; Baldani, S.; De Masi, N.; Mandurino, M.; Collivasone, G.; Scotti, N.; Gherlone, E.; Cantatore, G. Translucency of bulk-fill composite materials: A systematic review. J. Esthet. Restor. Dent. 2024, 36, 995–1009. [Google Scholar] [CrossRef]
- Lee, Y.-K. Influence of filler on the difference between the transmitted and reflected colors of experimental resin composites. Dent. Mater. 2008, 24, 1243–1247. [Google Scholar] [CrossRef]
- Flury, S.; Hayoz, S.; Peutzfeldt, A.; Hüsler, J.; Lussi, A. Depth of cure of resin composites: Is the ISO 4049 method suitable for bulk fill materials? Dent. Mater. 2012, 28, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Gallo, S.; Poggio, C.; Ricaldone, V.; Arciola, C.R.; Scribante, A. New Resin-Based Bulk-Fill Composites: In vitro Evaluation of Micro-Hardness and Depth of Cure as Infection Risk Indexes. Materials 2020, 13, 1308. [Google Scholar] [CrossRef] [PubMed]
- Hasanain, F.A.; Nassar, H.M.; Ajaj, R.A. Effect of Light Curing Distance on Microhardness Profiles of Bulk-Fill Resin Composites. Polymers 2022, 14, 528. [Google Scholar] [CrossRef] [PubMed]
- Alrahlah, A.; Silikas, N.; Watts, D.C. Post-cure depth of cure of bulk fill dental resin-composites. Dent. Mater. 2014, 30, 149–154. [Google Scholar] [CrossRef]
- Carrillo-Marcos, A.; Salazar-Correa, G.; Castro-Ramirez, L.; Ladera-Castañeda, M.; López-Gurreonero, C.; Cachay-Criado, H.; Aliaga-Mariñas, A.; Cornejo-Pinto, A.; Cervantes-Ganoza, L.; Cayo-Rojas, C.F. The Microhardness and Surface Roughness Assessment of Bulk-Fill Resin Composites Treated with and without the Application of an Oxygen-Inhibited Layer and a Polishing System: An In Vitro Study. Polymers 2022, 14, 3053. [Google Scholar] [CrossRef]
- Price RB, T.; Felix, C.A.; Andreou, P. Effects of resin composite composition and irradiation distance on the performance of curing lights. Biomaterials 2004, 25, 4465–4477. [Google Scholar] [CrossRef]
- Yılmaz Atalı, P.; Doğu Kaya, B.; Manav Özen, A.; Tarçın, B.; Şenol, A.A.; Tüter Bayraktar, E.; Korkut, B.; Bilgin Göçmen, G.; Tağtekin, D.; Türkmen, C. Assessment of micro-hardness, degree of conversion, and flexural strength for single-shade universal resin composites. Polymers 2022, 14, 4987. [Google Scholar] [CrossRef]
- El-Safty, S.; Akhtar, R.; Silikas, N.; Watts, D. Nanomechanical properties of dental resin-composites. Dent. Mater. 2012, 28, 1292–1300. [Google Scholar] [CrossRef]
- Jun, S.K.; Kim, D.A.; Goo, H.J.; Lee, H.H. Investigation of the correlation between the different mechanical properties of resin composites. Dent. Mater. J. 2013, 32, 48–57. [Google Scholar] [CrossRef]
- Lempel, E.; Czibulya, Z.; Kovács, B.; Szalma, J.; Tóth, Á; Kunsági-Máté, S.; Varga, Z.; Böddi, K. Degree of Conversion and BisGMA, TEGDMA, UDMA Elution from Flowable Bulk Fill Composites. Int. J. Mol. Sci. 2016, 17, 732. [Google Scholar] [CrossRef]
- Amirouche-Korichi, A.; Mouzali, M.; Watts, D.C. Effects of monomer ratios and highly radiopaque fillers on degree of conversion and shrinkage-strain of dental resin composites. Dent. Mater. 2009, 25, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.W. Dimethacrylate network formation and polymer property evolution as determined by the selection of monomers and curing conditions. Dent. Mater. 2012, 28, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.; Hefnawy, S.; Nagi, S. Degree of conversion and polymerization shrinkage of low shrinkage bulk-fill resin composites. Contemp. Clin. Dent. 2019, 10, 465. [Google Scholar] [CrossRef]
- Karabela, M.M.; Sideridou, I.D. Synthesis and study of properties of dental resin composites with different nanosilica particles size. Dent. Mater. 2011, 27, 825–835. [Google Scholar] [CrossRef]
- Paolone, G.; Mandurino, M.; Scotti, N.; Cantatore, G.; Blatz, M.B. Color stability of bulk-fill compared to conventional resin-based composites: A scoping review. J. Esthet. Restor. Dent. 2023, 35, 657–676. [Google Scholar] [CrossRef] [PubMed]
- Alshali, R.Z.; Salim, N.A.; Satterthwaite, J.D.; Silikas, N. Long-term sorption and solubility of bulk-fill and conventional resin-composites in water and artificial saliva. J. Dent. 2015, 43, 1511–1518. [Google Scholar] [CrossRef]
- Marovic, D.; Par, M.; Macan, M.; Klarić, N.; Plazonić, I.; Tarle, Z. Aging-Dependent Changes in Mechanical Properties of the New Generation of Bulk-Fill Composites. Materials 2022, 15, 902. [Google Scholar] [CrossRef]
- Althahban, S.; Alomari, A.S.; El-Din MSallam, H.; Jazaa, Y. An investigation of wear, mechanical, and water sorption/solubility behaviors of a commercial restorative composite containing nano-additives. J. Mater. Res. Technol. 2023, 23, 491–502. [Google Scholar] [CrossRef]
- Ferracane, J.L. Placing Dental Composites—A Stressful Experience. Oper. Dent. 2008, 33, 247–257. [Google Scholar] [CrossRef]
- Sampaio, C.S.; Arias, J.F.; Atria, P.J.; Cáceres, E.; Díaz, C.P.; Freitas, A.Z.; Hirata, R. Volumetric polymerization shrinkage and its comparison to internal adaptation in bulk fill and conventional composites: A μCT and OCT in vitro analysis. Dent. Mater. 2019, 35, 1568–1575. [Google Scholar] [CrossRef]
- Guan, X.; Zhu, T.; Zhang, D. Development in polymerization shrinkage control of dental light-cured resin composites: A literature review. J. Adhes. Sci. Technol. 2023, 37, 602–623. [Google Scholar] [CrossRef]
- Par, M.; Marovic, D.; Attin, T.; Tarle, Z.; Tauböck, T.T. Effect of rapid high-intensity light-curing on polymerization shrinkage properties of conventional and bulk-fill composites. J. Dent. 2020, 101, 103448. [Google Scholar] [CrossRef] [PubMed]
- Tauböck, T.T.; Jäger, F.; Attin, T. Polymerization shrinkage and shrinkage force kinetics of high- and low-viscosity dimethacrylate- and ormocer-based bulk-fill resin composites. Odontology 2019, 107, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Algamaiah, H.; Silikas, N.; Watts, D.C. Polymerization shrinkage and shrinkage stress development in ultra-rapid photo-polymerized bulk fill resin composites. Dent. Mater. 2021, 37, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Rizzante, F.A.P.; Mondelli, R.F.L.; Furuse, A.Y.; Borges, A.F.S.; Mendonça, G.; Ishikiriama, S.K. Shrinkage stress and elastic modulus assessment of bulk-fill composites. J. Appl. Oral Sci. 2019, 27, e20180132. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Xu, Y.-X.; Liu, Y.-S. Polymerization shrinkage and shrinkage stress of bulk-fill and non-bulk-fill resin-based composites. J. Dent. Sci. 2022, 17, 1212–1216. [Google Scholar] [CrossRef]
- Gonçalves, F.; Pfeifer, C.; Ferracane, J.; Braga, R. Contraction Stress Determinants in Dimethacrylate Composites. J. Dent. Res. 2008, 87, 367–371. [Google Scholar] [CrossRef]
- Eweis, A.; Yap, A.; Yahya, N. Comparison of Flexural Properties of Bulk-fill Restorative/Flowable Composites and Their Conventional Counterparts. Oper. Dent. 2020, 45, 41–51. [Google Scholar] [CrossRef]
- Dahri, W.M.; Kumar, N.; Altaf, N.; Mughal, W.; Zafar, M.S. Mechanical and Biomimetic Characteristics of Bulk-Fill Resin Dental Composites Following Exposure in a Simulated Acidic Oral Environment. Biomimetics 2023, 8, 19. [Google Scholar] [CrossRef]
- Ilie, N.; Moldovan, M.; Ionescu, A.C. Microstructure and Mechanical Behavior of Modern Universal-Chromatic and Bulk-Fill Resin-Based Composites Developed to Simplify Dental Restorative Procedures. J. Funct. Biomater. 2022, 13, 178. [Google Scholar] [CrossRef]
- Shah, P.K.; Stansbury, J.W. Role of filler and functional group conversion in the evolution of properties in polymeric dental restoratives. Dent. Mater. 2014, 30, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Goracci, C.; Cadenaro, M.; Fontanive, L.; Giangrosso, G.; Juloski, J.; Vichi, A.; Ferrari, M. Polymerization efficiency and flexural strength of low-stress restorative composites. Dent. Mater. 2014, 30, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Finan, L.; Palin, W.M.; Moskwa, N.; McGinley, E.L.; Fleming, G.J. The influence of irradiation potential on the degree of conversion and mechanical properties of two bulk-fill flowable RBC base materials. Dent. Mater. 2013, 29, 906–912. [Google Scholar] [CrossRef]
- Fronza, B.; Ayres, A.; Pacheco, R.; Rueggeberg, F.; Dias, C.; Giannini, M. Characterization of inorganic filler content, mechanical properties, and light transmission of Bulk-fill resin composites. Oper. Dent. 2017, 42, 445–455. [Google Scholar] [CrossRef]
- Szczesio-Wlodarczyk, A.; Sokolowski, J.; Kleczewska, J.; Bociong, K. Ageing of Dental Composites Based on Methacrylate Resins—A Critical Review of the Causes and Method of Assessment. Polymers 2020, 12, 882. [Google Scholar] [CrossRef]
- Ferracane, J.L.; Berge, H.X.; Condon, J.R. In vitro aging of dental composites in water—Effect of degree of conversion, filler volume, and filler/matrix coupling. J. Biomed. Mater. Res. 1998, 42, 465–472. [Google Scholar] [CrossRef]
- Osiewicz, M.A.; Werner, A.; Roeters, F.J.; Kleverlaan, C.J. Wear of bulk-fill resin composites. Dent. Mater. 2022, 38, 549–553. [Google Scholar] [CrossRef]
- Shimokawa, C.; Giannini, M.; André, C.; Sahadi, B.; Faraoni, J.; Palma-Dibb, R.; Soares, C.; Price, R. In Vitro Evaluation of Surface Properties and Wear Resistance of Conventional and Bulk-fill Resin-based Composites after Brushing with a Dentifrice. Oper. Dent. 2019, 44, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Marquis, P.M.; Shortall, A.C. Influence of filler loading on the two-body wear of a dental composite. J. Oral Rehabil. 2003, 30, 729–737. [Google Scholar] [CrossRef]
- Matsuura, T.; Komatsu, K.; Choi, K.; Suzumura, T.; Cheng, J.; Chang, T.-L.; Chao, D.; Ogawa, T. Conditional Mitigation of Dental-Composite Material-Induced Cytotoxicity by Increasing the Cure Time. J. Funct. Biomater. 2023, 14, 119. [Google Scholar] [CrossRef]
- Lim, S.M.; Yap, A.; Loo, C.; Ng, J.; Goh, C.Y.; Hong, C.; Toh, W.S. Comparison of cytotoxicity test models for evaluating resin-based composites. Hum. Exp. Toxicol. 2017, 36, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Wiertelak-Makała, K.; Szymczak-Pajor, I.; Bociong, K.; Śliwińska, A. Considerations about Cytotoxicity of Resin-Based Composite Dental Materials: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 152. [Google Scholar] [CrossRef] [PubMed]
- Demïrel, G.; Gür, G.; Demïrsoy, F.F.; Altuntaş, E.G.; Yener-Ilce, B.; Kiliçarslan, M.A. Cytotoxic effects of contemporary bulk-fill dental composites: A real-time cell analysis. Dent. Mater. J. 2020, 39, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Shajii, L.; Santerre, J.P. Effect of filler content on the profile of released biodegradation products in micro-filled Bis-GMA/TEGDMA dental composite resins. Biomaterials 1999, 20, 1897–1908. [Google Scholar] [CrossRef]




| DRC Experimental Groups | Components (wt%) | |||||
|---|---|---|---|---|---|---|
| UDMA | Bis-EFMA | TEGDMA | CQ | DMAEMA | Fillers | |
| UBT/Filler (30/70) | 10.65 | 7.10 | 11.83 | 0.21 | 0.21 | 70 |
| UBT/Filler (28/72) | 9.94 | 6.62 | 11.04 | 0.20 | 0.20 | 72 |
| UBT/Filler (26/74) | 9.23 | 6.15 | 10.26 | 0.18 | 0.18 | 74 |
| UBT/Filler (24/76) | 8.52 | 5.68 | 9.46 | 0.17 | 0.17 | 76 |
| DRCs | DOC (mm) n = 6 | WS and SL (μg/mm3) n = 6 | ||
|---|---|---|---|---|
| DOC20s (mm) (p = 0.451) | DOC40s (mm) (p = 0.011) | WS (μg/mm3) (p = 0.000) | SL (μg/mm3) (p = 0.000) | |
| FBF | 4.197 ± 0.1 a | 5.018 ± 0.2 b | 33.27 ± 1.8 a | 13.65 ± 1.9 a |
| UBT/Filler (30/70) | 4.289 ± 0.1 a | 5.589 ± 0.2 a | 24.27 ± 1.3 b | 6.53 ± 0.7 b |
| UBT/Filler (28/72) | 4.290 ± 0.3 a | 5.427 ± 0.3 a,b | 25.74 ± 2.2 b | 6.81 ± 1.1 b |
| UBT/Filler (26/74) | 4.194 ± 0.2 a | 5.398 ± 0.3 a,b | 21.53 ± 0.2 c | 5.79 ± 1.1 b |
| UBT/Filler (24/76) | 4.415 ± 0.2 a | 5.660 ± 0.2 a | 21.66 ± 1.3 c | 5.76 ± 1.1 b |
| Height, mm | DRCs | VHN n = 9 | ||||
|---|---|---|---|---|---|---|
| Top (HV0.5) | p-Value | Bottom (HV0.5) | p-Value | Bottom/Top (%) | ||
| 2 | FBF | 61.4 ± 0.2 a | p = 0.000 | 58.4 ± 1.2 b | p = 0.000 | 95.2 |
| UBT/Filler (30/70) | 51.3 ± 1.3 c | 50.3 ± 0.7 d | 98.0 | |||
| UBT/Filler (28/72) | 52.4 ± 0.5 c | 51.3 ± 0.9 d | 97.9 | |||
| UBT/Filler (26/74) | 55.6 ± 1.2 b | 55.6 ± 1.2 c | 100.0 | |||
| UBT/Filler (24/76) | 62.6 ± 1.4 a | 62.1 ± 1.7 a | 99.2 | |||
| 3 | FBF | 60.0 ± 1.2 b | p = 0.000 | 58.9 ± 1.2 b | p = 0.000 | 98.1 |
| UBT/Filler (30/70) | 49.6 ± 0.3 e | 50.2 ± 1.3 c | 101.2 | |||
| UBT/Filler (28/72) | 53.4 ± 2.0 d | 52.1 ± 1.5 c | 97.5 | |||
| UBT/Filler (26/74) | 57.1 ± 0.5 c | 58.6 ± 1.5 b | 102.6 | |||
| UBT/Filler (24/76) | 63.2 ± 1.6 a | 63.6 ± 0.1 a | 100.7 | |||
| 4 | FBF | 60.0 ± 1.2 b | p = 0.000 | 55.7 ± 0.7 b | p = 0.000 | 92.9 |
| UBT/Filler (30/70) | 49.6 ± 0.6 e | 49.3 ± 0.9 d | 99.2 | |||
| UBT/Filler (28/72) | 54.2 ± 1.1 d | 52.0 ± 1.5 c | 95.9 | |||
| UBT/Filler (26/74) | 57.8 ± 1.6 c | 56.7 ± 0.6 b | 98.1 | |||
| UBT/Filler (24/76) | 63.6 ± 0.1 a | 63.4 ± 0.8 a | 99.7 | |||
| 5 | FBF | 60.9 ± 1.7 b | p = 0.000 | 51.0 ± 2.8 c | p = 0.000 | 83.8 |
| UBT/Filler (30/70) | 49.5 ± 0.7 d | 46.9 ± 0.9 b | 94.9 | |||
| UBT/Filler (28/72) | 52.4 ± 0.7 c | 50.7 ± 0.7 c | 96.7 | |||
| UBT/Filler (26/74) | 58.7 ± 2.8 b | 56.7 ± 0.5 b | 96.6 | |||
| UBT/Filler (24/76) | 63.9 ± 1.0 a | 63.9 ± 1.0 a | 100.0 | |||
| DRCs | DC (%) | ||||
|---|---|---|---|---|---|
| 2 mm (p = 0.000) | 3 mm (p = 0.007) | 4 mm (p = 0.000) | 5 mm (p = 0.000) | p-Value | |
| FBF | 48.7 ± 1.7 b,A | 46.7 ± 1.9 b,A | 32.6 ± 7.7 b,A | 32.8 ± 2.1 b,A | p = 0.092 |
| UBT/Filler (30/70) | 53.4 ± 1.9 a,A | 53.5 ± 3.0 a,A | 52.3 ± 4.4 a,A | 51.3 ± 2.5 a,A | p = 0.743 |
| UBT/Filler (28/72) | 56.1 ± 1.0 a,A | 52.9 ± 2.6 a,A,B | 53.5 ± 3.5 a,A,B | 50.1 ± 3.5 a,B | p = 0.042 |
| UBT/Filler (26/74) | 55.2 ± 1.5 a,A | 55.9 ± 2.6 a,A | 53.0 ± 3.0 a,A,B | 50.1 ± 2.4 a,B | p = 0.013 |
| UBT/Filler (24/76) | 55.3 ± 1.0 a,A | 52.8 ± 2.7 a,A,B | 49.6 ± 2.3 a,B | 48.5 ± 4.0 a,B | p = 0.005 |
| DRCs | SS (MPa) (p = 0.002) | VS (%) (p = 0.009) |
|---|---|---|
| FBF | 1.60 ± 0.1 b | 4.91 ± 0.3 a,b |
| UBT/Filler (30/70) | 2.05 ± 0.2 a | 5.08 ± 0.6 a |
| UBT/Filler (28/72) | 1.93 ± 0.2 a | 4.86 ± 0.5 a,b |
| UBT/Filler (26/74) | 2.05 ± 0.2 a | 4.80 ± 0.8 b |
| UBT/Filler (24/76) | 2.16 ± 0.3 a | 4.65 ± 0.6 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, H.; Liu, F.; He, J. The Effect of Inorganic Filler Content on the Properties of BPA-Free Bulk-Fill Dental Resin Composites. Materials 2024, 17, 5040. https://doi.org/10.3390/ma17205040
Deng H, Liu F, He J. The Effect of Inorganic Filler Content on the Properties of BPA-Free Bulk-Fill Dental Resin Composites. Materials. 2024; 17(20):5040. https://doi.org/10.3390/ma17205040
Chicago/Turabian StyleDeng, Huilin, Fang Liu, and Jingwei He. 2024. "The Effect of Inorganic Filler Content on the Properties of BPA-Free Bulk-Fill Dental Resin Composites" Materials 17, no. 20: 5040. https://doi.org/10.3390/ma17205040
APA StyleDeng, H., Liu, F., & He, J. (2024). The Effect of Inorganic Filler Content on the Properties of BPA-Free Bulk-Fill Dental Resin Composites. Materials, 17(20), 5040. https://doi.org/10.3390/ma17205040







