Abstract
Recycling and reusing industrial waste and by-products are topics of great importance across all industries, but they hold particular significance in the metal industry. Aluminum, the most widely used non-ferrous metal globally, generates considerable waste during production, including dross, salt slag, spent carbon cathode and bauxite residue. Extensive research has been conducted to recycle and re-extract the remaining aluminum from these wastes. Given their varied environmental impacts, recycling these materials to maximize residue utilization is crucial. The components of dross, salt slag, and bauxite residue include aluminum and various oxides. Through recycling, alumina can be extracted using processes such as pyrometallurgy and hydrometallurgy, which involve leaching, iron oxide separation, and the production of alumina salt. Initially, the paper will provide a brief introduction to the generation of aluminum residues—namely, dross, salt slag, and bauxite residue—including their environmental impacts, followed by an exploration of their potential applications in sectors such as environmental management, energy, and construction materials.
Keywords:
aluminum; valorization; waste heat; dross; salt slag; spent carbon cathode; bauxite residue 1. Introduction
Aluminum (Al) and aluminum alloys are highly valued metals with diverse applications because of their superior thermal characteristics, electrical conductivity, low weight, and corrosion resistance [1]. As one of the most recycled materials, aluminum’s significant value and extensive use in the construction, automotive, packaging, and aerospace industries highlight its importance. Notably, aluminum used in building and automotive parts is recycled at rates up to 90% at the end of its useful life [2], and 75% of all aluminum ever produced remains in use today [3]. Ranking second only to steel in the amount of non-ferrous metal produced, aluminum’s production volume surpasses that of all other non-ferrous metals combined [4]. In particular, aluminum alloys are crucial in the aerospace and automotive industries, where they often replace steel due to their ability to naturally form a passive oxide layer when exposed to air, thereby preventing corrosion under various conditions [5].
The aluminum industry generates both non-solid residues (e.g., waste heat) and solid waste, including aluminum dross, salt slags, spent carbon cathode, and bauxite residues. These residues from both primary and secondary aluminum production pose a global challenge. The recycling and reuse of industrial waste and by-products are paramount in construction technology. Traditional industrial by-products like fly ash, granulated blast furnace slag, and silica fume have been extensively researched for their reactivity and effectiveness as active additions in cement and concrete manufacturing [6]. Less reactive wastes serve as additional cementitious materials for cement production or as inert aggregates for concrete and mortar. The residues from aluminum industries, rich in metal oxides and containing valuable rare earth elements, underscore the importance of valorizing aluminum production residues for their incorporation and reuse in everyday applications. This review begins with a brief overview of aluminum production and the residues it generates—namely, waste heat, aluminum dross, salt slag, spent carbon cathode, and bauxite residues. We then explore the valorization potential of each residue and present opportunities for large-scale residue reuse.
2. Overview of Aluminum
In the following sections, we describe the Hall–Héroult process and explain the technology applied during the production process to fully comprehend the operations of the global aluminum industry. Opportunities for the use of residues can then be determined, and we can identify constraints on integrating novel procedures for minimizing and reusing waste products.
2.1. The Hall–Héroult Process
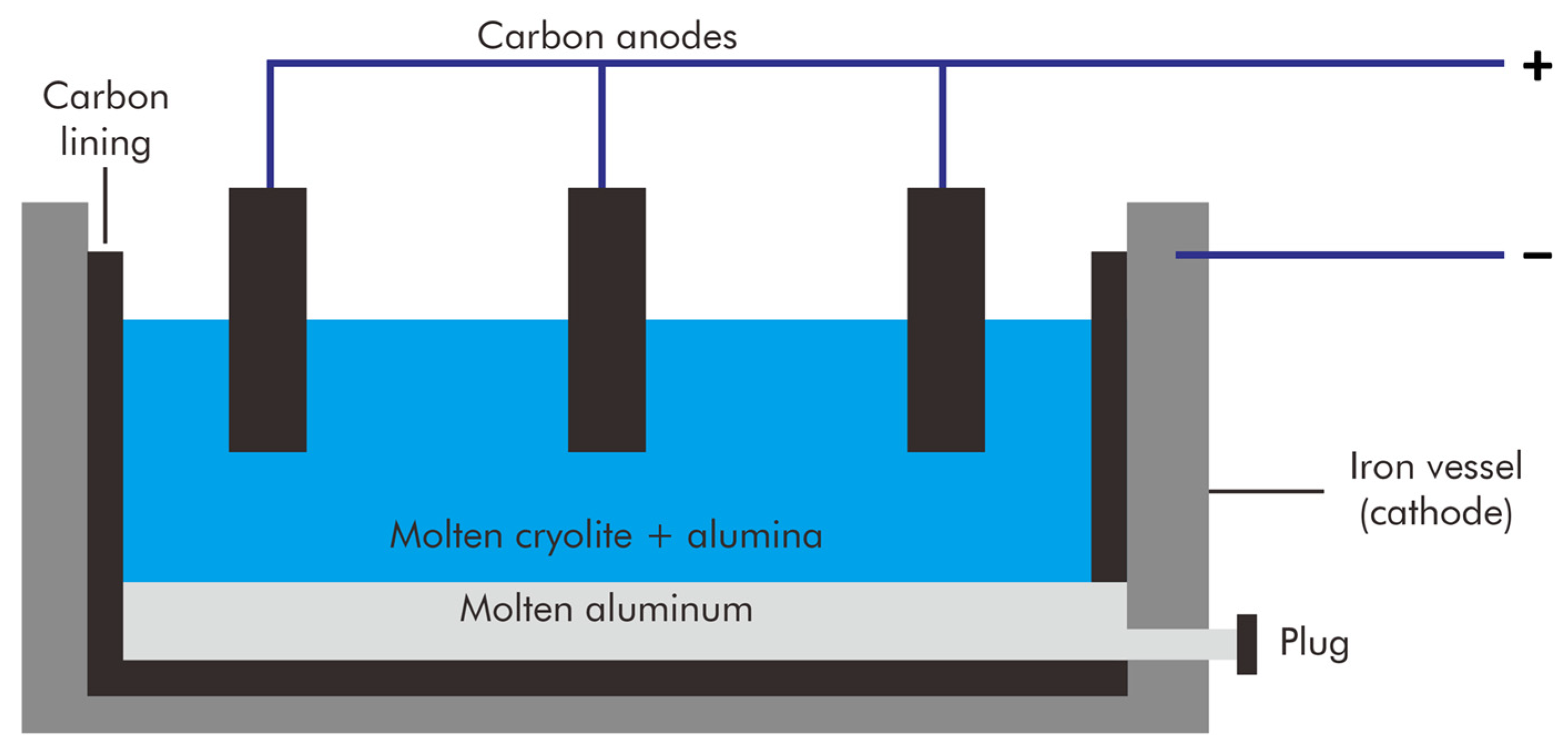
The current method of producing primary aluminum was established in 1886 when Paul Héroult and Charles Hall independently discovered that the reduction of molten aluminum oxide in cryolite (Na3AlF6) served as a more affordable means of aluminum production. As both shared the patent, this production technique is known as the Hall–Héroult process (Figure 1). In 1887, Karl Joseph Bayer discovered that alumina (Al2O3) could be extracted from bauxite, an ore named after the French province of Les Baux, and used as a cost-effective feedstock for the Hall–Héroult process.
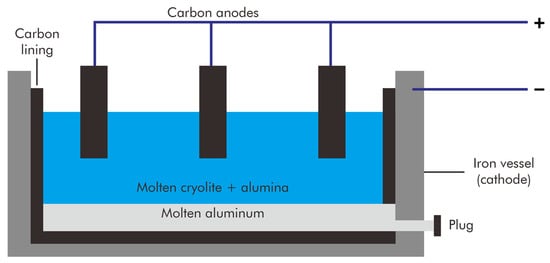
Figure 1.
Illustration of the Hall–Héroult process.
The Bayer process separates alumina from bauxite. Bauxite is dissolved in a hot caustic solution to break it down into a green liquor. This green liquor is then separated from the undissolved material and allowed to cool, where it precipitates pure alumina hydrate. The hydrate is subsequently calcined to produce alumina. The high-purity petroleum coke used to make the carbon anodes for aluminum reduction is ground, calcined, and combined with ground used carbon anodes and pitch, which serves as a binder, to create a paste. Although the Hall–Héroult process consumes a significant amount of electrical energy, manufacturers have gradually increased the efficiency of this technique. Nonetheless, the extraction process produces significant CO2 emissions from the anodes and relatively high heat loss from the electrolytic cells.
2.2. Current Production of Aluminum
Aluminum is the most abundant metallic element, constituting 7.96% of the Earth’s crust [7]. It is seldom found in its elemental form because of its high reactivity, especially when in contact with oxygen, necessitating its extraction from minerals in ores. Bauxite is the principal ore used for aluminum extraction, accounting for more than 99% of primary aluminum production [8]. Open-cast mines on the Earth’s surface are the main sites for bauxite extraction [9]. Bauxite contains three main types of aluminum hydroxide compounds: gibbsite (Al(OH)3), diaspore (α-AlO(OH)), and boehmite (γ-AlO(OH)) [10]. Typically, alumina constitutes 40–60% of bauxite’s weight, along with trace amounts of iron, silicon, and titanium compounds [3], necessitating refinement because of these trace compounds.
Advancements in the metallurgy of aluminum alloys, along with population growth and increased economic activity [11], have led to a rise in bauxite extraction. In 2023, the world’s aluminum production increased marginally to 70 million metric tons (MT) from 68.4 million MT in 2022. Of this total, 41 million metric tons, or more than half, came from China, followed by India and Russia [12]. According to official data, China’s primary aluminum output increased by 7.10% year over year to 14.24 Mt between January and April of this year. An unsustainable increase in alumina imports (+75% y/y to 1.06 Mt), the intermediate product between bauxite and metal in the primary aluminum production chain, is the main cause of this growth [13]. In this country, alumina is produced with an estimated 82 million metric tons produced, followed by Australia with about 19 million metric tons that year [14]. The largest producer of aluminum in India, Vedanta Aluminum, ranks among the top three global companies, with a production capacity of 3 million tonnes per annum (MTPA) [15]. The company announced the new 1.5 MTPA expansion at its alumina refinery in Lanjigarh, Odisha in early 2024. As a result, the alumina refinery’s production capacity has grown from its previous 2 MTPA to 3.5 MTPA. Global demand for aluminum is expected to double over the next decade [16], though more conservative estimates suggest a potential doubling or tripling of demand by 2050 [17]. Australia, China, Guinea, Brazil, India, and Indonesia are the six largest bauxite producers currently [18]. While bauxite ore is plentiful, the growth in secondary production and recycling of aluminum has also surged; hence, the estimated 55–75 Gt of global bauxite reserves are likely to meet demand for many centuries [19].
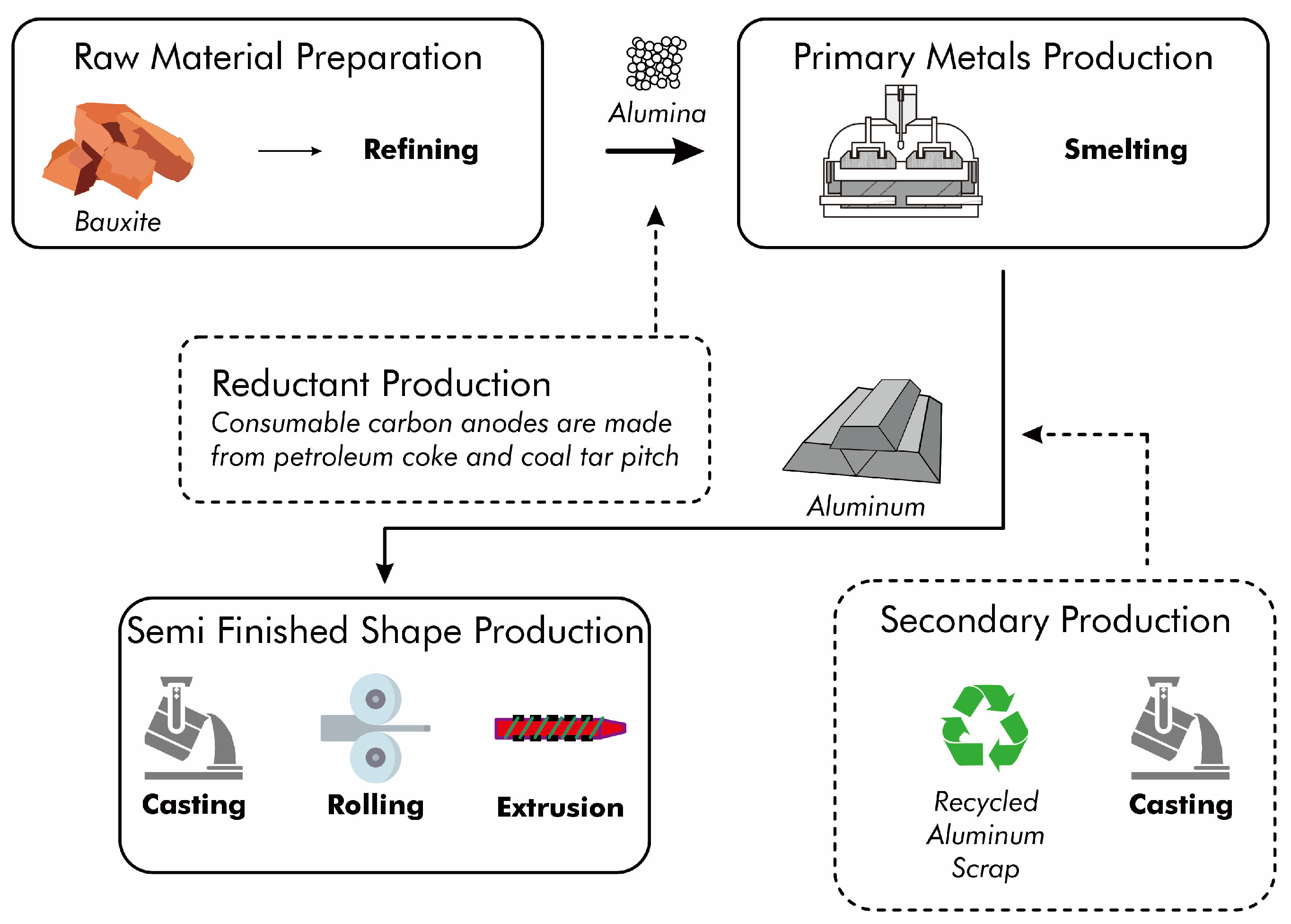
Primary aluminum is produced exclusively from mined ore. The refined ore undergoes electrolytic reduction (Figure 2) before being shaped through casting, rolling, or extrusion to produce bulk material. For products with specific requirements, manufacturers process this bulk material further. Secondary aluminum production involves scrap pretreatment as well as smelting and refining. Pretreatment operations include the processing, cleaning, and sorting of scrap. The smelting and refining processes encompass cleaning, melting, refining, alloying, and pouring of aluminum recovered from scrap.
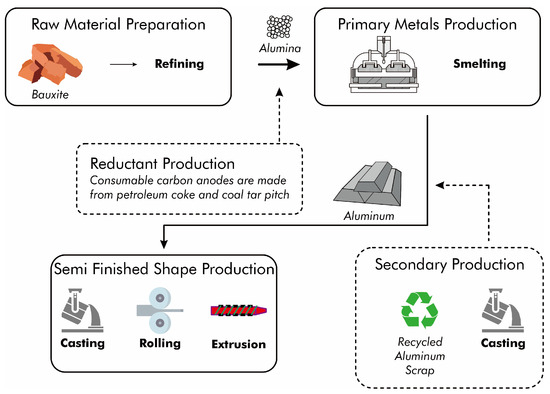
Figure 2.
Flow diagram of primary and secondary aluminum processing.
2.3. The Bayer Processes
The Bayer process is the most widely recognized refining method, though the nepheline-based process and the combined or parallel Bayer–Sinter process are used in some regions. The nepheline-based process uses nepheline syenite as an alternative to bauxite [20], prized for its high aluminum content. However, it presents challenges because of (a) the slow dissolution of aluminum-bearing phases and (b) the formation of silica gel at high pulp densities and low treatment temperatures [21,22].
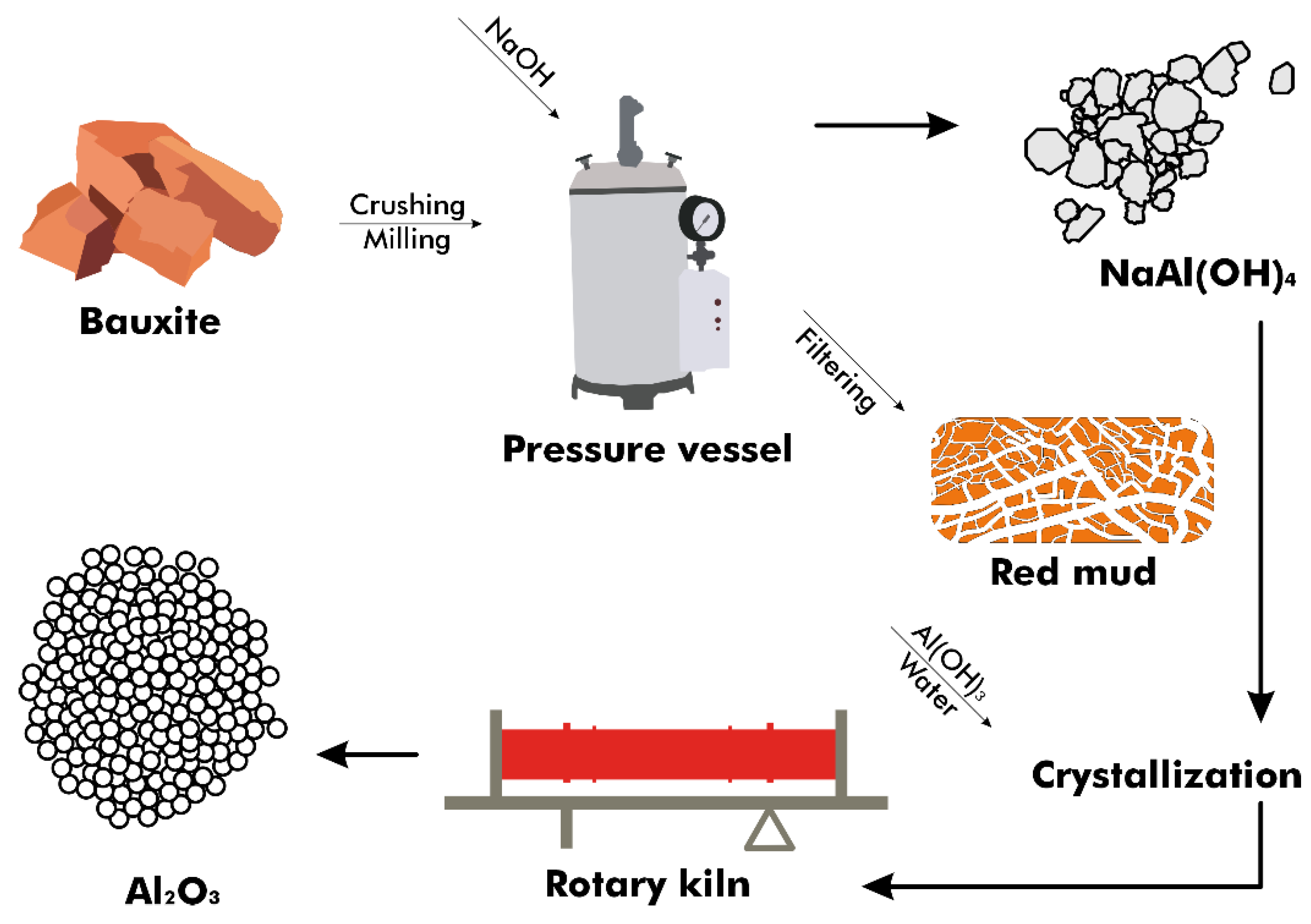
In the Bayer process, bauxite ore undergoes processing to produce purified alumina, necessitated by the impurities within the ore (Figure 3). High-porosity and reactive activated alumina are produced in situ from the bauxite rocks with minimal processing, without direct purification from the ore [23]. This process involves adding ground and blended bauxite and sodium hydroxide (NaOH) to a pressure vessel at temperatures ranging from 150 to 265 °C, depending on the concentration of aluminum compounds present [24]. The compounds dissolve, creating an equilibrium as described by Equations (1) and (2) [25], with conditions favoring the reaction’s shift to the right to produce hydrated NaAl(OH)4 in a step known as digestion.
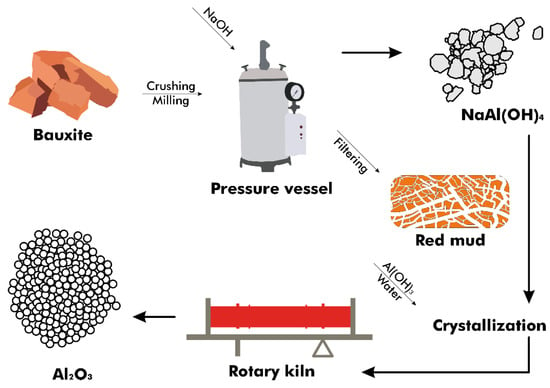
Figure 3.
Flow diagram of the Bayer process.
A supersaturated sodium aluminate liquor known as a slurry is produced by chemically treating the solution. The equilibrium is then shifted to the left by reversing Equation (3) and by cooling the mixture in the presence of seeded crystals of Al(OH)3. Aluminum hydroxide is heated at approximately 1000 °C in a rotary kiln or calciner to remove water and produce anhydrous aluminum oxide, while the sodium hydroxide is recycled [26].
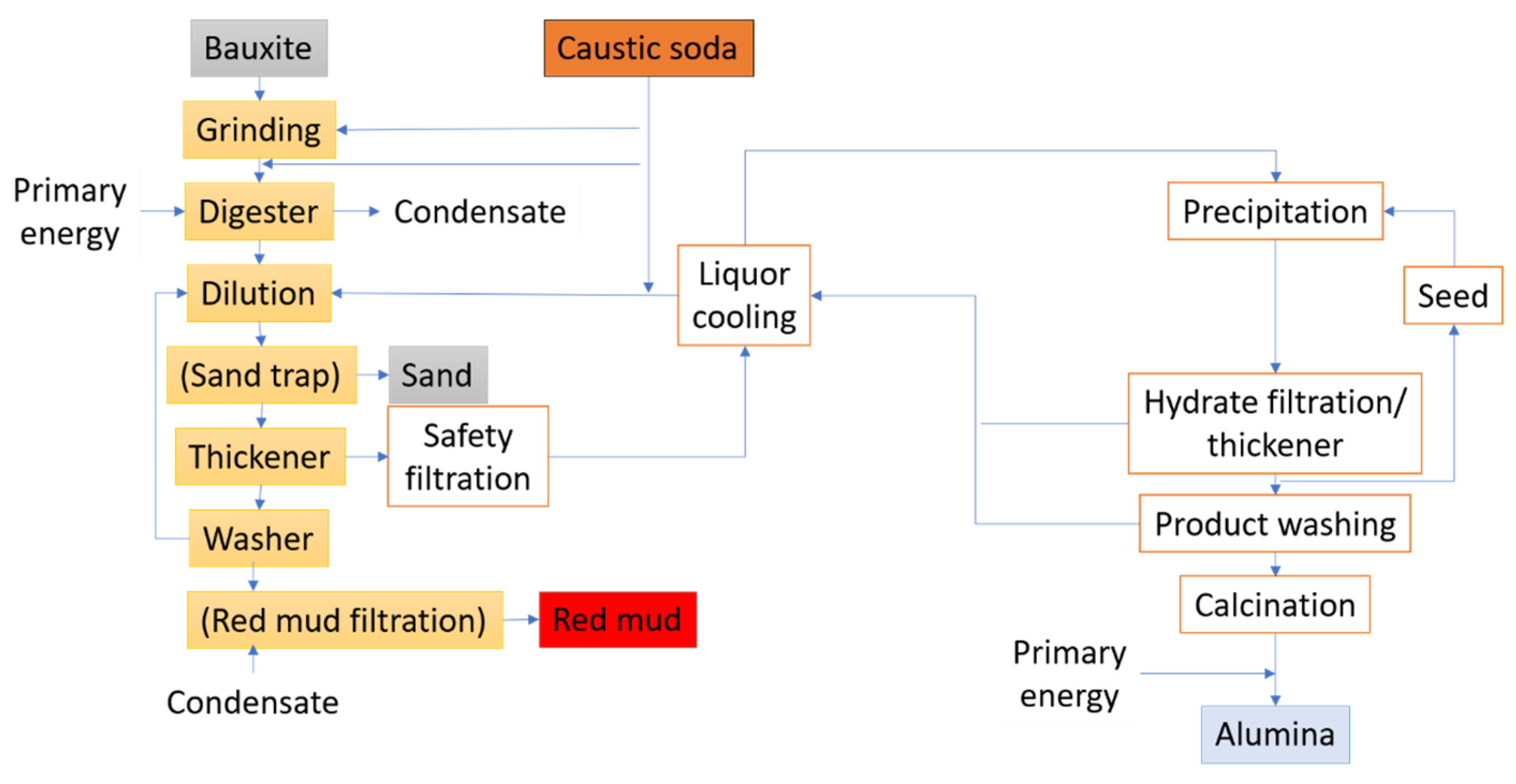
The primary smelters or other industries then acquire the anhydrous aluminum oxide for processing. Figure 4 presents the stages of a typical refining procedure.
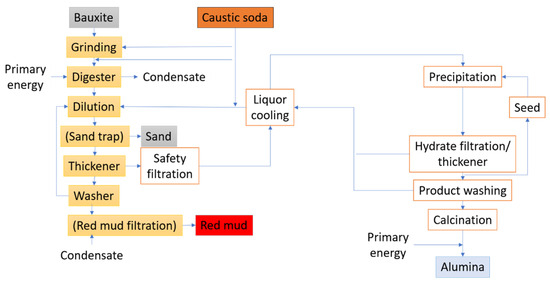
Figure 4.
Overview of the stages of refining bauxite to alumina.
3. Impact of Aluminum Production on the Environment
The manufacturing of aluminum involves numerous additional supporting activities and resources, all of which must be considered in any life cycle analysis of aluminum production. The impacts of aluminum production include the construction of roads and infrastructure, as well as the extraction and disposal of a large amount of rock. Once the mine site is depleted, the post-restoration soils exhibit low water-holding capacities, rendering them unsuitable for crop cultivation. Furthermore, aluminum production is associated with the damage and even loss of ecosystems through photochemical ozone formation, acidification, eutrophication, and ecotoxicity [27]. The aluminum industry emits between 0.45 and 5 Gt of carbon dioxide (CO2) equivalent annually [28]. Industrial facilities converting alumina into aluminum often rely on power plants powered by fossil fuels, thereby increasing greenhouse gas emissions. Power generation may also include hydroelectricity, which reduces carbon emissions but still poses environmental impacts related to dam construction and the flooding of large areas for reservoirs. Particulate and gaseous emissions during aluminum reduction processes include carbon monoxide (CO), CO2, sulfur oxides (SOx), aluminum fluoride (AlF3), calcium fluoride (CaF2), and volatile organic compounds. Polycyclic aromatic hydrocarbons (PAHs), pollutants created during the electrolytic process, are particularly concerning given their carcinogenic potential. Hence, any future sustainable plant design must prioritize the efficient use of natural resources, minimize the input of residues into the environment, and explore alternative, recyclable energy sources such as waste heat.
3.1. Waste Heat
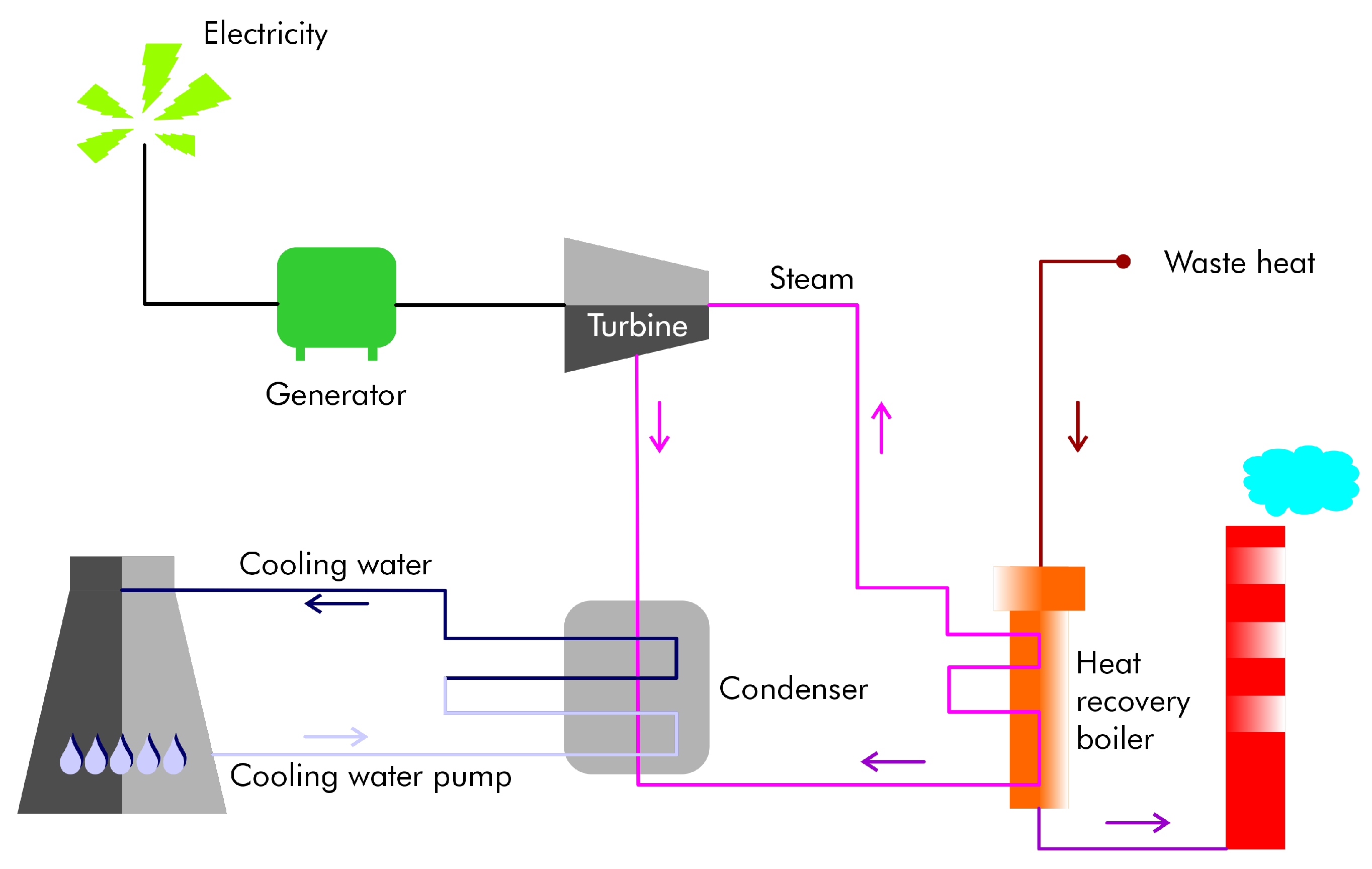
Primary aluminum production consumes significantly more energy and water than secondary production. The efficiency of existing plants can be improved using modern technologies. Recovering, reclaiming, and using generated waste heat are methods for reducing energy consumption. Heat recovery techniques, including the use of electrolytic cells, can help minimize heat loss and reduce the need for fuel and electricity. Most heat is lost through the cell sidewalls, with a smaller amount lost through off-gassing. In secondary aluminum production, scrap melting in high-temperature furnaces, a crucial step, only employs waste heat recovery in about one-third of existing high-capacity furnaces. Nevertheless, several methods for recovering waste heat are available (Figure 5).
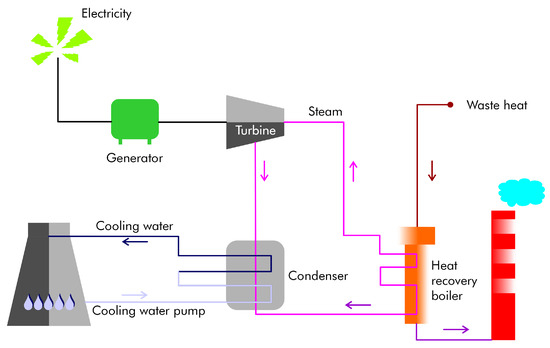
Figure 5.
A heat recovery model. The steam is fed into the highly efficient turbine/generator unit, which generates electrical energy.
3.2. Aluminum Dross
A significant challenge in producing pure aluminum is the generation of other solid wastes. Primary aluminum production generates considerably more atmospheric emissions and solid waste than secondary production. Materials with less than 45% aluminum content are classified as “dross”, whereas residues with more than 45% aluminum content are termed “skimmings” [29]. Dross can be further divided into “white dross”, from primary smelters without salt covers, and “black dross”, originating from secondary smelters. Metal beads, crystallized salt, and solid nonmetallic particles are found in the nonmetallic waste products from dross smelting processes. White dross comprises a fine powder obtained by skimming the molten aluminum, containing between 20% and 45% recoverable metallic aluminum. In the second stage of aluminum production, the flux-containing dross resulting from the melting operation of aluminum scrap in reverberatory furnaces is referred to as “black dross”, distinguished by its darker color [30]. Black dross typically contains aluminum oxide (20–50%), a salt–flux mixture (40–55%), and aluminum metal, with the recoverable aluminum content usually ranging between 10% and 20%. It has a much higher salt content (typically greater than 40%) than white dross [31]. In the separation process of aluminum production, white dross requires more energy and water than black dross and also produces more waste [32]. Globally, more than 95% of the four million tonnes of white dross and over a million tonnes of black dross produced annually are landfilled.
3.3. Salt Slag
The nonmetallic residue resulting from dross smelting operations is known as salt slag and typically contains 3–7% residual metallic aluminum, 15–30% aluminum oxide, 30–55% sodium chloride, and 15–30% potassium chloride, along with other materials that vary in abundance depending on the type of scrap [33]. Salt slag, also referred to as salt cake, is classified as toxic and hazardous waste with variable harmful properties [34]. The scrap/dross and salt flux are re-added to an oil or gas-fired furnace during the rotary salt furnace process. The salt facilitates the agglomeration and separation of the metal, thereby increasing metal recovery by shielding it from the atmosphere’s reactivity [35]. Additionally, it enhances heat transfer to the metal, prevents metal oxidation, and absorbs contaminants including oxides, nitrides, carbides, and other substances found in scrap or created by reactions during melting [36]. Aluminum metal and salt slag are removed from the furnace after melting. After tapping and cooling, the salt cake is formed, containing all the nonmetallic components from the raw mix [37]. The oxides in the dross (from the raw mixture) form a net-like structure, trapping aluminum. This structure is disrupted by the molten flux, which also facilitates the coalescence and sinking of aluminum droplets into the aluminum bath [38]. Carbon typically remains in the salt slag following the decomposition of organic contaminants. If insufficient amounts of salt are present, a high concentration of oxides and other contaminants may cause the molten salt to become highly viscous. In practice, a more viscous slag results in significant metal loss because of the entrapment of metal droplets.
Carbides, nitrides, and phosphates bound with aluminum, along with the oxides of alloying elements (e.g., Cu, Fe, Si, Zn), make up most of the nonmetallic compounds. When liquid aluminum comes into contact with finely dispersed carbon, Al4C3 is formed, which originates from organic contaminants in scrap such as paints, plastic coatings, and hybrid-sandwich components. The AlN-containing dross is fed into rotary furnaces, where it is captured by the salt slag. The high concentration of NaCl and KCl in salt slag causes the slag to release chlorides into water when in contact with a water source or groundwater. The presence of toxic, harmful, explosive, poisonous, and foul-smelling gases, e.g., NH3, CH4, PH3, H2, and H2S, means that the gaseous emissions resulting from the contact of the salt slag with water may also have a significant negative impact on the environment [39]. Depending on the type of scrap, it may contain carbides, nitrides, phosphides, and sulfides [40].
3.4. Spent Carbon Cathode
Another solid waste produced from aluminum is the spent carbon cathode. This carbon-rich material produced when electrolysis cells in the aluminum manufacturing process are overhauled [41]. Most aluminum electrolytic cells only last 5 to 8 years because high-temperature electrolytes continuously erode the cathode carbon during production [42]. The main way that SCC is currently disposed of is in landfills [43], which may release highly toxic cyanide and soluble fluoride into the environment, endangering ecological and environmental safety [44]. Moreover, it also has a significant effect on the wellbeing and ecological equilibrium of plants and animals [45]. While carbon recovery is relatively easy, recovering fluorine resources is typically difficult, primarily for innocuous detoxification [46]. Among the techniques used to treat spent SCC are high-temperature heating [47], chemical leaching [48], and physical separation [49]. The drawbacks of chemical leaching and physical separation are their lengthy processing times, complicated procedures, and high wastewater production. The combustion and boiling points of dangerous compounds, like fluorides, serve as the foundation for the high-temperature heating treatment. Moreover, these techniques have a number of drawbacks, including low leaching rates, lengthy leaching cycles, high energy consumption, low recovery rates, insufficiently pure carbon powder, and the production of new pollutants throughout the treatment process [50]. Therefore, finding a treatment method that is both effective and pollution-free is essential.
3.5. Bauxite Residue
The most significant waste created in the primary aluminum process is bauxite residue or “red mud”, generated during the Bayer process. This red-colored sludge is produced in the second stage of alumina production. The high concentration of Fe2O3 in bauxite residue gives it its red-colored appearance. To produce one ton of aluminum, four tons of bauxite are required, and two tons of bauxite residue are generated. Red mud consists primarily of iron (Fe), silicon (Si), and titanium (Ti) oxides, sodium hydroxide, unextracted aluminum oxide, zinc (Zn), phosphorus (P), nickel (Ni), and vanadium (V), as well as other oxides. The composition of red mud varies among aluminum production facilities (Table 1).

Table 1.
Oxide compositions of red mud produced globally.
The bauxite residue becomes extremely alkaline, with pH values from 9 to 13, when bauxite is treated with concentrated NaOH at high temperature and pressure [71]. Bauxite residue waste management typically involves controlled landfill disposal. Because sodium hydroxide is added during the Bayer process, the residue is extremely alkaline [72]. In Canada, red mud is considered a Class 8 hazardous good (corrosive material) under the dangerous goods transport regulation, Sections 2.40–2.42, in accordance with UN code No. 3244. Thus, specific packaging and the use of UN-compliant containers are required for all shipments. Transportation via airfreight is quite difficult, except in limited quantities. Meanwhile, sea freight is easier, and road transportation packaging standards are normally used. Moreover, given the high alkalinity of the water and soil (pH 10–13), the presence of heavy metals, and even traces of radioactive elements, there is a significant contamination risk [73]. Extensive treatment involving maintenance and monitoring is necessary, as is a sizable storage space for its disposal.
4. Valorization of Residue from Aluminum Production
Given its high pH and trace amounts of heavy metals, aluminum residue poses a serious environmental risk. The pursuit of producing cleaner bauxite residue has become a key focus for the aluminum industry, necessitating that governments and producers adopt a circular economy model for aluminum production, use, and reuse [74]. The circular economy model facilitates waste reduction by adding value to products [75]. Aluminum is one metal that benefits significantly from the circular economy; it can be recycled numerous times without losing its mechanical, physical, or chemical properties. The following subsections discuss strategies to promote the valorization of waste heat, white and black dross, salt slag, and bauxite residue from aluminum manufacturing.
4.1. Waste Heat Recovery
Several studies have focused on the use of waste heat from the aluminum industry, which could benefit from potential sources of recoverable heat, such as those from refining, smelting, recycling, and secondary melting processes. Heat recovery is also feasible from calciners and hot alumina. For calciners, various technologies are under consideration to recover latent heat from water vapor [25]. There is potential to use recoverable heat from hot alumina to produce heated water for generating electricity or for other processes within aluminum processing plants. However, the availability of hot water for plant operations is often limited.
Electrolysis pots have shown good potential for heat recovery. The steel-shell-protected wall can be fitted with a thermoelectric device. In Europe, heat exchangers on the exhaust gas stream are used to recover heat from aluminum smelting cells or reactors [26]. Hot exhaust gases from fuel combustion, produced during anode baking processes, contain significant amounts of tar vapor and volatiles, which are problematic. The primary cast furnace produces exhaust gas flows and temperatures. The gas may contain hydrogen fluoride [27] at concentrations of less than 1 to 10 ppm, which limits the use of conventional heat exchangers for waste heat recovery. The melting furnace emits a considerable quantity of exhaust gases at temperatures around 870 °C, which may include flux material vapors, organic vapors, and particulates. An option is to treat the gases with absorbents, eliminating the need to cool the gases and producing cleaner gases suitable for heat recovery in existing systems.
The gases from crucible heaters, gas generators, and reverberatory (reverb) furnaces—with temperatures ranging from 790 to 1090 °C—contain natural gas combustion products and are free of major contaminants. These gases can account for up to 60% of the total heat input for heating systems and are viable candidates for heat recovery. Rotary furnaces produce cold gases laden with a large number of contaminants, and the industry has yet to develop an approach for their recovery. Consequently, research is underway to develop high-temperature polymers or composites for gas cleaning applications in waste heat recovery. Delacquering systems remove volatile materials, such as paint coatings, from used beverage cans and preheat them to about 480 °C before releasing them from a rotary kiln. The unit emits relatively clean exhaust gases at a temperature of about 340 °C, yet these gases do not undergo any heat recovery. Nonrecoverable heat is also found in crucible heaters. Nowicki et al. presented a mapping of heat demands at Alcoa Deschambault Quebec (ADQ) [76]. Their conservative assumptions indicated that the ADQ facility had the potential to produce approximately 10 MW of electricity using extractable waste heat. An aluminum foundry in Romania evaluated a waste heat recovery system [77]. Uses for the waste heat in the foundry included direct heating during winter, conversion to steam during summer, and electricity generation during spring and autumn when both heating and cooling demands are low. Wang et al. analyzed the performance of the organic Rankine cycle (ORC) in recovering low-temperature waste heat from aluminum reduction cells [78], observing that the variation in heat source temperature significantly affects net power output. When wind and solar power are used as a major energy supply for industrial aluminum electrolysis, the heat-exchanging system is also suitable for aluminum cells. Waste heat can be used for the aluminum die-casting industry [79]. Egilegor et al. analyzed the potential of heat recovery in aluminum low-pressure die casting to ensure effective operation and prolonged lifetime under high temperatures [80]. They concluded that given the working temperature/pressure and fluids, selecting appropriate materials for the heat pipe shells was crucial.
4.2. Aluminum Dross
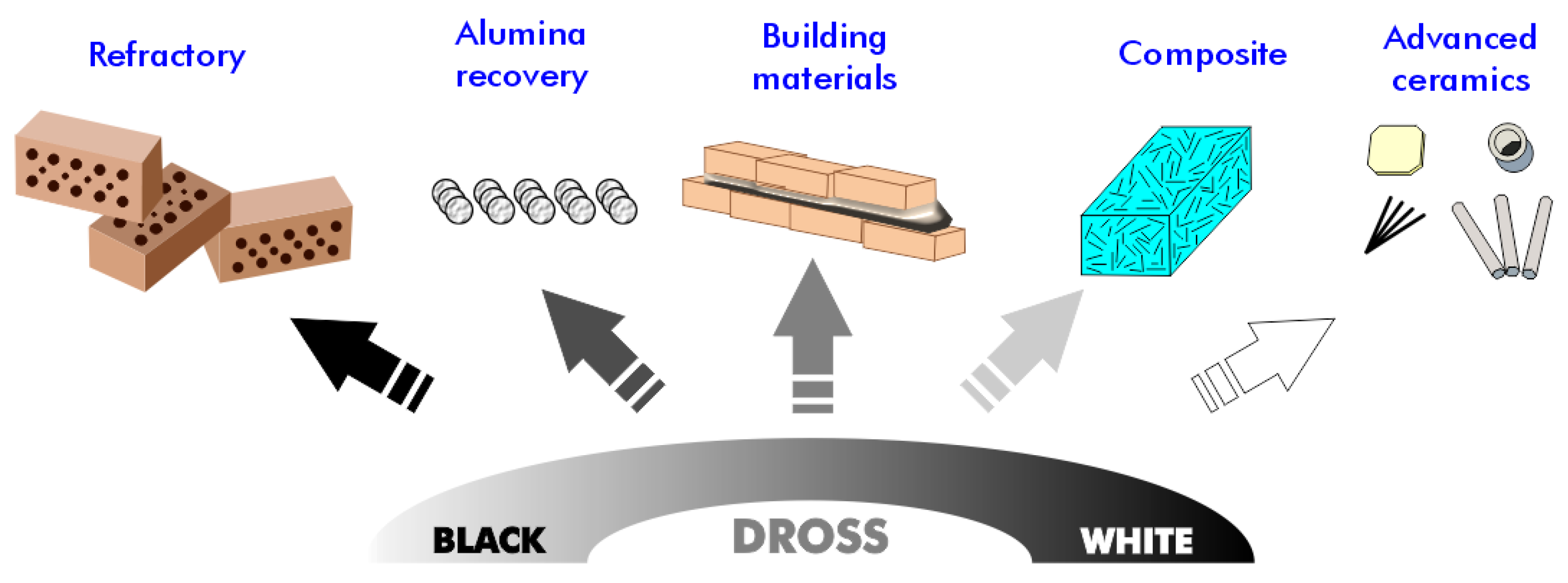
Given the significant variability in the composition of aluminum dross among batches, greater effort is required to identify potential applications for this material. Various techniques for recycling and reusing aluminum dross show promise for managing aluminum dross and its reuse in diverse applications, such as the development of construction and building materials, separation media and agents, alumina extraction, and hydrogen production (Figure 6). We explore several of these applications in the following subsections.
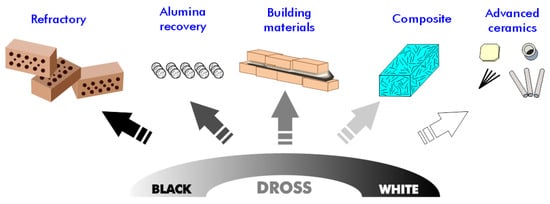
Figure 6.
Potential application of aluminum dross residues.
4.2.1. Aluminum Metal Recovery
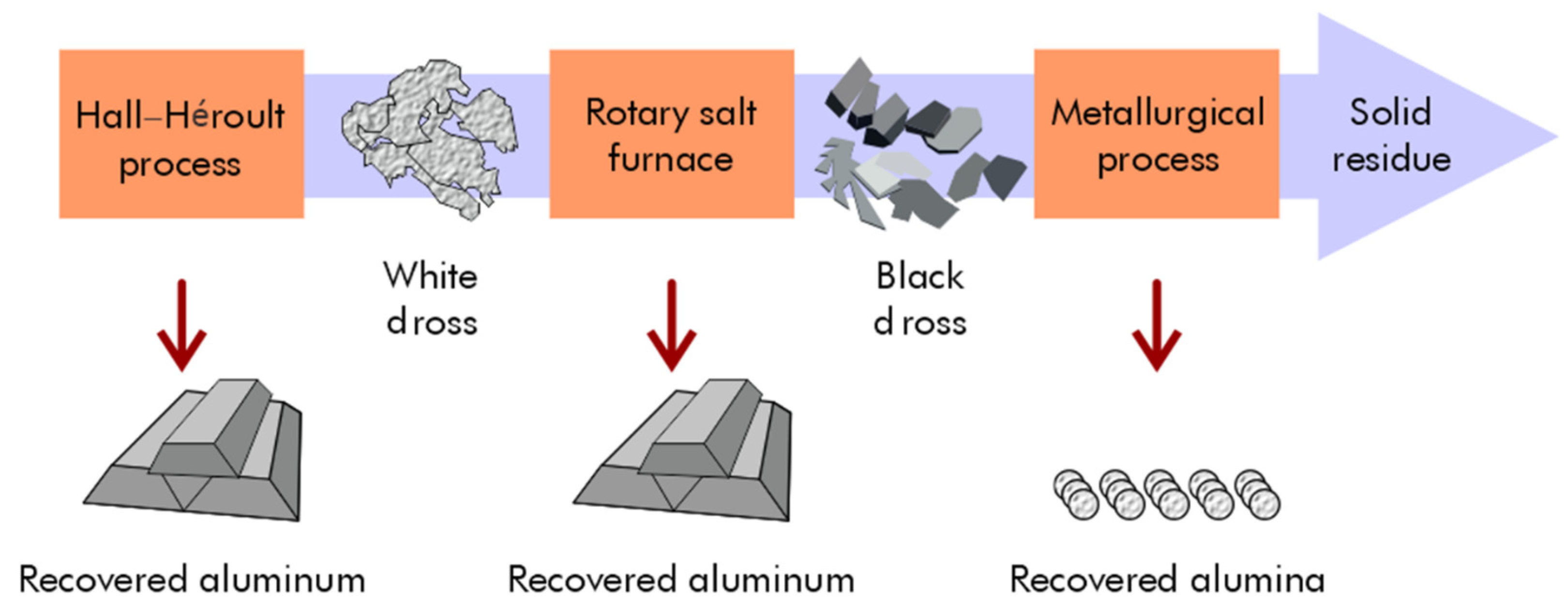
Aluminum metal can be recovered using cost-effective recovery processes, and aluminum oxides find applications in the metallurgical and construction industries. The recycling and reuse of materials are crucial for reducing waste generation and decreasing the economy’s dependence on the extraction of primary (virgin) raw materials. This circular economy conserves resources and energy while slowing the depletion of virgin natural resources. During the Hall–Héroult process, aluminum metal can be reclaimed from white dross, which can be recycled through a rapid slurry-forming (RSF) process (Figure 7).
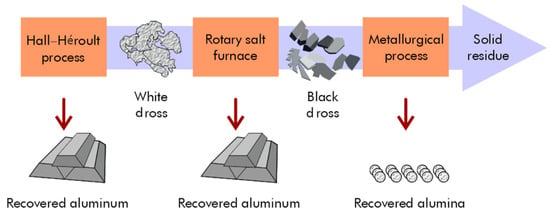
Figure 7.
Recovery of aluminum and alumina from white and black dross using RSF and metallurgical processes.
Several methods have been developed to recover dross while retaining the maximum amount of aluminum metal, such as spreading hot dross to accelerate cooling, combining dry pressing and alkaline roasting processes, and using an inert gas like argon to prevent oxidation [81]. The standard procedures for processing aluminum dross involve initially cooling the dross to room temperature using methods such as rotary drum coolers, stationary inert gas coolers, and floor spreading. The remaining metal can be recovered by reheating the raw dross (or a milled concentrate) with salt flux in a rotary furnace or, less commonly, in a side-well reverberatory furnace [82].
Manual separation and density-based separation methods like hydraulic and pneumatic classifiers are less effective. Zuo et al. demonstrated a method for recovering salts and ammonium hydroxide from black aluminum dross using catalytic hydrolysis, followed by filtration and drying before calcination [83]. Aluminum and other valuable elements can be recovered in the form of alloys, such as aluminum nitride (AlN), by adding alumina in electrochemical reduction. Salts are also reclaimed during the filtration of leachate followed by crystallization.
4.2.2. Applications in Construction Materials
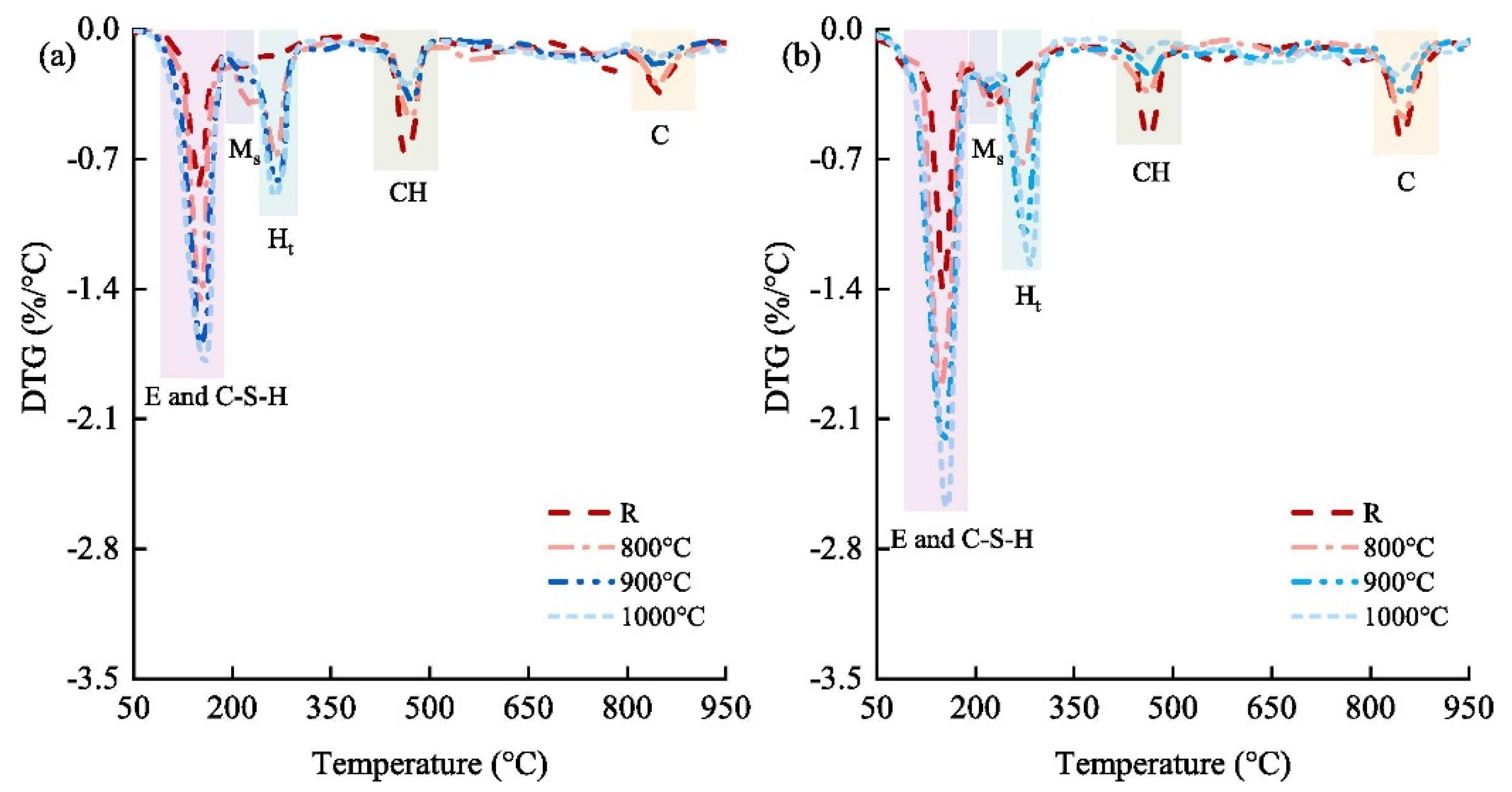
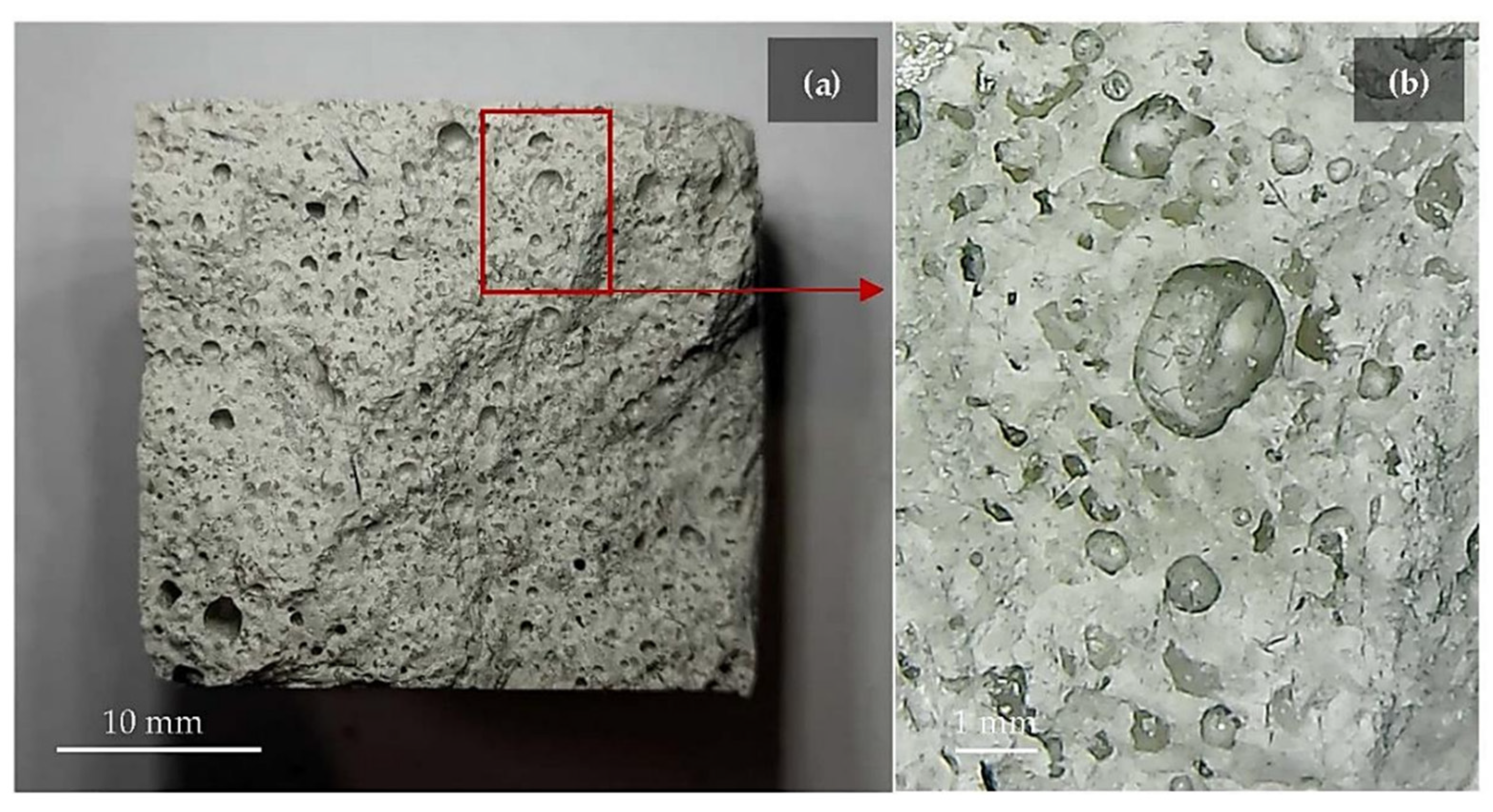
The use of white or black dross as a filler in the construction industry is common. Both white and black dross can serve as fillers in asphalt—when the particles are smaller than 700 μm—to improve stiffness and abrasion resistance and reduce microcracking [84]. Zhang et al. developed novel supplementary cementitious materials (SCMs) by mixing secondary aluminum dross with dolomite [85]. They investigated the effect of thermal activation on calcined mixes, the hydration properties of pastes containing novel SCMs, and the workability of cemented paste backfill (CPB). High-temperature calcination significantly increased SCM activity, and thermal activation enhanced the activity of SCMs (Figure 8).
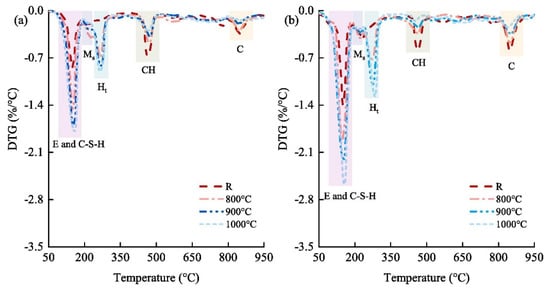
Figure 8.
Differential thermogravimetry (DTG) of pastes at curing times of (a) 3 days and (b) 28 days. E, ettringite (Ca6Al2S3H64O60); C-S-H, calcium silicate hydrate; Ms, monosulphate (Ca6Al2SH24O22); Ht, hydrotalcite (Mg6Al2CH24O52); CH, portlandite (CaO2H2) [85].
The black-dross-leached Al residue, produced during the hydrothermal treatment of Al black dross, can also be used as a raw material for producing Portland cement clinker [85]. Ercoli et al. evaluated the mechanical and thermal properties of geopolymer foams reinforced by carbon fibers, using by-products from the secondary aluminum industry [86]. The flexural and tensile strengths decreased when aluminum-rich by-products were added as additives to foam the geopolymers because of the gas bubbles formed in their structure during the consolidation process (Figure 9 illustrates the gas bubbles in the geopolymer foam, which are not homogeneous in size). However, the Charpy impact strength value was increased because chopped carbon fibers reinforced the geopolymer.
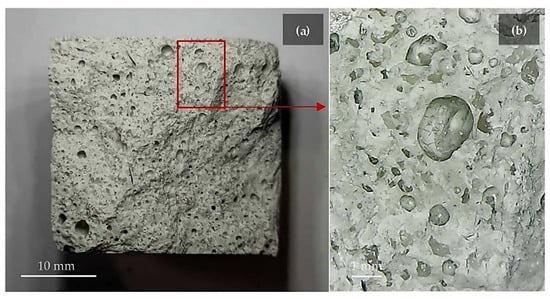
Figure 9.
(a) Geopolymer foam section and (b) magnified image of bubbles generated by the oxidation of the by-product [86]. The gas bubbles were generated during the consolidation process of by-product and alkali activator.
4.2.3. Dross for Engineering Composites
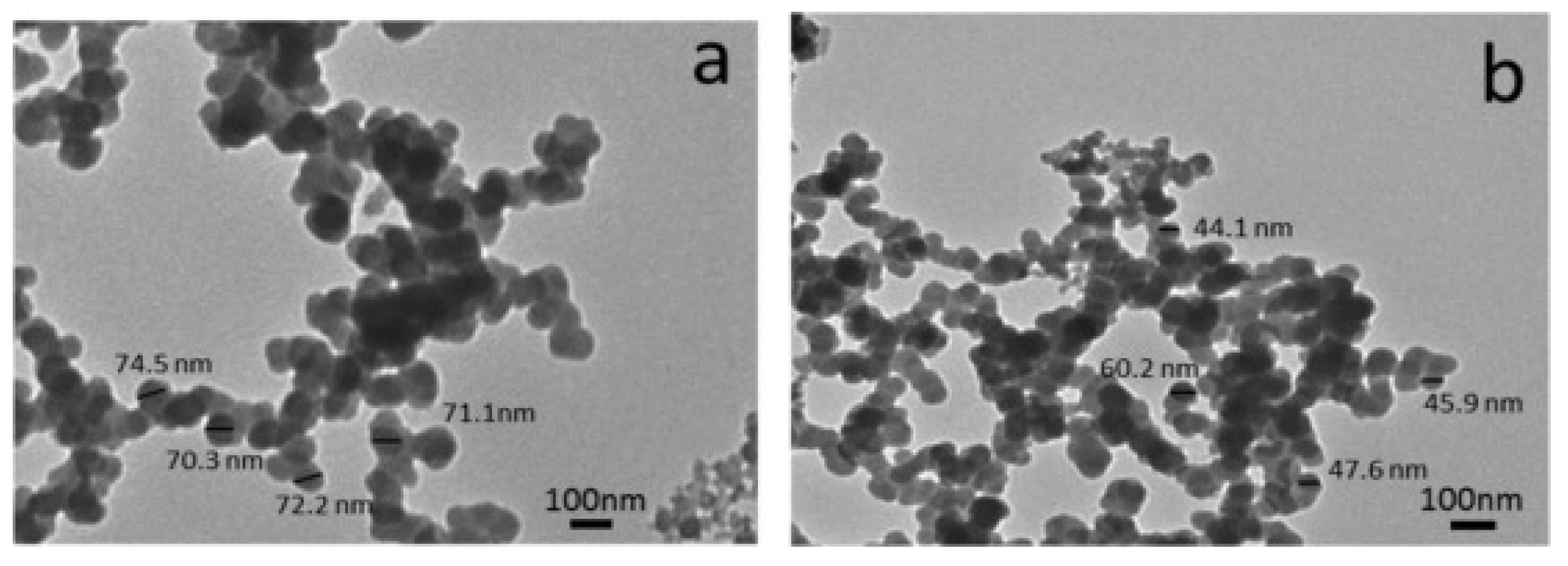
Dirisu et al. used uncarbonized egg shell [87] to reinforce aluminum dross, cement, silicate, and pulverized carbon graphite. They applied a molding process to produce a building ceiling composite and investigated how its composition affected thermal conductivity and microstructure. The sample comprised 30 wt% Al dross, 25 wt% cement, 30 wt% silicate, 5 wt% carbon graphite (CG), and 10% uncarbonized eggshell (UES). The composite could serve as a flame-retardant ceiling because of its lower thermal conductivity relative to existing ceiling tiles. Moreover, the produced composite exhibited a lower heat flux than other previously developed ceiling composites. The production of conventional materials from expensive raw materials is increasingly being replaced by more cost-effective composite materials [88]. Dross, SiC, and TiO2 nanopowders have been analyzed for use in ceramic composites [88]. SiC is known for its desirable properties, including high hardness, strength, and melting point; good chemical and thermal stability; and oxidation and erosion resistance. TiO2 can be added to SiC with the aim of improving the sinterability and properties of the composites [89]. TEM images of sintered composites, with and without added SiC and TiO2 (after being milled for 5 h), show no particle coarsening, and the particle size of the waste Al dross decreases as the weight percent of SiC and TiO2 (as reinforcement) increases (Figure 10).
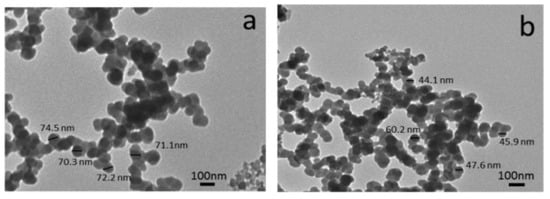
Figure 10.
TEM images of (a) Al-dross waste powder without reinforcement and (b) W15 powder that contains 7.5 wt% SiC and 7.5 wt% TiO2 [89].
4.2.4. Other Applications
Other uses of aluminum dross include its application in the steel, ceramic, and fertilizer industries. Heo and Park investigated Fe recovery from electric arc furnace (EAF) slag using aluminothermic smelting reduction (ASR) at 1773 K with Al dross as the reductant [90]. During the FeO reduction reaction, solid spinel (MgO·Al2O33) and MgO compounds precipitate from the slag. Moreover, the metallic Al in the Al dross reductant can reduce Mn from the EAF slags. The use of black dross to produce a ladle-fluxing agent for the steel industry has been studied [91]. Ewais and Besisa produced magnesium aluminate titanate (MAT)-based ceramics by sintering aluminum dross waste and combining it with rutile ore powders at 1300 °C for 6 h [92]. The obtained samples were thermally stable and did not decompose at high temperatures, presenting a promising method for producing a new advanced ceramic material. Zhang et al. synthesized MgAl2O4 spinel from secondary aluminum dross [93]. They doped the spinel with rare earth oxides (REO), including Y2O3, Eu2O3, La2O3, and CeO2, to improve the densification behavior. The densification of the MgAl2O4 doped with lanthanum oxide was higher when heated at 1473 to 1773 K Dangtungee et al. neutralized aluminum dross and used it as fertilizer [94]. The treated aluminum dross fertilizer enhanced the height and weight of Chinese cabbage relative to untreated plants and controls (Figure 11). Sweet corn, basil, and spring onions also exhibited improved growth with the aluminum treatment. Because no toxic heavy metals are present in the prepared fertilizer, aluminum dross—after neutralization—can be considered an environmentally friendly fertilizer.

Figure 11.
Untreated and dross-treated Chinese cabbage after 13 days [94]. The plants showed increased height and weight compared to the control plants.
Aside from metals and oxides, gaseous phases such as NH3 can be extracted during the synthesis of materials from aluminum dross. Gomes et al. used ammonia (NH3) released from Al dross as a nitrogen source for sprouting seeds [95]. Vegetable and fruit seeds were planted in soils directly infused with dross-produced NH3 for 2 h, followed by 8 to 12 h of self-diffusion time to ensure even distribution of the gas throughout the soil. Seeds that prefer acidic soils did not germinate well in soils infused with NH3, whereas seeds that thrive in alkaline conditions, such as ridge gourd and watermelon seeds, sprouted early and robustly. Shi et al. [96] created a polyaluminum chloride flocculant by treating secondary aluminum dross with hydrochloric acid. The new flocculant effectively removed impurities from wastewater and other waste liquids. Philipson et al. [97] produced a silicon alloy reducing silica in calcia–silica (CaO-SiO2) slag with aluminum dross as a reductant. The kinetics of reduction were investigated by comparing the Si-alloy and product slag compositions to simulated thermodynamic equilibrium. The Al dross was shown to be as effective a reductant as pure Al, and the results show rapid aluminothermic reduction and good Si-alloy/slag separation for both reductants.
4.3. Salt Slag
Salt slag, a material produced during the melting of aluminum scrap or dross, contains aluminum, aluminum oxide, alkaline chlorides, and impurities such as carbides, nitrides, sulfides, and phosphides [98,99]. The treatment of salt slag is typically carried out in the US, Canada, and Europe, where the landfilling of salt slag is prohibited by law. The treatment of salt cake results in a significant financial return. The salt fraction, including halite (NaCl) and sylvite (KCl) along with residual metallic aluminum, can be recovered, justifying the investment in salt cake recycling facilities by large refining companies [100]. The residues generated post-recycling are non-toxic, and the alumina-containing compounds can be used as new raw materials in other processes and applications, such as in the cement industry and as refractory materials (Figure 12).
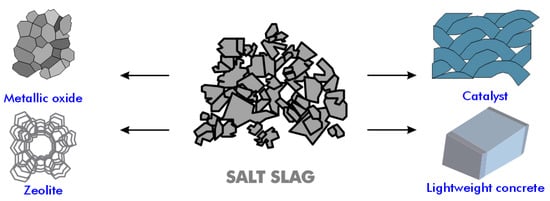
Figure 12.
Various applications of aluminum salt slag.
4.3.1. Synthesis of Zeolite
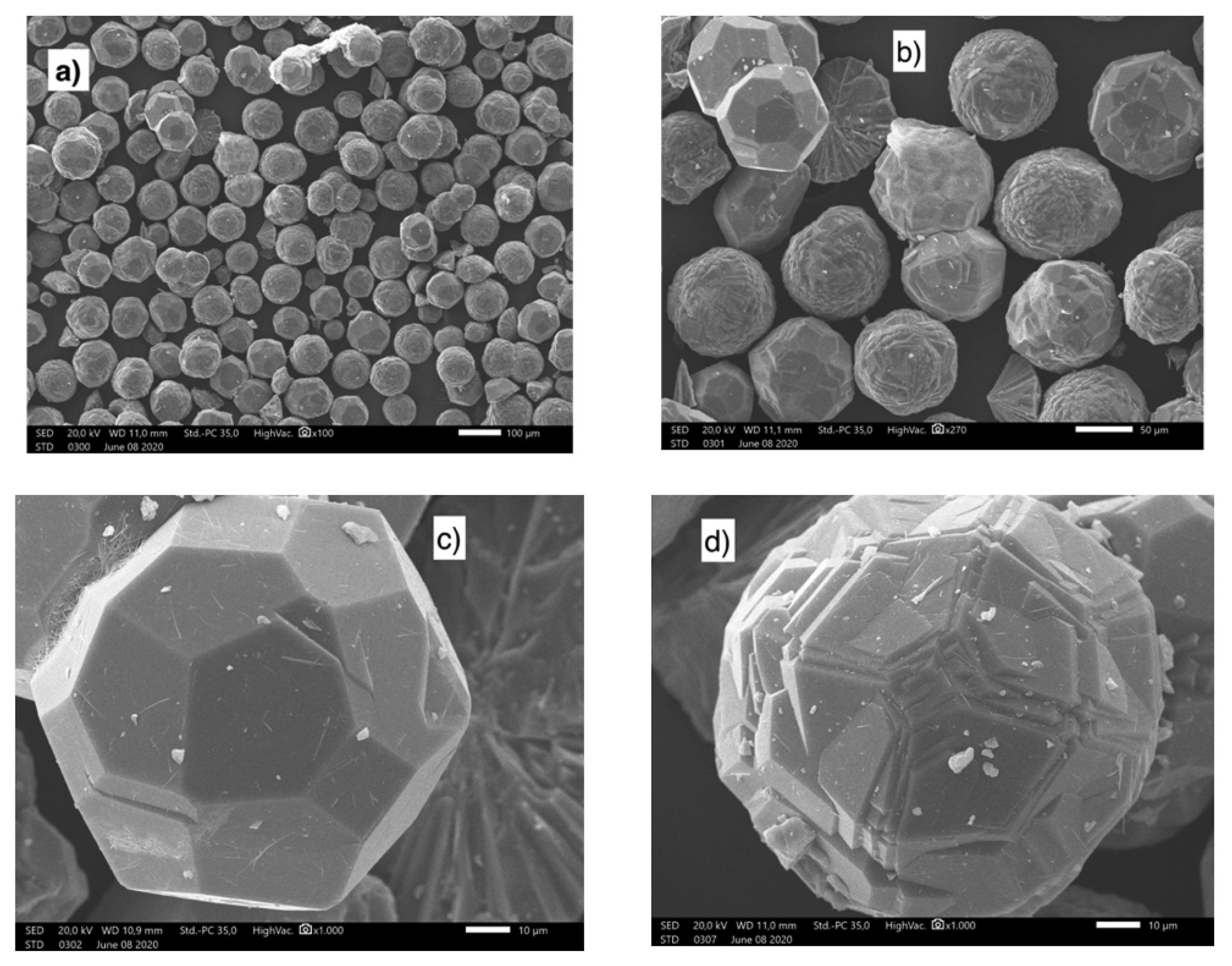
Sánchez-Hernández et al. used hydrolyzed salt as a reactant along with Na2SiO3 and NaOH solutions to synthesize zeolite [101]. Their single-step method produced zeolite-type NaP (Na6Al6Si10O32·12H2O) by adding the corresponding amount of silicate solution. The produced zeolite exhibited characteristics similar to those of conventional chemical reagent–based forms. Jimenez et al. synthesized pollucite and analcime zeolites by extracting aluminum from saline slag [102]. The structures of the synthesized materials are based on (Al,Si)O4 tetrahedra that share corners and feature pores, channels, and/or cavities at the molecular level. The extraction liquor contained aluminum and alkali metal cations. After adding the required amount of Si and undergoing hydrothermal synthesis at 200 °C for 24 h, zeolitic materials were produced (Figure 13 shows SEM images of this material). The crystallinity and water content of analcime were higher than those of pollucite. Morphological analyses revealed the formation of spherical particles, which are larger for analcime solids with pentagonal or polygonal faces. For pollucite, spheres are smaller, and it is possible that they are hollow.
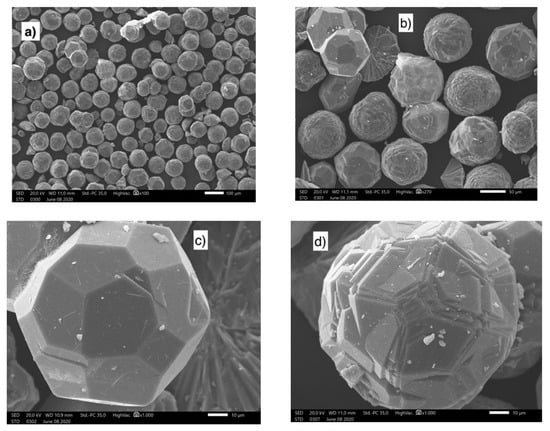
Figure 13.
SEM images at different magnifications of ANA zeolites obtained from the aluminum waste [102]. (a,b) demonstrated the existence of particles with a diameter of less than 100 μm. (c,d) showed pentagonal faces zeolite.
4.3.2. Application in Construction Materials
Lin et al. explored the use of recycled aluminum salt slag (RASS) as aggregate combined with recycled concrete through the alkali-activation method for stone column materials [103]. Both materials were stabilized by fly ash and ground granulated blast furnace slag to improve soft soils. Increasing the RASS content decreased the unconfined compressive strength [104] of the mixtures; however, the mixture of recycled concrete aggregate with 5% alkali-activated fly ash and slag met the minimum unconfined compressive strength [104] requirement for ground improvement.
4.4. Spent Carbon Cathode
Spent carbon cathode is considered as hazardous solid wastes from aluminum production. Therefore, studies are carried out to investigate cost-effective and environmentally friendly purification techniques for separating carbon and subsequently using it efficiently. Most of the research is on metal recovery (Figure 14), which will be elaborated on in the next subsection. Additionally, the use of spent carbon cathode for other applications will also be discussed.
Figure 14.
Recovery of various metal from spent carbon cathode.
4.4.1. Metal Recovery
Carbon recovery from spent carbon cathode is studied by several researchers. For example, Yuan et al. [105] synthesized silicon carbide with silicon dioxide at 1600 °C, giving the spent cathode carbon regeneration value, and obtained high-purity carbon powder through ultrasonic leaching. High-purity recovered carbon was also obtained from the research of Lu et al. [106] to be used directly as the anode for Na-ion batteries in order to benefit from these characteristics and reuse the high value-added carbon material. The capacitive nature of Na+ in recovered carbon material allows the electrode to demonstrate notable electrochemical performance in the electrolyte solvent. However, there is a challenge of physical leaching treatments that produced low carbon extraction [107]. Other metals, such as iron and silicon, have been recovered from spent carbon cathodes for the treatment of red mud [108]. Fluoride can also be recovered from spent carbon cathodes. Robshaw et al. [109] used chelating resin loaded with lanthanum ions. The resin showed great promise in recovering fluoride from aqueous waste streams and demonstrated a large defluoridation capacity.
4.4.2. Other Applications
A hydrothermal acid-leaching technique was employed by Xiao et al. [110] to separate the various components in spent carbon cathode. While carbon and silicon remained in the filter residue, impurity elements like Al, F, and Fe entered the solution. Additionally, they used the carbothermal reduction method to create silicon carbide products. Spent carbon cathode has a highly expanded graphitic structure by nature [111]. This can be advantageous in preparing the anodes of lithium-ion batteries [112]. The anode material used was the purified graphitized spent carbon cathode which has better cycle stability. Carbon anodes for aluminum electrolysis were made by Yao et al. [113] using the recovered carbon material from roasting molten salt. It was performed better compared to carbon anodes made entirely of petroleum coke. This suggested that SCC might be utilized as raw materials to make carbon anodes for aluminum electrolysis. Zhi et al. [114] treated the spent carbon cathode with water washing process, and recovered large amount of sodium fluoride (NaF). Li et al. [115] investigated the thermal behavior of spent carbon cathode block blended with molten salts. It was discovered that NaCl-Na2CO3 binary molten salts were efficient in lowering the fluoride content of spent carbon cathode blocks and preserving the energy needed for the thermal treatment procedure.
4.5. Bauxite Residue
The proposed solution to bauxite residue stockpiling involves creating comprehensive use technology or transforming it into a secondary resource. Since the 1950s, multiple research projects have focused on the disposal and use of bauxite, given its distinctive physical and chemical characteristics, such as high pH and the presence of trace amounts of heavy metals, which can pose a serious environmental risk [116]. The production of cleaner bauxite residue is now a primary focus for the aluminum industry. The subsequent subsections will discuss the waste management of bauxite residue, neutralization of impurities, extraction of metal oxide and rare earth elements and the use of this residue in environmental, energy, coating, construction, and geopolymer applications.
4.5.1. Waste Management Methods
Wet processing, dry processing, and semi-dry processing are the three standard methods for disposing of bauxite residue in landfills [117,118,119]. During wet processing, bauxite residue is first washed in a thickener to facilitate the sedimentation of solids and produce a residue slurry with a solid content of 15% to 30%. The resulting slurry is then pumped into a storage yard comprising earthworks, tailing ponds, and dams to allow the solids to settle. The liquid supernatant in the storage yard is collected and used to generate recovered caustic soda [120]. However, the disposal of the dilute residue slurry after wet processing poses environmental risks to nearby areas/communities because of the potential release of caustic and highly alkaline (pH >12) liquid into the subsoil water [121].
The dry-stacking method and dry cake disposal have been adopted by many aluminum production plants because of their reduced spatial footprint and lower potential for leaks, as landfills are becoming scarcer and space-limited [122]. In this process, the bauxite residue is pre-thickened to produce a residue slurry with a solid content of over 50%, which is then deposited in layers ranging in thickness from 0.4 to 0.7 m. Drum filters or plate and frame filter presses are used to achieve the high solid content of the residue slurry during filtration. Bulldozers turn up the bottom layer to expedite drying and minimize dust emissions. Successive layers of residue slurry are placed on top to expand the spatial storage capacity, thereby reducing the landfill space occupied by bauxite residues [123].
Semi-dry processing refers to the co-disposal of bauxite residue slurry generated by the Bayer and sintering processes. The residue typically exhibits a low permeability coefficient and high shear yield strength because of its hydration and hardening capabilities [124]. Thus, the dried residue from the sintering process can directly replace clay in the construction of dams.
4.5.2. Neutralization of Bauxite Residues
Efforts have been made to partially neutralize bauxite residues to mitigate the environmental impact of their high alkalinity. These approaches include seawater neutralization for plants near the sea, carbonation treatment, acid neutralization, sintering, and bioleaching. The addition of seawater to caustic red mud decreases the pH, leading to the precipitation of minerals such as hydroxycarbonate, carbonate, and hydroxide [125]. Seawater neutralization converts readily soluble, strongly caustic wastes into less soluble, weakly alkaline solids, although it does not completely eliminate hydroxide. The waste’s carbonate and bicarbonate alkalinity are primarily removed through reactions of hydroxide with calcium to form aragonite and calcite [126]. Neutralization is considered complete once the liquid from the treated bauxite residue has a pH below 9.0 and a total alkalinity of less than 200 mg/L (measured as calcium carbonate equivalent alkalinity), allowing the decantation of seawater-neutralized bauxite residue to be safely discharged into the marine environment [127].
Red mud neutralization by carbon dioxide gas has been studied [104]. Gaseous CO2 or flue gas containing CO32− is bubbled through aqueous slurries to form carbonic acid in the aqueous phase [128]. This carbonic acid reacts with the basic components of red mud, thereby lowering its pH. However, only a fraction of the alkaline material in red mud is neutralized by gaseous CO2 within the short contact times necessitated by industrial process rates. Consequently, although the pH of the aqueous phase decreases rapidly upon exposure to CO2, it quickly returns to unacceptable levels as more alkaline material leaches from the mud. Given that the pH of water exposed to gaseous CO2 is unlikely to fall below approximately 5.5, the neutralization rate of the solids in the aqueous slurry typically does not meet industrial requirements. Researchers have, therefore, explored the use of high-pressure liquid CO2 instead of its vapor phase for more effectively reducing the pH of red mud [129], including the approach of neutralizing red mud with CO2 over multiple cycles [130].
A variety of aqueous acidic solutions, including acidic industrial wastewater, have been considered for neutralizing residual alkalinity. Several studies have assessed the feasibility of treating bauxite residues, such as the Kwinana red mud slurry, with acid [131]. However, the complete neutralization of the residue requires large volumes of reagent, even when using spent (waste) acid, leading to relatively high costs. Additionally, acid treatment introduces significant quantities of impurities into the process’s water stream, such as sulfate in the case of sulfuric acid and chloride in the case of hydrochloric acid. Therefore, reintroducing any water from the residue deposits back into the production process is likely to be unacceptable without further treatments to eliminate these added impurities. The treatment of red mud with acidic spent pickling solutions (SPSs) derived from the steelmaking process produces a coagulant—a mixture of aluminum and iron salts—for wastewater treatment. Although sintering the residue can control all leachable soda, this process can be prohibitively expensive given the increased energy consumption required for high-temperature sintering of red mud. Alcoa of Australia has implemented a bioremediation of bauxite residue in Western Australia by adding organic substrate to the red mud to cultivate microorganisms. These microorganisms produce various organic acids and, in some cases, CO2, which neutralizes the mud [131].
Efforts to find viable solutions for bauxite residue disposal continue, including efforts to improve storage, monitoring, and safety standards to reduce environmental impacts, develop techniques to enhance storage yard reclamation potential, and promote reusability methods to decrease the volume of accumulated bauxite residue [132]. Despite these efforts, red mud still occupies a significant amount of landfill space, and sustainable waste management strategies are needed to use red mud as a secondary resource material.
4.5.3. Extraction of Metal Oxides from Bauxite Residue
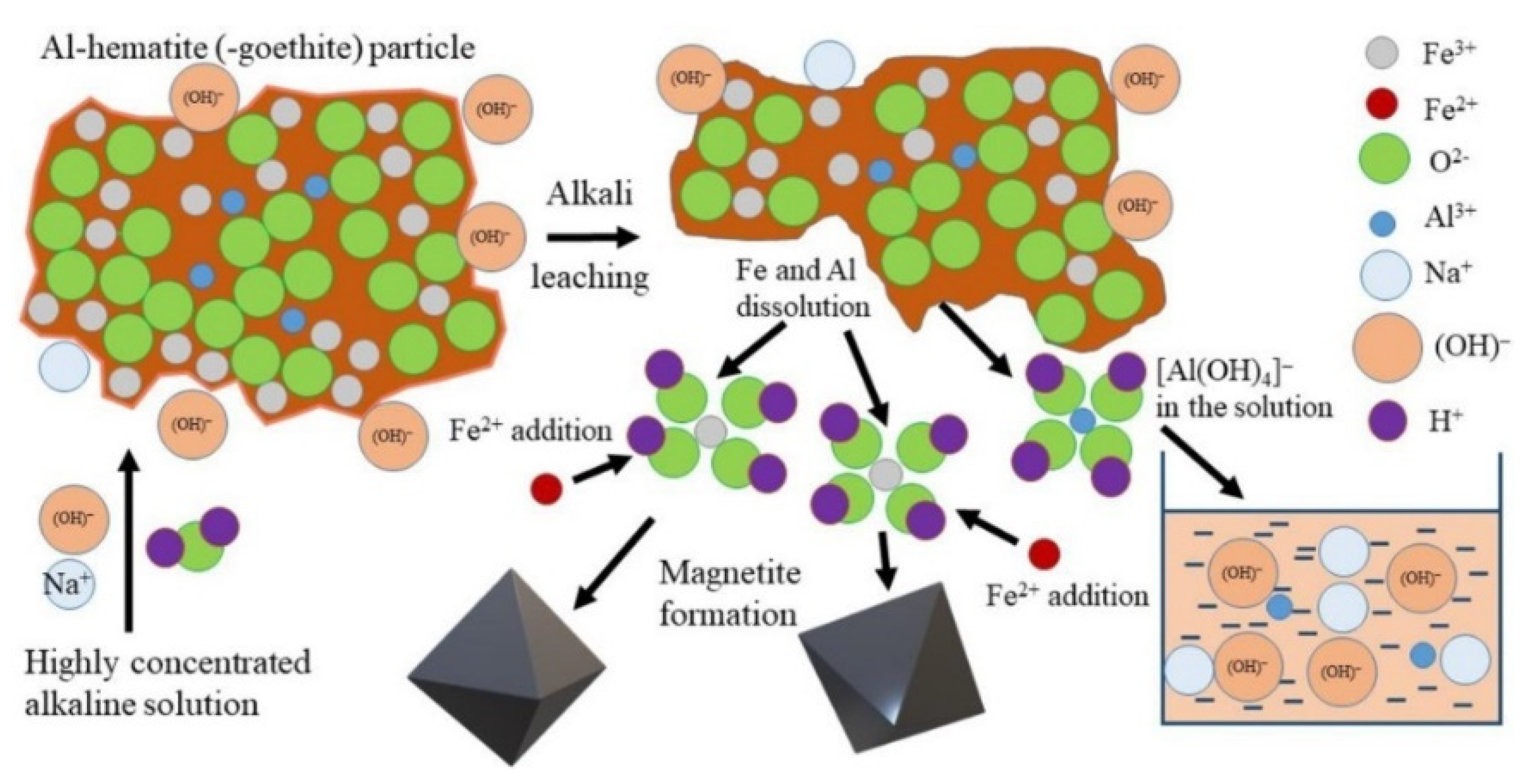
Bauxite residue primarily consists of Fe2O3, Al2O3, SiO2, TiO2, Na2O, and CaO (Table 1). Various methods exist for extracting these oxides. The high Na2O content limits the potential application of red mud for iron-containing products, as it is not easily soluble in water, resulting in high alkalinity. However, the hydrochemical conversion of goethite to magnetite has been proposed as a solution to this issue [133]. High-iron bauxite residue leaching can occur in the presence of Fe2+ (Figure 15), as this ion facilitates the extraction of Al from Al-hematite and Al-goethite via the precipitation of magnetite from the solution. The simultaneous extraction of Al and Na results in a product containing more than 96% Fe3O4.
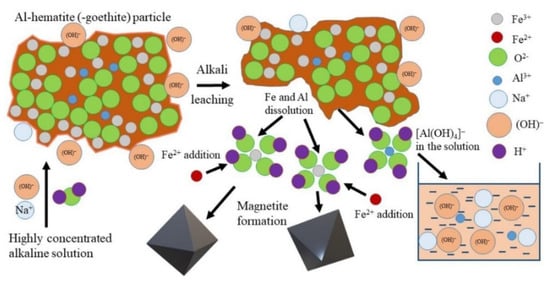
Figure 15.
The schematic mechanism of high-iron bauxite residue leaching by a highly concentrated NaOH solution in the presence of Fe2+ [133].
Li et al. proposed a method for extracting Al2O3 and TiO2 from red mud smelting separation slag through mineral phase reconstruction conducted under an air atmosphere [134]. Al2O3 and SiO2 are converted into alkaline-soluble NaAlO2 and Ca2SiO4 by alkaline hydroxide roasting. The resulting NaAlO2 solution can serve as a source for extracting alumina, with more than 80% of Al2O3 selectively dissolved in a 95 °C NaOH leaching solution. Subsequently, SiO2 is extracted from the residue using an HCl solution, yielding a solution containing SiO2 and a concentrated residue of undissolved CaO and TiO2. Hydrochloric acid leaching was used to recover the latter.
A novel process for the coupled treatment of coal gangue and red mud has also been developed [135]. Guo et al. demonstrated that red mud can adjust the Al/Si molar ratio of coal gangue to an appropriate level and that the Na2O in the red mud can partially substitute for the Na2CO3 required for coal gangue activation, thereby reducing the amount of Na2CO3 consumed from 100% to 12.1–20.5%.
4.5.4. Extraction of Rare Earth Metals from Red Mud
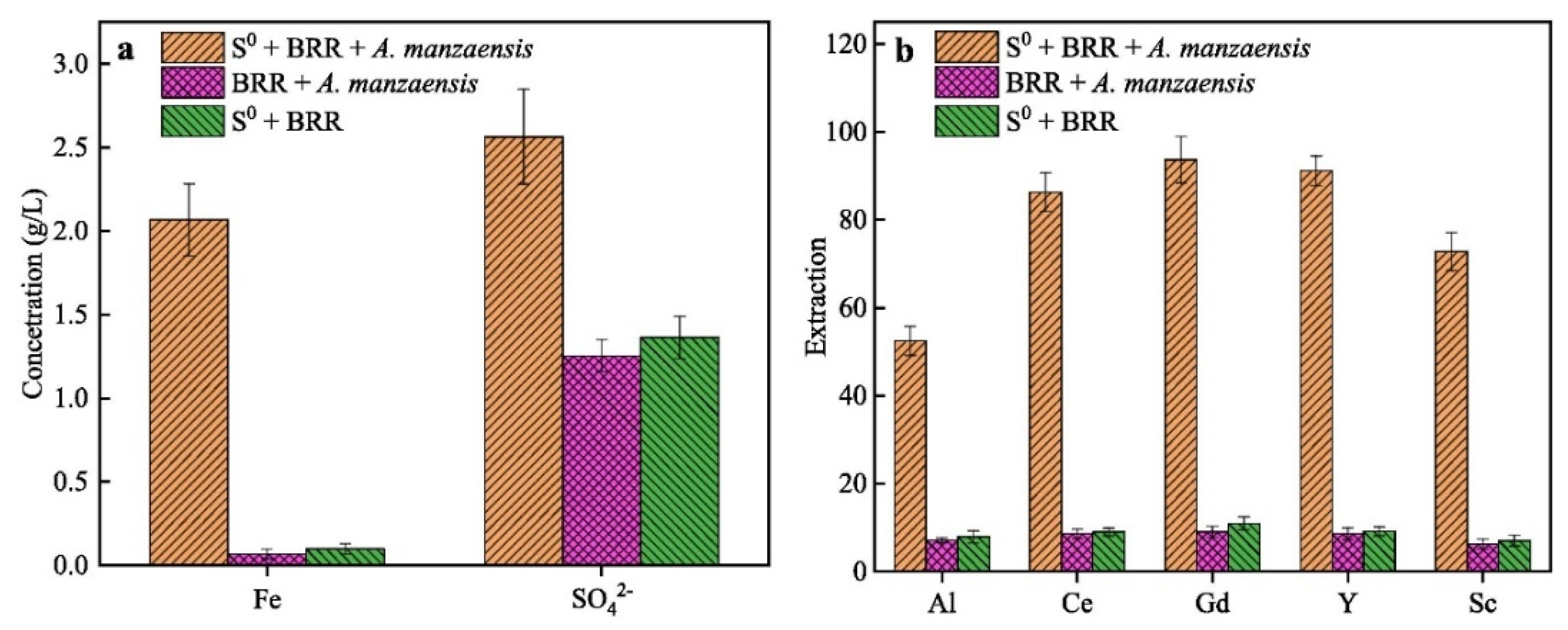
In addition to the extraction of metal oxides from red mud, several rare earth elements (REEs) may also be extracted [136]. Small amounts of Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu are found in red muds [137]. Red mud has been identified as one of the promising sources of scandium (Sc) [138]. Dai et al. developed a low-cost and environmentally friendly biosorbent for Sc extraction [139]. In their study, H3PO4-activated biochar (P40s) produced from pitaya peels was used for Sc adsorption and recovery from red mud. This biochar demonstrated optimal reusability after five adsorption/desorption cycles. The extraction of Al and REEs from red mud via aerobic and anaerobic bi-stage bioleaching has also been explored [140] by Zhang et al., which achieved the highest extraction rates for Al, Ce, Y, and Sc (Figure 16). Thus, the release of valuable elements from bioleached red mud residue can be enhanced by bio-oxidation under anaerobic conditions.
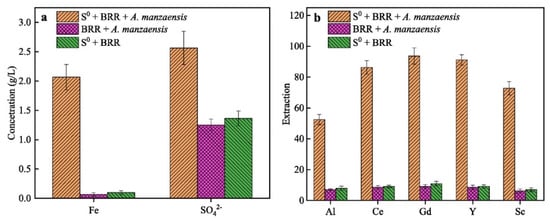
Figure 16.
(a) [Fe2+] and [SO42−] concentrations and (b) the extraction rates of Al, Ce, Gd, Y, and Sc during the anaerobic bioleaching of red mud using Acidianus manzaensis [140].
Roasting can also remove calcium and obtain rare earth element (REE) oxides from red mud; this process is followed by the selective mixing of sulfuric acid with REE oxides. Calcium, aluminum, and iron components can also be recovered at each stage [141].
4.5.5. Bauxite Residues for Environmental Applications
Carbon capture and storage (CCS) technologies are a significant area of research aimed at reducing greenhouse gas emissions. Direct mineral carbonation is a CCS technology that offers the benefits of permanence, affordability, and promising on-site options, while also enhancing the environmental quality of waste. In recent years, red mud has been explored for gas cleaning treatments.
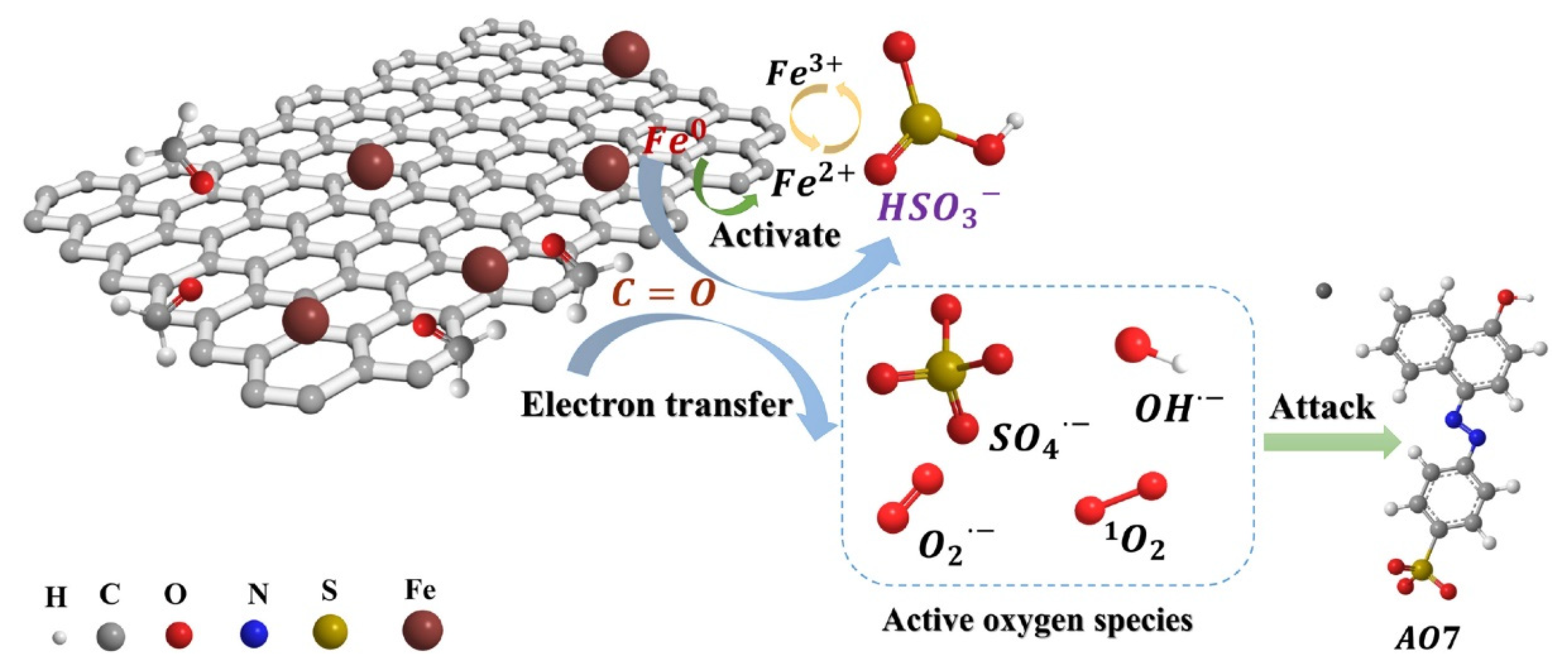
The use of red mud in reducing concentrations of residual antibiotics in composting has been investigated [142]. Composting has been found to decrease the concentrations of most residual antibiotics, as well as the abundance of antibiotic resistance genes and mobile genetic elements. In another application, Li et al. synthesized zero-valent iron (ZVI) biochar composites from red mud and ginkgo leaves [143]. Ginkgo leaves contain active groups that have a strong adsorption effect on heavy metal ions [144]. Iron phases in red mud were transformed from Fe2O3 to Fe0 after pyrolysis at 800 °C with a red mud:ginkgo leaf mass ratio of 2:1; the iron phase and ketonic functional groups (C=O) were identified as two key active sites (Figure 17). The synthesized composites were effective in removing bisulfate and acid orange 7.
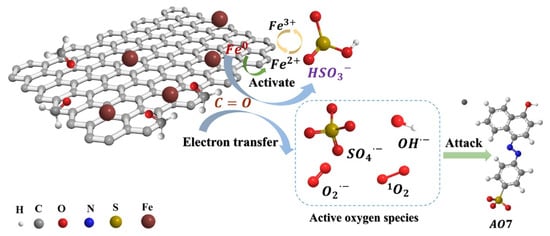
Figure 17.
Acid orange 7 (AO7) degradation in the biochar composites [143]; both radical and nonradical processes broke down the adsorbed AO7 molecules. Notably, SO42− played a significant part in the degradation process.
Bai et al. proposed the use of red mud for purifying heavy metal pollutants in water [145]. The adsorption strength for Pb, Cd, and Cu can be enhanced by increasing the liquid temperature. Guo et al. synthesized biochar-supported red mud catalysts for the degradation of COVID 19–related drugs such as arbidol and acyclovir [146]. The presence of functional groups in red mud catalysts, which contain oxygen and Fe species (Fe0 and Fe3O4) as well as Ca2+ ions, was crucial for the removal of arbidol.
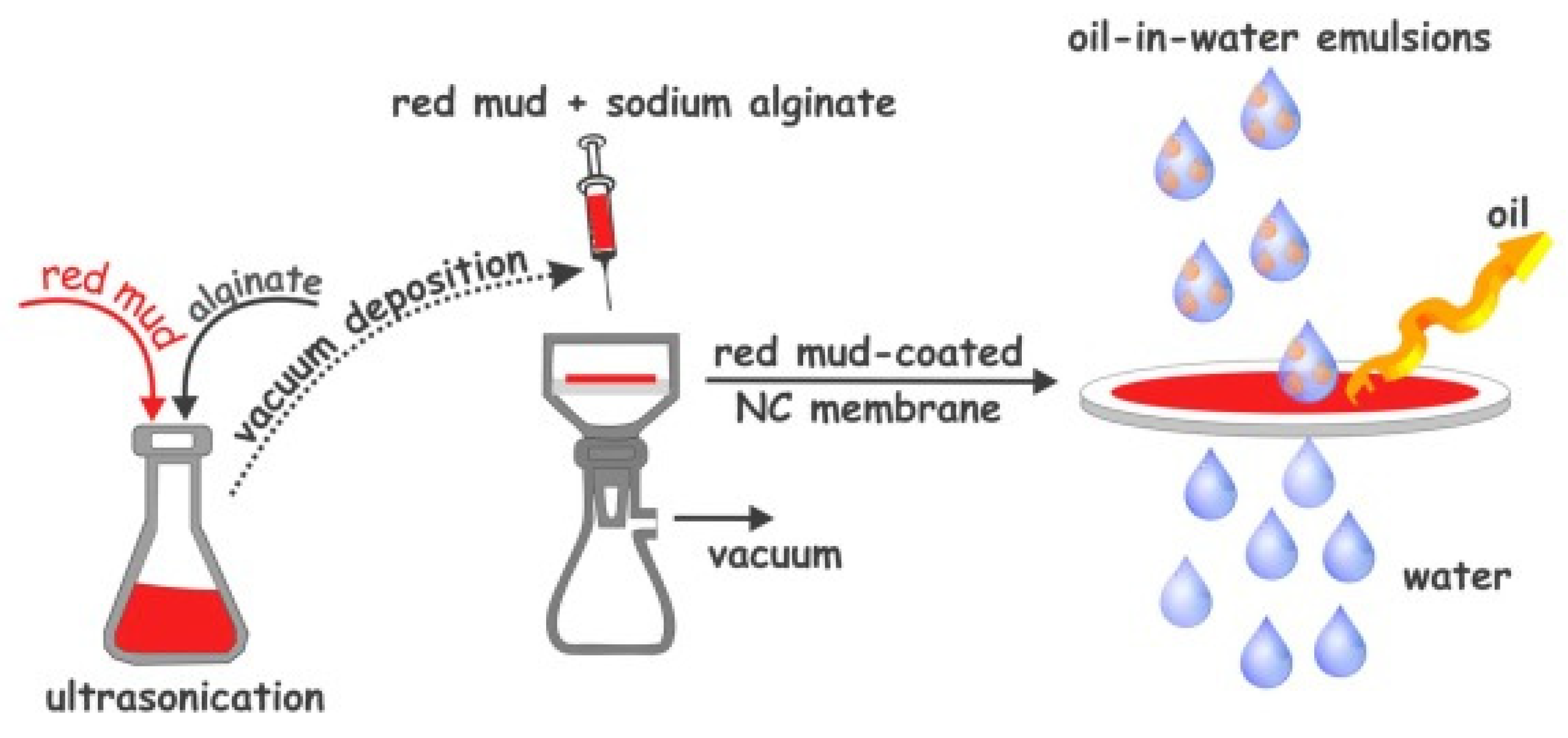
The efficient treatment of industrial oily wastewater before discharge into receiving bodies is critical for preventing environmental pollution and protecting human health. Membranes with superwettable surfaces have been regarded as promising materials for the separation of oil/water emulsions [147]. These surfaces include nitrocellulose membranes coated with red mud (Figure 18).
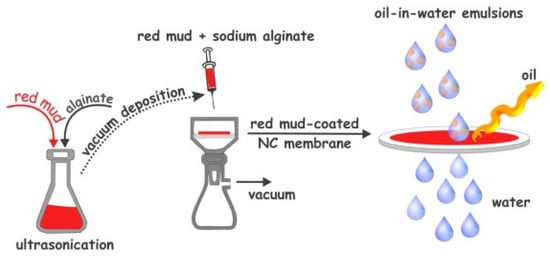
Figure 18.
Schematic illustration of the fabrication of red-mud-coated-nitrocellulose (NC) membrane for oil-in-water emulsion separation [147].
4.5.6. Bauxite Residue as Construction Material
Portland cement is the most widely used type of cement for general construction purposes. Most CO2 emissions from the cement industry occur through the process of decarbonizing limestone during the creation of clinker. Given the rising demand for infrastructure and sanitation, the primary method for reducing cement use (and thus greenhouse gas emissions) in the civil construction sector involves the partial or complete replacement of Portland cement clinker. Several studies have explored producing various types of cement from red mud [148,149]. The moderate amounts of SiO2 and Al2O3 in red mud allow for its potential use as a material for mortar, concrete, bricks, aggregates, and roofing. Zeng et al. discussed the production of a permeable red mud–based brick using a fully automatic production line for ecological RM permeable bricks (Figure 19) [150]. The company’s daily output was 3000 m2, and its production process could digest nearly 100,000 t of RM annually. The brick exhibited a bending strength of 6–8 MPa, exceeding the national standard of 3.2 MPa, with an RM content of up to 80%.

Figure 19.
Red mud–based permeable brick produced in Shandong Province, China [150].
Wang et al. incorporated red mud into road cement clinker [151]. For developing Portland cement for road clinker, they first de-alkalized the sintering red mud and then combined it with materials such as clay, fly ash, limestone, and sandstone. Each group of raw materials containing red mud exhibited a lower thermal decomposition temperature than the control materials; this reduction in decomposition temperature related to the accelerated carbonate decomposition process of the raw materials for roads facilitated by the addition of red mud.
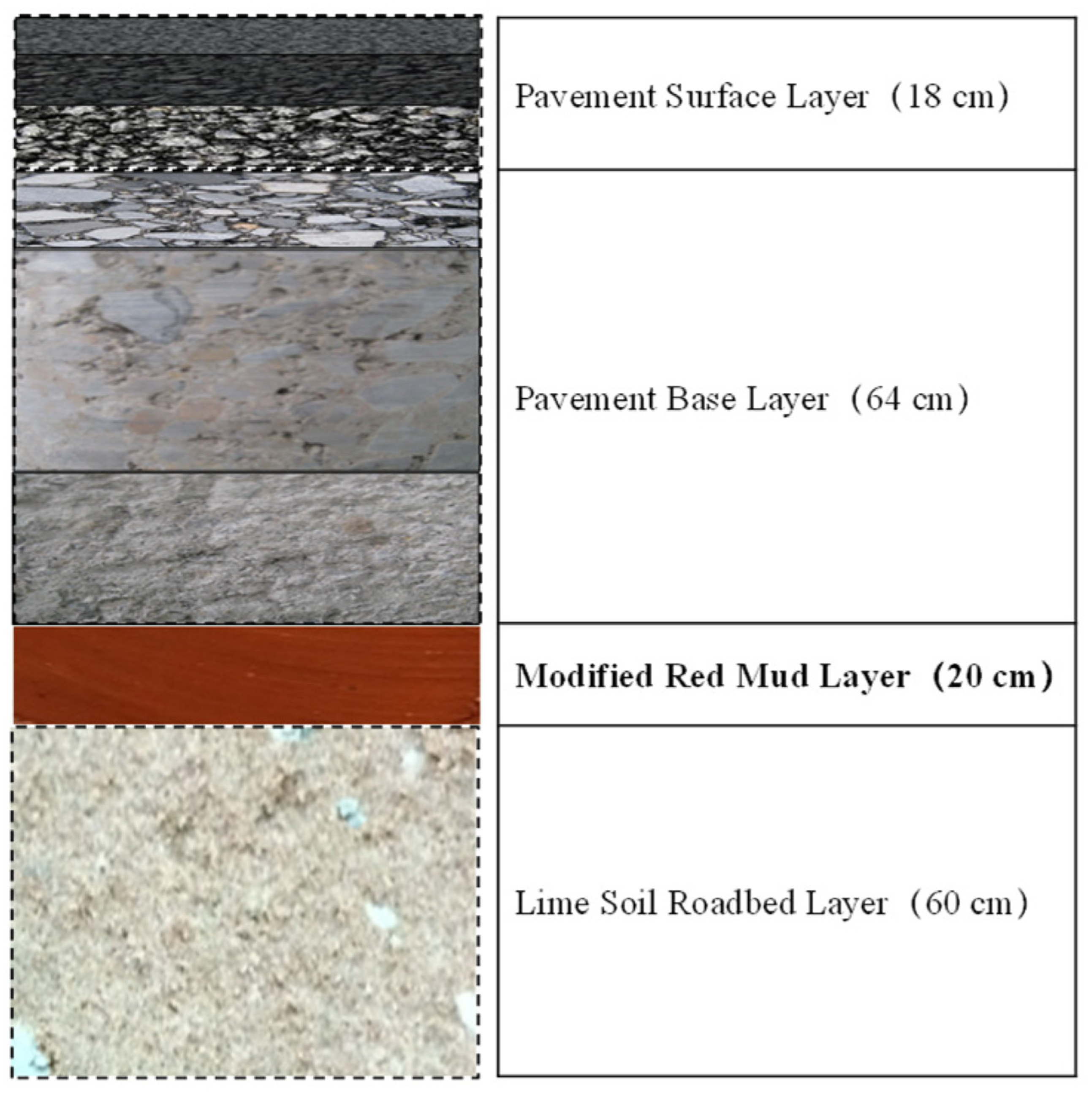
The structural characterization of red mud in roof tile manufacturing has also been investigated [152]. Increases in both firing temperature and RM content enhance the compressive strength of fired samples. Ma et al. studied modified Bayer red mud for use as a highway subgrade and assessed its performance in a large-scale application as part of a road trial for the Ji-Qing Highway Reconstruction and Expansion Project in China [153]. The emplaced subgrade spanned approximately 4900 m in length, 11 m in width, and 0.2 m in thickness (Figure 20). The modified red mud replaced the standard 0.2 m of lime-stabilized soil in the upper part of the roadbed. This modified Bayer red mud showed significant improvements in road performance. The pH of the leaching solution decreased, and the compressive strength reached up to 3 MPa within three days. Additionally, the concentration of the leached harmful components decreased by more than 70% relative to the pretreated red mud.
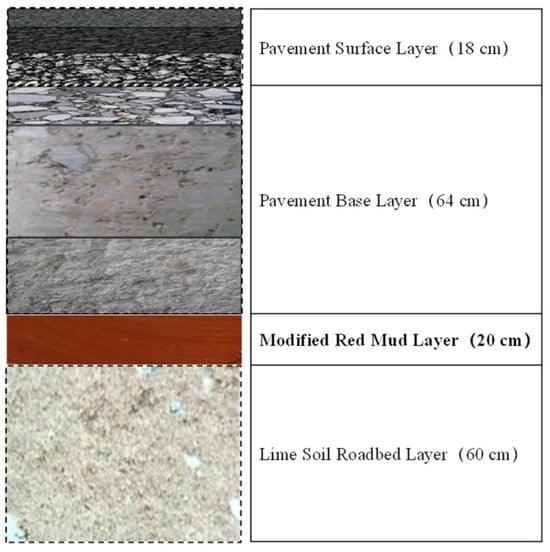
Figure 20.
Pavement structural diagram of a demonstration road in Jinan Qingdao Highway, China [153].
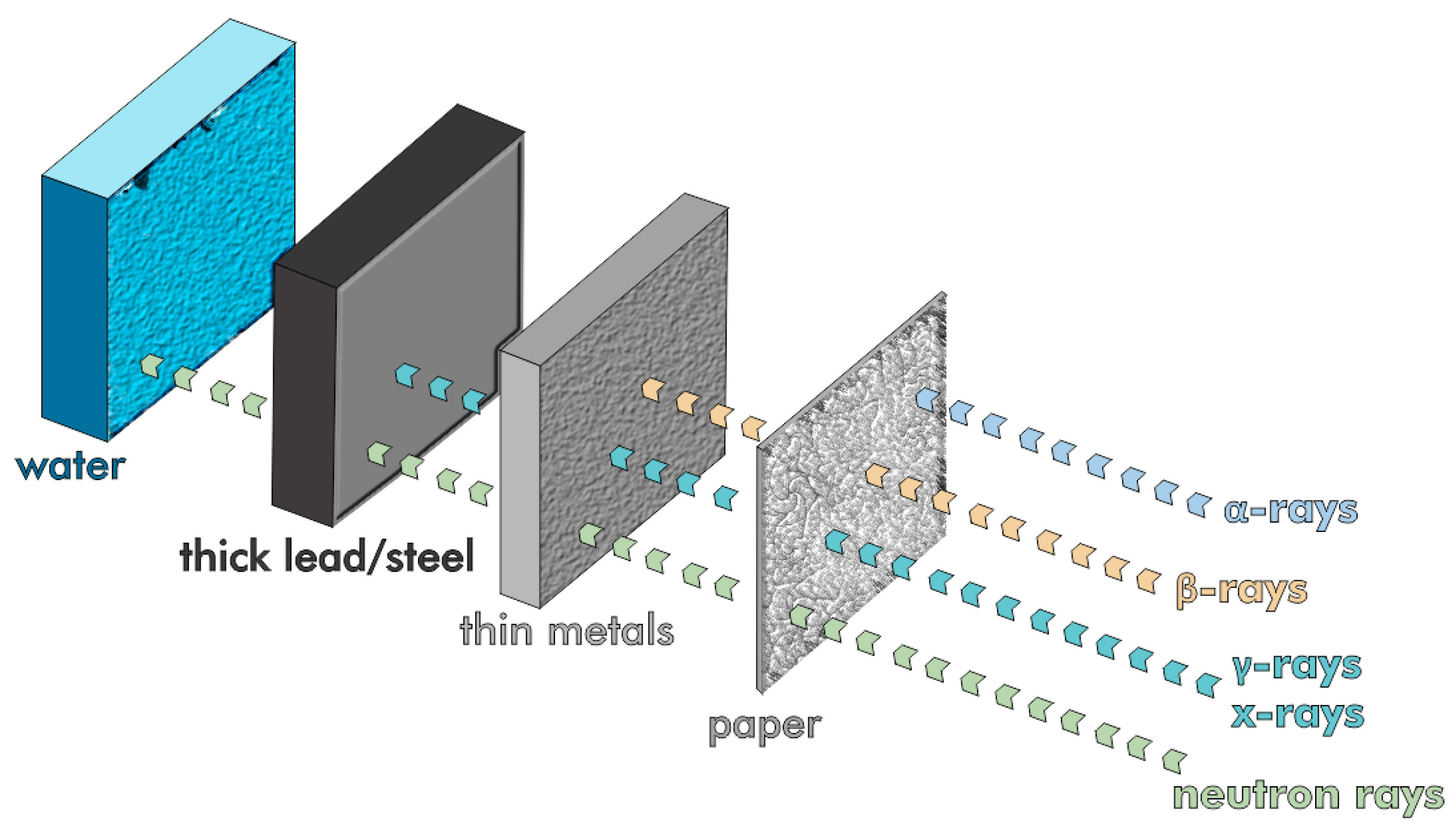
Iron and other high-atomic-number (Z) waste materials, such as steel slag and red mud, can be used to create X- and γ-ray shielding concrete [154,155]. Human skin itself blocks alpha particles, whereas beta radiation is stopped by thin sheets of wood or aluminum (Figure 21). The most challenging forms of radiation to attenuate are highly penetrative and dangerous uncharged gamma and neutron radiation.
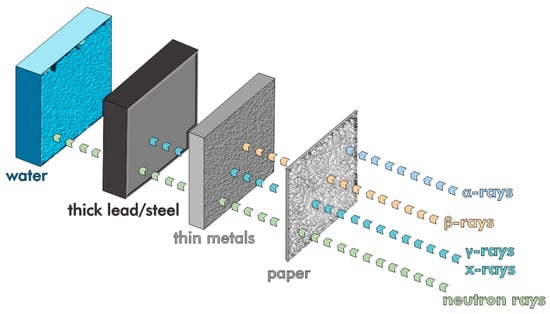
Figure 21.
The comparative penetrative power of various radiation sources.
Aside from concrete, lead is often used to shield against high-energy photons given its high atomic number and density [156]. The challenge with radiation shielding in concrete is predicting radiation attenuation because of variable moisture within the concrete. Although commonly used, lead poses significant health and environmental risks, and its heterogeneous nature complicates predictions of its effectiveness as a radiation barrier [157,158].
Agrawal et al. fabricated diagnostic X-ray shielding tiles from red mud and barium sulfate (BaSO4) with the addition of sodium hexametaphosphate (SHMP) and kaolin clay to enhance their mechanical strength [159]. The radiation attenuation of the tiles, measuring 10.3 mm and 14.7 mm in thickness, was equivalent to that of 2 mm and 2.3 mm of lead at 125 and 140 kilovoltage peak (kVp), respectively. These tiles are already being used in bone densitometry and catheterization laboratories.
4.5.7. Bauxite Residue for Geopolymers
Geopolymer is an inorganic polymer material composed of aluminosilicate and an alkali activator. The sources of Al2O3 and SiO2 can be derived from natural minerals such as metakaolin and clay, given their abundance in many regions of the world [160]. The drive for more eco-friendly materials has spurred interest in using this metal oxide-rich industrial waste. Such residual materials include fly ash, silica fume, blast furnace slag, and air-cooled slag [161,162]. Gonçalves et al. produced 3D-printed red mud/metakaolin-based geopolymers as methylene blue sorbents from water pollutants (Figure 22) [163]. Lattices with approximately 62% open porosity were achieved, showing no significant adsorption loss after 10 cycles of operation. These geopolymer composites exhibited low leachability with regard to contaminants, especially those with notable compressive strength. The development of geopolymer-based protective coverings has recently garnered increased attention. Fly ash–red mud geopolymers have been explored as coating materials for mild steel [164], with the developed material providing excellent corrosion protection, thereby helping to extend the service life of structures made of mild steel.
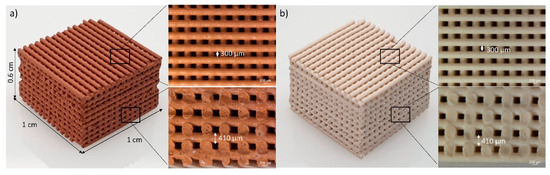
Figure 22.
Optical micrographs of 3D printed red mud with (a) 50% red mud and (b) 100% metakaolin [163].
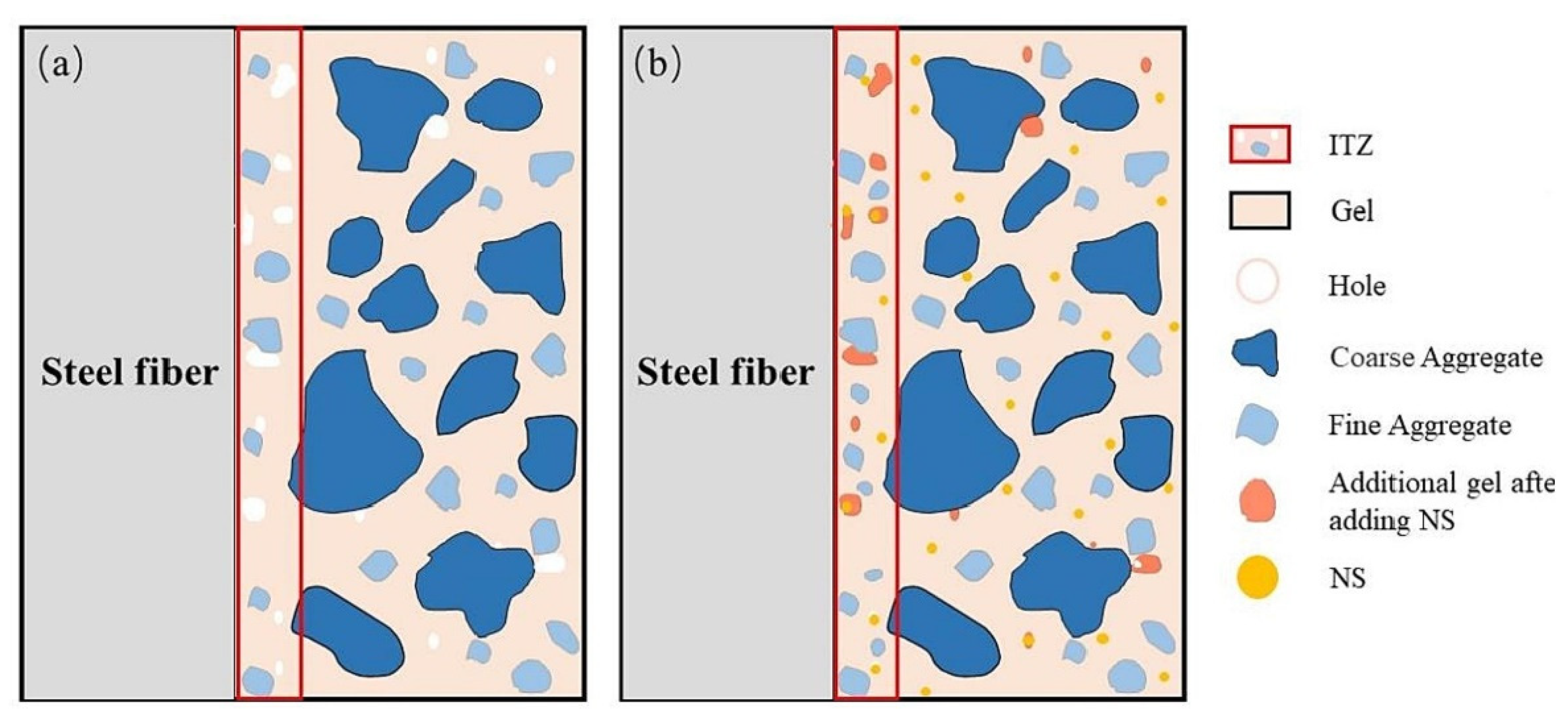
Xu et al. investigated the effects of nano-SiO2 (NS) and steel fiber on the properties of red mud–based geopolymer concrete [165]. They found that steel fibers and nano-SiO2 significantly enhanced the performance of the red mud geopolymer composite, with the most substantial improvements observed when both nano-SiO2 (NS) and steel fiber were combined. The interface transition zone of the NS-reinforced fiber matrix is depicted in Figure 23.
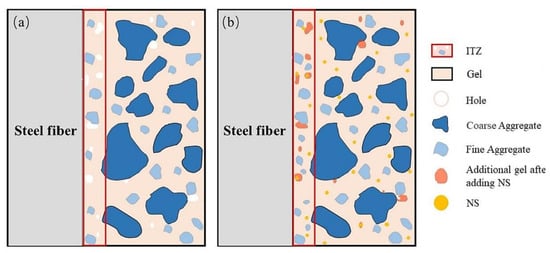
Figure 23.
A nano-SiO2-reinforced fiber matrix interfacial transition zone (ITZ); (a) without nano-SiO2 (NS) and (b) with NS [165].
The thermal-related performance of PCM (phase change material) geopolymer composites has also been evaluated [166] by Afolabi et al., which used expanded graphite (EG) to encapsulate PCMs as a composite, then vacuum-impregnated the composite into geopolymer aggregates to create an enhanced thermal wall material for use in buildings and construction. The outdoor thermal storage performance of the EG/PCM composite geopolymer wall material exceeded that of conventional materials such as cement, clay, and gypsum.
5. Conclusions
Despite recent technological advances, the disposal of aluminum dross, salt slag, and bauxite residue remains a challenge for the aluminum industry, with the problems with resource utilization of these residues and critical issues continuing to impact the feasibility of recycling and the economics of the process. Multiple applications must be implemented to reduce the volumes of generated waste. In this review, we discussed the use of these residues in several construction products, including their utility as materials for cement and concrete, as supplemental additives to Portland cement, and as geopolymers to replace cement materials. The valorization of this industrial waste requires controlled processing parameters to ensure that the finished products meet existing application standards. The effectiveness of aluminum industrial waste in new applications must be comparable to alternatives, such as steel and energy plant industrial waste, in terms of quality, price, and environmental risk. The aluminum industry must collaborate with aluminum associations and research institutions to develop and promote the potential use of residues and their large-scale reuse in a variety of applications.
Funding
This work was suppoeted by the Volet Soutien à la diffusion des travaux de recherche et de création (PSDrc 2024–2025) UQAC.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Authors thank Rio Tinto Saguenay for their support for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Davis, J.R. Alloying: Understanding the Basics; ASM International: Materials Park, OH, USA, 2001. [Google Scholar] [CrossRef]
- Aluminium. Available online: http://www.rsc.org/periodic-table/element/13/aluminium (accessed on 14 March 2023).
- Kvande, H. The Aluminum Smelting Process. J. Occup. Environ. Med. 2014, 56, S2–S4. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, M.E. Aluminum Recycling, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar] [CrossRef]
- Fridlyander, I.N.; Sister, V.G.; Grushko, O.E.; Berstenev, V.V.; Sheveleva, L.M.; Ivanova, L.A. Aluminum Alloys: Promising Materials in the Automotive Industry. Met. Sci. Heat Treat. 2002, 44, 365–370. [Google Scholar] [CrossRef]
- Purchase, C.K.; Al Zulayq, D.M.; O’Brien, B.T.; Kowalewski, M.J.; Berenjian, A.; Tarighaleslami, A.H.; Seifan, M. Circular Economy of Construction and Demolition Waste: A Literature Review on Lessons, Challenges, and Benefits. Materials 2022, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, R.L.; Gao, S. 4.1—Composition of the Continental Crust. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 1–51. [Google Scholar] [CrossRef]
- Kvande, H. Occurrence and Production of Aluminum. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Wiley Online Library: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Kamble, P.H.; Bhosale, S.M. Environmental Impact of Bauxite Mining: A Review. Int. J. Res. Appl. Sci. Eng. Technol. 2019, 7, 86–90. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Duong, L.V.; Wood, B.J.; Frost, R.L. XPS study of the major minerals in bauxite: Gibbsite, bayerite and (pseudo-)boehmite. J. Colloid Interface Sci. 2006, 296, 572–576. [Google Scholar] [CrossRef]
- Hamed, M.Y.; Silver, J.; Wilson, M.T. Studies on the reactions of ferric iron with glutathione and some related thiols. Part III. A study of the iron catalyzed oxidation of glutathione by molecular oxygen. Inorganica Chim. Acta 1983, 80, 237–244. [Google Scholar] [CrossRef]
- Investing News Network. Top 10 Aluminum-Producing Countries (Updated 2024); Investing News Network: Toronto, ON, Canada, 2024. [Google Scholar]
- China’s Aluminium Industry Now Has a Bauxite Tightness Headache. Available online: https://www.kpler.com/blog/chinas-aluminium-industry-now-has-a-bauxite-tightness-headache (accessed on 17 October 2024).
- Leading Countries Based on the Production of Alumina Worldwide in 2023. 2024. Available online: https://www.statista.com/statistics/264963/global-alumina-production-by-country/ (accessed on 17 October 2024).
- Vedanta Aluminium Expands Alumina Refining Capacity To 3.5 Million Tonnes per Annum (MTPA). Available online: https://vedantaaluminium.com/media/press-releases/list/vedanta-aluminium-expands-alumina-refining-capacity-to-3-5-million-tonnes-per-annum-mtpa/ (accessed on 17 October 2024).
- Zhao, R.; Nowicki, C.; Gosselin, L.; Duchesne, C. Energy and exergy inventory in aluminum smelter from a thermal integration point-of-view. Int. J. Energy Res. 2016, 40, 1321–1338. [Google Scholar] [CrossRef]
- Cullen, J.M.; Allwood, J.M. Mapping the Global Flow of Aluminum: From Liquid Aluminum to End-Use Goods. Environ. Sci. Technol. 2013, 47, 3057–3064. [Google Scholar] [CrossRef]
- Rodionova, I.A. The shifts in the spatial structure of the world bauxite industry and Guinea’s position in the industry. Rev. ESPACIOS 2020, 41, 11–21. [Google Scholar]
- Swain, B.; Lee, C.G.; Park, J.R. Assessment of bauxite residue as secondary resource for rare earth metal and valorization challenges: A perspective. Resour. Conserv. Recycl. Adv. 2022, 14, 200078. [Google Scholar] [CrossRef]
- Samantray, J.; Anand, A.; Dash, B.; Ghosh, M.K.; Behera, A.K. Nepheline Syenite—An Alternative Source for Potassium and Aluminium; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Bagani, M.; Balomenos, E.; Panias, D. Nepheline Syenite as an Alternative Source for Aluminum Production. Minerals 2021, 11, 734. [Google Scholar] [CrossRef]
- Terry, B. The acid decomposition of silicate minerals part I. Reactivities and modes of dissolution of silicates. Hydrometallurgy 1983, 10, 135–150. [Google Scholar] [CrossRef]
- Haddad, A.Z.; Pilgrim, C.D.; Sawvel, A.M.; Hohman, J.N.; Gadgil, A.J. On the Conversion of Bauxite Ores to Highly Activated Alumina Media for Water Remediation. Adv. Sustain. Syst. 2019, 3, 1900005. [Google Scholar] [CrossRef]
- BenSaad, L.; Hwi, K.K.; AlSereti, M.; Shahimi, M. Evaluation of the analgesic effects of Libyan fresh pomegranate fruit of Punica granatum. PharmaNutrition 2014, 2, 108–109. [Google Scholar] [CrossRef]
- Kaußen, F.M.; Friedrich, B. Methods for Alkaline Recovery of Aluminum from Bauxite Residue. J. Sustain. Metall. 2016, 2, 353–364. [Google Scholar] [CrossRef]
- Donoghue, A.M.; Frisch, N.; Olney, D. Bauxite mining and alumina refining: Process description and occupational health risks. J. Occup. Environ. Med. 2014, 56 (Suppl. S5), S12–S17. [Google Scholar] [CrossRef] [PubMed]
- Farjana, S.H.; Huda, N.; Mahmud, M.A.P. Impacts of aluminum production: A cradle to gate investigation using life-cycle assessment. Sci. Total Environ. 2019, 663, 958–970. [Google Scholar] [CrossRef]
- Crippa, M.; Solazzo, E.; Guizzardi, D.; Monforti-Ferrario, F.; Tubiello, F.N.; Leip, A. Food systems are responsible for a third of global anthropogenic GHG emissions. Nat. Food 2021, 2, 198–209. [Google Scholar] [CrossRef]
- Santamaría, L.; Korili, S.A.; Gil, A. Layered double hydroxides from slags: Closing the loop. J. Environ. Chem. Eng. 2022, 10, 106948. [Google Scholar] [CrossRef]
- Peterson, R.D.; Newton, L. Review of Aluminium Dross Processing. In Light Metals; TMS-AIME: Warrendale, PA, USA, 2002; p. 1029. [Google Scholar]
- Maung, K.N.; Yoshida, T.; Liu, G.; Lwin, C.M.; Muller, D.B.; Hashimoto, S. Assessment of secondary aluminum reserves of nations. Resour. Conserv. Recycl. 2017, 126, 34–41. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Allahverdi, A. Hazardous aluminum dross characterization and recycling strategies: A critical review. J. Environ. Manag. 2018, 223, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Wibner, S.; Antrekowitsch, H.; Meisel, T.C. Studies on the Formation and Processing of Aluminium Dross with Particular Focus on Special Metals. Metals 2021, 11, 1108. [Google Scholar] [CrossRef]
- Environmental Protection Agency An Gníomhaireacht um Chaomhnú Comhshaoil. European Waste Catalogue and Hazardous Waste List. Available online: https://v4r7y5k5.stackpathcdn.com/wp-content/uploads/2016/08/EWC_HWL.pdf (accessed on 14 March 2023).
- Zhang, L. State of the Art in Aluminum Recycling from Aluminum Dross; Light Metals: Proceedings of Sessions; TMS Annual Meeting: San Antonio, TX, USA, 2006; Volume 931. [Google Scholar]
- Das, B.R.; Dash, B.; Tripathy, B.C.; Bhattacharya, I.N.; Das, S.C. Production of η-alumina from waste aluminium dross. Miner. Eng. 2007, 20, 252–258. [Google Scholar] [CrossRef]
- Lazzaro, G.; Eltrudis, M.; Pranovi, F. Recycling of aluminium dross in electrolytic pots. Resour. Conserv. Recycl. 1994, 10, 153–159. [Google Scholar] [CrossRef]
- Stark, T.D.; Martin, J.W.; Gerbasi, G.T.; Thalhamer, T.; Gortner, R.E. Aluminum Waste Reaction Indicators in a Municipal Solid Waste Landfill. J. Geotech. Geoenvironmental Eng. 2012, 138, 252–261. [Google Scholar] [CrossRef]
- Abdulkadir, A.; Ajayi, A.; Hassan, M.I. Evaluating the Chemical Composition and the Molar Heat Capacities of a white Aluminum Dross. Energy Procedia 2015, 75, 2099–2105. [Google Scholar] [CrossRef]
- Hwang, J.; Huang, X.; Xu, Z. Recovery of Metals from Aluminum Dross and Saltcake. J. Miner. Mater. Charact. Eng. 2006, 5, 47–62. [Google Scholar] [CrossRef]
- Zhou, L.; Yao, Z.; Sun, K.; Tian, Z.; Li, J.; Zhong, Q. Methodological Review of Methods and Technology for Utilization of Spent Carbon Cathode in Aluminum Electrolysis. Energies 2024, 17, 4866. [Google Scholar] [CrossRef]
- Gao, L.; Mostaghel, S.; Ray, S.; Chattopadyay, K. Using SPL (spent pot-lining) as an alternative fuel in metallurgical furnaces. Metall. Mater. Trans. E 2016, 3, 179–188. [Google Scholar]
- Yong, Y.; Jianhang, H.; Yongkui, L.; Dapeng, Z.; Hua, W. A new method for simultaneous separation and solidification of arsenic from arsenic-bearing gypsum sludge using waste carbon cathodes. Sep. Purif. Technol. 2022, 291, 120656. [Google Scholar] [CrossRef]
- Lisbona, D.F.; Somerfield, C.; Steel, K.M. Leaching of spent pot-lining with aluminium nitrate and nitric acid: Effect of reaction conditions and thermodynamic modelling of solution speciation. Hydrometallurgy 2013, 134–135, 132–143. [Google Scholar] [CrossRef]
- Andrade-Vieira, L.; Davide, L.; Gedraite, L.; Campos, J.; Azevedo, H. Genotoxicity of SPL (spent pot lining) as measured by Tradescantia bioassays. Ecotoxicol. Environ. Saf. 2011, 74, 2065–2069. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Li, R.; Zhao, H.; Liu, W.; Lu, T.; Liu, F. Detoxification of spent cathode carbon blocks from aluminum smelters by joint controlling temperature-vacuum process. J. Clean. Prod. 2020, 249, 119370. [Google Scholar] [CrossRef]
- Yang, K.; Gong, P.; Tian, Z.; Lai, Y.; Li, J. Recycling spent carbon cathode by a roasting method and its application in Li-ion batteries anodes. J. Clean. Prod. 2020, 261, 121090. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, D.; Jiang, Y.; Zeng, B.; Zhao, C.; Zhang, M.; Zeng, B.; Zhu, X.; Su, X.; Romanovski, V.; et al. Recovery of carbon from spent carbon cathode by alkaline and acid leaching and thermal treatment and exploration of its application in lithium-ion batteries. Environ. Sci. Pollut. Res. 2023, 30, 114327–114335. [Google Scholar] [CrossRef]
- Yang, K.; Gong, P.; Tian, Z.; Peng, K.; Lai, Y. Carbon recovered from spent carbon cathode of aluminum reduction cell towards its valorisation as negative electrodes for lithium ion batteries. Diam. Relat. Mater. 2020, 109, 108062. [Google Scholar] [CrossRef]
- Wang, C.; Mao, S.; Li, L. Study on ultrasonic leaching and recovery of fluoride from spent cathode carbon of aluminum electrolysis. RSC Adv. 2023, 13, 16300–16310. [Google Scholar] [CrossRef]
- Wehr, J.B.; Fulton, I.; Menzies, N.W. Revegetation strategies for bauxite refinery residue: A case study of Alcan Gove in Northern Territory, Australia. Environ. Manag. 2006, 37, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Snars, K.; Gilkes, R.J. Evaluation of bauxite residues (red muds) of different origins for environmental applications. Appl. Clay Sci. 2009, 46, 13–20. [Google Scholar] [CrossRef]
- Cablik, V. Characterization and applications of red mud from bauxite processing. Gospod. Surowcami Miner. -Miner. Resour. Manag. 2007, 23, 27–38. [Google Scholar]
- Vachon, P.; Tyagi, R.D.; Auclair, J.C.; Wilkinson, K.J. Chemical and biological leaching of aluminum from red mud. Environ. Sci. Technol. 1994, 28, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Tsang, D.C.W.; Zhou, Y.; Rinklebe, J.; Song, H.; Kwon, E.E.; Baek, K.; Sik Ok, Y. Mechanistic insights into red mud, blast furnace slag, or metakaolin-assisted stabilization/solidification of arsenic-contaminated sediment. Environ. Int. 2019, 133, 105247. [Google Scholar] [CrossRef] [PubMed]
- Rivera, R.M.; Ulenaers, B.; Ounoughene, G.; Binnemans, K.; Van Gerven, T. Extraction of rare earths from bauxite residue (red mud) by dry digestion followed by water leaching. Miner. Eng. 2018, 119, 82–92. [Google Scholar] [CrossRef]
- Mohapatra, B.; Rao, M.; Rao, R.B.; Paul, A. Charactertstics of red mud generated at NALCO refinery, Damanjodi, India. Light Met. 2000, 2000, 161–165. [Google Scholar]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of Rare Earths and Major Metals from Bauxite Residue (Red Mud) by Alkali Roasting, Smelting, and Leaching. J. Sustain. Metall. 2017, 3, 393–404. [Google Scholar] [CrossRef]
- Agrawal, S.; Rayapudi, V.; Dhawan, N. Comparison of microwave and conventional carbothermal reduction of red mud for recovery of iron values. Miner. Eng. 2019, 132, 202–210. [Google Scholar] [CrossRef]
- Harmaji, A.; Sunendar, B. Utilization of Fly Ash, Red Mud, and Electric Arc Furnace Dust Slag for Geopolymer. Mater. Sci. Forum 2016, 841, 157–161. [Google Scholar] [CrossRef]
- Ramdhani, E.P.; Wahyuni, T.; Ni’mah, Y.L.; Suprapto, S.; Prasetyoko, D. Extraction of Alumina from Red Mud for Synthesis of Mesoporous Alumina by Adding CTABr as Mesoporous Directing Agent. Indones. J. Chem. 2018, 18, 337–343. [Google Scholar] [CrossRef]
- Deihimi, N.; Irannajad, M.; Rezai, B. Characterization studies of red mud modification processes as adsorbent for enhancing ferricyanide removal. J. Environ. Manag. 2018, 206, 266–275. [Google Scholar] [CrossRef]
- Xenidis, A.; Harokopou, A.D.; Mylona, E.; Brofas, G. Modifying alumina red mud to support a revegetation cover. JOM 2005, 57, 42–46. [Google Scholar] [CrossRef]
- Sglavo, V.M.; Campostrini, R.; Maurina, S.; Carturan, G.; Monagheddu, M.; Budroni, G.; Cocco, G. Bauxite ‘red mud’ in the ceramic industry. Part 1: Thermal behaviour. J. Eur. Ceram. Soc. 2000, 20, 235–244. [Google Scholar] [CrossRef]
- Boyarintsev, A.V.; Aung, H.Y.; Stepanov, S.I.; Shoustikov, A.A.; Ivanov, P.I.; Giganov, V.G. Evaluation of Main Factors for Improvement of the Scandium Leaching Process from Russian Bauxite Residue (Red Mud) in Carbonate Media. ACS Omega 2022, 7, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Chaikin, L.; Shoppert, A.; Valeev, D.; Loginova, I.; Napol’skikh, J. Concentration of Rare Earth Elements (Sc, Y, La, Ce, Nd, Sm) in Bauxite Residue (Red Mud) Obtained by Water and Alkali Leaching of Bauxite Sintering Dust. Minerals 2020, 10, 500. [Google Scholar] [CrossRef]
- Valeev, D.; Zinoveev, D.; Kondratiev, A.; Lubyanoi, D.; Pankratov, D. Reductive Smelting of Neutralized Red Mud for Iron Recovery and Produced Pig Iron for Heat-Resistant Castings. Metals 2020, 10, 32. [Google Scholar] [CrossRef]
- Boeva, N.M.; Slukin, A.D.; Makarova, M.A.; Shipilova, E.S.; Melnikov, P.P.; Vnuchkov, D.A.; Zhegallo, E.A.; Zaitseva, L.V.; Bortnikov, N.S. Bauxites of the Tatarka Deposit (Yenisei Ridge, Russia): The First Evidence of a Contact-Karst Origin. Dokl. Earth Sci. 2023, 513, 1173–1181. [Google Scholar] [CrossRef]
- Srikanth, S.; Ray, A.K.; Bandopadhyay, A.; Ravikumar, B.; Jha, A. Phase Constitution During Sintering of Red Mud and Red Mud–Fly Ash Mixtures. J. Am. Ceram. Soc. 2005, 88, 2396–2401. [Google Scholar] [CrossRef]
- Mikulionok, I.O. A state of art and prospects of red mud management. Energy Technol. Resour. Sav. 2024, 79, 62–87. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Wang, K.-T.; Tang, Q.; Cui, X.-M. Synthesis and characterization of low temperature (<800 °C) ceramics from red mud geopolymer precursor. Constr. Build. Mater. 2017, 131, 564–573. [Google Scholar] [CrossRef]
- Mathisen, A.; Sørensen, H.; Melaaen, M.; Müller, G.-I. Investigation into Optimal CO2 Concentration for CO2 Capture from Aluminium Production. Energy Procedia 2013, 37, 7168–7175. [Google Scholar] [CrossRef]
- D’Adamo, I.; Falcone, P.M.; Martin, M.; Rosa, P. A Sustainable Revolution: Let’s Go Sustainable to Get Our Globe Cleaner. Sustainability 2020, 12, 4387. [Google Scholar] [CrossRef]
- Falcone, P.M. Tourism-Based Circular Economy in Salento (South Italy): A SWOT-ANP Analysis. Soc. Sci. 2019, 8, 216. [Google Scholar] [CrossRef]
- Nowicki, C.; Gosselin, L. An Overview of Opportunities for Waste Heat Recovery and Thermal Integration in the Primary Aluminum Industry. JOM 2012, 64, 990–996. [Google Scholar] [CrossRef]
- Pocola, A.; Serban, A.; Balan, M. Complex and Efficient Waste Heat Recovery System in Aluminum Foundry. Energy Procedia 2017, 112, 504–509. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, N.; Jing, G. Performance analysis of ORC power generation system with low-temperature waste heat of aluminum reduction cell. Physics Procedia 2012, 24, 546–553. [Google Scholar] [CrossRef]
- Yang, Y.; Tao, W.; Wang, Z.; Shi, Z. A Numerical Approach on Waste Heat Recovery through Sidewall Heat-Exchanging in an Aluminum Electrolysis Cell. Adv. Mater. Sci. Eng. 2021, 2021, 3573306. [Google Scholar] [CrossRef]
- Jouhara, H.; Nieto, N.; Egilegor, B.; Zuazua, J.; González, E.; Yebra, I.; Igesias, A.; Delpech, B.; Almahmoud, S.; Brough, D.; et al. Waste heat recovery solution based on a heat pipe heat exchanger for the aluminium die casting industry. Energy 2023, 266, 126459. [Google Scholar] [CrossRef]
- Egilegor, B.; Jouhara, H.; Zuazua, J.; Al-Mansour, F.; Plesnik, K.; Montorsi, L.; Manzini, L. ETEKINA: Analysis of the potential for waste heat recovery in three sectors: Aluminium low pressure die casting, steel sector and ceramic tiles manufacturing sector. Int. J. Thermofluids 2020, 1–2, 100002. [Google Scholar] [CrossRef]
- Lv, H.; Xie, M.; Wu, Z.; Li, L.; Yang, R.; Han, J.; Liu, F.; Zhao, H. Effective Extraction of the Al Element from Secondary Aluminum Dross Using a Combined Dry Pressing and Alkaline Roasting Process. Materials 2022, 15, 5686. [Google Scholar] [CrossRef]
- Spoel, H.; Zebedee, W.A. The hot aluminum dross recycling (HDR) system. In Proceedings of the Conference: Annual Meeting and Exhibition of the Minerals, Metals and Materials Society (TMS), Anaheim, CA, USA, 4–8 February 1996. [Google Scholar]
- Zuo, Z.; Lv, H.; Li, R.; Liu, F.; Zhao, H. A new approach to recover the valuable elements in black aluminum dross. Resour. Conserv. Recycl. 2021, 174, 105768. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, K.; Li, H.; Zhang, X.; Jiang, Y. Novel SCMs produced by the calcination of secondary aluminium dross with dolomite and their potential usage in cemented paste backfill. Constr. Build. Mater. 2023, 365, 130119. [Google Scholar] [CrossRef]
- Ercoli, R.; Laskowska, D.; Nguyen, V.V.; Le, V.S.; Louda, P.; Łoś, P.; Ciemnicka, J.; Prałat, K.; Renzulli, A.; Paris, E.; et al. Mechanical and Thermal Properties of Geopolymer Foams (GFs) Doped with By-Products of the Secondary Aluminum Industry. Polymers 2022, 14, 703. [Google Scholar] [CrossRef]
- Dirisu, J.O.; Oyedepo, S.O.; Fayomi, O.S.I.; Akinlabi, E.T. Development of Silicate Aluminium Dross Composites for Sustainable Building Ceilings. Silicon 2021, 13, 1979–1991. [Google Scholar] [CrossRef]
- Shehab, E.; Meiirbekov, A.; Amantayeva, A.; Tokbolat, S. Cost Modelling for Recycling Fiber-Reinforced Composites: State-of-the-Art and Future Research. Polymers 2023, 15, 150. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.A.; Nassar, A.H.; Zawrah, M.F. In-situ formation of composite having hard outer layer based on aluminum dross reinforced by SiC and TiO2. Constr. Build. Mater. 2020, 248, 118638. [Google Scholar] [CrossRef]
- Heo, J.H.; Park, J.H. Thermochemical analysis for the reduction behavior of FeO in EAF slag via Aluminothermic Smelting Reduction (ASR) process: Part II. Effect of aluminum dross and lime fluxing on Fe and Mn recovery. Calphad 2017, 58, 229–238. [Google Scholar] [CrossRef]
- Beheshti, R.; Moosberg-Bustnes, J.; Akhtar, S.; Aune, R.E. Black Dross Processing: Utilization of Black Dross in the Production of a Ladle Fluxing Agent. J. Sustain. Metall. 2017, 3, 265–273. [Google Scholar] [CrossRef]
- Ewais, E.M.M.; Besisa, N.H.A. Tailoring of magnesium aluminum titanate based ceramics from aluminum dross. Mater. Des. 2018, 141, 110–119. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Z.; Han, Z.; Xiao, X. Effect of rare earth oxides doping on MgAl2O4 spinel obtained by sintering of secondary aluminium dross. J. Alloys Compd. 2018, 735, 2597–2603. [Google Scholar] [CrossRef]
- Dangtungee, R.; Vatcharakajon, P.; Techawinyutham, L. Aluminium dross neutralization and its application as plant fertilizer. Mater. Today Proc. 2022, 52, 2420–2426. [Google Scholar] [CrossRef]
- Gomes, S.A.; Reghu, V.R.; Ramaswamy, P. Sprouting in Seeds Aided by Nitrogen Sourced from Ammonia Fumes Leached from Aluminum Dross. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Shi, J.; Huang, Z.; Qin, Q.; Yuan, C.; Chen, J.; Zhou, Y. Experiment study on the preparation of polyaluminum chloride with secondary aluminum dross. Met. Mine 2021, 50, 206–210. [Google Scholar]
- 97. Philipson, H.; Wallin, M.; Einarsrud, K.E.; Tranell, G. Kinetics of Silicon Production by Aluminothermic Reduction of Silica Using Aluminum and Aluminum Dross as Reductants. In Proceedings of the 16th International Ferro-Alloys Congress (INFACON XVI), Trondheim, Norway, 27–29 September 2021. [Google Scholar]
- Grande, L.; Vicente, M.Á.; Korili, S.A.; Gil, A. Synthesis strategies of alumina from aluminum saline slags. Process Saf. Environ. Prot. 2023, 172, 1010–1028. [Google Scholar] [CrossRef]
- Feng, H.; Wang, L.; Li, G.; Jin, Q. Removal of impurities for the synthesis of high-property alumina-spinel ceramic from secondary aluminum dross. Ceram. Int. 2023, 49 Pt A, 34616–34626. [Google Scholar] [CrossRef]
- Teixeira, A.B.; Ambrós, W.M.; Sampaio, C.H.; Raposo, F.L.Q.; De Brum, I.A.S.; Moncunill, J.O. Optimization of Water Leaching of Chlorides from Aluminum Salt Slag. Minerals 2022, 12, 1141. [Google Scholar] [CrossRef]
- Pereira, D.A.; de Aguiar, B.; Castro, F.; Almeida, M.F.; Labrincha, J.A. Mechanical behaviour of Portland cement mortars with incorporation of Al-containing salt slags. Cem. Concr. Res. 2000, 30, 1131–1138. [Google Scholar] [CrossRef]
- Jiménez, A.; Misol, A.; Morato, Á.; Rives, V.; Vicente, M.A.; Gil, A. Synthesis of pollucite and analcime zeolites by recovering aluminum from a saline slag. J. Clean. Prod. 2021, 297, 126667. [Google Scholar] [CrossRef]
- Lin, Y.; Maghool, F.; Arulrajah, A.; Horpibulsuk, S. Alkali activation of recycled concrete and aluminum salt slag aggregates for semi-rigid column inclusions. Constr. Build. Mater. 2023, 366, 130106. [Google Scholar] [CrossRef]
- Mucsi, G.; Halyag, N.; Kurusta, T.; Kristály, F. Control of Carbon Dioxide Sequestration by Mechanical Activation of Red Mud. Waste Biomass Valorization 2021, 12, 6481–6495. [Google Scholar] [CrossRef]
- Yuan, J.; Xiao, J.; Li, F.; Wang, B.; Yao, Z.; Yu, B.; Zhang, L. Co-treatment of spent cathode carbon in caustic and acid leaching process under ultrasonic assisted for preparation of SiC. Ultrason. Sonochemistry 2018, 41, 608–618. [Google Scholar] [CrossRef]
- Lu, Y.; Hua, Z.; Chen, C.; Chen, Z.; Li, X.; Yu, H.; Peng, K.; Tian, Z. Stable and fast Na-ion storage of recovered carbon from the spent carbon cathode of aluminum electrolysis. Sustain. Energy Fuels 2024, 8, 1040–1047. [Google Scholar] [CrossRef]
- Li, N.; Xie, G.; Wang, Z.X.; Hou, Y.Q.; Li, R.X. Recycle of spent potlining with low carbon grade by floatation. Adv. Mater. Res. 2014, 881, 1660–1664. [Google Scholar] [CrossRef]
- Xie, W.; Zhou, F.; Liu, J.; Bi, X.; Huang, Z.; Li, Y.; Chen, D.; Zou, H.; Sun, S. Synergistic reutilization of red mud and spent pot lining for recovering valuable components and stabilizing harmful element. J. Clean. Prod. 2020, 243, 118624. [Google Scholar] [CrossRef]
- Robshaw, T.; Tukra, S.; Hammond, D.B.; Leggett, G.J.; Ogden, M.D. Highly efficient fluoride extraction from simulant leachate of spent potlining via La-loaded chelating resin. An equilibrium study. J. Hazard. Mater. 2019, 361, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, L.; Yuan, J.; Yao, Z.; Tang, L.; Wang, Z.; Zhang, Z. Co-utilization of spent pot-lining and coal gangue by hydrothermal acid-leaching method to prepare silicon carbide powder. J. Clean. Prod. 2018, 204, 848–860. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Dong, H.; Wang, J.; Fu, X.; Cui, X.; Li, S.; Li, C. Preparation of anode materials for lithium-ion batteries by spent carbon anode from electrolytic aluminum. J. Environ. Chem. Eng. 2021, 9, 105932. [Google Scholar] [CrossRef]
- Zheng, Z.; Xie, M.; Yu, G.; Wu, Z.; Zhong, J.; Wang, Y.; Zhao, H.; Liu, F. Preparation of lithium-ion battery anode materials from graphitized spent carbon cathode derived from aluminum electrolysis. Int. J. Miner. Metall. Mater. 2024, 31, 2466–2475. [Google Scholar] [CrossRef]
- Yao, Z.; Zhong, Q.; Xiao, J.; Ye, S.; Tang, L.; Zhang, Z. An environmental-friendly process for dissociating toxic substances and recovering valuable components from spent carbon cathode. J. Hazard. Mater. 2021, 404, 124120. [Google Scholar] [CrossRef]
- Zhi, P.; Ou, Y.; Li, C.; Wang, Y.; Zhao, D. Study on harmless and resource utilization of spent cathode carbon washing process. IOP Conf. Ser. Earth Environ. Sci. 2020, 467, 012177. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Jiao, Y.; Xu, F.; Wang, X.; Zheng, F.; Zou, J.; Gao, Q.; Hu, H. A Sustainable Strategy for Spent Cathode Carbon Blocks Hazardous Waste Recycling Using Binary Molten Salt Thermal Treatment. J. Therm. Sci. 2024, 33, 1082–1093. [Google Scholar] [CrossRef]
- Liu, D.Y.; Wu, C.S. Stockpiling and Comprehensive Utilization of Red Mud Research Progress. Materials 2012, 5, 1232–1246. [Google Scholar] [CrossRef]
- Ujaczki, É.; Feigl, V.; Molnár, M.; Cusack, P.; Curtin, T.; Courtney, R.; O’Donoghue, L.; Davris, P.; Hugi, C.; Evangelou, M.W.; et al. Re-using bauxite residues: Benefits beyond (critical raw) material recovery. J. Chem. Technol. Biotechnol. 2018, 93, 2498–2510. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, F.; Xie, M.; Liu, W.; Sohn, H.Y. Recycling and utilization of spent potlining by different high temperature treatments. J. Clean. Prod. 2021, 289, 125704. [Google Scholar] [CrossRef]
- Lyu, F.; Hu, Y.; Wang, L.; Sun, W. Dealkalization processes of bauxite residue: A comprehensive review. J. Hazard. Mater. 2021, 403, 123671. [Google Scholar] [CrossRef] [PubMed]
- Nikraz, H.R.; Bodley, A.J.; Cooling, D.J.; Kong, P.Y.L.; Soomro, M. Comparison of Physical Properties between Treated and Untreated Bauxite Residue Mud. J. Mater. Civ. Eng. 2007, 19, 2–9. [Google Scholar] [CrossRef]
- Mayes, W.M.; Jarvis, A.P.; Burke, I.T.; Walton, M.; Feigl, V.; Klebercz, O.; Gruiz, K. Dispersal and Attenuation of Trace Contaminants Downstream of the Ajka Bauxite Residue (Red Mud) Depository Failure, Hungary. Environ. Sci. Technol. 2011, 45, 5147–5155. [Google Scholar] [CrossRef]
- Xue, S.; Kong, X.; Zhu, F.; Hartley, W.; Li, X.; Li, Y. Proposal for management and alkalinity transformation of bauxite residue in China. Environ. Sci. Pollut. Res. 2016, 23, 12822–12834. [Google Scholar] [CrossRef]
- Rai, S.; Wasewar, K.L.; Lataye, D.H.; Mukhopadhyay, J.; Yoo, C.K. Feasibility of red mud neutralization with seawater using Taguchi’s methodology. Int. J. Environ. Sci. Technol. 2013, 10, 305–314. [Google Scholar] [CrossRef]
- McConchie, D.; Clark, M.; Hanahan, C.; Davies-McConchie, F. The Use of Seawater-neutralised Bauxite Refinery Residues in the Management of Acid Sulphate Soils, Sulphidic Mine Tailings and Acid Mine Drainage. In Proceedings of the 3rd Queensland Environment Conference, Brisbane, Australia, 1 January 2000. [Google Scholar]
- Dealing with Red Mud—By—Product of the Bayer Process for Refining Aluminium. Available online: https://www.azom.com/article.aspx?ArticleID=2071 (accessed on 14 March 2023).
- Khaitan, S.; Dzombak, D.A.; Lowry, G.V. Mechanisms of Neutralization of Bauxite Residue by Carbon Dioxide. J. Environ. Eng. 2009, 135, 433–438. [Google Scholar] [CrossRef]
- Kasturi, A.; Gabitto, J.; Tsouris, C.; Custelcean, R. Carbon dioxide capture with aqueous amino acids: Mechanistic study of amino acid regeneration by guanidine crystallization and process intensification. Sep. Purif. Technol. 2021, 271, 118839. [Google Scholar] [CrossRef]
- Sahu, R.C.; Patel, R.K.; Ray, B.C. Neutralization of red mud using CO2 sequestration cycle. J. Hazard. Mater. 2010, 179, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Wasewar, K.L.; Lataye, D.H.; Mishra, R.S.; Puttewar, S.P.; Chaddha, M.J.; Mahindiran, P.; Mukhopadhyay, J. Neutralization of red mud with pickling waste liquor using Taguchi’s design of experimental methodology. Waste Manag. Res. 2012, 30, 922–930. [Google Scholar] [CrossRef]
- Wang, P.; Liu, D.-Y. Physical and Chemical Properties of Sintering Red Mud and Bayer Red Mud and the Implications for Beneficial Utilization. Materials 2012, 5, 1800–1810. [Google Scholar] [CrossRef]
- Mussel, G.; Summers, J.; Sparling, G. Bioremediation of Bauxite Residue in Western Australia: An Initial Feasibility Study; Alcoa of Australia: Perth, Australia, 1993. [Google Scholar]
- Aranda-Usón, A.; Portillo-Tarragona, P.; Scarpellini, S.; Llena-Macarulla, F. The progressive adoption of a circular economy by businesses for cleaner production: An approach from a regional study in Spain. J. Clean. Prod. 2020, 247, 119648. [Google Scholar] [CrossRef]
- Shoppert, A.; Valeev, D.; Diallo, M.M.; Loginova, I.; Beavogui, M.C.; Rakhmonov, A.; Ovchenkov, Y.; Pankratov, D. High-Iron Bauxite Residue (Red Mud) Valorization Using Hydrochemical Conversion of Goethite to Magnetite. Materials 2022, 15, 8423. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, Z.; Pan, J.; Zhu, D.; Dong, T.; Lu, S. Extracting Al2O3 and TiO2 from Red Mud Smelting Separation Slag by Alkali and Acid Leaching Methods. Metals 2023, 13, 552. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Q.; Yan, K.; Cheng, F.; Lou, H.H. Novel Process for Alumina Extraction via the Coupling Treatment of Coal Gangue and Bauxite Red Mud. Ind. Eng. Chem. Res. 2014, 53, 4518–4521. [Google Scholar] [CrossRef]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef]
- Silva Filho, E.; Alves, M.; Da Motta, M. Lama vermelha da indústria de beneficiamento de alumina: Produção, características, disposição e aplicações alternativas. Matéria 2007, 12, 322–338. [Google Scholar] [CrossRef]
- Ding, W.; Bao, S.; Zhang, Y.; Xiao, J. Efficient Selective Extraction of Scandium from Red Mud. Miner. Process. Extr. Metall. Rev. 2022, 44, 304–312. [Google Scholar] [CrossRef]
- Dai, X.; Thi Hong Nhung, N.; Hamza, M.F.; Guo, Y.; Chen, L.; He, C.; Ning, S.; Wei, Y.; Dodbiba, G.; Fujita, T. Selective adsorption and recovery of scandium from red mud leachate by using phosphoric acid pre-treated pitaya peel biochar. Sep. Purif. Technol. 2022, 292, 121043. [Google Scholar] [CrossRef]
- Zhang, D.-R.; Chen, H.-R.; Nie, Z.-Y.; Xia, J.-L.; Li, E.-P.; Fan, X.-L.; Zheng, L. Extraction of Al and rare earths (Ce, Gd, Sc, Y) from red mud by aerobic and anaerobic bi-stage bioleaching. Chem. Eng. J. 2020, 401, 125914. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Wang, N.; Gu, H. Selective extraction of rare earth elements from red mud using oxalic and sulfuric acids. J. Environ. Chem. Eng. 2022, 10, 108650. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, X.; Li, J.; Niu, Q.; Tang, A.; Li, Q. Metagenomic insights into role of red mud in regulating fate of compost antibiotic resistance genes mediated by both direct and indirect ways. Environ. Pollut. 2023, 317, 120795. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Z.; Zhao, C.; Zhang, Y.; Peng, D.; Gong, Z. Facile fabrication of Zero-valent-iron biochar from red mud for bisulfite activation in wastewater treatment: Performance and mechanism. Environ. Technol. Innov. 2023, 30, 103110. [Google Scholar] [CrossRef]
- Cai, Y.-R.; Song, Y.; Chang, C. Adsorption properties and mechanism of ginkgo biloba leaf-based materials for Cd (II) in aqueous solution. Environ. Sci. Pollut. Res. 2022, 29, 78499–78508. [Google Scholar] [CrossRef]
- Bai, B.; Bai, F.; Li, X.; Nie, Q.; Jia, X.; Wu, H. The remediation efficiency of heavy metal pollutants in water by industrial red mud particle waste. Environ. Technol. Innov. 2022, 28, 102944. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Gan, S.; He, H.; Cai, N.; Xu, J.; Guo, P.; Chen, B.; Pan, X. Effective degradation of COVID-19 related drugs by biochar-supported red mud catalyst activated persulfate process: Mechanism and pathway. J. Clean Prod. 2022, 340, 130753. [Google Scholar] [CrossRef] [PubMed]
- Turk, B.; Kazak, O.; Akkaya, G.K.; Tor, A. A simple and green preparation of red mud-coated membrane for efficient separation of oil-in-water emulsions. J. Environ. Chem. Eng. 2022, 10, 106928. [Google Scholar] [CrossRef]
- Viyasun, K.; Anuradha, R.; Thangapandi, K.; Santhosh Kumar, D.; Sivakrishna, A.; Gobinath, R. Investigation on performance of red mud based concrete. Mater. Today Proc. 2021, 39, 796–799. [Google Scholar] [CrossRef]
- Vladić Kancir, I.; Serdar, M. Contribution to Understanding of Synergy between Red Mud and Common Supplementary Cementitious Materials. Materials 2022, 15, 1968. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Lyu, F.; Sun, W.; Zhang, H.; Wang, L.; Wang, Y. Progress on the Industrial Applications of Red Mud with a Focus on China. Minerals 2020, 10, 773. [Google Scholar] [CrossRef]
- Wang, X.; Sun, K.; Li, X.; Ma, J.; Luo, Z. The Effect of Red Mud on Sintering Processes and Minerals of Portland Cement for Roads. Crystals 2021, 11, 1267. [Google Scholar] [CrossRef]
- Pişkin, S.; Kantürk Figen, A.; özkan, E.; özçay, ü. Structural Characterization of Seydişehir Red Mud to Utilization in Roof Tile Manufacturing. IFAC Proc. Vol. 2013, 46, 484–487. [Google Scholar] [CrossRef]
- Ma, S.; Sun, Z.; Wei, J.; Zhang, X.; Zhang, L. Utilization of Modified Red Mud Waste from the Bayer Process as Subgrade and Its Performance Assessment in a Large-Sale Application. Coatings 2022, 12, 471. [Google Scholar] [CrossRef]
- Tyagi, G.; Singhal, A.; Routroy, S.; Bhunia, D.; Lahoti, M. Radiation Shielding Concrete with alternate constituents: An approach to address multiple hazards. J. Hazard. Mater. 2021, 404, 124201. [Google Scholar] [CrossRef]
- Tyagi, G.; Singhal, A.; Routroy, S.; Bhunia, D.; Lahoti, M. A review on sustainable utilization of industrial wastes in radiation shielding concrete. Mater. Today Proc. 2020, 32, 746–751. [Google Scholar] [CrossRef]
- Bijanu, A.; Arya, R.; Agrawal, V.; Tomar, A.S.; Gowri, V.S.; Sanghi, S.K.; Mishra, D.; Salammal, S.T. Metal-polymer composites for radiation protection: A review. J. Polym. Res. 2021, 28, 392. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Tian, X.; Liu, T.; Wang, Q.; Dilmurat, A.; Li, D.; Ziegmann, G. Recycling and remanufacturing of 3D printed continuous carbon fiber reinforced PLA composites. J. Clean. Prod. 2017, 142, 1609–1618. [Google Scholar] [CrossRef]
- Agrawal, V.; Paulose, R.; Arya, R.; Rajak, G.; Giri, A.; Bijanu, A.; Sanghi, S.K.; Mishra, D.; N, P.; Khare, A.K.; et al. Green conversion of hazardous red mud into diagnostic X-ray shielding tiles. J. Hazard. Mater. 2022, 424, 127507. [Google Scholar] [CrossRef] [PubMed]
- Sunendar, B.; Fathina, A.; Harmaji, A.; Mardhian, D.F.; Asri, L.; Widodo, H.B. The effect of CHA-doped Sr addition to the mechanical strength of metakaolin dental implant geopolymer composite. AIP Conf. Proc. 2017, 1887, 020020. [Google Scholar] [CrossRef]
- Harmaji, A.; Hardiansyah, A.; Widianingsih, N.A.; Jannah, R.M.; Soepriyanto, S. The effect of Basic Oxygen Furnace, Blast Furnace, and Kanbara Reactor Slag as Reinforcement to Cement Based Mortar. JPSE (J. Phys. Sci. Eng.) 2022, 7, 56–61. [Google Scholar] [CrossRef]
- Simatupang, P.H.; Harmaji, A.; Anggraini, A.N.; Pane, I.; Sunendar, B.; Imran, I. Utilization of Industrial Waste in Indonesia as Filler for Low and High Calcium Fly Ash based Geopolymer. In Proceedings of the 2nd International Conference on Materials and Metallurgical Technology 2015 (ICOMMET 2015), Surabaya, Indonesia, 4–6 October 2015. [Google Scholar]
- Gonçalves, N.P.F.; Olhero, S.M.; Labrincha, J.A.; Novais, R.M. 3D-printed red mud/metakaolin-based geopolymers as water pollutant sorbents of methylene blue. J. Clean. Prod. 2023, 383, 135315. [Google Scholar] [CrossRef]
- Singh Tomar, A.; Gupta, R.; Singh, A.; Thankaraj Salammal, S.; Akram Khan, M.; Mishra, D. Evaluation of corrosion protective properties of fly ash-red mud based geopolymer coating material for mild steel. Mater. Today Proc. 2022, 68, 181–186. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Q.; Long, H.; Deng, H.; Chen, Z.; Hui, D. Influence of nano-SiO2 and steel fiber on mechanical and microstructural properties of red mud-based geopolymer concrete. Constr. Build. Mater. 2023, 364, 129990. [Google Scholar] [CrossRef]
- Afolabi, L.O.; Ariff, Z.M.; Megat-Yusoff, P.S.M.; Al-Kayiem, H.H.; Arogundade, A.I.; Afolabi-Owolabi, O.T. Red-mud geopolymer composite encapsulated phase change material for thermal comfort in built-sector. Solar Energy 2019, 181, 464–474. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).