A New Feasible Opportunity for Recycling Lead and Silver from Zinc Plant Residues by Flotation
Abstract
:1. Introduction
2. Materials and Method
2.1. Flotation Experiments
2.2. Experimental Design
3. Results and Discussion
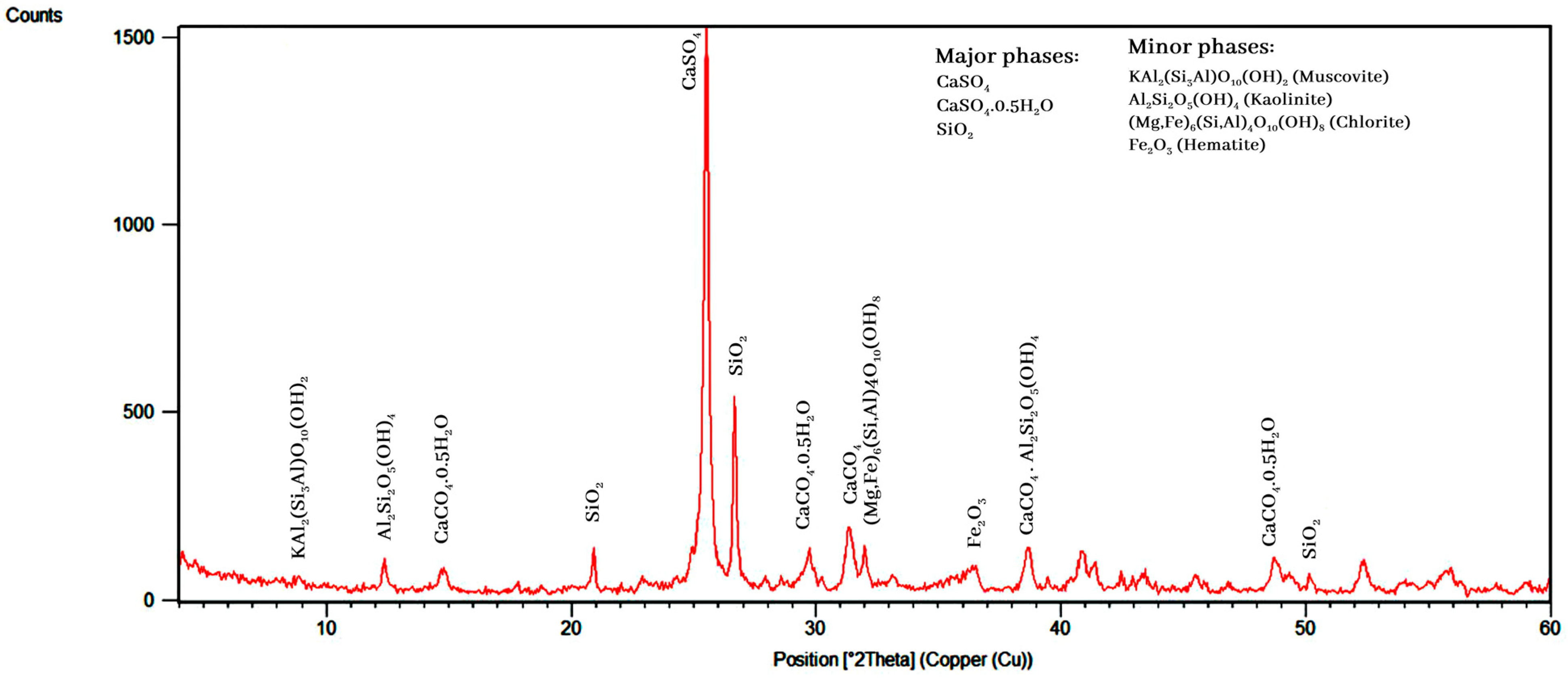
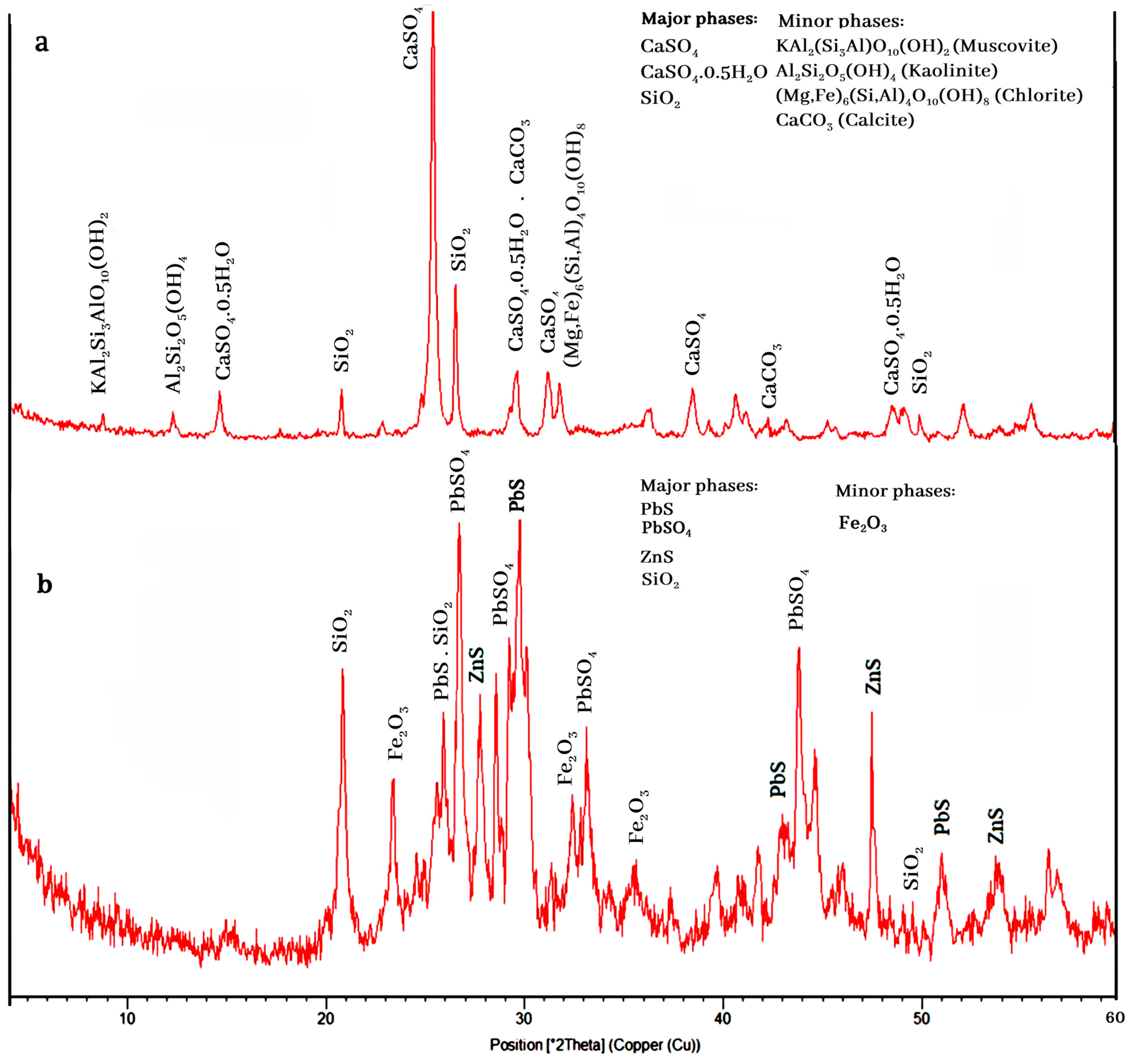
3.1. Characterization
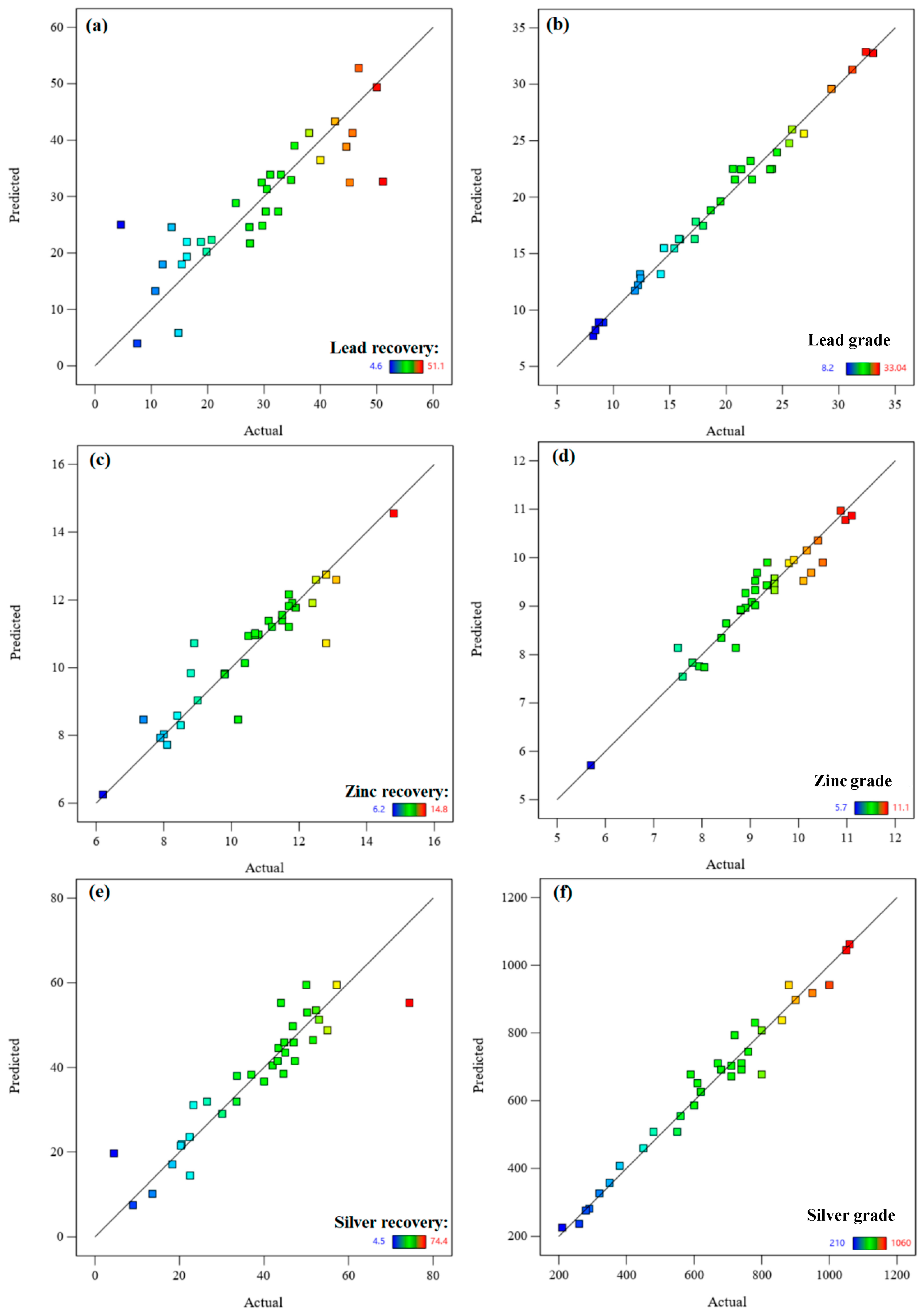
3.2. Model Performance
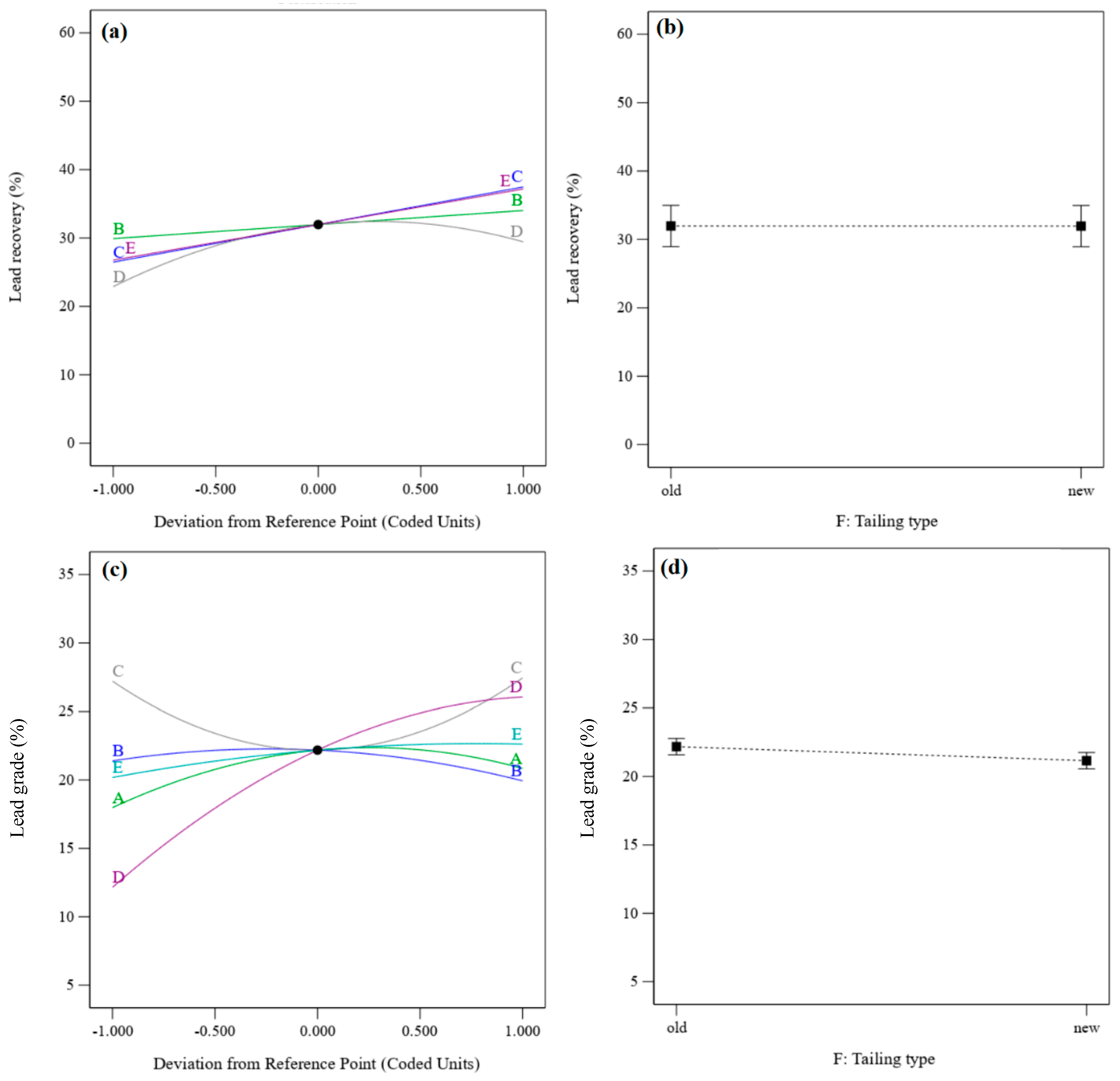
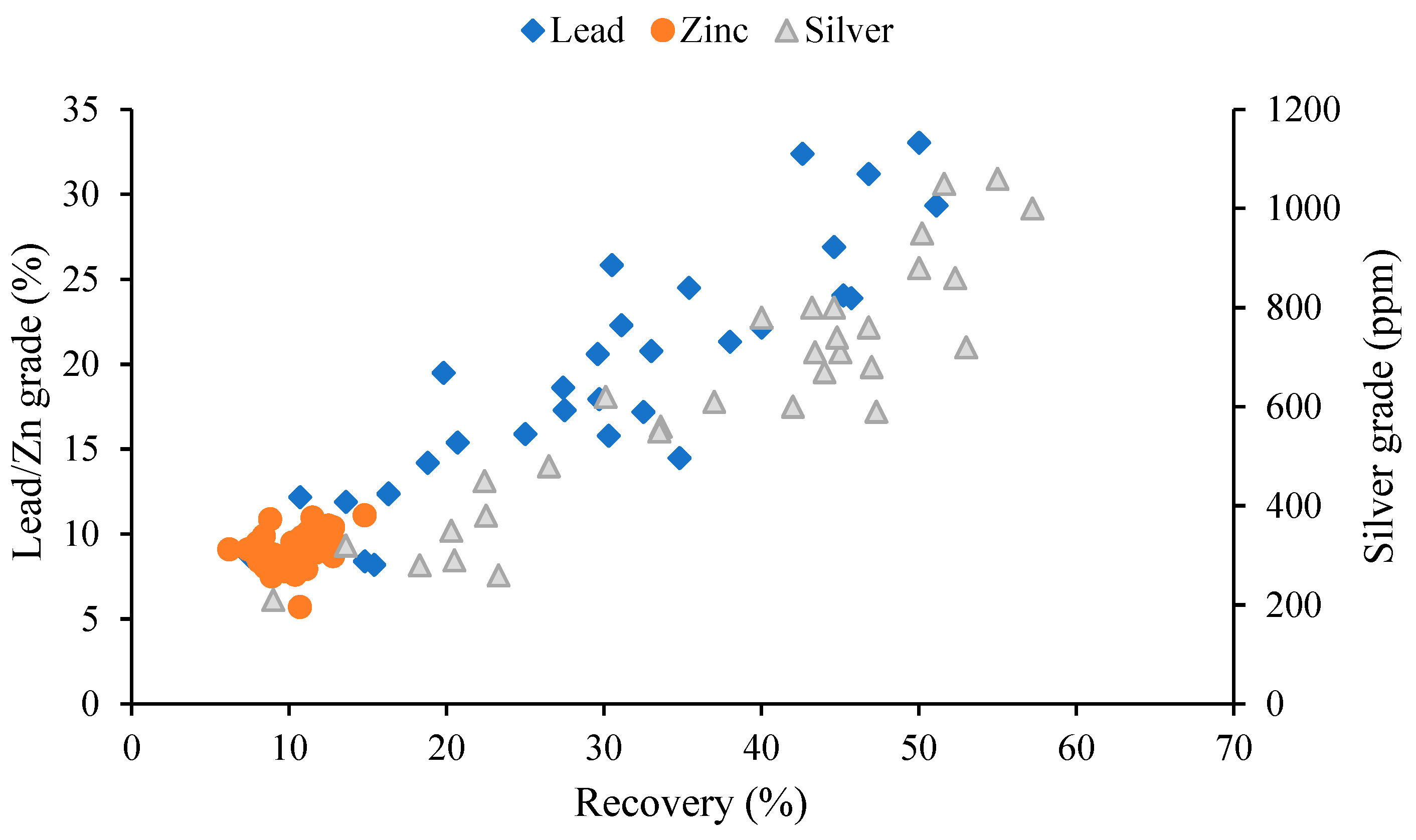
3.3. Effect of Parameters and Interactions
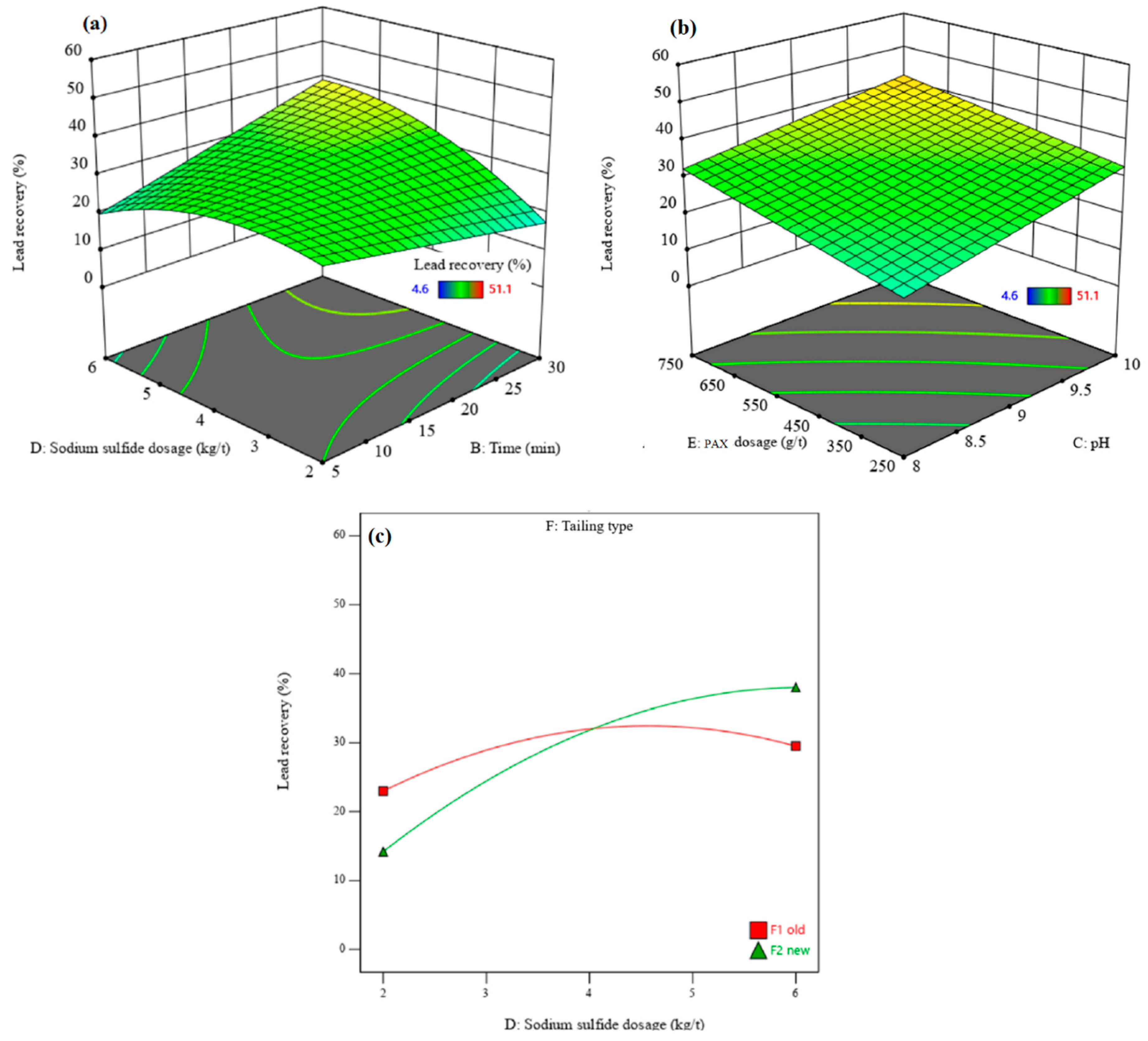
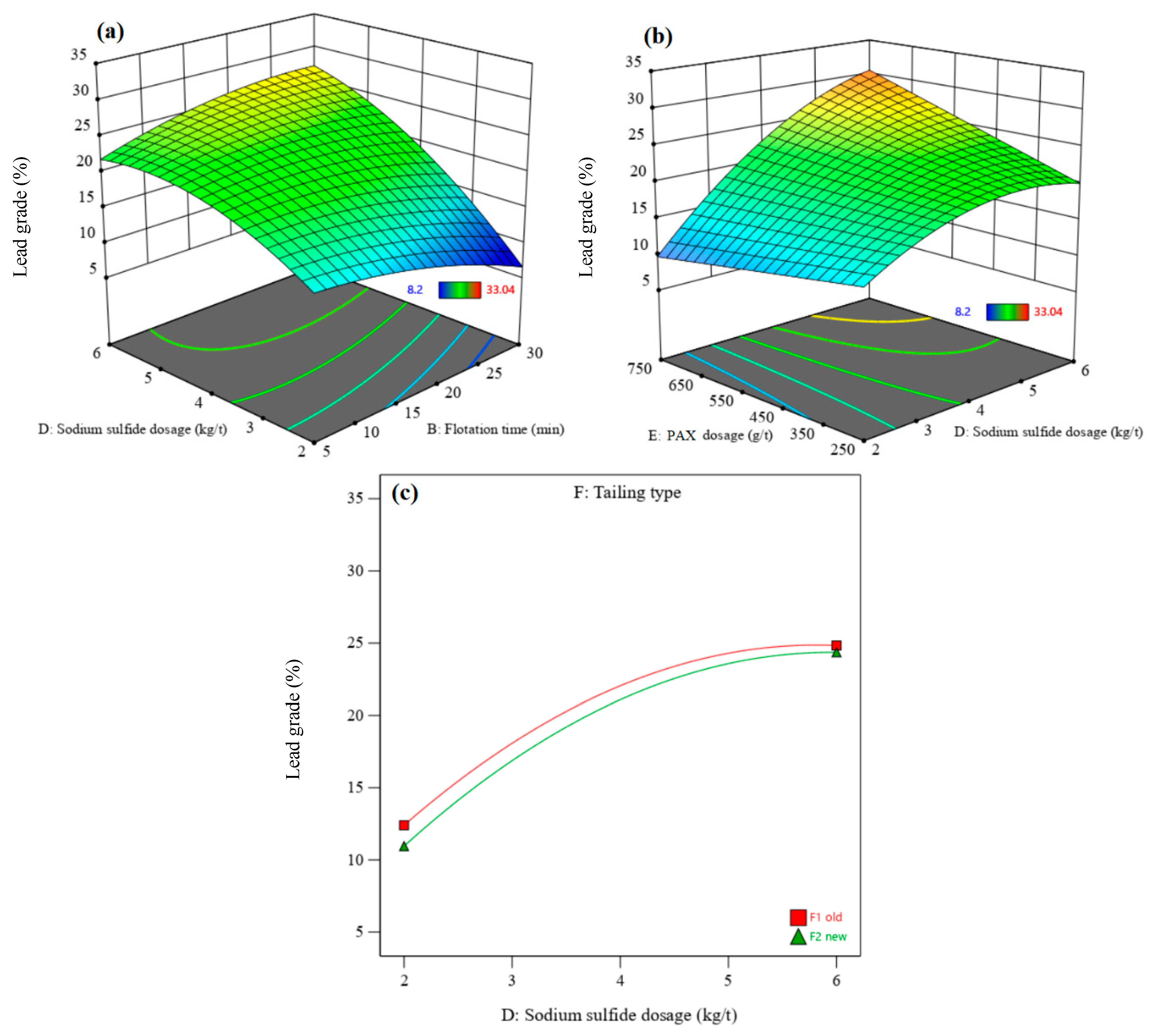
3.3.1. Recovery and Grade of Lead
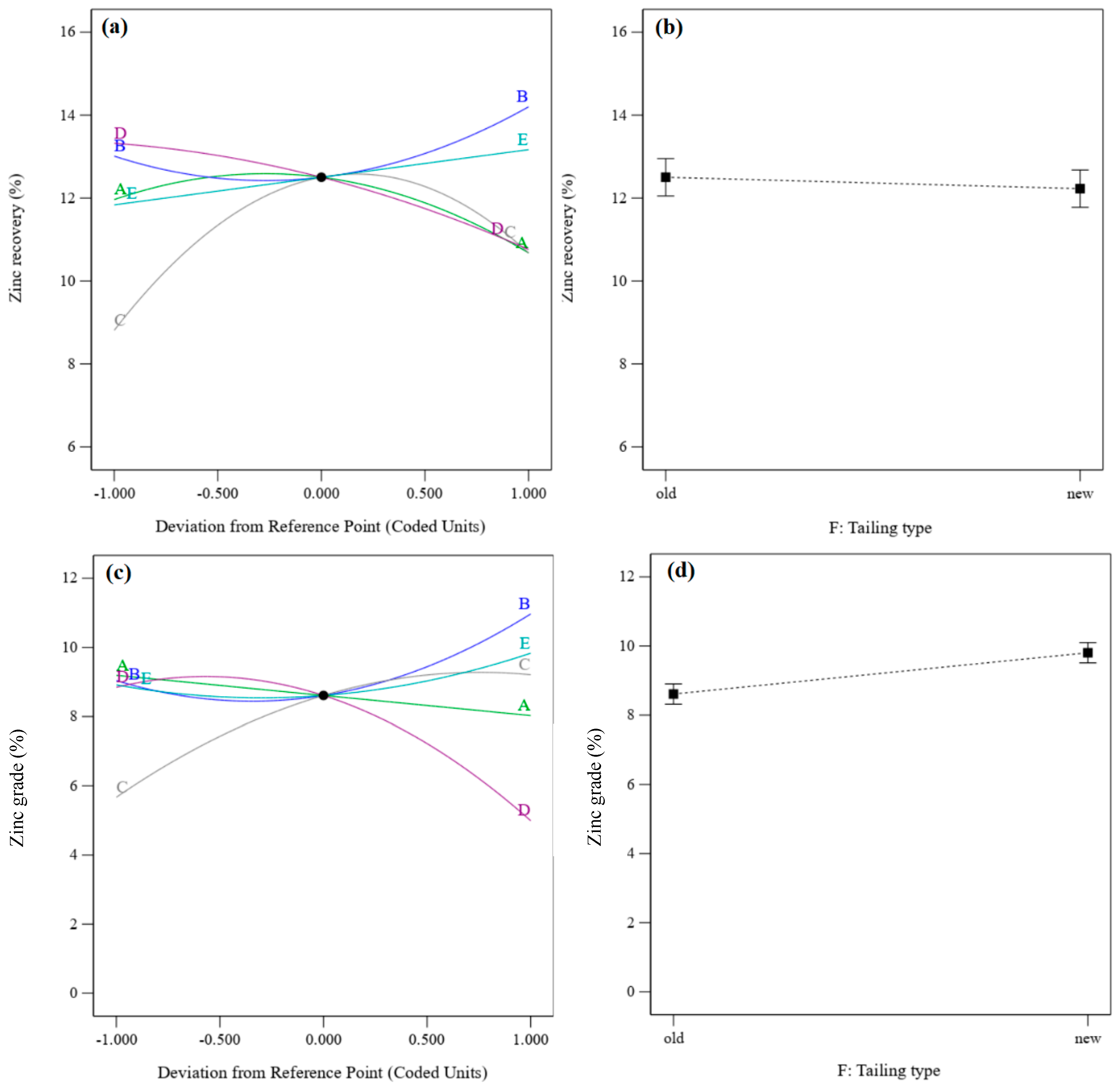
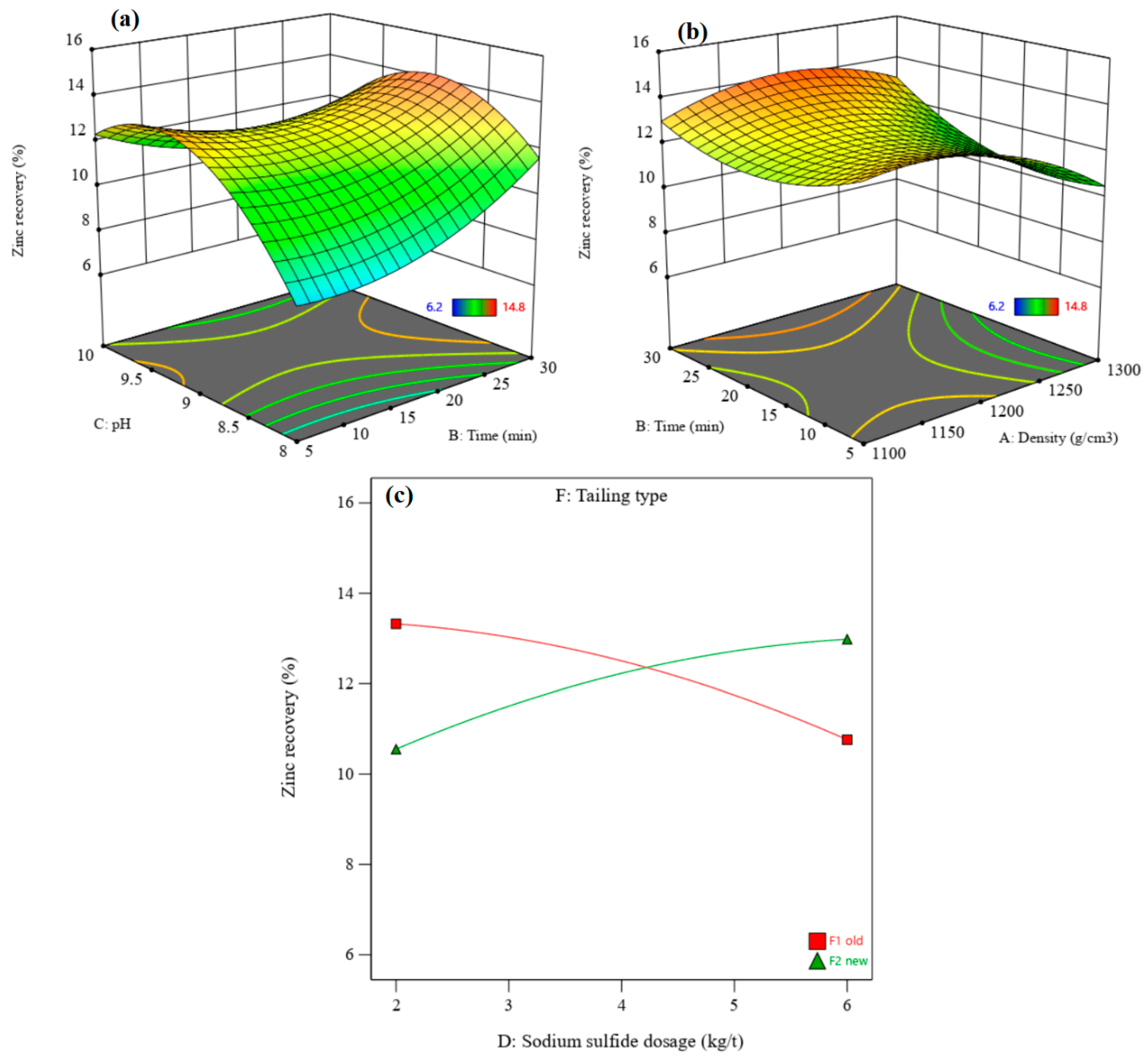
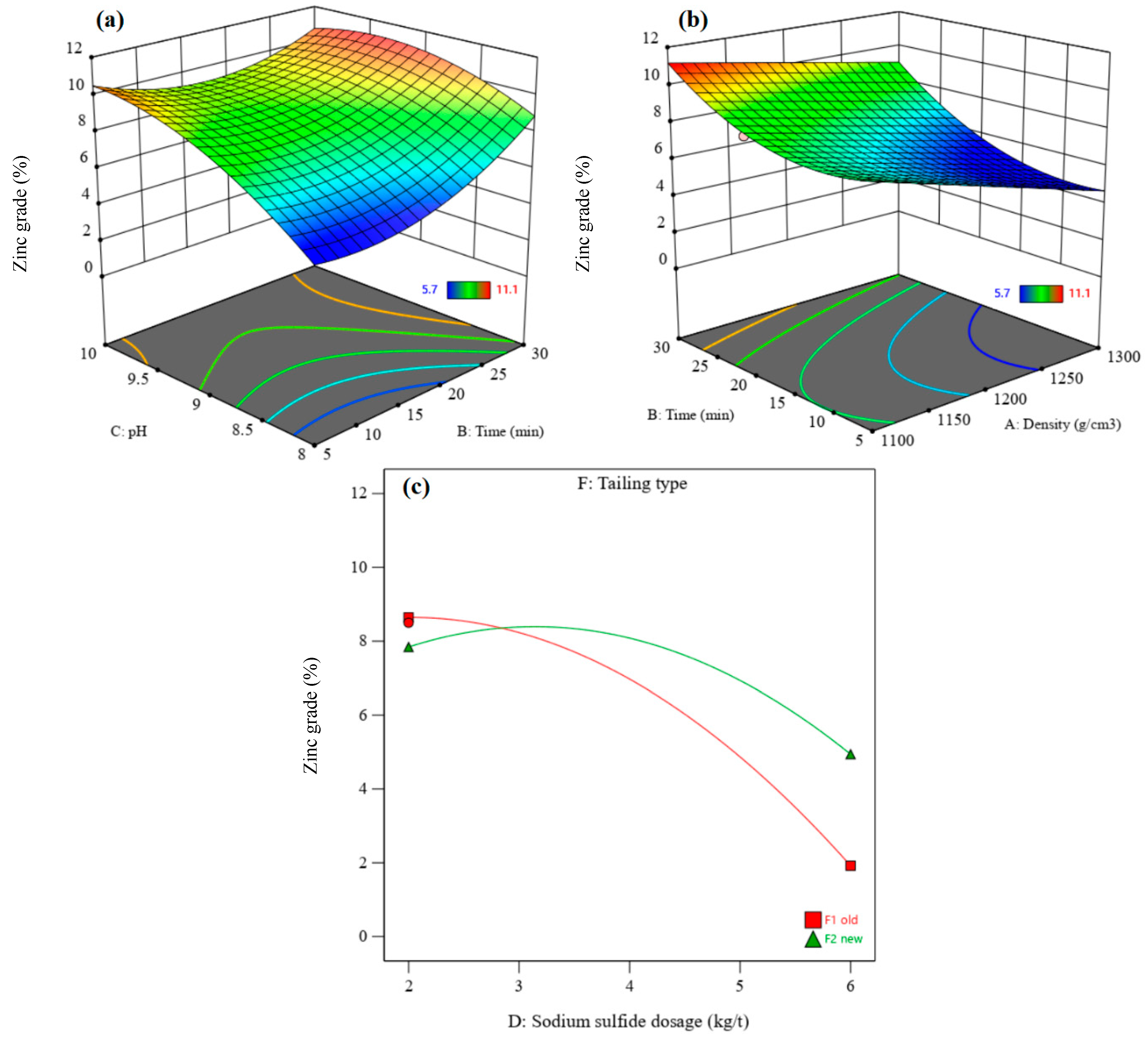
3.3.2. Recovery and Grade of Zinc
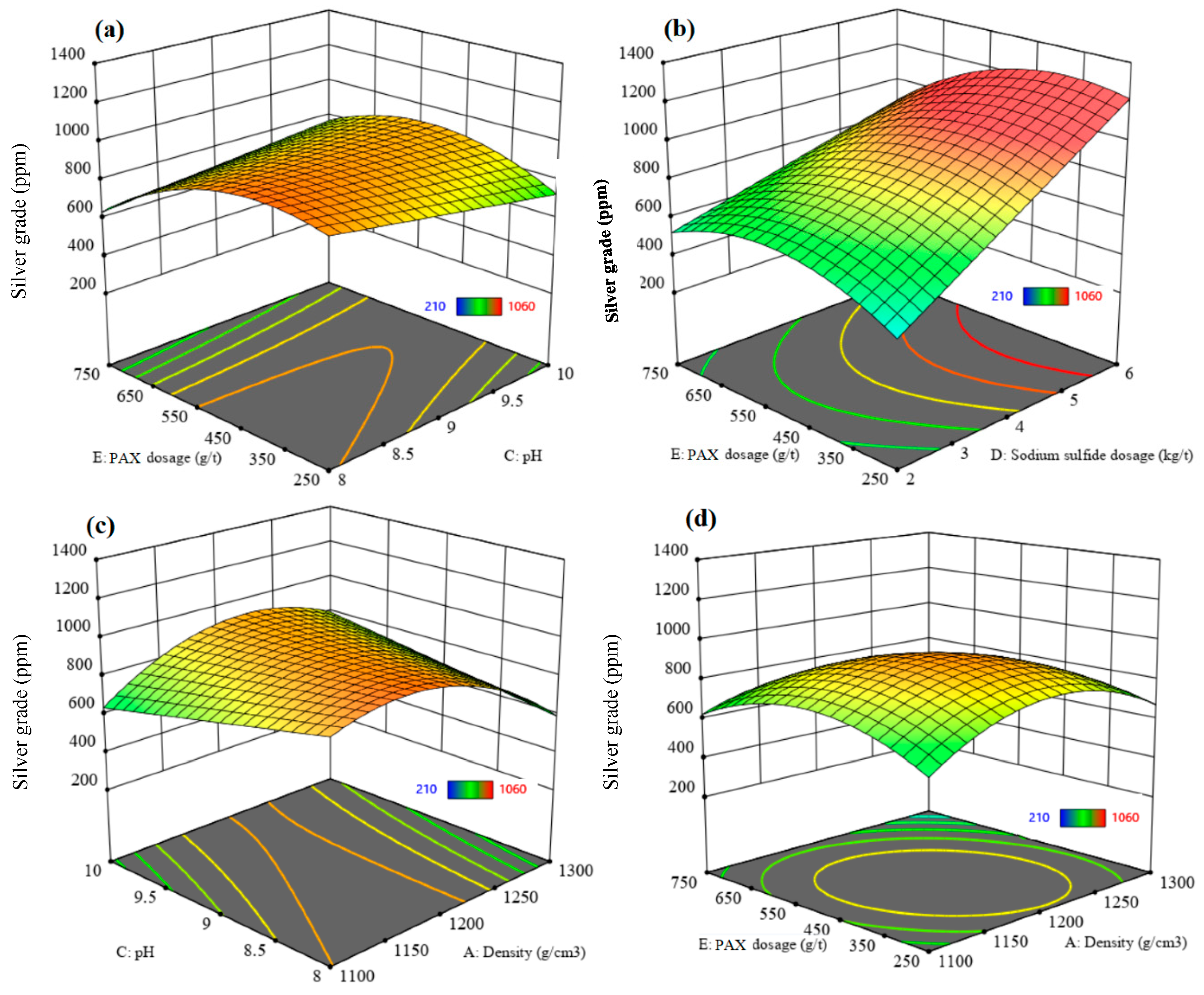
3.3.3. Recovery and Grade of Silver
3.4. Brief Economic Evaluation of a Lead and Silver Concentrate Production Plan by the Flotation Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Espiari, S.; Rashchi, F.; Sadrnezhaad, S. Hydrometallurgical treatment of tailings with high zinc content. Hydrometallurgy 2006, 82, 54–62. [Google Scholar] [CrossRef]
- Evdokimov, S.; Evdokimov, V. Metal recovery from old tailings. J. Min. Sci. 2014, 50, 800–808. [Google Scholar] [CrossRef]
- Falagán, C.; Grail, B.M.; Johnson, D.B. New approaches for extracting and recovering metals from mine tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Hajati, A.; Khodadadi, A.; Koleini, S. Flotation of zinc oxide minerals from low-grade tailings by oxine and dithizone using the Taguchi approach. Min. Metall. Explor. 2010, 27, 158–165. [Google Scholar] [CrossRef]
- Kitobo, W.; Gaydardzhiev, S.; Frenay, J.; Bastin, D.; Ndala, I. Separation of copper and zinc by solvent extraction during reprocessing of flotation tailings. Sep. Sci. Technol. 2010, 45, 535–540. [Google Scholar] [CrossRef]
- Lei, C.; Yan, B.; Chen, T.; Xiao, X.-M. Recovery of metals from the roasted lead-zinc tailings by magnetizing roasting followed by magnetic separation. J. Clean. Prod. 2017, 158, 73–80. [Google Scholar] [CrossRef]
- Maltrana, V.; Morales, J. The Use of Acid Leaching to Recover Metals from Tailings: A Review. Metals 2023, 13, 1862. [Google Scholar] [CrossRef]
- Muravyov, M.I.; Bulaev, A.G.; Kondrat’eva, T.F. Complex treatment of mining and metallurgical wastes for recovery of base metals. Miner. Eng. 2014, 64, 63–66. [Google Scholar] [CrossRef]
- Babel, B.; Penz, M.; Schach, E.; Boehme, S.; Rudolph, M. Reprocessing of a southern Chilean Zn tailing by flotation—A case study. Minerals 2018, 8, 295. [Google Scholar] [CrossRef]
- Asadollahfardi, G.; Sarmadi, M.S.; Rezaee, M.; Khodadadi-Darban, A.; Yazdani, M.; Paz-Garcia, J.M. Comparison of different extracting agents for the recovery of Pb and Zn through electrokinetic remediation of mine tailings. J. Environ. Manag. 2021, 279, 111728. [Google Scholar] [CrossRef]
- Andrews, W.J.; Moreno, C.J.G.; Nairn, R.W. Potential recovery of aluminum, titanium, lead, and zinc from tailings in the abandoned Picher mining district of Oklahoma. Miner. Econ. 2013, 26, 61–69. [Google Scholar] [CrossRef]
- Bilal, M.; Park, I.; Hornn, V.; Ito, M.; Hassan, F.U.; Jeon, S.; Hiroyoshi, N. The challenges and prospects of recovering fine copper sulfides from tailings using different flotation techniques: A review. Minerals 2022, 12, 586. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Bussière, B.; Kongolo, M.; McLaughlin, J.; Marion, P. Environmental desulphurization of four Canadian mine tailings using froth flotation. Int. J. Miner. Process. 2000, 60, 57–74. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Kongolo, M. Physico-chemical properties of tailing slurries during environmental desulphurization by froth flotation. Int. J. Miner. Process. 2003, 69, 221–234. [Google Scholar] [CrossRef]
- Nazari, S.; Hoseinian, F.S.; Li, J.; Safari, M.; Khoshdast, H.; Li, J.; He, Y. Synergistic effect of grinding time and submicron (nano) bubbles on the zeta potential state of spent lithium-ion batteries: A gene expression programming approach. J. Energy Storage 2023, 70, 107942. [Google Scholar] [CrossRef]
- Antonijević, M.; Dimitrijević, M.; Stevanović, Z.; Serbula, S.; Bogdanovic, G. Investigation of the possibility of copper recovery from the flotation tailings by acid leaching. J. Hazard. Mater. 2008, 158, 23–34. [Google Scholar] [CrossRef]
- Gibson, B.A.; Nwaila, G.; Manzi, M.; Ghorbani, Y.; Ndlovu, S.; Petersen, J. The valorisation of platinum group metals from flotation tailings: A review of challenges and opportunities. Miner. Eng. 2023, 201, 108216. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D.W. Rare earth and phosphorus leaching from a flotation tailings of Florida phosphate rock. Minerals 2018, 8, 416. [Google Scholar] [CrossRef]
- Kursunoglu, S.; Kursunoglu, N.; Hussaini, S.; Kaya, M. Selection of an appropriate acid type for the recovery of zinc from a flotation tailing by the analytic hierarchy process. J. Clean. Prod. 2021, 283, 124659. [Google Scholar] [CrossRef]
- Asadi, T.; Azizi, A.; Lee, J.-c.; Jahani, M. Leaching of zinc from a lead-zinc flotation tailing sample using ferric sulphate and sulfuric acid media. J. Environ. Chem. Eng. 2017, 5, 4769–4775. [Google Scholar] [CrossRef]
- Muravyov, M.I.; Fomchenko, N.V.; Usoltsev, A.V.; Vasilyev, E.A.; Kondrat’eva, T.F. Leaching of copper and zinc from copper converter slag flotation tailings using H2SO4 and biologically generated Fe2(SO4)3. Hydrometallurgy 2012, 119, 40–46. [Google Scholar] [CrossRef]
- Manca, P.P.; Massacci, G.; Pintus, D.; Sogos, G. The flotation of sphalerite mine tailings as a remediation method. Miner. Eng. 2021, 165, 106862. [Google Scholar] [CrossRef]
- Lorenzo-Tallafigo, J.; Romero-García, A.; Iglesias-González, N.; Mazuelos, A.; Romero, R.; Carranza, F. A novel hydrometallurgical treatment for the recovery of copper, zinc, lead and silver from bulk concentrates. Hydrometallurgy 2021, 200, 105548. [Google Scholar] [CrossRef]
- Ruşen, A.; Sunkar, A.; Topkaya, Y.A. Zinc and lead extraction from Çinkur leach residues by using hydrometallurgical method. Hydrometallurgy 2008, 93, 45–50. [Google Scholar] [CrossRef]
- Guo, Z.-h.; Pan, F.-K.; Xiao, X.-Y.; Zhang, L.; Jiang, K.-Q. Optimization of brine leaching of metals from hydrometallurgical residue. Trans. Nonferrous Metals Soc. China 2010, 20, 2000–2005. [Google Scholar] [CrossRef]
- Motamedizadeh, M.; Azizi, A.; Bahri, Z. Recycling lead from a zinc plant residue (ZPR) using brine leaching and cementation with aluminum powder. Environ. Sci. Pollut. Res. 2021, 28, 42121–42134. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Niu, L.; Zhang, W.; Zhang, T.-a. High purity metal lead recovery from zinc direct leaching residue via chloride leaching and direct electrolysis. Separation Purif. Technol. 2021, 263, 118329. [Google Scholar] [CrossRef]
- Persson, A. Understand Lithium Mining’s Environmental Impact. 2024. Available online: https://www.carbonchain.com/blog/understand-lithium-minings-environmental-impact/ (accessed on 8 March 2024).
- Kashani, A.N.; Rashchi, F. Separation of oxidized zinc minerals from tailings: Influence of flotation reagents. Miner. Eng. 2008, 21, 967–972. [Google Scholar] [CrossRef]
- Yang, X.; Huang, X.; Qiu, T. Recovery of zinc from cyanide tailings by flotation. Miner. Eng. 2015, 84, 100–105. [Google Scholar] [CrossRef]
- Bagheri, B.; Mehrabani, J.V.; Farrokhpay, S. Recovery of sphalerite from a high zinc grade tailing. J. Hazard. Mater. 2020, 381, 120946. [Google Scholar] [CrossRef] [PubMed]
- Rashchi, F.; Dashti, A.; Arabpour-Yazdi, M.; Abdizadeh, H. Anglesite flotation: A study for lead recovery from zinc leach residue. Miner. Eng. 2005, 18, 205–212. [Google Scholar] [CrossRef]
- Du, Y.; Tong, X.; Xie, X.; Zhang, W.; Yang, H.; Song, Q. Recovery of Zinc and Silver from Zinc Acid-Leaching Residues with Reduction of Their Environmental Impact Using a Novel Water Leaching-Flotation Process. Minerals 2021, 11, 586. [Google Scholar] [CrossRef]
- Zheng, Y.-X.; Lv, J.-F.; Liu, W.; Qin, W.-Q.; Wen, S.-M. An innovative technology for recovery of zinc, lead and silver from zinc leaching residue. Physicochem. Probl. Miner. Process. 2016, 52, 943–954. [Google Scholar]
- Yao, G.; Guo, Q.; Li, Y.; Xu, Z.; Han, Z.; He, M.; Qi, T. An innovation technology for recovering silver and valuable metals from hazardous zinc leaching residue through direct reduction. Miner. Eng. 2022, 188, 107857. [Google Scholar] [CrossRef]
- Pattanaik, A.; Rayasam, V. Analysis of reverse cationic iron ore fines flotation using RSM-D-optimal design—An approach towards sustainability. Adv. Powder Technol. 2018, 29, 3404–3414. [Google Scholar] [CrossRef]
- Hao, J.; Wang, F.; Wang, X.; Zhang, D.; Bi, Y.; Gao, Y.; Zhao, X.; Zhang, Q. Development and optimization of baicalin-loaded solid lipid nanoparticles prepared by coacervation method using central composite design. Eur. J. Pharm. Sci. 2012, 47, 497–505. [Google Scholar] [CrossRef]
- Ghaedi, M.; Mazaheri, H.; Khodadoust, S.; Hajati, S.; Purkait, M. Application of central composite design for simultaneous removal of methylene blue and Pb2+ ions by walnut wood activated carbon. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 479–490. [Google Scholar] [CrossRef]
- Ahmadi, M.; Vahabzadeh, F.; Bonakdarpour, B.; Mofarrah, E.; Mehranian, M. Application of the central composite design and response surface methodology to the advanced treatment of olive oil processing wastewater using Fenton’s peroxidation. J. Hazard. Mater. 2005, 123, 187–195. [Google Scholar] [CrossRef]
- Kamran Haghighi, H.; Jafarian Mohammadi, S.M.J.; Salarirad, M.M. Promoted Flotation of Lead and Silver Oxidized Minerals Using a Synergetic Combination of Collectors. J. Sustain. Metall. 2024, 10, 1507–1527. [Google Scholar] [CrossRef]
- Kamran Haghighi, H.; Jafarian Mohammadi, S.M.J.; Salarirad, M.M. Increasing the recovery of lead and silver in the flotation of Angouran oxidized lead and zinc ore. J. Sep. Sci. Eng. 2024, 16, 17–29. [Google Scholar] [CrossRef]
- Bruin, J. Newtest: Command to Compute New Test. Available online: https://stats.oarc.ucla.edu/stata/ado/analysis/ (accessed on 1 February 2011).
- Bulatovic, S.M. Handbook of Flotation Reagents: Flotation of Sulfide Ores; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Herrera-Urbina, R.; Sotillo, F.J.; Fuerstenau, D.W. Effect of sodium sulfide additions on the pulp potential and amyl xanthate flotation of cerussite and galena. Int. J. Miner. Process. 1999, 55, 157–170. [Google Scholar] [CrossRef]
- Newell, A.J.H.; Bradshaw, D.J.; Harris, P.J. The effect of heavy oxidation upon flotation and potential remedies for Merensky type sulfides. Miner. Eng. 2006, 19, 675–686. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, A.; Liao, Y.; Wang, L.; Cao, Y. Combined Column and Mechanical Flotation Cell Process for the Beneficiation of Sanshandao Gold Ore. ACS Omega 2021, 6, 33607–33613. [Google Scholar] [CrossRef]
- Shahverdi, M.; Khodadadi Darban, A.; Abdollahy, M.; Yamini, Y. Investigation of effect of sulfate ion on xanthate consumption in galena flotation based on thermodynamic diagrams. J. Min. Environ. 2018, 9, 1035–1048. [Google Scholar]

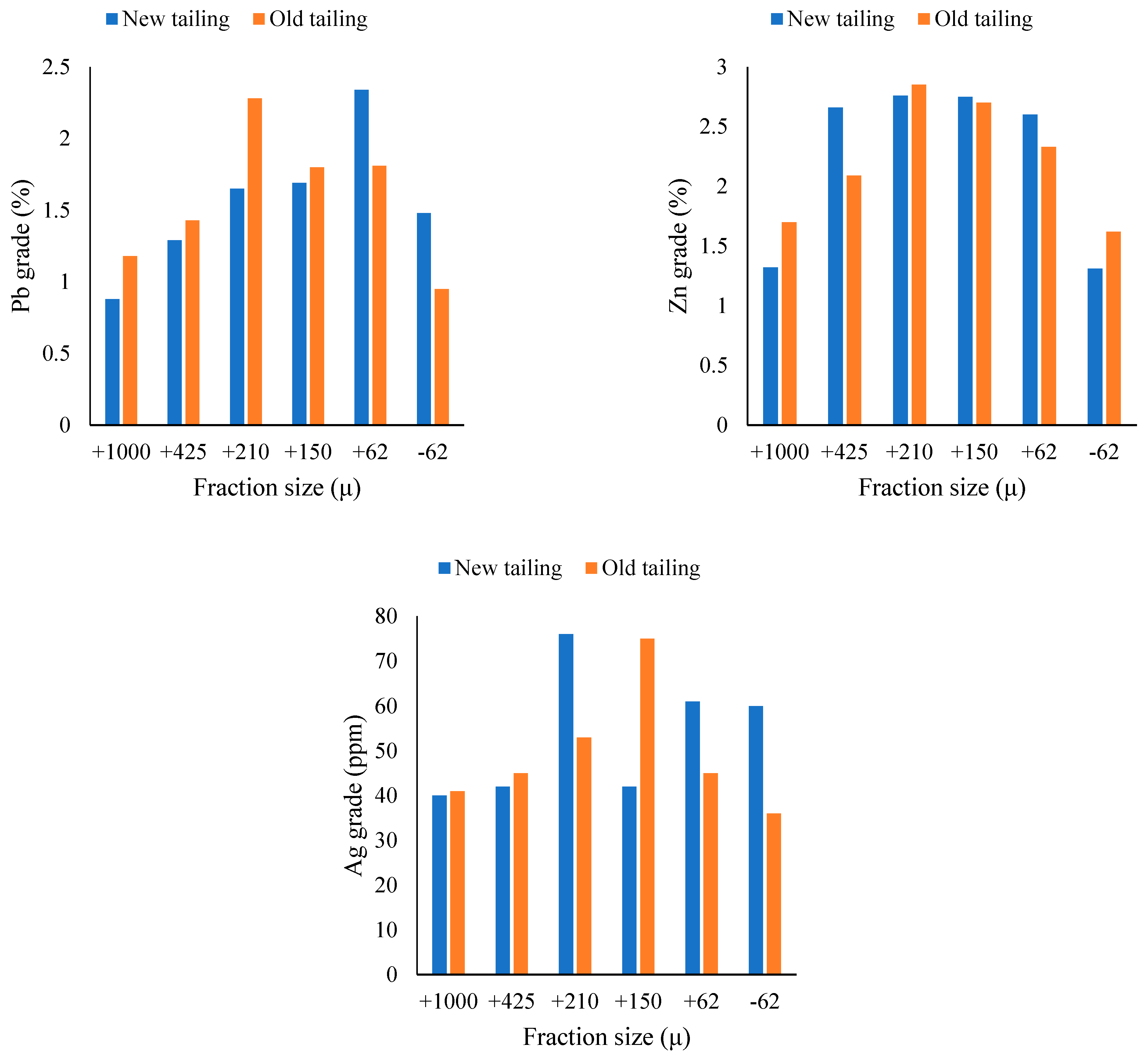


| Old | 3 < Pb < 4 | 2 < Pb < 3 | 1 < Pb < 2 | Pb < 1 |
| New | 3 < Pb < 4 | 2 < Pb < 3 | 1 < Pb < 2 | Pb < 1 |
| Sample Category | Ag (ppm) | Zn (%) | Pb (%) | Moisture (%) |
|---|---|---|---|---|
| Old—Pb (3–4) | 61 | 2.38 | 2.20 | 8.53 |
| New—Pb (3–4) | 51 | 1.20 | 1.71 | 11.95 |
| New—Pb (2–3) | 83 | 1.61 | 1.70 | 4.40 |
| Old—Pb (2–3) | 50 | 2.20 | 1.72 | 9.10 |
| Old—Pb (1–2) | 40 | 1.51 | 1.06 | 4.40 |
| New—Pb (1–2) | 66 | 1.75 | 1.51 | 4.80 |
| Variables | Residue Type | PAX Dosage (g/t) | Na2S Dosage (kg/t) | pH | Flotation Time (min) | Density (kg/m3) |
|---|---|---|---|---|---|---|
| Levels | Old/new | 250–700 | 2–6 | Pb < 1 | 5–30 | 110–1300 |
| Run | A: Density (g/cm3) | B: Time (min) | C: pH | D: Sodium Sulfide Dosage (kg/t) | E: Amyl Xanthate Dosage (g/t) | F: Ore Type |
|---|---|---|---|---|---|---|
| 1 | 1100 | 20 | 8 | 2 | 577.50 | old |
| 2 | 1224 | 30 | 10 | 4.36 | 425 | new |
| 3 | 1100 | 30 | 10 | 2 | 750 | new |
| 4 | 1100 | 15 | 10 | 6 | 250 | new |
| 5 | 1100 | 30 | 9 | 3.96 | 250 | old |
| 6 | 1300 | 20 | 9 | 4.22 | 549.05 | old |
| 7 | 1152 | 30 | 10 | 6 | 727.50 | old |
| 8 | 1300 | 30 | 8 | 2 | 535 | new |
| 9 | 1100 | 5 | 9 | 4.52 | 725 | old |
| 10 | 1195 | 15 | 10 | 2 | 687.50 | new |
| 11 | 1206 | 10 | 8.5 | 2.26 | 741.64 | new |
| 12 | 1300 | 30 | 9 | 6 | 750 | new |
| 13 | 1198 | 5 | 9 | 6 | 454.61 | new |
| 14 | 1264 | 5 | 8.5 | 2.18 | 275 | old |
| 15 | 1300 | 5 | 10 | 2 | 362.50 | new |
| 16 | 1300 | 15 | 8 | 5 | 250 | new |
| 17 | 1290 | 5 | 10 | 6 | 250 | old |
| 18 | 1100 | 5 | 8 | 2.60 | 250 | new |
| 19 | 1181 | 25 | 9 | 2 | 250 | new |
| 20 | 1300 | 30 | 10 | 2 | 250 | old |
| 21 | 1218 | 30 | 8 | 3.6 | 750 | old |
| 22 | 1206 | 10 | 8.5 | 2.26 | 741.64 | new |
| 23 | 1210 | 10 | 10 | 4.94 | 750 | new |
| 24 | 1150 | 15 | 10 | 2.80 | 427.5 | old |
| 25 | 1224 | 30 | 10 | 4.36 | 425 | new |
| 26 | 1198 | 5 | 9 | 6 | 454.61 | new |
| 27 | 1100 | 20 | 9.5 | 4.36 | 577.5 | new |
| 28 | 1300 | 20 | 9 | 4.22 | 549.05 | old |
| 29 | 1295 | 5 | 10 | 2 | 750 | old |
| 30 | 1125.63 | 5 | 10 | 6 | 484.80 | old |
| 31 | 1210 | 15 | 9 | 6 | 750 | old |
| 32 | 1150 | 15 | 10 | 2. | 427.50 | old |
| 33 | 1100 | 5 | 9 | 4.17 | 272.50 | old |
| Model Type | F-Value of Model | p-Value of Model | Lack of Fit (p-Value) | C.V.% | R2 | Adequate Precision | Significant Variables * |
|---|---|---|---|---|---|---|---|
| Lead recovery | 7.88 | <0.0001 | 0.1925 | 27.38 | 0.7243 | 11.8333 | C, D, E, BD, DF |
| Lead grade | 36.46 | <0.0001 | 0.7676 | 7.31 | 0.9877 | 21.4947 | D, E, AB, AD, AE. AF, BC, BD, BE, BF, DE, EF, A2, B2, C2, D2 |
| Zinc recovery | 4.83 | 0.0029 | 0.9919 | 10.13 | 0.8758 | 10.0156 | A, B, C, AE, BC, DF, EF, A2, B2, C2 |
| Zinc grade | 5.63 | 0.0037 | 0.9549 | 6.05 | 0.9253 | 11.3558 | B, C, D, F, AD, AE, AF, BC, BF, CD, CF, DF, B2, C2, D2, E2 |
| Silver recovery | 5.58 | 0.0007 | 0.7867 | 22.90 | 0.8481 | 8.3676 | A, C, E, AE, BC, CF, DF, C2 |
| Silver grade | 19.76 | <0.0001 | 0.3297 | 10.22 | 0.9705 | 15.8459 | A, B, D, AB, AC, AD, AE, AF, BC, BD, BF, CD, CE, DE, EF, A2, E2 |
| 1.8 | Feed Lead Grade (%) | 340 | Number of Working Days per Year (Days) |
| 1.55 | Feed zinc grade (%) | 3 | Number of shifts per day |
| 35 | Feed silver grade (ppm) | 8 | Working hours per shift (hours) |
| 40 | Product lead grade (%) | 2120 | Daily input capacity (tons) |
| 5 | (%) Zinc grade in the product | 17,820 | Annual production of lead concentrate (tons) |
| 800 | Silver grade of the product (ppm) | 52.40 | Daily production (tons of concentrate) |
| 1.48 | The amount of zinc (%) left in the tailing | 875 | Price per ton of concentrate (USD) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamran Haghighi, H.; Hoseinian, F.S.; Maria Sastre, A. A New Feasible Opportunity for Recycling Lead and Silver from Zinc Plant Residues by Flotation. Materials 2024, 17, 5218. https://doi.org/10.3390/ma17215218
Kamran Haghighi H, Hoseinian FS, Maria Sastre A. A New Feasible Opportunity for Recycling Lead and Silver from Zinc Plant Residues by Flotation. Materials. 2024; 17(21):5218. https://doi.org/10.3390/ma17215218
Chicago/Turabian StyleKamran Haghighi, Hossein, Fatemeh Sadat Hoseinian, and Ana Maria Sastre. 2024. "A New Feasible Opportunity for Recycling Lead and Silver from Zinc Plant Residues by Flotation" Materials 17, no. 21: 5218. https://doi.org/10.3390/ma17215218
APA StyleKamran Haghighi, H., Hoseinian, F. S., & Maria Sastre, A. (2024). A New Feasible Opportunity for Recycling Lead and Silver from Zinc Plant Residues by Flotation. Materials, 17(21), 5218. https://doi.org/10.3390/ma17215218





