Electrochemical Impedance Spectroscopy Study of Ceria- and Zirconia-Based Solid Electrolytes for Application Purposes in Fuel Cells and Gas Sensors
Abstract
:1. Introduction
1.1. The Aim of the Research
1.2. Literature Review
2. Materials and Methods
2.1. Materials Used
2.2. Samples Preparation
2.3. Characterisation of Sintered Discs
2.4. Electrochemical Property Examination by Electrochemical Impedance Spectroscopy (EIS)
3. Results
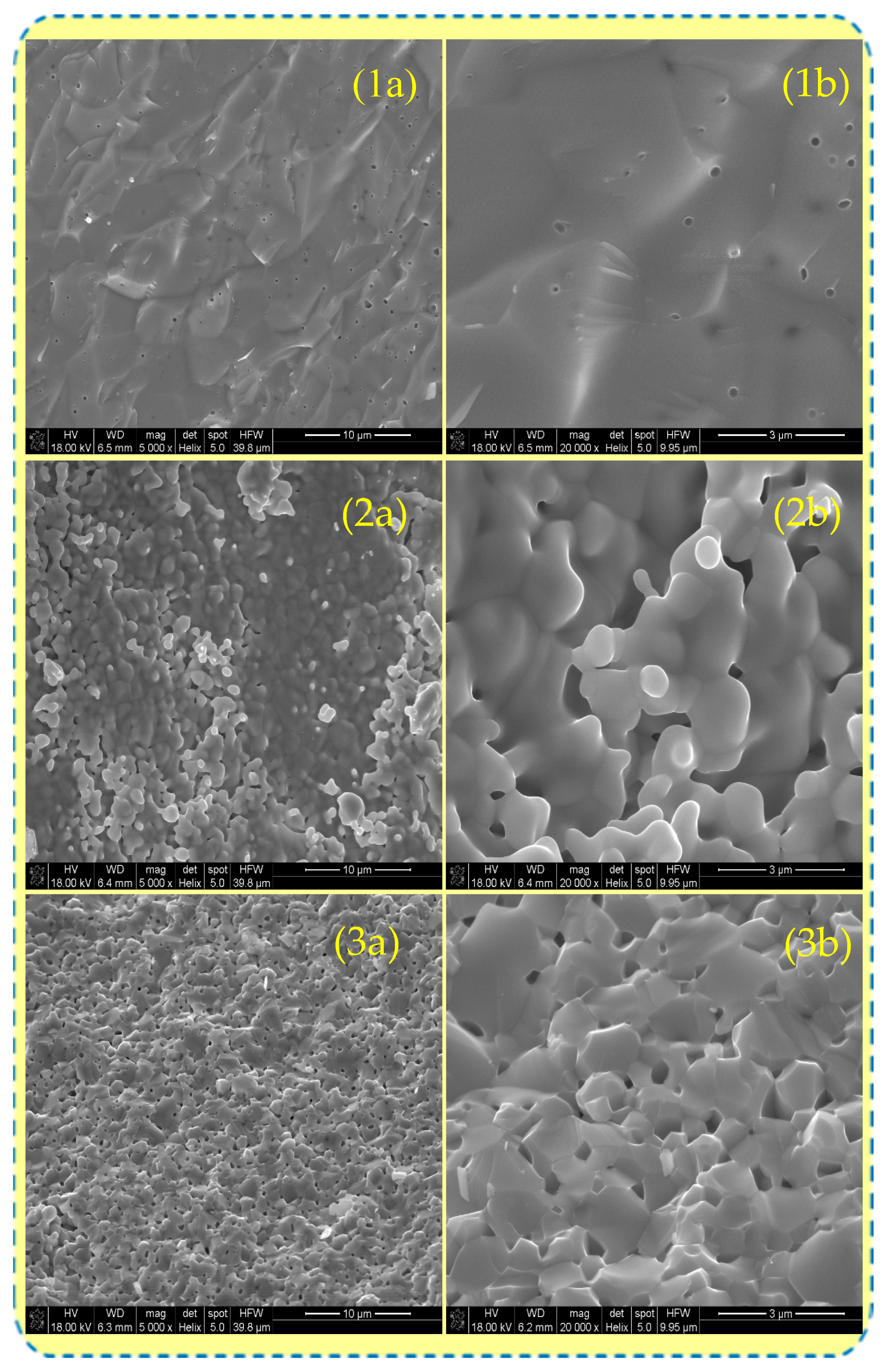
3.1. Morphological Features of the Sintered Samples
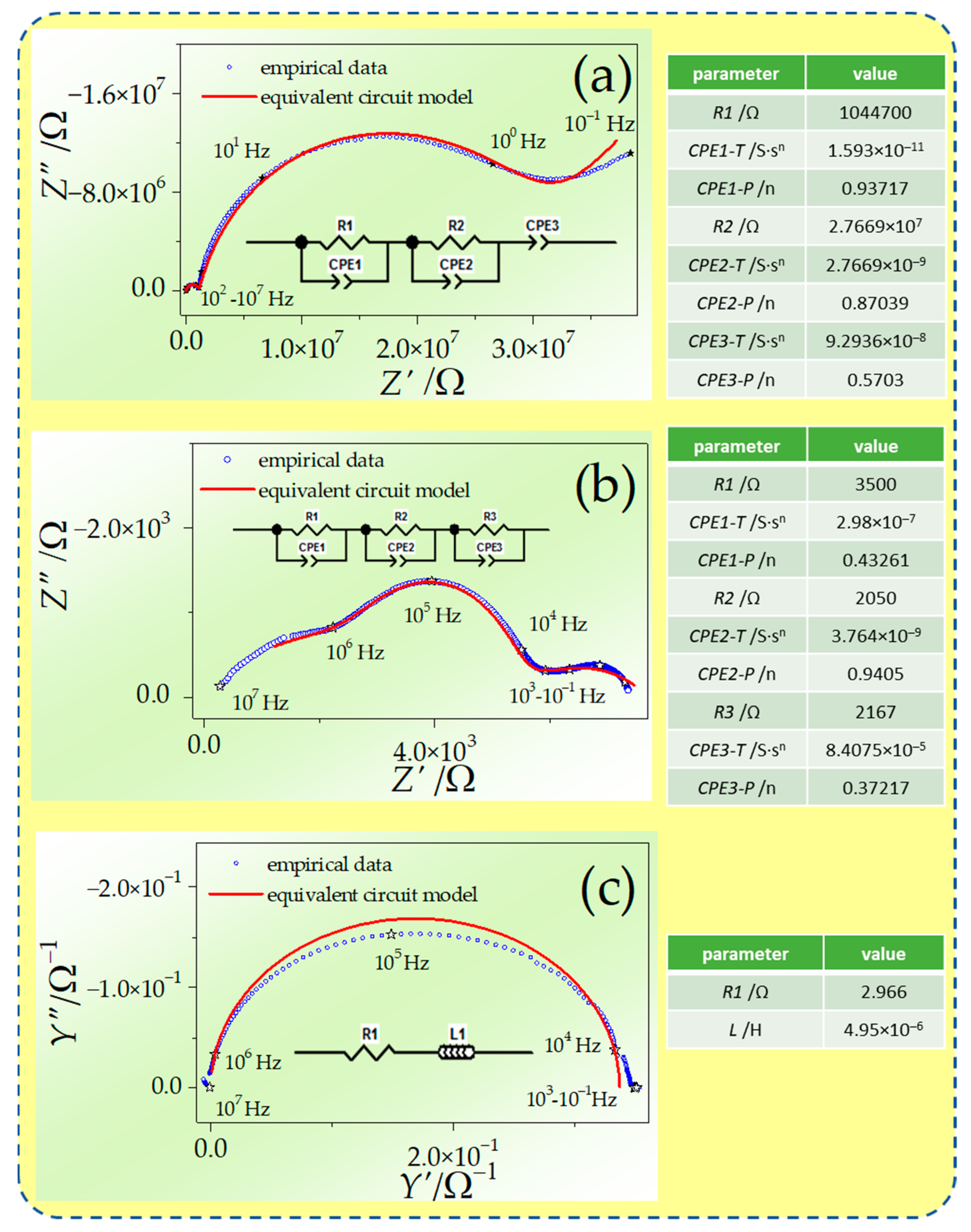
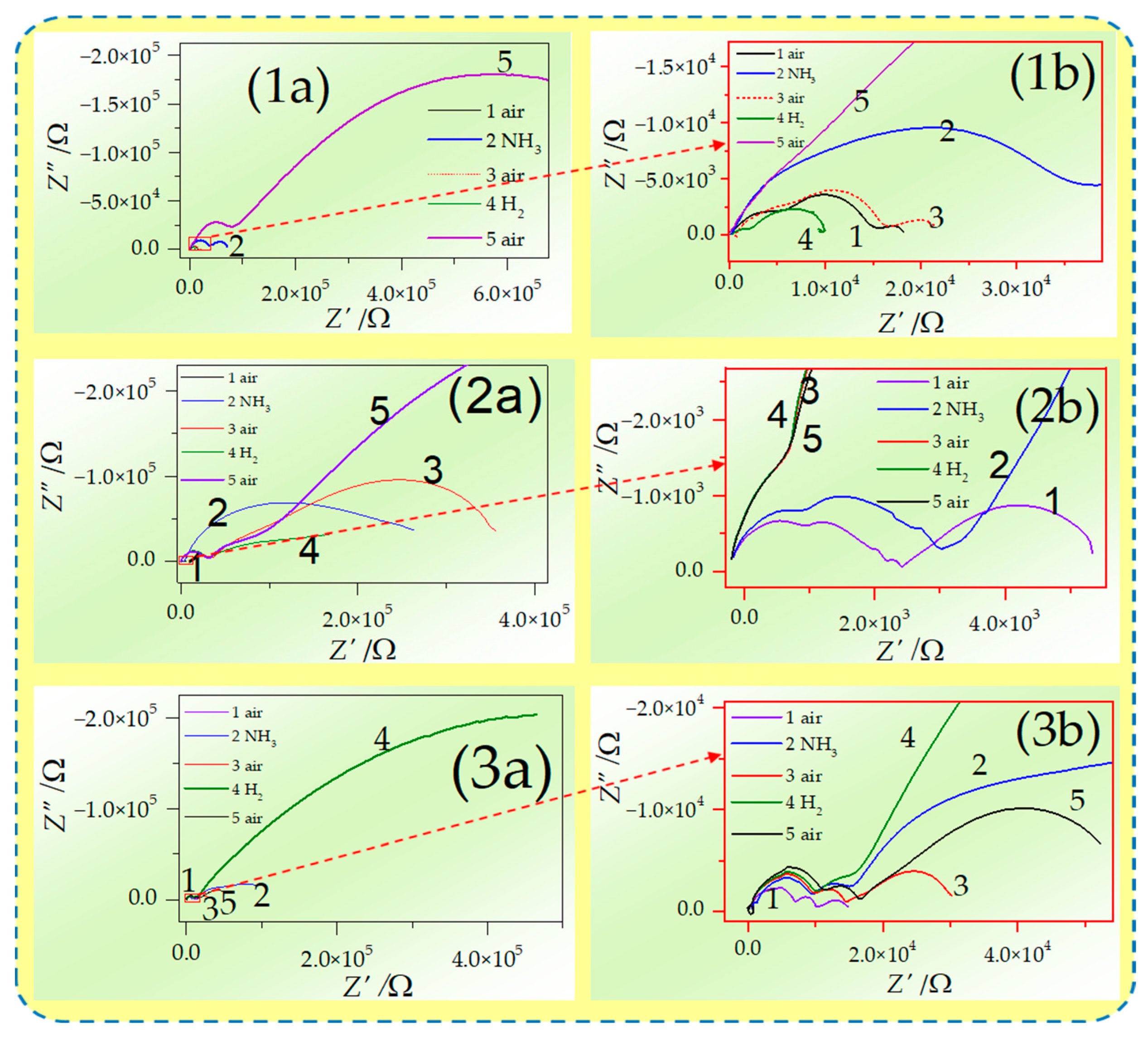
3.2. Electrochemical Properties of the Sintered Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| List of Abbreviations (In Order of Appearance) | |
| YSZ | yttria-stabilized zirconia |
| CGO10 | Ce0.9Gd0.1O2−δ |
| CGO20 | Ce0.8Gd0.2O2−δ |
| GDC | Ce0.8Gd0.2O1.9 |
| ScCeSZ | Sc0.1Ce0.01Zr0.89O1.91 |
| ScYbSZ | Sc0.09Yb0.01Zr0.9O1.95 |
| XRD | X-ray diffraction spectroscopy |
| EDS | energy-dispersive X-ray spectroscopy |
| EIS | electrochemical impedance spectroscopy |
| SOFC | solid oxide fuel cell |
| List of Symbols (In Order of Appearance) | |
| Zr4+ | zirconium cation |
| MeO | metal in the second oxidation state oxide |
| zirconia in the fourth oxidation state ion sitting on metal in the second oxidation state lattice site with double positive charge | |
| double positively charged oxygen vacancy | |
| an oxygen ion sitting on lattice site with neutral charge | |
| metal in the third oxidation state oxide | |
| a rare earth oxide | |
| Ce4+ | ceria cation in the fourth oxidation state |
| Ce3+ | ceria cation in the third oxidation state |
| δ | oxygen deficiency in metal-doped oxide ceramic |
| X | Vegard’s Slope |
| ri | difference between the ionic radii of the dopant metal and Ce4+ for the coordination number equal to 8 |
| zi | charge difference between the introduced ion and Ce4+ ions |
| σ | specific conductivity |
| 2Θ | angle between incident beam and the crystallographic reflection plane in XRD |
| A | lattice constant |
| R1, R2, R3 | resistor elements |
| CPE1, CPE2, CPE3 | constant phase elements |
| L1 | inductance element |
| R1, R2, R3 | resistance parameters of resistors |
| CPE1-T, CPE2-T, CPE3-T | time parameter in constant phase elements |
| CPE1-P, CPE2-T, CPE3-T | phase parameter in constant phase element |
| L | inductance element parameter |
| Z′ | real impedance component |
| Z″ | imaginary impedance component |
| Y′ | real admittance component |
| Y″ | imaginary admittance component |
| T | absolute temperature |
| t | temperature in degrees Celsius |
| q | valency of mobile ions |
| c | relative concentration of mobile ions to the number of possible positions in the lattice |
| μ | mobility of the ions |
| electron | |
| n-time negatively charged oxygen vacancy | |
| O2− | oxygen anion |
| A | a constant in Arrhenius law |
| EA | the activation energy for conduction |
| k | Boltzman’s constant |
| oxygen partial pressure | |
| m | a parameter determined by both the type of the carrier (n or p) and the defects (e.g., oxygen vacancy) in the semiconductor |
| hydroxide ion | |
| n | numbers of electrons |
| proton in interstitial site | |
| hydroxide ion sitting on oxygen lattice site with positive charge | |
| List of Chemical Formulas (In Order of Appearance) | |
| Ce0.8Gd0.2O1.9 | assumed composition of commercial material (specimen GDC) |
| Sc0.1Ce0.01Zr0.89O1.95 | assumed composition of commercial material (specimen ScCeSZ) |
| Sc0.09Yb0.01Zr0.9O1.95 | assumed composition of commercial material (specimen ScYbSZ) |
| Ce(Re)O2−δ | rare earth doped ceria |
| NH3 | gaseous ammonia |
| H2 | gaseous hydrogen |
| ZrO2 | zirconia |
| CO | carbon monoxide |
| SiO2 | silica |
| MnO2 | manganese oxide |
| Al2O3 | aluminum oxide |
| CaO | calcium oxide |
| MgO | magnesium oxide |
| Y2O3 | ytrria |
| Sc2O3 | scandia |
| Yb2O3 | ytterbium oxide |
| Si3N4 | a silicon nitride |
| AlN | aluminum nitride |
| CuO | copper (II) oxide |
| c-ZrO2 | cubic zirconia |
| N2O | nitrous oxide |
| H2O | water |
References
- Song, X.; Ding, Y.; Zhang, J.; Jiang, C.; Liu, Z.; Lin, C.; Zheng, W.; Zeng, Y. Thermophysical and Mechanical Properties of Cubic, Tetragonal and Monoclinic ZrO2. J. Mater. Res. Technol. 2023, 23, 648–655. [Google Scholar] [CrossRef]
- Guan, S.H.; Liu, Z.P. Theoretical Aspects on Doped-Zirconia for Solid Oxide Fuel Cells: From Structure to Conductivity. Chin. J. Chem. Phys. 2021, 34, 125–136. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Wang, X.; Yu, J.; Li, L. A Review of Zirconia-Based Solid Electrolytes. Ionics 2016, 22, 2249–2262. [Google Scholar] [CrossRef]
- Coutinho, M.M.; De Paula Nascimento, A.C.; Amarante, J.E.V.; Dos Santos, C.; De Almeida Ferreira, J.L.; Da Silva, C.R.M. Four-Point Bending Fatigue Behavior of Al2O3-ZrO2Ceramic Biocomposites Using CeO2 as Dopant. Mater. Res. 2022, 25, e20220199. [Google Scholar] [CrossRef]
- Dresvyannikov, A.F.; Petrova, E.V.; Kashfrazyeva, L.I. Electrochemical Synthesis of Precursors of Al2O3-ZrO2 Ceramic Stabilized with Cerium Oxide and Magnesium Aluminate. Inorganics 2022, 10, 57. [Google Scholar] [CrossRef]
- Inserra, B.; Coppola, B.; Montanaro, L.; Tulliani, J.M.; Palmero, P. Preparation and Characterization of Ce-ZrO2/Al2O3 Composites by DLP-Based Stereolithography. J. Eur. Ceram. Soc. 2023, 43, 2907–2916. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Jung, I.H. Thermodynamic Assessment of the Al2O3–ZrO2, CaO–Al2O3–ZrO2, and Al2O3–SiO2–ZrO2 Systems. Ceram. Int. 2022, 48, 5413–5427. [Google Scholar] [CrossRef]
- Liu, S.; Song, Z.; Wang, J.; Han, B.; Sun, Y. In Situ Self-Assembly Preparation and Characterization of CaO–ZrO2 Nanopowders under Vacuum. Vacuum 2023, 213, 112089. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Yigit, N.; Wicht, T.; Stöger-Pollach, M.; Lucrédio, A.F.; Marcos, F.C.F.; Assaf, E.M.; Rupprechter, G. Partial Oxidation of Bio-Methane over Nickel Supported on MgO–ZrO2 Solid Solutions. Top. Catal. 2023, 66, 1539–1552. [Google Scholar] [CrossRef]
- Lu, N.; He, G.; Yang, Z.; Yang, X.; Li, Y.; Li, J. Fabrication and Reaction Mechanism of MgO-Stabilized ZrO2 Powders by Combustion Synthesis. Ceram. Int. 2022, 48, 7261–7264. [Google Scholar] [CrossRef]
- Li, F.; Yang, H.; Zhang, J.; Gu, C.; Huo, J.; Jiang, L.; Wang, X.; Zhao, D. OH-PLIF Investigation of Y2O3-ZrO2 Coating Improving Flame Stability in a Narrow Channel. Chem. Eng. J. 2021, 405, 126708. [Google Scholar] [CrossRef]
- Cao, W.; Zhou, J.; Ren, C.; Omran, M.; Gao, L.; Tang, J.; Zhang, F.; Chen, G. Research on the Drying Kinetics for the Microwave Drying of Y2O3–ZrO2 Ceramic Powder. J. Mater. Res. Technol. 2023, 26, 4563–4580. [Google Scholar] [CrossRef]
- Komissarenko, D.A.; Sokolov, P.S.; Evstigneeva, A.D.; Slyusar, I.V.; Nartov, A.S.; Volkov, P.A.; Lyskov, N.V.; Evdokimov, P.V.; Putlayev, V.I.; Dosovitsky, A.E. DLP 3D Printing of Scandia-Stabilized Zirconia Ceramics. J. Eur. Ceram. Soc. 2021, 41, 684–690. [Google Scholar] [CrossRef]
- Mosiałek, M.; Hanif, M.B.; Šalkus, T.; Kežionis, A.; Kazakevičius, E.; Orliukas, A.F.; Socha, R.P.; Łasocha, W.; Dziubaniuk, M.; Wyrwa, J.; et al. Synthesis of Yb and Sc Stabilized Zirconia Electrolyte (Yb0.12Sc0.08Zr0.8O2−δ) for Intermediate Temperature SOFCs: Microstructural and Electrical Properties. Ceram. Int. 2023, 49, 15276–15283. [Google Scholar] [CrossRef]
- Luo, C.; Li, N.; Deng, T. Enhancement of Si3N4 Ceramics via Evolution of Grain Interface between ZrO2 and Si3N4 under Pressureless Sintering. J. Am. Ceram. Soc. 2023, 106, 7043–7056. [Google Scholar] [CrossRef]
- Parveen, A.; Chauhan, N.R.; Suhaib, M. Influence of Compaction Pressure and Si3N4/ZrO2 Reinforcement on the Properties of Aluminium Hybrid Composites. Adv. Mater. Process. Technol. 2022, 8, 2905–2917. [Google Scholar] [CrossRef]
- Traipanya, K.; Wasanapiarnpong, T.; Mongkolkachit, C. Fabrication and Characterizations of High Density Si3N4 - ZrO2 Ceramics. J. Met. Mater. Miner. 2023, 33, 1621. [Google Scholar] [CrossRef]
- Mu, T.; Xu, W.; Ling, J.; Dong, T.; Qin, Z.; Zhou, Y. Microstructure and Properties of ZrO2-AlN Composite Ceramics by Microwave Sintering. Wuji Cailiao Xuebao/J. Inorg. Mater. 2021, 36, 1231–1236. [Google Scholar] [CrossRef]
- Zang, X.; Lu, Y. Preparation and Dielectric Properties at High Frequency of AlN-Based Composited Ceramic. J. Mater. Sci. Mater. Electron. 2020, 31, 2826–2832. [Google Scholar] [CrossRef]
- Xing, Y.Z.; Men, Y.N.; Feng, X.; Geng, J.H.; Zou, Z.R.; Chen, F.H. Evolutions in the Microstructure and Ionic Conductivity of CuO-Doped Yttria-Stabilized Zirconia. J. Solid. State Chem. 2022, 315, 123497. [Google Scholar] [CrossRef]
- Farid, M.A.; Ijaz, S.; Ashiq, M.N.; Ehsan, M.F.; Gul, F.; Batool, S.R.; Athar, M.; ul Hassan, S. Synthesis of Mesoporous Zirconium Manganese Mixed Metal Oxide Nanowires for Photocatalytic Reduction of CO2. J. Mater. Res. 2022, 37, 522–532. [Google Scholar] [CrossRef]
- Anandan, K.; Rajendran, V. Size and Magnetic Effect of Manganese (Mn) Doped Zirconia (Zro2) Nanoparticles. Int. J. Innov. Technol. Explor. Eng. 2020, 9, 257–260. [Google Scholar] [CrossRef]
- Gao, L.; Guan, R.; Zhang, S.; Zhi, H.; Jin, C.; Jin, L.; Wei, Y.; Wang, J. As-Sintered Manganese-Stabilized Zirconia Ceramics with Excellent Electrical Conductivity. Crystals 2022, 12, 620. [Google Scholar] [CrossRef]
- Chang, C.H.; Lin, C.Y.; Chang, C.H.; Liu, F.H.; Huang, Y.T.; Liao, Y.S. Enhanced Biomedical Applicability of ZrO2–SiO2 Ceramic Composites in 3D Printed Bone Scaffolds. Sci. Rep. 2022, 12, 6845. [Google Scholar] [CrossRef] [PubMed]
- Akbarpour, S.; Khoshandam, B.; Maroufi, S. The Synthesis and Crystal Phase Evolution of SiO2-Stabilized Zirconia Nanocomposites at Low Temperatures for the Production of Zircon. J. Mater. Eng. Perform. 2023, 32, 6214–6225. [Google Scholar] [CrossRef]
- Zheng, X.; Hong, X.; Qiao, X.; Yang, Y.; Jiao, S. Highly Sensitive NO2 Sensor Based on Mesoporous ZrO2–WO3 Nanotubes Composite. Mater. Res. Bull. 2023, 167, 112394. [Google Scholar] [CrossRef]
- Pal, N.; Dutta, G.; Kharashi, K.; Murray, E.P. Investigation of an Impedimetric LaSrMnO3-Au/Y2O3-ZrO2-Al2O3 Composite NOx Sensor. Materials 2022, 15, 1165. [Google Scholar] [CrossRef]
- Pandiyan, R.; Vinothkumar, V.; Chen, T.W.; Chen, S.M.; Abinaya, M.; Rwei, S.P.; Hsu, H.Y.; Huang, C.W.; Yu, M.C. Synthesis of Ag@ZrO2 Nanoparticles: A Sensitive Electrochemical Sensor for Determination of Antibiotic Drug Tinidazole. Int. J. Electrochem. Sci. 2022, 17, 220414. [Google Scholar] [CrossRef]
- Feng, G.; Che, Y.; Wang, S.; Wang, S.; Hu, J.; Xiao, J.; Song, C.; Jiang, L. Sensitivity Enhancement of In2O3/ZrO2 Composite Based Acetone Gas Sensor: A Promising Collaborative Approach of ZrO2 as the Heterojunction and Dopant for in-Situ Grown Octahedron-like Particles. Sens. Actuators B Chem. 2022, 367, 132087. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Espro, C.; Iannazzo, D.; Moulaee, K.; Neri, G. A Novel Yttria-Doped ZrO2 Based Conductometric Sensor for Hydrogen Leak Monitoring. Int. J. Hydrogen Energy 2022, 47, 9819–9828. [Google Scholar] [CrossRef]
- Dudek, M. Composite Oxide Electrolytes for Electrochemical Devices. Adv. Mater. Sci. 2008, 8, 15–30. [Google Scholar] [CrossRef]
- Escudero, M.J.; Valero, C.; Cauqui, M.Á.; Goma, D.; Yeste, M.P. Ni-Ce-ZrO2 System as Anode Material for Direct Internal Reforming Biogas Solid Oxide Fuel Cells. Fuel 2022, 322, 124247. [Google Scholar] [CrossRef]
- Li, S.; Guo, R.; Li, J.; Chen, Y.; Liu, W. Synthesis of NiO-ZrO2 Powders for Solid Oxide Fuel Cells. Ceram. Int. 2003, 29, 883–886. [Google Scholar] [CrossRef]
- Maiti, T.K.; Majhi, J.; Maiti, S.K.; Singh, J.; Dixit, P.; Rohilla, T.; Ghosh, S.; Bhushan, S.; Chattopadhyay, S. Zirconia- and Ceria-Based Electrolytes for Fuel Cell Applications: Critical Advancements toward Sustainable and Clean Energy Production. Environ. Sci. Pollut. Res. 2022, 29, 64489–64512. [Google Scholar] [CrossRef]
- Li, Z.; Men, Y.; Liu, S.; Wang, J.; Qin, K.; Tian, D.; Shi, T.; Zhang, L.; An, W. Boosting CO2 Hydrogenation Efficiency for Methanol Synthesis over Pd/In2O3/ZrO2 Catalysts by Crystalline Phase Effect. Appl. Surf. Sci. 2022, 603, 154420. [Google Scholar] [CrossRef]
- Mu, S.; Liu, K.; Li, H.; Zhao, Z.; Lyu, X.; Jiao, Y.; Li, X.; Gao, X.; Fan, X. Microwave-Assisted Synthesis of Highly Dispersed ZrO2 on CNTs as an Efficient Catalyst for Producing 5-Hydroxymethylfurfural (5-HMF). Fuel Process. Technol. 2022, 233, 107292. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, M.; Xie, Z.; Tian, D.; Sheng, G.; Tang, X.; Li, H.; Wu, Y.; Song, C.; Gao, X.; et al. Insights into the Interfacial Structure of Cu/ZrO2 Catalysts for Methanol Synthesis from CO2 Hydrogenation: Effects of Cu-Supported Nano-ZrO2 Inverse Interface. Chem. Eng. J. 2023, 470, 144006. [Google Scholar] [CrossRef]
- Ge, Y.; Zou, T.; Martín, A.J.; Pérez-Ramírez, J. ZrO2-Promoted Cu-Co, Cu-Fe and Co-Fe Catalysts for Higher Alcohol Synthesis. ACS Catal. 2023, 13, 9946–9959. [Google Scholar] [CrossRef]
- Sasaki, K.; Natsukoshi, K.; Yamada, K.; Ikegawa, K.; Yasutake, M.; Tachikawa, Y.; Lyth, S.M.; Matsuda, J.; Yildiz, B.; Tuller, H.L. Reversible Solid Oxide Cells: Selection of Fuel Electrode Materials for Improved Performance and Durability. ECS Trans. 2023, 111, 1901. [Google Scholar] [CrossRef]
- Yusupandi, F.; Devianto, H.; Widiatmoko, P.; Nurdin, I.; Yoon, S.P.; Lim, T.H.; Arif, A.F. Performance Evaluation of An Electrolyte-Supported Intermediate-Temperature Solid Oxide Fuel Cell (IT-SOFC) with Low-Cost Materials. Int. J. Renew. Energy Dev. 2022, 11, 1037–1042. [Google Scholar] [CrossRef]
- Svitlyk, V.; Weiss, S.; Hennig, C. Stability of Doped Zirconia under Extreme Conditions: Toward Long-Term and Secure Storage of Radioactive Waste. J. Am. Ceram. Soc. 2022, 105, 7831–7839. [Google Scholar] [CrossRef]
- Abdala, P.M.; Craievich, A.F.; Fantini, M.C.A.; Temperini, M.L.A.; Lamas, D.G. Metastable Phase Diagram of Nanocrystalline ZrO2-Sc2O3 Solid Solutions. J. Phys. Chem. C 2009, 113, 18661–18666. [Google Scholar] [CrossRef]
- Arachi, Y.; Sakai, H.; Yamamoto, O.; Takeda, Y.; Imanishai, N. Electrical Conductivity of the ZrO2-Ln2O3 (Ln = Lanthanides) System. Solid. State Ion. 1999, 121, 133–139. [Google Scholar] [CrossRef]
- Salomone, F.; Sartoretti, E.; Ballauri, S.; Castellino, M.; Novara, C.; Giorgis, F.; Pirone, R.; Bensaid, S. CO2 Hydrogenation to Methanol over Zr- and Ce-Doped Indium Oxide. Catal. Today 2023, 423, 114023. [Google Scholar] [CrossRef]
- Nan, Y.; Mao, Y.; Zha, F.; Yang, Z.; Ma, S.; Tian, H. ZrO2–ZnO–CeO2 Integrated with Nano-Sized SAPO-34 Zeolite for CO2 Hydrogenation to Light Olefins. React. Kinet. Mech. Catal. 2022, 135, 2959–2972. [Google Scholar] [CrossRef]
- Suarez Anzorena, R.; Muñoz, F.F.; Toscani, L.M.; Bonelli, P.; Cukierman, A.L.; Larrondo, S.A. Ce–Zr-Sm Ternary Oxides Synthesized via a Template Free Urea-Hydrothermal Method. Ceram. Int. 2022, 48, 25714–25722. [Google Scholar] [CrossRef]
- Fazl, F.; Gholivand, M.B. High Performance Electrochemical Method for Simultaneous Determination Dopamine, Serotonin, and Tryptophan by ZrO2–CuO Co-Doped CeO2 Modified Carbon Paste Electrode. Talanta 2022, 239, 122982. [Google Scholar] [CrossRef]
- Arfaoui, J.; Ghorbel, A.; Petitto, C.; Delahay, G. New MoO3-CeO2-ZrO2 and WO3-CeO2-ZrO2 Nanostructured Mesoporous Aerogel Catalysts for the NH3-SCR of NO from Diesel Engine Exhaust. J. Ind. Eng. Chem. 2021, 95, 182–189. [Google Scholar] [CrossRef]
- Kurapova, O.Y.; Shugurov, S.M.; Vasil’eva, E.A.; Savelev, D.A.; Konakov, V.G.; Lopatin, S.I. Thermal Prehistory, Structure and High-Temperature Thermodynamic Properties of Y2O3-CeO2 and Y2O3-ZrO2-CeO2 Solid Solutions. Ceram. Int. 2021, 47, 11072–11079. [Google Scholar] [CrossRef]
- Kurapova, O.Y.; Glukharev, A.G.; Glumov, O.V.; Konakov, V.G. The Effect of the Sintering Parameters on the Structure and Oxygen Ion Conductivity of Y2O3–ZrO2–CeO2 Ceramics. Open Ceram. 2021, 5, 100086. [Google Scholar] [CrossRef]
- Mileva, A.; Tsoncheva, T.; Issa, G.; Dimitrov, M.; Kovacheva, D.; Henych, J. Nanosized CuO-CeO2 -TiO2 and CuO-ZrO2-TiO2 Composites as Catalysts for Methanol Decomposition: Effect of Modification Procedure. J. Chem. Technol. Metall. 2021, 56, 782–790. [Google Scholar]
- Kornienko, O.A.; Andrievskaya, O.R.; Barshchevskaya, H.K. Phase Relations in the System Ternary Based on Ceria, Zirconia and Ytterbia at 1500 °C. J. Chem. Technol. 2021, 28, 142–152. [Google Scholar] [CrossRef]
- Demchenko, I.N.; Nikiforow, K.; Chernyshova, M.; Melikhov, Y.; Syryanyy, Y.; Korsunska, N.; Khomenkova, L.; Brodnikovskyi, Y.; Brodnikovskyi, D. X-Ray Photoelectron Spectroscopy Analysis of Scandia-Ceria-Stabilized Zirconia Composites with Different Transport Properties. Materials 2023, 16, 5504. [Google Scholar] [CrossRef] [PubMed]
- Yuze, I.L.; Ng, C.K.; Poon, K.L.; Ting, C.H.; Ramesh, S.; Chuah, Y.D.; Tan, C.Y.; Sara Lee, K.Y.; Sutharsini, U. Effects of Sintering on the Properties of Ceria Co-Doped Scandia Stabilised Zirconia. In Proceedings of the AIP Conference Proceedings; AIP Press: New York, NY, USA, 2023; Volume 2643. [Google Scholar]
- Arifin, N.A.; Afifi, A.A.; Samreen, A.; Hafriz, R.S.R.M.; Muchtar, A. Characteristic and Challenges of Scandia Stabilized Zirconia as Solid Oxide Fuel Cell Material—In Depth Review. Solid. State Ion. 2023, 399, 116302. [Google Scholar] [CrossRef]
- Agarkov, D.; Borik, M.; Komarov, B.; Korableva, G.; Kulebyakin, A.; Kuritsyna, I.; Lomonova, E.; Milovich, F.; Myzina, V.; Tabachkova, N. Long-Term Conductivity Stability of Electrolytic Membranes of Scandia Stabilized Zirconia Co-Doped with Ytterbia. Membranes 2023, 13, 586. [Google Scholar] [CrossRef]
- Taniguchi, S.; Miyara, K.; Kawabata, T.; Uryu, C.; Inoue, Y.; Chou, J.-T.; Sasaki, K. Stability of Nickel/Scandia-Doped-Stabilized-Zirconia Composite Anode Under High Fuel Utilization Conditions. ECS Trans. 2021, 103, 1879–1883. [Google Scholar] [CrossRef]
- Aflaki, M.; Davar, F.; Loghman Estarki, M.R.; Wang, R.; Guo, L. Sc3+:Ce4+:Y3+ Doped Zirconia Nanopowders (ScCeYSZ): Synthesis, Thermal Phase Stability and Hot Corrosion Behavior of Spark Plasma Sintered Body. Arab. J. Chem. 2023, 16, 105160. [Google Scholar] [CrossRef]
- Kishimoto, A.; Umemura, T.; Kondo, S.; Teranishi, T. Ceria-Based Solid Electrolyte Exhibits Superior Mechanical and Electric Properties Compared to Zirconia-Based Solid Electrolyte. Ceram. Int. 2022, 48, 21824–21831. [Google Scholar] [CrossRef]
- Momin, N.; Manjanna, J.; Aruna, S.T.; Senthil Kumar, S.; Anjaneya, K.C. Effect of 20 mol % Gadolinium Doping on Oxide Ion Conductivity of Ceria as Electrolyte for Intermediate Temperature Solid Oxide Fuel Cells. Ceram. Int. 2022, 48, 35867–35873. [Google Scholar] [CrossRef]
- Acharya, S.A.; Gaikwad, V.M. Understanding of Dynamics of Electrical Processes in Nanostructured Gd-Doped Ceria. Ferroelectrics 2022, 588, 164–179. [Google Scholar] [CrossRef]
- Kemp, D.; Tarancón, A.; De Souza, R.A. Recipes for Superior Ionic Conductivities in Thin-Film Ceria-Based Electrolytes. Phys. Chem. Chem. Phys. 2022, 24, 12926–12936. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.J.M.; Loureiro, F.J.A.; Holz, L.I.V.; Grilo, J.P.F.; Macedo, D.A.; Paskocimas, C.A.; Fagg, D.P. Composite of Ca-Cobaltite with Pr-Doped Ceria as Oxygen Electrode for Solid Oxide Electrochemical Cells. ECS Meet. Abstr. 2021, MA2021-03, 129. [Google Scholar] [CrossRef]
- Nicholas, J.D.; De Jonghe, L.C. Prediction and evaluation of sintering aids for Cerium Gadolinium Oxide. Solid State Ionics 2007, 178, 1187–1194. [Google Scholar] [CrossRef]
- Ramesh, S. Electrical Properties of a Nanosynthesized PGDC Electrolyte Material. J. Nano-Electron. Phys. 2022, 14, 02017. [Google Scholar] [CrossRef]
- Durgesh, R.; Pal, K. Mohan Kant Comprehensive Analysis of Ce1−xSmxO2−δ Solid Electrolytes: Structural, Microstructural, and Electrochemical Characterization for Intermediate Temperature Solid Oxide Fuel Cells. ECS J. Solid. State Sci. Technol. 2023, 12, 083012. [Google Scholar]
- Gager, E.; Nino, J.C. Processing, Phase Stability, and Conductivity of Multication-Doped Ceria. Inorganics 2023, 11, 299. [Google Scholar] [CrossRef]
- Madhual, S.; Kumar, P.P. Insights on Oxide Ion Transport in Yttria-Doped Ceria from Molecular Dynamics Simulations. J. Mater. Sci. 2023, 58, 4499–4512. [Google Scholar] [CrossRef]
- Artini, C.; Viviani, M.; Presto, S.; Massardo, S.; Carnasciali, M.M.; Gigli, L.; Pani, M. Correlations between Structure, Microstructure and Ionic Conductivity in (Gd,Sm)-Doped Ceria. Phys. Chem. Chem. Phys. 2022, 24, 23622–23633. [Google Scholar] [CrossRef]
- Ajith Kumar, S.; Kuppusami, P.; Amirthapandian, S.; Fu, Y.P. Effect of Sm Co-Doping on Structural, Mechanical and Electrical Properties of Gd Doped Ceria Solid Electrolytes for Intermediate Temperature Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2020, 45, 29690–29704. [Google Scholar] [CrossRef]
- Bocardo-Roldán, C.; Díaz-Guillén, J.A.; Díaz-Guillén, J.C.; Fuentes, A.F.; Padmadas, P.K. The Electrical Properties of Zr Doped Ce0.6Y0.4O2−δ Solid Electrolytes for Solid Oxide Fuel Cell Applications. ECS Trans. 2023, 110, 215–223. [Google Scholar] [CrossRef]
- Kaur, T.; Singh, K.; Kolte, J. Effect of Intrinsic and Extrinsic Oxygen Vacancies on the Conductivity of Gd-Doped CeO2 Synthesized by a Sonochemical Route. J. Phys. Chem. C 2022, 126, 18018–18028. [Google Scholar] [CrossRef]
- Vinchhi, P.; Patel, R.; Mukhopadhyay, I.; Ray, A.; Pati, R. Effect of Doping Concentration on Grain Boundary Conductivity of Samaria Doped Ceria Composites. J. Electrochem. Soc. 2021, 168, 124515. [Google Scholar] [CrossRef]
- Anirban, S.K.; Dutta, A. Revisiting Ionic Conductivity of Rare Earth Doped Ceria: Dependency on different factors. Int. J. Hydrogen Energy 2020, 45, 25139. [Google Scholar] [CrossRef]
- Steele, B.C.H. Appraisal of Ce1−yGdyO2−y/2 Electrolytes for IT-SOFC Operation at 500 °C. Solid. State Ion. 2000, 129, 95–110. [Google Scholar] [CrossRef]
- Terio Corporation. Available online: http://www.terio.cn/product (accessed on 13 October 2024).
- Cubic Zirconia. Available online: https://rruff.info/tmp_rruff/Cubiczirconia__R110112-9__Powder__Xray_Data_XY_RAW__10945.rruff (accessed on 13 October 2024).
- Cerianite. Available online: https://rruff.info/repository/sample_child_record_powder/by_minerals/CerianiteCe__R050379-9__Powder__Xray_Data_XY_Processed__2482.txt (accessed on 13 October 2024).
- Kežionis, A.; Šalkus, T.; Dudek, M.; Madej, D.; Mosiałek, M.; Napruszewska, B.D.; Łasocha, W.; Hanif, M.B.; Motola, M. Investigation of Alumina- and Scandia-Doped Zirconia Electrolyte for Solid Oxide Fuel Cell Applications: Insights from Broadband Impedance Spectroscopy and Distribution of Relaxation Times Analysis. J. Power Sources 2024, 591, 233846. [Google Scholar] [CrossRef]
- Shirbhate, S.; Gaikwad, V.; Acharya, S. Oxygen vacancies disordering and oxy-ion diffusion mechanism in doped ceria electrolytes under IT-SOFC operating conditions. J. Solid. State Electrochem. 2022, 26, 133–148. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Dutta, P.K.; Akbar, S.A. Oxygen sensors: Materials, methods, designs and applications. J. Mater. Sci. 2003, 38, 4271. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Chieli, S.; Palombari, R. Use of solid state protonic conductors for oxygen potentiometric sensor at room temperature. Solid. State Ion. 1991, 46, 183. [Google Scholar] [CrossRef]
- Xie, L.; Li, Z.; Sun, L.; Dong, B.; Fatima, Q.; Wang, Z.; Yao, Z.; Haidry, A.A. Sol-Gel Synthesis of TiO2 with p-Type Response to Hydrogen Gas at Elevated Temperature. Front. Mater. 2019, 6, 96. [Google Scholar] [CrossRef]
- Ciftyurek, E.; Li, Z.; Schierbaum, K. Adsorbed Oxygen Ions and Oxygen Vacancies: Their Concentration and Distribution in Metal Oxide Chemical Sensors and Influencing Role in Sensitivity and Sensing Mechanisms. Sensors 2023, 23, 29. [Google Scholar] [CrossRef]
- Yu, Y.; Shah, M.A.K.Y.; Wang, H.; Cheng, X.; Guo, L.; Huang, J.; Lund, P.; Zhu, B. Synergistic Proton and Oxygen Ion Transport in Fluorite Oxide-Ion Conductor. Energy Mater. Adv. 2024, 5, 81. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia Gas Sensors: A Comprehensive Review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Gorbova, E.; Tzorbatzoglou, F.; Molochas, C.; Chloros, D.; Demin, A.; Tsiakaras, P. Fundamentals and Principles of Solid-State Electrochemical Sensors for High Temperature Gas Detection. Catalysts 2022, 12, 1. [Google Scholar] [CrossRef]



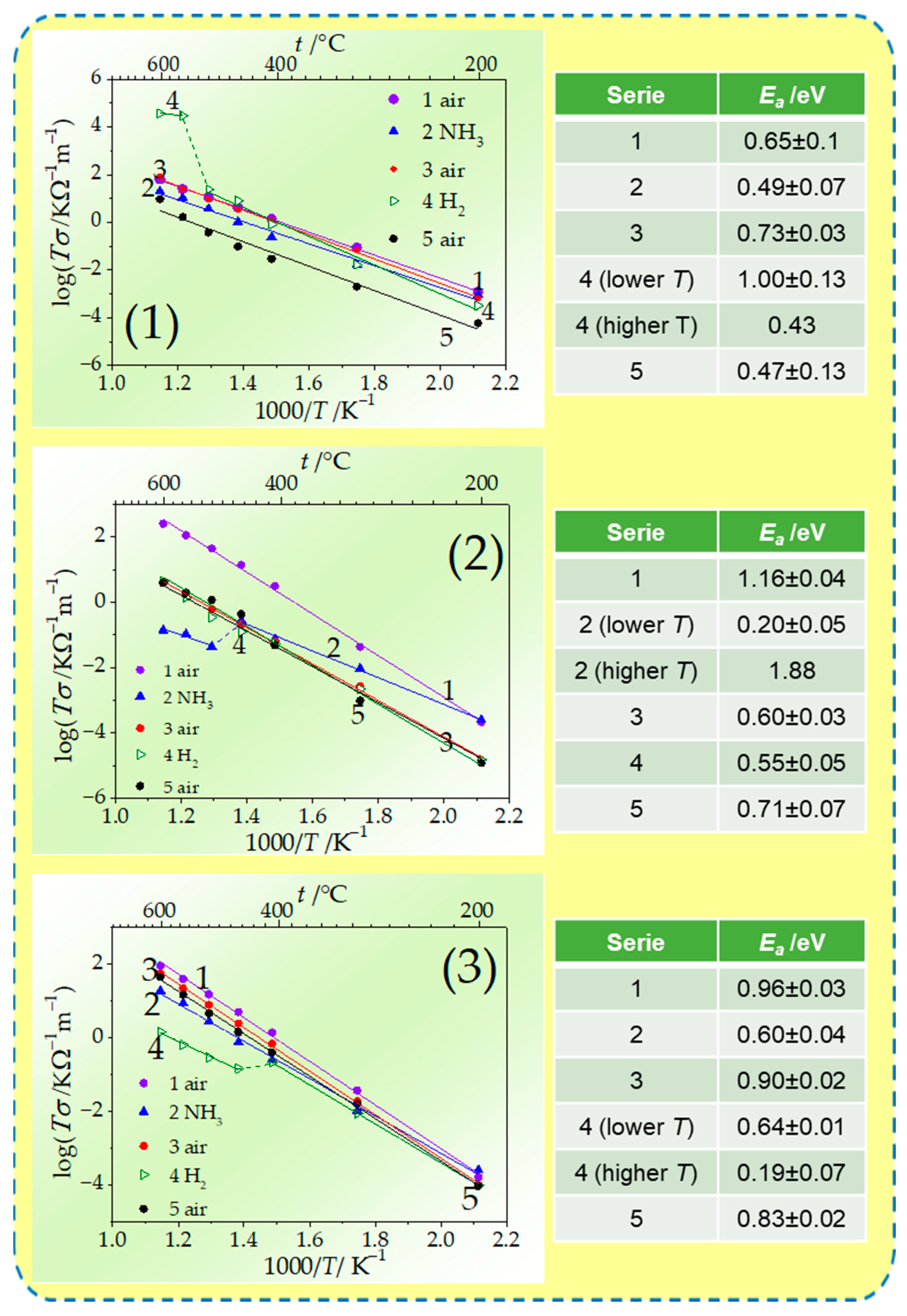
| Composition | σ/Ω−1 m−1 500 °C | σ/Ω−1 m−1 600 °C | σ/Ω−1 m−1 700 °C |
|---|---|---|---|
| Ce0.9Gd0.1O2−δ | 9.5 × 10−1 | 2.53 × 100 | 5.44 × 101 |
| Ce0.9Sm0.1O2−δ | 3.3 × 100 | 9.0 × 10−1 | 2.00 × 100 |
| Ce0.9Y0.1O2−δ | 8.7 × 10−1 | 3.44 × 100 | 1.015 × 101 |
| Ce0.8Gd0.2O2−δ | 5.3 × 10−1 | 1.8 × 100 | 4.700 × 100 |
| Specimen Label | Assumed Composition | Composition by XRD with Lattice Constant | Element | Experimental Content/%wt | Theoretical Content/%wt |
|---|---|---|---|---|---|
| GDC | Ce0.8Gd0.2O1.9 | CeO2 100% a = 0.54547 nm | O | 12.94 | 17.49 |
| Ce | 67.38 | 64.42 | |||
| Gd | 19.67 | 18.09 | |||
| ScCeSZ | Sc0.1Ce0.01Zr0.89O1.95 | c-ZrO2 96.3% a = 0.51254 nm CeO2 3.7% | O | 13.6 | 26.38 |
| Y | 1.51 | 0.00 | |||
| Zr | 72.04 | 68.64 | |||
| Sc | 9.69 | 3.80 | |||
| Ce | 3.16 | 1.18 | |||
| ScYbSZ | Sc0.09Yb0.01Zr0.9O1.95 | c-ZrO2 100% a = 0.50906 nm | O | 17.05 | 26.20 |
| Zr | 57.90 | 68.95 | |||
| Sc | 2.83 | 3.40 | |||
| Yb | 22.21 | 1.45 |
| Temperature/°C/°C | Specimen Conduction/Ω−1 m−1 | ||
|---|---|---|---|
| GDC | ScCeSZ | ScYbSZ | |
| 200 | 2.55 × 10−6 | 4.57 × 10−7 | 3.48 × 10−7 |
| 300 | 1.51 × 10−4 | 7.44 × 10−5 | 6.42 × 10−5 |
| 400 | 2.04 × 10−3 | 4.67 × 10−3 | 2.07 × 10−3 |
| 450 | 5.61 × 10−3 | 1.91 × 10−2 | 6.88 × 10−3 |
| 500 | 1.38 × 10−2 | 5.64 × 10−2 | 1.97 × 10−2 |
| 550 | 3.01 × 10−2 | 1.37 × 10−1 | 4.74 × 10−2 |
| 600 | 7.32 × 10−2 | 2.90 × 10−1 | 1.04 × 10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziubaniuk, M.; Piech, R.; Paczosa-Bator, B. Electrochemical Impedance Spectroscopy Study of Ceria- and Zirconia-Based Solid Electrolytes for Application Purposes in Fuel Cells and Gas Sensors. Materials 2024, 17, 5224. https://doi.org/10.3390/ma17215224
Dziubaniuk M, Piech R, Paczosa-Bator B. Electrochemical Impedance Spectroscopy Study of Ceria- and Zirconia-Based Solid Electrolytes for Application Purposes in Fuel Cells and Gas Sensors. Materials. 2024; 17(21):5224. https://doi.org/10.3390/ma17215224
Chicago/Turabian StyleDziubaniuk, Małgorzata, Robert Piech, and Beata Paczosa-Bator. 2024. "Electrochemical Impedance Spectroscopy Study of Ceria- and Zirconia-Based Solid Electrolytes for Application Purposes in Fuel Cells and Gas Sensors" Materials 17, no. 21: 5224. https://doi.org/10.3390/ma17215224
APA StyleDziubaniuk, M., Piech, R., & Paczosa-Bator, B. (2024). Electrochemical Impedance Spectroscopy Study of Ceria- and Zirconia-Based Solid Electrolytes for Application Purposes in Fuel Cells and Gas Sensors. Materials, 17(21), 5224. https://doi.org/10.3390/ma17215224








