Transparent and Flexible Hierarchical Porous Structure of Polyvinyl Alcohol Aerogel: A Microstructure Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Gelling Methods
2.2.2. Drying Methods
2.3. Characterizations
2.3.1. Scanning Electron Microscope (SEM)
2.3.2. The Analysis for Recording Weight Loss During Heating
2.3.3. Dynamic Mechanical Analyzer (DMA)
2.3.4. Spectrophotometer (V-770)
3. Results and Discussion
3.1. Mechanical Property
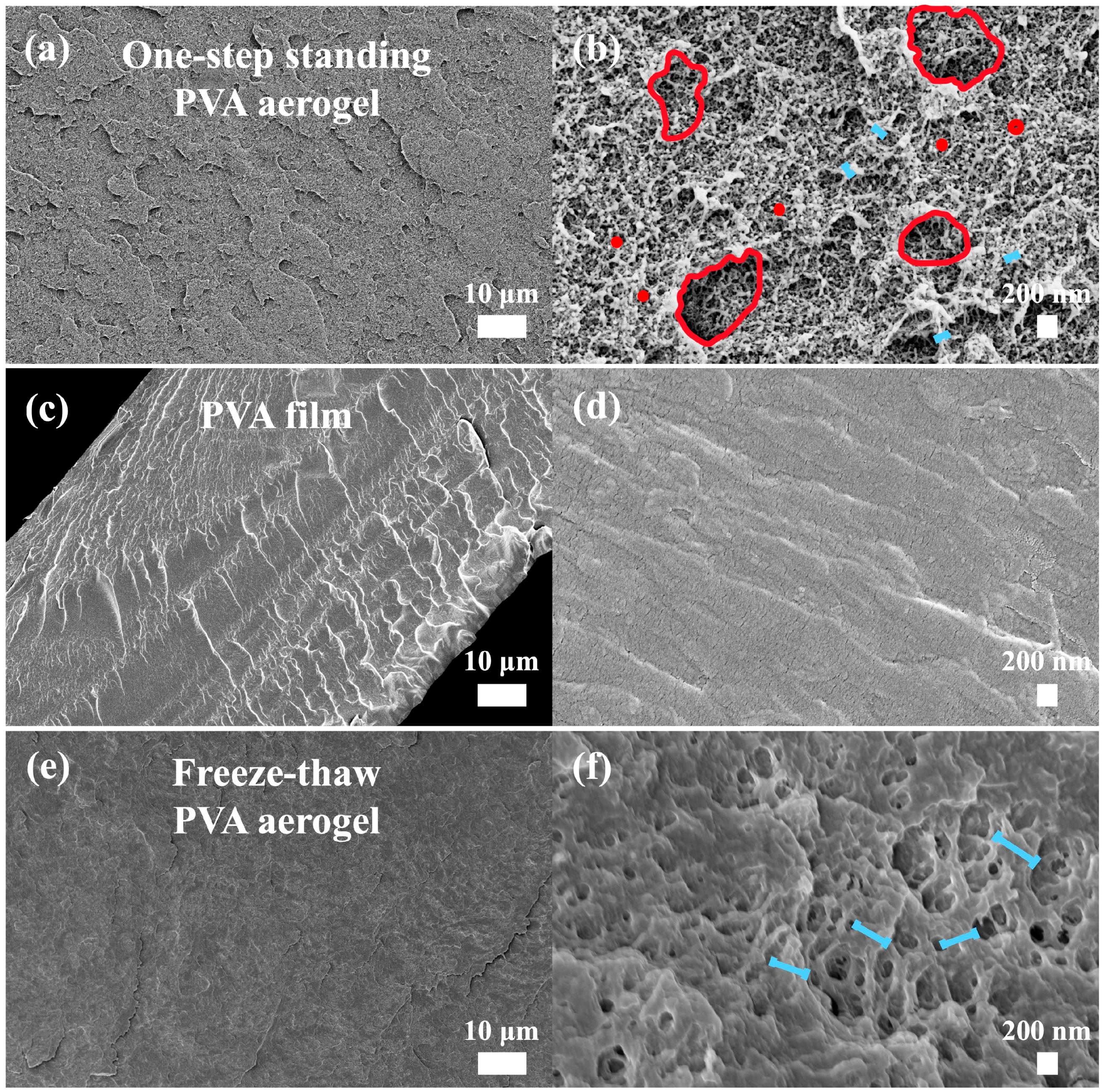
3.2. Morphology
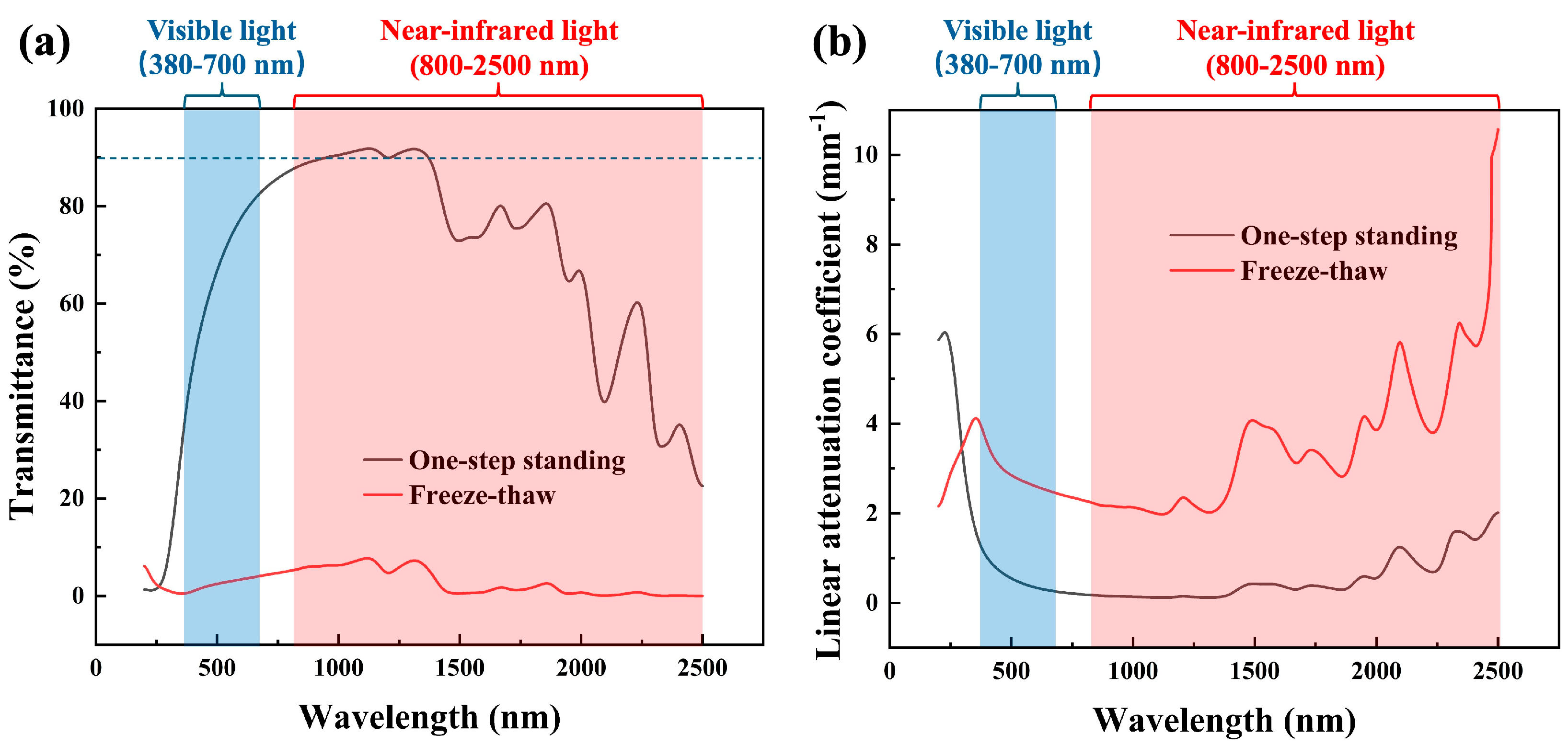
3.3. Transmittance
3.4. Thermal Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kistler, S.S. Coherent expanded-aerogels. J. Phys. Chem. 2002, 36, 52–64. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Şahin, İ.; Özbakır, Y.; Inönü, Z.; Ulker, Z.; Erkey, C. Kinetics of supercritical drying of gels. Gels 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.; Camino-Rey, M.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- Li, F.; Huang, X.; Liu, J.-X.; Zhang, G.-J. Sol-gel derived porous ultra-high temperature ceramics. J. Adv. Ceram. 2020, 9, 1–16. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Bokov, D.; Jalil, A.T.; Chupradit, S.; Suksatan, W.; Ansari, M.J.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by sol-gel method: Synthesis and application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, A.; Kaur, P.; Singh, L. Unveiling the high-temperature dielectric relaxation and conduction mechanisms in sol-gel derived LaFeO3 modified Sodium Bismuth Titanate ceramics. J. Alloys Compd. 2023, 941, 169023. [Google Scholar] [CrossRef]
- Cai, H.; Jiang, Y.; Feng, J.; Zhang, S.; Peng, F.; Xiao, Y.; Li, L.; Feng, J. Preparation of silica aerogels with high temperature resistance and low thermal conductivity by monodispersed silica sol. Mater. Des. 2020, 191, 108640. [Google Scholar] [CrossRef]
- Vareda, J.P.; Lamy-Mendes, A.; Durães, L. A reconsideration on the definition of the term aerogel based on current drying trends. Microporous Mesoporous Mater. 2018, 258, 211–216. [Google Scholar] [CrossRef]
- He, Y.-L.; Xie, T. Advances of thermal conductivity models of nanoscale silica aerogel insulation material. Appl. Therm. Eng. 2015, 81, 28–50. [Google Scholar] [CrossRef]
- Giannella, V.; Branda, F.; Passaro, J.; Petrone, G.; Barbarino, M.; Citarella, R. Acoustic improvements of aircraft headrests based on Electrospun Mats evaluated through Boundary Element Method. Appl. Sci. 2020, 10, 5712. [Google Scholar] [CrossRef]
- Rao, A.V.; Hegde, N.D.; Hirashima, H. Absorption and desorption of organic liquids in elastic superhydrophobic silica aerogels. J. Colloid Interface Sci. 2007, 305, 124–132. [Google Scholar]
- Reim, M.; Reichenauer, G.; Körner, W.; Manara, J.; Arduini-Schuster, M.; Korder, S.; Beck, A.; Fricke, J. Silica-aerogel granulate–Structural, optical and thermal properties. J. Non-Cryst. Solids 2004, 350, 358–363. [Google Scholar] [CrossRef]
- Dorcheh, A.S.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Yang, H.; Kong, X.; Zhang, Y.; Wu, C.; Cao, E. Mechanical properties of polymer-modified silica aerogels dried under ambient pressure. J. Non-Crystalline Solids 2011, 357, 3447–3453. [Google Scholar] [CrossRef]
- Parmenter, K.E.; Milstein, F. Mechanical properties of silica aerogels. J. Non-Crystalline Solids 1998, 223, 179–189. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; Zheng, Y.; Zhang, D.; Zhao, Y.; Wang, C.; Pan, C.; Liu, C.; Shen, C. Lightweight, superelastic, and hydrophobic polyimide nanofiber/MXene composite aerogel for wearable piezoresistive sensor and oil/water separation applications. Adv. Funct. Mater. 2021, 31, 2008006. [Google Scholar] [CrossRef]
- Hou, X.; Mao, Y.; Zhang, R.; Fang, D. Super-flexible polyimide nanofiber crosslinked polyimide aerogel membranes for high efficient flexible thermal protection. Chem. Eng. J. 2021, 417, 129341. [Google Scholar] [CrossRef]
- Zaghlol, S.; Amer, W.A.; Shaaban, M.H.; Ayad, M.M.; Bober, P.; Stejskal, J. Conducting macroporous polyaniline/poly (vinyl alcohol) aerogels for the removal of chromium (VI) from aqueous media. Chem. Pap. 2020, 74, 3183–3193. [Google Scholar] [CrossRef]
- Guastaferro, M.; Reverchon, E.; Baldino, L. Polysaccharide-based aerogel production for biomedical applications: A comparative review. Materials 2021, 14, 1631. [Google Scholar] [CrossRef]
- Karimi, A.; Navidbakhsh, M. Mechanical properties of PVA material for tissue engineering applications. Mater. Technol. 2014, 29, 90–100. [Google Scholar] [CrossRef]
- Hassan, C.M.; Trakampan, P.; Peppas, N.A. Water solubility characteristics of poly (vinyl alcohol) and gels prepared by freezing/thawing processes. In Water Soluble Polymers: Solutions Properties and Applications; Springer: Boston, MA, USA, 2002; pp. 31–40. [Google Scholar]
- Alexandre, N.; Ribeiro, J.; Gaertner, A.; Pereira, T.; Amorim, I.; Fragoso, J.; Costa, E.; Santos-Silva, A.; Rodrigues, M.; Santos, J.D.; et al. Biocompatibility and hemocompatibility of polyvinyl alcohol hydrogel used for vascular grafting—In vitro and in vivo studies. J. Biomed. Mater. Res. Part A 2014, 102, 4262–4275. [Google Scholar]
- Elhosiny Ali, H.; Abdel-Aziz, M.; Mahmoud Ibrahiem, A.; Sayed, M.A.; Abd-Rabboh, H.S.M.; Awwad, N.S.; Algarni, H.; Shkir, M.; Yasmin Khairy, M. Microstructure study and linear/nonlinear optical performance of Bi-embedded PVP/PVA films for optoelectronic and optical cut-off applications. Polymers 2022, 14, 1741. [Google Scholar] [CrossRef] [PubMed]
- Minus, M.L.; Chae, H.G.; Kumar, S. Interfacial crystallization in gel-spun poly (vinyl alcohol)/single-wall carbon nanotube composite fibers. Macromol. Chem. Phys. 2009, 210, 1799–1808. [Google Scholar] [CrossRef]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Sapuan, S.; Ilyas, R. Highly transparent and antimicrobial PVA based bionanocomposites reinforced by ginger nanofiber. Polym. Test. 2020, 81, 106186. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Yin, X.; Utomo, H.D.; Tao, N.F.; Huang, H. Silica aerogel as super thermal and acoustic insulation materials. J. Environ. Prot. 2018, 9, 295–308. [Google Scholar] [CrossRef]
- Jung, S.M.; Preston, D.J.; Jung, H.Y.; Deng, Z.; Wang, E.N.; Kong, J. Porous Cu nanowire aerosponges from one-step assembly and their applications in heat dissipation. Adv. Mater. 2016, 28, 1413–1419. [Google Scholar] [CrossRef]
- Bo, Y.; Yu, A.; Liu, H.; Chen, S.; Xu, W.; Diao, S.; Zhang, C. Preparation of elastic graphene aerogel and its adsorption of oil. J. Porous Mater. 2021, 28, 39–56. [Google Scholar] [CrossRef]
- Mohanan, J.L.; Brock, S.L. A new addition to the aerogel community: Unsupported CdS aerogels with tunable optical properties. J. Non-Crystalline Solids 2004, 350, 1–8. [Google Scholar] [CrossRef]


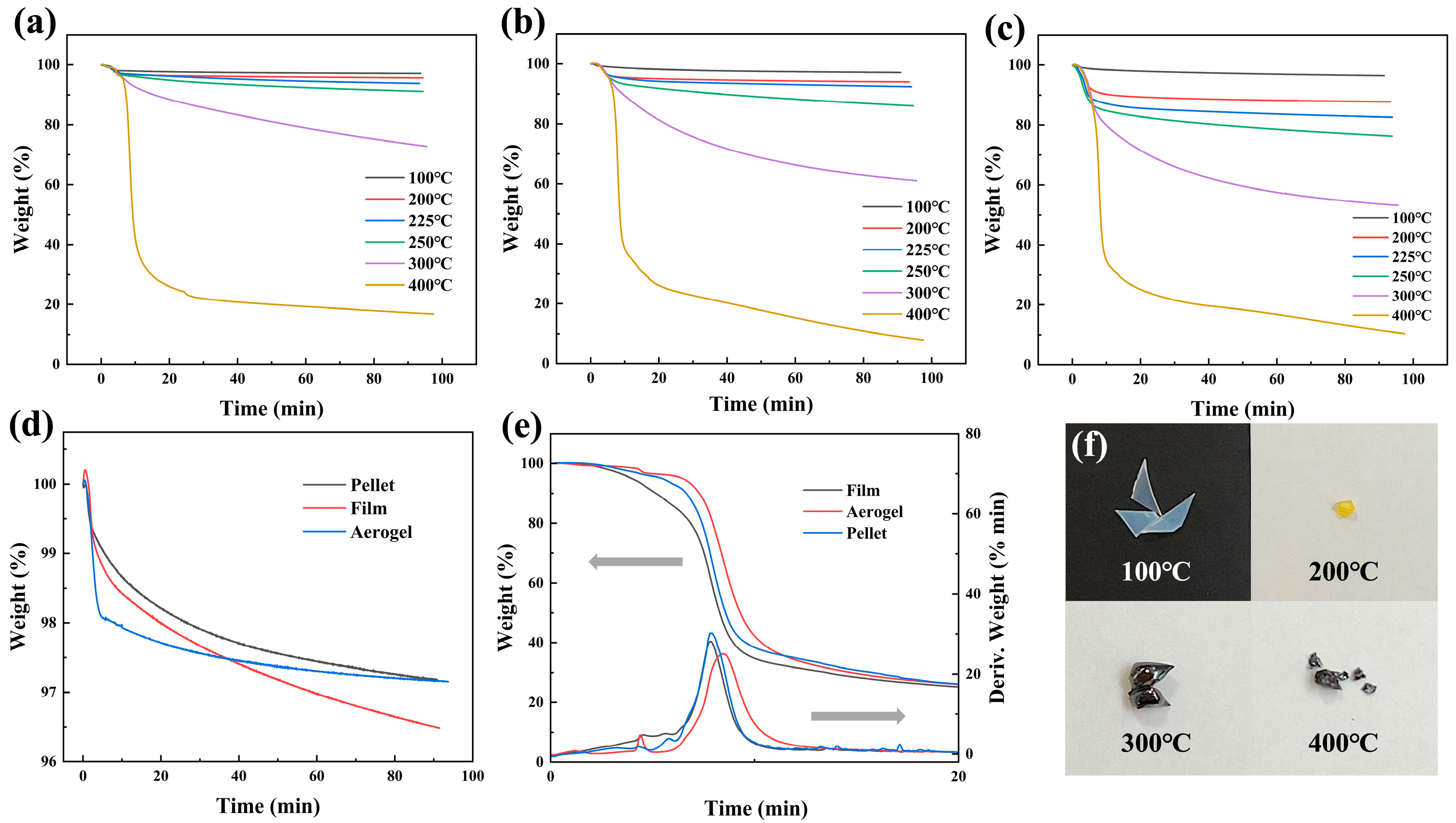

| Samples | 100 °C | 200 °C | 225 °C | 250 °C | 300 °C | 400 °C |
|---|---|---|---|---|---|---|
| PVA aerogel | 97.15% | 95.68% | 93.80% | 91.11% | 72.79% | 16.77% |
| PVA pellet | 97.17% | 94.01% | 92.39% | 86.02% | 61.05% | 7.91% |
| PVA film | 96.48% | 87.75% | 82.57% | 76.29% | 53.16% | 10.38% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, X.; Zhang, H.; Sun, X.; Mu, Y.; Barrett, T.; Doyle, C.; Minus, M.L.; Zheng, Y. Transparent and Flexible Hierarchical Porous Structure of Polyvinyl Alcohol Aerogel: A Microstructure Study. Materials 2024, 17, 5312. https://doi.org/10.3390/ma17215312
Li X, Zhang X, Zhang H, Sun X, Mu Y, Barrett T, Doyle C, Minus ML, Zheng Y. Transparent and Flexible Hierarchical Porous Structure of Polyvinyl Alcohol Aerogel: A Microstructure Study. Materials. 2024; 17(21):5312. https://doi.org/10.3390/ma17215312
Chicago/Turabian StyleLi, Xiaoli, Xuguang Zhang, Hexiang Zhang, Xiao Sun, Ying Mu, Thomas Barrett, Conor Doyle, Marilyn L. Minus, and Yi Zheng. 2024. "Transparent and Flexible Hierarchical Porous Structure of Polyvinyl Alcohol Aerogel: A Microstructure Study" Materials 17, no. 21: 5312. https://doi.org/10.3390/ma17215312
APA StyleLi, X., Zhang, X., Zhang, H., Sun, X., Mu, Y., Barrett, T., Doyle, C., Minus, M. L., & Zheng, Y. (2024). Transparent and Flexible Hierarchical Porous Structure of Polyvinyl Alcohol Aerogel: A Microstructure Study. Materials, 17(21), 5312. https://doi.org/10.3390/ma17215312






