Evaluation of Thermal and Mechanical Properties of Bi-In-Sn/WO3 Composites for Efficient Heat Dissipation
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
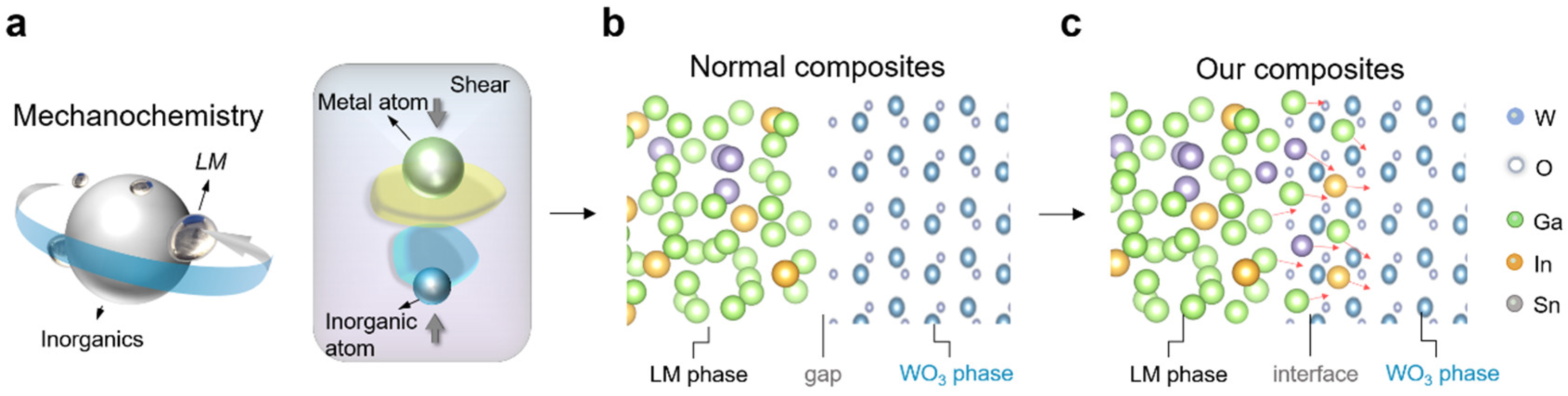
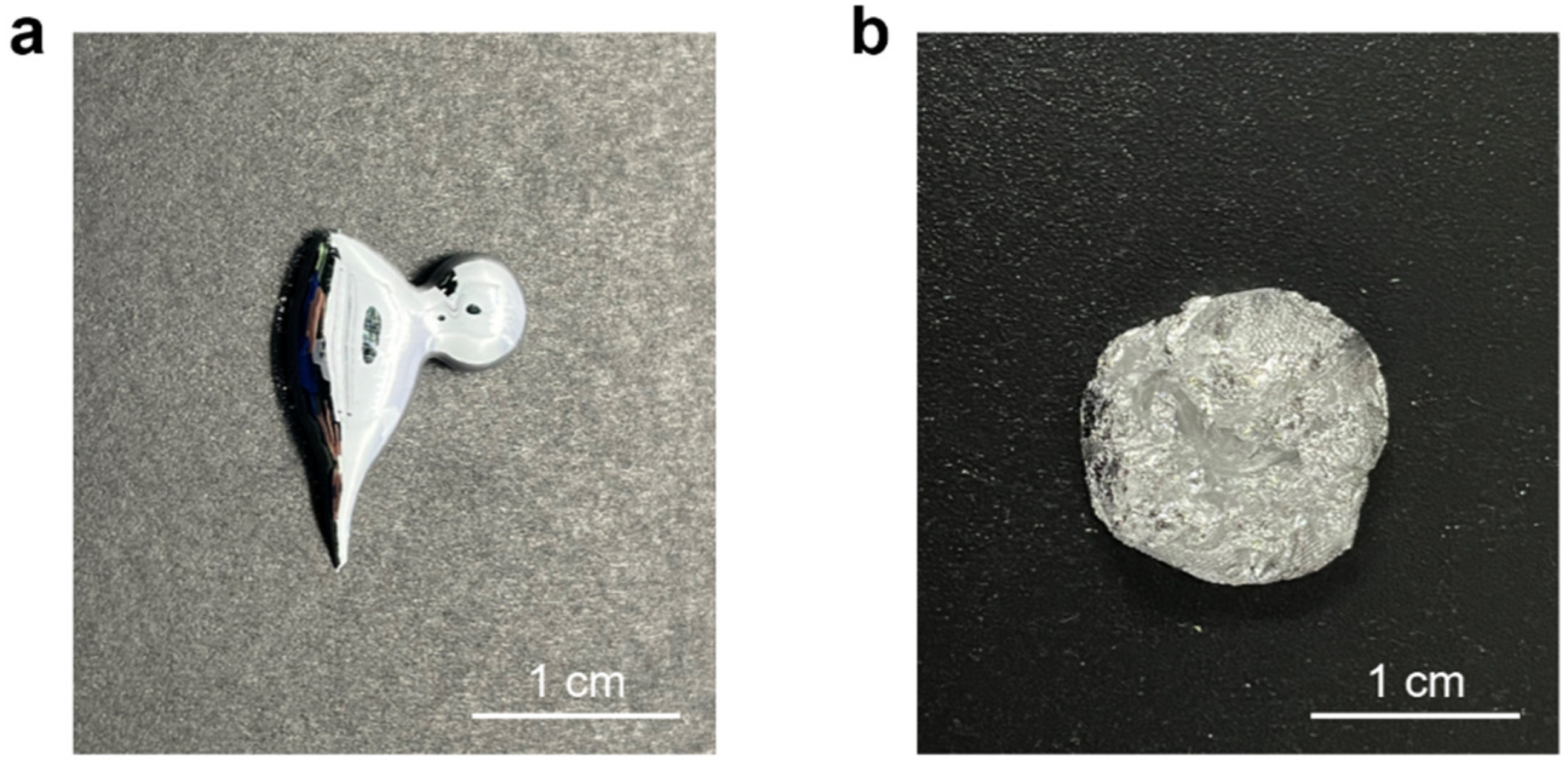
3.1. Synthesis of Bi-In-Sn/WO3 via Mechanochemistry
3.2. Microstructure and Physical State of Bi-In-Sn/WO3 Composite
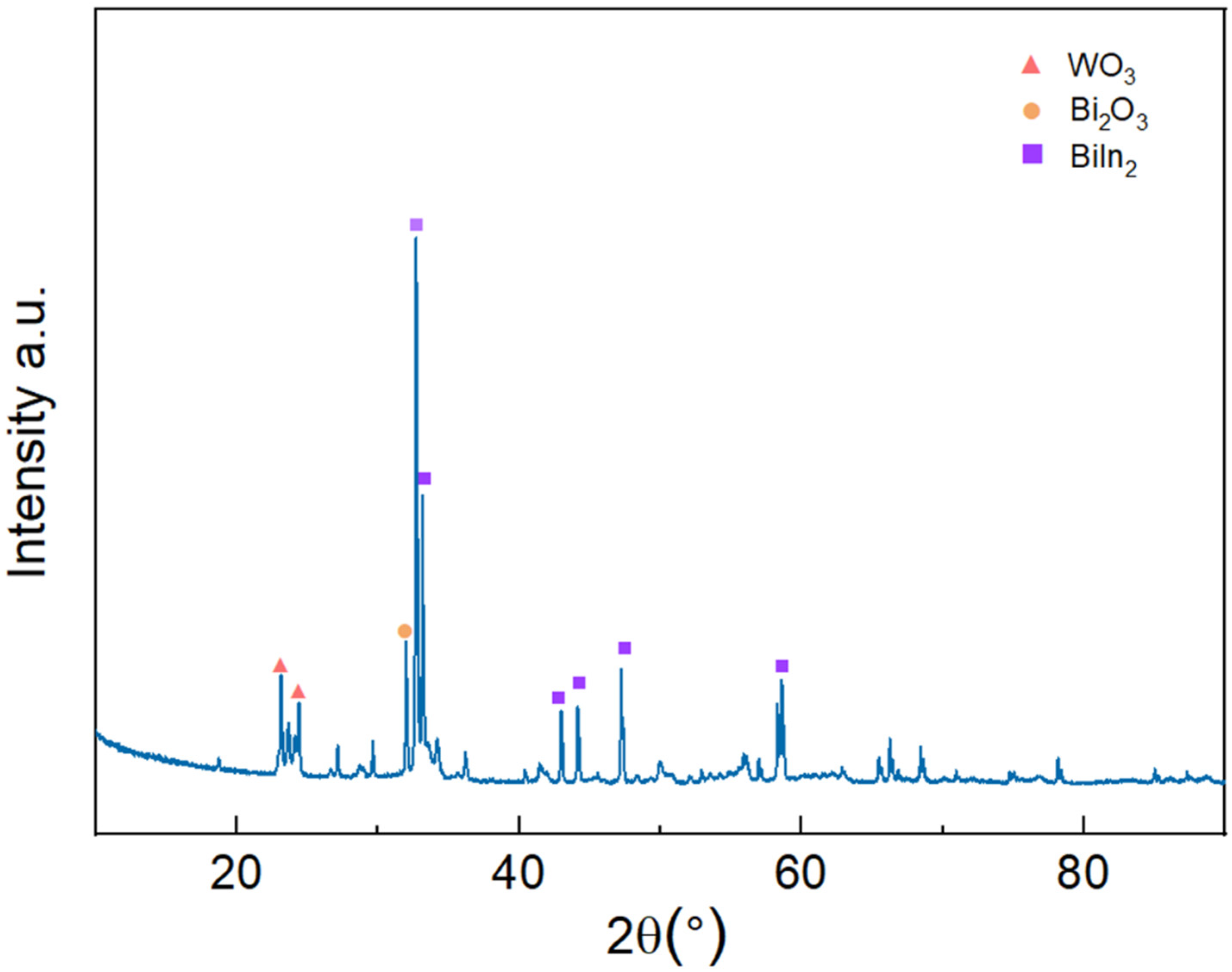
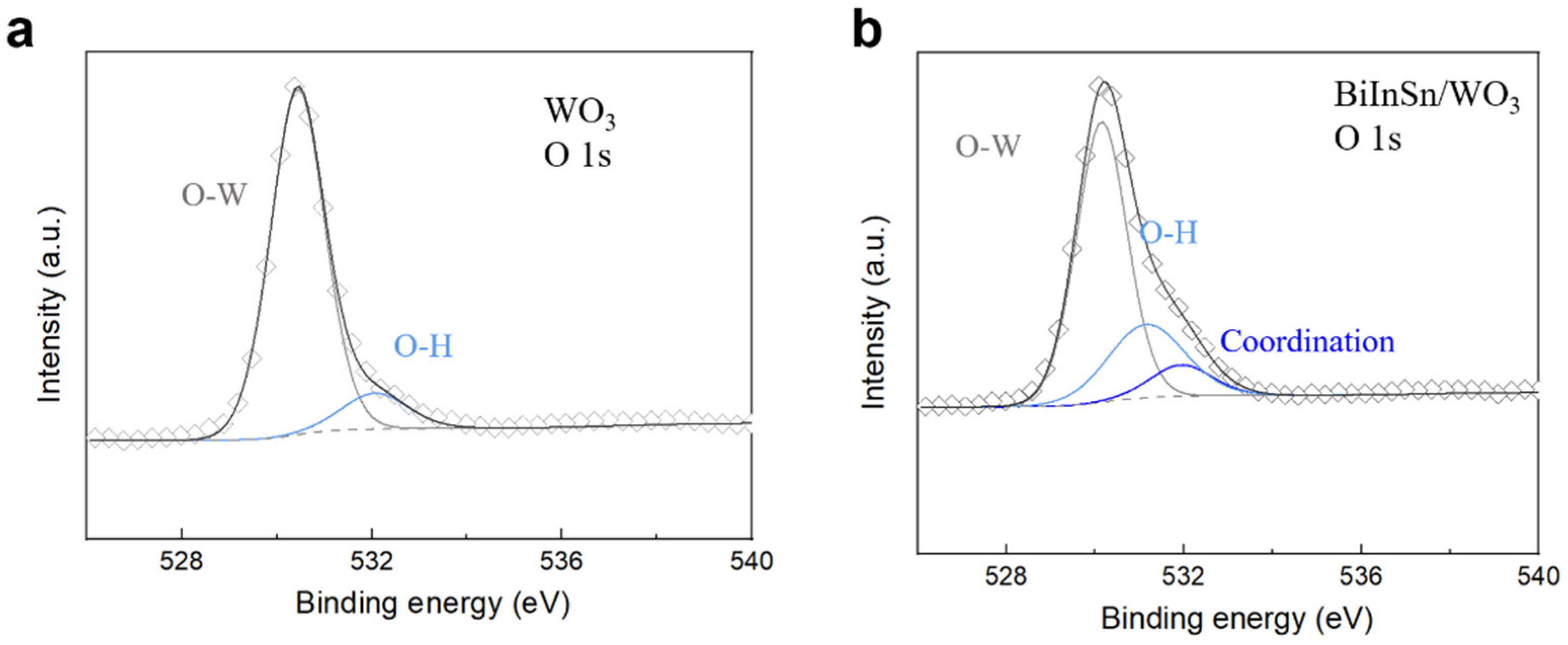
3.3. Phase Analysis and Bonding in Bi-In-Sn/WO3 Composite
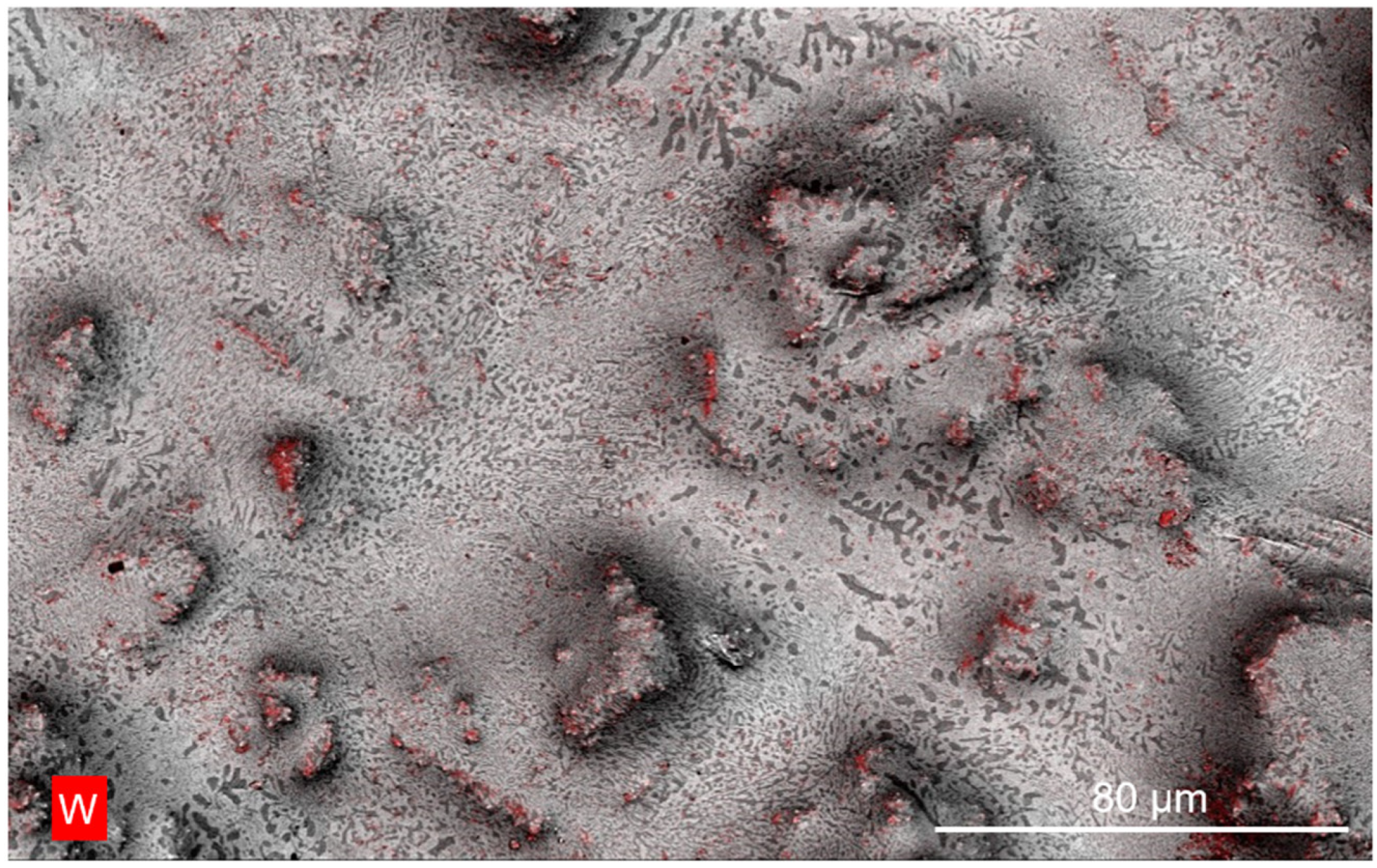
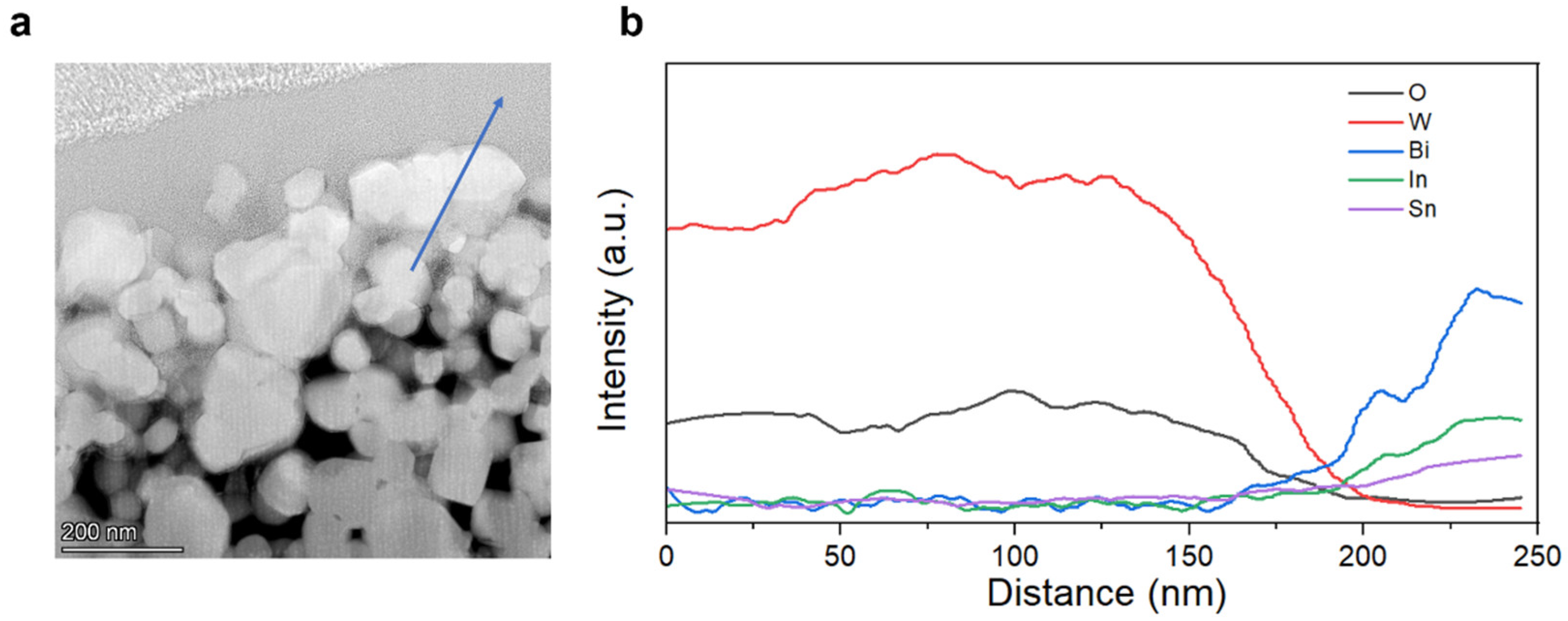
3.4. Interface Analysis of Bi-In-Sn/WO3 Composite
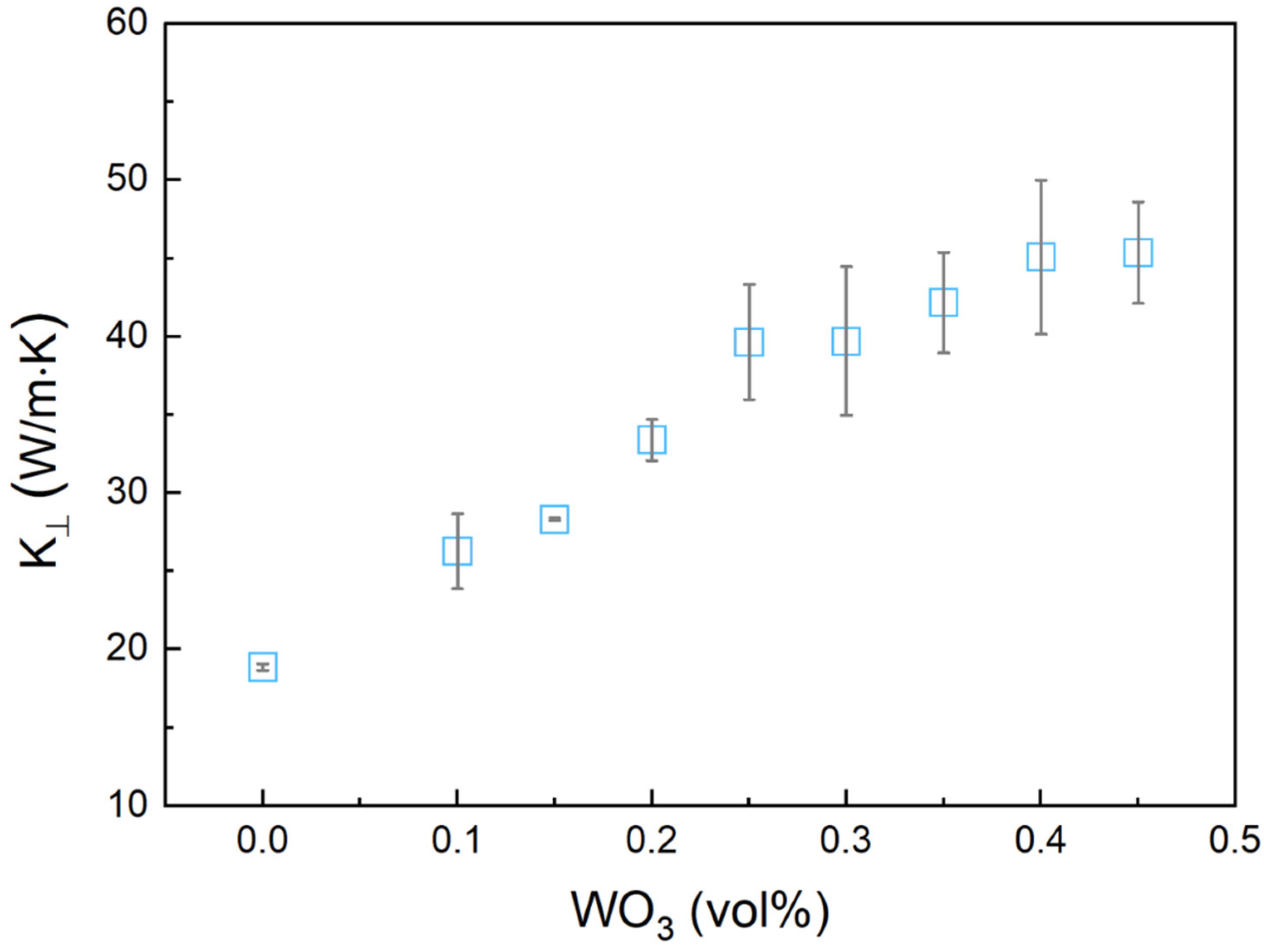
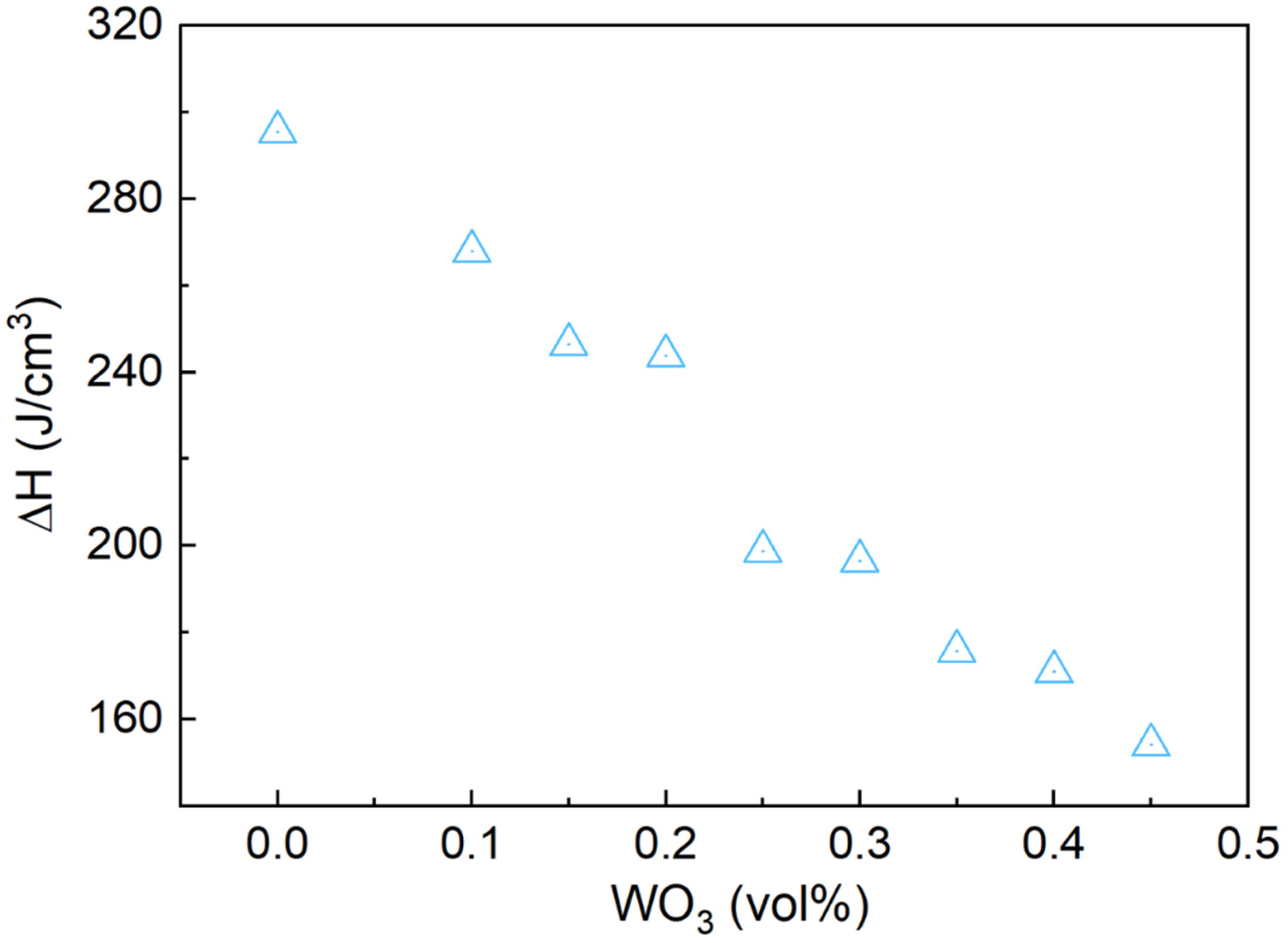
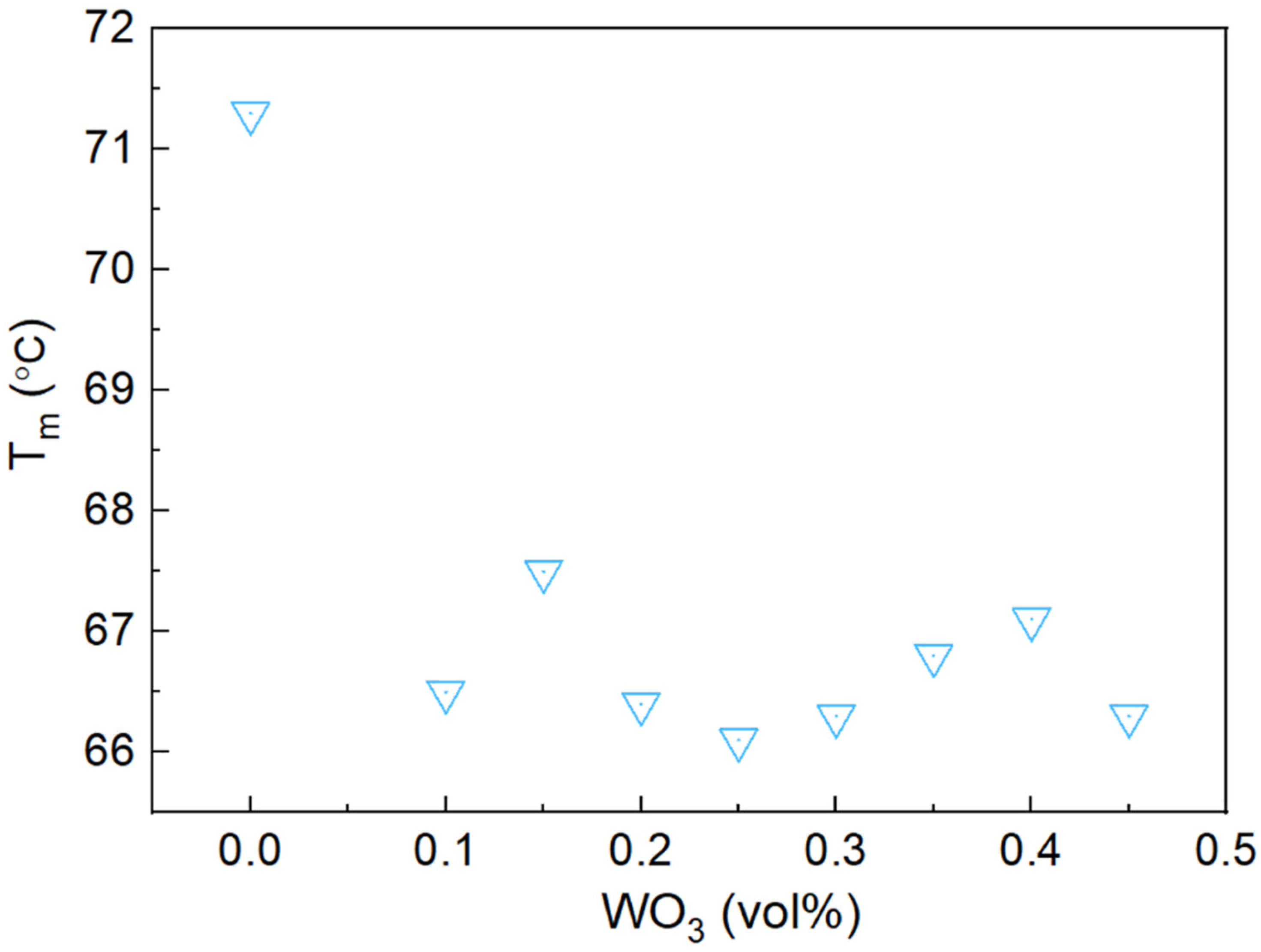
3.5. Thermal Properties of Bi-In-Sn/WO3 Composite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Liu, J.; Deng, Z. Bismuth-based liquid metals: Advances, applications, and prospects. Mater. Horiz. 2024, 11, 1369–1394. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xu, J.; Li, Y.; Zhang, Y.; Kuang, C. Study of the Phase-Change Thermal-Storage Characteristics of a Solar Collector. Materials 2022, 15, 7497. [Google Scholar] [CrossRef]
- Ge, H.; Li, H.; Mei, S.; Liu, J. Low melting point liquid metal as a new class of phase change material: An emerging frontier in energy area. Renew. Sustain. Energy Rev. 2013, 21, 331–346. [Google Scholar] [CrossRef]
- Lv, P.; Cheng, H.; Ji, C.; Wei, W. Graphitized-rGO/Polyimide Aerogel as the Compressible Thermal Interface Material with Both High in-Plane and through-Plane Thermal Conductivities. Materials 2021, 14, 2350. [Google Scholar] [CrossRef] [PubMed]
- Shu, D.; Sun, J.; Huang, F.; Qin, W.; Wang, C.; Yue, W. Boron Nitride/Carbon Fiber High-Oriented Thermal Conductivity Material with Leaves–Branches Structure. Materials 2024, 17, 2183. [Google Scholar] [CrossRef]
- Zhao, L.; Xing, Y.; Wang, Z.; Liu, X. The passive thermal management system for electronic device using low-melting-point alloy as phase change material. Appl. Therm. Eng. 2017, 125, 317–327. [Google Scholar] [CrossRef]
- Handschuh-Wang, S.; Wang, T.; Zhang, Z.; Liu, F.; Han, P.; Liu, X. Long-Term Corrosion of Eutectic Gallium, Indium, and Tin (EGaInSn) Interfacing with Diamond. Materials 2024, 17, 2683. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, W.; Liu, C.; Xie, H.; Xu, H. Phase change mediated graphene hydrogel-based thermal interface material with low thermal contact resistance for thermal management. Compos. Sci. Technol. 2022, 219, 109223. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, J.; Wei, N.; Yang, J.; Pei, Q.-X. Recent progress in the development of thermal interface materials: A review. Phys. Chem. Chem. Phys. 2021, 23, 753–776. [Google Scholar] [CrossRef]
- Yang, X.-H.; Tan, S.-C.; He, Z.-Z.; Zhou, Y.-X.; Liu, J. Evaluation and optimization of low melting point metal PCM heat sink against ultra-high thermal shock. Appl. Therm. Eng. 2017, 119, 34–41. [Google Scholar] [CrossRef]
- Togun, H.; Sultan, H.S.; Mohammed, H.I.; Sadeq, A.M.; Biswas, N.; Hasan, H.A.; Homod, R.Z.; Abdulkadhim, A.H.; Yaseen, Z.M.; Talebizadehsardari, P. A critical review on phase change materials (PCM) based heat exchanger: Different hybrid techniques for the enhancement. J. Energy Storage 2024, 79, 109840. [Google Scholar] [CrossRef]
- Widjajana, M.S.; Chiu, S.-H.; Chi, Y.; Baharfar, M.; Zheng, J.; Ghasemian, M.B.; Bhattacharyya, S.K.; Tang, J.; Rahim, M.A.; Kalantar-Zadeh, K. A liquid metal-based process for tuning the thermoelectric properties of bismuth indium systems. J. Mater. Chem. C 2023, 11, 10299–10309. [Google Scholar] [CrossRef]
- Chen, K.; Wei, X.; Ding, J.; Wang, W.; Lu, J. Bi-Sn-In phase change material with low melting point and high cyclic stability for thermal energy storage and management. Chem. Eng. J. 2022, 435, 135055. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Xie, H.; Zhao, L.; Zhang, Z.; Li, Z.; Han, B.; Zhao, Y. Ultrasound-assisted preparation of uniform BiInSn phase change microparticles for enhanced thermal interface materials through in-situ low-temperature soldering. Mater. Chem. Phys. 2023, 309, 128408. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Wang, Z.; Zhang, Y.; Wang, H.; Zou, D. Micro-encapsulation of a low-melting-point alloy phase change material and its application in electronic thermal management. J. Clean. Prod. 2023, 417, 138058. [Google Scholar] [CrossRef]
- Huang, K.; Qiu, W.; Ou, M.; Liu, X.; Liao, Z.; Chu, S. An anti-leakage liquid metal thermal interface material. RSC Adv. 2020, 10, 18824–18829. [Google Scholar] [CrossRef]
- Shchegolkov, A.V.; Lipkin, M.S.; Shchegolkov, A.V. Preparation of WO3 Films on Titanium and Graphite Foil for Fuel Cell and Supercapacitor Applications by Electrochemical (Cathodic) Deposition Method. Russ. J. Gen. Chem. 2022, 92, 1161–1167. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, C.-C.; Wang, Q.; Chu, W. Enhancement on heat transfer and reliability of low melting temperature alloy based thermal interface materials. Int. Commun. Heat Mass Transf. 2024, 151, 107215. [Google Scholar] [CrossRef]
- Zhao, L.; Xing, Y.; Liu, X. Experimental investigation on the thermal management performance of heat sink using low melting point alloy as phase change material. Renew. Energy 2020, 146, 1578–1587. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, F.; Liu, B.; Wang, S.; Wang, Q.; Kuang, G.; Xie, W.; Meng, S. Ablation behavior of gradient ceramic-polymer composites in wind tunnel environment. J. Eur. Ceram. Soc. 2024, 44, 6160–6168. [Google Scholar] [CrossRef]
- Abdul Jaleel, S.A.; Kim, T.; Baik, S. Covalently Functionalized Leakage-Free Healable Phase-Change Interface Materials with Extraordinary High-Thermal Conductivity and Low-Thermal Resistance. Adv. Mater. 2023, 35, 2300956. [Google Scholar] [CrossRef] [PubMed]
- Gilman, J.J. Mechanochemistry. Science 1996, 274, 65. [Google Scholar] [CrossRef]
- Hu, Y.; Li, B.; Yu, C.; Fang, H.; Li, Z. Mechanochemical preparation of single atom catalysts for versatile catalytic applications: A perspective review. Mater. Today 2023, 63, 288–312. [Google Scholar] [CrossRef]
- Wu, D.; Liu, D.; Tian, X.; Lei, C.; Chen, X.; Zhang, S.; Chen, F.; Wu, K.; Fu, Q. A Universal Mechanochemistry Allows On-Demand Synthesis of Stable and Processable Liquid Metal Composites. Small Methods 2022, 6, e2200246. [Google Scholar] [CrossRef]
- Wang, C.; Gong, Y.; Cunning, B.V.; Lee, S.; Le, Q.; Joshi, S.R.; Buyukcakir, O.; Zhang, H.; Seong, W.K.; Huang, M.; et al. A general approach to composites containing nonmetallic fillers and liquid gallium. Sci. Adv. 2021, 7, eabe3767. [Google Scholar] [CrossRef]
- Kumar, M.R.; Mohan, S.; Behera, C.K. Measurements of Mixing Enthalpy for a Lead-Free Solder Bi-In-Sn System. J. Electron. Mater. 2019, 48, 8096–8106. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Yao, R.; Lai, W.; Liu, R.; Yu, R.; Chen, X.; Li, J. Thermal Contact Resistance Optimization of Press-Pack IGBT Device Based on Liquid Metal Thermal Interface Material. IEEE Trans. Power Electron. 2022, 37, 5411–5421. [Google Scholar] [CrossRef]
- Liu, J.; Feng, H.; Dai, J.; Yang, K.; Chen, G.; Wang, S.; Jin, D.; Liu, X. A Full-component recyclable Epoxy/BN thermal interface material with anisotropy high thermal conductivity and interface adaptability. Chem. Eng. J. 2023, 469, 143963. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Z.-Y.; Wang, Y.; Du, X.-Y.; Liu, J.-D.; Zhang, C.; Li, W.; Zhao, Z.-B. Graphene/Polyolefin Elastomer Films as Thermal Interface Materials with High Thermal Conductivity, Flexibility, and Good Adhesion. Chem. Mater. 2023, 35, 2486–2494. [Google Scholar] [CrossRef]
- Li, J.; Ma, Q.; Gao, S.; Liang, T.; Pang, Y.; Zeng, X.; Li, Y.-y.; Zeng, X.; Sun, R.; Ren, L. Liquid bridge: Liquid metal bridging spherical BN largely enhances the thermal conductivity and mechanical properties of thermal interface materials. J. Mater. Chem. C 2022, 10, 6736–6743. [Google Scholar] [CrossRef]
- ASTM D5470; Standard Test Method for Thermal Transmission Properties of Thermally Conductive Electrical Insulation Materials. ASTM: West Conshohocken, PA, USA, 2017.
- Wang, H.; Xing, W.; Chen, S.; Song, C.; Dickey, M.D.; Deng, T. Liquid Metal Composites with Enhanced Thermal Conductivity and Stability Using Molecular Thermal Linker. Adv. Mater. 2021, 33, 2103104. [Google Scholar] [CrossRef] [PubMed]
- Manciu, F.S.; Enriquez, J.L.; Durrer, W.G.; Yun, Y.; Ramana, C.V.; Gullapalli, S.K. Spectroscopic analysis of tungsten oxide thin films. J. Mater. Res. 2011, 25, 2401–2406. [Google Scholar] [CrossRef]
- Mazur, M.; Wojcieszak, D.; Wiatrowski, A.; Kaczmarek, D.; Lubańska, A.; Domaradzki, J.; Mazur, P.; Kalisz, M. Analysis of amorphous tungsten oxide thin films deposited by magnetron sputtering for application in transparent electronics. Appl. Surf. Sci. 2021, 570, 151151. [Google Scholar] [CrossRef]
- Terohid, S.A.A.; Heidari, S.; Jafari, A.; Asgary, S. Effect of growth time on structural, morphological and electrical properties of tungsten oxide nanowire. Appl. Phys. A 2018, 124, 567. [Google Scholar] [CrossRef]
- Li, G.; Jiang, W.; Guan, F.; Zhu, J.; Zhang, Z.; Fan, Z. Microstructure, mechanical properties and corrosion resistance of A356 aluminum/AZ91D magnesium bimetal prepared by a compound casting combined with a novel Ni-Cu composite interlayer. J. Mater. Process. Technol. 2021, 288, 116874. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Ning, Z.; Zhu, Y.; Yuan, R. Evaluation of Thermal and Mechanical Properties of Bi-In-Sn/WO3 Composites for Efficient Heat Dissipation. Materials 2024, 17, 5315. https://doi.org/10.3390/ma17215315
Wu D, Ning Z, Zhu Y, Yuan R. Evaluation of Thermal and Mechanical Properties of Bi-In-Sn/WO3 Composites for Efficient Heat Dissipation. Materials. 2024; 17(21):5315. https://doi.org/10.3390/ma17215315
Chicago/Turabian StyleWu, Die, Zhen Ning, Yanlin Zhu, and Rui Yuan. 2024. "Evaluation of Thermal and Mechanical Properties of Bi-In-Sn/WO3 Composites for Efficient Heat Dissipation" Materials 17, no. 21: 5315. https://doi.org/10.3390/ma17215315
APA StyleWu, D., Ning, Z., Zhu, Y., & Yuan, R. (2024). Evaluation of Thermal and Mechanical Properties of Bi-In-Sn/WO3 Composites for Efficient Heat Dissipation. Materials, 17(21), 5315. https://doi.org/10.3390/ma17215315





