Argon Ion Implantation as a Method of Modifying the Surface Properties of Wood–Plastic Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. WPC Preparation
2.2. Modelling and Ion Implantation
- -
- 100% polyethylene (HDPE);
- -
- 100% recycled polyethylene (PE recycled);
- -
- 40% sawdust + 60% HDPE;
- -
- 50% sawdust + 50% HDPE;
- -
- 60% sawdust + 40% HDPE.
2.3. Determination of Colour Change
- 0 < ΔE ≤ 1—unnoticeable difference;
- 1 < ΔE ≤ 2—difference noticed by an experienced observer;
- 2 < ΔE ≤ 3.5—difference noticed by an inexperienced observer;
- 3.5 < ΔE ≤ 5—noticeable difference;
- 5 < ΔE—significant colour change.
2.4. Determination of the Wetting Angle and Surface Free Energy
2.5. Statistical Analysis
3. Results and Discussion
3.1. Modelling and Ion Implantation Results
3.2. Colour Change Results
3.3. Wetting Angle and Surface Free Energy Results
3.4. Statistical Analysis of Results
4. Conclusions
- During this study, it was found that the most significant influence on the analysed features is exerted by the dose of ions and the share of lignocellulosic raw material in the composition of the composites;
- Studies of the ion penetration depth profile indicate that the layer on which the changes occur reaches 200 nm. Additionally, the surface is damaged, which was visible, especially at the highest doses used;
- It seems that surface damage can be minimised through modification with a lower-current ion beam;
- The implantation of Ar ions on the WPC surface led to a slight decrease in the wetting angle and an increase in the free surface energy compared to non-implanted samples.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Zhang, Z.; Zhang, D.; Qi, F.; Dai, Y.; Wei, W.; Ouyang, X. Effects of metal ion implantation (Fe, Ti, Zn and Zr) on mechanical properties, corrosion resistance and biocompatibility of WE43 Mg alloy. J. Magnes. Alloy. 2024. [Google Scholar] [CrossRef]
- Bai, W.; Zhou, Y.; Xiao, M.; Xu, L.; Xiao, H.; Tong, Y.; He, C.; Pang, J.; Xie, Q.; Yang, C. Effects of Cu ion implantation on the microstructure, dielectric and impedance properties of SrTiO3 ceramics prepared by reduction-reoxidation method. Ceram. Int. 2024, 50, 46279–46287. [Google Scholar] [CrossRef]
- Williams, J.S. Ion implantation of semiconductors. Mater. Sci. Eng. A 1998, 253, 8–15. [Google Scholar] [CrossRef]
- Resende, R.C.; Ribeiro, R.P.; Waldman, W.R.; Cruz, N.C.; Araujo, J.R.; Rangel, E.C. Improvement of thermoplastic elastomer degradation resistance by low-energy plasma immersion ion bombardment. Mater. Chem. Phys. 2020, 242, 122467. [Google Scholar] [CrossRef]
- Karthikeyan, K.R.; Thanigai Arul, K.; Ramana Ramya, J.; Nabhiraj, P.Y.; Menon, R.; Krishna, J.B.M.; Narayana Kalkura, S. Novel microporous surface and blue emission of argon ion implanted polyvinylacohol/bionanohydroxyapatite coatings. Radiat. Phys. Chem. 2020, 171, 108678. [Google Scholar] [CrossRef]
- Prakrajang, K.; Wanichapichart, P.; Anuntalabhochai, S.; Pitakrattananukool, S.; Yu, L.D. Ion beam modification of chitosan and cellulose membranes for simulation of ion bombardment of plant cell envelope. Nucl. Instrum. Methods Phys. Res. B Beam Interact. Mater. At. 2009, 267, 1645–1649. [Google Scholar] [CrossRef]
- Barlak, M.; Wilkowski, J.; Werner, Z. Ion implantation changes of tribological and corrosion resistance properties of materials used in wood industry. Ann. Wars. Univ. Life Sci.-SGGW For. Wood Technol. 2016, 94, 19–27. [Google Scholar]
- Barlak, M.; Wilkowski, J.; Boruszewski, P.; Werner, Z.; Pałubicki, B. Changes of functional properties of materials used in wood industry after ion implantation processes. Ann. Wars. Univ. Life Sci.-SGGW For. Wood Technol. 2017, 97, 133–139. [Google Scholar]
- Wilkowski, J.; Barlak, M.; Bottger, R.; Werner, Z.; Konarski, P.; Pisarek, M.; Wachowicz, J.; Von Borany, J.; Auriga, A. Effect of nitrogen ion implantation on the life time of WC-Co tools used in particleboard milling. Wood Mater. Sci. Eng. 2021, 17, 521–532. [Google Scholar] [CrossRef]
- Kondyurin, A.; Gan, B.K.; Bilek, M.M.M.; McKenzie, D.R.; Mizuno, K.; Wuhrer, R. Argon plasma immersion ion implantation of polystyrene films. Nucl. Instrum. Methods Phys. Res. B Beam Interact. Mater. At. 2008, 266, 1074–1084. [Google Scholar] [CrossRef]
- Sun, C.; Sprouster, D.J.; Zhang, Y.; Chen, D.; Wang, Y.; Ecker, L.E.; Gan, J. Formation window of gas bubble superlattice in molybdenum under ion implantation. Phys. Rev. Mater. 2019, 3, 103607. [Google Scholar] [CrossRef]
- Narula, C.; Chauhan, R.P.; Garg, A.; Rana, P.; Panchal, S.; Gupta, R. Modification of the Properties of CdSe Nanowires by Argon Ion Implantation. J. Electron. Mater. 2024, 53, 5073–5082. [Google Scholar] [CrossRef]
- Lu, N.; Chen, Z.; Zhang, W.; Yang, G.; Liu, Q.; Böttger, R.; Zhou, S.; Liu, Y. Effect of silver ion implantation on antibacterial ability of polyethylene food packing films. Food Packag. Shelf Life 2021, 28, 100650. [Google Scholar] [CrossRef]
- Shanthini, G.M.; Sakthivel, N.; Menon, R.; Nabhiraj, P.Y.; Gómez-Tejedor, J.A.; Meseguer-Dueñas, J.M.; Gómez Ribelles, J.L.; Krishna, J.B.M.; Narayana Kalkura, S. Surface stiffening and enhanced photoluminescence of ion implanted cellulose—Polyvinyl alcohol—Silica composite. Carbohydr. Polym. 2016, 153, 619–630. [Google Scholar] [CrossRef]
- Chan, K.F.; Ho, J.P.Y.; Li, W.Y.; Lau, B.M.F.; Tse, A.K.W.; Fong, W.F.; Bilek, M.M.M.; McKenzie, D.R.; Chu, P.K.; Yu, K.N. Investigation of cytocompatibility of surface-treated cellulose nitrate films by using plasma immersion ion implantation. Surf. Coat. Technol. 2007, 201, 6897–6900. [Google Scholar] [CrossRef]
- Betlej, I.; Barlak, M.; Wilkowski, J.; Werner, Z.; Zagórski, J.; Lipska, K.; Boruszewski, P. Wettability of the surface of bacterial cellulose film modified with the ion implantation. Ann. Wars. Univ. Life Sci.-SGGW. For. Wood Technol. 2022, 118, 15–21. [Google Scholar] [CrossRef]
- Chmielnicki, B.; Konieczny, J. Properties of WPC composites with polyethylene matrix filled with nutshell flour. Przetwórstwo Tworzyw 2014, 1, 12–20. [Google Scholar]
- Borysiuk, P.; Auriga, R.; Wilkowski, J.; Auriga, A.; Trocinński, A.; Seng Hua, L. A Study on the Susceptibility of PLA Biocomposites to Drilling. Forests 2022, 13, 1950. [Google Scholar] [CrossRef]
- Borysiuk, P.; Boruszewski, P.; Auriga, R.; Danecki, L.; Auriga, A.; Rybak, K.; Nowacka, M. Influence of a bark-filler on the properties of PLA biocomposites. J. Mater. Sci. 2021, 56, 9196–9208. [Google Scholar] [CrossRef]
- SRIM. Available online: http://www.srim.org/ (accessed on 19 September 2024).
- Barlak, M.; Wilkowski, J.; Szymanowski, K.; Czarniak, P.; Podziewski, P.; Werner, Z.; Zagórski, J.; Staszkiewicz, B. Influence of the ion implantation of nitrogen and selected metals on the lifetime of WC-Co indexable knives during MDF machining. Ann. Wars. Univ. Life Sci.-SGGW. For. Wood Technol. 2019, 108, 45–52. [Google Scholar] [CrossRef]
- Otwarta Encyklopedia Leśna. Available online: https://www.encyklopedia.lasypolskie.pl/doku.php?id=b:budowa-i-sklad-chemiczny-drewna.products (accessed on 19 September 2024).
- Barette, J.P.; Hazard, C.; Mayer, J. Mémotech—Bois et Matériaux Associés; Éditions Casteilla: Paris, France, 1996; p. 22. ISBN 978-2-7135-1645-0. [Google Scholar]
- Kraszkiewicz, A. Analiza wybranych właściwości chemicznych drewna i kory robinii akacjowej (Robinia pseudoacacia L.). Inżynieria Rol. 2009, 117, 69–75. [Google Scholar]
- Clementi, E.; Raimondi, D.L.; Reinhardt, W.P. Atomic screening constants from SCF functions. II. Atoms with 37 to 86 electrons. J. Chem. Phys. 1967, 47, 1300–1307. [Google Scholar] [CrossRef]
- ISO 7724-3:2003; Paints And Varnishes—Colorimetry—Part 3: Calculation of Colour Differences. Polish Committee for Standardization: Warsaw, Poland, 2003.
- Persze, L.; Tolvaj, L. Photodegradation of wood at elevated temperature: Colour change. J. Photochem. Photobiol. B Biol. 2012, 108, 44–47. [Google Scholar] [CrossRef]
- Yang, H.; Matinrad, H.; Goldbaum, M.H.; Bu, J.J.; Fukuoka, H.; Afshari, N.A. Refractive Changes After Implantation of Reversed Intraocular Lens in Cataract Surgery: A Mathematical Model. J. Refract. Surg. 2023, 39, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, J.; Sohn, C.; Choi, B. Manufacturing Method of Colored Damond by on Miplantation and Heat Treatment. U.S. Patent 7,604,846 B2, 20 October 2009. [Google Scholar]
- Chen, R.; Lu, W.; Lu, J.; Pu, R.; Lin, J.; Yu, J. The color mechanism of iron on quartz by ion implantation. Phys. B Condens. Matter 2022, 627, 413550. [Google Scholar] [CrossRef]
- Rao, K.S.; Sahoo, R.K.; Dash, T.; Magudapathy, P.; Panigrahi, B.K.; Nayak, B.B.; Mishra, B.K. N and Cr ion implantation of natural ruby surfaces and their characterization. Nucl. Instrum. Methods Phys. Res. B Beam Interact. Mater. At. 2016, 373, 70–75. [Google Scholar] [CrossRef]
- de Meijer, M.; Haemers, S.; Cobben, W.; Militz, H. Surface Energy Determinations of Wood: Comparison of Methods and Wood Species. Langmuir 2000, 16, 9352–9359. [Google Scholar] [CrossRef]
- Gérardin, P.; Petrič, M.; Petrissans, M.; Lambert, J.; Ehrhrardt, J.J. Evolution of wood surface free energy after heat treatment. Polym. Degrad. Stab. 2007, 92, 653–657. [Google Scholar] [CrossRef]


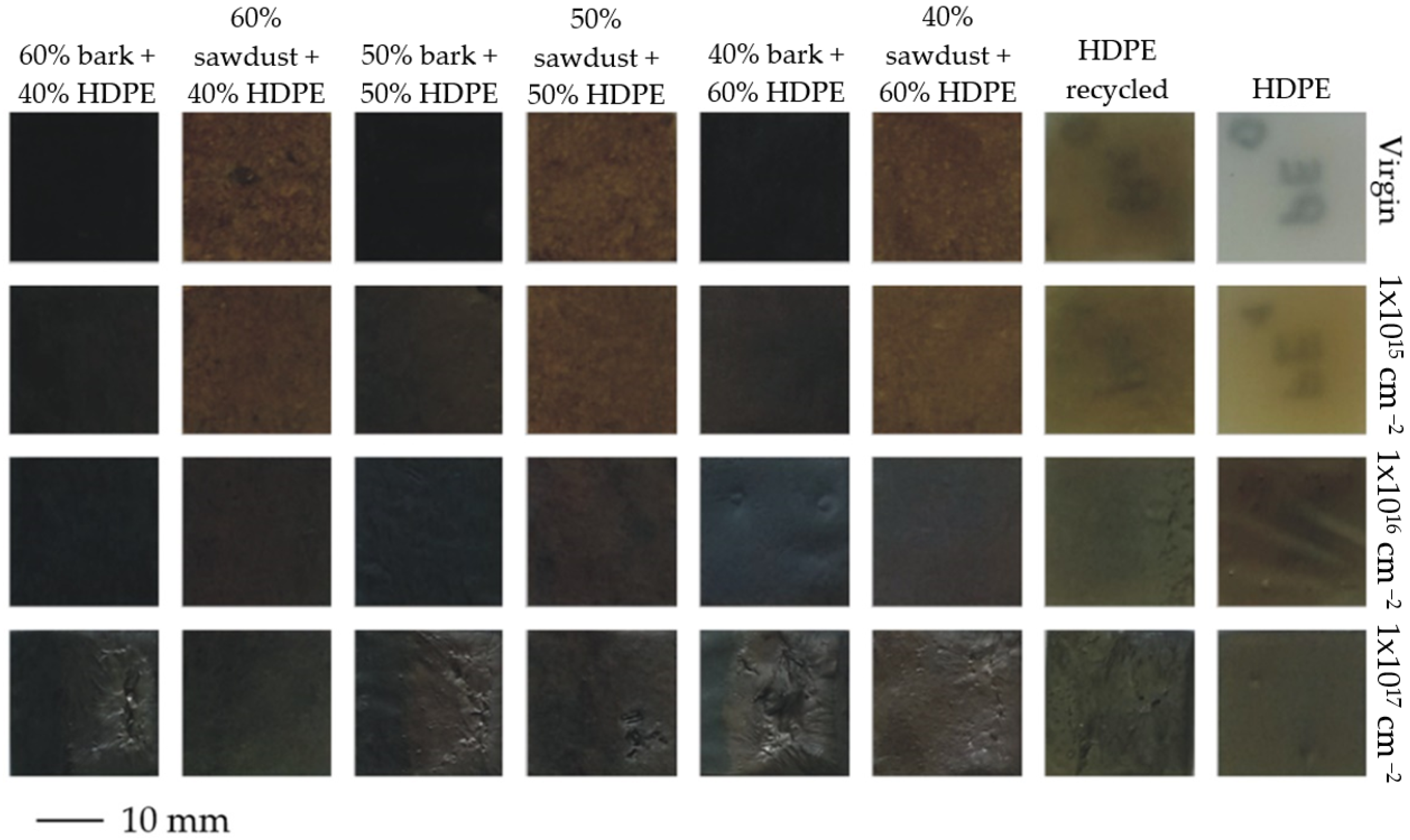

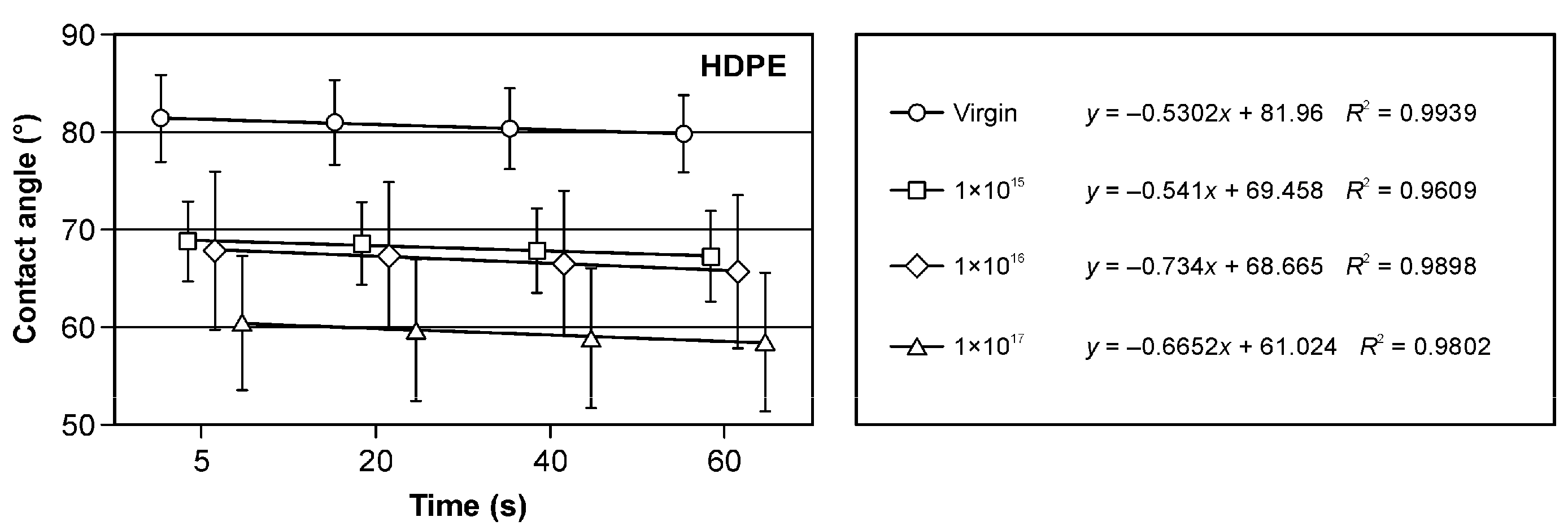
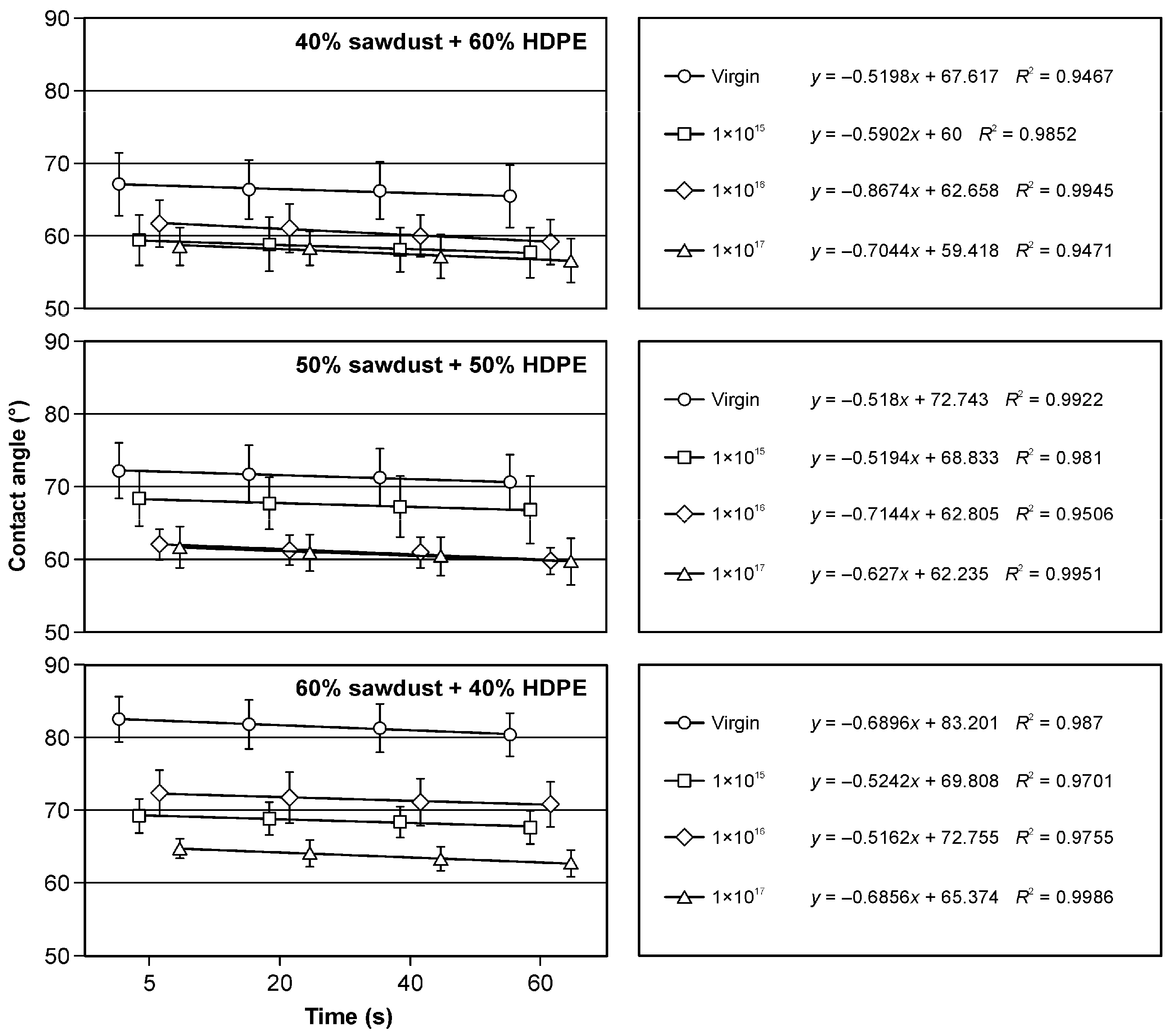
| Samples | HDPE (%) | Sawdust (%) | Bark (%) |
|---|---|---|---|
| 40% sawdust + 60% HDPE | 60 | 40 | - |
| 40% bark + 60% HDPE | 60 | - | 40 |
| 50% sawdust + 50% HDPE | 50 | 50 | - |
| 50% bark + 50% HDPE | 50 | - | 50 |
| 60% sawdust + 40% HDPE | 40 | 60 | - |
| 60% bark + 40% HDPE | 40 | - | 60 |
| Recycled HDPE | 100 | - | - |
| HDPE | 100 | - | - |
| Substrate | at.% C | at.% H | at.% O | at.% N | Density (g/cm3) |
|---|---|---|---|---|---|
| HDPE | 33.33 | 66.67 | - | - | 0.867 |
| Recycled HDPE | 33.33 | 66.67 | - | - | 0.874 |
| 40% sawdust + 60% HDPE | 32.56 | 59.02 | 8.38 | 0.03 | 0.977 * |
| 50% sawdust + 50% HDPE | 32.37 | 57.11 | 10.48 | 0.03 | 1.052 * |
| 60% sawdust + 40% HDPE | 32.18 | 55.2 | 12.58 | 0.04 | 1.100 * |
| Parameter | HDPE | HDPE Recycled | 40% Sawdust + 60% HDPE | 50% Sawdust + 50% HDPE | 60% Sawdust + 40% HDPE |
|---|---|---|---|---|---|
| SRIMmax (atoms/cm3)/(atoms/cm2) | 1.58 × 105 | 1.61 × 105 (2%) | 1.47 × 105 (−7.1%) | 1.52 × 105 (−3.49%) | 1.58 × 105 (0.25%) |
| Nmax (cm−3) | 1.58 × 1020 1.58 × 1021 1.58 × 1022 | 1.61 × 1020 (2%) 1.61 × 1021 (2%) 1.61 × 1022 (2%) | 1.47 × 1020 (−7.1%) 1.47 × 1021 (−7.1%) 1.47 × 1022 (−7.1%) | 1.52 × 1020 (−3.49%) 1.52 × 1021 (−3.49%) 1.53 × 1022 (−3.49%) | 1.58 × 1020 (0.25%) 1.58 × 1021 (0.25%) 1.58 × 1022 (0.25%) |
| Rp (nm) | 111.8 | 110.9 (−0.81%) | 108.8 (−2.68%) | 103.1 (−7.78%) | 100.4 (−10.2%) |
| ΔRp (nm) | 50.6 | 50.2 (−0.79%) | 54.2 (7.11%) | 52.2 (3.16%) | 51.4 (1.58%) |
| Skewness | −0.2337 | −0.2391 (2.31%) | −0.1718 (−26.49%) | −0.1445 (−38.17%) | −0.1353 (−42.11%) |
| Kurtosis | 2.8273 | 2.8392 (0.42%) | 2.7826 (−1.58%) | 2.7531 (−2.62%) | 2.7531 (−2.62%) |
| YC (atoms/ion) | 0.1863 | 0.1855 (−0.43%) | 0.2167 (16.32%) | 0.2235 (19.97%) | 0.2313 (24.15%) |
| YH (atoms/ion) | 1.16 | 1.15 (−0.86%) | 1.11 (−4.31%) | 1.09 (−6.03) | 1.07 (−7.76%) |
| YO (atoms/ion) | - | - | 0.1421 | 0.183 | 0.228 |
| YN (atoms/ion) | - | - | 0.00054 | 0.00049 | 0.00071 |
| Ytotal (atoms/ion) | 1.3463 | 1.3355 (−0.8%) | 1.46934 (9.14%) | 1.49699 (11.19%) | 1.53001 (13.65%) |
| Filler Type | Filler Amount (%) | Fluence (cm−2) | L* | a* | b* | ΔE |
|---|---|---|---|---|---|---|
| bark | 0 | 0 | 52.046 (±3.355) | 1.878 (±1.695) | −4.021 (±2.084) | - |
| 40 | 26.096 (±0.291) | 1.680 (±0.483) | 1.713 (±0.367) | |||
| 50 | 24.180 (±0.410) | 1.664 (±0.372) | 1.980 (±0.496) | |||
| 60 | 25.024 (±0.519) | 0.705 (±0.314) | 0.904 (±0.400) | |||
| sawdust | 0 | 0 | 52.046 (±3.355) | 1.878 (±1.695) | −4.021 (±2.084) | - |
| 40 | 46.631 (±0.981) | 7.375 (±0.841) | 20.087 (±0.750) | |||
| 50 | 47.980 (±1.308) | 8.929 (±1.336) | 18.996 (±1.582) | |||
| 60 | 43.946 (±0.641) | 8.644 (±2.383) | 19.233 (±1.152) | |||
| bark | 0 | 1 × 1015 | 48.174 (±1.880) | 0.255 (±1.140) | 9.003 (±0.742) | 13.684 |
| 40 | 34.216 (±0.168) | 0.184 (±0.306) | 2.979 (±0.475) | 8.353 | ||
| 50 | 36.940 (±0.546) | 1.939 (±0.442) | 5.326 (±0.560) | 13.194 | ||
| 60 | 36.285 (±0.401) | 1.944 (±0.297) | 4.672 (±0.720) | 11.939 | ||
| sawdust | 0 | 1 × 1015 | 48.174 (±1.880) | 0.255 (±1.140) | 9.003 (±0.742) | 13.684 |
| 40 | 47.808 (±0.562) | 6.013 (±1.121) | 18.692 (±0.896) | 2.227 | ||
| 50 | 47.594 (±0.401) | 7.056 (±0.501) | 17.498 (±0.412) | 2.429 | ||
| 60 | 45.138 (±0.422) | 7.152 (±0.958) | 16.585 (±0.612) | 3.269 | ||
| bark | 0 | 1 × 1016 | 43.573 (±0.742) | 1.510 (±1.309) | 1.692 (±0.807) | 10.226 |
| 40 | 39.417 (±0.216) | −1.346 (±0.941) | −0.71 (±0.579) | 13.874 | ||
| 50 | 37.687 (±0.460) | −0.839 (±0.551) | −1.009 (±0.505) | 14.085 | ||
| 60 | 38.793 (±0.323) | −0,405 (±1.096) | −1.622 (±0.636) | 14.043 | ||
| sawdust | 0 | 1 × 1016 | 43.573 (±0.742) | 1.510 (±1.309) | 1.692 (±0.807) | 10.226 |
| 40 | 42.535 (±0.183) | 2.309 (±0.321) | 3.529 (±0.707) | 17.794 | ||
| 50 | 43.181 (±0.232) | 4.263 (±1.193) | 4.238 (±0.920) | 16.205 | ||
| 60 | 41.786 (±0.181) | 3.456 (±0.438) | 3.631 (±0.491) | 16.583 | ||
| bark | 0 | 1 × 1017 | 45.318 (±0.193) | 2.327 (±0.314) | 3.681 (±0.226) | 10.237 |
| 40 | 42.815 (±2.733) | −0.317 (±3.904) | 0.791 (±2.398) | 16.863 | ||
| 50 | 40.361 (±4.162) | 3.393 (±1.887) | 2.001 (±1.687) | 16.273 | ||
| 60 | 44.733 (±2.138) | 2.751 (±2.493) | 0.461 (±1.360) | 19.820 | ||
| sawdust | 0 | 1 × 1017 | 45.318 (±0.193) | 2.327 (±0.314) | 3.681 (±0.226) | 10.237 |
| 40 | 44.933 (±1.175) | 3.243 (±0.829) | 2.992 (±0.427) | 17.669 | ||
| 50 | 43.396 (±0.948) | −0.437 (±1.554) | 5.759 (±1.972) | 16.851 | ||
| 60 | 42.299 (±1.803) | 2.272 (±1.089) | 3.459 (±0.948) | 17.092 |
| Filler Type | Filler Amount (%) | Fluence (cm−2) | |||
|---|---|---|---|---|---|
| 0 | 1 × 1015 | 1 × 1016 | 1 × 1017 | ||
| Surface Free Energy (mJ/m2) | |||||
| bark | 0 | 44.5 | 51.4 | 51.1 | 54.4 |
| 40 | 44.6 | 49.9 | 51.8 | 55.3 | |
| 50 | 46.9 | 52.0 | 53.1 | 52.4 | |
| 60 | 48.1 | 48.0 | 50.3 | 54.0 | |
| sawdust | 0 | 44.5 | 51.4 | 51.2 | 54.4 |
| 40 | 48.9 | 56.7 | 55.7 | 56.1 | |
| 50 | 46.6 | 51.8 | 55.4 | 54.5 | |
| 60 | 43.8 | 51.5 | 48.8 | 52.8 | |
| Factors | p | X (%) |
|---|---|---|
| Filler type (A) | 1.39 × 10−10 | 2.07 |
| Filler amount (B) | <1.00 × 10−17 | 11.01 |
| Implanted fluencies of argon (C) | <1.00 × 10−17 | 45.07 |
| Time after placing a droplet (D) | 5.89 × 10−4 | 0.85 |
| A × B | <1.00 × 10−17 | 4.51 |
| A × C | 6.08 × 10−4 | 0.85 |
| B × C | <1.00 × 10−17 | 6.40 |
| A × D | 1.00 | <0.01 |
| B × D | 1.00 | 0.01 |
| C × D | 1.00 | 0.01 |
| A × B × C | 1.61 × 10−14 | 4.44 |
| A × B × D | 1.00 | <0.01 |
| A × C × D | 1.00 | <0.01 |
| B × C × D | 1.00 | 0.01 |
| A × B × C × D | 1.00 | 0.01 |
| Error | 24.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betlej, I.; Barlak, M.; Lipska, K.; Borysiuk, P.; Boruszewski, P. Argon Ion Implantation as a Method of Modifying the Surface Properties of Wood–Plastic Composites. Materials 2024, 17, 5347. https://doi.org/10.3390/ma17215347
Betlej I, Barlak M, Lipska K, Borysiuk P, Boruszewski P. Argon Ion Implantation as a Method of Modifying the Surface Properties of Wood–Plastic Composites. Materials. 2024; 17(21):5347. https://doi.org/10.3390/ma17215347
Chicago/Turabian StyleBetlej, Izabela, Marek Barlak, Karolina Lipska, Piotr Borysiuk, and Piotr Boruszewski. 2024. "Argon Ion Implantation as a Method of Modifying the Surface Properties of Wood–Plastic Composites" Materials 17, no. 21: 5347. https://doi.org/10.3390/ma17215347
APA StyleBetlej, I., Barlak, M., Lipska, K., Borysiuk, P., & Boruszewski, P. (2024). Argon Ion Implantation as a Method of Modifying the Surface Properties of Wood–Plastic Composites. Materials, 17(21), 5347. https://doi.org/10.3390/ma17215347








