Improvement Schemes for Bacteria in MICP: A Review
Abstract
:1. Introduction
2. Mechanism of Urease Action and the Role of Bacteria in MICP
2.1. The Mechanism of MICP Reaction at the Molecular Level: The Effect and Source of Urease

2.2. The Role of Bacteria in the MICP Process
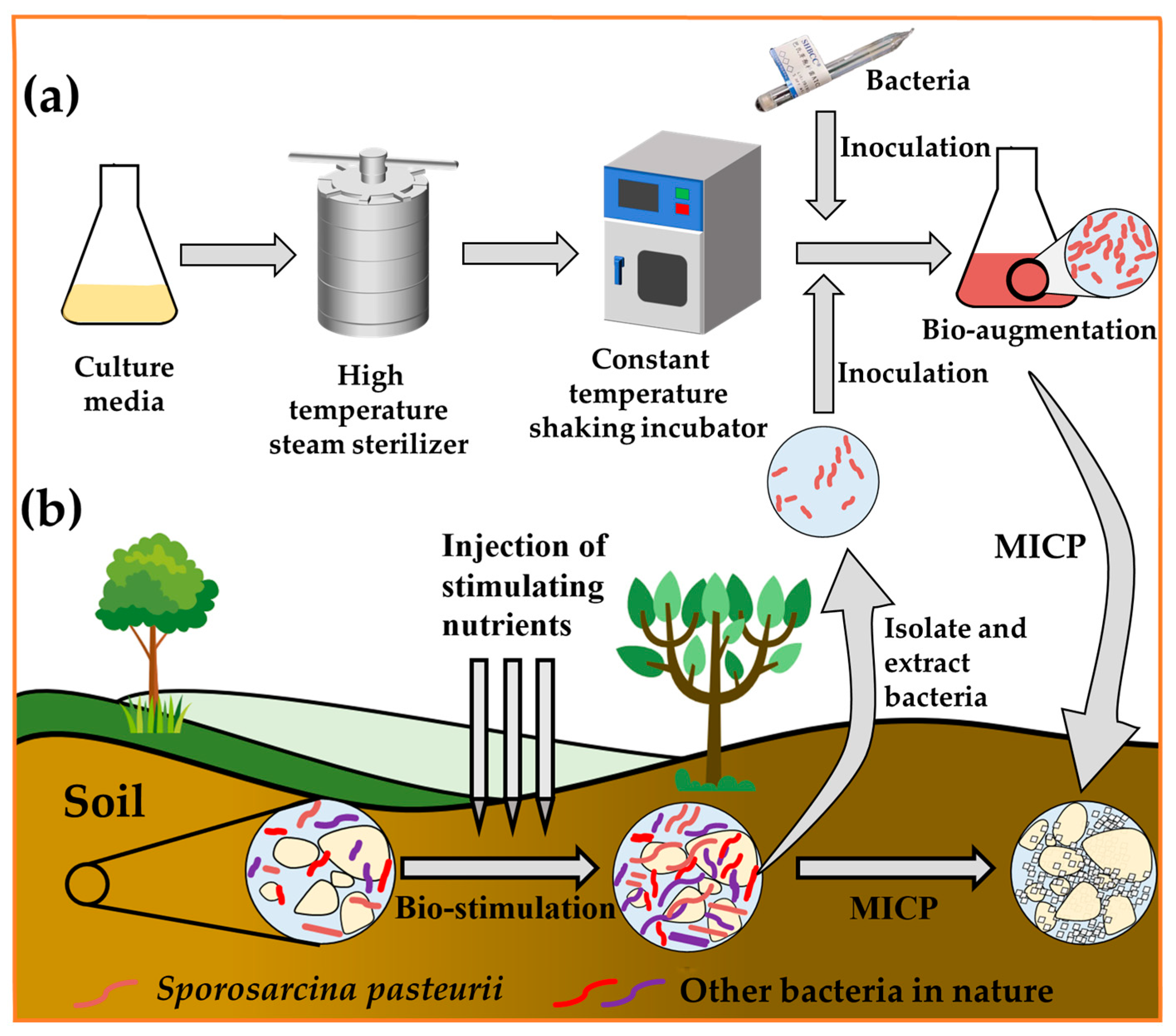
2.3. Different Treatments of Bacteria in MICP: Bio-Stimulation and Bio-Augmentation
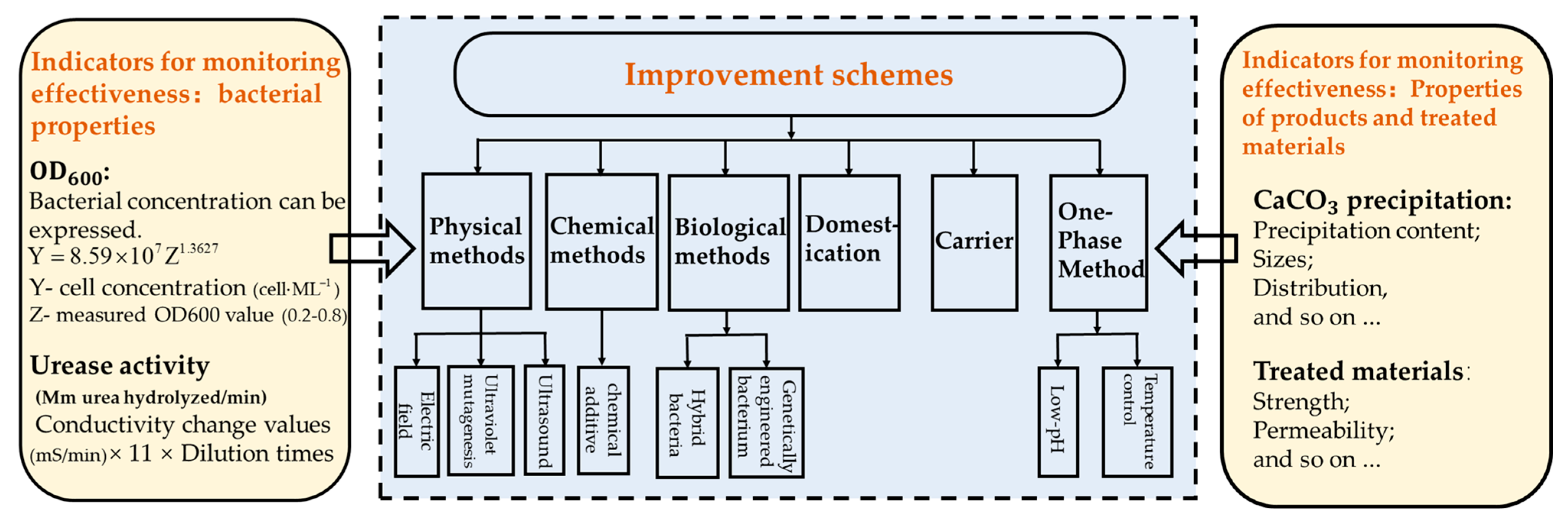
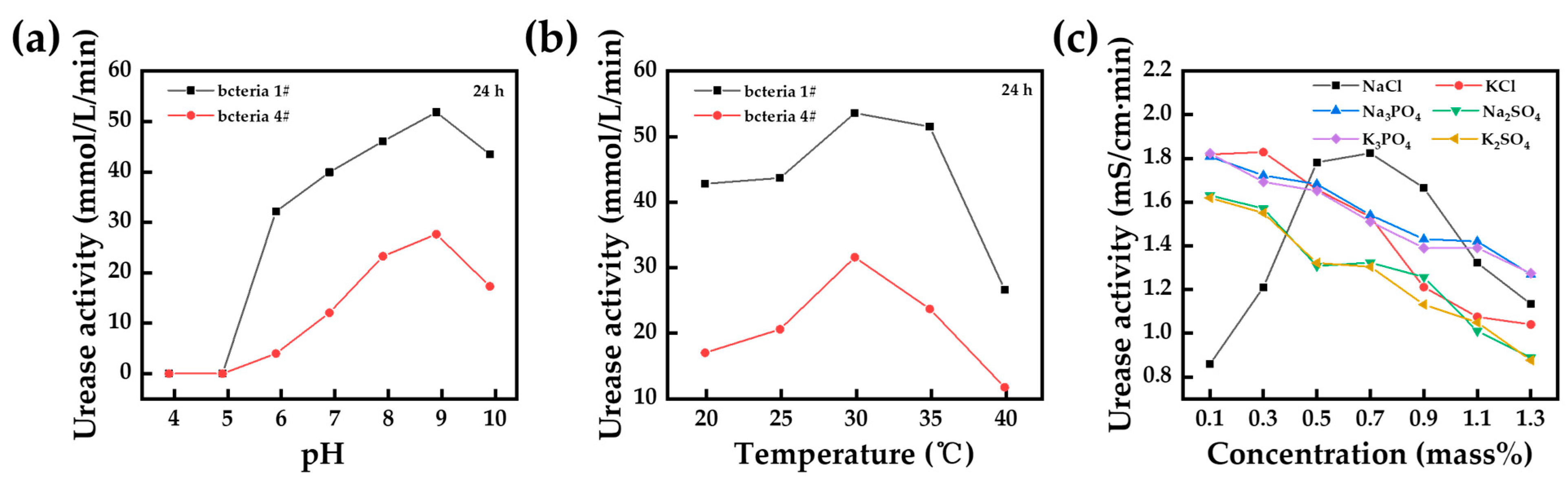
2.4. Assessment Indicators for Efficiency Improvements in MICP
3. Physical, Chemical, and Biological Methods for Improving Bacterial Performance
3.1. Physical Method
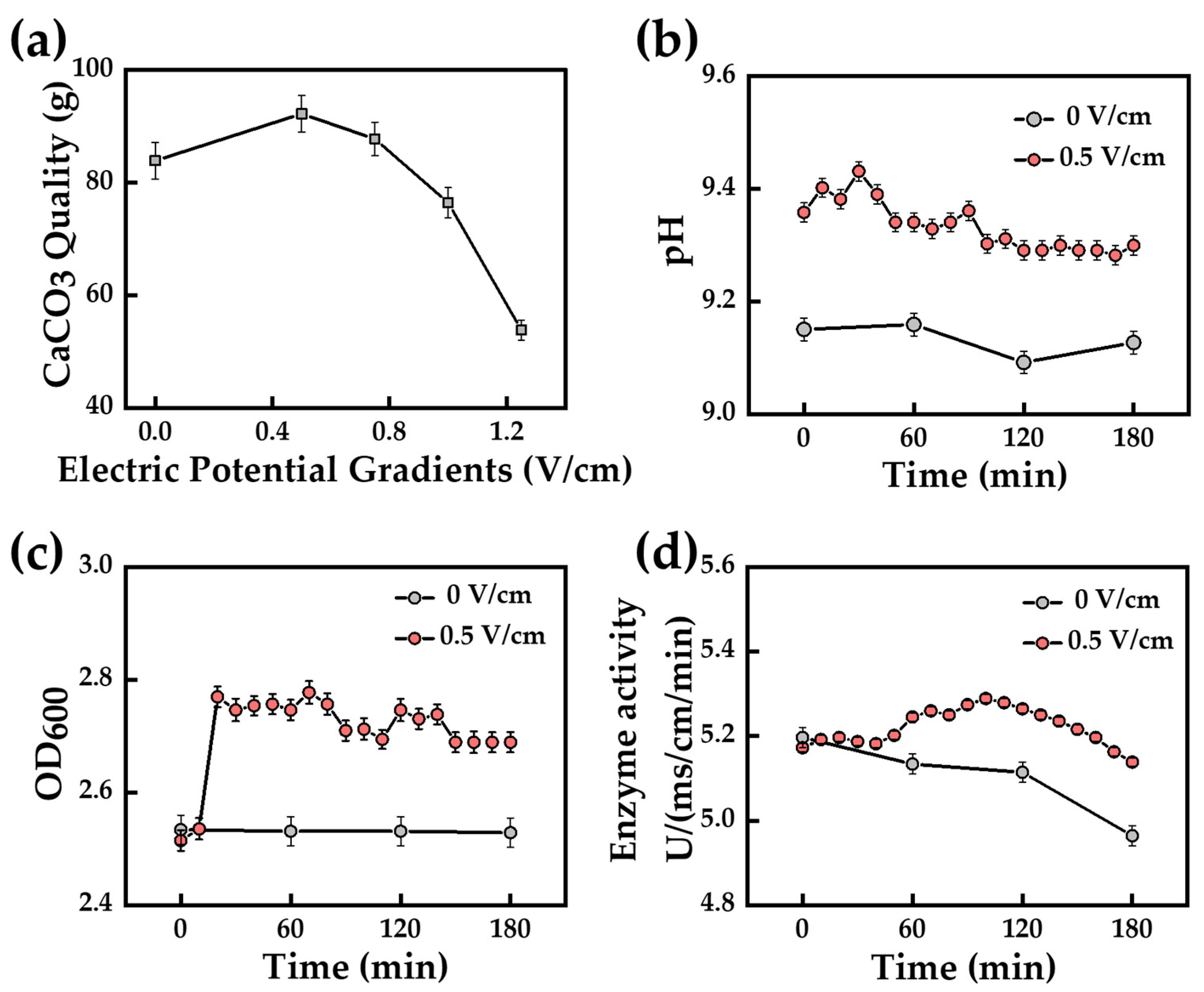
3.1.1. Electric Field
3.1.2. Ultraviolet Radiation Mutagenesis
3.1.3. Ultrasound
3.2. Chemical Method
3.3. Biological Method
3.3.1. Hybrid Bacteria
3.3.2. Genetically Engineered Bacterium
4. New Approaches to Address the Challenges in MICP
4.1. Improvements to Address Adverse Environmental Factors
4.2. One-Phase Injection Method to Improve Homogeneity of Bio-Reinforced Sand
5. Perspectives
6. Conclusions
- (1)
- In this paper, we describe the urease composition at the genetic level and review the sources of urease. Urease decomposes urea and induces calcium carbonate precipitation. In MICP, bacteria act as calcium carbonate nucleation sites in addition to secreting urease for the biomineralization process, and their secreted EPS also influence calcium carbonate production. Besides the bio-augmentation method, the MICP process can also be conducted by bio-stimulation as well as extraction and enrichment methods. Different schemes are compared and analyzed from the perspectives of environmental suitability, time cost, environmental dependence, and raw material consumption.
- (2)
- Physical (electric field, UV mutagenesis, and ultrasound), chemical (inorganic and organic additives), and biological (hybrid bacteria and DNA recombination) methods to enhance bacterial performance were summarized, and the performance of bacteria was enhanced in different ways by different treatments.
- (3)
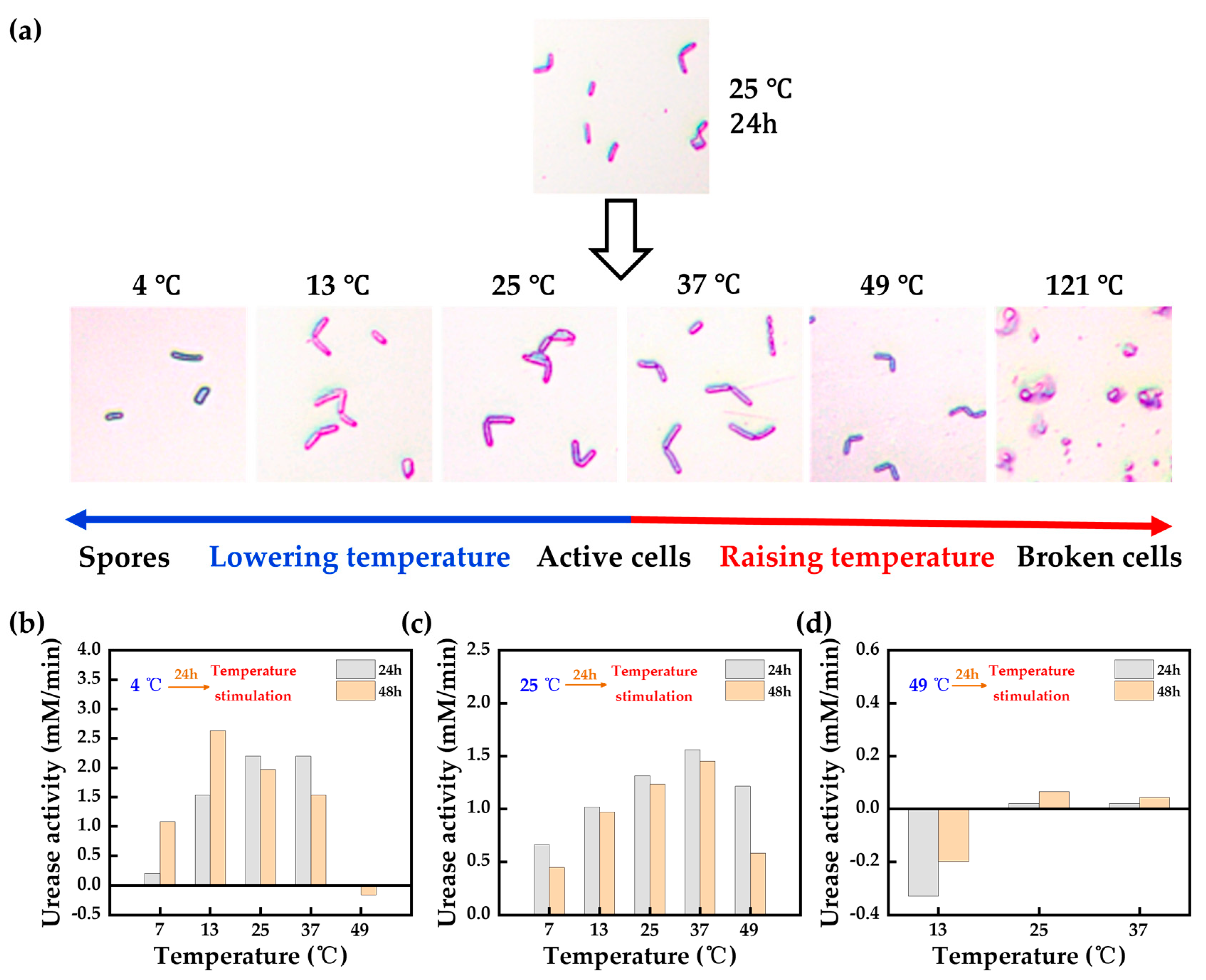
- The effects of different environmental factors on bacterial growth (e.g., pH, temperature, salinity, etc.) are described, and improvement methods are proposed for adverse environmental factors, such as the domestication of bacteria to cope with adverse environments and the protection of bacteria through carriers. Although unfavorable environmental factors can adversely affect bacterial urease, they can also provide new MICP treatment methods; that is, one-phase injection methods based on low temperatures or low pHs, which can significantly improve the efficiency of MICP reinforcement.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griesshaber, E.; Schmahl, W. Special Issue on Biomineralization: From Cells to Biomaterials. Acta Biomater. 2021, 120, 1–3. [Google Scholar] [CrossRef]
- Liu, H.L. Biogenic construction: The new era of civil engineering. Biogeotechnics 2024, 100130. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Miao, L.; Wang, H.; Wu, L.; Shi, W.; Kawasaki, S. State-of-the-art review of soil erosion control by MICP and EICP techniques: Problems, applications, and prospects. Sci. Total Environ. 2024, 912, 169016. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Stabnikov, V.; Ivanov, V. Microbially Induced Calcium Carbonate Precipitation on Surface or in the Bulk of Soil. Geomicrobiol. J. 2012, 29, 544–549. [Google Scholar] [CrossRef]
- Dong, Z.-H.; Pan, X.-H.; Zhu, C.; Tang, C.-S.; Lv, C.; Liu, B.; Wang, D.-L.; Li, H.; Cheng, Y.-J.; Shi, B. Bio-mediated geotechnology and its application in geoengineering: Mechanism, approach, and performance. Environ. Earth Sci. 2024, 83, 348. [Google Scholar] [CrossRef]
- Ishara, S.; Anand, R.; Parihar, A.; Reddy, M.S.; Goyal, S. Suitability and Challenges of Biomineralization Techniques for Ground Improvement. Int. J. Environ. Res. 2024, 18, 52. [Google Scholar] [CrossRef]
- Jiang, L.; Xia, H.; Wang, W.J.; Zhang, Y.; Li, Z. Applications of microbially induced calcium carbonate precipitation in civil engineering practice: A state-of-the-art review. Constr. Build. Mater. 2023, 404, 133227. [Google Scholar] [CrossRef]
- Badiee, H.; Sabermahani, M.; Javadi, A.S.; Bouazza, A. Comparison of Microbially Induced Carbonate Precipitation with Ordinary Portland Cement Producing Macroporous Pervious Concrete. J. Mater. Civ. Eng. 2021, 33, 04021070. [Google Scholar] [CrossRef]
- Wei, R.J.; Peng, J.; He, J.; Li, L.L.; Jiang, Z.; Tang, J.H. Effects of adding aluminum ion flocculant on MICP reinforcement of sand. Acta Geotech. 2024, 19, 3505–3517. [Google Scholar] [CrossRef]
- Jiang, N.-J.; Wang, Y.-J.; Chu, J.; Kawasaki, S.; Tang, C.-S.; Cheng, L.; Du, Y.-J.; Shashank, B.S.; Singh, D.N.; Han, X.-L.; et al. Bio-mediated soil improvement: An introspection into processes, materials, characterization and applications. Soil Use Manag. 2022, 38, 68–93. [Google Scholar] [CrossRef]
- Cui, M.-J.; Zheng, J.-J.; Zhang, R.-J.; Lai, H.-J.; Zhang, J. Influence of cementation level on the strength behaviour of bio-cemented sand. Acta Geotech. 2017, 12, 971–986. [Google Scholar] [CrossRef]
- Li, S.H.; Li, C.; Yao, D.; Wang, S. Feasibility of microbially induced carbonate precipitation and straw checkerboard barriers on desertification control and ecological restoration. Ecol. Eng. 2020, 152, 105883. [Google Scholar] [CrossRef]
- Dagliya, M.; Satyam, N.; Sharma, M.; Garg, A. Experimental study on mitigating wind erosion of calcareous desert sand using spray method for microbially induced calcium carbonate precipitation. J. Rock Mech. Geotech. Eng. 2022, 14, 1556–1567. [Google Scholar] [CrossRef]
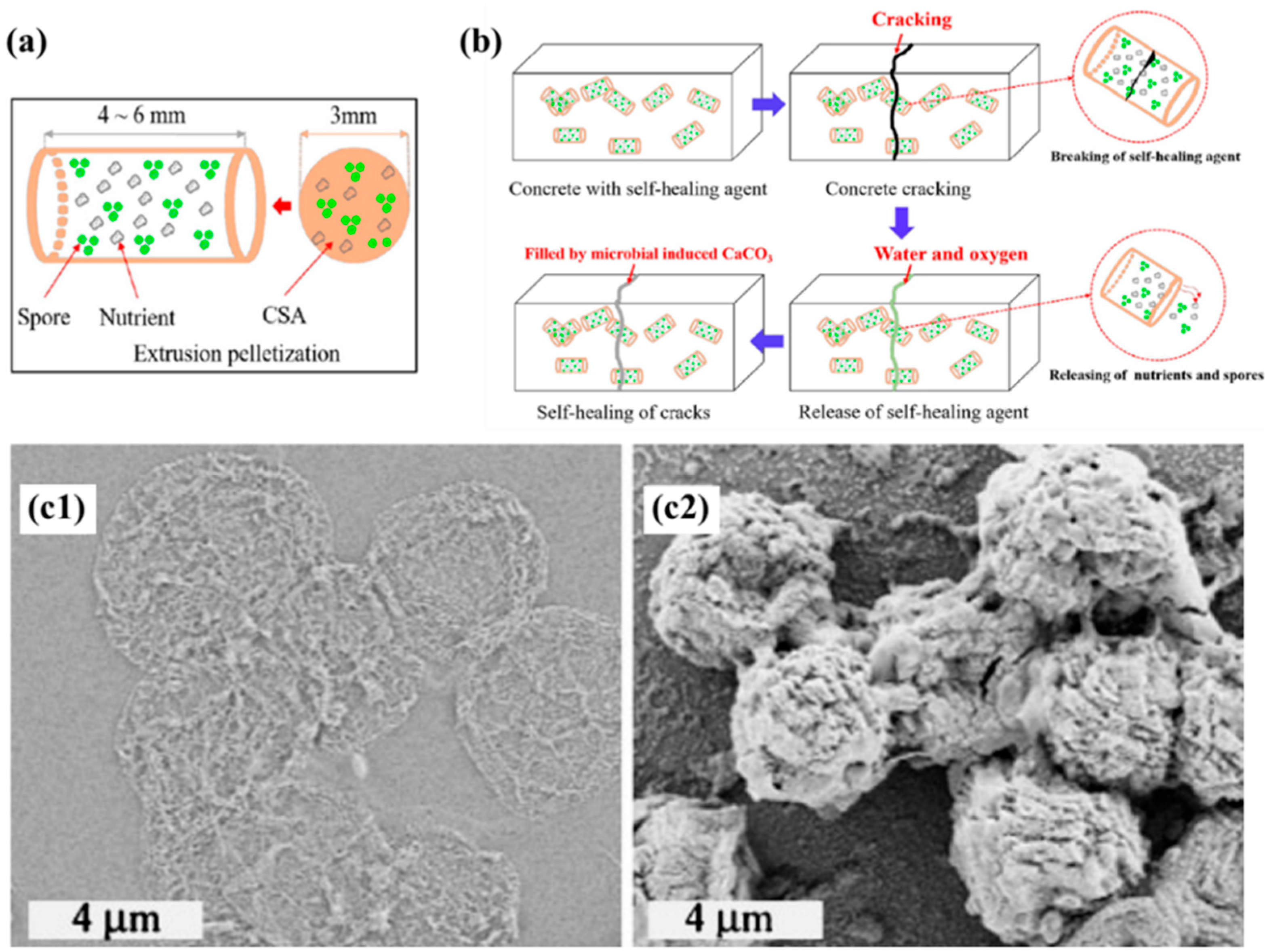
- Jiang, L.; Li, P.; Wang, W.; Zhang, Y.; Li, Z. A self-healing method for concrete cracks based on microbial-induced carbonate precipitation: Bacteria, immobilization, characterization, and application. J. Sustain. Cem.-Based Mater. 2024, 13, 222–242. [Google Scholar] [CrossRef]
- Qian, C.; Zheng, T.; Zhang, X.; Su, Y. Application of microbial self-healing concrete: Case study. Constr. Build. Mater. 2021, 290, 123226. [Google Scholar] [CrossRef]
- Garg, R.; Garg, R.; Eddy, N.O. Microbial induced calcite precipitation for self-healing of concrete: A review. J. Sustain. Cem.-Based Mater. 2023, 12, 317–330. [Google Scholar] [CrossRef]
- Guo, L.; Wang, B.; Guo, J.; Guo, H.; Jiang, Y.; Zhang, M.; Dai, Q. Experimental study on improving hydraulic characteristics of sand via microbially induced calcium carbonate precipitation. Geomech. Energy Environ. 2024, 37, 100519. [Google Scholar] [CrossRef]
- Xiao, P.; Liu, H.; Xiao, Y.; Stuedlein, A.W.; Evans, T.M. Liquefaction resistance of bio-cemented calcareous sand. Soil Dyn. Earthq. Eng. 2018, 107, 9–19. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, J.; Bian, H.; Yue, J.; Xiao, J.; Wei, Y. Study on Liquefaction-Resistance Performance of MICP-Cemented Sands: Applying Centrifuge Shake Table Tests. J. Geotech. Geoenviron. Eng. 2024, 150, 06024004. [Google Scholar] [CrossRef]
- Mu, B.; Gui, Z.; Lu, F.; Petropoulos, E.; Yu, Y. Microbial-Induced Carbonate Precipitation Improves Physical and Structural Properties of Nanjing Ancient City Walls. Materials 2021, 14, 5665. [Google Scholar] [CrossRef]
- Wu, H.R.; Shi, J.Q.; Xiao, Y.; He, J.L.; Chu, J. Microbial mineralization: A promising approach for stone cultural relics restoration. Biogeotechnics 2023, 1, 100053. [Google Scholar] [CrossRef]
- Cui, J.; Xie, Y.; Sun, T.; Chen, L.; Zhang, W. Deciphering and engineering photosynthetic cyanobacteria for heavy metal bioremediation. Sci. Total Environ. 2021, 761, 144111. [Google Scholar] [CrossRef] [PubMed]
- Landa-Marban, D.; Tveit, S.; Kumar, K.; Gasda, S.E. Practical approaches to study microbially induced calcite precipitation at the field scale. Int. J. Greenh. Gas Control 2021, 106, 103256. [Google Scholar] [CrossRef]
- Nething, C.; Smirnova, M.; Groening, J.A.D.; Haase, W.; Stolz, A.; Sobek, W. A method for 3D printing bio-cemented spatial structures using sand and urease active calcium carbonate powder. Mater. Des. 2020, 195, 109032. [Google Scholar] [CrossRef]
- Tang, C.-S.; Yin, L.-Y.; Jiang, N.-J.; Zhu, C.; Zeng, H.; Li, H.; Shi, B. Factors affecting the performance of microbial-induced carbonate precipitation (MICP) treated soil: A review. Environ. Earth Sci. 2020, 79, 94. [Google Scholar] [CrossRef]
- Liu, Y.; Ali, A.; Su, J.-F.; Li, K.; Hu, R.-Z.; Wang, Z. Microbial-induced calcium carbonate precipitation: Influencing factors, nucleation pathways, and application in waste water remediation. Sci. Total Environ. 2023, 860, 160439. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, N.; Strieth, D. Influencing factors on ureolytic microbiologically induced calcium carbonate precipitation for biocementation. World J. Microbiol. Biotechnol. 2023, 39, 61. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Liu, Z.-M.; Hu, B.-R.; Wu, W.-J. Progress on Mineralization Mechanism and Application Research of Sporosarcina pasteurii. Prog. Biochem. Biophys. 2020, 47, 467–482. [Google Scholar]
- Liu, X.; Zhang, Q.; Zhou, N.; Tian, Y. Expression of an Acid Urease with Urethanase Activity in E-coli and Analysis of Urease Gene. Mol. Biotechnol. 2017, 59, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Won, H.S.; Lee, B.J. Nickel-binding properties of the C-terminal tail peptide of Bacillus pasteurii UreE. J. Biochem. 2004, 136, 635–641. [Google Scholar] [CrossRef]
- Lee, Y.H.; Won, H.S.; Lee, M.H.; Lee, B.J. Effects of salt and nickel ion on the conformational stability of Bacillus pasteurii UreE. FEBS Lett. 2002, 522, 135–140. [Google Scholar] [CrossRef]
- Mulrooney, S.B.; Ward, S.K.; Hausinger, R.P. Purification and properties of the Klebsiella aerogenes UreE metal-binding domain, a functional metallochaperone of urease. J. Bacteriol. 2005, 187, 3581–3585. [Google Scholar] [CrossRef] [PubMed]
- Tobler, D.J.; Cuthbert, M.O.; Phoenix, V.R. Transport of Sporosarcina pasteurii in sandstone and its significance for subsurface engineering technologies. Appl. Geochem. 2014, 42, 38–44. [Google Scholar] [CrossRef]
- Saif, A.; Cuccurullo, A.; Gallipoli, D.; Perlot, C.; Bruno, A.W. Advances in Enzyme Induced Carbonate Precipitation and Application to Soil Improvement: A Review. Materials 2022, 15, 950. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Kayastha, A.M.; Srivastava, P.K. Purification and characterization of urease from dehusked pigeonpea (Cajanus cajan L.) seeds. Phytochemistry 2002, 61, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, Y.; Geng, W.; Song, J.; Zhou, Y.; Li, C. Comparison of jack bean and soybean crude ureases on surface stabilization of desert sand via enzyme-induced carbonate precipitation. Geoderma 2023, 435, 116504. [Google Scholar] [CrossRef]
- Dilrukshi, R.A.N.; Nakashima, K.; Kawasaki, S. Soil improvement using plant-derived urease-induced calcium carbonate precipitation. Soils Found. 2018, 58, 894–910. [Google Scholar] [CrossRef]
- Yan, B.; Zhou, Y.; Li, C.; Shu, S.; Gao, Y. Modified SICP method to mitigate the effect of bio-clogging by excess protein from soybean crude urease extracts for biocementation process. Acta Geotech. 2023, 18, 5047–5062. [Google Scholar] [CrossRef]
- Miao, L.; Wu, L.; Sun, X.; Li, X.; Zhang, J. Method for solidifying desert sands with enzyme-catalysed mineralization. Land Degrad. Dev. 2020, 31, 1317–1324. [Google Scholar] [CrossRef]
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Beecham, S. Enzyme induced calcium carbonate precipitation and its engineering application: A systematic review and meta-analysis. Constr. Build. Mater. 2021, 308, 125000. [Google Scholar] [CrossRef]
- Cui, M.-J.; Lai, H.-J.; Hoang, T.; Chu, J. One-phase-low-pH enzyme induced carbonate precipitation (EICP) method for soil improvement. Acta Geotech. 2021, 16, 481–489. [Google Scholar] [CrossRef]
- Hoang, T.; Alleman, J.; Cetin, B.; Ikuma, K.; Choi, S.-G. Sand and silty-sand soil stabilization using bacterial enzyme-induced calcite precipitation (BEICP). Can. Geotech. J. 2019, 56, 808–822. [Google Scholar] [CrossRef]
- Yi, H.; Zheng, T.; Jia, Z.; Su, T.; Wang, C. Study on the influencing factors and mechanism of calcium carbonate precipitation induced by urease bacteria. J. Cryst. Growth 2021, 564, 126113. [Google Scholar] [CrossRef]
- Zhang, J.X.; Xiao, Y.; Cui, H.; He, X.; Liu, H.L. Dancing with crystals: Bacterial functions and interactions in biomineralization. Biogeotechnics 2024, 2, 100084. [Google Scholar] [CrossRef]
- De Philippis, R.; Colica, G.; Micheletti, E. Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: Molecular basis and practical applicability of the biosorption process. Appl. Microbiol. Biotechnol. 2011, 92, 697–708. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Green, E.; Okoh, A.I. Bacterial Exopolysaccharides: Functionality and Prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef]
- Jittawuttipoka, T.; Planchon, M.; Spalla, O.; Benzerara, K.; Guyot, F.; Cassier-Chauvat, C.; Chauvat, F. Multidisciplinary Evidences that Synechocystis PCC6803 Exopolysaccharides Operate in Cell Sedimentation and Protection against Salt and Metal Stresses. PLoS ONE 2013, 8, e55564. [Google Scholar] [CrossRef]
- Kim, H.J.; Shin, B.; Lee, Y.S.; Park, W. Modulation of calcium carbonate precipitation by exopolysaccharide in Bacillus sp JH7. Appl. Microbiol. Biotechnol. 2017, 101, 6551–6561. [Google Scholar] [CrossRef]
- Shao, P.P.; Comolli, L.R.; Bernier-Latmani, R. Membrane Vesicles as a Novel Strategy for Shedding Encrusted Cell Surfaces. Minerals 2014, 4, 74–88. [Google Scholar] [CrossRef]
- Zhu, T.; Dittrich, M. Carbonate Precipitation through Microbial Activities in Natural environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Oggerin, M.; Tornos, F.; Rodriguez, N.; del Moral, C.; Sanchez-Roman, M.; Amils, R. Specific jarosite biomineralization by Purpureocillium lilacinum, an acidophilic fungi isolated from Rio Tinto. Environ. Microbiol. 2013, 15, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.G.; Anderson, C.M.; Graddy, C.M.R.; DeJong, J.T.; Nelson, D.C.; Ginn, T.R. Large-Scale Comparison of Bioaugmentation and Biostimulation Approaches for Biocementation of Sands. J. Geotech. Geoenviron. Eng. 2017, 143, 04016124. [Google Scholar] [CrossRef]
- Graddy, C.M.R.; Gomez, M.G.; DeJong, J.T.; Nelson, D.C. Native Bacterial Community Convergence in Augmented and Stimulated Ureolytic MICP Biocementation. Environ. Sci. Technol. 2021, 55, 10784–10793. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.G.; DeJong, J.T.; Anderson, C.M. Effect of bio-cementation on geophysical and cone penetration measurements in sands. Can. Geotech. J. 2018, 55, 1632–1646. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Cord-Ruwisch, R. Surface Percolation for Soil Improvement by Biocementation Utilizing In Situ Enriched Indigenous Aerobic and Anaerobic Ureolytic Soil Microorganisms. Geomicrobiol. J. 2017, 34, 546–556. [Google Scholar] [CrossRef]
- Wang, X.R.; Li, C.; He, J. A highly effective strain screened from soil and applied in cementing fine sand based on MICP-bonding technology. J. Biotechnol. 2022, 350, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Harkes, M.P.; van Paassen, L.A.; Booster, J.L.; Whiffin, V.S.; van Loosdrecht, M.C.M. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol. Eng. 2010, 36, 112–117. [Google Scholar] [CrossRef]
- Ramachandran, S.K.; Ramakrishnan, V.; Bang, S.S. Remediation of concrete using micro-organisms. ACI Mater. J. 2001, 98, 3–9. [Google Scholar]
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Wei, R.; Peng, J.; Li, L.; Jiang, Z.; Tang, J. Accelerated Reinforcement of Calcareous sand via Biomineralization with Aluminum Ion Flocculant. Appl. Biochem. Biotechnol. 2023, 195, 7197–7213. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Chu, J. Soil bio-cementation using a new one-phase low-pH injection method. Acta Geotech. 2019, 14, 615–626. [Google Scholar] [CrossRef]
- Berg, H. Problems of weak electromagnetic field effects in cell biology. Bioelectrochem. Bioenerg. 1999, 48, 355–360. [Google Scholar] [CrossRef]
- Gao, F.; Feng, J.; Yan, Z.; Zhang, M.; Qin, J.; Zhang, Y.; Yang, M. Weak electric field strengthens the β-oxidation degradation of fatty acids by activated sludge to produce micro-nano CaCO3. J. Environ. Chem. Eng. 2024, 12, 113219. [Google Scholar] [CrossRef]
- Zhu, X.; Lin, F.; Sun, J.; Li, X.; Zhu, G.; Lu, Y.; Sun, L.; Wang, H. Effects of Weak Electric Fields on the Denitrification Performance of Pseudomonas stutzeri: Insights into Enzymes and Metabolic Pathways. Microorganisms 2024, 12, 1218. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, M.; Tian, Y.; Zhang, Z.; Wu, L.; Hu, L. Using Electric Field to Improve the Effect of Microbial-Induced Carbonate Precipitation. Sustainability 2023, 15, 5901. [Google Scholar] [CrossRef]
- Shao, G.; Huang, R.; Liu, P. Application of electric fields to improve cementation homogeneity of biogrouted sands. Soil Use Manag. 2024, 40, e12943. [Google Scholar] [CrossRef]
- Deng, J.; Li, M.; Tian, Y.; Wu, L.; Hu, L.; Zhang, Z.; Zheng, H. Experimental study on solidification of uranium tailings by microbial grouting combined with electroosmosis. Nucl. Eng. Technol. 2023, 55, 4527–4542. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Basu, P.C.; Reddy, M.S. Strain improvement of Sporosarcina pasteurii for enhanced urease and calcite production. J. Ind. Microbiol. Biotechnol. 2009, 36, 981–988. [Google Scholar] [CrossRef]
- Xu, K.; Huang, M.; Zhang, J.; Cui, M.; Xu, C. Bio-cementation of tailings sands using ultraviolet-induced urease-producing bacteria. Environ. Geotech. 2023. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, C.; Yin, Y.; Shi, L.; Bian, H.; Han, Z. Experimental study on solidification of silt through urease-producing strains induced by ultraviolet mutagenesis. Chin. J. Geotech. Eng. 2023, 45, 2500–2509. [Google Scholar]
- Hawrylik, E. The usage of ultrasounds to disintegrate escherichia Coli becteria contained in treated wastewater. Archit. Civ. Eng. Environ. 2019, 12, 131–136. [Google Scholar]
- Liu, Z.; Peng, J.; Li, J.; Song, E. Experimental study on improving effect of microorganism solidifying sand by ultrasonic. J. Zhejiang Univ. Eng. Sci. 2020, 54, 1411–1417. [Google Scholar]
- Haystead, J.; Gilmour, K.; Sherry, A.; Dade-Robertson, M.; Zhang, M. Effect of (in)organic additives on microbially induced calcium carbonate precipitation. J. Appl. Microbiol. 2024, 135, lxad309. [Google Scholar] [CrossRef] [PubMed]
- Benini, S.; Rypniewski, W.R.; Wilson, K.S.; Miletti, S.; Ciurli, S.; Mangani, S. A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: Why urea hydrolysis casts two nickels. Structure 1999, 7, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Won, H.S.; Lee, Y.H.; Kim, J.H.; Shin, I.S.; Lee, M.H.; Lee, B.J. Structural characterization of the nickel-binding properties of Bacillus pasteurii urease accessory protein (Ure)E in solution. J. Biol. Chem. 2004, 279, 17466–17472. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, H.; Nordlander, E. Computational Modeling of the Mechanism of Urease. Bioinorg. Chem. Appl. 2010, 2010, 364891. [Google Scholar] [CrossRef]
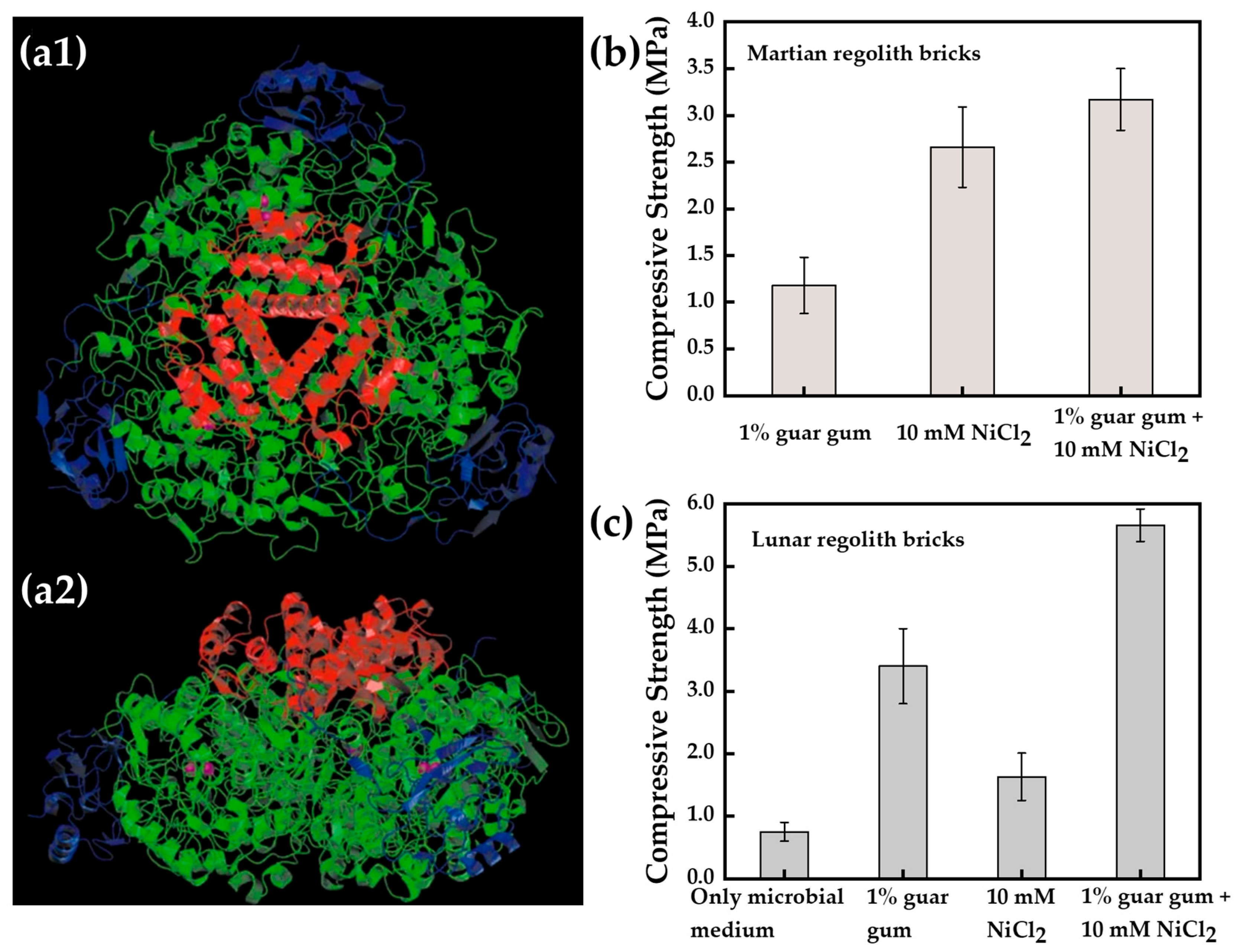
- Dikshit, R.; Gupta, N.; Dey, A.; Viswanathan, K.; Kumar, A. Microbial induced calcite precipitation can consolidate martian and lunar regolith simulants. PLoS ONE 2022, 17, e0266415. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Miao, L.; Tong, T.; Wang, C. Comparison between microbiologically-induced calcium carbonate precipitation and magnesium carbonate precipitation. Chin. J. Geotech. Eng. 2018, 40, 1309–1315. [Google Scholar]
- Lv, C.; Tang, C.S.; Zhang, J.Z.; Pan, X.H.; Liu, H. Effects of calcium sources and magnesium ions on the mechanical behavior of MICP-treated calcareous sand: Experimental evidence and precipitated crystal insights. Acta Geotech. 2023, 18, 2703–2717. [Google Scholar] [CrossRef]
- Wei, R.; Peng, J.; Chen, Y.; Xu, P.; Li, L. Study on Methods and Effects of Coral Sand Reinforcement by MICP Combined with Reef Resources in South China Sea. J. Disaster Prev. Mitig. Eng. 2023, 43, 1255–1265. [Google Scholar]
- Li, M.; Cheng, X.; Yang, Z.; Guo, H. Breeding High-yield Urease-producing Sporosarcina pasteurii Strain by NTG Mutation. J. Agric. Sci. Technol. 2013, 15, 130–134. [Google Scholar]
- Xiao, Y.; Deng, H.; Li, J.; Xiong, Y.; Cheng, L. Effect of Triton X-100 on Improving Urease Activity of Sporosarcina pasteurii. Mater. Rev. 2024, 38, 209–215. [Google Scholar]
- Xu, W.; Zheng, J.; Chu, J.; Zhang, R.; Cui, M.; Lai, H.; Zeng, C. New method for using N-(N-butyl)-thiophosphoric triamide to improve the effect of microbial induced carbonate precipitation. Constr. Build. Mater. 2021, 313, 125490. [Google Scholar] [CrossRef]
- Xu, W.; Zheng, J.; Cui, M.; Lai, H. Study on the effect of MICP with NBPT on calcium carbonate deposition. J. Huazhong Univ. Sci. Technol. Nat. Sci. 2022, 50, 1–6. [Google Scholar]
- Gu, Z.R.; Li, X.J.; Wu, J.; Niu, S.; Wang, P.B.; Zheng, J.J.; Yan, J.Y.; Xu, L.; Yang, M.; Yan, Y.J. A novel strategy for reinforcing cementation process coupling microbially induced carbonate precipitation (MICP) with cross-linked silk fibroin. J. Environ. Chem. Eng. 2023, 11, 109871. [Google Scholar] [CrossRef]
- Xue, J.L.; Wu, Y.N.; Shi, K.; Xiao, X.F.; Gao, Y.; Li, L.; Qiao, Y.L. Study on the degradation performance and kinetics of immobilized cells in straw-alginate beads in marine environment. Bioresour. Technol. 2019, 280, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-H.; Kwon, Y.-J.; So, J.-S. Bioremediation of heavy metals by using bacterial mixtures. Ecol. Eng. 2016, 89, 64–69. [Google Scholar] [CrossRef]
- Zhu, S.; Hu, X.; Zhao, Y.; Fan, Y.; Wu, M.; Cheng, W.; Wang, P.; Wang, S. Coal Dust Consolidation Using Calcium Carbonate Precipitation Induced by Treatment with Mixed Cultures of Urease-Producing Bacteria. Water Air Soil Pollut. 2020, 231, 442. [Google Scholar] [CrossRef]
- Gat, D.; Tsesarsky, M.; Shamir, D.; Ronen, Z. Accelerated microbial-induced CaCO3 precipitation in a defined coculture of ureolytic and non-ureolytic bacteria. Biogeosciences 2014, 11, 2561–2569. [Google Scholar] [CrossRef]
- Harnpicharnchai, P.; Mayteeworakoon, S.; Kitikhun, S.; Chunhametha, S.; Likhitrattanapisal, S.; Eurwilaichitr, L.; Ingsriswang, S. High level of calcium carbonate precipitation achieved by mixed culture containing ureolytic and nonureolytic bacterial strains. Lett. Appl. Microbiol. 2022, 75, 888–898. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, C.; Zhou, A.; Yang, C.; Zhao, L.; Li, Z. Aragonite formation induced by open cultures of microbial consortia to heal cracks in concrete: Insights into healing mechanisms and crystal polymorphs. Constr. Build. Mater. 2019, 224, 815–822. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, J.; Pan, H.; Zhang, X.; Zhang, Y. Genetically engineered bacterium: Principles, practices, and prospects. Front. Microbiol. 2022, 13, 997587. [Google Scholar] [CrossRef]
- Connolly, J.; Kaufman, M.; Rothman, A.; Gupta, R.; Redden, G.; Schuster, M.; Colwell, F.; Gerlach, R. Construction of two ureolytic model organisms for the study of microbially induced calcium carbonate precipitation. J. Microbiol. Methods 2013, 94, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Bergdale, T.E.; Pinkelman, R.J.; Hughes, S.R.; Zambelli, B.; Ciurli, S.; Bang, S.S. Engineered biosealant strains producing inorganic and organic biopolymers. J. Biotechnol. 2012, 161, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, X.; Ye, L.; Liu, X.; Zhu, J.; Yang, S.; Yan, Y.; Xu, L.; Yan, J. A genetically engineered composite biofilm for microbial induced calcium carbonate precipitation by synergic effect of urease, protein adhesive and xanthan gum. J. Environ. Chem. Eng. 2022, 10, 108431. [Google Scholar] [CrossRef]
- Gorospe, C.M.; Han, S.-H.; Kim, S.-G.; Park, J.-Y.; Kang, C.-H.; Jeong, J.-H.; So, J.-S. Effects of different calcium salts on calcium carbonate crystal formation by Sporosarcina pasteurii KCTC 3558. Biotechnol. Bioprocess Eng. 2013, 18, 903–908. [Google Scholar] [CrossRef]
- Lauchnor, E.G.; Topp, D.M.; Parker, A.E.; Gerlach, R. Whole cell kinetics of ureolysis by Sporosarcina Pasteurii. J. Appl. Microbiol. 2015, 118, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.S.; Lee, M.; Hii, S.L. An Overview of the Factors Affecting Microbial-Induced Calcite Precipitation and its Potential Application in Soil Improvement. J. Civ. Environ. Eng. 2012, 6, 188–194. [Google Scholar]
- Omoregie, A.I.; Khoshdelnezamiha, G.; Senian, N.; Ong, D.E.L.; Nissom, P.M. Experimental optimisation of various cultural conditions on urease activity for isolated Sporosarcina pasteurii strains and evaluation of their biocement potentials. Ecol. Eng. 2017, 109, 65–75. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, Z.; Wang, D.; Di, J.; Guo, X.; Yang, Z.; Li, Y.; Wang, Y.; Wang, Y. Optimization of growth conditions and biological cementation effect of Sporosarcina pasteurii. Constr. Build. Mater. 2023, 395, 132288. [Google Scholar] [CrossRef]
- Han, Q.; Xiao, Y.; Li, P. Study on the effects of different polymicrobial environments on b. Pasteurii survivability, urease activity, and crack healing. Constr. Build. Mater. 2023, 408, 133716. [Google Scholar] [CrossRef]
- Shi, W.B.; Wang, M.; Wu, L.; Xie, X.H.; Wang, M.; Lu, T.J. Study of Concrete Crack Repair using Bacillus megaterium. Adv. Mater. Sci. Eng. 2022, 2022, 6188680. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Tong, T.; Wang, C. Effect of methods of adding urea in culture media on sand solidification tests. Chin. J. Geotech. Eng. 2018, 40, 939–944. [Google Scholar]
- Sun, X.; Miao, L.; Wu, L.; Wang, C.; Chen, R. Experimental study on precipitation rate of MICP under low temperatures. Chin. J. Geotech. Eng. 2019, 41, 1133–1138. [Google Scholar]
- Peng, J.; Cao, T.; He, J.; Dai, D.; Tian, Y. Improvement of Coral Sand with MICP Using Various Calcium Sources in Sea Water Environment. Front. Phys. 2022, 10, 825409. [Google Scholar] [CrossRef]
- Xiao, Y.; Deng, H.; Li, J.; Cheng, L.; Zhu, W. Study on the domestication of Sporosarcina pasteurii and strengthening effect of calcareous sand in seawater environment. Rock Soil Mech. 2022, 43, 395–404. [Google Scholar]
- Guo, S.J.; Zhang, J.X.; Li, M.; Zhou, N.; Song, W.J.; Wang, Z.J.; Qi, S.M. A preliminary study of solid-waste coal gangue based biomineralization as eco-friendly underground backfill material: Material preparation and macro-micro analyses. Sci. Total Environ. 2021, 770, 145241. [Google Scholar] [CrossRef]
- Farmani, F.; Bonakdarpour, B.; Ramezanianpour, A.A. pH reduction through amendment of cement mortar with silica fume enhances its biological treatment using bacterial carbonate precipitation. Mater. Struct. 2015, 48, 3205–3215. [Google Scholar] [CrossRef]
- Su, Y.; Zheng, T.; Qian, C. Application potential of Bacillus megaterium encapsulated by low alkaline sulphoaluminate cement in self-healing concrete. Constr. Build. Mater. 2021, 273, 121740. [Google Scholar] [CrossRef]
- Antipov, A.; Shchukin, D.; Fedutik, Y.; Zanaveskina, I.; Klechkovskaya, V.; Sukhorukov, G.; Möhwald, H. Urease-catalyzed carbonate precipitation inside the restricted volume of polyelectrolyte capsules. Macromol. Rapid Commun. 2003, 24, 274–277. [Google Scholar] [CrossRef]
- Seifan, M.; Sarmah, A.K.; Ebrahiminezhad, A.; Ghasemi, Y.; Samani, A.K.; Berenjian, A. Bio-reinforced self-healing concrete using magnetic iron oxide nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 2167–2178. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; De Belie, N.; Verstraete, W. Diatomaceous earth as a protective vehicle for bacteria applied for self-healing concrete. J. Ind. Microbiol. Biotechnol. 2012, 39, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Jia, G.; Jiang, C.; Li, Z. Sugar-coated expanded perlite as a bacterial carrier for crack-healing concrete applications. Constr. Build. Mater. 2020, 232, 117222. [Google Scholar] [CrossRef]
- Wang, J.Y.; Snoeck, D.; Van Vlierberghe, S.; Verstraete, W.; De Belie, N. Application of hydrogel encapsulated carbonate precipitating bacteria for approaching a realistic self-healing in concrete. Constr. Build. Mater. 2014, 68, 110–119. [Google Scholar] [CrossRef]
- Soysal, A.; Milla, J.; King, G.M.; Hassan, M.; Rupnow, T. Evaluating the Self-Healing Efficiency of Hydrogel-Encapsulated Bacteria in Concrete. Transp. Res. Rec. 2020, 2674, 113–123. [Google Scholar] [CrossRef]
- Cui, M.-J.; Zheng, J.-J.; Zhang, R.-J.; Lai, H.-J. Soil bio-cementation using an improved 2-step injection method. Arab. J. Geosci. 2020, 13, 1270. [Google Scholar] [CrossRef]
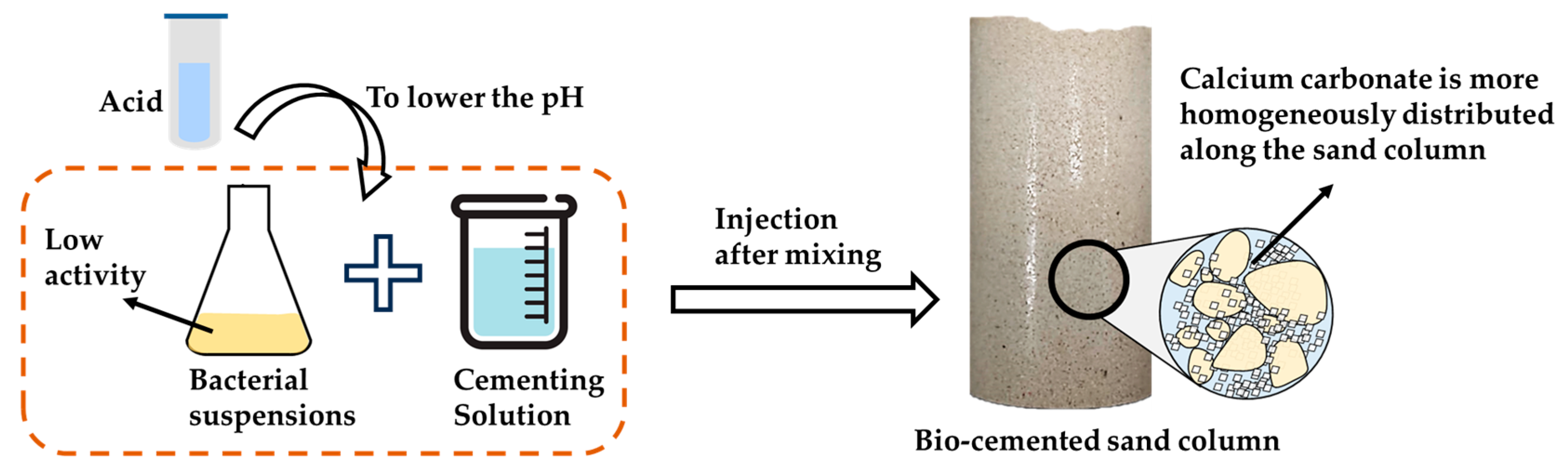
- Yang, Y.; Chu, J.; Liu, H.; Cheng, L. Improvement of uniformity of biocemented sand column using CH3COOH-buffered one-phase-low-pH injection method. Acta Geotech. 2023, 18, 413–428. [Google Scholar] [CrossRef]
- Yu, X.; Yang, H. One-phase MICP and two-phase MISP composite cementation. Constr. Build. Mater. 2023, 409, 133724. [Google Scholar] [CrossRef]
- Lai, Y.; Liu, S.; Cai, Y.; Yu, J. Reinforcement of Different Sands by Low-pH Bio-Mineralization. Materials 2023, 16, 6211. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.-J.; Lai, H.-J.; Hoang, T.; Chu, J. Modified one-phase-low-pH method for bacteria or enzyme-induced carbonate precipitation for soil improvement. Acta Geotech. 2022, 17, 2931–2941. [Google Scholar] [CrossRef]
- Lai, Y.; Yu, J.; Liu, S.; Liu, J.; Wang, R.; Dong, B. Experimental study to improve the mechanical properties of iron tailings sand by using MICP at low pH. Constr. Build. Mater. 2021, 273, 121729. [Google Scholar] [CrossRef]
- Lai, H.-J.; Cui, M.-J.; Chu, J. Effect of pH on soil improvement using one-phase-low-pH MICP or EICP biocementation method. Acta Geotech. 2023, 18, 3259–3272. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Guan, D.; Zhou, Y.; Cheng, L.; Zheng, J. Influence of different bacterial grouting strategies on MICP one-phase injection method. J. Hohai Univ. Nat. Sci. 2020, 48, 222–230. [Google Scholar]
- Li, L.; Liu, T.; Jiang, G.; Fang, C.; Qu, B.; Zheng, S.; Yang, G.; Tang, C. Insight into the temperature stimulation on the self-healing properties of cement-based materials. Constr. Build. Mater. 2022, 361, 129704. [Google Scholar] [CrossRef]
- Kalantary, F.; Kahani, M. Optimization of the biological soil improvement procedure. Int. J. Environ. Sci. Technol. 2019, 16, 4231–4240. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Wang, S.; Evans, T.M.; Stuedlein, A.W.; Chu, J.; Zhao, C.; Wu, H.; Liu, H. Homogeneity and mechanical behaviors of sands improved by a temperature-controlled one-phase MICP method. Acta Geotech. 2021, 16, 1417–1427. [Google Scholar] [CrossRef]
- Xiao, P.; Liu, H.; Stuedlein, A.W.; Evans, T.M.; Xiao, Y. Effect of relative density and biocementation on cyclic response of calcareous sand. Can. Geotech. J. 2019, 56, 1849–1862. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Desai, C.S.; Jiang, X.; Liu, H. Strength and Deformation Responses of Biocemented Sands Using a Temperature-Controlled Method. Int. J. Geomech. 2019, 19, 04019120. [Google Scholar] [CrossRef]
- Lv, C.; Tang, C.-S.; Zhang, J.-Z.; Liu, H.; Pan, X.-H. Regulating the Process of Microbially Induced Calcium Carbonate Precipitation through Applied Electric Fields: Evidence and Insights Using Microfluidics. J. Geotech. Geoenviron. Eng. 2024, 150, 04024087. [Google Scholar] [CrossRef]
- Wang, Y.; Soga, K.; Dejong, J.T.; Kabla, A. A microfluidic chip and its use in characterising the particle-scale behaviour of microbial-induced calcium carbonate precipitation (MICP). Geotechnique 2019, 69, 1086–1094. [Google Scholar] [CrossRef]
- Xiao, Y.; He, X.; Stuedlein, A.W.; Chu, J.; Evans, T.M.; van Paassen, L.A. Crystal Growth of MICP through Microfluidic Chip Tests. J. Geotech. Geoenviron. Eng. 2022, 148, 06022002. [Google Scholar] [CrossRef]
- Su, F.; Yang, Y.; Qi, Y.; Zhang, H. Combining microbially induced calcite precipitation (MICP) with zeolite: A new technique to reduce ammonia emission and enhance soil treatment ability of MICP technology. J. Environ. Chem. Eng. 2022, 10, 107770. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, Q.; Hu, X.; Wu, M.; Zhao, Y.; Feng, Y.; Shen, D. Application of zeolite as a bacterial carrier in the self-healing of cement mortar cracks. Constr. Build. Mater. 2022, 331, 127324. [Google Scholar] [CrossRef]
- Lajevardi, S.H.; Shafiei, H. Investigating the biological treatment effect on fine-grained soil resistance against wind erosion: An experimental case study. Aeolian Res. 2023, 60, 100841. [Google Scholar] [CrossRef]
- Meng, H.; Gao, Y.; He, J.; Qi, Y.; Hang, L. Microbially induced carbonate precipitation for wind erosion control of desert soil: Field-scale tests. Geoderma 2021, 383, 114723. [Google Scholar] [CrossRef]



| Processing Method | Environmental Suitability | Time Cost | Environmental Dependence | Raw Material Consumption |
|---|---|---|---|---|
| Bio-augmentation | ★ | ★★ | ★ | ★★ |
| Bio-stimulation | ★★★ | ★ | ★★★ | ★ |
| Isolation and Enrichment | ★★★ | ★★★ | ★★★ | ★★★ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Wei, R.; Peng, J.; Dai, D. Improvement Schemes for Bacteria in MICP: A Review. Materials 2024, 17, 5420. https://doi.org/10.3390/ma17225420
Zhu J, Wei R, Peng J, Dai D. Improvement Schemes for Bacteria in MICP: A Review. Materials. 2024; 17(22):5420. https://doi.org/10.3390/ma17225420
Chicago/Turabian StyleZhu, Jin, Renjie Wei, Jie Peng, and Di Dai. 2024. "Improvement Schemes for Bacteria in MICP: A Review" Materials 17, no. 22: 5420. https://doi.org/10.3390/ma17225420
APA StyleZhu, J., Wei, R., Peng, J., & Dai, D. (2024). Improvement Schemes for Bacteria in MICP: A Review. Materials, 17(22), 5420. https://doi.org/10.3390/ma17225420






