Abstract
Solution-processable hole-transporting materials (HTMs) that form highly soluble films and thermally stable amorphous states are essential for advancing optoelectronic devices. However, the currently commercialized HTM, N,N-bis(3-methylphenyl)-N,N0-bis(phenyl)benzidine (TPD), exhibits poor solubility and limited carrier transport when spin-coated into thin films. Herein, to address these issues, a fluorenyl group was ingeniously incorporated into a series of molecules structurally similar to TPD. The resulting compounds, namely, 2,7-di-(N,N-diphenylamino)-9,9-dimethyl-9H-fluorene (DDF), 2,7-di-p-tolyl-(N,N-diphenylamino)-9,9-dimethyl-9H-fluorene (2M-DDF), and 2,7-di-tetra-p-tolyl-(N,N-diphenylamino)-9,9-dimethyl-9H-fluorene (4M-DDF), offered tunable energy levels, carrier transport, crystallinity, and steric configuration via adjustment of the number of terminal methyl groups. Owing to its satisfactory performance, 2M-DDF can serve as an effective alternative to TPD in OLED devices as well as a guest molecule in host–guest systems for long-afterglow materials. Devices incorporating 2M-DDF as the HTM, with an Alq3 emitter, achieved a maximum CE of 4.78 cd/A and a maximum L (Lmax) of 21,412 cd m−2, with a turn-on voltage (Von) of 3.8 V. The luminous efficiency of 2M-DDF was approximately five times that of TPD (4106 cd m−2). Furthermore, when 2M-DDF and TPD were utilized as guest molecules in afterglow materials, the afterglow duration of 2M-DDF (10 s) was 2.5 times that of TPD (4 s). This study provides a theoretical basis for the development of high-performance HTMs and long-afterglow materials, establishing a framework for the application of fluorene-based compounds in emerging fields such as long-afterglow materials.
1. Introduction
Efficient organic optoelectronic devices such as solar cells, large-area displays, long-afterglow materials [1,2,3,4], phosphorescent room-temperature materials [5,6,7,8,9], organic light-emitting diodes (OLEDs) [10,11,12,13,14], and organic perovskite solar cells [15,16,17,18,19] rely on organic materials with high charge transport ability. OLEDs and phosphorescent materials with a long afterglow at room temperature are especially meritorious [20,21] for portable display devices and can even realize large-area display panels. However, the difficulty of designing and synthesizing thermally robust emissive materials with high hole-transport mobility and improved multi-functional properties has limited the development of next-generation high-performance optoelectronic devices [22,23,24,25,26]. Some widely used and relatively inexpensive hole-transporting materials [27,28,29,30,31] such as triphenylamine, carbazole (CA), and their derivatives demonstrate excellent hole mobility and high chemical stability. The common HTMs are N,N-diphenyl-N,N-bis(1-naphthyl)-(1,10-biphenyl)-4,40-diamine (NPB), and TPD, which possess high charge-carrier mobility and are easily sublimated [32,33,34]. However, the low solubility of these materials limits their large-scale application [35,36].
Fluorene-based conjugated compounds exhibit significantly enhanced solubility due to the presence of methyl groups linked to the 9-position of fluorine, this is mainly because the alkyl chains have the ability to hinder intermolecular packing [37], along with a high energy band gap (HOMO: −5.5~−5.2 eV, LUMO: −2.6~−2.0 eV) and superior electron and charge double transport capacity (10−4~10−5 cm2V−1S−1), which has garnered increasing attention from scholars [38,39]. For instance, Okumoto and Shiroto et al. [39] employed the Suzuki coupling method to synthesize a symmetrical fluorene compound with biphenyl as the core, which serves as a hole-transport material exhibiting high hole mobility and excellent thermal stability. However, due to the compound’s symmetrical structure, it tends to form a regular and tightly packed arrangement during film formation or device utilization, leading to crystallization issues that ultimately compromise device performance, efficiency, and lifespan. Consequently, the practical application of this material is limited.
Inspired by the structure of TPD and combined with the advantages of fluorene-based compounds, the DDF compound was synthesized. We further introduced a methyl group through the end group to control the crystallization performance, so 2,7-di-p-tolyl-(N,N-diphenylamino)-9,9-dimethyl-9H-fluorene (2M-DDF), and 2,7-di-tetra-p-tolyl-(N,N-diphenylamino)-9,9-dimethyl-9H-fluorene (4M-DDF) were synthesized in this study. The thermal, electrochemical properties and carrier mobility of the three compounds were investigated through thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), cyclic voltammetry (CV), and space charge limited current (SCLC). The DDF, 2M-DDF, and 4M-DDF compounds have good solubility, thermal stability, and are easily fabricated as thin films by spin coating. More advantageous is the high carrier mobility in the hole-transport layers of the 2M-DDF prepared via spin coating. Consequently, an OLED device with 2M-DDF as the hole-transport layer and Alq3 as the emitter achieved superior performance, with a maximum current efficiency (CE) of 4.78 cd/A, a Von of 3.80 V, and a luminance (L) of 21,412 cd/m2. Compared with traditional OLED hole-transport materials TPD and poly[(9,9-dioctylfluorenyl-2,7-diyl)-alt-(4,4′-(N-(4-butylphenyl) (TFB) materials with similar structure, the maximum luminous brightness is increased by nearly five times (4106 cd m−2) and 1.3 times(15,211 cd m−2), respectively, and the luminous performance is greatly improved, indicating that these materials have a good application prospect. When 2M-DDF was employed as the guest molecules in an afterglow material, the afterglow time of 2M-DDF was 2.5 times that of TPD. Notably, the afterglow persisted for >10 s. The structure–activity relationship of these molecules was systematically described, providing a theoretical foundation for broadening their application in photophysical devices.
2. Results and Discussion
2.1. Fluorene-Based Amorphous Compounds with Good Solubility and High Thermal Stability Facilitate the Preparation of Flexible Films via Solution-Processable Materials Methods
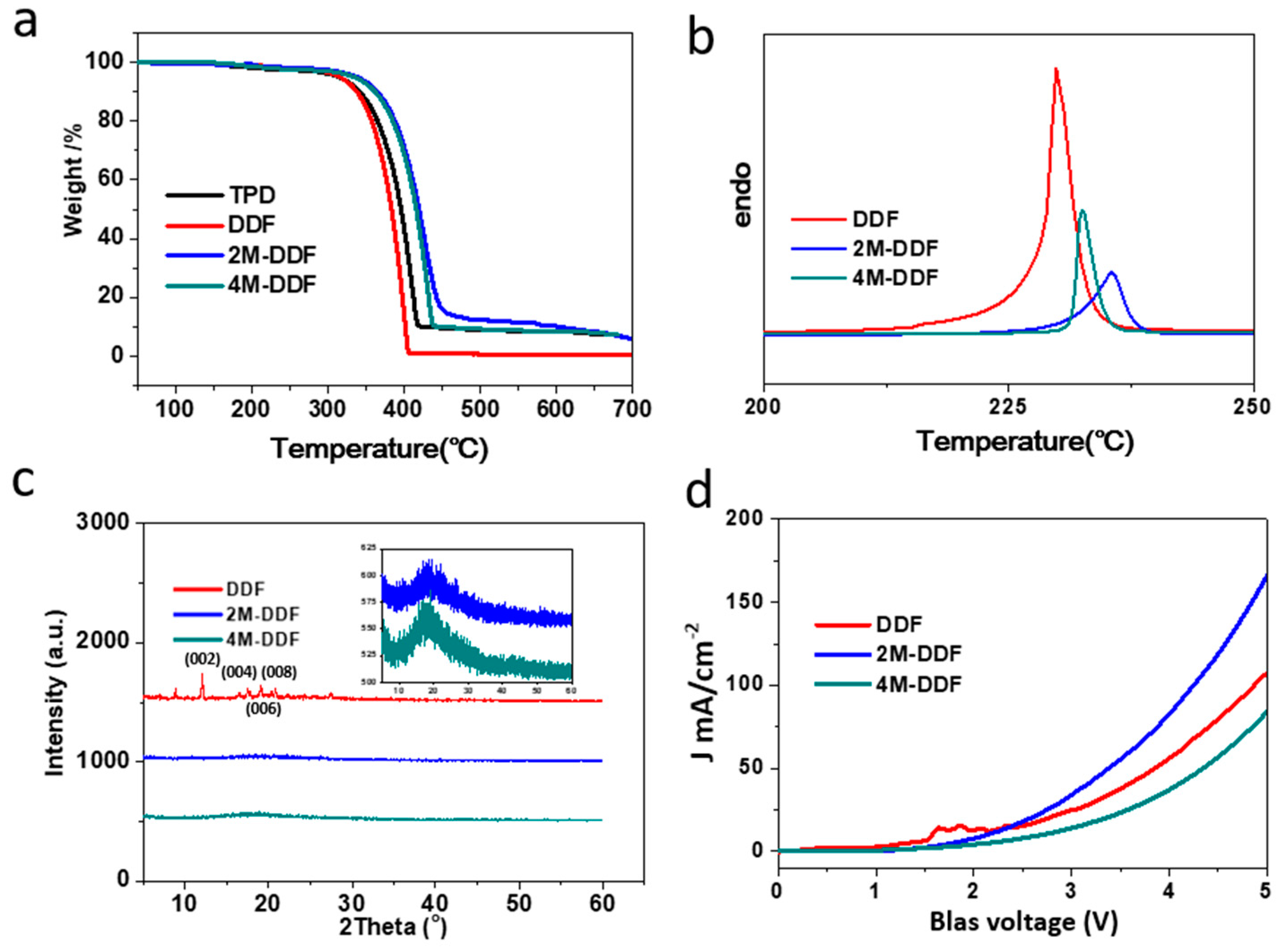
The chemical structure and synthetic routes of DDF, 2M-DDF, and 4M-DDF are shown in Scheme S1 of the Supporting Information. Solubility is an important property of a compound and directly affects the performance of a device. Good solubility is a prerequisite for the preparation of high-performance devices [40,41]. Thermodynamic stability is critical to the performance of materials. The TGA and DSC properties of fluorene-based small molecules DDF, 2M-DDF, 4M-DDF were tested at a heating rate of 10 °C min−1 in a nitrogen atmosphere. From the TGA curves in Figure 1a, it can be seen that the temperatures corresponding to 5% mass loss of DDF, 2M-DDF, and 4M-DDF compounds are 319, 329, 327, and 348 °C, respectively, indicating good thermal stability. The DSC curves in Figure 1b show that the melting points of DDF, 2M-DDF, and 4M-DDF are 185, 230, 236, and 232 °C, respectively, indicating high melting temperatures and good thermal stability for the fluorene-based small molecules.
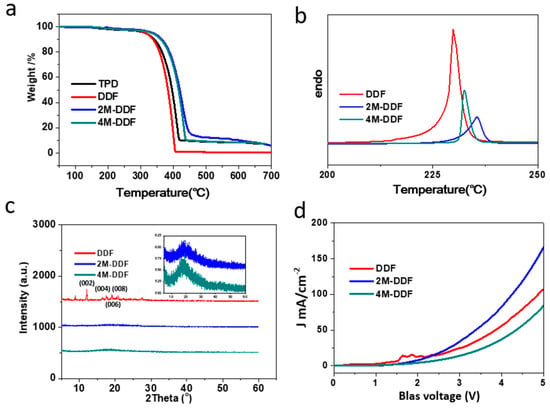
Figure 1.
TGA (a), DSC (b), XRD (c), and SCLC (d) curves of DDF, 2M-DDF, and 4M-DDF. The space charge limited current (SCLC) of hole-only devices with the configuration of indium tin oxide (ITO)/poly(3,4-ethylenedioxythiophene)–polystyrene sulfonate (PEDOT:PSS)/DDF (red), 2M-DDF (blue), 4M-DDF (cyan)/Au.
The synthesized compounds were easily crystallized during the spin-coating preparation of thin films. Grain boundaries that impede the carrier transport were easily formed. Introducing a methyl group at the end group of the molecule increased the steric hindrance, effectively inhibiting molecular crystallization as shown by the X-ray diffraction (XRD) patterns in Figure 1c. The XRD spectrum of DDF displays obvious crystalline peaks but those of 2M-DDF and 4M-DDF show diffuse low peaks (Figure 1c inset), confirming their amorphous nature. As hoped, the methyl group strongly inhibited the crystallization properties of the compound. A smooth, amorphous 2M-DDF film was prepared using the solution method [42]. The well-formed 2M-DDF film is a promising solution-processable HTM for photovoltaic devices such as OLEDs and organic photoconductors.
2.2. Superior Electrochemical Properties of Fluorene Compounds Facilitate Hole Injection and Hence the Carrier-Transport Efficiency
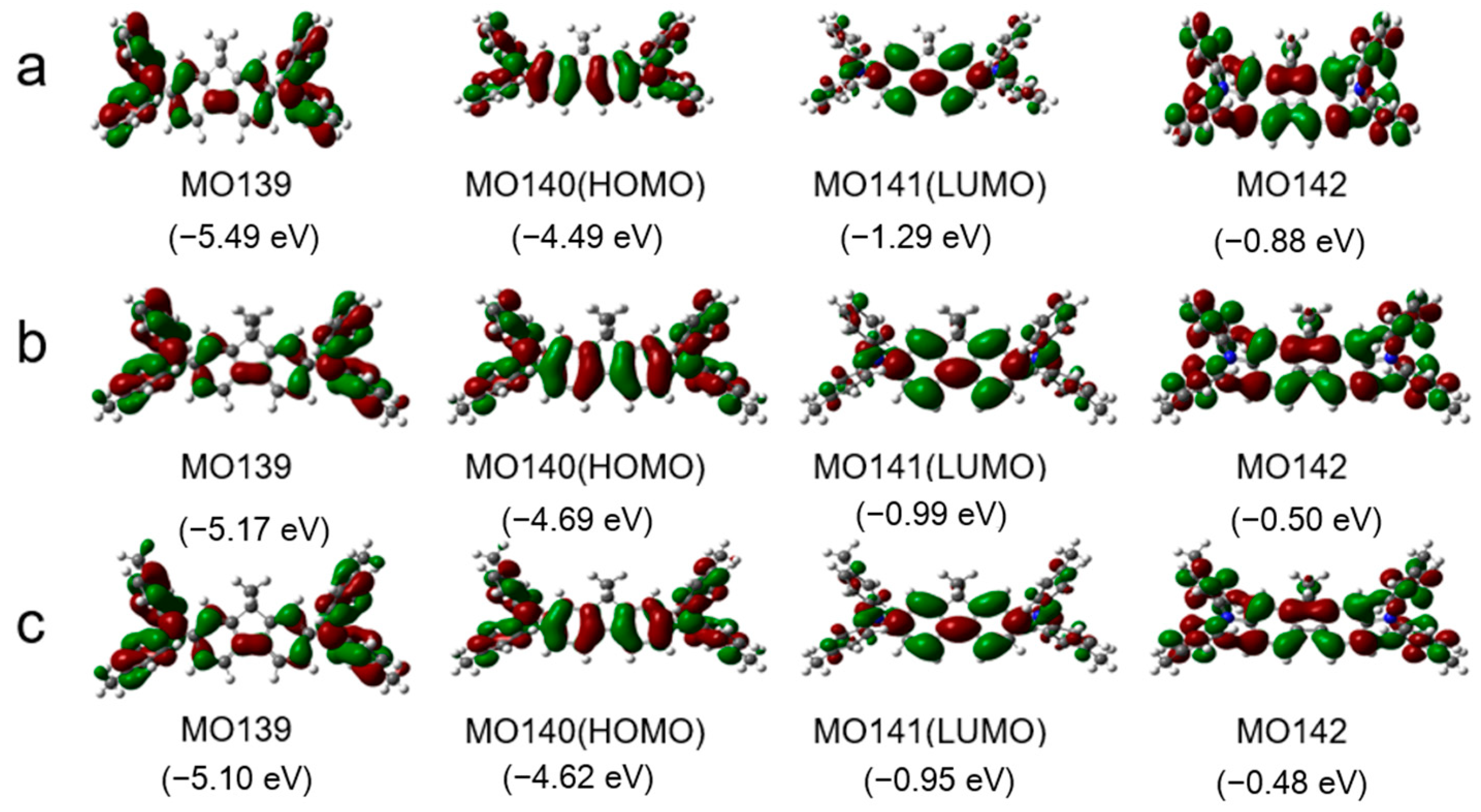
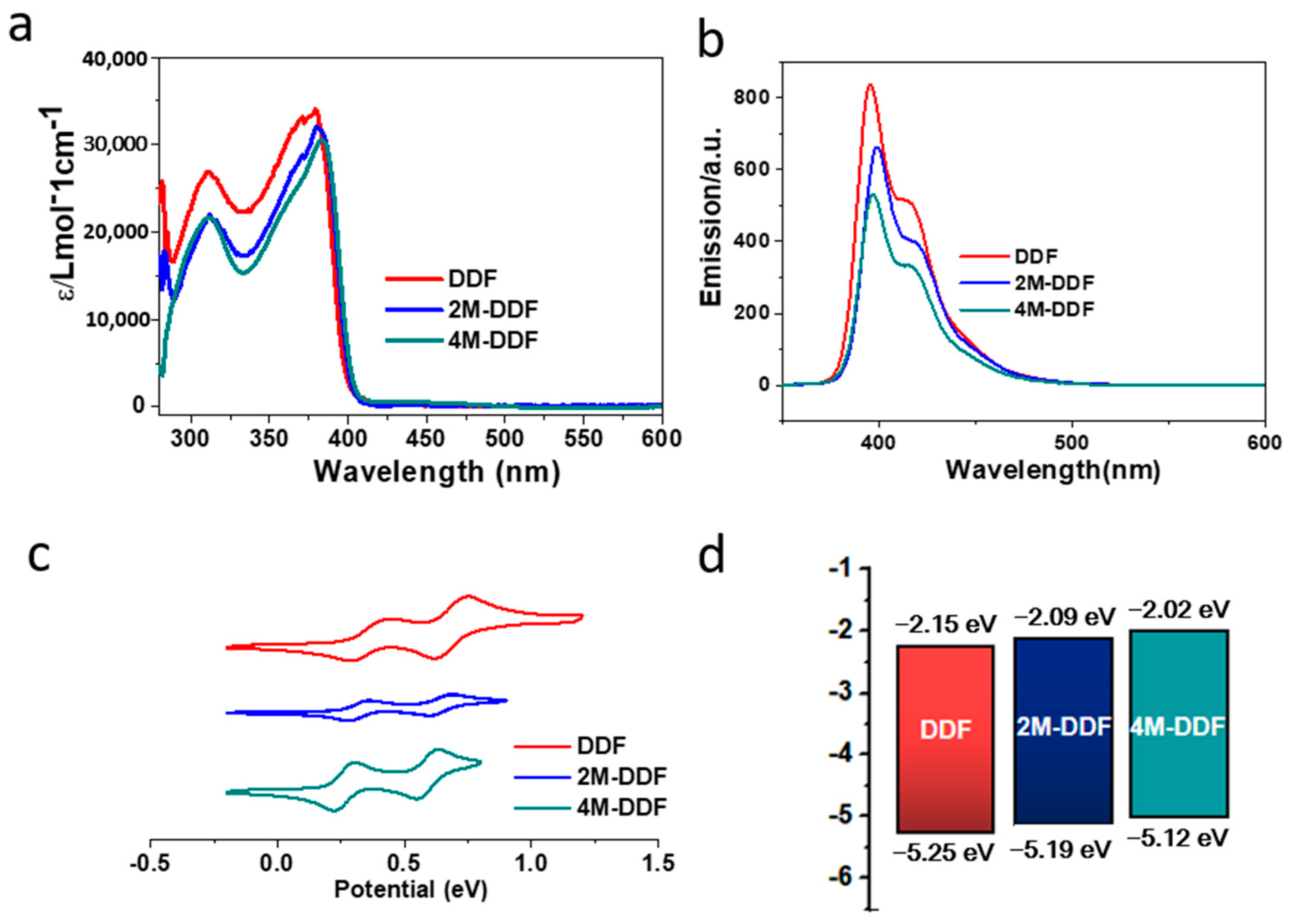
Figure 2 shows the frontier orbitals of the arylamines calculated using density functional theory (DFT) at the B3LYP/6-31G(d) level. Introducing the fluorene group and adjusting the end groups largely affected the energy level of the compound. The HOMO energy levels of DDF, 2M-DDF, and 4M-DDF were −4.99, −4.69, and −4.62 eV, respectively. The LUMO energy levels of DDF, 2M-DDF, and 4M-DDF were −1.29, −0.99, and −0.95 eV, respectively, indicating that the energy level can be adjusted by changing the molecular structure. Figure 3a depicts the UV–vis absorption and fluorescence (FL) spectra of DDF, 2M-DDF, and 4M-DDF in toluene solution (5 × 10−6 M). The spectra of all compounds show a strong absorption peak around 380–400 nm assignable to n–π* transitions of the fluorene core [43]. From the onset of absorption, the optical band gap Eg was calculated as 3.10 eV for DDF, 2M-DDF, and 4M-DDF. The fluorescence maxima of the fluorene-based compounds appeared at 410 nm (Figure 3b). Figure 3c shows the electrochemical measurements (CV curves) of DDF, 2M-DDF, and 4M-DDF. From the onset potentials of oxidation, the HOMO energy levels of DDF, 2M-DDF, and 4M-DDF were estimated as −5.25, −5.19, and −5.12 eV, respectively. Note that all fluorine-based compounds exhibited shallower HOMO energies than TPD (−5.50 eV) [16,17]. The HOMO energies of 2M-DDF (−5.19 eV) and 4M-DDF (−5.12 eV) approached the work function energy of indium tin oxide (ITO) (−4.80 eV), implying smaller hole-injection barriers from ITO to 2M-DDF and 4M-DDF (0.39 and 0.32 eV, respectively) than from ITO to TPD [16,17]. The reduced injection barrier of holes is conducive to hole injection. From the HOMO energy levels and Egop, the lowest unoccupied molecular orbital (LUMO) energy levels of DDF, 2M-DDF, and 4M-DDF were calculated as −2.15, −2.09, and −2.02 eV, respectively. The low LUMO energies of 2M-DDF and 4M-DDF indicate that these materials can prevent electron leakage from the emissive layer into the hole-transport layer. Judging from the energy levels, all compounds are suitable hole-transport materials for optoelectronic devices. 2M-DDF and 4M-DDF with higher HOMO energies are especially expected to improve the carrier-transport performance.
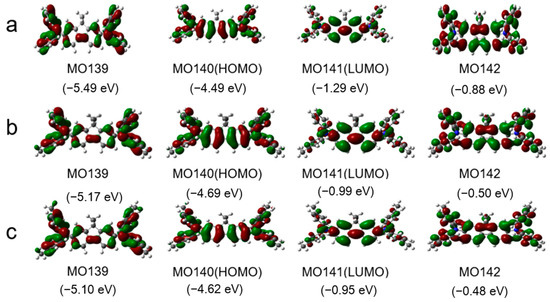
Figure 2.
Some molecular orbitals and the corresponding energies of DDF (a), 2M-DDF (b), and 4M-DDF (c) using B3LYP/6-311G (d, p) in toluene. The grey, blue, and white spheres represent carbon, nitrogen, and hydrogen atoms, respectively.
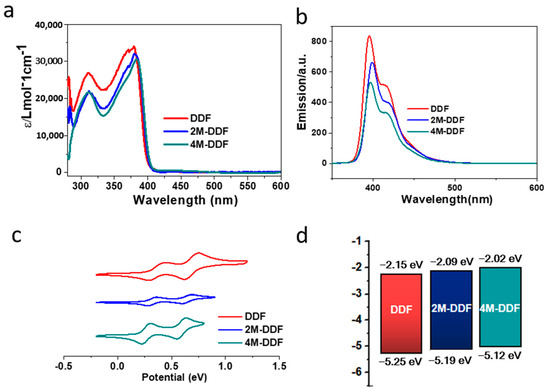
Figure 3.
UV–vis absorption spectra (a) and fluorescence spectra (b) of DDF, 2M-DDF, and 4M-DDF in toluene (excitation wavelength: λmax of absorption; concentration: 5 × 10−6 mol L−1). The UV–vis absorption and fluorescence were measured at room temperature. (c) Electrochemical curves of DDF, 2M-DDF, and 4M-DDF in dichloromethane vs. Ag/Ag+, with the concentration of 5 × 10−3 mol L−1 and orbital energy levels of those determined (d). The HOMO energy was deduced from the oxidation onset potential and calculated by the equation: EHOMO = −Eonset − 4.93 (eV), ELUMO = EHOMO + Egop. The optical band gap (Egop) was calculated using Tauc’s relation in toluene [43,44].
2.3. Superior Carrier-Transport Performance and High Energy Level Greatly Improve the Luminous Efficiency of OLED Devices
The electroluminescent efficiency of an HTM critically depends on the hole-injection/transporting capability of the material. To measure the hole mobilities of the HTMs, we fabricated hole-only devices with the configuration ITO/PEDOT:PSS/HTMs/Au (PEDOT:PSS = poly(3,4-ethylenedioxythiophene) polystyrene sulfonate). The hole mobilities of TPD, DDF, 2M-DDF, and 4M-DDF were measured using the space charge limited current method. The HTM layers were prepared by spin coating. The current density–voltage (J–V) characteristics of the single-carrier devices are shown in Figure 1d. All fluorine-based compounds generated much higher hole-current densities than TPD, owing to the hole-predominant transporting ability of triphenylamine. The hole mobilities of DDF, 2M-DDF, and 4M-DDF were 2.35 × 10−4, 4.65 × 10−4, and 1.55 × 10−4 cm2 V−1 s−1, respectively (Figure 1d), far exceeding that of TPD (1 × 10−4 cm2 V−1 s−1) [16,17]. The especially high mobility of 2M-DDF (five times that of TPD) can be explained partly by the good film-forming properties of the fluorene-based 2M-DDF molecule. The smaller the interface barrier, the more favorable is the carrier transport. Another contributing factor is the higher HOMO energy of 2M-DDF (−5.19 eV) compared to TPD (−5.50 eV) [16,17]. The close HOMO energy levels of 2M-DDF and ITO (−4.80 eV) reduce the injection barrier of holes, facilitating hole injection [13]. Owing to their superior transmission properties, 2M-DDF thin films fabricated via solution-engineering spin coating were chosen as the hole-transport material in OLED devices.
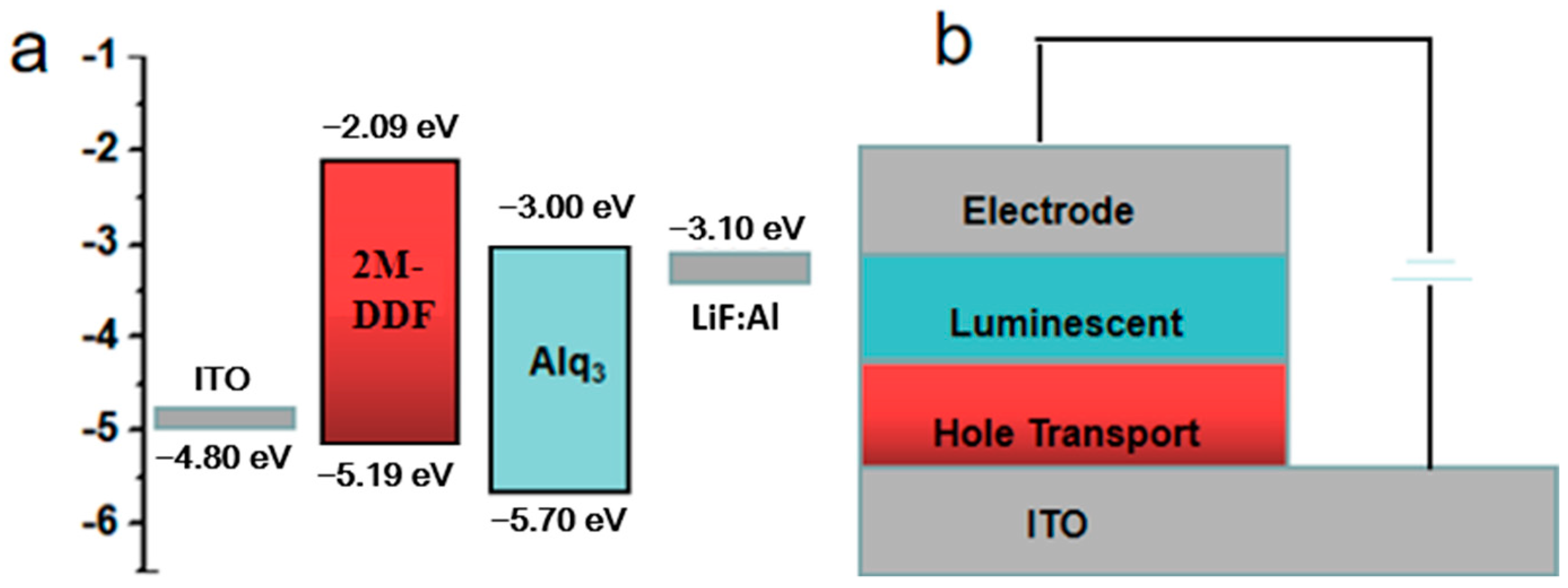
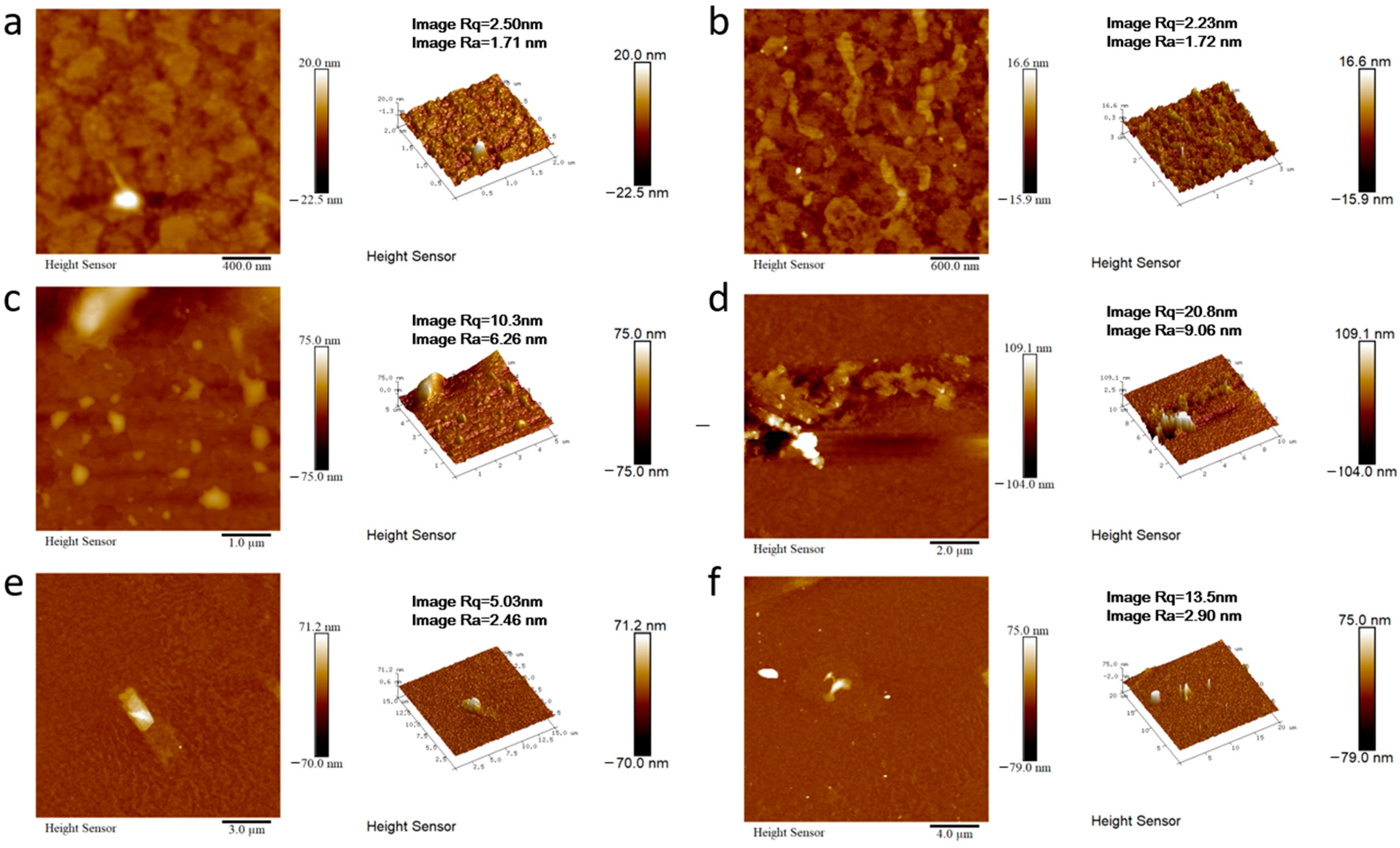
In order to better expand the application field of this kind of materials, we take the materials of 2M-DDF with the highest carrier mobility as an example to explore its application in the field of OLED. 2M-DDF was inserted as the hole-transport layer in OLED devices (ITO/HTL/Alq3/LiF/Al). The energy levels in the materials and the device structures of the OLEDs are displayed in Figure 4a and Figure 4b, respectively. The relevant parameters of the fluorescent OLEDs are summarized in Table 1. With 2M-DDF as an HTM, the OLED device achieved a maximum L (Lmax) of 21,412 cd m−2 and a maximum CE (CEmax) of 4.78 cd A−1, with a turn-on voltage of Von = 3.8 V. It is widely believed that the film morphology of the functional layer is critical for the performance of multilayered optoelectronic devices. To further verify the film morphology of 2M-DDF as HTMs, we performed atomic force microscopy (AFM) measurements on the film samples of ITO/2M-DDF. As shown in Figure 5, tapping-mode AFM images of the ITO/2M-DDF show smooth surface morphology. The advantages of this material were better demonstrated by comparing it with the similarly structured compound TPD as a hole-transport layer device [45]. The Lmax and CEmax of the TPD device were 4106 cd m−2 and 3.70 cd A−1, respectively, far below those of 2M-DDF, and the Von increased to 4.3 V. The OLED device primarily utilizes evaporation for the hole-transport layer, with TPD being the chosen material. To better showcase the advantages of a solution-prepared hole-transport layer, we have selected poly(9-vinylcarbazole) (PVK) and poly[(9,9-dioctylfluorenyl-2,7-diyl)-alt-(4,4′-(N-(4-butylphenyl) (TFB) as contrast materials for performance comparison. The Lmax, CEmax, and Von of the PVK device were 2288.9 cd m−2, 1.10 cd A−1, and 3.4 V, respectively. It is due to the low HOMO energy level of PVK, which leads to a large hole injection barrier between ITO and PVK. In addition, PVK also has a low charge mobility of 2.5 × 10−6/V⋅s [12,15,46,47,48,49]. The Lmax, CEmax and Von of the TFB device were 15,211 cd m−2, 2.90 cd A−1, and 2.4 V, respectively. The especially high luminous brightness of 2M-DDF (nearly five times that of TPD and nearly 1.3 times that of TFB) is further substantiated by the fact that this particular hole-transport material exhibits significant potential for application within the realm of OLED technology. Moreover, it provides a theoretical basis for how to design molecules with higher mobility.
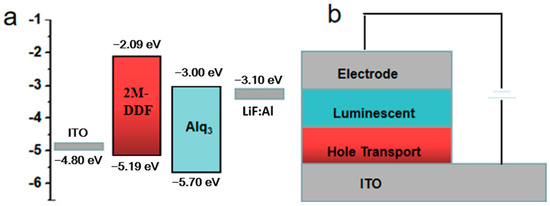
Figure 4.
(a) Schematic energy level diagram of the fabricated OLED devices. (b) The device structure of OLEDs.

Table 1.
EL performance of the fabricated OLED devices.
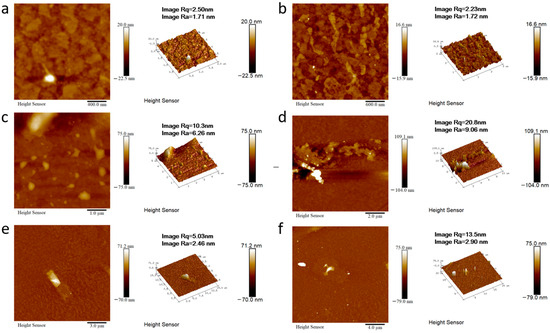
Figure 5.
AFM height images of ITO/2M-DDF with different multiples ((a): 400 nm, (b): 600 nm, (c): 1 μm, (d): 2 μm, (e): 3 μm, (f): 4 μm).
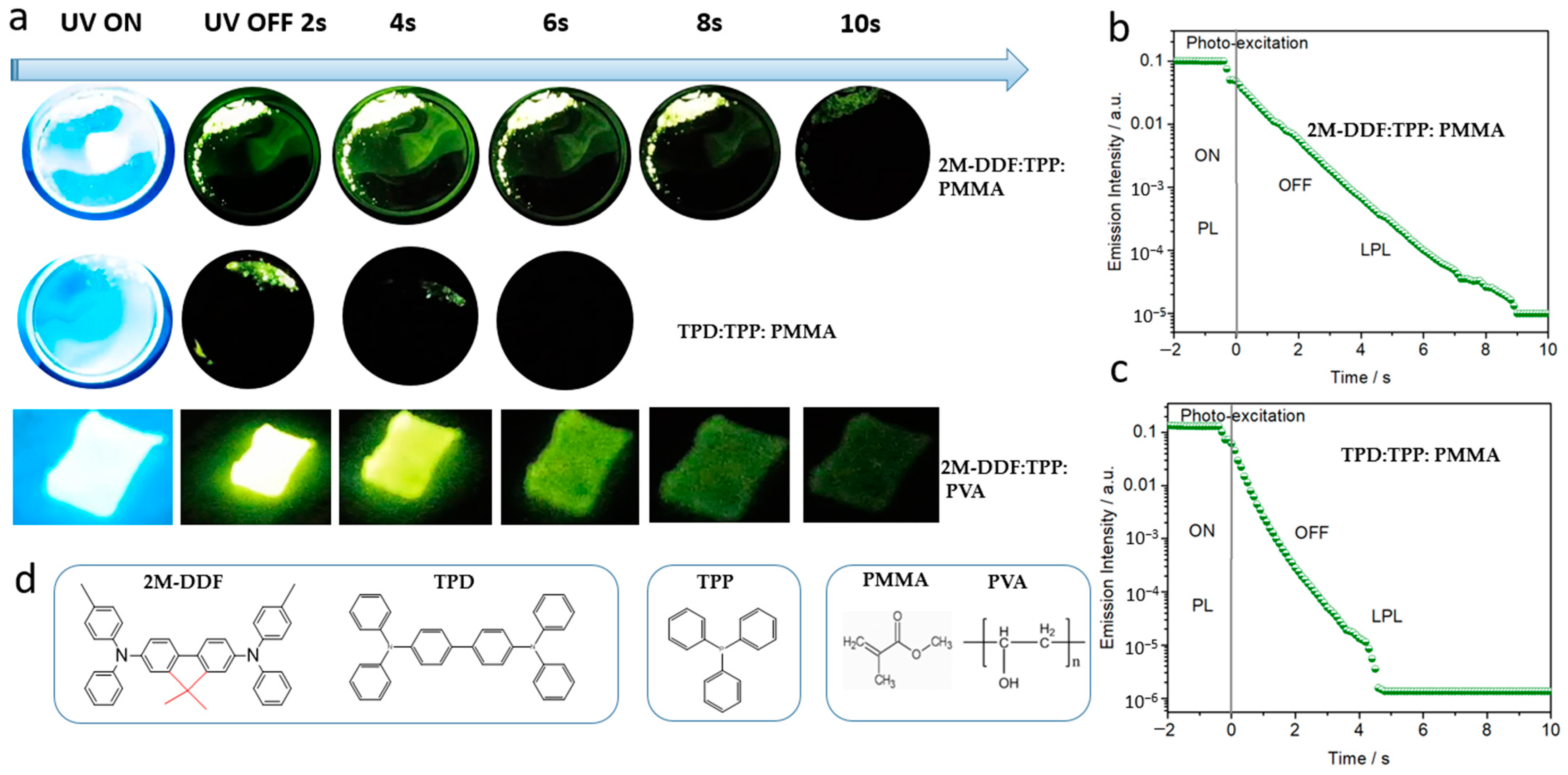
To better reflect the advantages of these kinds of effective carrier-transport materials based on 2M-DDF, we used 2M-DDF/TPD and triphenylphosphine (TPP) materials as guests and hosts and poly (methyl methacrylate (PMMA) polymers as rigid matrices to prepare rigid 2M-DDF:TPP:PMMA and TPD:TPP:PMMA. The 2M-DDF:TPP:PMMA afterglow materials were irradiated under a 365-nm ultraviolet lamp for 5 s and the light source was then removed. The 2M-DDF:TPP:PMMA emitted a green afterglow for 10 s (upper row of Figure 6a), which was clearly visible to the naked eye. TPD:TPP:PMMA also emitted a visible green afterglow, but its lifetime was only 4 s (lower row of Figure 6a). Figure 6b shows the semi-logarithmic plots of the emission decay profiles of 2M-DDF:TPP:PMMA and TPD:TPP:PMMA from −2 s to 10 s. Both 2M-DDF:TPP:PMMA and TPD:TPP:PMMA exhibit photoluminescent characteristics under light excitation (−2 to 0 s), and long afterglow characteristics after turning off the excitation light source (0–12 s). The long-persistent luminescence emissions of 2M-DDF:TPP:PMMA and TPD:TPP:PMMA visibly endured for approximately 10 s and 4 s, respectively, consistent with the afterglow times under naked eye observation. At present, there are still few afterglow materials with more than 10 s, and this research provides a basis for the development of materials with longer afterglow (as shown in Table 2) [50,51,52,53]. The comparison with TPD as a hole-transport material with high carrier mobility reveals that 2M-DDF is more suitable for the preparation of afterglow materials.
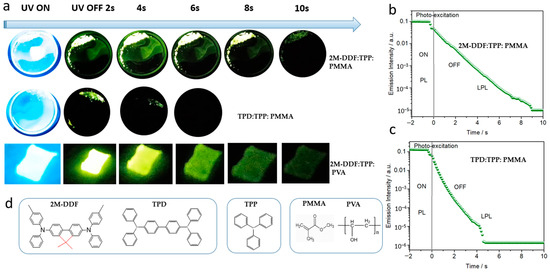
Figure 6.
(a) LPL photographs under ambient conditions of 2M-DDF:TPP:PMMA, TPD:TPP:PMMA, and 2M-DDF:TPP:PVA (excitation: 365 nm). The semi-logarithmic plot of the emission decay profile of the 2M-DDF:TPP:PMMA (b) and TPD:TPP:PMMA (c) from −2 s to 10 s, which exhibited photoluminescence (PL) upon photo-excitation (from −2 s to 0 s) and LPL after the excitation turned off (excitation wavelength: 365 nm; excitation power: 10 mW; excitation time: 2 s; sample temperature: 300 K). (d) Chemical structure of 2M-DDF, TPD, TPP, PMMA, and PVA.

Table 2.
A summary of properties of organic LPL materials.
The material prepared on the PMMA substrate is a rigid film suitable for applications requiring greater hardness. We also prepared polymer films on substrates of polyvinyl alcohol (PVA) (2M-DDF:TPP:PVA and TPD:TPP:PVA) and polyethylene glycol (PEG) (2M-DDF:TPP:PEG and TPD:TPP:PEG) (Figure 6a). The films were simply and quickly prepared by heating the compounds. As expected, the PVA substrate realized a flexible film with similar afterglow times as PMMA (9 s for 2M-DDF:TPP:PVA; see upper row of Figure 6a). Clearly, the polymer-substrate characteristics strongly influence the afterglow time, implying a wide application range of the 2M-DDF material.
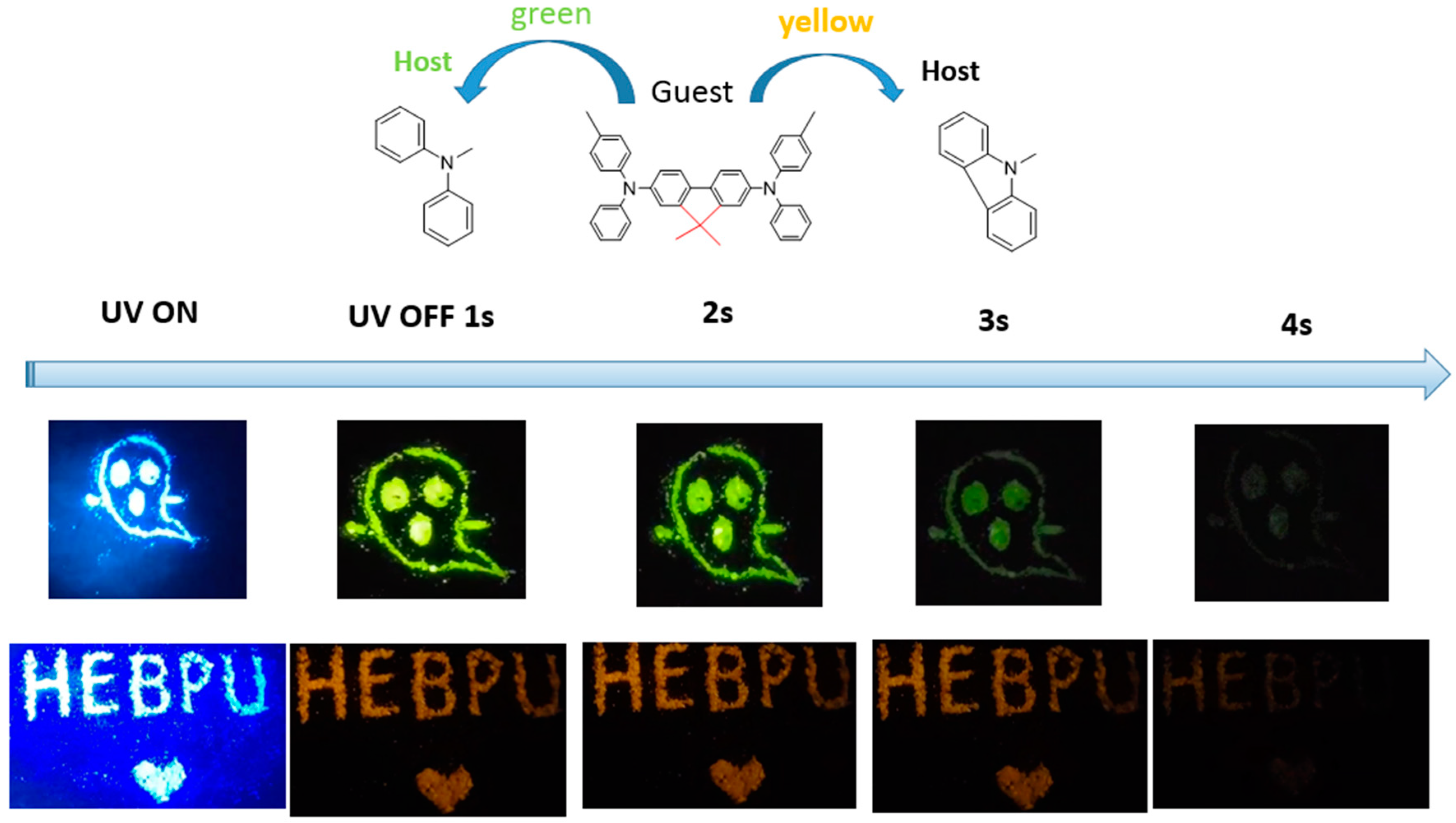
To further expand the application field of the proposed materials, we doped crystal materials with different hosts of different sizes, preparing multi-color tunable afterglow materials in a rigid crystal-mode environment. When diphenylamine (DPA) and CA host materials were doped with 2M-DDF guest molecules (forming doped crystalline 2M-DDF: DPA), we were delighted to observe a deep yellow afterglow for 3 s and a deep green afterglow for 3.4 s under 365 nm ultraviolet excitation (see Figure 7). Such multi-color tunable afterglow materials can potentially be prepared as anti-counterfeiting materials with high encryption degrees.
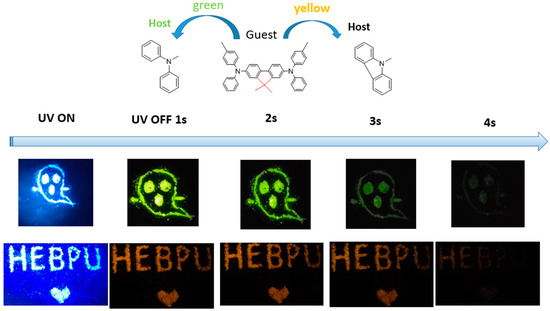
Figure 7.
The LPL photograph under ambient conditions of 2M-DDF:DPA (up) and 2M-DDF:CA (down) (excitation: 365 nm).
In order to better expand the application of materials, we replaced the spiro-OMeTAD hole-transport layer with 2M-DDF in the fabrication of perovskite solar cells. Unfortunately, the efficiency of the replaced spiro-OMeTAD hole-transport layer with 2M-DDF in the fabrication of perovskite solar cells is lower 2M-DDF(1)~2M-DDF(3) (as shown in Table S1). Analysis of the test data shows that the relatively high FF% of the replaced spiro-OMeTAD hole-transport layer with 2M-DDF suggests suitable mobility of 2M-DDF (as shown Table S1); however, the unsuitable energy levels and the suboptimal PVK/HTM interface increase charge recombination, thereby reducing device efficiency. We attempted to incorporate 2M-DDF as an intermediate layer between the perovskite and hole-transport layer. The corresponding test results are presented in rows 2M-DDF(4)~2M-DDF(6) of Table S1. However, the results remained suboptimal, with considerably low photoelectric conversion efficiency. This limitation is primarily attributed to the mismatch between the perovskite and 2M-DDF layers. As reported in the literature, both high hole mobility and resistance to morphological perturbations are critical for effective HTMs [54,55,56].
3. Conclusions
This study analyzed the properties of three newly synthesized solution-processable HTMs (DDF, 2M-DDF, and 4M-DDF). The energy level, solubility, crystallinity, and molecular steric configuration could be simultaneously tuned by changing the number of terminal methyl groups. Among the synthesized compounds, 2M-DDF exhibited the highest hole mobility (4.35 × 10−4 cm2 V−1 s−1) compared to DDF (2.35 × 10−4) and 4M-DDF (1.55 × 10−4 cm2 V−1 s−1). Consequently, devices fabricated with 2M-DDF as HTMs with an Alq3 emitter delivered a maximum CE of 4.78 cd/A and a maximum L (Lmax) of 21,412 cd m−2 with a turn-on voltage of Von = 3.8 V. The luminous efficiency of 2M-DDF was approximately five times that of TPD and 1.3 times that of TFB. The proposed strategy can simply design solution-processable HTMs for efficient OLEDs. Moreover, 2M-DDF and TPD can be incorporated as guest molecules in afterglow materials. The afterglow time of 2M-DDF was 2.5 times that of TPD and endured for a surprisingly long time (10 s). This study provides a theoretical basis for developing high-performance organic photoelectric devices and long-afterglow materials. The afterglow time and brightness of green afterglow and deep yellow afterglow materials have been significantly enhanced; however, further advancements are required in the development of other color afterglow materials. For example, one of the common applications of organic RTP materials is biological imaging, where the beneficial emission wavelength is in the near-infrared region. However, there are only a few related studies at present, so that finding a suitable host matrix is an important way to activate near-infrared RTP emission. On the other hand, in future studies, a new material with enhanced hole mobility and a suitable energy level should be developed to replace the conventional spiro-OMeTAD in perovskite solar cells. By refining the structure of the compound to optimize its energy level, we can better support efficient hole transport in perovskite applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma17225417/s1, Scheme S1. Synthetic routes and chemical structure of 1 and 2a–c (1) CH3I, t-BuOK, THF, rf, 12 h, Ar; (2) K2CO3, Cu, NaHSO3, Toluene, 210 °C, 10 h; The star to showcase the binding site (N-group) to the fluorene. Table S1. Summary of the photovoltaic performance of PSC devices.
Author Contributions
Conceptualization, M.M.; Validation, M.Z.; Formal analysis, Y.L.; Investigation, M.Z.; Resources, Y.L.; Data curation, Y.L.; Writing—original draft, M.M. and Y.L.; Writing—review & editing, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by S&T Program of Chengde (No. 202304B068).
Institutional Review Board Statement
Not application.
Informed Consent Statement
Not application.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, Z.-Y.; Chen, Z.; Zhu, Y.-Q.; Zhuo, M.-Z.; Yang, G.-Y.; Wang, X.-H.; Wu, M.-X. Ultralong Blue Organic Room-Temperature Phosphorescence Promoted by Green Assembly. Adv. Funct. Mater. 2024, 2408023. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhao, X.; Bai, X.; Cheng, Q.W.; Liu, Y. Thermal Activated Reversible Phosphorescence Behavior of Solid Supramolecule Mediated by β-Cyclodextrin. Adv. Funct. Mater. 2024, 34, 2400898. [Google Scholar] [CrossRef]
- Cui, J.Y.; Ali, S.H.; Shen, Z.Y.; Xu, W.S.; Liu, J.Y.; Li, P.X.; Li, Y.; Chen, L.G.; Wang, B.W. 3-Polylysine organic ultra-long room-temperature phosphorescent materials based on phosphorescent molecule doping. Chem. Sci. 2024, 15, 4171. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-J.; Yan, S.R.; Chen, L.; Qiao, L.; Xu, S.H.; Qi, T.F.; Liu, B.; Peng, H.Q. Isomeric Engineering of Organic Luminophores for Multicolor Room Temperature Phosphorescence Including Red Afterglow. Adv. Funct. Mater. 2024, 2406888. [Google Scholar] [CrossRef]
- Chen, J.W.; Zhang, S.G.; Liu, G.Y.; Zhang, Y.F.; Xue, S.F.; Sun, Q.K.; Yang, W.J. Regulating Organic Dopant Dispersion in Polymer Matrices for Concentration-Controlled Color-Tunable Organic RTP Emissions. Adv. Opt. Mater. 2023, 11, 2301163. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, Y.X.; Ma, X.R.; Fan, W.W.; Cheng, Y.; He, R.Y.; Meng, X.; Shi, Y.G.; Cao, Q.; Zheng, L.Y. Luminophore with Multiple Emission Centers for Fluorescence/Phosphorescence Dual Ratiometric Chemical Sensing in Aqueous Solution. Adv. Opt. Mater. 2024, 12, 2303107. [Google Scholar] [CrossRef]
- Zhang, X.H.; Chong, K.C.; Xie, Z.L.; Liu, B. Color-tunable dual-mode organic afterglow for white-light emission and information encryption based on carbazole doping. Angew. Chem. Int. Ed. 2023, 62, e202310335. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.H.; Hu, P.T.; Zhang, H.Q.; Yang, Q.C.; Wei, H.S.; Chen, R.T.; Yu, J.H.; Liu, C.; Wang, Y.H.; Luo, S.L.; et al. Enabling Highly Robust Full-Color Ultralong Room-Temperature Phosphorescence and Stable White Organic Afterglow from Polycyclic Aromatic Hydrocarbons. Chem. Int. Ed. 2024, 63, e202318516. [Google Scholar] [CrossRef]
- Qiao, W.G.; Yao, M.; Xu, J.W.; Peng, H.Y.; Xia, J.L.; Xie, X.L.; Li, Z.A. Naphthyl Substituted Impurities Induce Efficient Room Temperature Phosphorescence. Angew. Chem. 2023, 135, e202315911. [Google Scholar] [CrossRef]
- Jang, E.; Jang, H. Quantum dot light-emitting diodes. R Chem. Rev. 2023, 123, 4663–4692. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, S.H.; Lee, J.Y. Concentration quenching resistant donor-acceptor molecular structure for high efficiency and long lifetime thermally activated delayed fluorescent organic light-emitting diodes via suppressed non-radiative channel. Chem. Eng. J. 2020, 395, 125159. [Google Scholar] [CrossRef]
- Chen, J.; Song, D.; Zhao, S.; Qiao, B.; Zheng, W.; Xu, Z. Highly efficient all-solution processed blue quantum dot light-emitting diodes based on balanced charge injection achieved by double hole transport layers. Org. Electron. 2021, 94, 106169. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Ye, T.; Ding, S.; Wang, K.; Peng, Z.; Sun, X.W. High-performance perovskite light-emitting diodes based on double hole transport layers. J. Mater. Chem. C 2021, 9, 2115. [Google Scholar] [CrossRef]
- Zhong, Z.; Quan, H.; Zhang, J.; Peng, F.; Zhong, W.; Ying, L. Improving the Performance of Quantum Dot Light-Emitting Diodes by the Enrichment of a Fluorinated Component on Top of a Hole Transport Layer. ACS Appl. Electron. Mater. 2023, 5, 6452–6458. [Google Scholar] [CrossRef]
- Su, H.; Xu, Z.; He, X.; Yao, Y.; Zheng, X.; She, Y.; Zhu, Y.; Zhang, J.; Liu, S.F. Surface energy engineering of buried interface for highly stable perovskite solar cells with efficiency over 25%. Adv. Mater. 2024, 36, 2306724. [Google Scholar] [CrossRef]
- Liu, C.; Yang, T.; Cai, W.; Wang, Y.; Chen, X.; Wang, S.; Huang, W.; Du, Y.; Wu, N.; Wang, Z.; et al. Flexible Indoor Perovskite Solar Cells by In Situ Bottom-Up Crystallization Modulation and Interfacial Passivation. Adv. Mater. 2024, 36, 2311562. [Google Scholar] [CrossRef]
- Shen, L.; Song, P.; Zheng, L.; Wang, L.; Zhang, X.; Liu, K.; Liang, Y.; Tian, W.; Luo, Y.; Qiu, J.; et al. Ion-Diffusion Management Enables All-Interface Defect Passivation of Perovskite Solar Cells. Adv. Mater. 2023, 35, 2301624. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Meng, X.; Liu, G.; Duan, S.; Hu, D.; Shen, B.; Kang, B.; Silva, S.R.P. SnO2 Surface Modification and Perovskite Buried Interface Passivation by 2, 5-Furandicarboxylic Acid for Flexible Perovskite Solar Cells. Adv. Funct. Mater. 2024, 2404686. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, Q.; Li, Y.; Chen, Y.; He, D.; Ma, D.; Han, X.; Li, R.; Yang, K.; Yang, Y.; et al. Polydentate Ligand Reinforced Chelating to Stabilize Buried Interface toward High-Performance Perovskite Solar Cells. Angew. Chem. Int. Ed. 2024, 63, e202317185. [Google Scholar] [CrossRef]
- Salehi, A.; Fu, X.; Shin, D.H.; So, F. Recent advances in OLED optical design. Adv. Funct. Mater. 2019, 29, 1808803. [Google Scholar] [CrossRef]
- Song, X.; Zhang, D.; Lu, Y.; Yin, C.; Duan, L. Understanding and Manipulating the Interplay of Wide-Energy-Gap Host and TADF Sensitizer in High-Performance Fluorescence OLEDs. Adv. Mater. 2019, 31, 1901923. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Pan, F.; Chen, J.; Li, J.; Yang, Y.; Sun, Y.; Zhu, X.; Li, P.; Cao, X.; Xi, J.; et al. Optimizing the Buried Interface in Flexible Perovskite Solar Cells to Achieve Over 24% Efficiency and Long-Term Stability. Adv. Mater. 2024, 36, 2308039. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Li, Y.; Fan, X.J. Significant breakthroughs in interface engineering for high-performance colloidal OLEDs: A mini review. Phys. D Appl. Phys. 2023, 56, 343001. [Google Scholar] [CrossRef]
- Tsai, K.W.; Hung, M.K.; Mao, Y.H.; Chen, S.A. Solution—Processed thermally activated delayed fluorescent OLED with high EQE as 31% using high triplet energy crosslinkable hole transport materials. Adv. Funct. Mater. 2019, 29, 1901025. [Google Scholar] [CrossRef]
- Zhang, X.; Li, D.; Zhang, Z.; Liu, H.; Wang, S. Constructing Effective Hole Transport Channels in Cross-Linked Hole Transport Layer by Stacking Discotic Molecules for High Performance Deep Blue QLEDs. Adv. Sci. 2022, 9, 2200450. [Google Scholar] [CrossRef] [PubMed]
- Krucaite, G.; Volyniuk, D.; Simokaitiene, J.; Grigalevicius, S.; Linb, C.H.; Shao, C.M.; Chang, C.H. Naphthyl substituted triphenylamine derivatives as hole transporting materials for efficient red PhOLEDs. Dye. Pigment. 2019, 162, 196–202. [Google Scholar] [CrossRef]
- Li, X.; Cui, J.; Ba, Q.; Zhang, Z.; Chen, S.; Yin, G.; Wang, Y.; Li, B.; Xiang, G.; Kim, K.S.; et al. Multiphotoluminescence from a Triphenylamine Derivative and Its Application in White Organic Light-Emitting Diodes Based on a Single Emissive Layer. Adv. Mater. 2019, 31, 1900613. [Google Scholar] [CrossRef]
- Braveenth, R.; Bae, I.J.; Wang, Y.; Kim, S.H.; Kim, M.; Chai, K.Y. Acridine-Triphenylamine Based Hole-Transporting and Hole-Injecting Material for Highly Efficient Phosphorescent-Based Organic Light Emitting Diodes. Appl. Sci. 2018, 8, 1168. [Google Scholar] [CrossRef]
- Zhou, G.; Wong, W.Y.; Yao, B.; Xie, Z.; Wang, L. Triphenylamine-dendronized pure red iridium phosphors with superior OLED efficiency/color purity trade-offs. Angew. Chem. Int. Ed. 2007, 46, 1149–1151. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Q.; Shang, Y.; Yang, C.; Ao, L.; Qin, J.; Ma, D.; Shuai, Z. Multifunctional bipolar triphenylamine/oxadiazole derivatives: Highly efficient blue fluorescence, red phosphorescence host and two-color based white OLEDs. Chem. Commun. 2009, 77–79. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, K.; Huang, Z.; Wang, Z.; Duttwyler, S.; Wang, Y.; Lu, P.J. Emissions from a triphenylamine–benzothiadiazole–monocarbaborane triad and its applications as a fluorescent chemosensor and a white OLED component. Mater. Chem. C 2019, 7, 2430–2435. [Google Scholar] [CrossRef]
- Braveenth, R.; Bae, I.J.; Han, J.H.; Qiong, W.; Seon, G.; Raagulan, K.; Yang, K.; Park, Y.H.; Kim, M.; Chai, K.Y. Utilizing a Spiro Core with Acridine- and Phenothiazine-Based New Hole Transporting Materials for Highly Efficient Green Phosphorescent Organic Light-Emitting Diodes. Molecules 2018, 23, 713. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Xu, B.; Jiang, B.; Fu, H.; Zhu, W.; Jiang, X.; Zhang, Z. A novel fluorene derivative containing four triphenylamine groups: Highly thermostable blue emitter with hole-transporting ability for organic light-emitting diode (OLED). Synth. Met. 2005, 155, 206–210. [Google Scholar] [CrossRef]
- Gmelch, M.; Thomas, H.; Fries, F.; Reineke, S. Programmable transparent organic luminescent tags. Sci. Adv. 2019, 5, 7310. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim-Ouali, M.; Dumur, F. Recent advances on metal-based near-infrared and infrared emitting OLEDs. Molecules 2019, 24, 1412. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Feng, Q.; Yang, M.; Wang, Q.; Xu, H.; Liu, J.; Bo, S.; Zhang, X.L.; Li, Z.H. Synthesis and properties study of a X-type dendrimer based on triphenylamine. Mater. Lett. 2017, 193, 112–114. [Google Scholar] [CrossRef]
- Li, W.; Liu, B.; Sun, M.J. Synthesis of 4, 5-diazofluorene photoelectric materials the application of thin film devices. Nanjing Univ. Posts Telecommun. Nat. Sci. 2013, 33, 1673–5439. (In Chinese) [Google Scholar]
- Luo, X.; Chang, G.; Zhang, L. Synthesis and characterization of polyimidone containing fluorine. Chin. J. Synth. Chem. 2009, 17, 15–18. (In Chinese) [Google Scholar]
- Wua, S.; Liua, H.; Suna, W.; Lia, X.; Wang, S. Regulation of peripheral tert-butyl position: Approaching efficient blue OLEDs based on solution-processable hole-transporting materials. Org. Electron. 2019, 71, 85–92. [Google Scholar] [CrossRef]
- Cho, Y.J.; Yook, K.S.; Lee, J.Y. High Efficiency in a Solution-Processed Thermally Activated Delayed-Fluorescence Device Using a Delayed-Fluorescence Emitting Material with Improved Solubility. Adv. Mater. 2014, 26, 6642–6646. [Google Scholar] [CrossRef] [PubMed]
- Ayobi, A.; Mirnia, S.N.; Rezaee Roknabadi, M.; Bahari, A. The effects of MoO3/TPD multiple quantum well structures on the performance of organic light emitting diodes (OLEDs). J. Mater. Sci. Mater. Electron. 2019, 30, 3952–3958. [Google Scholar] [CrossRef]
- Bian, M.Y.; Zhang, D.D.; Wang, Y.X.; Chung, Y.H.; Liu, Y.; Ting, H.; Duan, L.; Chen, Z.J.; Bian, Z.Q.; Liu, Z.W.; et al. Long—Lived and Highly Efficient TADF—PhOLED with “(A) n-D-(A) n” Structured Terpyridine Electron—Transporting Material. Adv. Funct. Mater. 2018, 28, 1800429. [Google Scholar] [CrossRef]
- Wang, T.; Weerasinghe, K.C.; Liu, D.; Li, W.; Yan, X.; Zhou, X.; Wang, L. Ambipolar organic semiconductors with cascades of energy levels for generating long-lived charge separated states: A donor–acceptor1–acceptor2 architectural triarylamine dye. J. Mater. Chem. C 2014, 2, 5466–5470. [Google Scholar] [CrossRef]
- Sun, H.; Li, P.; Liu, D.; Wang, T.; Li, W.; Hu, W.; Wang, L.; Zhou, X. Tuning photophysical properties via alkoxyl groups in charge-separated triphenylamine sensitizers for dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2019, 368, 233–241. [Google Scholar] [CrossRef]
- Shang, Z.; Liu, D.; Wang, T.; Yu, X.; Li, B.; Li, W.; Hu, W.; Zhou, X. Enhanced Hole-Injection Property in an OLED with a Self-assembled Monolayer of Hole-Transporting TPD on Thin Au as the Anode. Trans. Tianjin Univ. 2018, 24, 580–586. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, W.L.W.; Wang, T.; Liu, D.; Zhou, X. Synthesis and Properties of a Novel Triphenylamine Hole Transport Material. Chem. Ind. Eng. 2020, 37, 3. [Google Scholar]
- Wei, Q.; Fei, N.; Islam, A.; Lei, T.; Hong, L.; Peng, R.; Fan, X.; Chen, L.; Gao, P.; Ge, Z. Small-molecule emitters with high quantum efficiency: Mechanisms, structures, and applications in OLED devices. Adv. Opt. Mater. 2018, 6, 1800512. [Google Scholar] [CrossRef]
- Jankus, V.; Data, P.; Graves, D.; McGuinness, C.; Santos, J.; Bryce, M.R.; Dias, F.B.; Monkman, A.P. Highly efficient TADF OLEDs: How the emitter–host interaction controls both the excited state species and electrical properties of the devices to achieve near 100% triplet harvesting and high efficiency. Adv. Funct. Mater. 2014, 24, 6178–6186. [Google Scholar] [CrossRef]
- Duan, L.; Qiao, J.; Sun, Y.; Qiu, Y. Strategies to design bipolar small molecules for OLEDs: Donor—Acceptor structure and non-donor—Acceptor structure. Adv. Mater. 2011, 23, 1137–1144. [Google Scholar] [CrossRef]
- Song, J.W.; Muleta, D.Y.; Feng, W.H.; Song, Y.K.; Zhou, X.Q.; Li, W.; Wang, L.C.; Liu, D.Z.; Wang, T.Y.; Hu, W. Photophysical tuning of small-molecule-doped organic crystals with long-persistent luminescence by variation of dopants. Dye. Pigment. 2021, 193, 109501. [Google Scholar] [CrossRef]
- Han, J.; Feng, W.; Muleta, D.Y.; Bridgmohan, C.N.; Dang, Y.; Xie, G.; Zhang, H.; Zhou, X.; Li, W.; Wang, L.; et al. Small-molecule—Doped organic crystals with long—Persistent luminescence. Adv. Funct. Mater. 2019, 29, 1902503. [Google Scholar] [CrossRef]
- Wang, T.; Liu, M.; Feng, W.; Cao, R.; Sun, Y.; Wang, L.; Liu, D.; Wang, Y.; Wang, T.; Hu, W. Long-Lived Charge Separation Induced Organic Long—Persistent Luminescence with Circularly Polarized Characteristic. Adv. Opt. Mater. 2023, 11, 2202613. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Zhang, X.; Mei, J.; Tian, H. Multimode Stimuli-Responsive Room-Temperature Phosphorescence Achieved by Doping Butterfly-like Fluorogensinto Crystalline Small-Molecular Hosts. JACS Au 2024, 4, 1954–1965. [Google Scholar] [CrossRef] [PubMed]
- Jacak, J.E.; Jacak, W.A. Routes for metallization of perovskite solar cells. Materials 2022, 15, 2254. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Gao, F.; Wang, X.; Zhan, C.; Zhang, X.; Zheng, G.; Zhang, X.; Gao, X.; He, Z.; Zhao, Q. Eliminating performance loss from perovskite films to solar cells. Sci. Adv. 2024, 10, eadp0790. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, C.; Xu, J.; Maxwell, A.; Zhou, W.; Yang, Y.; Zhou, Q.; Bati, A.S.R.; Wan, H.; Wang, Z.; et al. Improved charge extraction in inverted perovskite solar cells with dual-site-binding ligands. Science 2024, 384, 189–193. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).