Dissolution of Lithium Contained in Lepidolite Using Ascorbic Acid: Kinetic and Modeling Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
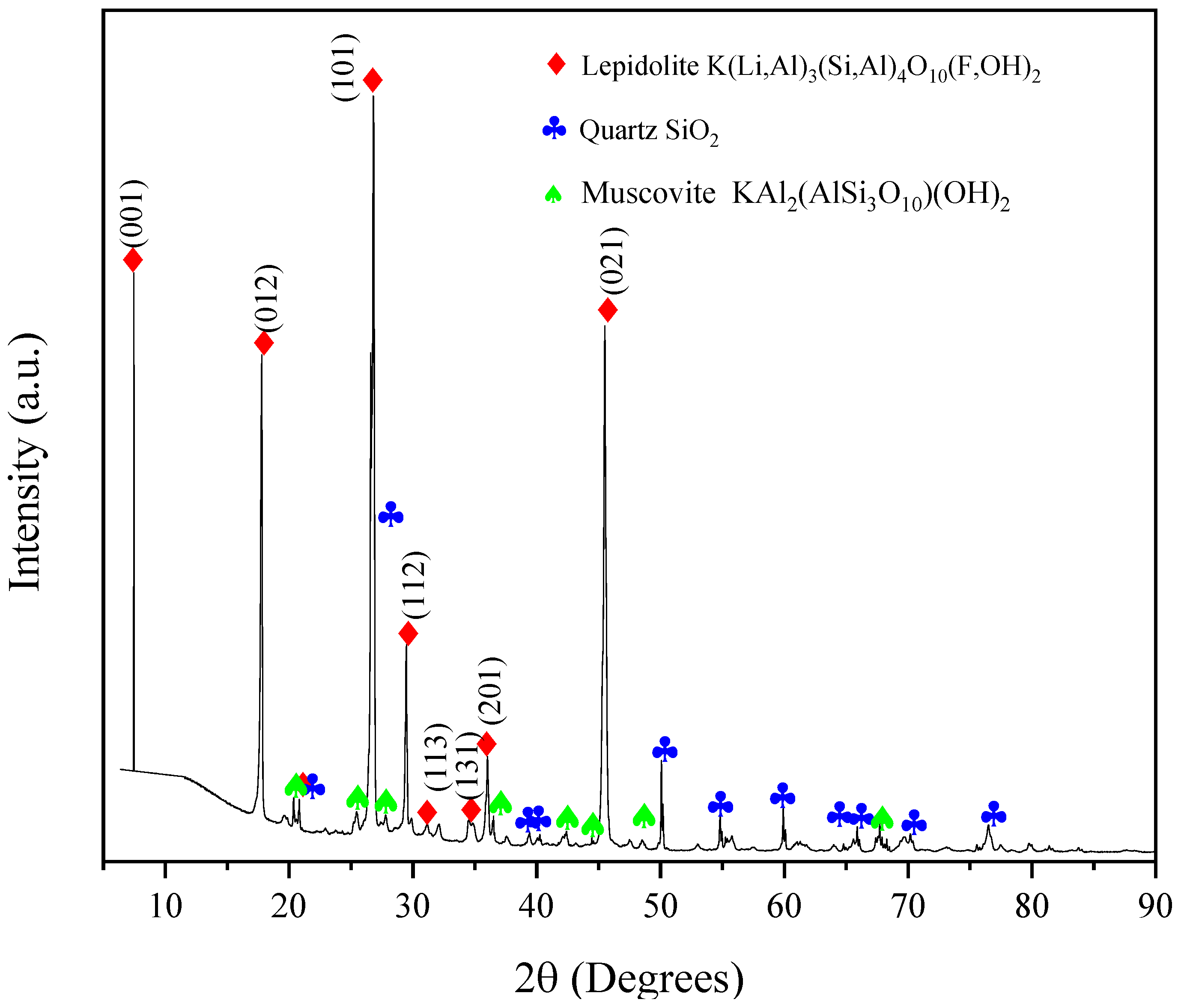
3.1. Mineral Characterization
3.2. Reaction Nature of Lepidolite in Ascorbic Acid
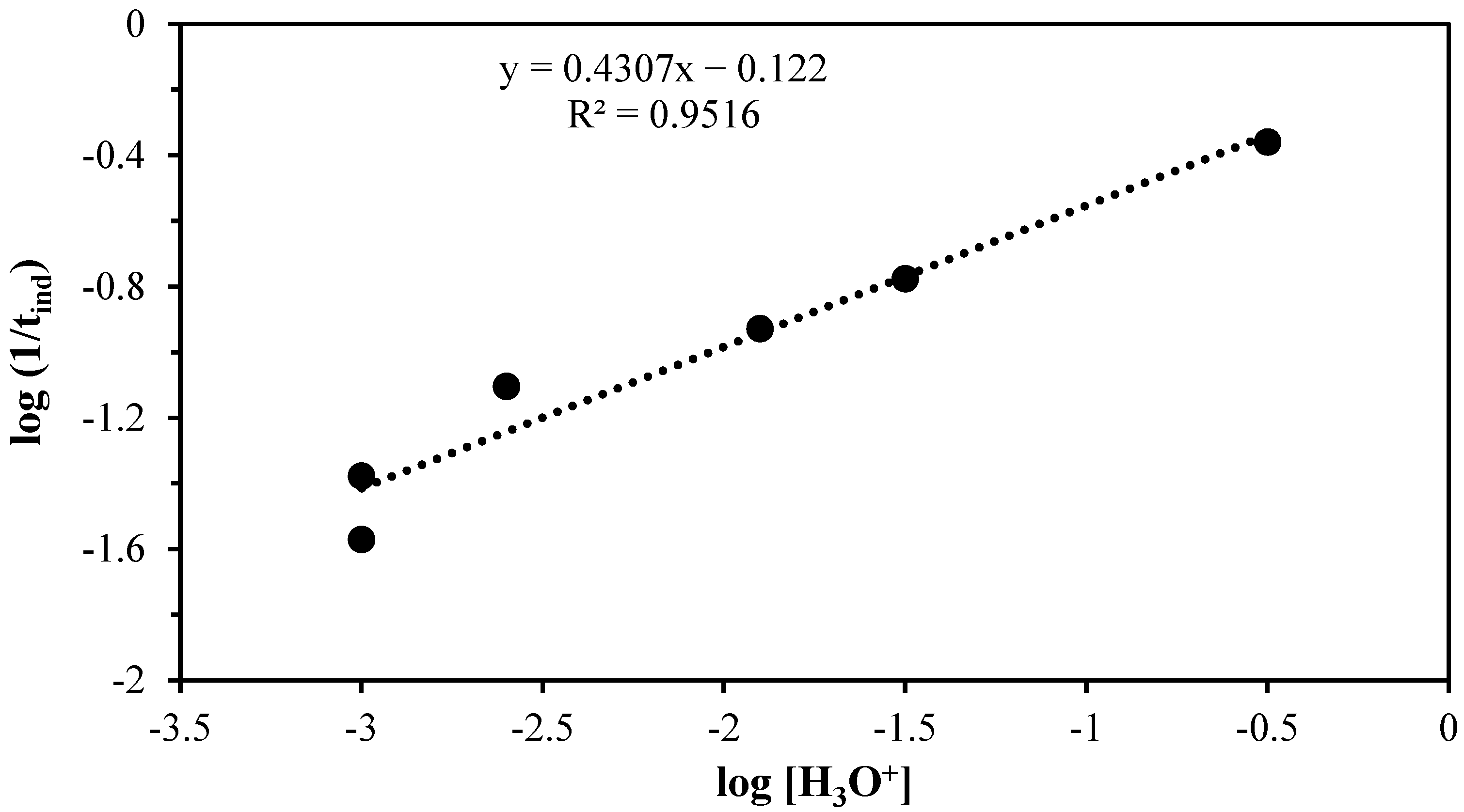
3.3. Acid Decomposition Kinetics
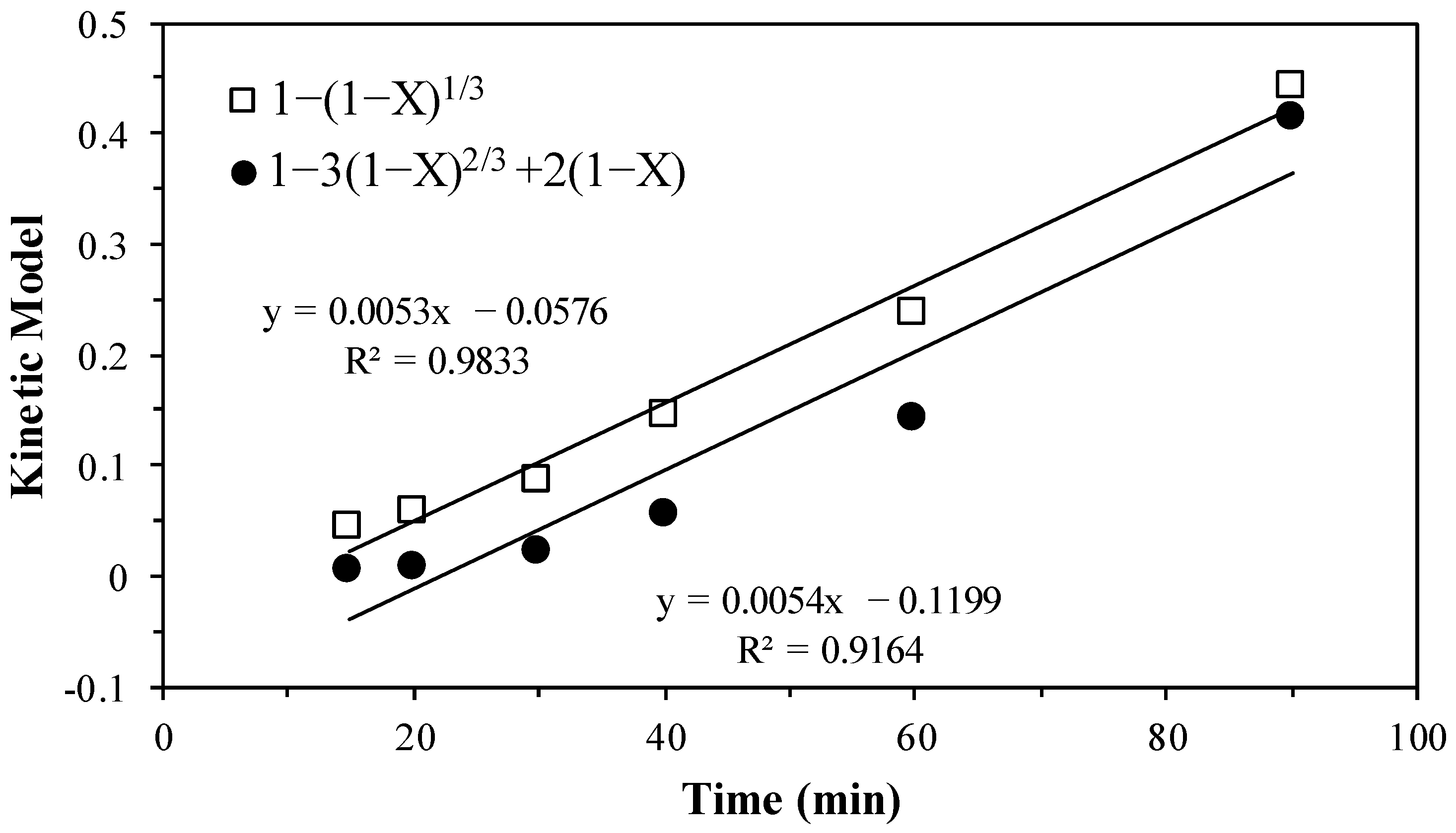
3.4. Kinetic Modeling
3.5. Advantages of the Studied Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, C.; Li, J.; Lu, Y.; Zhu, D. The influence of industrial solid waste in conjuntion with lepidolite tailings on the mechanical properties and microstructure of cemented backfill materials. Constr. Build. Mater. 2024, 419, 135422. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Arshadi, F.; Mofidi, Z.; Mohseni, D.M.; Kök, C.; Assefi, M.; Soozanipour, A.; Zargar, M.; Asadnia, M.; Boroumand, Y.; et al. Direct lithium extraction: A new paradigm for lithium production and resource utilization. Desalination 2024, 575, 117249. [Google Scholar] [CrossRef]
- Lappalainen, H.; Rinne, M.; Elomaa, H.; Aromaa, J.; Lundström, M. Environmental impacts of lithium hydroxide monohydrate production from spodumene concéntrate—A simulation-based life cycle assessment. Miner. Eng. 2024, 209, 108632. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, W.; Liu, D.; He, L.; Liu, X.; Zhao, Z. Ionic transport kinetics of selective electrochemical lithium extraction from brines. Electrochim. Acta 2024, 475, 143519. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Wang, Y.; Yun, R.Y.; Xiang, X. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine. Sep. Purif. Technol. 2021, 256, 117807. [Google Scholar] [CrossRef]
- Olaoluwa, D.T.; Baba, A.A.; Oyewole, A.L. Beneficiation of a Nigerian lepidolite ore by sulfuric acid leaching. Miner. Process. Extr. Metall. Rev. 2023, 132, 134–140. [Google Scholar] [CrossRef]
- Gao, T.; Fan, N.; Chen, W.; Dai, T. Lithium extraction from hard rock lithium ores (spodumene, lepidolite, zinnwaldite, petalite): Technology, Resources, environment and cost. China Geol. 2023, 6, 137–153. [Google Scholar] [CrossRef]
- Kallitsis, E.; Lindsay, J.J.; Chordia, M.; Wu, B.; Offer, G.J.; Edge, J.S. Think global act local: The dependency of global lithim−ion battery emissions on production location and material sources. J. Clean. Prod. 2024, 449, 141725. [Google Scholar] [CrossRef]
- Kanagasundaram, T.; Murphy, O.; Haji, M.N.; Wilson, J.J. The recovery and separation of lithium by using solvent extraction methods. Coord. Chem. Rev. 2024, 509, 215727. [Google Scholar] [CrossRef]
- Kolahchian, T.M.; Bonalumi, D.; Lozza, G.G. Analyzing the global warming potential of the production and utilization of lithium−batteries with nickel−manganese−cobalt cathode chemistries in European Gigafactories. Energy 2024, 288, 129622. [Google Scholar] [CrossRef]
- Wang, J.; Hu, H. Microbubble−assisted pressure carbonation for preparation of high purity lithium carbonate. J. Mater. Res. Technol. 2020, 9, 9498–9505. [Google Scholar] [CrossRef]
- Zhu, Q.; Yu, X.; Liu, Y.; Wang, Y.; Yang, P.; Liu, K. Electrochemical lithium extraction with continuous flow electrodes. Desalination 2024, 574, 117250. [Google Scholar] [CrossRef]
- Zhou, É.; Li, W.; Pouletc, T.; Basarir, H.; Karrech, A. Life cycle assessment of recycling lithium−battery related mineral processing by–products: A review. Miner. Eng. 2024, 208, 108600. [Google Scholar] [CrossRef]
- Duan, J.; Kang, K.; Li, P.; Zhang, W.; Li, X.; Wang, J.; Liu, Y. The design and regulation of porous silicon−carbon composites for enhanced electrochemical lithium storage performance. J. Ind. Eng. Chem. 2024, 131, 410–421. [Google Scholar] [CrossRef]
- Gu, J.; Chen, L.; Li, X.; Luo, G.; Fan, L.; Chao, Y.; Ji, H.; Zhu, W. Multifunctional AlPO4 reconstructed LiMn2O4 surface for electrochemical lithium extraction from brine. J. Energy Chem. 2024, 89, 410–421. [Google Scholar] [CrossRef]
- Zhai, J.; Chen, P.; Long, J.; Fan, C.; Chen, Z.; Sun, W. Recent advances on beneficial management of lithium refinery residue in China. Miner. Eng. 2024, 208, 108556. [Google Scholar] [CrossRef]
- Boroumand, Y.; Razmjou, A. Adsorption–type aluminium–based direct lithium extraction: The effect of heat, salinity and lithium content. Desalination 2024, 577, 117406. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, X.; Xiong, X. Extraction of lithium and phosphorus from amblygonite using calcium sulfate roasting and water leaching. Hydrometallurgy 2024, 225, 106282. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Zhou, Y.; Zhang, X.; Xu, C.; Duan, H.; Wang, R.; Lu, X. Research on the decomposition mechanisms of lithium silicate ores with different crystal structures by autotrophic and heterotrophic bacteria. Sci. Total Environ. 2024, 925, 171762. [Google Scholar] [CrossRef]
- Korbel, C.; Filippova, I.V.; Filippov, L.O. Froth flotation of lithium micas—A review. Miner. Eng. 2023, 192, 107986. [Google Scholar] [CrossRef]
- Wang, J.; Koenig, G.M., Jr. Direct Lithium Extraction Using Intercalation Materials. Chem. Eur. J. 2024, 30, e202302776. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lv, M.; Kuang, G.; Cao, Y.; Wang, H. Stepwise heat treatment for fluorine removal on selective leachability of Li from lepidolite using HF/H2SO4 as lixiviant. Sep. Purif. Technol. 2021, 259, 118194. [Google Scholar] [CrossRef]
- Choe, G.; Kim, H.; Kwon, J.; Jung, W.; Park, K.; Kim, Y. Re–evaluation of batterry–grade lithium purity toward sustainable batteries. Nat. Commun. 2024, 15, 1185. [Google Scholar] [CrossRef]
- Guo, H.; Kuang, G.; Wan, H.; Yang, Y.; Yu, H.; Wang, H. Enhanced acid treatment to extract lithium from lepidolite with a fluorine−based chemical method. Hidrometallurgy 2019, 183, 9–19. [Google Scholar] [CrossRef]
- Nie, W.; Wen, S.; Xian, Y.; Li, Y.; Han, G.; Jiang, Y. Leaching rubidium from a low–grade rubidium–bearing aluminosilicate ore. J. Mater. Res. Technol. 2021, 13, 1546–1554. [Google Scholar] [CrossRef]
- Resentera, A.C.; Rosales, G.D.; Esquivel, M.R.; Rodriguez, M.H. Lithium fluoride dissolution in sulfuric acid solution: Optimization and application in the extraction of lithium from fluorinated α-spodumene. Hidrometallurgy 2023, 217, 106027. [Google Scholar] [CrossRef]
- Necke, T.; Stein, H.K.; Joachim, H.K.; Grünewald, B.B. Lithium Extraction and Zeolite Synthesis via Mechanochemical Treatment of the Silicate Minerals Lepidolite, Spodumene, and Petalite. Minerals 2023, 13, 1030. [Google Scholar] [CrossRef]
- Mulwanda, G.; Senananyake, G.; Oskierski, H.C.; Altarawneh, M.; Dlugogorski, B.Z. Extracction of lithium from lepidolite by sodium bisulphate roasting, water leaching and precipitation as lithium phosphate from purified leach liquors. Hidrometallurgy 2023, 222, 106139. [Google Scholar] [CrossRef]
- Vieceli, N.; Nogueira, C.A.; Pereira, M.F.C.; Soares, D.A.P.; Durão, F.O.; Guimarães, C.; Margarido, F. Effects of mechanical activation on lithium extraction from a lepidolite ore concentrate. Miner. Eng. 2017, 102, 1–14. [Google Scholar] [CrossRef]
- Taejun, P.; Junho, S.; Sunkyung, K.; Taegong, R.; Byungsu, K.; Hankwon, C. An effective lithium extraction route from lepidolite. Hydrometallurgy 2023, 222, 106202. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, J.; Fu, L.; Zuo, Y.; Zhang, G. Microwave−enhanced sulfate roasting for lithium extraction from lepidolite: A comprehensive study. J. Clean. Prod. 2024, 434, 140248. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Liu, W.; Wang, C.; Wang, D.; Qin, W. A novel approach for extracting lithium from overhaul slag by low temperature roasting−water leaching. Chem. Eng. J. 2024, 481, 148571. [Google Scholar] [CrossRef]
- Ivanets, A.; Bicheva, E.; Prozorovich, V.; Kouznetsona, T.; Aimbetova, I.O.; Su, X. Effect of Ti−containing precursors on structure andadsorption performance of Li4Ti5O12 and Li2TiO3 oxides to Li+ ions. Sep. Purif. Technol. 2024, 335, 125986. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.V.; Santhosh, G.; Manjanna, J. Dissolution of cathode active material of spent Li−ion batteries using tartaric acid and ascorbic acid mixture to recover Co. Hydrometallurgy 2016, 161, 54–57. [Google Scholar] [CrossRef]
- Bae, H.; Kim, Y. Technologies of lithium recycling from waste lithium ion batteries: A review. Mater. Adv. 2021, 2, 3234–3250. [Google Scholar] [CrossRef]
- Levenspiel, O. Ingeniería de las Reacciones Químicas, 2nd ed.; Reverté: Barcelona, Spain, 2002; pp. 108–146. [Google Scholar]
- Ballester, A.; Verdeja, F.; Sancho, J. Metalurgia Extractiva, 1st ed.; Síntesis: Madrid, Spain, 2000; pp. 154–196. [Google Scholar]
- Juárez, J.C.; Patiño, F.; Flores, M.U.; Méndez, J.E.; Reyes, I.A.; Ordoñez, S.; Reyes, M. Kinetics and modelling of the decomposition of a solid solution of potassium–ammonium arsenojarosite in NaOH and Ca(OH)2 media. Reac. Kinet. Mech. Cat. 2017, 121, 387–402. [Google Scholar] [CrossRef]
- Ordoñez, S.; Flores, M.U.; Patiño, F.; Reyes, I.A.; Islas, H.; Reyes, M.; Méndez, E.; Palacios, E. Kinetic Analysis of the Decomposition Reaction of the mercury jarosite in NaOH medium. Int. J. 2017, 49, 798–809. [Google Scholar] [CrossRef]
- Upadhyaya, G.S.; Dube, R.K. Problemas de Termodinámica y Cinética en Metalurgia, 1st ed.; Géminis: Ituzaingó, Argentina, 1979; pp. 121–154. [Google Scholar]
- Bursten, B.L. Química la Ciencia Central, 7th ed.; Pearson Prentice Hall: Ciudad de México, Mexico, 1998. [Google Scholar]
- Flores, M.U.; Reyes, I.A.; Palacios, E.G.; Patiño, F.; Juarez, J.C.; Reyes, M.; Teja, A.M.; Islas, H.; Gutiérrez, E.J. Kinetic analysis of the thermal decomposition of a synthetic mercury jarosite. Minerals 2019, 9, 200. [Google Scholar] [CrossRef]
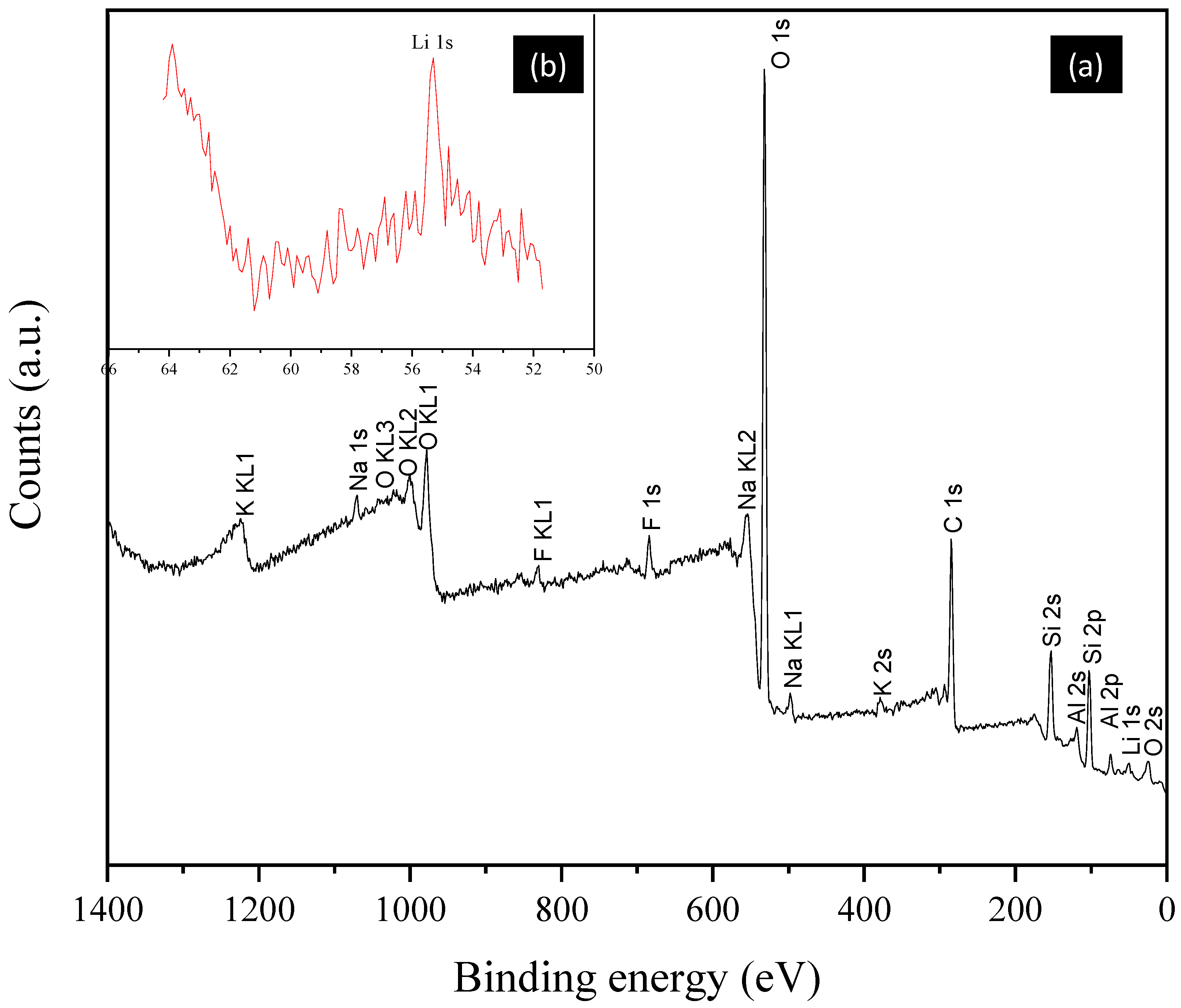





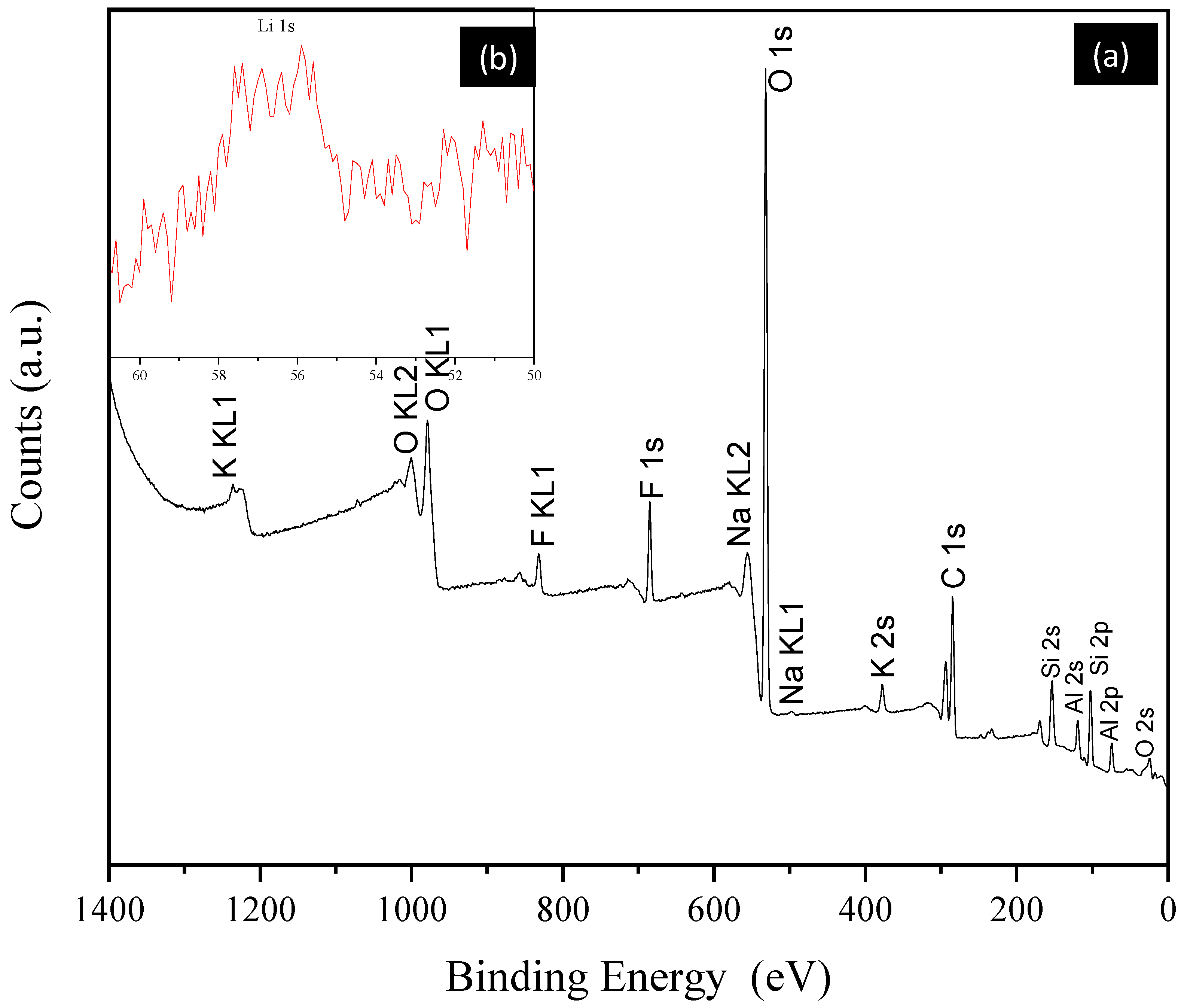
| Element | Start BE 1 (eV) | Peak BE (eV) | End BE (eV) | Atomic % | PP At. % |
|---|---|---|---|---|---|
| Li 1s | 60 | 55.53 | 51.73 | 3.38 | 3.22 |
| Si 2p | 108 | 102.76 | 98.44 | 17.12 | 19.33 |
| C 1s | 299.13 | 284.73 | 281 | 31.76 | 28.76 |
| K 2s | 391.10 | 377.08 | 359.52 | 0.95 | 1.86 |
| Al 2p | 75.3 | 71.41 | 68.24 | 0.05 | 0.15 |
| O 1s | 540 | 531.97 | 527.03 | 44.12 | 44.98 |
| F 1s | 691.82 | 685.71 | 673.21 | 1.23 | 1.30 |
| Fe 2p | 733.76 | 712.76 | 704.65 | 0.52 | 0.26 |
| Na 1s | 1077 | 1071.73 | 1067.48 | 0.87 | 0.14 |
| Component | Li | Al | Si | Na | K | Fe | Mg |
|---|---|---|---|---|---|---|---|
| Weight (w/w) | 3.76 | 8.82 | 39.64 | 5.31 | 4.94 | 0.12 | 1.02 |
| Time (min) | Mass Fraction of Li |
|---|---|
| 0 | 0 |
| 5 | 0.02967033 |
| 10 | 0.088111888 |
| 15 | 0.125974026 |
| 20 | 0.163036963 |
| 30 | 0.237262737 |
| 40 | 0.375624376 |
| 60 | 0.557442557 |
| 90 | 0.827172827 |
| 120 | 1 |
| 150 | 0.989010989 |
| 180 | 0.978021978 |
| [C6H8O6] (mol L−1) | pH | [H3O+] (mol L−1) | kexp (min−1) | tind (min) |
|---|---|---|---|---|
| 0.114 | 0.5 | 0.31623 | 0.0305 | 2.29 |
| 0.085 | 1.5 | 0.03162 | 0.0154 | 5.97 |
| 0.057 | 1.9 | 0.01259 | 0.0135 | 8.48 |
| 0.028 | 2.6 | 0.00251 | 0.009 | 12.7 |
| 0.011 | 4 | 0.00100 | 0.0060 | 23.82 |
| 0.003 | 6.1 | 0.00100 | 0.004 | 37.19 |
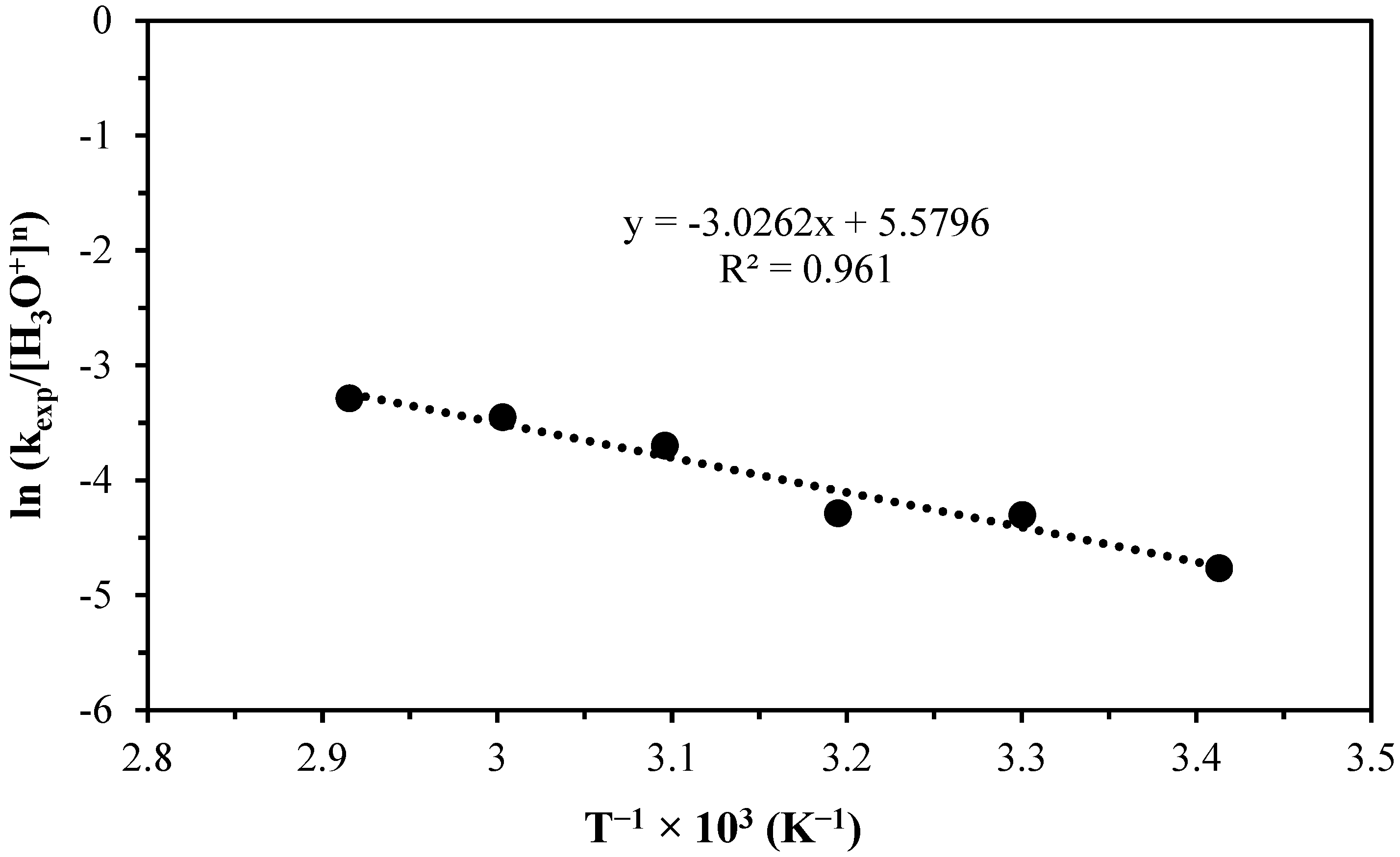
| Temperature (°C/K) | pH | [H3O+] (mol L−1) | kexp (min−1) | tind (min) |
|---|---|---|---|---|
| 20/293.15 | 2.84 | 0.00145 | 0.0032 | 15.48 |
| 30/303.15 | 2.83 | 0.00148 | 0.0051 | 8.48 |
| 40/313.15 | 2.45 | 0.00355 | 0.0059 | 5.23 |
| 50/323.15 | 2.25 | 0.00562 | 0.01139 | 3.62 |
| 60/333.15 | 2.15 | 0.00708 | 0.0151 | 1.79 |
| 70/343.15 | 2.1 | 0.00794 | 0.0181 | 0.71 |
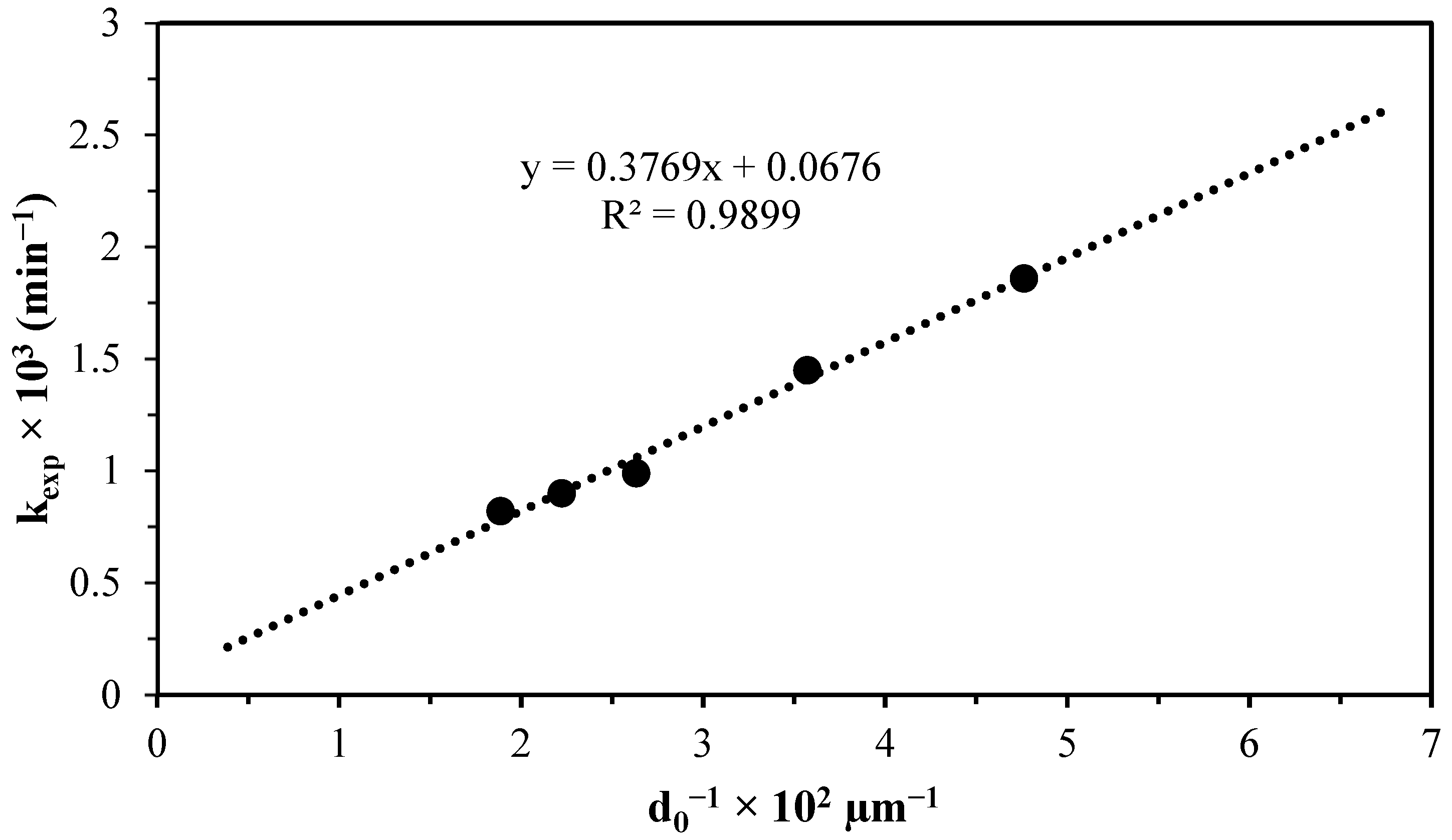
| Particle Size (µm) | Initial Ratio (µm) | pH | [H3O+] (mol L−1) | kexp (min−1) | tind (min) |
|---|---|---|---|---|---|
| 53 | 26.5 | 1.89 | 0.01288 | 0.0082 | 8.45 |
| 45 | 22.5 | 1.91 | 0.01230 | 0.009 | 8.48 |
| 38 | 19 | 1.95 | 0.01122 | 0.0099 | 8.92 |
| 28 | 14 | 1.97 | 0.01071 | 0.0145 | 8.95 |
| 21 | 10.5 | 1.95 | 0.01122 | 0.0186 | 8.95 |
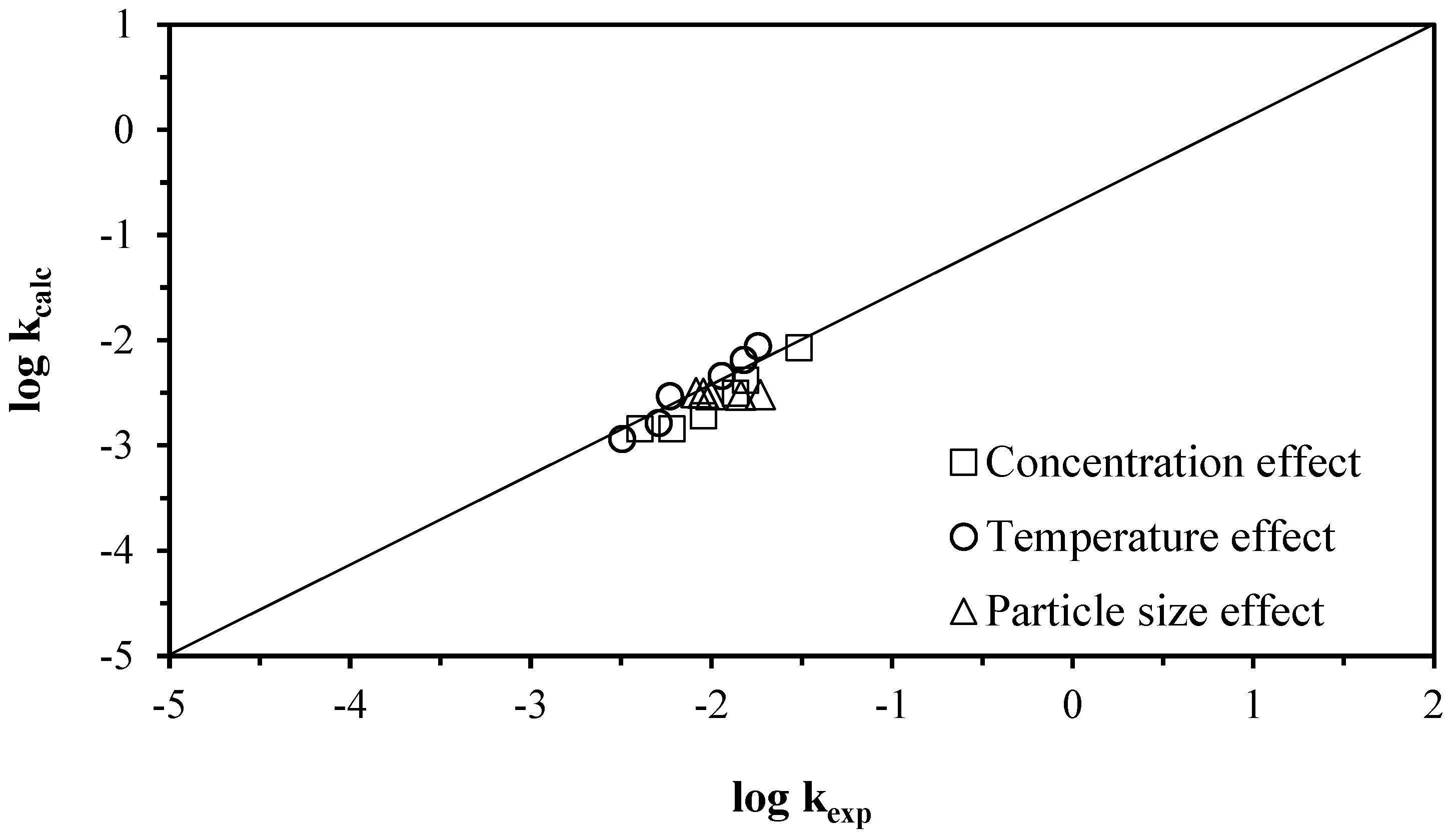
| Effect | pH | [C6H8O6] (mol L−1) | [H3O] (mol L−1) | T K | d0 µm | Tind (min) | kexp (min−1) | ||
|---|---|---|---|---|---|---|---|---|---|
| Concentration | 0.5 | 0.114 | 0.31623 | 303.15 | 38 | 2.29 | 0.0305 | ||
| 1.5 | 0.085 | 0.03162 | 303.15 | 38 | 5.97 | 0.0154 | |||
| 1.9 | 0.057 | 0.01259 | 303.15 | 38 | 8.48 | 0.0135 | |||
| 2.6 | 0.028 | 0.00251 | 303.15 | 38 | 12.7 | 0.009 | |||
| 4 | 0.011 | 0.00100 | 303.15 | 38 | 23.82 | 0.0060 | |||
| 6.1 | 0.003 | 0.00100 | 303.15 | 38 | 37.19 | 0.004 | |||
| Temperature | 2.84 | 0.057 | 0.00145 | 293.15 | 38 | 15.48 | 0.0032 | ||
| 2.83 | 0.057 | 0.00148 | 303.15 | 38 | 8.48 | 0.0051 | |||
| 2.45 | 0.057 | 0.00355 | 313.15 | 38 | 5.23 | 0.0059 | |||
| 2.25 | 0.057 | 0.00562 | 323.15 | 38 | 3.62 | 0.01139 | |||
| 2.15 | 0.057 | 0.00708 | 333.15 | 38 | 1.79 | 0.0151 | |||
| 2.1 | 0.057 | 0.00794 | 343.15 | 38 | 0.71 | 0.0181 | |||
| Particle size | 1.89 | 0.057 | 0.01288 | 303.15 | 53 | 8.45 | 0.0082 | ||
| 1.91 | 0.057 | 0.01230 | 303.15 | 45 | 8.48 | 0.009 | |||
| 1.95 | 0.057 | 0.01122 | 303.15 | 38 | 8.92 | 0.0099 | |||
| 1.97 | 0.057 | 0.01071 | 303.15 | 28 | 8.95 | 0.0145 | |||
| 1.95 | 0.057 | 0.01122 | 303.15 | 21 | 8.95 | 0.0186 | |||
| Induction period | n = 0.4307 | Ea = 48,580 J mol−1 | A = 2.57 × 107 | ||||||
| Progressive conversion period | n = 0.309 | Ea = 25,161 J mol−1 | A = 264.96 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordoñez, S.; Reyes, I.A.; Patiño, F.; Islas, H.; Reyes, M.; Pérez, M.; Juárez, J.C.; Flores, M.U. Dissolution of Lithium Contained in Lepidolite Using Ascorbic Acid: Kinetic and Modeling Analysis. Materials 2024, 17, 5447. https://doi.org/10.3390/ma17225447
Ordoñez S, Reyes IA, Patiño F, Islas H, Reyes M, Pérez M, Juárez JC, Flores MU. Dissolution of Lithium Contained in Lepidolite Using Ascorbic Acid: Kinetic and Modeling Analysis. Materials. 2024; 17(22):5447. https://doi.org/10.3390/ma17225447
Chicago/Turabian StyleOrdoñez, Sayra, Iván A. Reyes, Francisco Patiño, Hernán Islas, Martín Reyes, Miguel Pérez, Julio C. Juárez, and Mizraim U. Flores. 2024. "Dissolution of Lithium Contained in Lepidolite Using Ascorbic Acid: Kinetic and Modeling Analysis" Materials 17, no. 22: 5447. https://doi.org/10.3390/ma17225447
APA StyleOrdoñez, S., Reyes, I. A., Patiño, F., Islas, H., Reyes, M., Pérez, M., Juárez, J. C., & Flores, M. U. (2024). Dissolution of Lithium Contained in Lepidolite Using Ascorbic Acid: Kinetic and Modeling Analysis. Materials, 17(22), 5447. https://doi.org/10.3390/ma17225447










