The Effects of Phosphate Compounds on the Microstructure and Mechanical Properties of Fly Ash Geopolymer Mortars
Abstract
:1. Introduction
- Bottom ash, characterized by a coarse fraction (predominantly quartz), unburned particles, and mineral impurities derived from the biomass;
2. Materials and Methods
2.1. Fly Ashes
2.1.1. Density
- Vm—volume of pulverized sample, dm3;
- Vch—volume of sealed chamber, dm3;
- Pch—the pressure within the sample chamber, Pa;
- Vr—reference chamber of known volume, dm3;
- Pr—charged pressure, Pa;
- Psys—system pressure, Pa.
- mm—mass of pulverized sample, g.
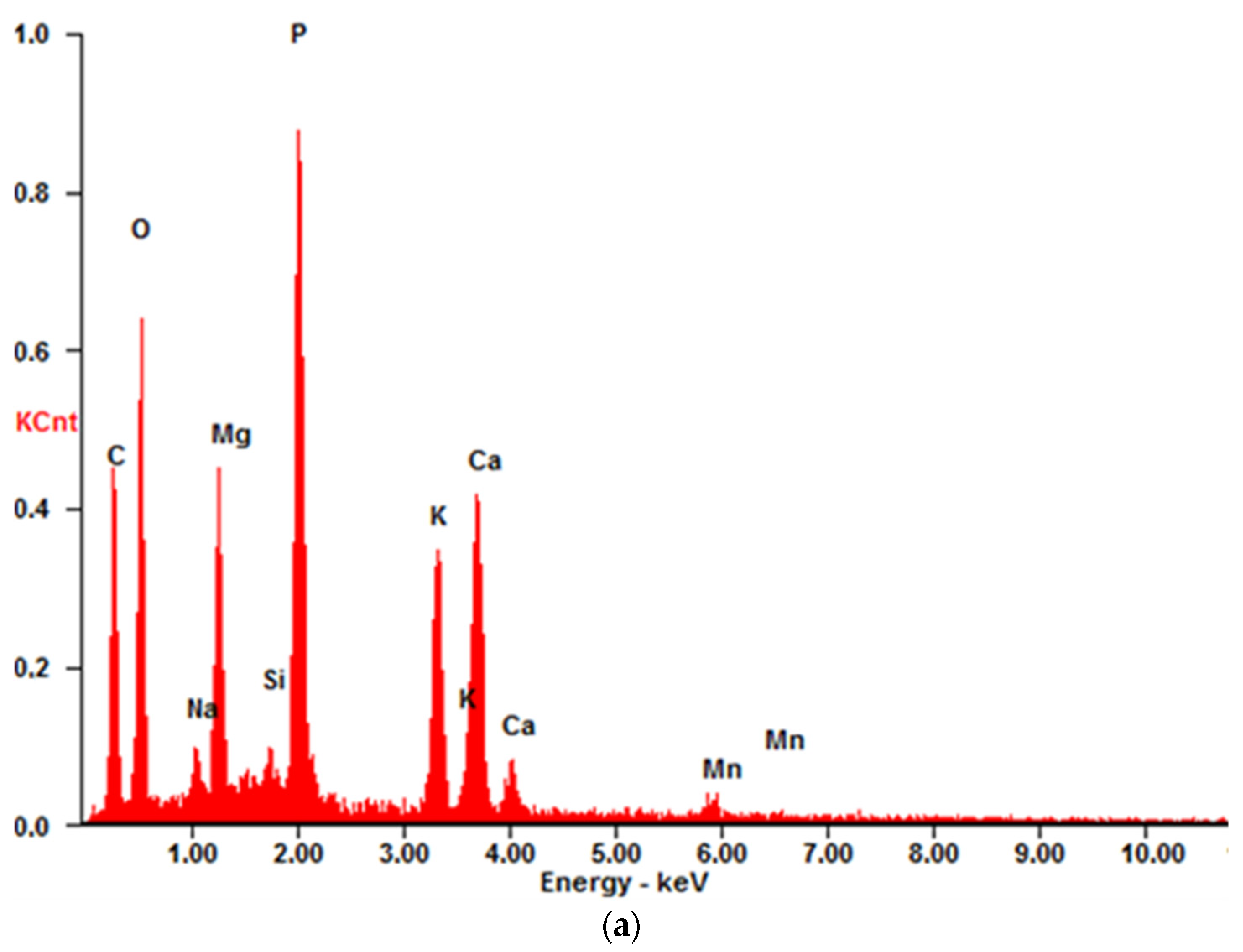
2.1.2. XRF and XRD
2.1.3. Loss on Ignition (LOI)
- m1—mass of sample before heating, g;
- m2—the constant mass of examined sample, g.
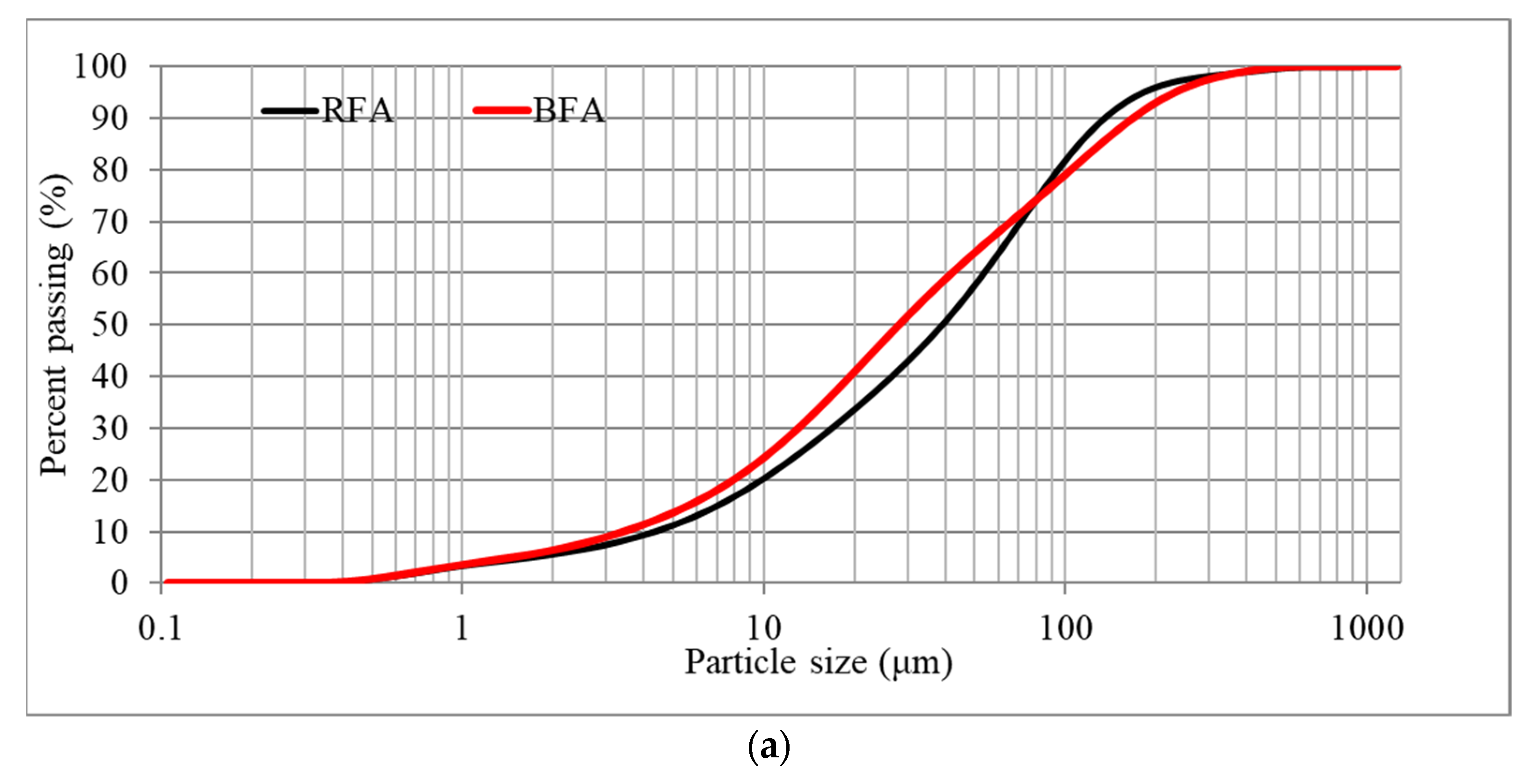
2.1.4. Particle Size Distributions (PSDs)
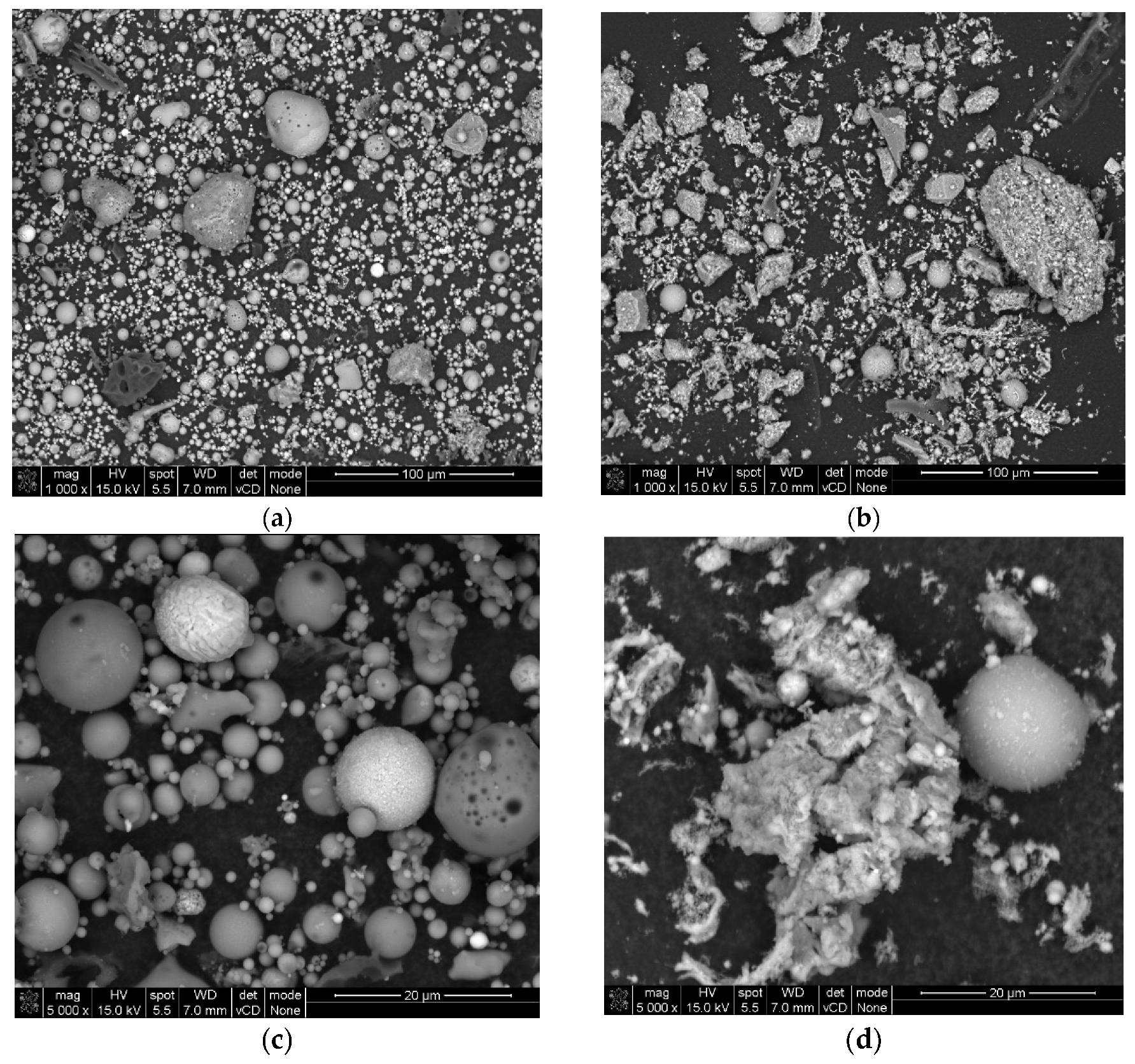
2.1.5. SEM Image Analysis
2.2. Alkali Activated Mortars
- Aluminosilicate precursors: mix of bituminous coal combustion fly ash (RFA) and wooden biomass combustion fly ash (BFA) from Polish Power Heat and Power Plant Siekierki, Warsaw, Poland;
- Alkaline activators: 30% sodium hydroxide solution—7.5 M (PCC, Brzeg Dolny), sodium silicate with SiO2/Na2O ratio between 1.9 and 2.1 (R150, Z.Ch. Rudniki S.A., Rudniki k. Częstochowy, Poland);
- Phosphate compound: Tetraphosphorus decaoxide—P4O10 (Chempur, Piekary Śląskie, Poland);
- Fine aggregate: Standard Sand according to EN 196-1 [32].
- Introduce geopolymerization precursor into the mixer and maintain low rpm (140 ± 5 rpm);
- Introduce sand uniformly for the initial 30 s of mixing;
- Incorporate alkaline activator solution steadily for the subsequent 30 s;
- Shift the mixer to high rpm (285 ± 10 rpm) and continue mixing for an additional 30 s.
2.2.1. Compressive and Flexural Strengths
2.2.2. Porosity
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
- Approximately 0.1 to 1.0% of the sample was amalgamated with 200 to 250 mg of finely ground KBr powder;
- The resultant mixture was meticulously pulverized and subsequently placed into a die designated for pellet formation;
- A compressive force of approximately 8 tons was exerted under a vacuum of several mm Hg for a duration of several minutes to yield transparent pellets.
3. Results and Discussion
3.1. Fly Ashes
3.1.1. Chemical Compositions, Mineral Phase Characterizations, and Physical Characteristics
3.1.2. PSDs
3.1.3. SEM Analysis
3.2. Fly Ash Mortars
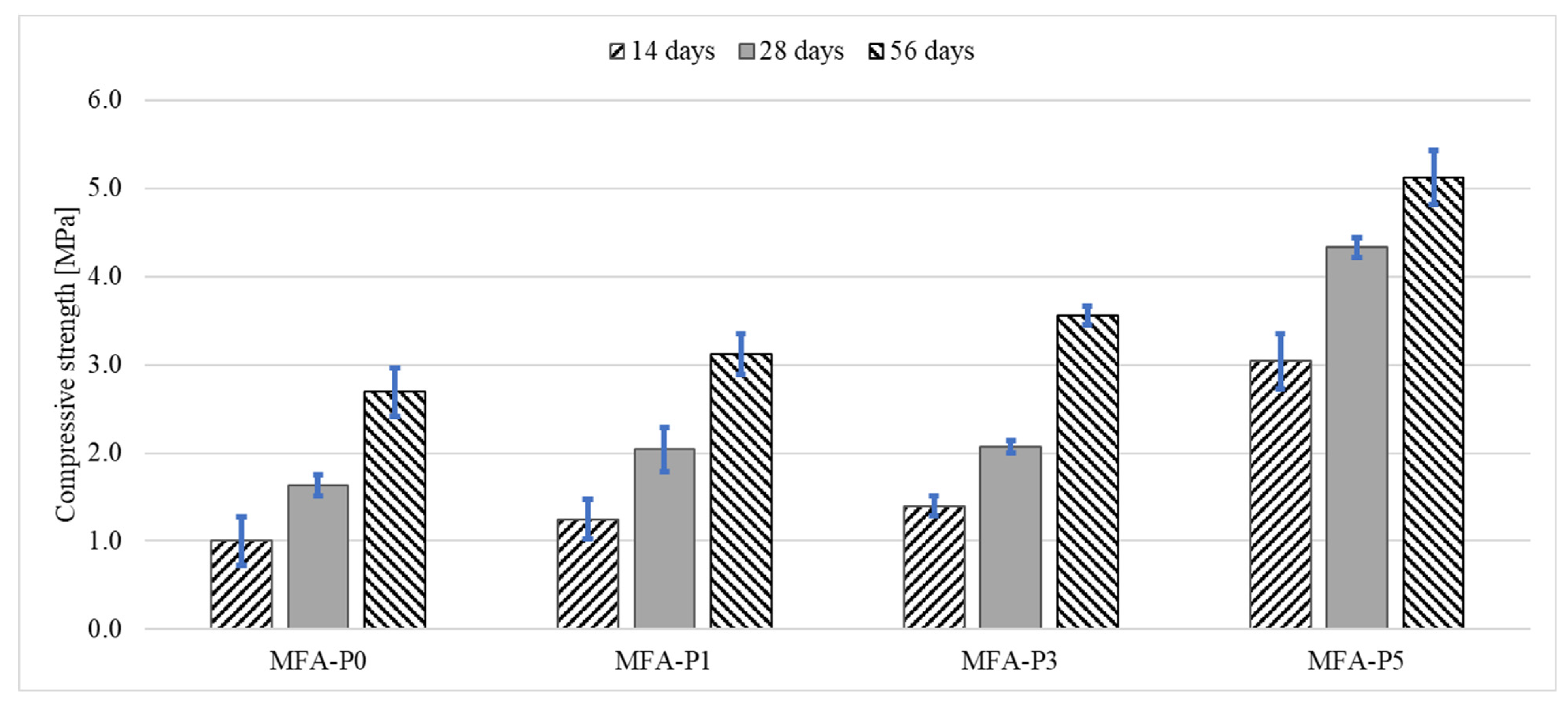
3.2.1. Flexural and Compressive Strengths
3.2.2. Porosity
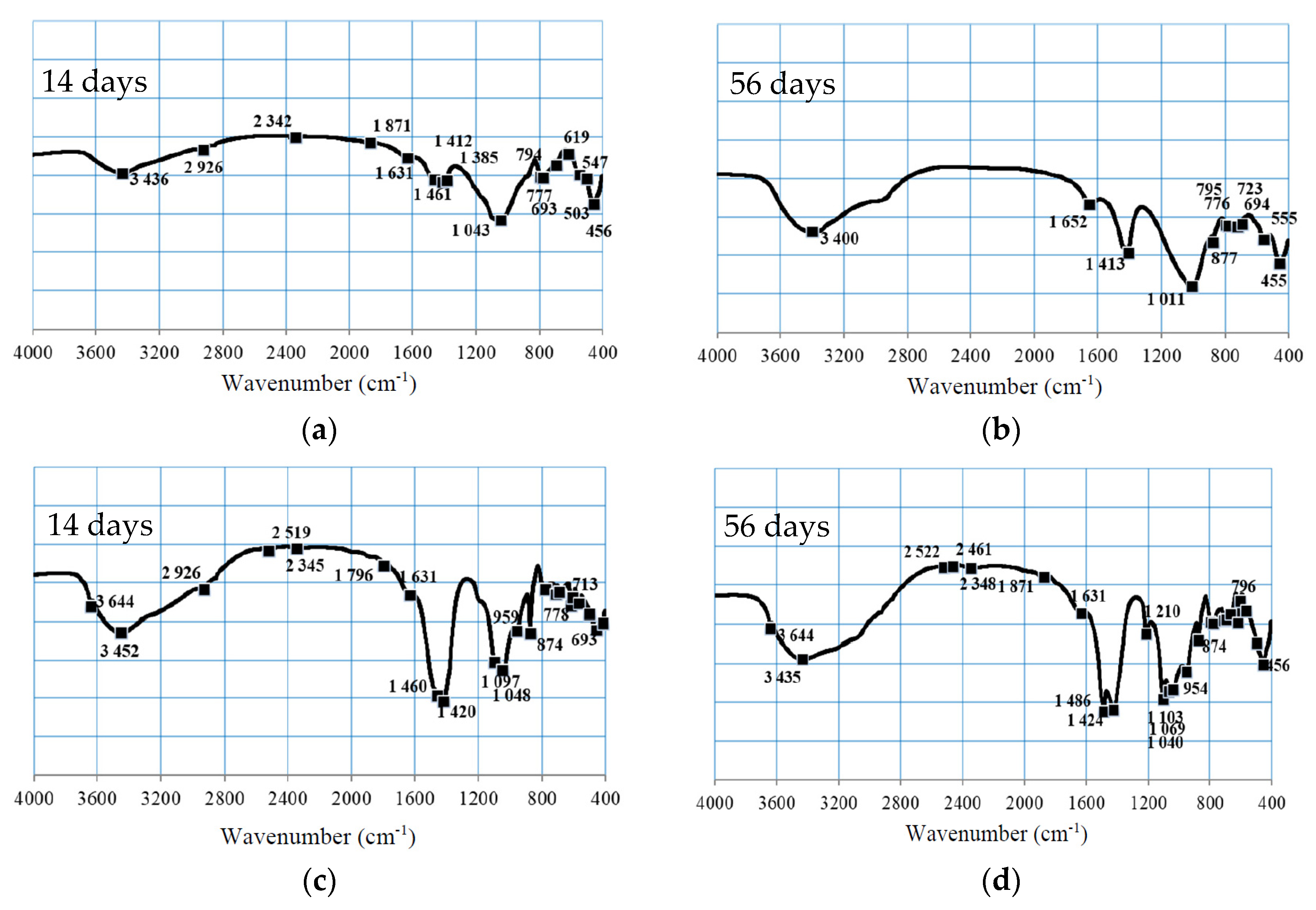
3.2.3. FTIR
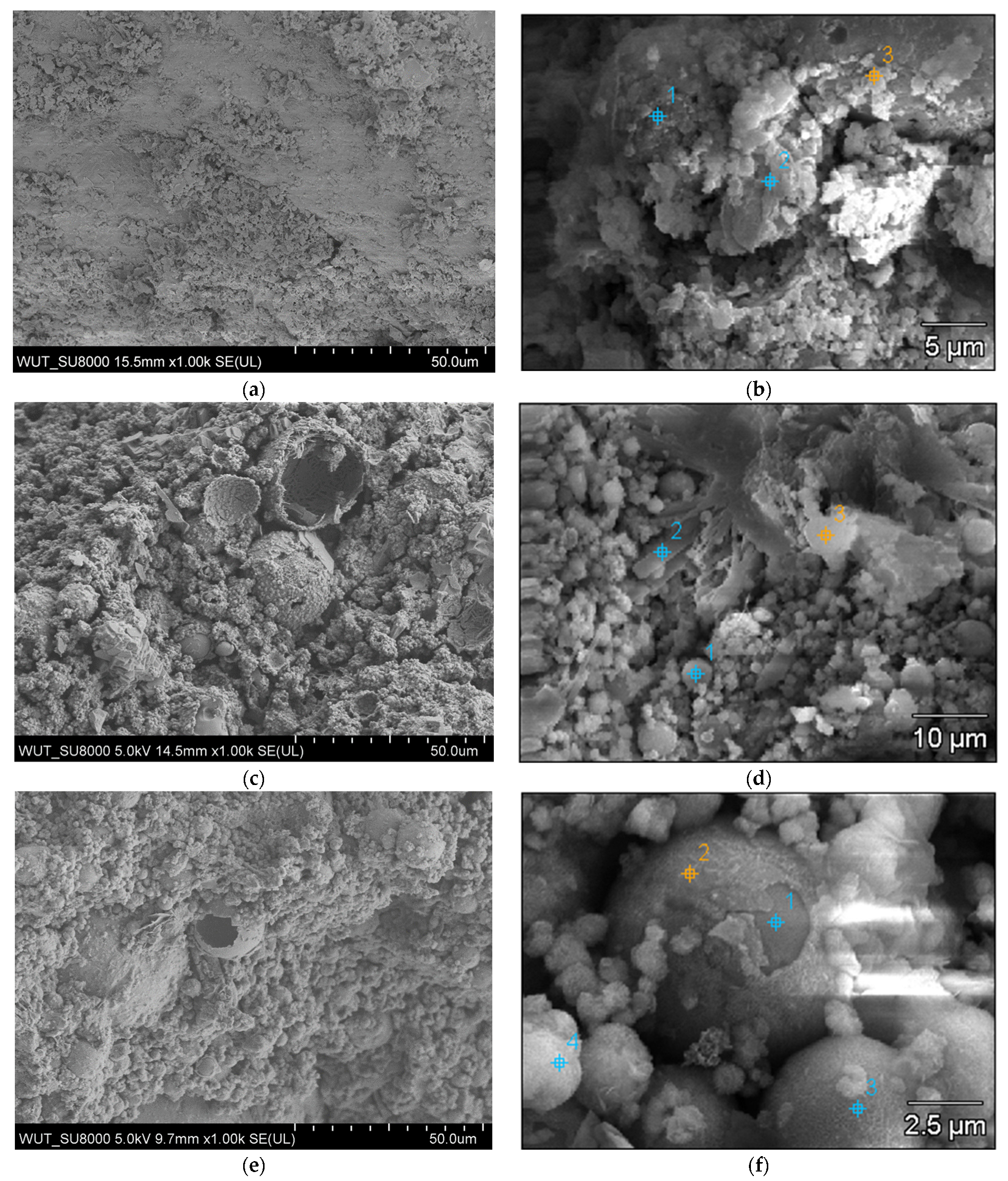
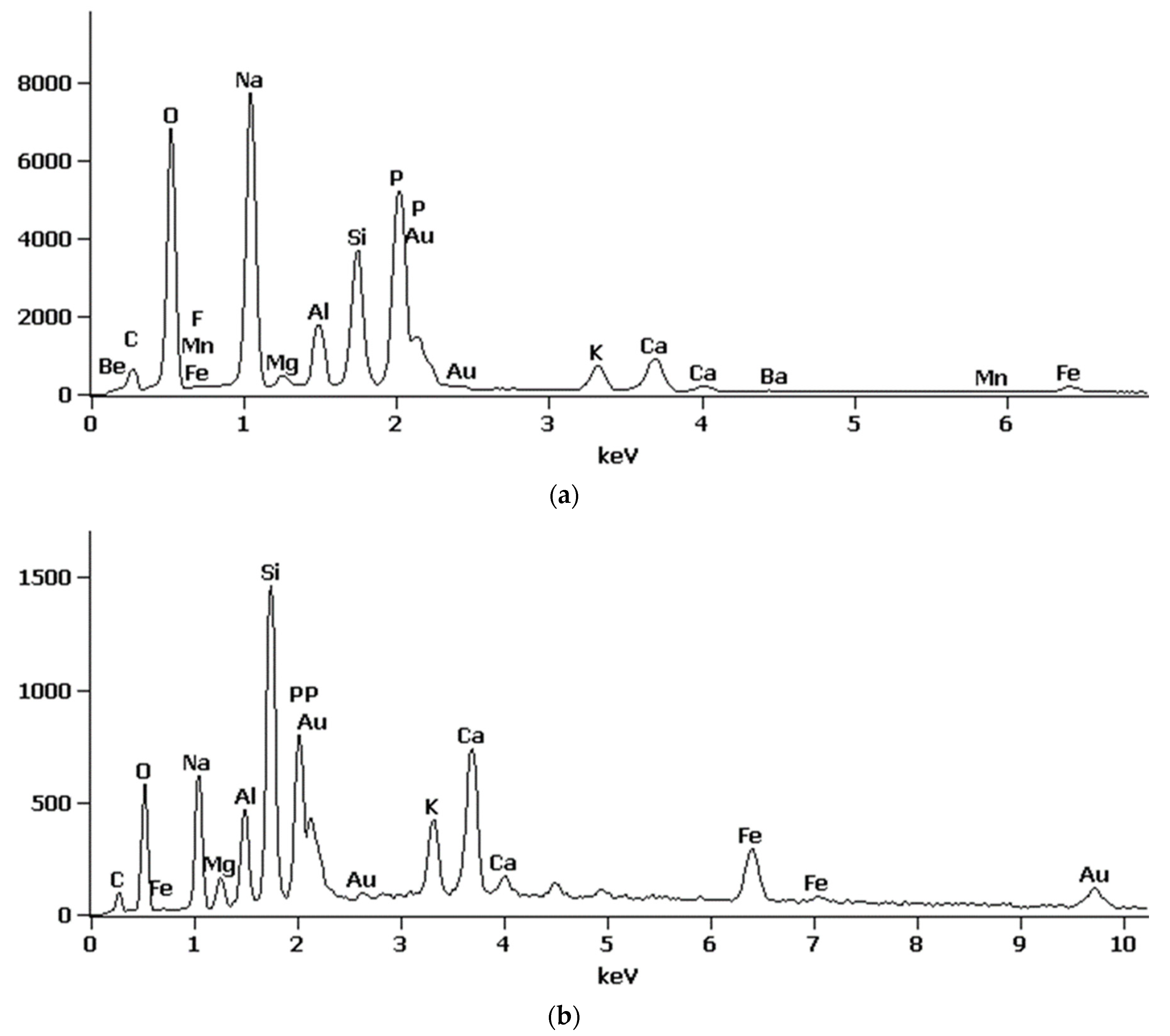
3.2.4. SEM
4. Conclusions
- The concentration of P4O10 present within the samples exhibits a positive relationship with both flexural and compressive strength across all assessed temporal intervals (7, 28, and 56 days).
- The incorporation of P4O10 leads to a marked increase in porosity of geopolymer mortars.
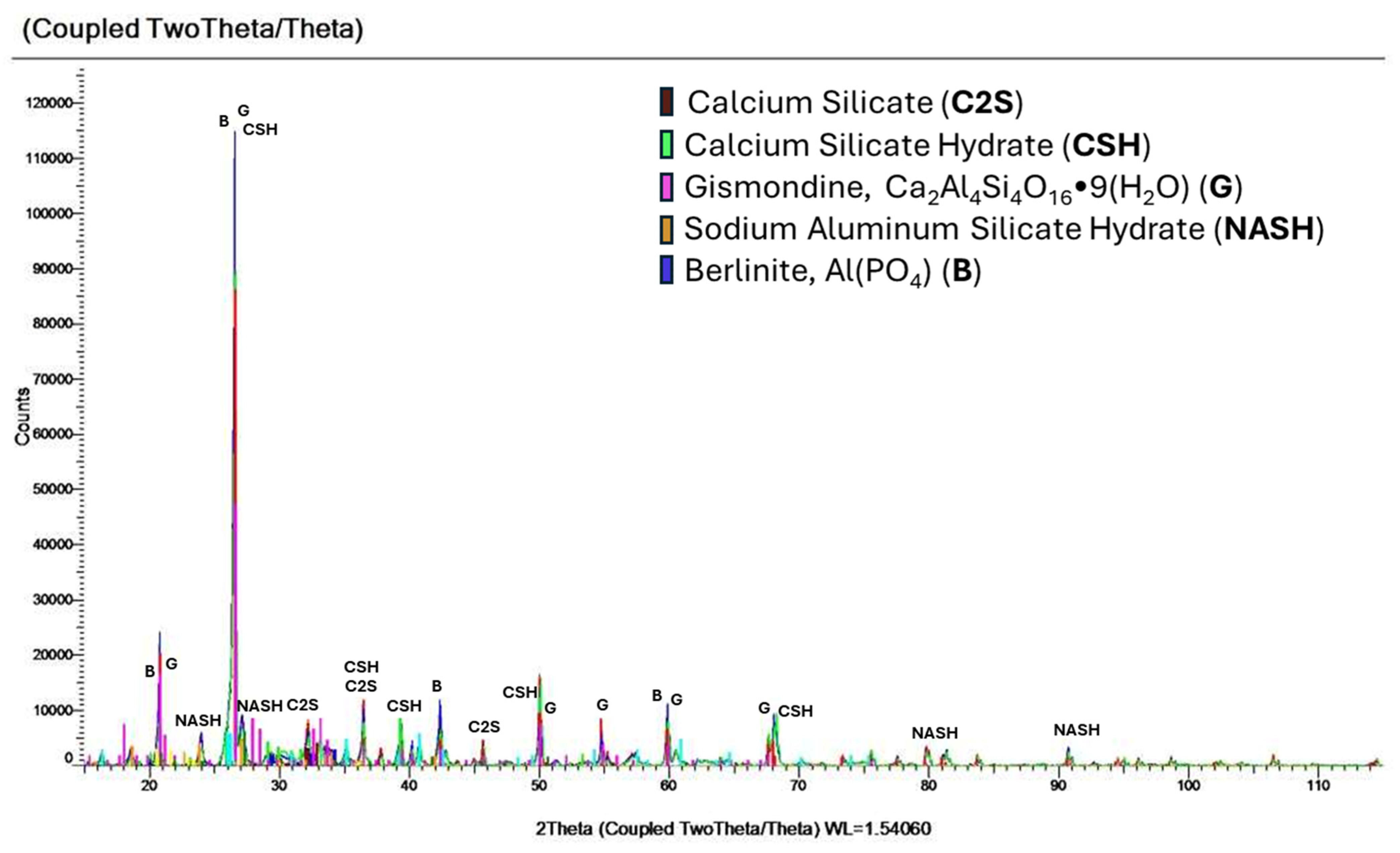
- The addition of 5% P4O10 facilitated the emergence of a new crystalline phase, identified as berlinite.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swora, M. Polish Pathway to Just Transition: Energy Law and Policy Trapped Between Sustainability and Security of Supply in Handbook of Energy Law in the Low-Carbon Transition; Bellantuono, G., Godden, L., Eds.; De Gruyter: Boston, MA, USA; Berlin, Germany, 2023; pp. 467–492. [Google Scholar] [CrossRef]
- OECD. OECD Inventory of Support Measures for Fossil Fuels: Country Notes; OECD: Paris, France, 2023. [Google Scholar] [CrossRef]
- James, A.K.; Thring, R.W.; Rutherford, P.M.; Helle, S.S. Characterization of biomass bottom ash from an industrial scale fixed-bed boiler by fractionation. Energy Environ. Res. 2013, 3, 21–32. [Google Scholar] [CrossRef]
- Modolo, R.C.E.; Silva, T.; Senff, L.; Tarelho, L.A.C.; Labrincha, J.A.; Ferreira, V.M.; Silva, L. Bottom ash from biomass combustion in BFB and its use in adhesive-mortars. Fuel Process. Technol. 2015, 129, 192–202. [Google Scholar] [CrossRef]
- Rajamma, R.; Ball, R.J.; Tarelho, L.A.C.; Allen, G.C.; Labrincha, J.A.; Ferreira, V.M. Characterisation and use of biomass fly ash in cement-based materials. J. Hazard. Mater. 2009, 172, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Masiá, A.A.T.; Buhre, B.J.P.; Gupta, R.P.; Wall, T.F. Characterizing ash of biomass and waste Fuel. Proc. Technol. 2007, 88, 1071–1081. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Agrela, F.; Cabrera, M.; Morales, M.M.; Zamorano, M.; Alshaaer, M. 2—Biomass fly ash and biomass bottom ash. In Woodhead Publishing Series in Civil and Structural Engineering, New Trends in Eco-Efficient and Recycled Concrete; Woodhead Publishing: Sawston, UK, 2019; pp. 23–58. [Google Scholar] [CrossRef]
- Alves, B.I.A.; Marvila, M.T.; Linhares Júnior, J.A.T.; Vieira, C.M.F.; Alexandre, J.; de Azevedo, A.R.G. Alkaline Activation of Binders: A Comparative Study. Materials 2024, 17, 667. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Yuan, J.; He, P.; Jia, D.; Yang, C.; Zhang, Y.; Yan, S.; Yang, Z.; Duan, X.; Wang, S.; Zhou, Y. Effect of curing temperature and SiO2/K2O molar ratio on the performance of metakaolin-based geopolymers. Ceram. Int. 2016, 42, 16184–16190. [Google Scholar] [CrossRef]
- Tuyan, M.; Andiç-Çakir, Ö.; Ramyar, K. Effect of alkali activator concentration and curing condition on strength and microstructure of waste clay brick powder-based geopolymer. Compos. Part B Eng. 2018, 135, 242–252. [Google Scholar] [CrossRef]
- Cho, Y.-K.; Yoo, S.-W.; Jung, S.-H.; Lee, K.-M.; Kwon, S.-J. Effect of Na2O content, SiO2/Na2O molar ratio, and curing conditions on the compressive strength of FA-based geopolymer. Constr. Build. Mater. 2017, 145, 253–260. [Google Scholar] [CrossRef]
- Sharmin, A.; Alengaram, U.J.; Jumaat, M.Z.; Yusuf, M.O.; Kabir, S.A.; Bashar, I.I. Influence of source materials and the role of oxide composition on the performance of ternary blended sustainable geopolymer mortar. Constr. Build. Mater. 2017, 144, 608–623. [Google Scholar] [CrossRef]
- Nath, S.K.; Maitra, S.; Mukherjee, S.; Kumar, S. Microstructural and morphological evolution of fly ash based geopolymers. Constr. Build. Mater. 2016, 111, 758–765. [Google Scholar] [CrossRef]
- Lee, B.; Kim, G.; Kim, R.; Cho, B.; Lee, S.; Chon, C.-M. Strength development properties of geopolymer paste and mortar with respect to amorphous Si/Al ratio of fly ash. Constr. Build. Mater. 2017, 151, 512–519. [Google Scholar] [CrossRef]
- Reig, L.; Sanz, M.; Borrachero, M.; Monzó, J.; Soriano, L.; Payá, J. Compressive strength and microstructure of alkali-activated mortars with high ceramic waste content. Ceram. Int. 2017, 43, 13622–13634. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Sobrados, I.; Sanz, J. The role played by the reactive alumina content in the alkaline activation of fly ashes, Microporous and Mesoporous. Materials 2016, 91, 111–119. [Google Scholar] [CrossRef]
- Alves, B.I.A.; Marvila, M.T.; Alexandre, J.; de Azevedo, A.R.G.; Linhares Júnior, J.A.T.; Vieira, C.M.F. Activated alkali cement based on blast furnace slag: Effect of curing type and concentration of Na2O. J. Mater. Res. Technol. 2023, 23, 4551–4565. [Google Scholar] [CrossRef]
- He, P.; Wang, M.; Fu, S.; Jia, D.; Yan, S.; Yuan, J.; Xu, J.; Wang, P.; Zhou, Y. Effects of Si/Al ratio on the structure and properties of metakaolin based geopolymer. Ceram. Int. 2016, 42, 14416–14422. [Google Scholar] [CrossRef]
- Hadi, N.S.M.; Al-Azzawi, M.; Yu, T. Effects of fly ash characteristics and alkaline activator components on compressive strength of fly ash-based geopolymer mortar. Constr. Build. Mater. 2018, 175, 41–54. [Google Scholar] [CrossRef]
- Vassilev, V.S.; Vassileva, G.C.; Vassilev, S.V. Advantages and disadvantages of composition and properties of biomass in comparison with coal: An overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- Sadangi, J.K.; Muduli, S.D.; Nayak, B.D.; Mishra, B.K. Effect of phosphate ions on preparation of fly ash based geopolymer. Int. J. Appl. Chem. 2013, 4, 20–26. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Dai, J.-G.; Ding, Z.; Xu, W.-T. Phosphate-based geopolymer: Formation mechanism and thermal stability. Mater. Lett. 2017, 190, 209–212. [Google Scholar] [CrossRef]
- Moukannaa, S.; Loutou, M.; Benzaazoua, M.; Vitola, L.; Alami, J.; Hakkou, R. Recycling of phosphate mine tailings for the production of geopolymers. J. Clean. Prod. 2018, 185, 891–903. [Google Scholar] [CrossRef]
- Boughanmi, S.; Labidi, I.; Megriche, A.; Tiss, H.; Nonat, A. Does phosphorus affect the industrial Portland cement reactivity? Constr. Build. Mater. 2018, 188, 599–606. [Google Scholar] [CrossRef]
- do Carmo Holanda, F.; Schmidt, H.; Quarcioni, A.V. Influence of phosphorus from phosphogypsum on the initial hydration of Portland cement in the presence of superplasticizers. Cem. Concr. Compos. 2017, 83, 384–393. [Google Scholar] [CrossRef]
- Tkaczewska, E.; Kłosek-Wawrzyn, E. Wpływ jonów fosforanowych PO3-/4 na proces hydratacji cementu. Cem. Wapno Beton 2012, 17, 401–408. [Google Scholar]
- Wang, Y.; Xiao, R.; Hu, W.; Jiang, X.; Zhang, X.; Huang, B. Effect of granulated phosphorus slag on physical, mechanical and microstructural characteristics of Class F fly ash based geopolymer. Constr. Build. Mater. 2021, 291, 123287. [Google Scholar] [CrossRef]
- EN 196-2; Method of Testing Cement—Part 2: Chemical Analysis of Cement. European Standard: Plzen, Czech Republic, 2013.
- EN 450-1; Fly Ash for Concrete—Part 1: Definition, Specifications and Conformity Criteria. European Standard: Plzen, Czech Republic, 2012.
- EN 196-1; Methods of Testing Cement—Part 1: Determination of Strength. European Standard: Plzen, Czech Republic, 2016.
- EN 998-2; Specification for Mortar for Masonry—Part 2: Masonry Mortar. European Standard: Plzen, Czech Republic, 2016.
- PN-B-10104; Wymagania Dotyczące Zapraw Murarskich Ogólnego Przeznaczenia. Zaprawy o Określonym Składzie Materiałowym, Wytwarzane na Miejscu Budowy. Polski Komitet Normalizacyjny: Warsaw, Poland, 2005.
- EN 1015-3; Methods of Test for Mortar for Masonry—Part 3: Determination of Consistence of Fresh Mortar (by Flow Table). European Standard: Plzen, Czech Republic, 1999.
- ASTM C618; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM: West Conshohocken, PA, USA, 2023.
- Fomenko, E.V.; Rogovenko, E.S.; Anshits, N.N.; Solovyov, L.A.; Anshits, A.G. Characterization of Silicate Glass/Mullite Composites Based on Coal Fly Ash Cenospheres as Effective Gas Separation Membranes. Materials 2023, 16, 6913. [Google Scholar] [CrossRef]
- Odzijewicz, J.I.; Wołejko, E.; Wydro, U.; Wasil, M.; Jabłońska-Trypuć, A. Utilization of Ashes from Biomass Combustion. Energies 2022, 15, 9653. [Google Scholar] [CrossRef]
- Hubert, J.; Grigoletto, S.; Michel, F.; Zhao, Z.; Courard, L. Development and Properties of Recycled Biomass Fly Ashes Modified Mortars. Recycling 2024, 9, 46. [Google Scholar] [CrossRef]
- Maschowski, C.; Kruspan, P.; Garra, P.; Arif, A.T.; Trouvé, G.; Gieré, R. Physicochemical and mineralogical characterization of biomass ash from different power plants in the Upper Rhine Region. Fuel 2019, 258, 116020. [Google Scholar] [CrossRef]
- Fuller, A.; Omidiji, Y.; Viefhaus, T. The impact of an additive on fly ash formation/transformation from wood dust combustion in a lab-scale pulverized fuel reactor. Renew. Energy 2019, 136, 732–745. [Google Scholar] [CrossRef]
- Szostek, M.; Szpunar-Krok, E.; Jańczak-Pieniążek, M.; Ilek, A. Short-Term Effect of Fly Ash from Biomass Combustion on Spring Rape Plants Growth, Nutrient, and Trace Elements Accumulation, and Soil Properties. Int. J. Environ. Res. Public Health 2022, 20, 455. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cooke, R.A.; Wang, L.; Ma, F.; Bhattarai, R. Characterization of fly ash ceramic pellet for phosphorus removal. J. Environ. Manag. 2017, 189, 67–74. [Google Scholar] [CrossRef]
- Sevim, Ö.; Demir, İ. Optimization of fly ash particle size distribution for cementitious systems with high compactness. Constr. Build Mater. 2019, 195, 104–114. [Google Scholar] [CrossRef]
- Hasler, P.; Nussbaumer, T. Particle Size Distribution of the Fly Ash from Biomass Combustion, Biomass for Energy and Industry. In Proceedings of the 10th European Conference and Technology Exhibition, Würzburg, Germany, 8–11 June 1998. [Google Scholar]
- Ma, J.; Wang, D.; Zhao, S.; Duan, P.; Yang, S. Influence of Particle Morphology of Ground Fly Ash on the Fluidity and Strength of Cement Paste. Materials 2021, 14, 283. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.; Jervis, W.; Fishburn, B.; Numata, T.; Joe, W.; Rawal, A.; Sorrell, C.C.; Koshy, P. Long-Term Strength Evolution in Ambient-Cured Solid-Activator Geopolymer Compositions. Minerals 2021, 11, 143. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Rao, F.; Rao, F.; Yin, W. Mechanical property and structural evolution of alkali-activated slag-phosphate mine tailings mortars. Chemosphere 2020, 251, 126367. [Google Scholar] [CrossRef]
- Moukannaa, S.; Bagheri, A.; Benzaazoua, M.; Sanjayan, J.; Pownceby, M.; Hakkou, R. Elaboration of alkali activated materials using a non-calcined red clay from phosphate mines amended with fly ash or slag: A structural study. Mater. Chem. Phys. 2020, 256, 123678. [Google Scholar] [CrossRef]
- You, D.; Fang, Y.; Zhu, C.; Gong, Y.; Gu, Y. Preparation and properties of alkali-activated cement containing phosphorous slag and fly ash. Ceram.-Silik. 2016, 60, 63–67. [Google Scholar] [CrossRef]
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Rożek, P.; Król, M.; Mozgawa, W. Spectroscopic studies of fly ash-based geopolymers, Spectrochimica. Acta Part A Mol. Biomol. Spectrosc. 2018, 198, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, C.; Zuo, L.; Wang, L.; Zhu, Q.; Wang, M. Investigation into the effect of calcium on the existence form of geopolymerized gel product of fly ash based geopolymers. Cem. Concr. Compos. 2019, 103, 279–292. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using class F fly ash elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Rüscher, C.H. Mechanical and microstructural properties of metakaolin-based geopolymer cements from sodium waterglass and phosphoric acid solution as hardeners: A comparative study. Appl. Clay Sci. 2017, 140, 81–87. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L. Designing precursors for geopolymer cements. J. Am. Ceram. Soc. 2008, 91, 3864–3869. [Google Scholar] [CrossRef]
- Cyr, M.; Pouhet, R. Carbonation in the pore solution of metakaolin-based geopolymer. Cem. Concr. Res. 2016, 88, 227–235. [Google Scholar] [CrossRef]
- Król, M.; Minkiewicz, J.; Mozgawa, W. IR spectroscopy studies of zeolites in geopolymeric materials derived from kaolinite. J. Mol. Struct. 2016, 1126, 200–206. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. Effects of Anions on the Formation of Aluminosilicate Gel in Geopolymers. Ind. Eng. Chem. Res. 2002, 41, 4550–4558. [Google Scholar] [CrossRef]
- Cuneyt, A. Amorphous Calcium Phosphate (Acp) Nanoparticles: Biomimetic Synthesis. Mater. Sci. Eng. C Mater. 2012, 32, 1097–1106. [Google Scholar] [CrossRef]
- Fauzi, A.; Nuruddin, M.F.; Malkawi, A.B.; Abdullah, M.M.A.B. Study of fly ash characterization as a cementitious material. Procedia Eng. 2016, 148, 487–493. [Google Scholar] [CrossRef]




| Mix Type | RFA (g) | BFA (g) | Alkaline Activator (g) | Sand (g) | P4O10 (g) | Water (g) |
|---|---|---|---|---|---|---|
| MFA-P0 | 1575 | 675 | 1464 | 3375 | 0 | 0 |
| MFA-P1 | 1575 | 675 | 1464 | 3375 | 22.5 | 0 |
| MFA-P3 | 1575 | 675 | 1464 | 3375 | 67.5 | 0 |
| MFA-P5 | 1575 | 675 | 1464 | 3375 | 112.5 | 0 |
| Chemical Composition (%) and Physical Characteristic | RFA | BFA |
|---|---|---|
| SiO2 | 51.73 | 50.98 |
| Al2O3 | 26.20 | 3.38 |
| Fe2O3 | 6.41 | 1.80 |
| MnO | 0.09 | 0.68 |
| MgO | 2.88 | 3.98 |
| CaO | 3.51 | 17.40 |
| Na2O | 1.18 | 0.54 |
| K2O | 2.92 | 7.96 |
| TiO2 | 0.20 | 1.05 |
| P2O5 | 0.34 | 2.43 |
| Loss On Ignition, LOI (%) | 5.50 | 7.88 |
| Total of XRF | 100.18 | 97.28 |
| ∑(SiO2 + Al2O3 + Fe2O3) | 84.34 | 56.16 |
| Real density (g/cm3) | 2.200 | 2.352 |
| Mean particle size (μm) | 54.32 | 58.50 |
| Mineral Composition (%) | ||
| Quartz | 13.4 | 38.2 |
| Mullite | 14.8 | – |
| Calcite | – | 8.9 |
| Portlandite | – | 1.4 |
| Hematite | – | 1.0 |
| Orthoclase | – | – |
| Microcline | – | – |
| Alunite | – | 2.4 |
| Anhydrite | – | 0.3 |
| Arcanite | – | 8.2 |
| Leucite | – | 1.9 |
| Archerite | – | - |
| Amor | 67.8 | 38.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prochon, P.; Piotrowski, T.; Kępniak, M. The Effects of Phosphate Compounds on the Microstructure and Mechanical Properties of Fly Ash Geopolymer Mortars. Materials 2024, 17, 5451. https://doi.org/10.3390/ma17225451
Prochon P, Piotrowski T, Kępniak M. The Effects of Phosphate Compounds on the Microstructure and Mechanical Properties of Fly Ash Geopolymer Mortars. Materials. 2024; 17(22):5451. https://doi.org/10.3390/ma17225451
Chicago/Turabian StyleProchon, Piotr, Tomasz Piotrowski, and Maja Kępniak. 2024. "The Effects of Phosphate Compounds on the Microstructure and Mechanical Properties of Fly Ash Geopolymer Mortars" Materials 17, no. 22: 5451. https://doi.org/10.3390/ma17225451
APA StyleProchon, P., Piotrowski, T., & Kępniak, M. (2024). The Effects of Phosphate Compounds on the Microstructure and Mechanical Properties of Fly Ash Geopolymer Mortars. Materials, 17(22), 5451. https://doi.org/10.3390/ma17225451









