Optimizing Suitable Mechanical Properties for a Biocompatible Beta-Titanium Alloy by Combining Plastic Deformation with Solution Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Selected for Study Alloy
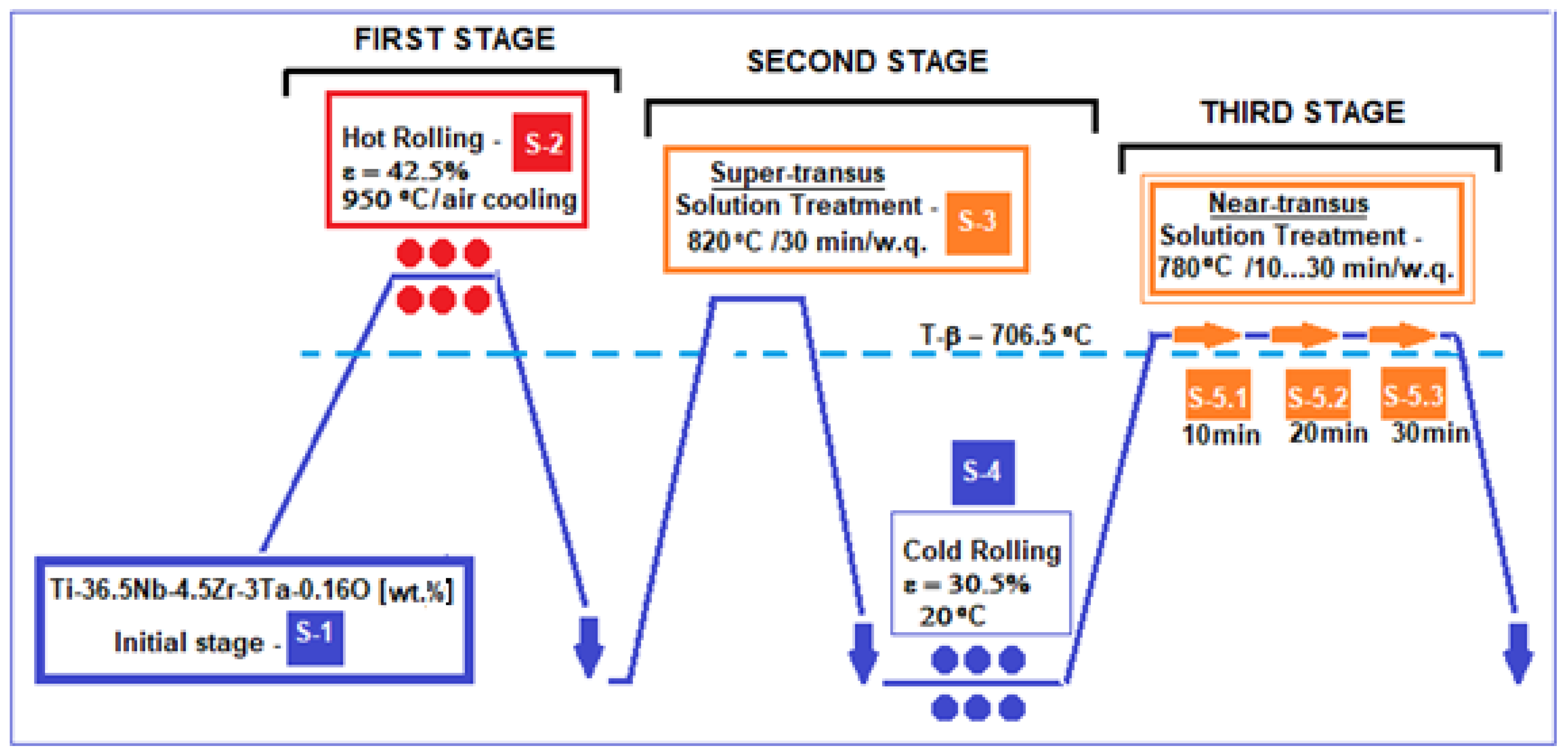
2.2. The Designing of the Thermo-Mechanical Processing Schema of the Alloy
- S-1 initial state, corresponding to the initially obtained alloy ingot, is considered to be the starting microstructure for the experimental program.
- S-2 state, which corresponding to that obtained from hot rolling (HR) at 950 °C, with a relative reduction in ε = 42.5%, followed by air cooling, and using a Mario di Maio LQR120AS rolling-mill (Mario di Maio Inc., Milan, Italy); the rolling speed was about 3 m/min. This first stage of the thermo-mechanical treatment scheme provides the highest heating temperature (950 °C) to ensure the simultaneous development of both microstructural processes: granulation reduction through plastic deformation, and recrystallization through high temperatures.
- S-3 state, corresponding to solution treatment above the β-transus temperature, therefore named a super-transus solution treatment: at 820 °C, with a holding time of 30 min (enough by calculation to solubilize the 2 × 15 × 45 mm sample), followed by water quenching (w.q.) in order to preserve the β-bcc microstructure obtained through this heat treatment. A GERO SR 100 × 500-type oven (Carbolite-Gero Inc., Neuhausen, Germany) under a high vacuum was used.
- S-4 state, corresponding to cold rolling (CR) with the relative reduction in ε = 30.5% using the same Mario di Maio LQR120AS rolling-mill as for HR. No lubricant was used. For this operation, an ultrasonic bath at 60 °C in ethylic alcohol was used for cleaning the samples.
- Obtaining samples S3 and S4 corresponded to the second stage of the experimental program, also aiming for a high reduction in the grain size like for the first stage, this time with texturing of the microstructure at the end, after the applied CR. This step involved first obtaining a homogeneous β-type microstructure (S-3), which then allows for easier cold rolling processing (S-4).
- S-5 state, corresponding to solution treatment very close to β-transus temperature, named near-transus solution treatment, at 780 °C, with three variants of holding time: S-5.1, 10 min; S-5.2, 20 min; S-5.3, 30 min. It follows water quenching (w.q.) to preserve the obtained microstructure. For this stage, the same equipment as for S-3 was used. The purpose of this third stage was to try three different holding times to see which one can achieve a more suitable microstructure with optimized mechanical properties at a temperature much closer to β-transus (780 °C) to save energy.
2.3. Analysis of the Alloy Microstructure and of Mechanical Properties
3. Results
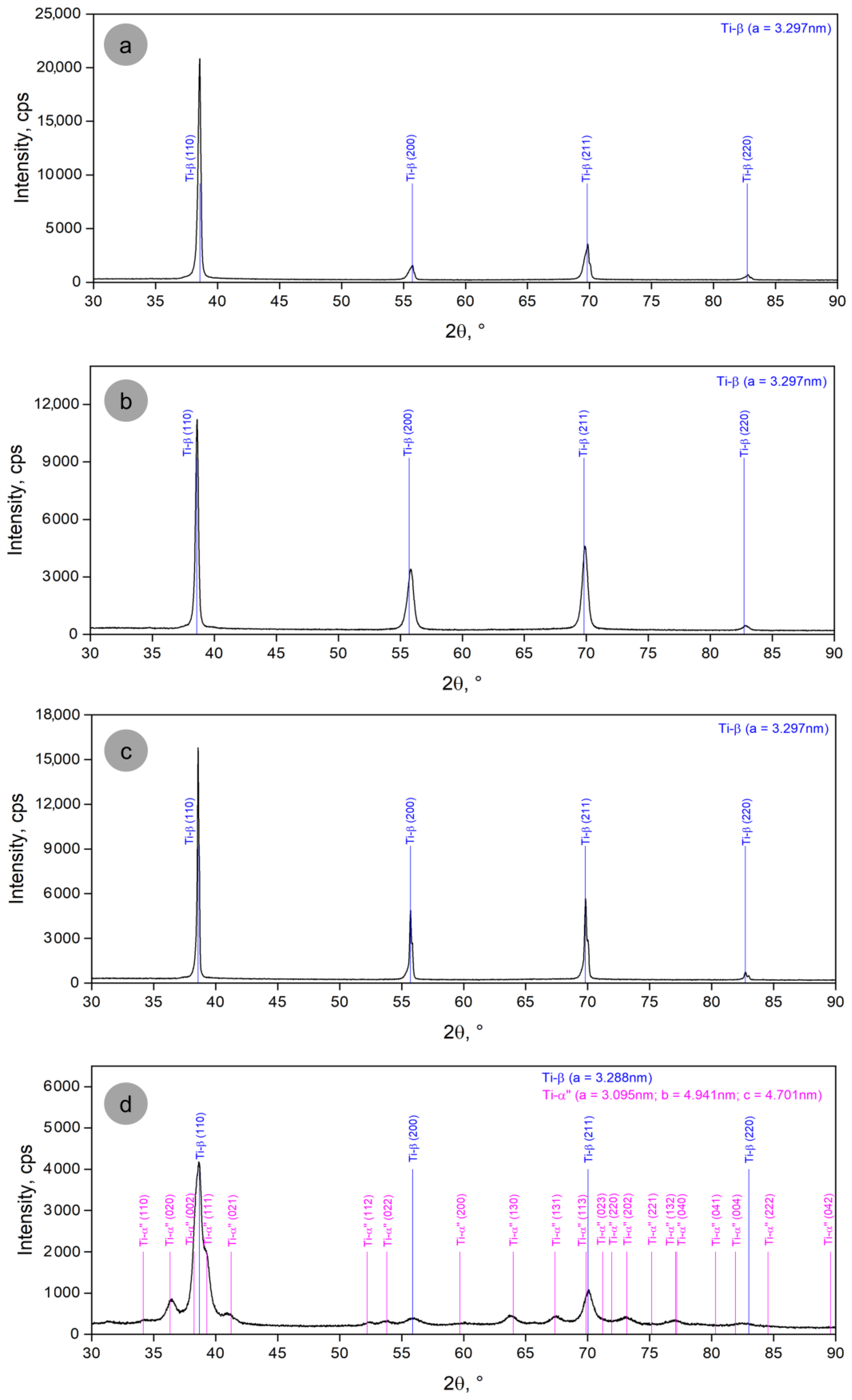
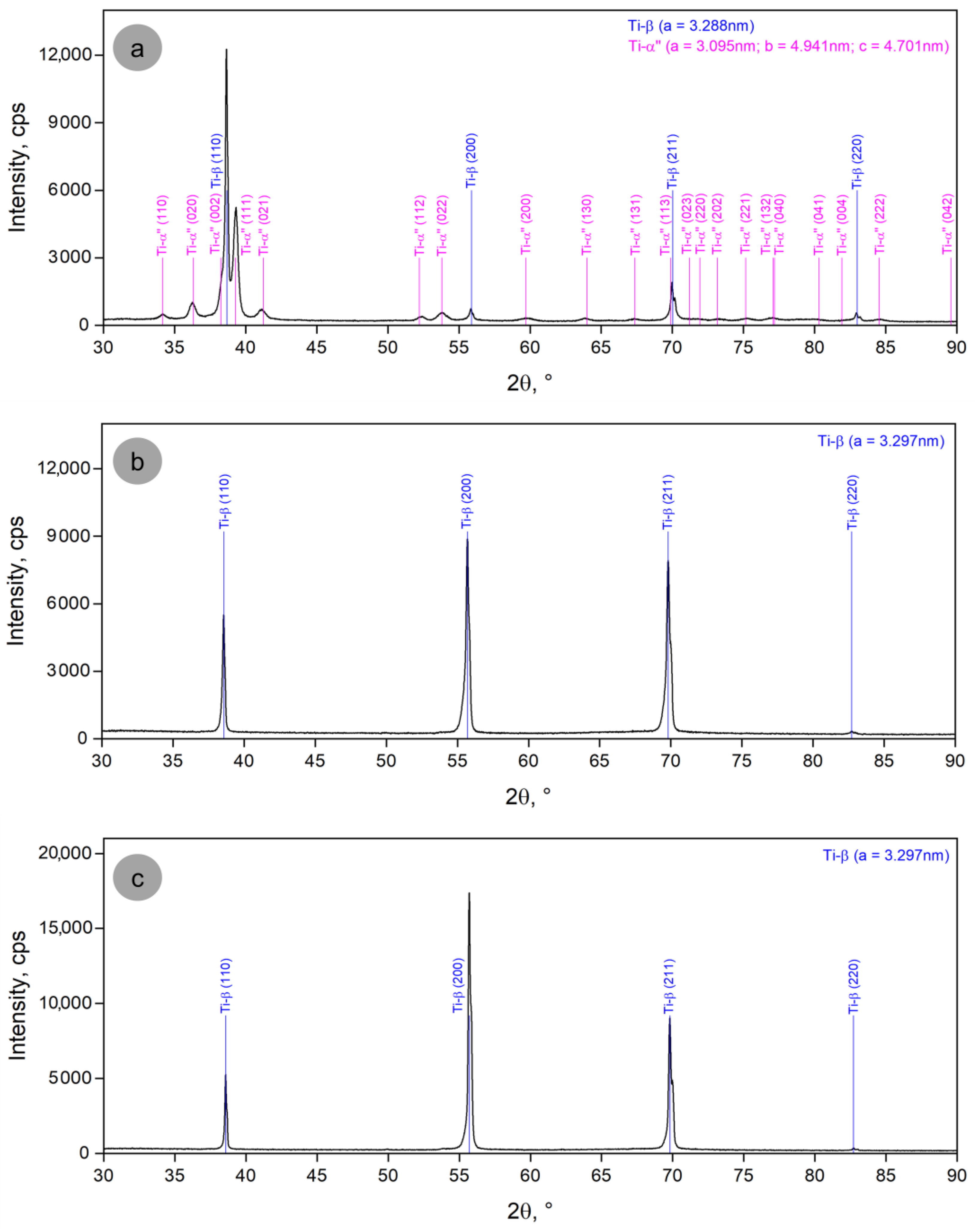
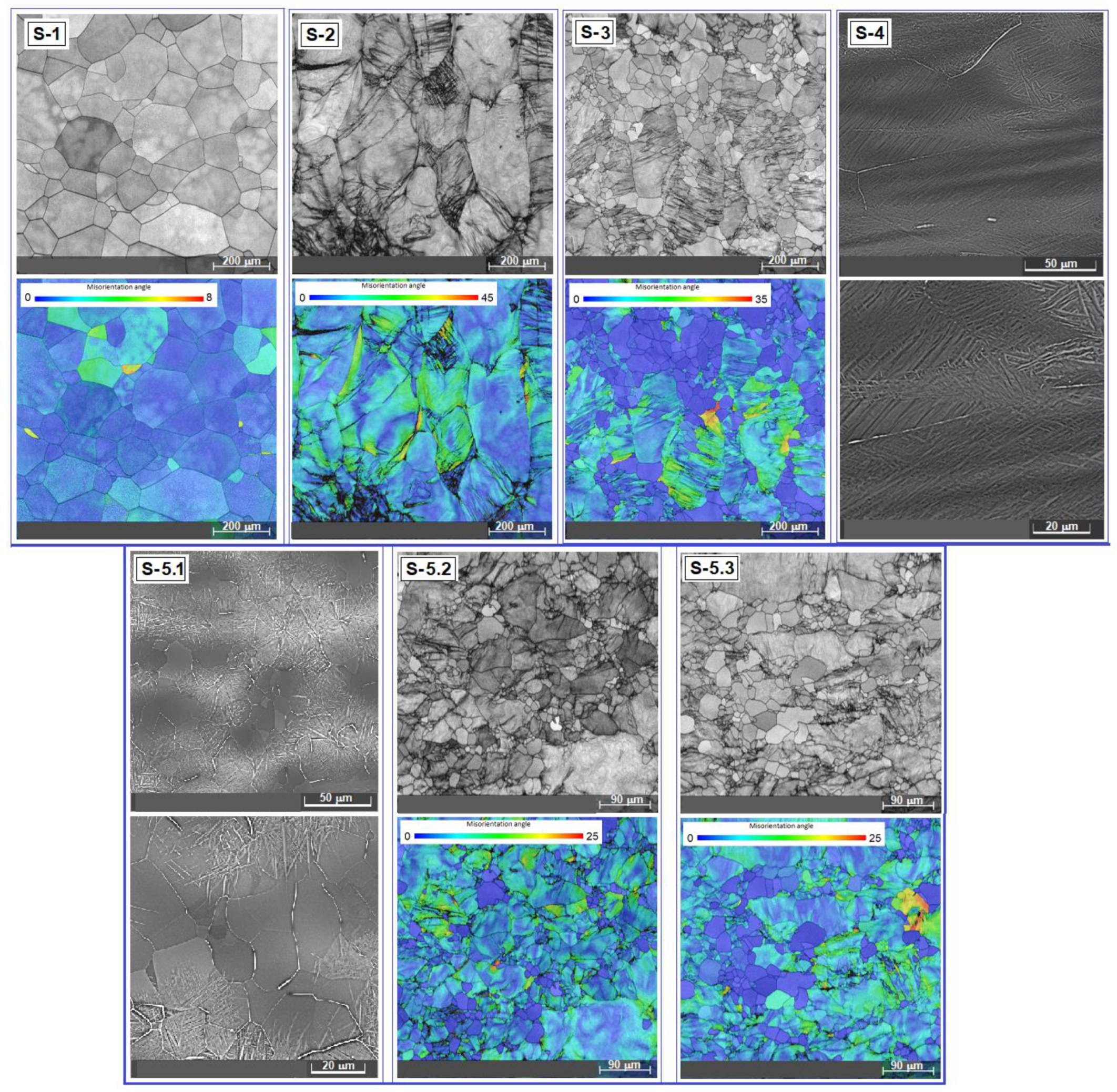
3.1. Analysis of the Alloy Microstructure Evolution
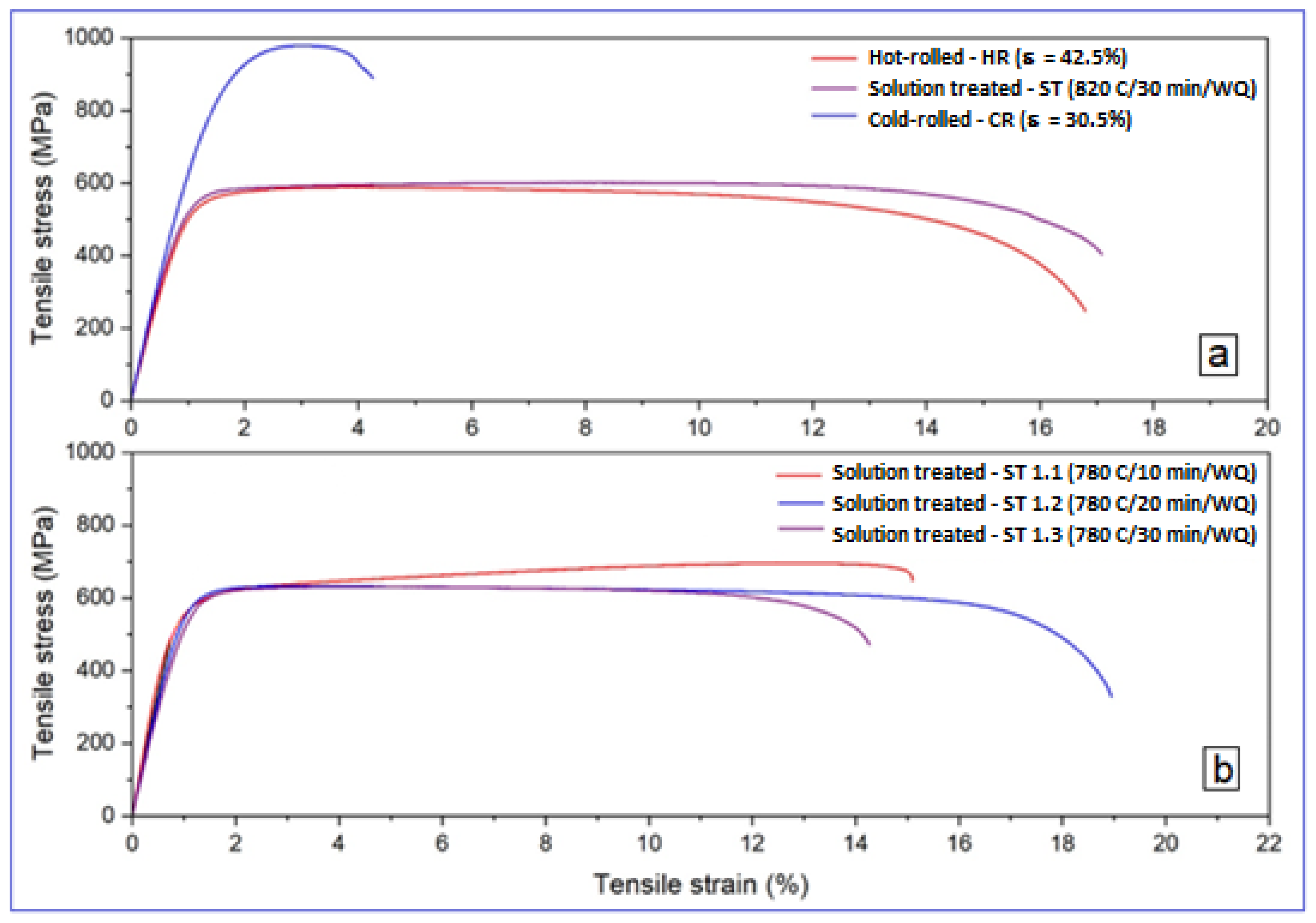
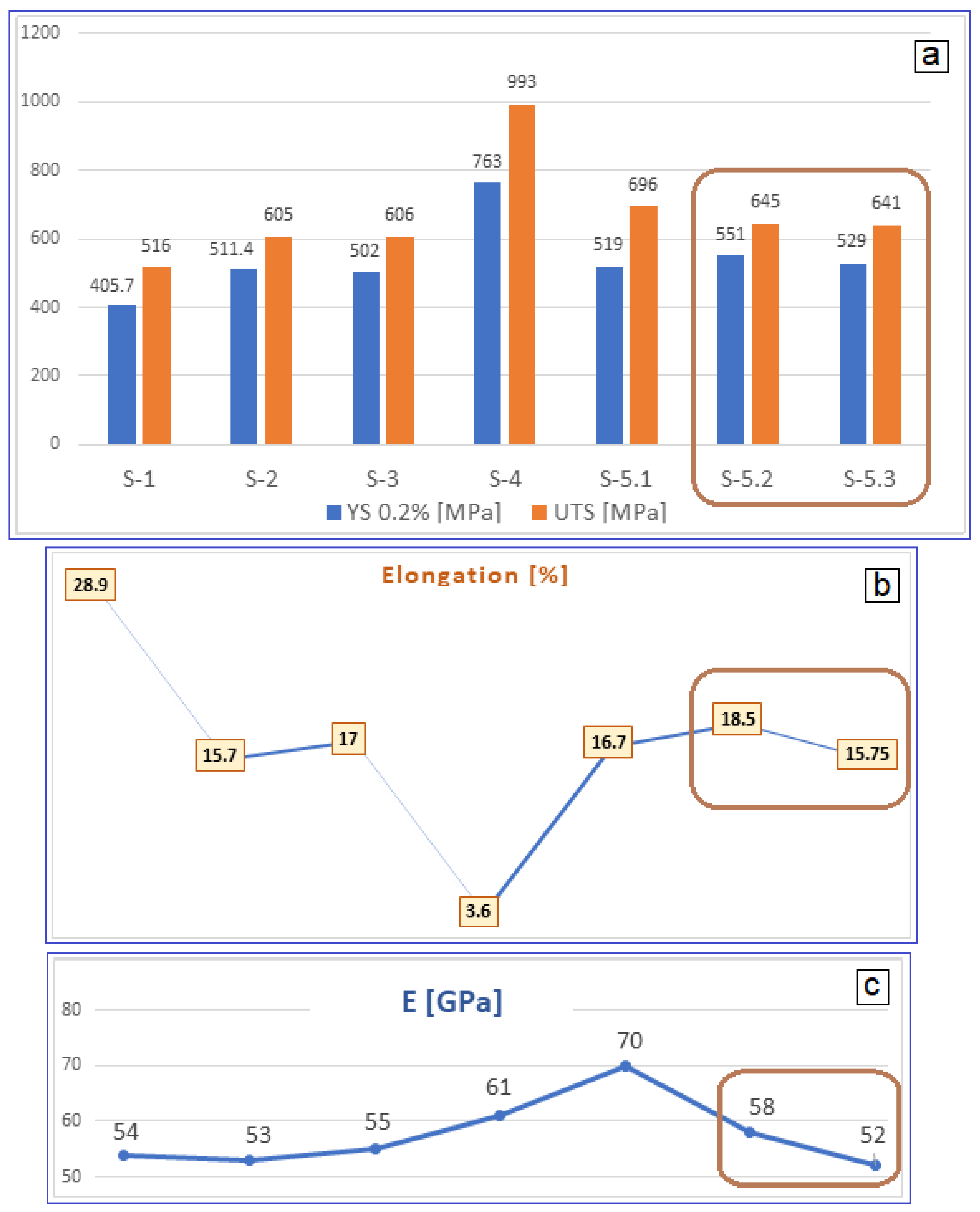
3.2. Analysis of the Alloy Mechanical Property Evolution
- (a)
- UTS = 500 MPa; E = 63 GPa for the Ti-35.3Nb-7.1Zr-5.1Ta wt.% [53];
- (b)
- UTS = 500 MPa; E = 65 GPa for the Ti-41.1Nb-7.1Zr wt.% [4];
- (c)
- UTS = 641 MPa; E = 52 GPa for the Ti-36.5Nb-4.5Zr-3Ta-0.16O wt.% [present case];
- (d)
- UTS = 755 MPa; for the Ti-39Nb-6Zr-0.26O wt.% [19];
- (e)
- UTS = 851 MPa; E = 60 GPa for the Ti-25Nb-17Ta-1Fe-0.25O wt.% [5].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.-Y.; Cui, Y.-W.; Zhang, L.-C. Recent Development in Beta Titanium Alloys for Biomedical Applications. Metals 2020, 10, 1139. [Google Scholar] [CrossRef]
- Anene, F.A.; Jaafar, C.N.A.; Zainol, I.; Hanim, M.A.A.; Suraya, M.T. Biomedical materials: A review of titanium based alloys. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2020, 235, 3792–3805. [Google Scholar] [CrossRef]
- Nomura, Y.; Okada, M.; Manaka, T.; Tsuchiya, T.; Iwasaki, M.; Matsuda, K.; Ishimoto, T. Effect of Partial Substitution of Zr for Ti Solvent on Young’s Modulus, Strength, and Biocompatibility in Beta Ti Alloy. Materials 2024, 17, 2548. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K. Review on Titanium and Titanium Based Alloys as Biomaterials for Orthopaedic Applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, L.Y. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef]
- Jimenez-Marcos, C.; Mirza-Rosca, J.C.; Baltatu, M.S.; Vizureanu, P. Experimental Research on New Developed Titanium Alloys for Biomedical Applications. Bioengineering 2022, 9, 686. [Google Scholar] [CrossRef]
- Intravaia, J.T.; Graham, T.; Kim, H.S.; Nanda, H.S.; Kumbar, S.G.; Nukavarapu, S.P. Smart orthopedic biomaterials and implants. Curr. Opin. Biomed. Eng. 2023, 25, 100439. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A. Biomedical Applications of Titanium Alloys: A Comprehensive Review. Materials 2024, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Walunj, G.; Desai, J.; Bohara, S.; Contieri, R.; Kothapalli, C.; Ivanov, E.; Borkar, T. Light Weight-Low Modulus Biocompatible Titanium Alloys Processed via Spark Plasma Sintering. J. Alloys Metall. Syst. 2023, 3, 100018. [Google Scholar] [CrossRef]
- Marković, G.; Manojlović, V.; Ružić, J.; Sokić, M. Predicting Low-Modulus Biocompatible Titanium Alloys Using Machine Learning. Materials 2023, 16, 6355. [Google Scholar] [CrossRef] [PubMed]
- Spataru, M.C.; Butnaru, M.; Sandu, A.V.; Vulpe, V.; Vlad, M.D.; Baltatu, M.S.; Geanta, V.; Voiculescu, I.; Vizureanu, P.; Solcan, C. In-depth assessment of new Ti-based biocompatible materials. Mater. Chem. Phys. 2021, 258, 123959. [Google Scholar] [CrossRef]
- Bergmann, G.; Bender, A.; Graichen, F.; Dymke, J.; Rohlmann, A.; Trepczynski, A.; Heller, M.O.; Kutzner, I. Standardized Loads Acting in Knee Implants. PLoS ONE 2014, 9, e86035. [Google Scholar] [CrossRef] [PubMed]
- Pesode, P.; Barve, S. A Review—Metastable β Titanium Alloy for Biomedical Applications. J. Eng. Appl. Sci. 2023, 70, 25. [Google Scholar] [CrossRef]
- Florea, A.; Stancel, C.D.; Butu, M.; Buzatu, M.; Serban, N.; Ursu, V. Improvement of Mechanical Properties for some Ti-Mo-Fe Alloys by Heat Treatment. U.P.B. Sci. Bull. Ser. B 2024, 86, 325–336. [Google Scholar]
- Cojocaru, V.D.; Dan, A.; Șerban, N.; Cojocaru, E.M.; Zărnescu-Ivan, N.; Gălbinașu, B.M. Effect of Cold-Rolling Deformation on the Microstructural and Mechanical Properties of a Biocompatible Ti-Nb-Zr-Ta-Sn-Fe Alloy. Materials 2024, 17, 2312. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Q.; Yu, Z.; Ren, L.; Zhao, X.; Wang, J. Study on Osseointegration Capability of β-Type Ti–Nb–Zr–Ta–Si Alloy for Orthopedic Implants. Materials 2024, 17, 472. [Google Scholar] [CrossRef]
- Ishimoto, T.; Ozasa, R.; Nakano, K.; Weinmann, M.; Schnitter, C.; Stenzel, M.; Matsugaki, A.; Nagase, T.; Matsuzaka, T.; Todai, M.; et al. Development of TiNbTaZrMo bio-high entropy alloy (BioHEA) super-solid solution by selective laser melting, and its improved mechanical property and biocompatibility. Scr. Mater. 2021, 194, 113658. [Google Scholar] [CrossRef]
- Gurau, C.; Gurau, G.; Mitran, V.; Dan, A.; Cimpean, A. The Influence of Severe Plastic Deformation on Microstructure and In Vitro Biocompatibility of the New Ti-Nb-Zr-Ta-Fe-O Alloy Composition. Materials 2020, 13, 4853. [Google Scholar] [CrossRef]
- Han, C.-B.; Lee, D.-G. Effect of Oxygen on Static Recrystallization Behaviors of Biomedical Ti-Nb-Zr Alloys. Metals 2024, 14, 333. [Google Scholar] [CrossRef]
- Slokar, L.; Štrkalj, A.; Glavaš, Z. Synthesis of Ti-Zr alloy by powder metallurgy. Eng. Rev. 2019, 39, 115–123. [Google Scholar] [CrossRef]
- Medvedev, A.E.; Molotnikov, A.; Lapovok, R.; Zeller, R.; Berner, S.; Habersetzer, P.; Torre, F.D. Microstructure and mechanical properties of Ti–15Zr alloy used as dental implant material. J. Mech. Behav. Biomed. Mater. 2016, 62, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Grandin, H.M.; Berner, S.; Dard, M. A review of Titanium Zirconium (TiZr) alloys for use in endosseous dental implants. Materials 2012, 5, 1348–1360. [Google Scholar] [CrossRef]
- Pang, E.L.; Pickering, E.J.; Baik, S.I.; Seidman, D.N.; Jones, N.G. The effect of zirconium on the omega phase in Ti-24Nb-[0–8]Zr (at.%) alloys. Acta Mater. 2018, 153, 62–70. [Google Scholar] [CrossRef]
- Niinomi, M.; Liu, Y.; Nakai, M.; Liu, H.; Li, H. Biomedical titanium alloys with Young’s moduli close to that of cortical bone. Regen. Biomater. 2016, 3, 173–185. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Kozlík, J.; Preisler, D.; Stráský, J.; Veselý, J.; Veverková, A.; Chráska, T.; Janeček, M. Phase transformations in a heterogeneous Ti-xNb-7Zr-0.8O alloy prepared by a field-assisted sintering technique. Mater. Des. 2021, 198, 109308. [Google Scholar] [CrossRef]
- Sochacka, P.; Jurczyk, M.U.; Kowalski, K.; Wirstlein, P.K.; Jurczyk, M. Ultrafine-Grained Ti-31Mo-Type Composites with HA and Ag, Ta2O5 or CeO2 Addition for Implant Applications. Materials 2021, 14, 644. [Google Scholar] [CrossRef]
- Strasky, J.; Harcuba, P.; Vaclavova, K.; Horvath, K.; Landa, M.; Srba, O.; Janecek, M. Increasing strength of a biomedical Ti-Nb-Ta-Zr alloy by alloying with Fe, Si and O. J. Mech. Behav. Biomed. Mater. 2017, 71, 329–336. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, J. The quantitative understanding on the influence of α″ phase on mechanical behavior of Ti-Nb-Ta-Zr-O alloy. J. Alloys Compd. 2018, 768, 914–923. [Google Scholar] [CrossRef]
- Acharya, S.; Panicker, A.G.; Laxmi, D.V.; Suwas, S.; Chatterjee, K. Study of the influence of Zr on the mechanical properties and functional response of Ti-Nb-Ta-Zr-O alloy for orthopedic applications. Mater. Des. 2019, 164, 107555. [Google Scholar] [CrossRef]
- Yokota, K.; Bahador, A.; Shitara, K.; Umeda, J.; Kondoh, K. Mechanisms of tensile strengthening and oxygen solid solution in single β-phase Ti-35 at.%Ta+O alloys. Mater. Sci. Eng. A 2021, 802, 140677. [Google Scholar] [CrossRef]
- Stráský, J.; Janeček, M.; Harcuba, P.; Preisler, D.; Landa, M. 4.2—Biocompatible beta-Ti alloys with enhanced strength due to increased oxygen content. In Titanium in Medical and Dental Applications; Froes, F.H., Qian, M., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 371–392. [Google Scholar] [CrossRef]
- Preisler, D. Oxygen-Strengthened Biomedical Beta Titanium Alloys. Master’s Thesis, Charles University, Prague, Czech Republic, 2018. Available online: https://hdl.handle.net/20.500.11956/98671 (accessed on 24 September 2024).
- Hussein, A.H.; Gepreel, M.A.H.; Gouda, M.K.; Hefnawy, A.M.; Kandil, S.H. Biocompatibility of new Ti–Nb–Ta base alloys. Mater. Sci. Eng. C 2016, 61, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Nocivin, A.; Raducanu, D.; Vasile, B.; Trisca-Rusu, C.; Cojocaru, E.M.; Dan, A.; Irimescu, R.; Cojocaru, V.D. Tailoring a Low Young Modulus for a Beta Titanium Alloy by Combining Severe Plastic Deformation with Solution Treatment. Materials 2021, 14, 3467. [Google Scholar] [CrossRef]
- Silva, M.R.D.; Gargarella, P.; Plaine, A.H.; Pauly, S.; Bolfarini, C. Influence of oxygen and plastic deformation on the microstructure and the hardness of a Ti–Nb–Ta–Zr–O Gum Metal. Mater. Sci. Eng. A 2021, 828, 142122. [Google Scholar] [CrossRef]
- Ji, X.; Gutierrez-Urrutia, I.; Emura, S.; Tsuchiya, K. Deformation mechanisms and effect of oxygen addition on mechanical properties of Ti-7.5Mo alloy with α” martensite. MATEC Web Conf. 2020, 321, 11059. [Google Scholar] [CrossRef]
- Ishiguro, Y.; Tsukada, Y.; Koyama, T. Phase-field study of the spinodal decomposition rate of β phase in oxygen added Ti–Nb alloys. Comput. Mater. Sci. 2020, 174, 109471. [Google Scholar] [CrossRef]
- Sun, S.Y.; Deng, C. Accurate calculation of (α+β)/β phase transition of titanium alloys based on binary phase diagrams. Titan. Ind. Prog. 2011, 28, 21–25. [Google Scholar]
- Bania, P.J. Beta titanium alloys and their role in the titanium industry. JOM 1994, 46, 16–19. [Google Scholar] [CrossRef]
- Kolli, R.; Devaraj, A. A review of metastable beta titanium alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef]
- Li, P.; Ma, X.; Wang, D.; Zhang, H. Microstructural and Mechanical Properties of β-Type Ti–Nb–Sn Biomedical Alloys with Low Elastic Modulus. Metals 2019, 9, 712. [Google Scholar] [CrossRef]
- Soundararajan, S.R.; Vishnu, J.; Manivasagam, G.; Muktinutalapati, N.R. Heat Treatment of Metastable Beta Titanium Alloys. In Welding—Modern Topics; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/chapters/72078 (accessed on 26 September 2024).
- Santhosh, R.; Geetha, M.; Nageswara Rao, M. Recent developments in heat treatment of Beta titanium alloys for aerospace applications. Trans. Indian Inst. Met. 2016, 70, 1681–1688. [Google Scholar] [CrossRef]
- Cullity, B.D. Stock, Elements of X-Ray Diffraction, 3rd ed.; Pearson: London, UK, 2001. [Google Scholar]
- Yuan, Y.; Wu, Y.; Yang, Z.; Liang, X.; Lei, Z.; Huang, H.; Wang, H.; Liu, X.; An, K.; Wu, W.; et al. Formation, structure and properties of biocompatible TiZrHfNbTa high-entropy alloys. Mater. Res. Lett. 2019, 7, 225–231. [Google Scholar] [CrossRef]
- Zhang, L.; Xiang, Y.; Han, J.; Srolovitz, D.J. The effect of randomness on the strength of high-entropy alloys. Acta Mater. 2019, 166, 424–434. [Google Scholar] [CrossRef]
- Neelakantan, S.; Rivera-Diaz-del-Castillo, R.; van der Zwaag, S. Prediction of the martensite start temperature for beta titanium alloys as a function of composition. Scr. Mater. 2009, 60, 611–614. [Google Scholar] [CrossRef]
- Tane, M.; Akita, S.; Nakano, T.; Hagihara, K.; Umakoshi, Y.; Niinomi, M.; Mori, H.; Nakajima, H. Low Young’s modulus of Ti–Nb–Ta–Zr alloys caused by softening in shear moduli c0 and c44 near lower limit of body-centered cubic phase stability. Acta Mater. 2010, 58, 6790–6798. [Google Scholar] [CrossRef]
- You, L.; Song, X. A study of low Young’s modulus Ti-Nb-Zr alloys using d electrons alloy theory. Scr. Mater. 2012, 67, 57–60. [Google Scholar] [CrossRef]
- Raabe, D.; Sander, B.; Friak, M.; Ma, D.; Neugebauer, J. Theory-guided bottom-up design of β-titanium alloys as biomaterials based on first principles calculations: Theory and experiments. Acta Mater. 2007, 55, 4475–4487. [Google Scholar] [CrossRef]
- Lee, T.; Lee, S.; Kim, I.S.; Moon, Y.H.; Kim, H.S.; Park, C.H. Breaking the limit of Young’s modulus in low-cost Ti–Nb–Zr alloy for biomedical implant applications. J. Alloys Compd. 2020, 828, 154401. [Google Scholar] [CrossRef]
- Bahl, S.; Suwas, S.; Chatterjee, K. Comprehensive review on alloy design, processing, and performance of β Titanium alloys as biomedical materials. Int. Mater. Rev. 2020, 66, 114–139. [Google Scholar] [CrossRef]
- Wei, L.S.; Kim, H.Y.; Miyazaki, S. Effects of oxygen concentration and phase stability on nano-domain structure and thermal expansion behavior of Ti–Nb–Zr–Ta–O alloys. Acta Mater. 2015, 100, 313. [Google Scholar] [CrossRef]
- Nakai, M.; Niinomi, M.; Akahori, T.; Tsutsumi, H.; Ogawa, M. Effect of Oxygen Content on Microstructure and Mechanical Properties of Biomedical Ti-29Nb-13Ta-4.6Zr Alloy under Solutionized and Aged Conditions. Mater. Trans. 2009, 50, 2716–2720. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hendrickson, M.; Nandwana, P.; Alam, T.; Choudhuri, D.; Banerjee, R. Influence of oxygen on omega phase stability in the Ti-29Nb-13Ta-4.6Zr alloy. Scr. Mater. 2016, 123, 144. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, H.Y.; Hosoda, H.; Miyazaki, S. Shape Memory Behavior of Ti-22Nb-(0.5–2.0) O(at%). Mater. Trans. 2005, 46, 852. [Google Scholar] [CrossRef]
| Alloying Element | T(βi) [°C] | Applicable Concentration Range [%] |
|---|---|---|
| Niobium | −12.1312 × [Nb] + 0.08178 × [Nb]2 − 0.000334771 × [Nb]3 | 0–40 |
| Tantalum | −7.4877 × [Ta] + 0.13494 × [Ta]2 − 0.00175 × [Ta]3 | 0–30 |
| Zirconium | −3.53793 × [Zr] − 0.04004 × [Zr]2 − 0.00037309 × [Zr]3 | 0–40 |
| Oxygen | 134.88076 × [O] + 21.00293 × [O]2 + 8.39629 × [O]3 | 0–1 |
| Sample | UTS [MPa] | YS [MPa] | E [GPa] | ε [%] | |
|---|---|---|---|---|---|
| S-1 | Test 1 | 537.16 | 412.54 | 55.17 | 35.48 |
| Test 2 | 495.40 | 398.94 | 53.51 | 22.46 | |
| Average | 516.28 ± 20.88 | 405.74 ± 6.80 | 54.34 ± 0.83 | 28.97 ± 6.51 | |
| S-2 | Test 1 | 588.09 | 498.31 | 53.30 | 16.79 |
| Test 2 | 622.26 | 524.50 | 54.23 | 14.61 | |
| Average | 605.18 ± 17.09 | 511.41 ± 13.10 | 53.77 ± 0.47 | 15.70 ± 1.09 | |
| S-3 | Test 1 | 600.51 | 499.86 | 56.39 | 17.08 |
| Test 2 | 611.19 | 504.13 | 53.62 | 16.99 | |
| Average | 605.85 ± 5.34 | 502.00 ± 2.13 | 55.01 ± 1.39 | 17.04 ± 0.04 | |
| S-4 | Test 1 | 978.65 | 731.13 | 59.21 | 4.25 |
| Test 2 | 1.009.20 | 796.57 | 63.84 | 3.03 | |
| Average | 993.93 ± 15.28 | 763.85 ± 32.72 | 61.53 ± 2.32 | 3.64 ± 0.61 | |
| S-5.1 | Test 1 | 694.72 | 517.46 | 69.81 | 15.10 |
| Test 2 | 697.27 | 521.56 | 70.35 | 18.32 | |
| Average | 696.00 ± 1.27 | 519.51 ± 2.05 | 70.08 ± 0.27 | 16.71 ± 1.61 | |
| S-5.2 | Test 1 | 632.02 | 541.97 | 57.41 | 18.94 |
| Test 2 | 657.34 | 561.17 | 58.65 | 18.04 | |
| Average | 644.68 ± 12.66 | 551.57 ± 9.60 | 58.03 ± 0.62 | 18.49 ± 0.45 | |
| S-5.3 | Test 1 | 629.32 | 525.56 | 52.08 | 14.25 |
| Test 2 | 652.50 | 532.34 | 53.39 | 17.25 | |
| Average | 640.91 ± 11.59 | 528.95 ± 3.39 | 52.74 ± 0.66 | 15.75 ± 1.50 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irimescu, R.E.; Raducanu, D.; Nocivin, A.; Cojocaru, E.M.; Cojocaru, V.D.; Zarnescu-Ivan, N. Optimizing Suitable Mechanical Properties for a Biocompatible Beta-Titanium Alloy by Combining Plastic Deformation with Solution Treatment. Materials 2024, 17, 5828. https://doi.org/10.3390/ma17235828
Irimescu RE, Raducanu D, Nocivin A, Cojocaru EM, Cojocaru VD, Zarnescu-Ivan N. Optimizing Suitable Mechanical Properties for a Biocompatible Beta-Titanium Alloy by Combining Plastic Deformation with Solution Treatment. Materials. 2024; 17(23):5828. https://doi.org/10.3390/ma17235828
Chicago/Turabian StyleIrimescu, Raluca Elena, Doina Raducanu, Anna Nocivin, Elisabeta Mirela Cojocaru, Vasile Danut Cojocaru, and Nicoleta Zarnescu-Ivan. 2024. "Optimizing Suitable Mechanical Properties for a Biocompatible Beta-Titanium Alloy by Combining Plastic Deformation with Solution Treatment" Materials 17, no. 23: 5828. https://doi.org/10.3390/ma17235828
APA StyleIrimescu, R. E., Raducanu, D., Nocivin, A., Cojocaru, E. M., Cojocaru, V. D., & Zarnescu-Ivan, N. (2024). Optimizing Suitable Mechanical Properties for a Biocompatible Beta-Titanium Alloy by Combining Plastic Deformation with Solution Treatment. Materials, 17(23), 5828. https://doi.org/10.3390/ma17235828






