Corrosion Tendency of S235 Steel in 3.5% NaCl Solution and Drinking Water During Six Months of Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Ex Situ Characterizations
2.2. In Situ Characterizations
2.3. Using Physico-Chemical Parameters to Monitor Corrosive Solution During Corrosion Tests
3. Results and Discussion
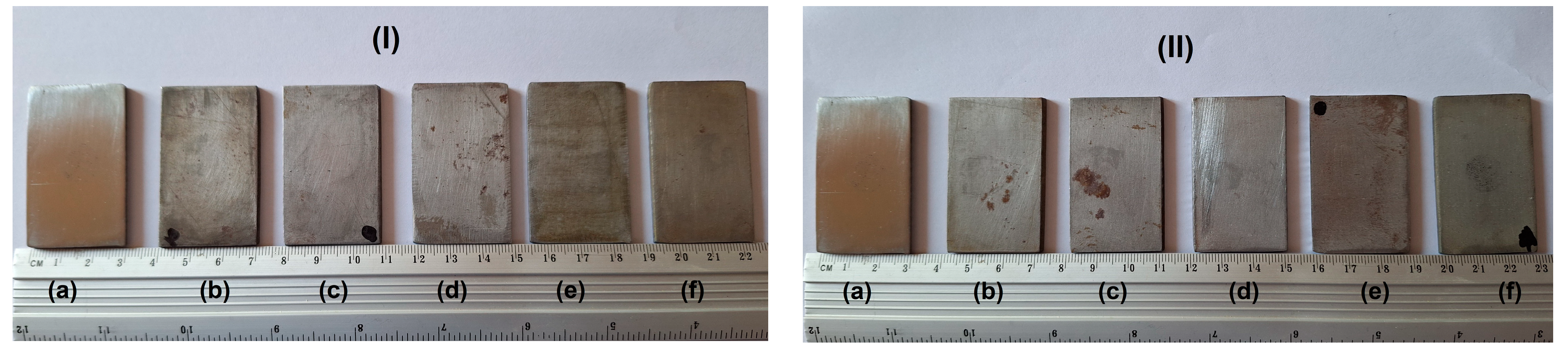
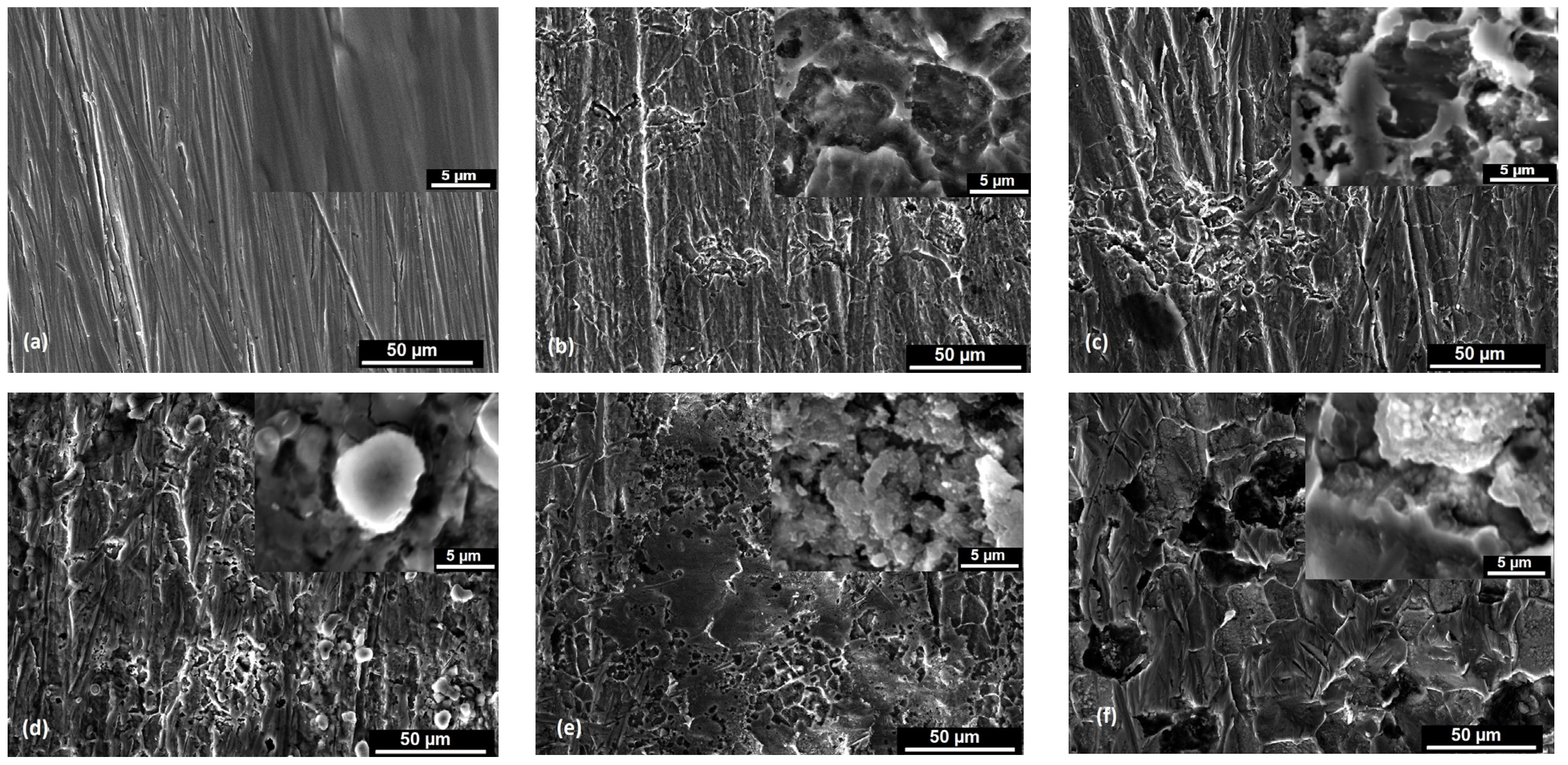
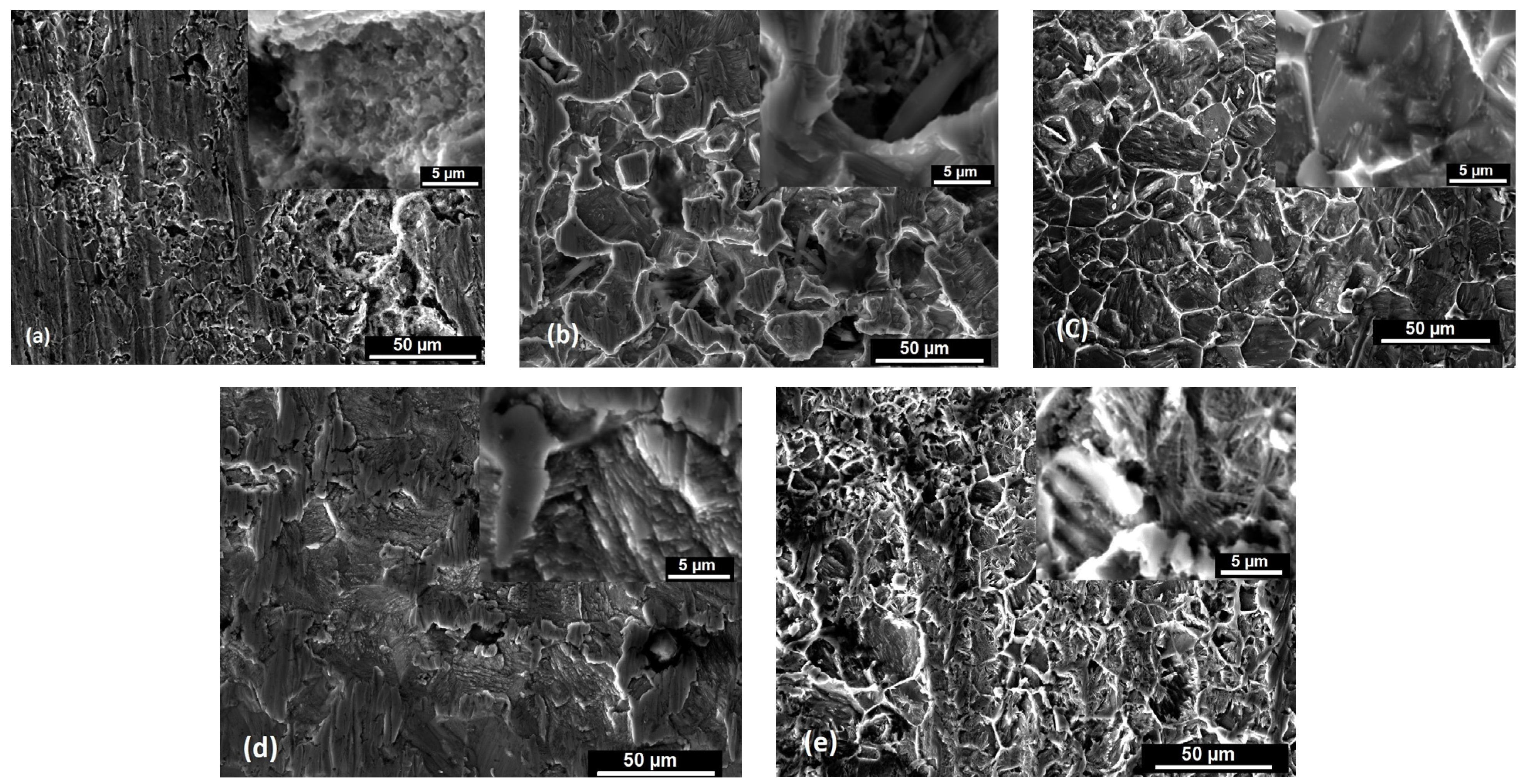
3.1. Morphological Aspects
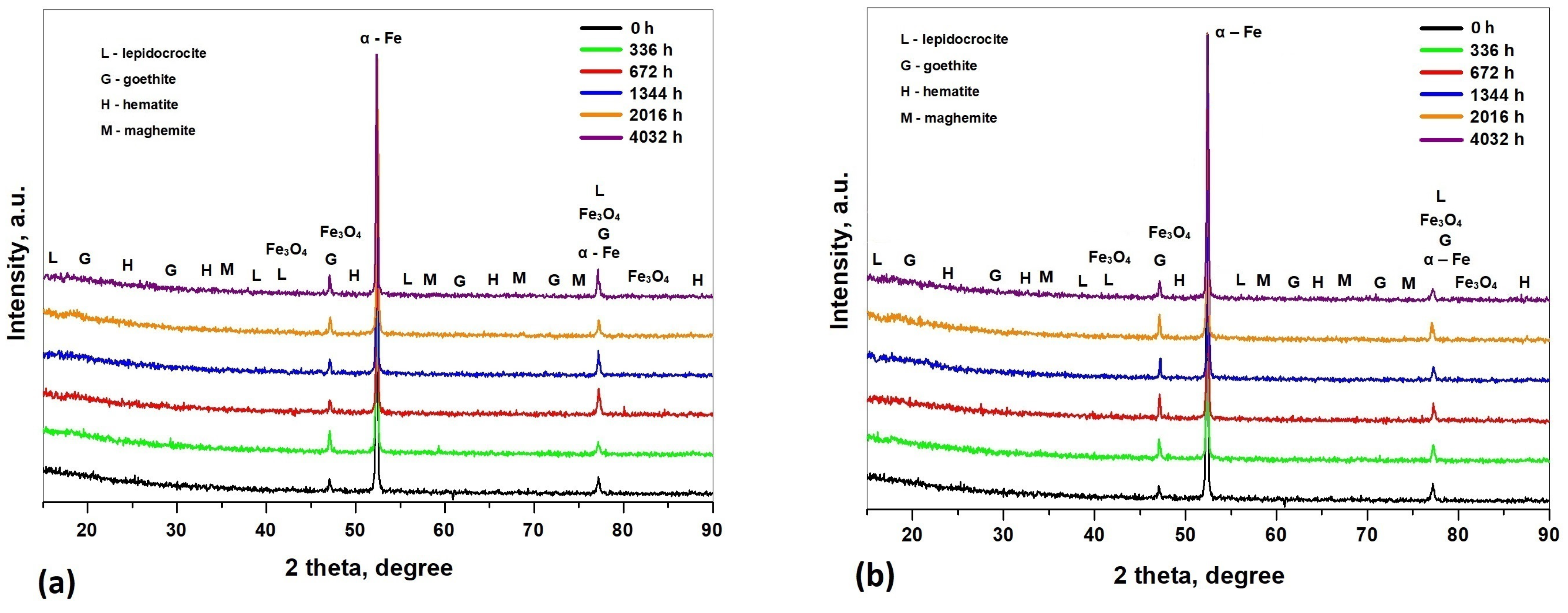
3.2. Analysis of Corrosion Products
3.3. Mechanical Properties
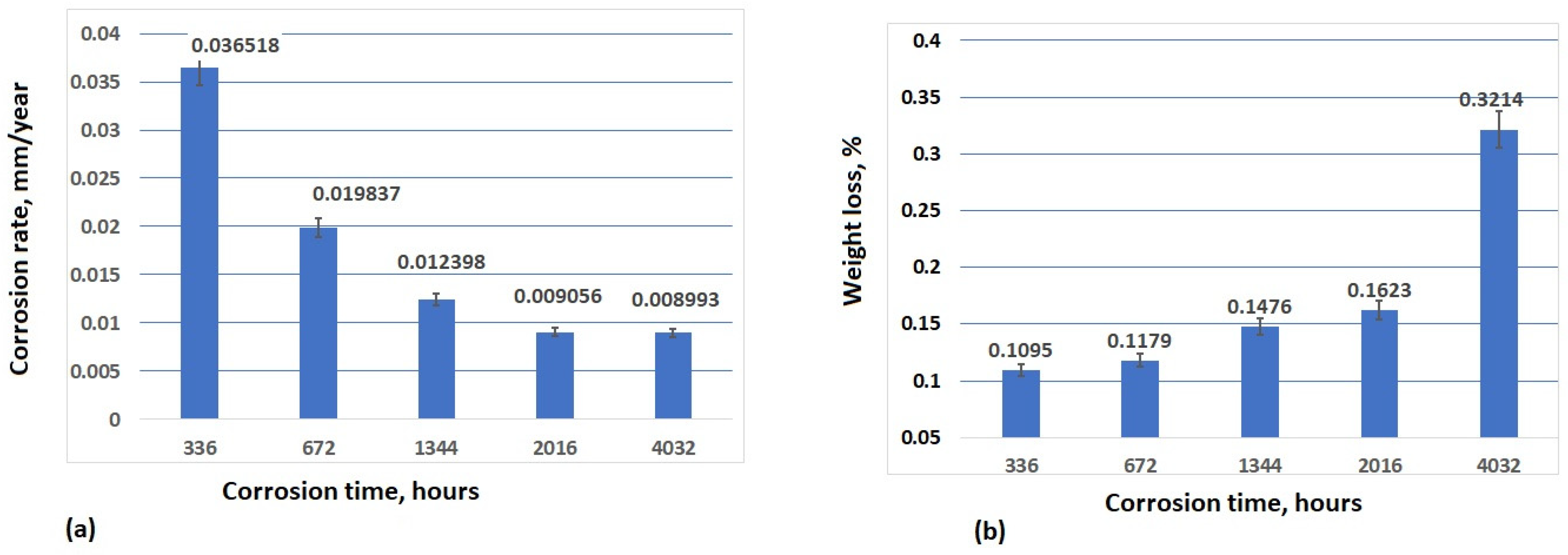
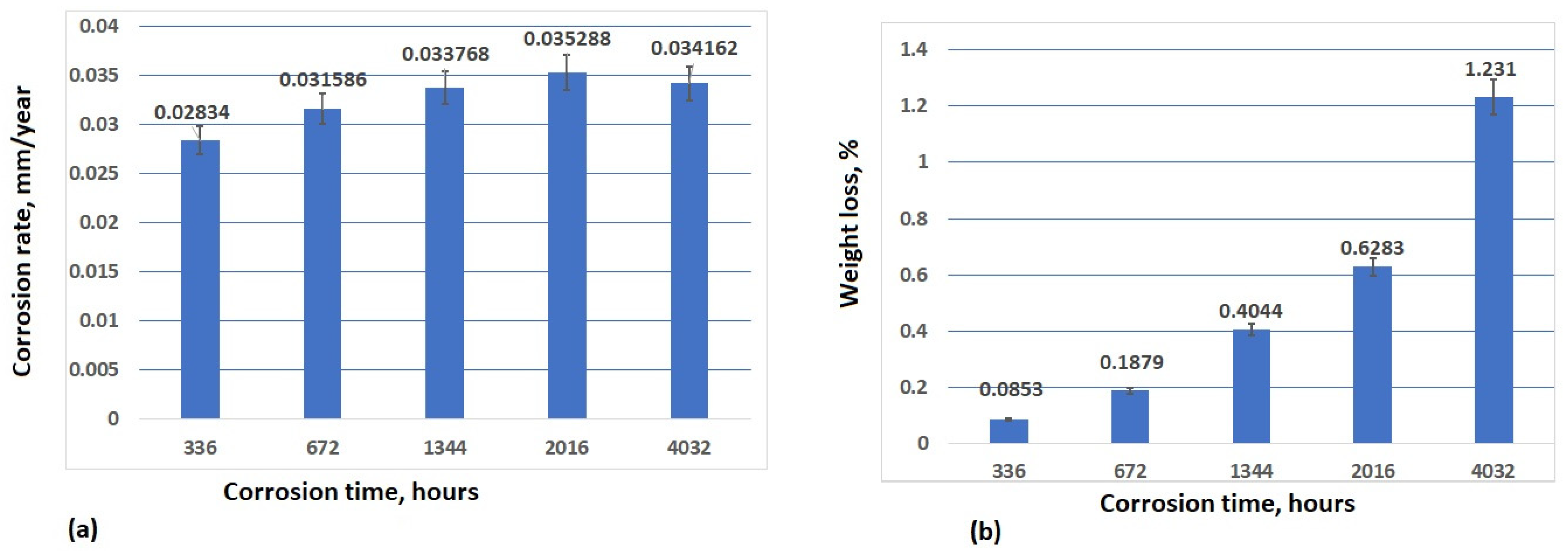
3.4. Analysis of Corrosion Weight Loss
3.5. Electrochemical Measurements
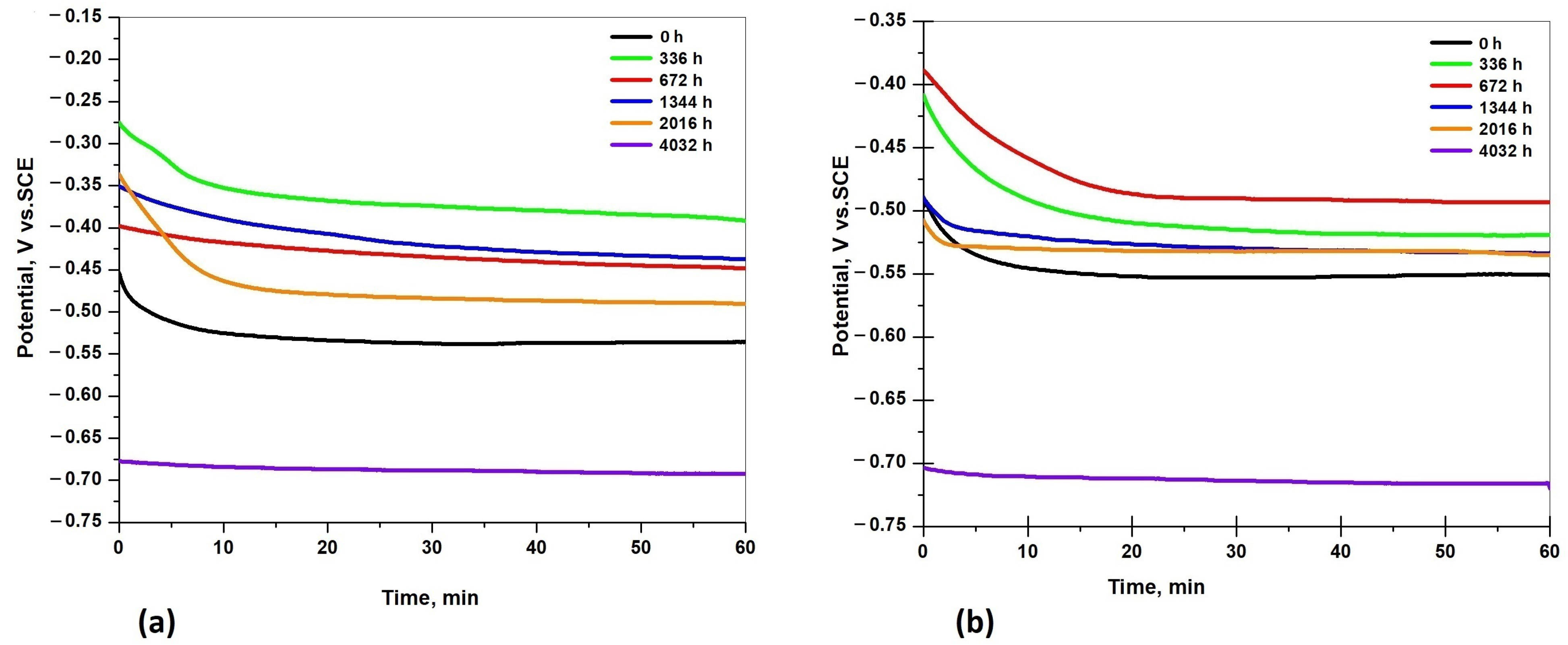
3.5.1. Open Circuit Potential
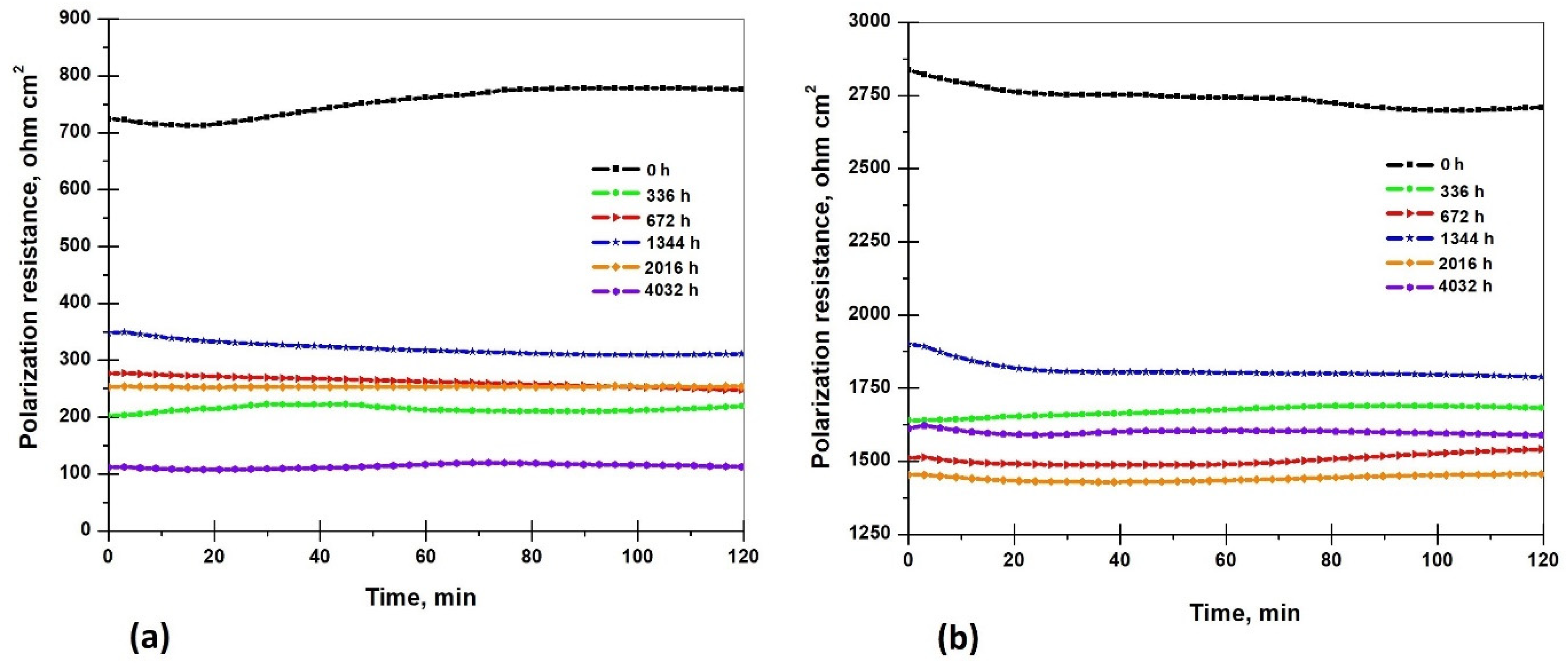
3.5.2. Polarization Resistance
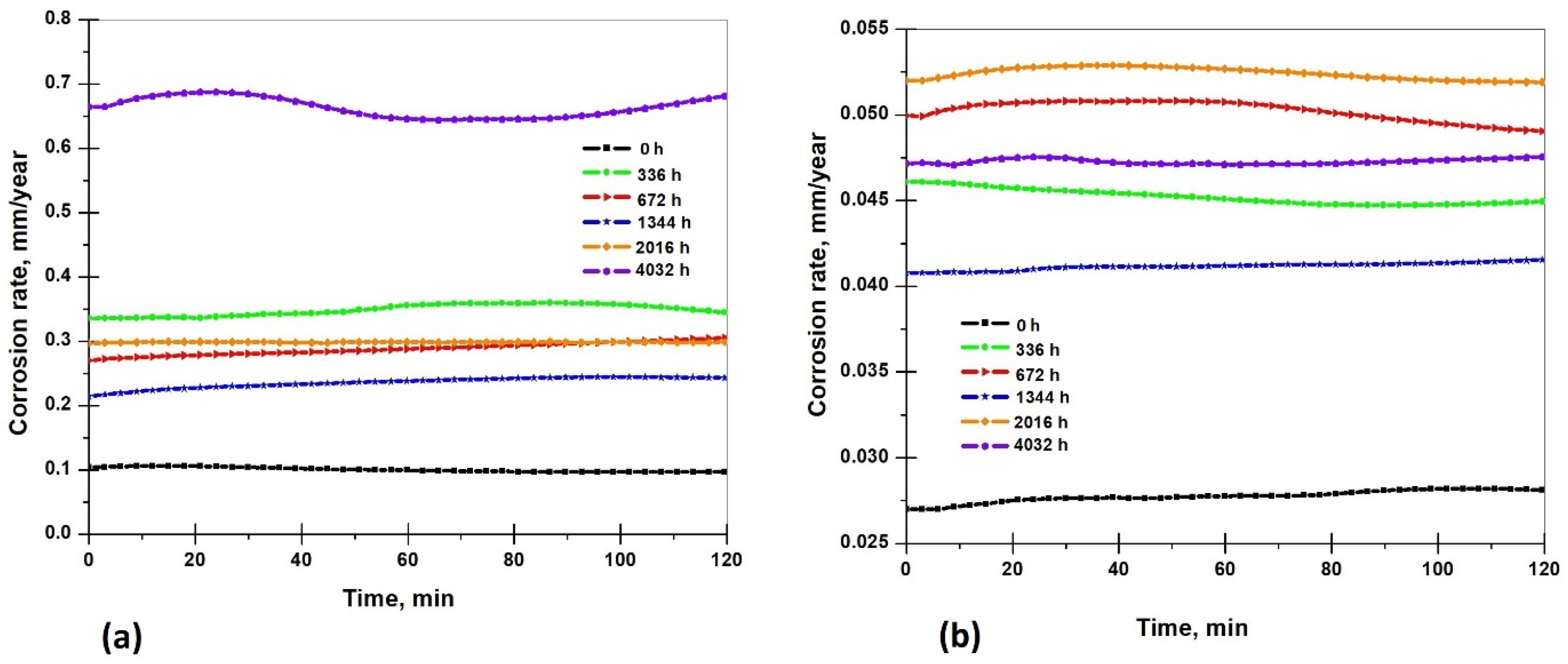
3.5.3. Corrosion Rate
3.6. Physico-Chemical Parameters of Corrosive Solutions During Corrosion Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, H.; Wang, K.; Song, S.; Zhang, X.; Gao, Q.; Liu, Y. Corrosion behavior research and corrosion prediction of structural steel in marine engineering. Anti-Corros. Methods Mater. 2022, 69, 636–650. [Google Scholar] [CrossRef]
- Lu, S.; He, Y.; Xu, R.; Wang, N.; Chen, S.; Dou, W.; Cheng, X.; Liu, G. Inhibition of microbial extracellular electron transfer corrosion of marine structural steel with multiple alloy elements. Bioelectrochemistry 2023, 151, 108377. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, C.; Qian, H.; Luo, Z.; Gao, Q. Corrosion behavior of Q235 steel by synergistic action of high concentration Cl− and complex scale in mixed salt flooding. Vacuum 2022, 204, 111365. [Google Scholar] [CrossRef]
- Al Hasan, N.H.J.; Alaradi, H.J.; Al Mansor, Z.A.K.; Al Shadood, A.H.J. The dual effect of stem extract of Brahmi (Bacopamonnieri) and Henna as a green corrosion inhibitor for low carbon steel in 0.5 M NaOH solution. Case. Stud. Constr. Mater. 2019, 11, e00300. [Google Scholar] [CrossRef]
- Mansha, M.; Kumar, A.M.; Adesina, A.Y.; Obot, I.B.; Khan, M. A novel trans-esterified water soluble hyperbranched polymer for surface protection of X60 steel: Experimental and theoretical approach. J. Mol. Liq. 2022, 349, 118091. [Google Scholar] [CrossRef]
- DNV GL—Report No. OAPUS310GKOCH (PP110272)-1, Rev. 3. Available online: http://impact.nace.org/economic-impact.aspx (accessed on 10 September 2024).
- Irsel, G. Study of the microstructure and mechanical property relationships of shielded metal arc and TIG welded S235JR steel joints. Mater. Sci. Eng. A 2022, 830, 142320. [Google Scholar] [CrossRef]
- Huang, L.; Wang, S.S.; Li, H.J.; Wang, J.Y.; Li, Z.G.; Wu, Y.C. Highly effective Q235 steel corrosion inhibition in 1M HCl solution by novel green strictosamide from Uncaria laevigata: Experimental and theoretical approaches. J. Environ. Chem. Eng. 2022, 10, 107581. [Google Scholar] [CrossRef]
- Quan, B.L.; Li, J.Q.; Chen, C.Y. Effect of sulfur on corrosion behavior of Q235 and 16Mn steel in sodium aluminate solutions. Mater. Res. Express 2020, 7, 035602. [Google Scholar] [CrossRef]
- Quan, B.; Li, J.; Chen, C. Effect of Corrosion Temperature on the Corrosion of Q235 Steel and 16Mn Steel in Sodium Aluminate Solutions. ACS Omega 2021, 6, 25904–25915. [Google Scholar] [CrossRef]
- Li, W.; Chen, H. Tensile performance of normal and high-strength structural steels at high strain rates. Thin-Walled Struct. 2023, 184, 110457. [Google Scholar] [CrossRef]
- Kossakowski, P.G. Analysis of the Void Volume Fraction for S235JR Steel at Failure for Low Initial Stress Triaxiality. Arch. Civ. Eng. 2018, 64, 101–115. [Google Scholar] [CrossRef]
- Hejwowski, T.; Szala, M. Wear-Fatigue Study of Carbon Steels. J. Adv. Sci. Tecnol. Res. 2021, 15, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pan, P.; Wu, Z.; Wang, Z. A Method for Predicting the Corrosion Behavior of Structural Steel under Atmosphere. Buildings 2023, 13, 253–270. [Google Scholar] [CrossRef]
- Chelaru, J.D.; Muresan, L.M. Study of S235 steel corrosion process in wastewater from the petrochemical industry. Stud. Univ. Babeș-Bolyai Chem. 2019, 64, 323–333. [Google Scholar] [CrossRef]
- Krella, A.K.; Zakrzewska, D.E.; Marchewicz, A. The resistance of S235JR steel to cavitation erosion. Wear 2020, 452–453, 203295. [Google Scholar] [CrossRef]
- Karlsdóttira, S.N.; Hjaltasona, S.M.; Ragnarsdóttirb, K.R. Corrosion behavior of materials in hydrogen sulfide abatement system at Hellisheiði geothermal power plant. Geothermics 2017, 70, 222–229. [Google Scholar] [CrossRef]
- Faes, W.; Lecompte, S.; Bael, J.V.; Salenbien, R.; Bäßler, R.; Bellemans, I.; Cools, P.; De Geyter, N.; Morent, R.; Verbeken, K.; et al. Corrosion behaviour of different steel types in artificial geothermal fluids. Geothermics 2019, 82, 182–189. [Google Scholar] [CrossRef]
- Lipinski, T. Corrosion Effect of 20% NaCl Solution on Basic Carbon Structural S235JR Steel. Eng. Rural. Dev. 2017, 16, 1069–1074. [Google Scholar]
- Lipinski, T. Corrosion of S235JR steel in NaCl environment at 3 °C. In Proceedings of the 26th International Conference on Metallurgy and Materials, Brno, Czech Republic, 24–26 May 2017. [Google Scholar]
- Quan, B.; Xie, Z. Study on Corrosion Behavior of Q235 Steel and 16Mn Steel by Electrochemical and Weight Loss Method. Arch. Metall. Mater. 2023, 2, 531–540. [Google Scholar] [CrossRef]
- Shuo, C.; Ji, S.; Zhu, F.; Pei, W.; Xiao, W.; Tang, X. Research on the Corrosion Behavior of Q235 Pipeline Steel in an Atmospheric Environment through Experiment. Materials 2022, 15, 6502. [Google Scholar] [CrossRef]
- Dwivedi, D.; Lepková, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef]
- Katayama, H. Corrosion simulation of carbon steels in atmospheric environment. Corr. Sci. 2005, 47, 2599–2606. [Google Scholar] [CrossRef]
- Slaimana, Q.J.M.; Hasan, B.O. Study on corrosion rate of carbon steel pipe under turbulent flow conditions. Can. J. Chem. Eng. 2010, 88, 1114–1120. [Google Scholar] [CrossRef]
- Liu, X.Y.; Sheng, K.; Zhao, T. Microstructure and texture evolution during the direct extrusion and bending–Shear deformation of AZ31 magnesium alloy. Acta Metall. Sin. Engl. Lett. 2019, 32, 710–718. [Google Scholar] [CrossRef]
- ISO 4287-1997; Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters. ISO: Geneva, Switzerland, 1997.
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Tech. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Malaret, F. Semi-Quantitative Categorization Method for the Corrosion Behavior of Metals Based on Immersion Test. Metals 2024, 14, 409. [Google Scholar] [CrossRef]
- ASTM NACE/ASTMG31-12a; Standard Guide for Laboratory Immersion Corrosion Testing of Metals. ASTM International: Houston, TX, USA, 2012.
- ISO 11845:2020; Corrosion of Metals and Alloys-General Principles for Corrosion Testing. British Standards Institution: Herndon, VA, USA, 2020.
- Ma, L.; Li, W.; Zhu, S.; Wang, L.; Guan, S. Corrosion inhibition of Schiff bases for Mg-Zn-Y-Nd alloy in normal saline: Experimental and theoretical investigations. Corr. Sci. 2021, 184, 109268. [Google Scholar] [CrossRef]
- Bogatu, N.; Muresan, A.C.; Mardare, L.; Ghisman, V.; Ravoiu, A.; Dima, F.M.; Buruiana, D.L. The Influence of Different Type Materials of Grit Blasting on the Corrosion Resistance of S235JR Carbon Steel. Inventions 2022, 8, 39. [Google Scholar] [CrossRef]
- ASTM G1-90 (1999); Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test. ASTM International: West Conshohocken, PA, USA, 1999.
- Hu, E.; Xu, Y.; Hu, X.; Pan, L.; Jiang, S. Corrosion behaviors of metals in biodiesel from rapeseed oil and methanol. Renew. Energy 2012, 37, 371–378. [Google Scholar] [CrossRef]
- Valcarce, M.B.; Vazquez, M.; Sanchez, R.S. Determination of the speed of corrosion of aluminum brass in drinking water. In Proceedings of the Congress CONAMET/SAM, La Serena, Chile, 3–5 November 2004. [Google Scholar]
- Mwakalesi, A.J.; Nyangi, M. Effective Corrosion Inhibition of Mild Steel in an Acidic Environment Using an Aqueous Extract of Macadamia Nut Green Peel Biowaste. Eng. Proc. 2023, 31, 41. [Google Scholar]
- Loto, R.T. Study of the corrosion resistance of Type 304L and 316 Austenitic Stainless Steels in Acid Chloride Solution. Orient. J. Chem. 2017, 33, 1090–1096. [Google Scholar] [CrossRef]
- Xiao, K.; Dong, C.F.; Li, X.G.; Wang, F.M. Corrosion Products and Formation Mechanism During Initial Stage of Atmospheric Corrosion of Carbon Steel. J. Iron Steel Res. Int. 2008, 15, 42–48. [Google Scholar] [CrossRef]
- Shi, L.; Song, Y.; Dong, K.; Wang, H.; Shan, D.; Han, E.H. The change of cathode/anode roles and corrosion forms in 2024/Q235/304 tri-metallic couple with the variation of oxygen concentrations and area ratios. Corr. Sci. 2021, 184, 109400. [Google Scholar] [CrossRef]
- Yujie, L.; Chang, H.; Qi, G.; Han, P.; He, B. The Electrochemical Corrosion Behaviour of Q235 Steel in Soil Containing Sodium Chloride. Int. J. Electrochem. Sci. 2021, 16, 210925. [Google Scholar]
- Morcillo, M.; Diaz, I.; Chico, B.; Cano, H.; de la Fuente, D. Weathering steels: From empirical development to scientific design. A review. Corros. Sci. 2014, 83, 6–31. [Google Scholar] [CrossRef]
- Sundjono, G.P.; Nuraini, L.; Prifiharni, S. Corrosion Behavior of Mild Steel in Seawater from Northern Coast of Java and Southern Coast of Bali. Indonesia. J. Eng. Technol. Sci. 2017, 49, 770–784. [Google Scholar] [CrossRef]
- Royani, A.; Prifiharni, S.; Nuraini, L.; Priyotomo, G.; Sundjono, G.P.; Purawiardi, I.; Gunawan, H. Corrosion of carbon steel after exposure in the river of Sukabumi. West Java. IOP Conf. Ser. Mater. Sci. Eng. 2019, 541, 012031. [Google Scholar] [CrossRef]
- Gedge, G. Corrosion of Cast Iron in Potable Water Service. Process, Corrosion and Related Aspects of Materials for Potable Water Supplies. In Proceedings of the Institute of Materials Conference, London, UK, 8–9 December 1992. [Google Scholar]
- Dong, X.; Zhai, X.; Zhang, Y.; Yang, J.; Guan, F.; Duan, J.; Sun, J.; Zhang, R.; Hou, B. Steel rust layers immersed in the South China Sea with a highly corrosive Desulfovibrio strain. NPJ Mater. Degrad. 2022, 6, 91. [Google Scholar] [CrossRef]
- He, C.G.; Song, Z.B.; Gan, Y.Z.; Ye, R.W.; Zhu, R.Z.; Liu, J.H.; Xu, Z.B. Study on the Corrosion Behavior and Mechanism of ER8 Wheel Steel in Neutral NaCl Solution. Coatings 2022, 12, 713. [Google Scholar] [CrossRef]
- Yang, J.; Lu, Y.; Guo, Z.; Gu, J.; Gu, C. Corrosion behaviour of a quenched and partitioned medium carbon steel in 3.5 wt.% NaCl solution. Corros. Sci. 2018, 130, 64–75. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Y.; Cai, F.; Lu, D.; Wang, X. Study on corrosion behavior and mechanism of AISI 4135 steel in marine environments based on field exposure experiment. Sci. Total Environ. 2022, 830, 154864. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, B.; Cao, J. Corrosion Mechanism of Q235A under 3.5% NaCl Salt Spray. Mater. Trans. 2020, 61, 2342–2347. [Google Scholar] [CrossRef]
- Liu, B.; Mu, X.; Yang, Y.; Hao, L.; Ding, X.; Dong, J.; Zhang, Z.; Hou, H.; Ke, W. Effect of tin addition on corrosion behavior of a low-alloy steel in simulated costal-industrial atmosphere. J. Mater. Sci. Technol. 2019, 35, 1228–1239. [Google Scholar] [CrossRef]
- Seechurn, Y.; Surnam, B.Y.R.; Wharton, J.A. Marine atmospheric corrosion of carbon steel in the tropical microclimate of Port Louis. Mater. Corros. 2022, 73, 1474–1489. [Google Scholar] [CrossRef]
- Evgeny, B.; Hughes, T.; Eskin, D. Effect of surface roughness on corrosion behaviour of low carbon steel in inhibited 4 M hydrochloric acid under laminar and turbulent flow conditions. Corros. Sci. 2016, 103, 196–205. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, M.; Razavi, S.M.; Beni, B.H.; Vashaee, D.; Tayebi, L. Controlling the degradation rate of bioactive magnesium implants by electrophoretic deposition of akermanite coating. Ceram. Int. 2014, 40, 3865–3872. [Google Scholar] [CrossRef]
- Li, L.; Wang, D.; Chen, S.; Yang, X. Investigation of oscillatory electro dissolution processes by chloride ion perturbation. In Proceedings of the 16th International Corrosion Congress, Beijing, China, 19–24 September 2005. [Google Scholar]
- Li, L.; Luo, J.; Lu, B.; Chen, S. Effect of interface chloride ion perturbation on oscillatory electrodissolution. Electrochim. Acta 2005, 50, 3524–3535. [Google Scholar] [CrossRef]
- You, D.; Pebere, N.; Dabosi, F. An investigation of the corrosion of pure iron by electrochemical techniques and in situ observations. Corr. Sci. 1993, 34, 5–15. [Google Scholar] [CrossRef]
- Huang, J.; Meng, X.; Huang, Y.; Jiang, W.; Chen, R.; Zheng, Z.; Gao, Y. Atmospheric corrosion of carbon steels in tropical and subtropical climates in Southern China. Mater. Corros. 2020, 71, 1400–1406. [Google Scholar] [CrossRef]
- Muresan, A.C.; Istrate, G.G. Elemente de Electrochimie şi Coroziune. Note de Curs (Elements of Electrochemistry and Corrosion. Course Notes); Galati University Press: Galati, Romania, 2021; pp. 167–180. [Google Scholar]
- Stratmann, M.; Müller, J. The mechanism of the oxygen reduction on rust covered metal substrates. Corros. Sci. 1994, 36, 327–359. [Google Scholar] [CrossRef]
- Deyab, M.A.; Keera, S.T. On corrosion and corrosion inhibition of carbon steel in stored biodiesel: Electrochemical (AC and DC) studies. J. Taiwan Inst. Chem. Eng. 2016, 68, 187–191. [Google Scholar] [CrossRef]



| Corrosive Environment | pH | Potential | Conductivity | Total Dissolved Solids | Salinity |
|---|---|---|---|---|---|
| 3.5% NaCl | 5.77 ± 0.02 | 73.82 ± 0.12 mV | 57 ± 0.06 mS/cm | 41.4 ± 0.07 ppt | 38.1 ± 0.40 g/L |
| Drinking water | 7.94 ± 0.03 | −52.2 ± 0.08 mV | 1057 ± 53 μS/cm | 751 ± 15.30 ppm | 0.523 ± 0.06 g/L |
| Tested Solutions | Immersion Time, h | Roughness Parameters, µm | |||
|---|---|---|---|---|---|
| Ra | Rz | Rq | Rt | ||
| 0 | 0.833 ± 0.003 | 8.276 ± 0.213 | 1.098 ± 0.006 | 9.509 ± 0.231 | |
| 3.5% NaCl | 336 | 1.869 ± 0.016 | 13.692 ± 0.371 | 2.5360 ± 0.026 | 17.471 ± 0.744 |
| 672 | 1.401 ± 0.014 | 10.443 ± 0.456 | 1.9190 ± 0.014 | 13.078 ± 0.658 | |
| 1344 | 1.121 ± 0.015 | 8.151 ± 0.195 | 1.439 ± 0.008 | 9.160 ± 0.314 | |
| 2016 | 0.982 ± 0.006 | 6.998 ± 0.054 | 1.252 ± 0.007 | 8.611 ± 0.206 | |
| 4032 | 1.442 ± 0.018 | 9.155 ± 0.202 | 1.816 ± 0.014 | 12.804 ± 0.473 | |
| Drinking water | 336 | 1.110 ± 0.009 | 8.151 ± 0.081 | 1.424 ± 0.012 | 10.158 ± 0.362 |
| 672 | 1.096 ± 0.005 | 8.820 ± 0.116 | 1.497 ± 0.013 | 11.531 ± 0.381 | |
| 1344 | 0.985 ± 0.004 | 7.662 ± 0.065 | 1.284 ± 0.026 | 10.832 ± 0.288 | |
| 2016 | 1.002 ± 0.006 | 6.908 ± 0.044 | 1.262 ± 0.025 | 8.860 ± 0.186 | |
| 4032 | 1.303 ± 0.012 | 8.750 ± 0.231 | 1.628 ± 0.038 | 10.058 ± 0.315 | |
| Vickers hardness, HV 0.5 | Tested solutions | Immersion time, h | |||||
| 0 | 336 | 672 | 1344 | 2016 | 4032 | ||
| 3.5% NaCl | 149.5 ± 1.5 | 119.0 ± 1.3 | 112.4 ± 1.1 | 112.0 ± 0.9 | 109.0 ± 0.7 | 102.9 ± 0.9 | |
| Drinking water | 136.2 ± 1.2 | 127.9 ± 0.8 | 105.2 ± 0.9 | 104.0 ± 0.7 | 102.1 ± 1.1 | ||
| Immersion Time | pH | Potential, mV | Conductivity, mS/cm | Total Dissolved Solids, ppt | Salinity, g/L |
|---|---|---|---|---|---|
| 336 h | 7.16 ± 0.5 | −13.5 ± 0.1 | 57.5 ± 0.3 | 40.9 ± 0.2 | 37.3 ± 0.3 |
| 672 h | 6.26 ± 0.2 | 38.6 ± 0.3 | 57.9 ± 0.2 | 41.0 ± 0.1 | 37.4 ± 0.3 |
| 1344 h | 9.40 ± 0.3 | 438.2 ± 2.7 | 58.2 ± 0.4 | 41.3 ± 0.3 | 37.7 ± 0.2 |
| 2016 h | 9.53 ± 0.3 | 448.5 ± 3.2 | 57.4 ± 0.2 | 40.7 ± 0.2 | 37.1 ± 0.1 |
| 4032 h | 10.06 ± 0.4 | 481.2 ± 3.1 | 57.2 ± 0.1 | 40.7 ± 0.2 | 37.1 ± 0.1 |
| Immersion Time | pH | Potential, mV | Conductivity, µS/cm | Total Dissolved Solids, ppm | Salinity, mg/L |
|---|---|---|---|---|---|
| 336 h | 7.94 ± 0.6 | −52.2 ± 1.6 | 1057 ± 10.6 | 751 ± 3.4 | 523 ± 2.3 |
| 672 h | 7.77 ± 0.6 | −44.0 ± 1.4 | 933 ± 8.6 | 663 ± 3.1 | 456 ± 1.9 |
| 1344 h | 8.16 ± 0.5 | −64.8 ± 1.6 | 832 ± 5.6 | 590 ± 2.3 | 405 ± 1.7 |
| 2016 h | 7.86 ± 0.4 | −50.9 ± 1.7 | 874 ± 6.2 | 527 ± 3.7 | 361 ± 1.3 |
| 4032 h | 8.06 ± 0.6 | −63.7 ± 1.8 | 704 ± 5.8 | 501 ± 2.9 | 342 ± 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buruiană, D.L.; Mureşan, A.C.; Bogatu, N.; Ghisman, V.; Herbei, E.E.; Başliu, V. Corrosion Tendency of S235 Steel in 3.5% NaCl Solution and Drinking Water During Six Months of Exposure. Materials 2024, 17, 5979. https://doi.org/10.3390/ma17235979
Buruiană DL, Mureşan AC, Bogatu N, Ghisman V, Herbei EE, Başliu V. Corrosion Tendency of S235 Steel in 3.5% NaCl Solution and Drinking Water During Six Months of Exposure. Materials. 2024; 17(23):5979. https://doi.org/10.3390/ma17235979
Chicago/Turabian StyleBuruiană, Daniela Laura, Alina Crina Mureşan, Nicoleta Bogatu, Viorica Ghisman, Elena Emanuela Herbei, and Vasile Başliu. 2024. "Corrosion Tendency of S235 Steel in 3.5% NaCl Solution and Drinking Water During Six Months of Exposure" Materials 17, no. 23: 5979. https://doi.org/10.3390/ma17235979
APA StyleBuruiană, D. L., Mureşan, A. C., Bogatu, N., Ghisman, V., Herbei, E. E., & Başliu, V. (2024). Corrosion Tendency of S235 Steel in 3.5% NaCl Solution and Drinking Water During Six Months of Exposure. Materials, 17(23), 5979. https://doi.org/10.3390/ma17235979






