DSSCs Sensitized with Phenothiazine Derivatives Containing 1H-Tetrazole-5-acrylic Acid as an Anchoring Unit
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Fabrication of Solar Cells
2.3.1. Fabrication of DSSCs
2.3.2. Fabrication of Tandem Dye-Sensitized Solar Cells
2.3.3. Dye Loading Analysis
3. Experimental
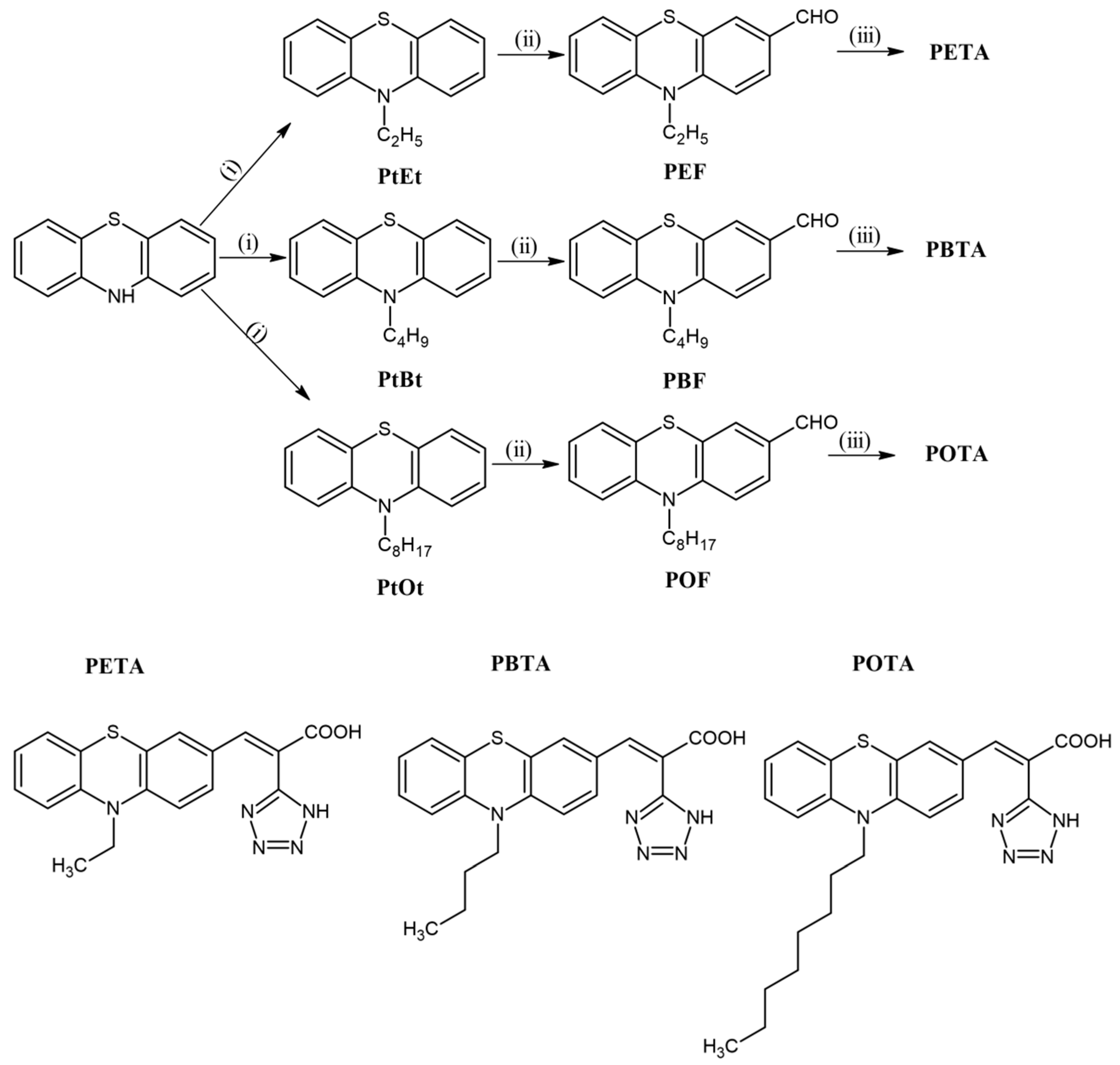
3.1. General Synthesis of (E)-3-(10-Alkyl-10H-phenothiazin-3-yl)-2-(1H-tetrazol-5-yl)acrylic Acid
3.1.1. (E)-3-(10-Ethyl-10H-phenothiazin-3-yl)-2-(1H-tetrazol-5-yl)acrylic Acid (PETA)
3.1.2. (E)-3-(10-Butyl-10H-phenothiazin-3-yl)-2-(1H-tetrazol-5-yl)acrylic acid (PBTA)
3.1.3. (E)-3-(10-Octyl-10H-phenothiazin-3-yl)-2-(1H-tetrazol-5-yl)acrylic acid (POTA)
4. Results and Discussion
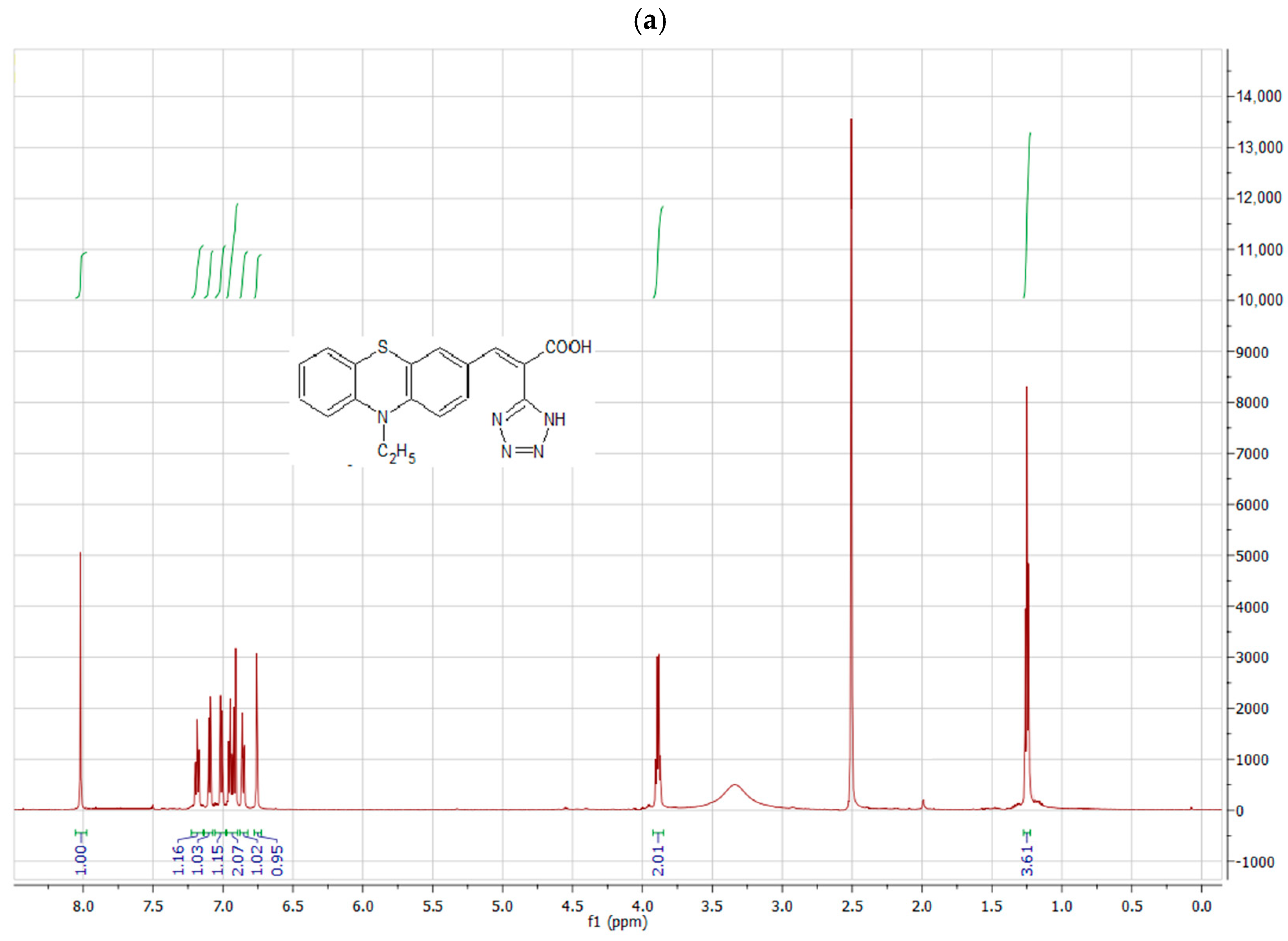
4.1. Synthesis and Structural Characterization
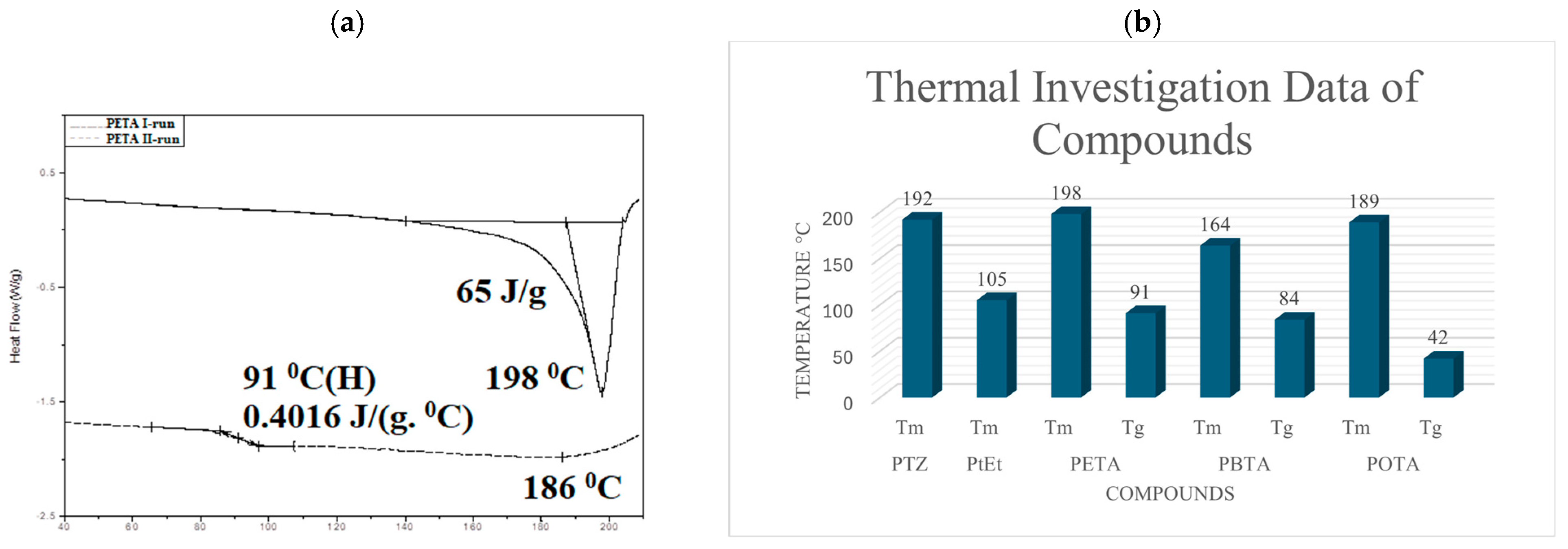
4.2. Thermal Properties
4.3. Electrochemical Investigations
4.4. Theoretical Calculations
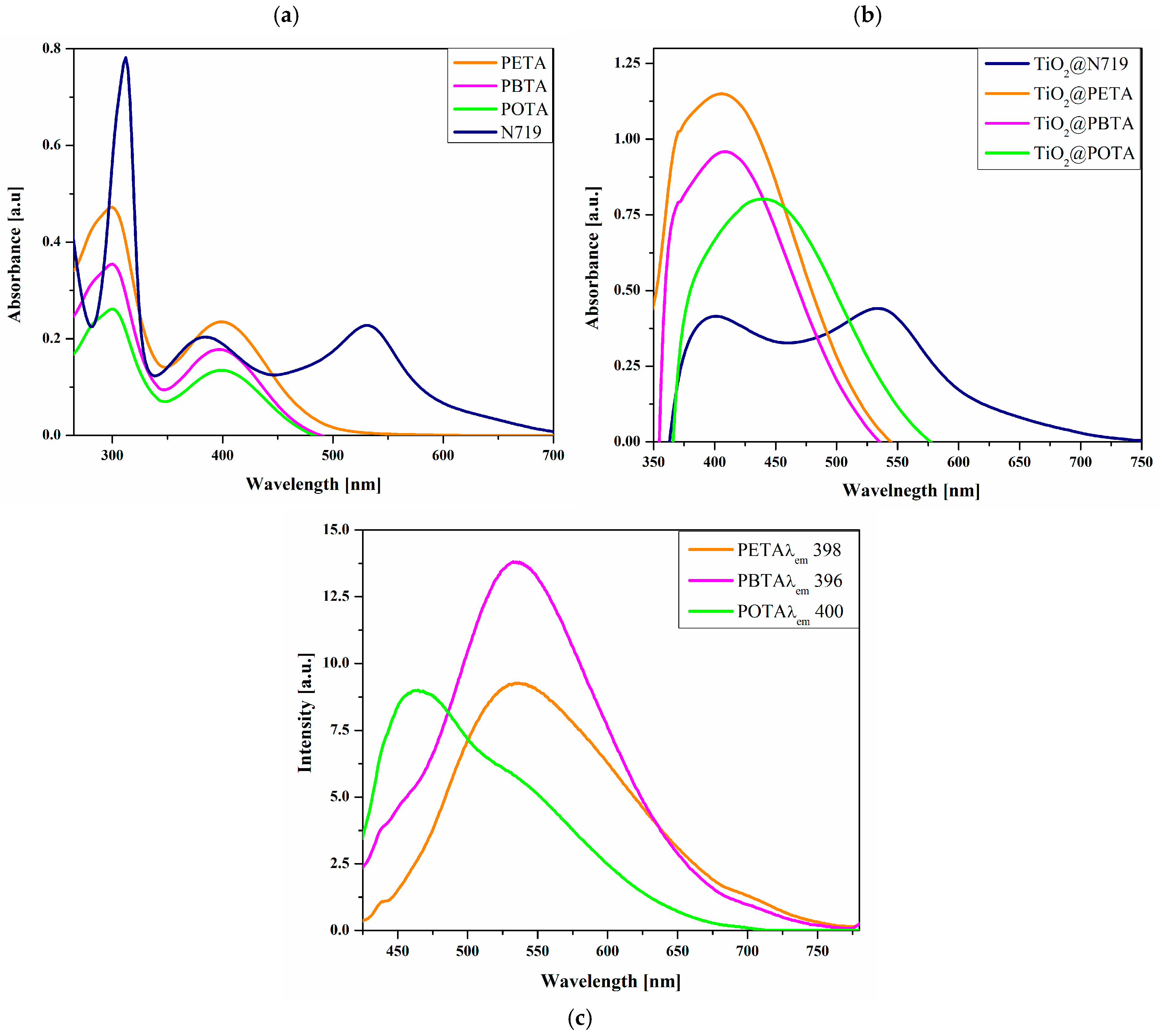
4.5. Photophysical Properties
| a Dye | UV–Vis | PL | ||
|---|---|---|---|---|
| λmax (nm), (b ε × 103) | c TiO2 | λem (nm) | Stokes Shifts ∆ (cm−1) | |
| N719 | 311 (70), 382 (25), 532 (20) | 402, 534 | 570 | 8634 |
| PETA | 300 (24), 398 (12) | 408 | 535 | 6434 |
| PBTA | 300 (20), 396 (10) | 410 | 535 | 6560 |
| POTA | 300 (7), 400 (13) | 434 | 463 | 3401 |
4.6. Photovoltaic Studies
| Photoanode | VOC [mV] | JSC [mA cm−2] | FF | PCE [%] | Dye Loading [10−7 mol cm−2] |
|---|---|---|---|---|---|
| FTO/TiO2@N719 | 720 ± 8.50 | 13.9 ± 0.17 | 0.53 ± 0.03 | 5.30 ± 0.40 | 0.86 |
| FTO/TiO2@PETA | 615 ± 8.08 | 3.66 ± 0.98 | 0.54 ± 0.01 | 1.18 ± 0.09 | 5.4 |
| FTO/TiO2@PBTA | 654 ± 1.53 | 3.92 ± 0.30 | 0.55 ± 0.03 | 1.34 ± 0.03 | 4.1 |
| FTO/TiO2@POTA | 654 ± 3.51 | 7.37 ± 0.63 | 0.52 ± 0.03 | 2.50 ± 0.09 | 3.2 |
| FTO/BL/TiO2@POTA | 679 ± 8.19 | 10.59 ± 0.36 | 0.42 ± 0.02 | 3.03 ± 0.25 | 3.2 |
| FTO/BL/TiO2@POTA:CDCA | 708 ± 5.03 | 7.42 ± 0.66 | 0.59 ± 0.03 | 3.10 ± 0.15 | 2.9 |
| FTO/TiO2@N719:POTA | 723 ± 8.62 | 15.04 ± 0.74 | 0.37 ± 0.02 | 4.00 ± 0.06 | - |
| FTO/BL/TiO2@N719:POTA | 734 ± 8.96 | 16.93 ± 1.54 | 0.37 ± 0.03 | 4.56 ± 0.01 | - |
| FTO/BL/TiO2@N719:POTA:CDCA | 758 ± 5.03 | 13.13 ± 2.39 | 0.52 ± 0.05 | 5.17 ± 0.78 | - |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Wahed, H.M.; Fadda, A.A.; Abdel-Latif, E.; Abdelmageed, S.M.; Elmorsy, M.R. Novel triphenylamine-based porphyrins: Synthesis, structural characterization, and theoretical investigation for dye-sensitized solar cell applications. J. Mol. Struct. 2023, 1281, 135147. [Google Scholar] [CrossRef]
- Elmorsy, M.R.; Badawy, S.A.; Abdel-Latif, E.; Assiri, M.A.; Ali, T.E. Significant improvement of dye-sensitized solar cell performance using low-band-gap chromophores based on triphenylamine and carbazole as strong donors. Dyes Pigments 2023, 214, 111206. [Google Scholar] [CrossRef]
- Souilah, M.; Hachi, M.; Fitri, A.; Benjelloun, A.; Benzakour, M.; Mcharfi, M.; Zgou, H. Efficient tuning of various coumarin based donor dyes with diketopyrrolopyrrole by forming DA′-π-A structure for high-efficiency solar cells: A DFT/TD-DFT study. Chem. Data Collect. 2023, 45, 101017. [Google Scholar] [CrossRef]
- Ammasi, A.; Munusamy, A.P.; Shkir, M. Computational investigations on acceptor substituent influence of metal-free efficient chromophores for optoelectronic properties. J. Mol. Model. 2022, 28, 349. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Huang, S.; Shao, W.; Kong, X.; Liu, B.; Hu, Z.; Wu, W.; Tan, H. The application of a novel D−A−π−A phenothiazine-based organic dye with N719 in efficient parallel tandem dye-sensitized solar cells. Synth. Met. 2023, 295, 117344. [Google Scholar] [CrossRef]
- Tian, H.; Yang, X.; Chen, R.; Pan, Y.; Li, L.; Hagfeldt, A.; Sun, L. Phenothiazine derivatives for efficient organic dye-sensitized solar cells. Chem. Commun. 2007, 3741–3743. [Google Scholar] [CrossRef]
- Hua, Y.; Chang, S.; Huang, D.; Zhou, X.; Zhu, X.; Zhao, J.; Chen, T.; Wong, W.-Y.; Wong, W.-K. Significant improvement of dye-sensitized solar cell performance using simple phenothiazine-based dyes. Chem. Mater. 2013, 25, 2146–2153. [Google Scholar] [CrossRef]
- Choi, H.; Baik, C.; Kang, S.O.; Ko, J.; Kang, M.S.; Nazeeruddin, M.K.; Grätzel, M. Highly efficient and thermally stable organic sensitizers for solvent-free dye-sensitized solar cells. Angew. Chem. Int. Ed. 2008, 47, 327–330. [Google Scholar] [CrossRef]
- Ito, S.; Zakeeruddin, S.M.; Humphry-Baker, R.; Liska, P.; Charvet, R.; Comte, P.; Nazeeruddin, M.K.; Péchy, P.; Takata, M.; Miura, H. High-efficiency organic-dye-sensitized solar cells controlled by nanocrystalline-TiO2 electrode thickness. Adv. Mater. 2006, 18, 1202–1205. [Google Scholar] [CrossRef]
- Wang, Z.-S.; Koumura, N.; Cui, Y.; Takahashi, M.; Sekiguchi, H.; Mori, A.; Kubo, T.; Furube, A.; Hara, K. Hexylthiophene-functionalized carbazole dyes for efficient molecular photovoltaics: Tuning of solar-cell performance by structural modification. Chem. Mater. 2008, 20, 3993–4003. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, J.M. Anchoring groups for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2015, 7, 3427–3455. [Google Scholar] [CrossRef]
- Urbani, M.; Grätzel, M.; Nazeeruddin, M.K.; Torres, T. Meso-substituted porphyrins for dye-sensitized solar cells. Chem. Rev. 2014, 114, 12330–12396. [Google Scholar] [CrossRef]
- Lu, J.; Xu, X.; Cao, K.; Cui, J.; Zhang, Y.; Shen, Y.; Shi, X.; Liao, L.; Cheng, Y.; Wang, M. D–π–A structured porphyrins for efficient dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 10008–10015. [Google Scholar] [CrossRef]
- Costa, J.C.; Santos, L.M. Hole transport materials based thin films: Topographic structures and phase transition thermodynamics of triphenylamine derivatives. J. Phys. Chem. C 2013, 117, 10919–10928. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Wei, X.; Li, X.; Ågren, H.; Wu, W.; Xie, Y. 2-Diphenylaminothiophene as the donor of porphyrin sensitizers for dye-sensitized solar cells. New J. Chem. 2014, 38, 3227–3235. [Google Scholar] [CrossRef]
- Ripolles-Sanchis, T.; Guo, B.-C.; Wu, H.-P.; Pan, T.-Y.; Lee, H.-W.; Raga, S.R.; Fabregat-Santiago, F.; Bisquert, J.; Yeh, C.-Y.; Diau, E.W.-G. Design and characterization of alkoxy-wrapped push–pull porphyrins for dye-sensitized solar cells. Chem. Commun. 2012, 48, 4368–4370. [Google Scholar] [CrossRef] [PubMed]
- Chermahini, Z.J.; Chermahini, A.N.; Dabbagh, H.A.; Teimouri, A. New tetrazole-based organic dyes for dye-sensitized solar cells. J. Energy Chem. 2015, 24, 770–778. [Google Scholar] [CrossRef]
- Massin, J.; Ducasse, L.; Toupance, T.; Olivier, C.l. Tetrazole as a new anchoring group for the functionalization of TiO2 nanoparticles: A joint experimental and theoretical study. J. Phys. Chem. C 2014, 118, 10677–10685. [Google Scholar] [CrossRef]
- da Silva, L.; Sanchez, M.; Freeman, H.S. New tetrazole based dyes as efficient co-sensitizers for dsscs: Structure-properties relationship. Org. Electron. 2020, 87, 105964. [Google Scholar] [CrossRef]
- Huang, S.; Schlichthörl, G.; Nozik, A.; Grätzel, M.; Frank, A. Charge recombination in dye-sensitized nanocrystalline TiO2 solar cells. J. Phys. Chem. B 1997, 101, 2576–2582. [Google Scholar] [CrossRef]
- Palomares, E.; Clifford, J.N.; Haque, S.A.; Lutz, T.; Durrant, J.R. Control of charge recombination dynamics in dye sensitized solar cells by the use of conformally deposited metal oxide blocking layers. J. Am. Chem. Soc. 2003, 125, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Park, N.-G.; Kang, M.; Kim, K.; Ryu, K.; Chang, S.; Kim, D.-K.; Van de Lagemaat, J.; Benkstein, K.; Frank, A. Morphological and photoelectrochemical characterization of core− shell nanoparticle films for dye-sensitized solar cells: Zn−O type shell on SnO2 and TiO2 cores. Langmuir 2004, 20, 4246–4253. [Google Scholar] [CrossRef]
- Wu, M.-S.; Yang, R.-S. Post-treatment of porous titanium dioxide film with plasmonic compact layer as a photoanode for enhanced dye-sensitized solar cells. J. Alloys Compd. 2018, 740, 695–702. [Google Scholar] [CrossRef]
- Xu, F.; Testoff, T.T.; Wang, L.; Zhou, X. Cause, regulation and utilization of dye aggregation in dye-sensitized solar cells. Molecules 2020, 25, 4478. [Google Scholar] [CrossRef] [PubMed]
- Yum, J.-H.; Jang, S.-R.; Humphry-Baker, R.; Grätzel, M.; Cid, J.-J.; Torres, T.; Nazeeruddin, M.K. Effect of coadsorbent on the photovoltaic performance of zinc pthalocyanine-sensitized solar cells. Langmuir 2008, 24, 5636–5640. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kurchania, R.; Pockett, A.; Ball, R.; Koukaras, E.; Cameron, P.; Sharma, G. Characterization of metal-free D-(π-A)2 organic dye and its application as cosensitizer along with N719 dye for efficient dye-sensitized solar cells. Indian J. Phys. 2015, 89, 1041–1050. [Google Scholar] [CrossRef]
- Althagafi, I.; El-Metwaly, N. Enhancement of dye-sensitized solar cell efficiency through co-sensitization of thiophene-based organic compounds and metal-based N-719. Arab. J. Chem. 2021, 14, 103080. [Google Scholar] [CrossRef]
- Iqbal, Z.; Wu, W.-Q.; Kuang, D.-B.; Wang, L.; Meier, H.; Cao, D. Phenothiazine-based dyes with bilateral extension of π-conjugation for efficient dye-sensitized solar cells. Dyes Pigments 2013, 96, 722–731. [Google Scholar] [CrossRef]
- De Rossi, U.; Moll, J.; Kriwanek, J.; Daehne, S. Influence of the N-alkyl chain length on the J-aggregation behavior of a cyanine dye. J. Fluoresc. 1994, 4, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.F.; Gnida, P.; Małecki, J.G.; Kotowicz, S.; Pająk, A.K.; Siwy, M.; Schab-Balcerzak, E. Effect of Anchoring Unit, N-Alkyl Chain Length, and Thickness of Titanium Dioxide Layer on the Efficiency of Dye-Sensitized Solar Cells (DSSCs) and Tandem DSSCs. Ind. Eng. Chem. Res. 2024, 63, 19994–20008. [Google Scholar] [CrossRef]
- Fabiańczyk, A.; Gnida, P.; Chulkin, P.; Kula, S.; Filapek, M.; Szlapa-Kula, A.; Janeczek, H.; Schab-Balcerzak, E. Effect of heterocycle donor in 2-cyanoacrylic acid conjugated derivatives for DSSC applications. Sol. Energy 2021, 220, 1109–1119. [Google Scholar] [CrossRef]
- Kim, S.H.; Sakong, C.; Chang, J.B.; Kim, B.; Ko, M.J.; Kim, D.H.; Hong, K.S.; Kim, J.P. The effect of N-substitution and ethylthio substitution on the performance of phenothiazine donors in dye-sensitized solar cells. Dyes Pigments 2013, 97, 262–271. [Google Scholar] [CrossRef]
- Nagarajan, B.; Kushwaha, S.; Elumalai, R.; Mandal, S.; Ramanujam, K.; Raghavachari, D. Novel ethynyl-pyrene substituted phenothiazine based metal free organic dyes in DSSC with 12% conversion efficiency. J. Mater. Chem. A 2017, 5, 10289–10300. [Google Scholar] [CrossRef]
- Slodek, A.; Zych, D.; Szafraniec-Gorol, G.; Gnida, P.; Vasylieva, M.; Schab-Balcerzak, E. Investigations of new phenothiazine-based compounds for dye-sensitized solar cells with theoretical insight. Materials 2020, 13, 2292. [Google Scholar] [CrossRef]
- Nhari, L.M.; El-Shishtawy, R.M.; Bouzzine, S.M.; Hamidi, M.; Asiri, A.M. Phenothiazine-based dyes containing imidazole with π-linkers of benzene, furan and thiophene: Synthesis, photophysical, electrochemical and computational investigation. J. Mol. Struct. 2022, 1251, 131959. [Google Scholar] [CrossRef]
- Joo, Y.H.; Shreeve, J.M. Energetic Mono-, Di-, and Trisubstituted Nitroiminotetrazoles. Angew. Chem. Int. Ed. 2009, 48, 564–567. [Google Scholar] [CrossRef]
- Rams-Baron, M.; Jędrzejowska, A.; Jurkiewicz, K.; Matussek, M.; Musiał, M.; Paluch, M. The dielectric response of phenothiazine-based glass-formers with different molecular complexity. Sci. Rep. 2021, 11, 15816. [Google Scholar] [CrossRef] [PubMed]
- Drzewicz, A.; Juszyńska-Gałązka, E.; Deptuch, A.; Kula, P. Effect of alkyl chain length on the phase situation of glass-forming liquid crystals. Crystals 2022, 12, 1401. [Google Scholar] [CrossRef]
- Xu, K. Silicon MOS optoelectronic micro-nano structure based on reverse-biased PN junction. Phys. Status Solidi (A) 2019, 216, 1800868. [Google Scholar] [CrossRef]
- Gnida, P.; Zimosz, S.; Glinka, A.; Ziółek, M.; Zych, D.; Kotowicz, S.; Amin, M.F.; Chulkin, P.; Kulesza-Matlak, G.Y.; Slodek, A. Unexpected Impact of N-Alkyl Chain Length in Bis-2-cyanoacrylic Acid Substituted Phenothiazines on the Photovoltaic Response of DSSCs. Ind. Eng. Chem. Res. 2024, 63, 7133–7153. [Google Scholar] [CrossRef]
- Allab, Y.; Chikhi, S.; Zaater, S.; Brahimi, M.; Djebbar, S. Impact of the functionalized tetrazole ring on the electrochemical behavior and biological activities of novel nickel (II) complexes with a series of tetrazole derivatives. Inorg. Chim. Acta 2020, 504, 119436. [Google Scholar] [CrossRef]
- Leal, J.G.; Sauer, A.C.; Mayer, J.C.; Stefanello, S.T.; Gonçalves, D.F.; Soares, F.A.; Iglesias, B.A.; Back, D.F.; Rodrigues, O.E.; Dornelles, L. Synthesis and electrochemical and antioxidant properties of chalcogenocyanate oxadiazole and 5-heteroarylchalcogenomethyl-1 H-tetrazole derivatives. New J. Chem. 2017, 41, 5875–5883. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.N.; Al-Ghamdi, H.A.; El-Shishtawy, R.M.; Asiri, A.M. Advances in phenothiazine and phenoxazine-based electron donors for organic dye-sensitized solar cells. Dyes Pigments 2021, 194, 109638. [Google Scholar] [CrossRef]
- Słodek, A.; Zych, D.; Kotowicz, S.; Szafraniec-Gorol, G.K.; Zimosz, S.K.; Schab-Balcerzak, E.; Siwy, M.; Grzelak, J.; Maćkowski, S. “Small in size but mighty in force”—The first principle study of the impact of A/D units in A/D-phenyl-π-phenothiazine-π-dicyanovinyl systems on photophysical and optoelectronic properties. Dyes Pigments 2021, 189, 109248. [Google Scholar] [CrossRef]
- Zimosz, S.; Zych, D.; Szafraniec-Gorol, G.; Kotowicz, S.; Malarz, K.; Musioł, R.; Slodek, A. Does the change in the length of the alkyl chain bring us closer to the compounds with the expected photophysical and biological properties?—Studies based on D-π-DA imidazole-phenothiazine system. J. Mol. Liq. 2022, 365, 120076. [Google Scholar] [CrossRef]
- Data, P.; Zassowski, P.; Lapkowski, M.; Grazulevicius, J.; Kukhta, N.; Reghu, R. Electrochromic behaviour of triazine based ambipolar compounds. Electrochim. Acta 2016, 192, 283–295. [Google Scholar] [CrossRef]
- Nagarajan, B.; Athrey, C.; Elumalai, R.; Chandran, S.; Raghavachari, D. Naphthalimide-phenothiazine based A’-π-D-π-A featured organic dyes for dye sensitized solar cell applications. J. Photochem. Photobiol. A Chem. 2021, 404, 112820. [Google Scholar] [CrossRef]
- Fujisawa, J.-I.; Eda, T.; Hanaya, M. Comparative study of conduction-band and valence-band edges of TiO2, SrTiO3, and BaTiO3 by ionization potential measurements. Chem. Phys. Lett. 2017, 685, 23–26. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16 Revision C. 01, 2016; Gaussian Inc.: Wallingford, CT, USA, 2016; Volume 1, p. 572. [Google Scholar]
- Eichkorn, K.; Weigend, F.; Treutler, O.; Ahlrichs, R. Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor. Chem. Acc. 1997, 97, 119–124. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- O’boyle, N.M.; Tenderholt, A.L.; Langner, K.M. Cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Law, K.Y. Squaraine chemistry: Effects of structural changes on the absorption and multiple fluorescence emission of bis [4-(dimethylamino) phenyl] squaraine and its derivatives. J. Phys. Chem. 1987, 91, 5184–5193. [Google Scholar] [CrossRef]
- Sil, M.C.; Chen, L.-S.; Lai, C.-W.; Chang, C.-C.; Chen, C.-M. Enhancement in the solar efficiency of a dye-sensitized solar cell by molecular engineering of an organic dye incorporating N-alkyl-attached 1, 8-naphthalamide derivative. J. Mater. Chem. C 2020, 8, 11407–11416. [Google Scholar] [CrossRef]
- Planells, M.; Pellejà, L.; Clifford, J.N.; Pastore, M.; De Angelis, F.; López, N.; Marder, S.R.; Palomares, E. Energy levels, charge injection, charge recombination and dye regeneration dynamics for donor–acceptor π-conjugated organic dyes in mesoscopic TiO2 sensitized solar cells. Energy Environ. Sci. 2011, 4, 1820–1829. [Google Scholar] [CrossRef]
- Pandey, S.S.; Inoue, T.; Fujikawa, N.; Yamaguchi, Y.; Hayase, S. Substituent effect in direct ring functionalized squaraine dyes on near infra-red sensitization of nanocrystalline TiO2 for molecular photovoltaics. J. Photochem. Photobiol. A Chem. 2010, 214, 269–275. [Google Scholar] [CrossRef]
- Miyashita, M.; Sunahara, K.; Nishikawa, T.; Uemura, Y.; Koumura, N.; Hara, K.; Mori, A.; Abe, T.; Suzuki, E.; Mori, S. Interfacial electron-transfer kinetics in metal-free organic dye-sensitized solar cells: Combined effects of molecular structure of dyes and electrolytes. J. Am. Chem. Soc. 2008, 130, 17874–17881. [Google Scholar] [CrossRef]
- Gnida, P.; Slodek, A.; Schab-Balcerzak, E. Effect of photoanode structure and sensitization conditions on the photovoltaic response of dye-sensitized solar cells. Opto-Electron. Rev. 2022, e140739. [Google Scholar] [CrossRef]
- Gnida, P.; Slodek, A.; Chulkin, P.; Vasylieva, M.; Pająk, A.K.; Seweryn, A.; Godlewski, M.; Witkowski, B.S.; Szafraniec-Gorol, G.; Schab-Balcerzak, E. Impact of blocking layer on DSSC performance based on new dye-indolo [3,2,1-jk] carbazole derivative and N719. Dyes Pigments 2022, 200, 110166. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, A.; Huang, G.; Yang, M.; Li, D.; Teng, M.; Han, D. Enhanced efficiency with CDCA co-adsorption for dye-sensitized solar cells based on metallosalophen complexes. Sol. Energy 2020, 209, 316–324. [Google Scholar] [CrossRef]

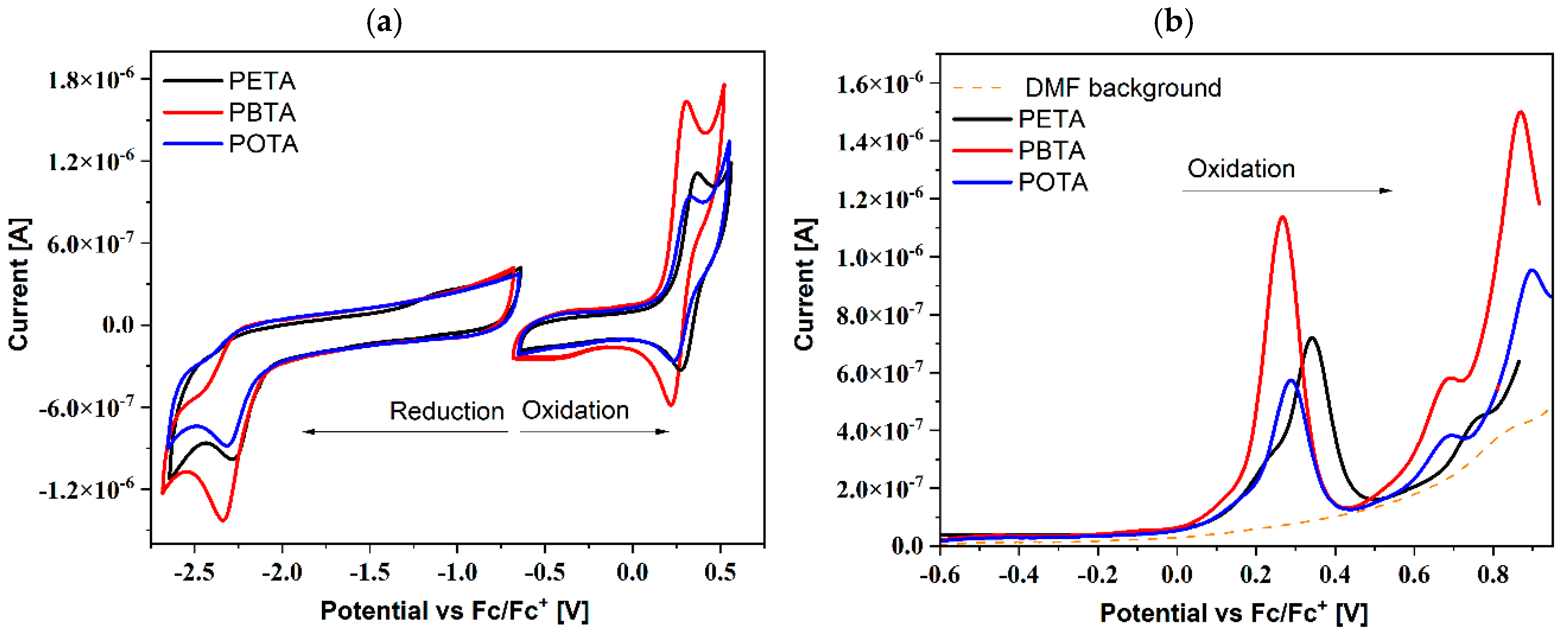
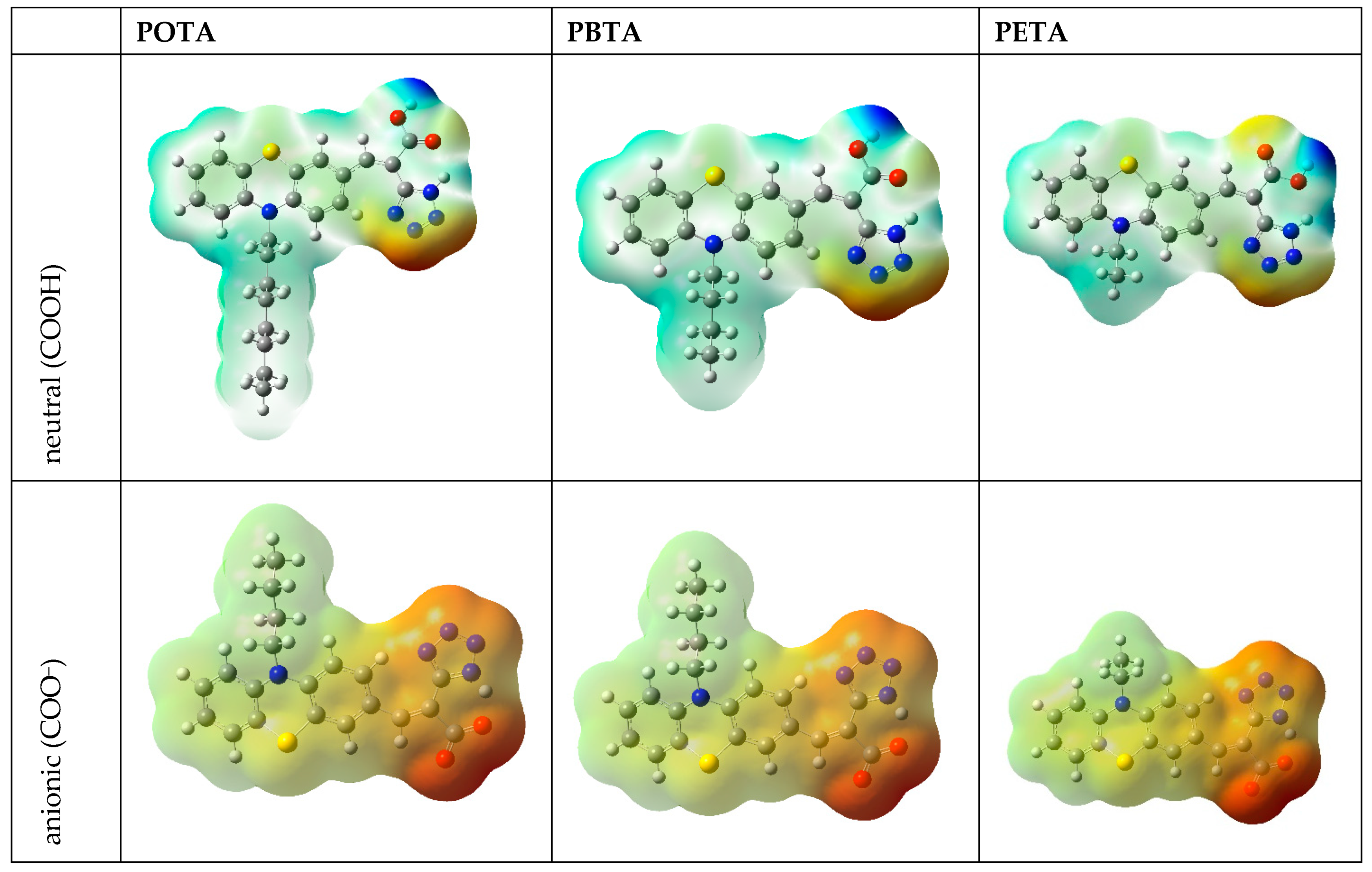


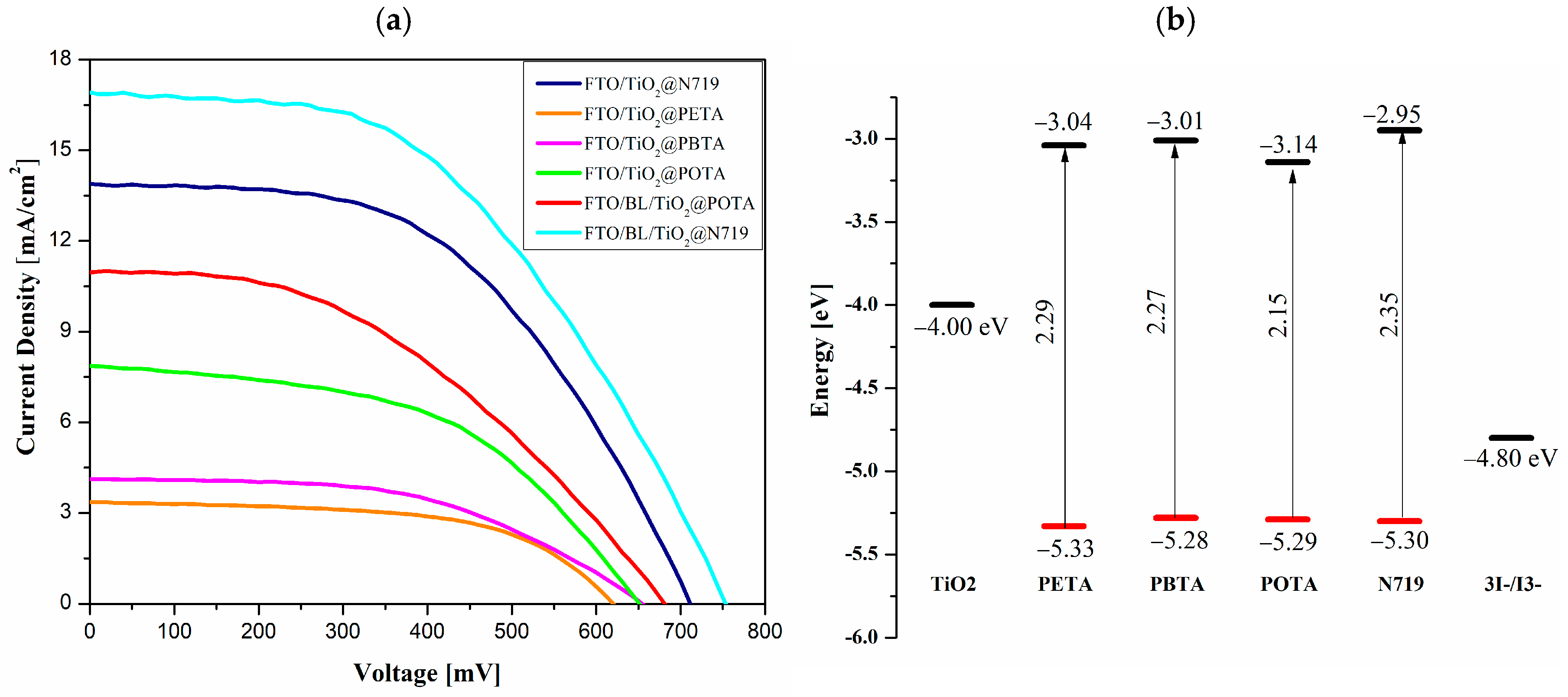
| Molecule | Method | Ered1 | Ered1(onset) | Eox1 | Eox1(onset) | EA | LUMO c | IP | HOMO c | Eg |
|---|---|---|---|---|---|---|---|---|---|---|
| [V] | [V] | [V] | [V] | [eV] | [eV] | [eV] | [eV] | [eV] | ||
| PETA | CV | −2.28 a | −2.06 | 0.36 b | 0.23 | −3.04 | −3.03 | −5.33 | −5.53 | 2.29 |
| DPV | −2.23 | −2.09 | 0.33 | 0.19 | −3.01 | −5.29 | 2.28 | |||
| PBTA | CV | −2.34 a | −2.09 | 0.30 b | 0.18 | −3.01 | −3.07 | −5.28 | −5.53 | 2.27 |
| DPV | −2.30 | −2.02 | 0.27 | 0.16 | −3.08 | −5.26 | 2.18 | |||
| POTA | CV | −2.32 a | −1.96 | 0.33 b | 0.19 | −3.14 | −3.07 | −5.29 | −5.52 | 2.15 |
| DPV | −2.30 | −1.94 | 0.27 | 0.19 | −3.16 | −5.29 | 2.13 | |||
| N719 * [40] | CV | −2.26 | −2.15 | 0.27 | 0.20 | −2.65 | −2.95 | −5.00 | −5.30 | 2.35 |
| DPV | −2.18 | −2.09 | 0.22 | 0.14 | −2.71 | −4.94 | 2.23 |
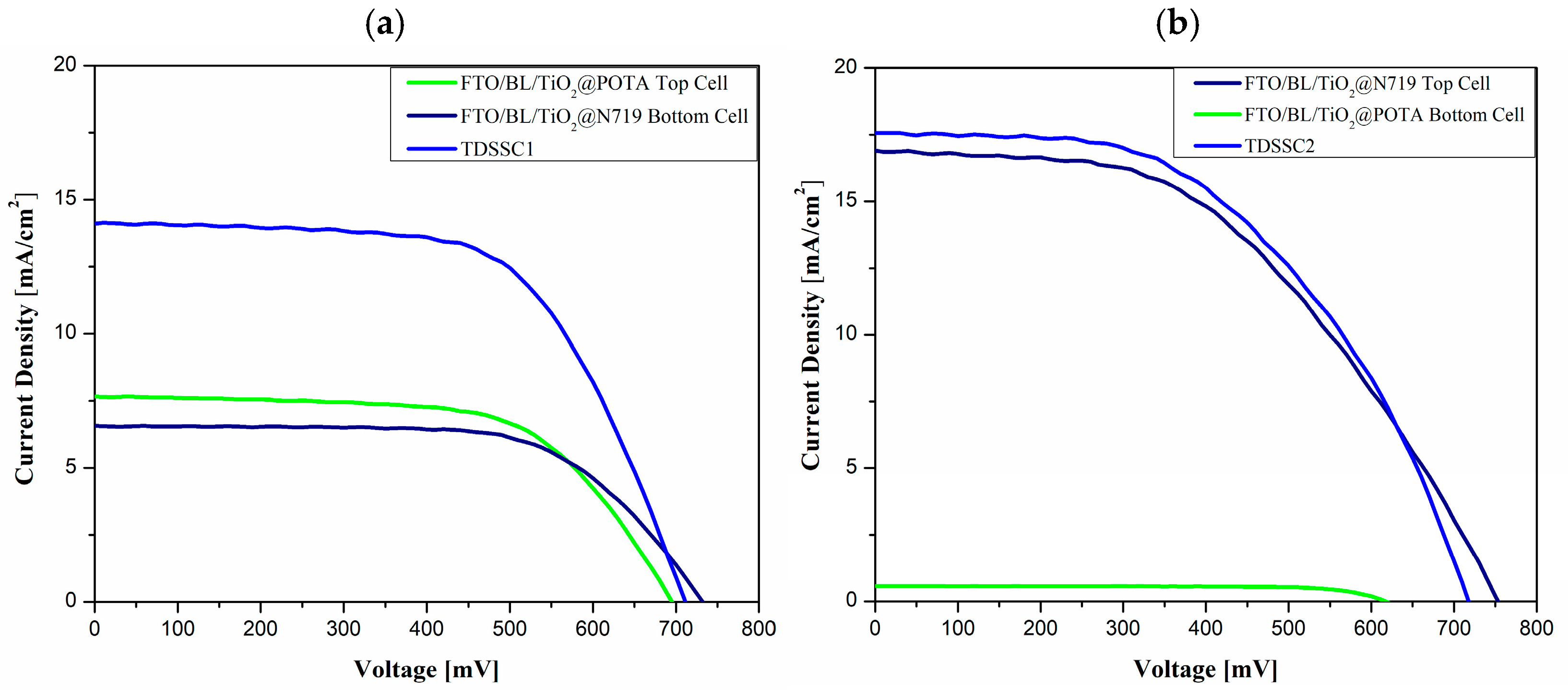
| T-Device | Photoanode | VOC [mV] | JSC [mA cm−2] | FF [−] | PCE [%] |
|---|---|---|---|---|---|
| TDSSC1 | FTO/BL/TiO2@POTA Top | 695 | 7.67 | 0.63 | 3.34 |
| FTO/BL/TiO2@N719 Bottom | 732 | 6.55 | 0.65 | 3.10 | |
| Tandem | 711 | 14.12 | 0.62 | 6.20 | |
| TDSSC2 | FTO/BL/TiO2@N719 Top | 753 | 16.92 | 0.48 | 6.08 |
| FTO/BL/TiO2@POTA Bottom | 617 | 0.58 | 0.75 | 0.27 | |
| Tandem | 717 | 17.59 | 0.51 | 6.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, M.F.; Gnida, P.; Małecki, J.G.; Kotowicz, S.; Schab-Balcerzak, E. DSSCs Sensitized with Phenothiazine Derivatives Containing 1H-Tetrazole-5-acrylic Acid as an Anchoring Unit. Materials 2024, 17, 6116. https://doi.org/10.3390/ma17246116
Amin MF, Gnida P, Małecki JG, Kotowicz S, Schab-Balcerzak E. DSSCs Sensitized with Phenothiazine Derivatives Containing 1H-Tetrazole-5-acrylic Acid as an Anchoring Unit. Materials. 2024; 17(24):6116. https://doi.org/10.3390/ma17246116
Chicago/Turabian StyleAmin, Muhammad Faisal, Paweł Gnida, Jan Grzegorz Małecki, Sonia Kotowicz, and Ewa Schab-Balcerzak. 2024. "DSSCs Sensitized with Phenothiazine Derivatives Containing 1H-Tetrazole-5-acrylic Acid as an Anchoring Unit" Materials 17, no. 24: 6116. https://doi.org/10.3390/ma17246116
APA StyleAmin, M. F., Gnida, P., Małecki, J. G., Kotowicz, S., & Schab-Balcerzak, E. (2024). DSSCs Sensitized with Phenothiazine Derivatives Containing 1H-Tetrazole-5-acrylic Acid as an Anchoring Unit. Materials, 17(24), 6116. https://doi.org/10.3390/ma17246116





