Internal Adaptation of Composite Fillings Made Using Universal Adhesives—A Micro-Computed Tomography Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
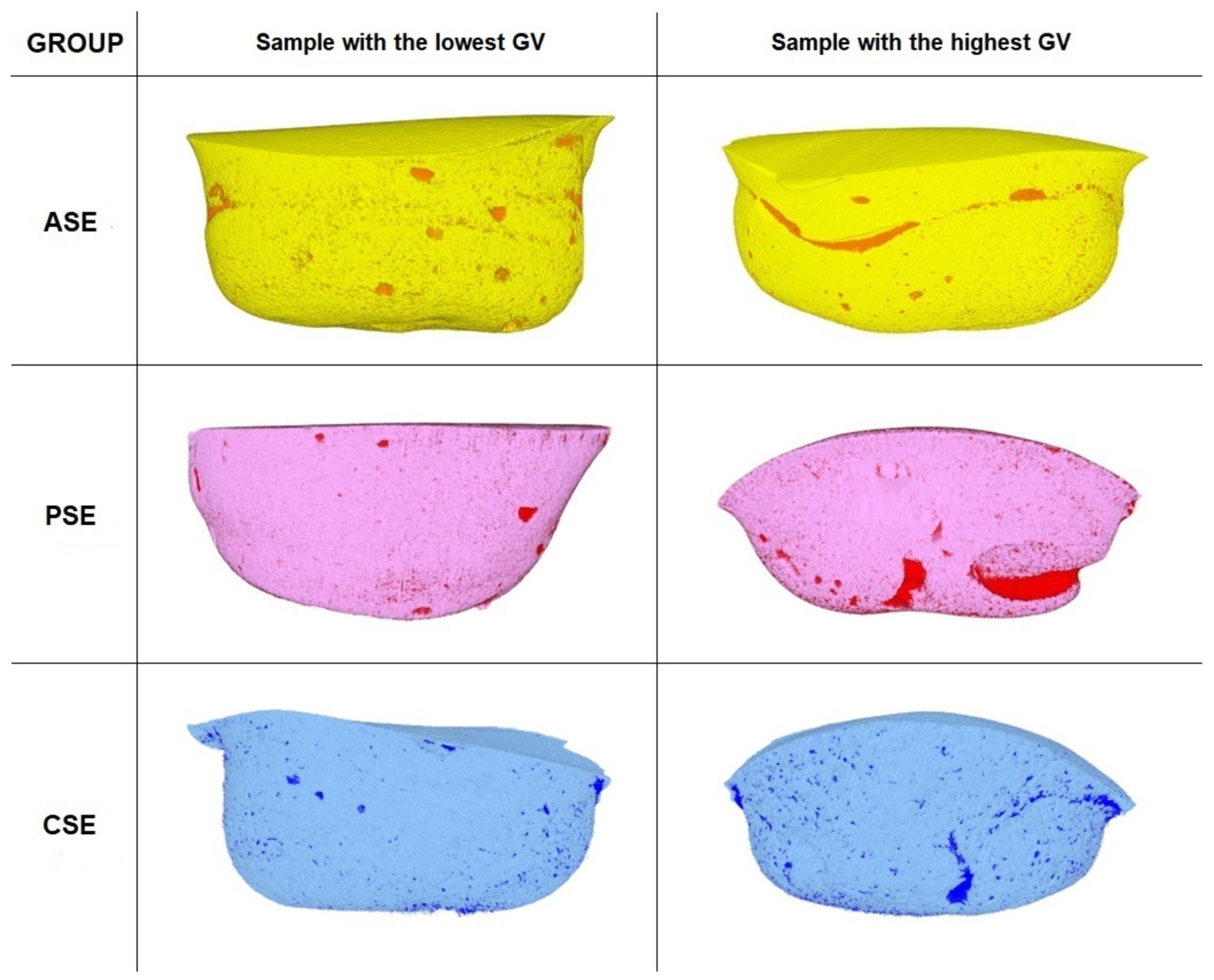
3.1. Gap Volume to Filling Volume (GV/FV) and Gap Volume to Cavity Volume (GV/CV) Ratios
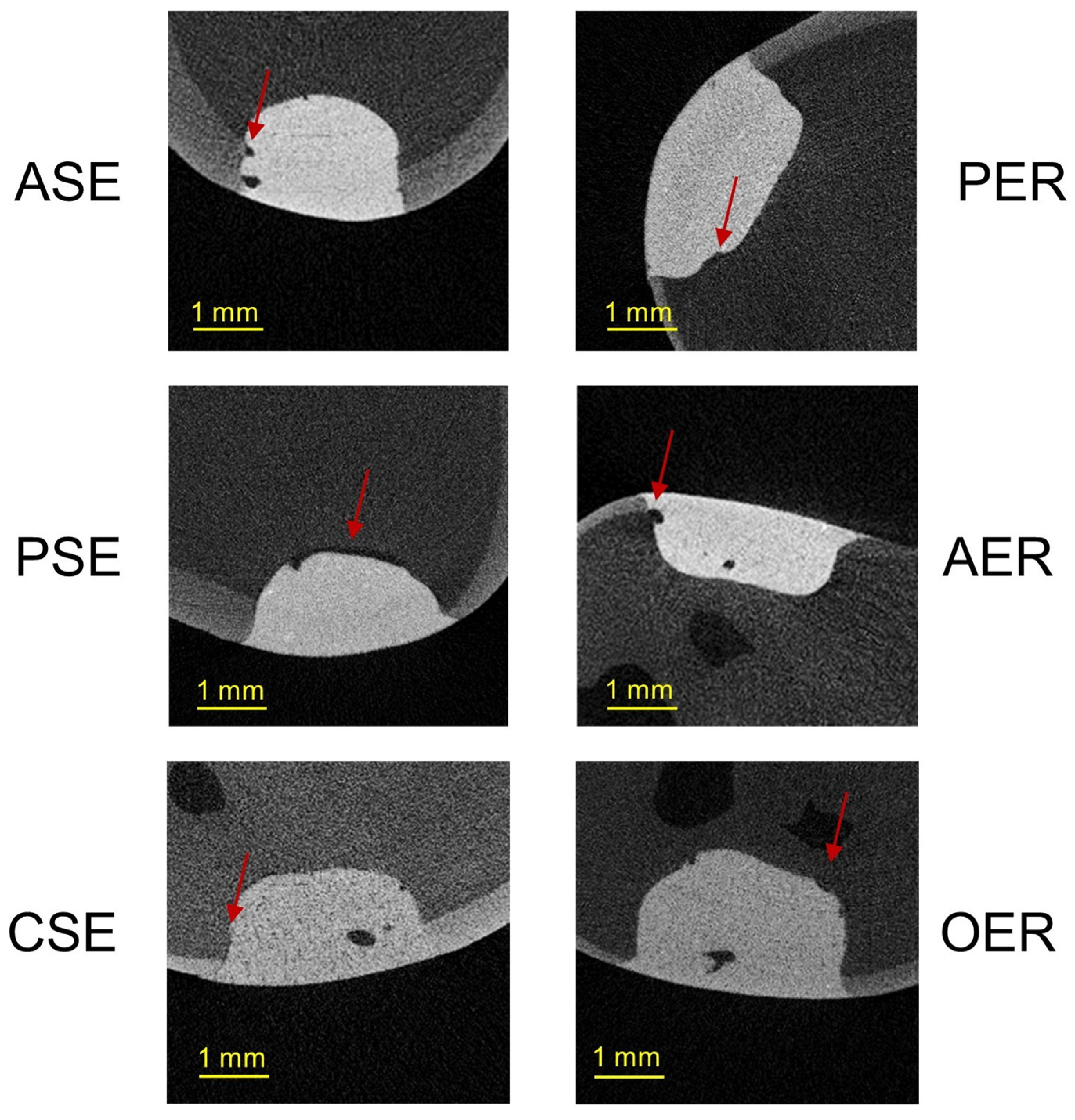
3.2. Gap Volumes at the Tooth–Filling Interface of the External (EGV) and Internal Parts (IGV) of Cavities
4. Discussion
4.1. Thermomechanical Loading (TML) and Water Degradation Analysis
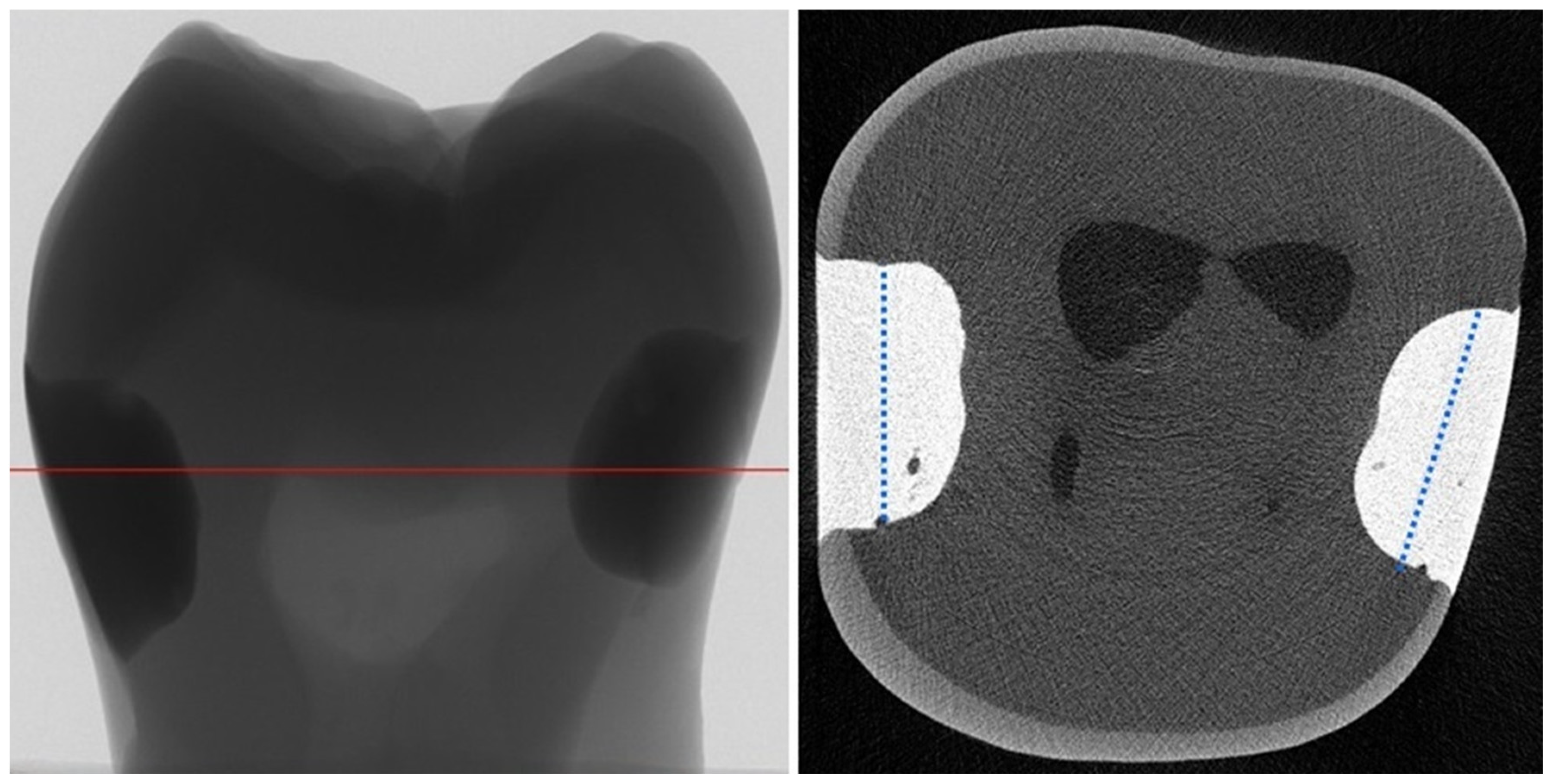
4.2. Micro-Computed Tomography (µCT) Analysis
4.3. Gap Volume to Filling Volume (GV/FV) and Gap Volume to Cavity Volume (GV/CV) Ratios
4.4. Gap Volumes at the Tooth–Filling Interface of the External (EGV) and Internal Parts (IGV) of Cavities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perdigão, J.; Araujo, E.; Ramos, R.Q.; Gomes, G.; Pizzolotto, L. Adhesive dentistry: Current concepts and clinical considerations. J. Esthet. Restor. Dent. 2021, 33, 51–68. [Google Scholar] [CrossRef]
- Hanabusa, M.; Mine, A.; Kuboki, T.; Momoi, Y.; Van End, A.; Van Meerbeek, B.; De Munck, J. Bonding effectiveness of a new “multi-mode” adhesive to enamel and dentine. J. Dent. 2012, 40, 475–484. [Google Scholar] [CrossRef]
- Perdigão, J.; Swift, E.J., Jr. Universal adhesives. J. Esthet. Restor. Dent. 2015, 27, 331–334. [Google Scholar] [CrossRef]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; De Stefano Dorigo, E. Dental adhesion review: Aging and stability of the bonded interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Khvostenko, D.; Salehi, S.; Naleway, S.E.; Hilton, T.J.; Ferracane, J.L.; Mitchell, J.C.; Kruzic, J.J. Cyclic mechanical loading promotes bacterial penetration along composite restoration marginal gaps. Dent. Mater. 2015, 31, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.; Ye, Q.; Park, J.; Topp, E.M.; Misra, A.; Marangos, O.; Wang, Y.; Bohaty, B.S.; Singh, V.; Sene, F.; et al. Adhesive/dentin interface: The weak link in the composite restoration. Ann. Biomed. Eng. 2010, 38, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Kaczor-Wiankowska, K.; Lipa, S.; Krasowski, M.; Sokołowski, J.; Lewusz-Butkiewicz, K.; Nowicka, A. Evaluation of gap formation at the composite resin-tooth interface after using universal adhesives: In vitro SEM study using the replica technique. Microsc. Res. Tech. 2020, 83, 176–185. [Google Scholar] [CrossRef]
- Peutzfeldt, A.; Asmussen, E. Determinants of in vitro gap formation of resin composites. J. Dent. 2004, 32, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Rosatto, C.M.; Bicalho, A.A.; Veríssimo, C.; Bragança, G.F.; Rodrigues, M.P.; Tantbirojn, D.; Versluis, A.; Soares, C.J. Mechanical properties, shrinkage stress, cuspal strain and fracture resistance of molars restored with bulk-fill composites and incremental filling technique. J. Dent. 2015, 43, 1519–1528. [Google Scholar] [CrossRef]
- Ferracane, J.L.; Hilton, T.J. Polymerization stress--is it clinically meaningful? Dent. Mater. 2016, 32, 1–10. [Google Scholar] [CrossRef]
- Massaro, H.; Zambelli, L.F.A.; Britto, A.A.d.; Vieira, R.P.; Ligeiro-de-Oliveira, A.P.; Andia, D.C.; Oliveira, M.T.; Lima, A.F. Solvent and HEMA Increase Adhesive Toxicity and Cytokine Release from Dental Pulp Cells. Materials 2019, 12, 2750. [Google Scholar] [CrossRef]
- Krifka, S.; Spagnuolo, G.; Schmalz, G.; Schweikl, H. A review of adaptive mechanisms in cell responses towards oxidative stress caused by dental resin monomers. Biomaterials. 2013, 34, 4555–4563. [Google Scholar] [CrossRef]
- Tuncer, S.; Demirci, M.; Schweikl, H.; Erguven, M.; Bilir, A.; Kara Tuncer, A. Inhibition of cell survival, viability and proliferation by dentin adhesives after direct and indirect exposure in vitro. Clin. Oral. Investig. 2012, 16, 1635–1646. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Yiu, C.; Hashimoto, M.; Breschi, L.; Carvalho, R.M.; Ito, S. Collagen degradation by host-derived enzymes during aging. J. Dent. Res. 2004, 83, 216–221. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.L.; Colucci, V.; Palma-Dibb, R.G.; Corona, S.A. Assessment of in vitro methods used to promote adhesive interface degradation: A critical review. J. Esthet. Restor. Dent. 2007, 19, 340–354. [Google Scholar] [CrossRef] [PubMed]
- De Munck, J.; Van Landuyt, K.; Peumans, M.; Poitevin, A.; Lambrechts, P.; Braem, M.; Van Meerbeek, B. A critical review of the durability of adhesion to tooth tissue: Methods and results. J. Dent. Res. 2005, 84, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Berge, H.X.; Condon, J.R. In vitro aging of dental composites in water--effect of degree of conversion, filler volume, and filler/matrix coupling. J. Biomed. Mater. Res. 1998, 42, 465–472. [Google Scholar] [CrossRef]
- Gale, M.S.; Darvell, B.W. Thermal cycling procedures for laboratory testing of dental restorations. J. Dent. 1999, 27, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Călinoiu, Ș.G.; Bîcleșanu, C.; Florescu, A.; Stoia, D.I.; Dumitru, C.; Miculescu, M. Comparative Study on Interface Fracture of 4th Generation 3-Steps Adhesive and 7th Generation Universal Adhesive. Materials 2023, 16, 5834. [Google Scholar] [CrossRef] [PubMed]
- Rengo, C.; Goracci, C.; Ametrano, G.; Chieffi, N.; Spagnuolo, G.; Rengo, S.; Ferrari, M. Marginal leakage of class v composite restorations assessed using microcomputed tomography and scanning electron microscope. Oper. Dent. 2015, 40, 440–448. [Google Scholar] [CrossRef]
- Kaczor, K.; Krasowski, M.; Lipa, S.; Sokołowski, J.; Nowicka, A. How do the etching mode and thermomechanical loading influence the marginal integrity of universal adhesives? Oper. Dent. 2020, 45, 306–317. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.H. Measurement of the internal adaptation of resin composites using micro-CT and its correlation with polymerization shrinkage. Oper. Dent. 2014, 39, E57–E70. [Google Scholar] [CrossRef]
- Ersen, K.A.; Gürbüz, Ö.; Özcan, M. Evaluation of polymerization shrinkage of bulk-fill resin composites using microcomputed tomography. Clin. Oral. Investig. 2020, 24, 1687–1693. [Google Scholar] [CrossRef]
- Putignano, A.; Tosco, V.; Monterubbianesi, R.; Vitiello, F.; Gatto, M.L.; Furlani, M.; Giuliani, A.; Orsini, G. Comparison of three different bulk-filling techniques for restoring class II cavities: μCT, SEM-EDS combined analyses for margins and internal fit assessments. J. Mech. Behav. Biomed. Mater. 2021, 124, 104812. [Google Scholar] [CrossRef]
- Borges, A.L.S.; Dal Piva, A.M.d.O.; Moecke, S.E.; de Morais, R.C.; Tribst, J.P.M. Polymerization Shrinkage, Hygroscopic Expansion, Elastic Modulus and Degree of Conversion of Different Composites for Dental Application. J. Compos. Sci. 2021, 5, 322. [Google Scholar] [CrossRef]
- Sampaio, C.S.; Barbosa, J.M.; Cáceres, E.; Rigo, L.C.; Coelho, P.G.; Bonfante, E.A.; Hirata, R. Volumetric shrinkage and film thickness of cementation materials for veneers: An in vitro 3D microcomputed tomography analysis. J. Prosthet. Dent. 2017, 117, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Meleo, D.; Manzon, L.; Pecci, R.; Zuppante, F.; Bedini, R. A proposal of microtomography evaluation for restoration interface gaps. Ann. Ist. Super. Sanita. 2012, 48, 83–88. [Google Scholar]
- Condon, J.R.; Ferracane, J.L. Assessing the effect of composite formulation on polymerization stress. J. Am. Dent. Assoc. 2000, 131, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Demarco, F.F.; Cenci, M.S.; Montagner, A.F.; de Lima, V.P.; Correa, M.B.; Moraes, R.R.; Opdam, N.J.M. Longevity of composite restorations is definitely not only about materials. Dent. Mater. 2023, 39, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Ferracane, J.; Lee, I.B. Effect of layering methods, composite type, and flowable liner on the polymerization shrinkage stress of light cured composites. Dent. Mater. 2012, 28, 801–809. [Google Scholar] [CrossRef]
- Braga, R.R.; Ballester, R.Y.; Ferracane, J.L. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent. Mater. 2005, 21, 962–970. [Google Scholar] [CrossRef]
- Morresi, A.L.; D’Amario, M.; Capogreco, M.; Gatto, R.; Marzo, G.; D’Arcangelo, C.; Monaco, A. Thermal cycling for restorative materials: Does a standardized protocol exist in laboratory testing? A literature review. J. Mech. Behav. Biomed. Mater. 2014, 29, 295–308. [Google Scholar] [CrossRef]
- Kitasako, Y.; Burrow, M.F.; Nikaido, T.; Tagami, J. The influence of storage solution on dentin bond durability of resin cement. Dent. Mater. 2000, 16, 1–6. [Google Scholar] [CrossRef]
- Masarwa, N.; Mohamed, A.; Abou-Rabii, I.; Abu Zaghlan, R.; Steier, L. Longevity of self-etch dentin bonding adhesives compared to etch-and-rinse dentin bonding adhesives: A systematic review. J. Evid. Based. Dent. Pract. 2016, 16, 96–106. [Google Scholar] [CrossRef]
- Sabatini, C. Effect of a chlorhexidine-containing adhesive on dentin bond strength stability. Oper. Dent. 2013, 38, 609–617. [Google Scholar] [CrossRef]
- Sezinando, A.; Luque-Martinez, I.; Muñoz, M.A.; Reis, A.; Loguercio, A.D.; Perdigão, J. Influence of a hydrophobic resin coating on the immediate and 6-month dentin bonding of three universal adhesives. Dent. Mater. 2015, 31, e236–e246. [Google Scholar] [CrossRef] [PubMed]
- Brackett, M.G.; Tay, F.R.; Brackett, W.W.; Dib, A.; Dipp, F.A.; Mai, S.; Pashley, D.H. In vivo chlorhexidine stabilization of hybrid layers of an acetone-based dentin adhesive. Oper. Dent. 2009, 34, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, A.D.; Hass, V.; Gutierrez, M.F.; Luque-Martinez, I.V.; Szezs, A.; Stanislawczuk, R.; Bandeca, M.C.; Reis, A. Five-year effects of chlorhexidine on the in vitro durability of resin/dentin interfaces. J. Adhes. Dent. 2016, 18, 35–42. [Google Scholar] [PubMed]
- Kiyomura, M. Bonding strength to bovine dentin with 4-METAMMA-TBB resin: Long term stability and influence of water. J. Jpn. Sot. Dent. Mater. 1987, 6, 860–872. [Google Scholar]
- Ghavami-Lahiji, M.; Davalloo, R.T.; Tajziehchi, G.; Shams, P. Micro-computed tomography in preventive and restorative dental research: A review. Imaging. Sci. Dent. 2021, 51, 341–350. [Google Scholar] [CrossRef]
- Nahedh, H.A.; Sibai, N.S. Evaluation of Interfacial Gap Volume of Two Low-shrinkage Composites Using Micro-Computed Tomography. Oper. Dent. 2017, 42, 658–668. [Google Scholar] [CrossRef]
- Tosco, V.; Monterubbianesi, R.; Furlani, M.; Giuliani, A.; Putignano, A.; Orsini, G. Micro-computed tomography for assessing the internal and external voids of bulk-fill composite restorations: A technical report. Imaging. Sci. Dent. 2022, 52, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Li, S.B.; Gu, L.J.; Li, Y. Detection of marginal leakage of Class V restorations in vitro by micro-computed tomography. Oper. Dent. 2014, 39, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Zaharia, C.; Duma, V.-F.; Sinescu, C.; Socoliuc, V.; Craciunescu, I.; Turcu, R.P.; Marin, C.N.; Tudor, A.; Rominu, M.; Negrutiu, M.-L. Dental Adhesive Interfaces Reinforced with Magnetic Nanoparticles: Evaluation and Modeling with Micro-CT versus Optical Microscopy. Materials 2020, 13, 3908. [Google Scholar] [CrossRef] [PubMed]
- Hirata, R.; Clozza, E.; Giannini, M.; Farrokhomanesh, E.; Janal, M.; Tovar, N.; Bonfante, E.A.; Coelho, P.G. Shrinkage assessment of low shrinkage composites using micro-computed tomography. J. Biomed. Mater. Res. Part. B. 2015, 103B, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Algamaiah, H.; Sampaio, C.S.; Rigo, L.C.; Janal, M.N.; Giannini, M.; Bonfante, E.A.; Coelho, P.G.; Reis, A.F.; Hirata, R. Microcomputed Tomography Evaluation of Volumetric Shrinkage of Bulk-Fill Composites in Class II Cavities. J. Esthet. Restor. Dent. 2017, 29, 118–127. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.B.; Trevelin, L.T.; Silva, B.T.F.D.; Francisconi-Dos-Rios, L.F.; Siriani, L.K.; Cardoso, M.V. Bonding efficiency and durability: Current possibilities. Braz. Oral. Res. 2017, 31, e57. [Google Scholar] [CrossRef]
- Perdigão, J.; Reis, A.; Loguercio, A.D. Dentin adhesion and MMPs: A comprehensive review. J. Esthet. Restor. Dent. 2013, 25, 219–241. [Google Scholar] [CrossRef]
- Fehrenbach, J.; Lacerda-Santos, R.; Machado, L.S.; Miotti, L.L.; de Carvalho, F.G.; Münchow, E.A. Which self-etch acidic composition may result in higher dental bonds at the long-term? A network meta-analysis review of in vitro studies. J. Dent. 2022, 126, 104283. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, E.; Cardoso, M.; Marques Ferreira, M.; Marto, C.M.; Paula, A.; Coelho, A.S. 10-MDP Based Dental Adhesives: Adhesive Interface Characterization and Adhesive Stability—A Systematic Review. Materials 2019, 12, 790. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Aggressiveness of contemporary self-etching systems. I: Depth of penetration beyond dentin smear layers. Dent. Mater. 2001, 17, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Dietschi, D.; Herzfeld, D. In vitro evaluation of marginal and internal adaptation of class II resin composite restorations after thermal and occlusal stressing. Eur. J. Oral. Sci. 1998, 106, 1033–1034. [Google Scholar] [CrossRef] [PubMed]


| Group | Adhesive (Manufacturer; Batch Number) | Etching Technique | Composition | Composite Resin (Manufacturer; Batch Number) | Type of Composite Resin; Particle Size; Filler Content (wt/vol.%); Volumetric Polymerization Shrinkage | |
|---|---|---|---|---|---|---|
| Experimental | PER | Peak Universal (Ultradent, South Jordan, UT, USA; 5135 BBBXD) | Etch and rinse | Ethyl alcohol, HEMA, methacrylic acid, 0.2% chlorhexidine di (acetate) | Amelogen Plus (Ultradent, South Jordan, UT, USA; 9031 BB5BJ) | Microhybrid; 0.7 μm, 76/61, 2.97% [26] |
| PSE | Self-etch | |||||
| AER | Adhese Universal (Ivoclar Vivadent; Schaan, Lichtenstein; U23288) | Etch and rinse | MDP, bis-GMA, HEMA, MCAP, D3MA, ethanol, water, initiator, stabilizers, silicon dioxide | IPS Empress Direct (Ivoclar Vivadent; Schaan, Lichtenstein; T39377) | Nanohybrid; 40 nm–3 μm; 75–79/52–59, 2.13% [27] | |
| ASE | Self-etch | |||||
| Control | OER | OptiBond FL (Kerr, Orange, CA, USA; 5457273) | Etch and rinse | Primer: HEMA, glycerol phosphate dimethacrylate, mono-2-methacryloyloxyethyl phthalate, water, ethanol Bond: bis-GMA, HEMA, glycerol dimethacrylate, filler particles (fumed SiO2, barium aluminoborosilicate, Na2SiF6) | Herculite XRV Ultra (Kerr, Orange, CA, USA; 5136056) | Nanohybrid; 40 nm–30 μm; 78/59, 2.7% |
| CSE | Clearfil SE (Kuraray Noritake Dental; Tokyo, Japan; 000147) | Self-etch | Primer: water, 10-MDP, HEMA, hydrophilic aliphatic dimethacrylate, accelerators, dl-camphorquinone Bond: 10-MDP, bis-GMA, HEMA, initiators, colloidal silica, dl-camphorquinone, accelerator | Clearfil Majesty ES-2 (Kuraray Noritake Dental; Tokyo, Japan; 3J0009) | Nanohybrid, 370 nm–1.5 μm, 78/66; no information | |
| Group | GV (mm3) | FV (mm3) | CV (mm3) | GV/FV (%) | GV/CV (%) | |
|---|---|---|---|---|---|---|
| Etch and rinse | PER | 0.369 ± 0.107 | 11.930 ± 1.415 | 12.299 ± 1.444 | 0.031 ± 0.009 de Min. 0.022 Max. 0.044 | 0.030 ± 0.008 DE Min. 0.021 Max. 0.042 |
| AER | 0.269 ± 0.106 | 14.073 ± 1.777 | 14.342 ± 1.777 | 0.019 ± 0.005 bcdg Min. 0.013 Max. 0.028 | 0.018 ± 0.005 BCDG Min. 0.013 Max. 0.027 | |
| OER | 0.426 ± 0.049 | 14.688 ± 1.435 | 15.114 ± 1.466 | 0.029 ± 0.003 cf Min. 0.025 Max. 0.033 | 0.028 ± 0.002 CF Min. 0.024 Max. 0.032 | |
| Self-etch | PSE | 0.322 ± 0.070 | 13.496 ± 1.795 | 13.818 ± 1.804 | 0.024 ± 0.006 ac Min. 0.016 Max. 0.040 | 0.024 ± 0.006 AC Min. 0.016 Max. 0.038 |
| ASE | 0.215 ± 0.076 | 14.137 ± 1.972 | 14.352 ± 2.015 | 0.015 ± 0.004 abef Min. 0.010 Max. 0.026 | 0.015 ± 0.004 ABEF Min. 0.010 Max.0.025 | |
| CSE | 0.461 ± 0.137 | 14.306 ± 1.620 | 14.767 ± 1.708 | 0.032 ± 0.009 ag Min. 0.013 Max. 0.043 | 0.031 ± 0.008 AG Min. 0.013 Max. 0.041 | |
| Group | EGV (mm3) | IGV (mm3) | GV (mm3) | |
|---|---|---|---|---|
| Etch and rinse | PER | 0.181 ± 0.069 | 0.187 ± 0.083 | 0.369 ± 0.107 |
| AER | 0.123 ± 0.037 | 0.146 ± 0.078 | 0.269 ± 0.106 | |
| OER | 0.279 ± 0.074 * | 0.147 ± 0.075 * | 0.426 ± 0.049 | |
| Self-etch | PSE | 0.189 ± 0.079 | 0.132 ± 0.087 | 0.322 ± 0.070 |
| ASE | 0.114 ± 0.040 | 0.101 ± 0.075 | 0.215 ± 0.076 | |
| CSE | 0.321 ± 0.121 * | 0.140 ± 0.043 * | 0.461 ± 0.137 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczor-Wiankowska, K.; Puszkarz, A.K.; Palczewska-Komsa, M.; Lipa, S.; Krasowski, M.; Sokołowski, J.; Lewusz-Butkiewicz, K.; Ulacha, K.; Nowicka, A. Internal Adaptation of Composite Fillings Made Using Universal Adhesives—A Micro-Computed Tomography Analysis. Materials 2024, 17, 636. https://doi.org/10.3390/ma17030636
Kaczor-Wiankowska K, Puszkarz AK, Palczewska-Komsa M, Lipa S, Krasowski M, Sokołowski J, Lewusz-Butkiewicz K, Ulacha K, Nowicka A. Internal Adaptation of Composite Fillings Made Using Universal Adhesives—A Micro-Computed Tomography Analysis. Materials. 2024; 17(3):636. https://doi.org/10.3390/ma17030636
Chicago/Turabian StyleKaczor-Wiankowska, Kinga, Adam K. Puszkarz, Mirona Palczewska-Komsa, Sebastian Lipa, Michał Krasowski, Jerzy Sokołowski, Katarzyna Lewusz-Butkiewicz, Katarzyna Ulacha, and Alicja Nowicka. 2024. "Internal Adaptation of Composite Fillings Made Using Universal Adhesives—A Micro-Computed Tomography Analysis" Materials 17, no. 3: 636. https://doi.org/10.3390/ma17030636







