Polishing Mechanism of CMP 4H-SiC Crystal Substrate (0001) Si Surface Based on an Alumina (Al2O3) Abrasive
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Orthogonal Design Experiment
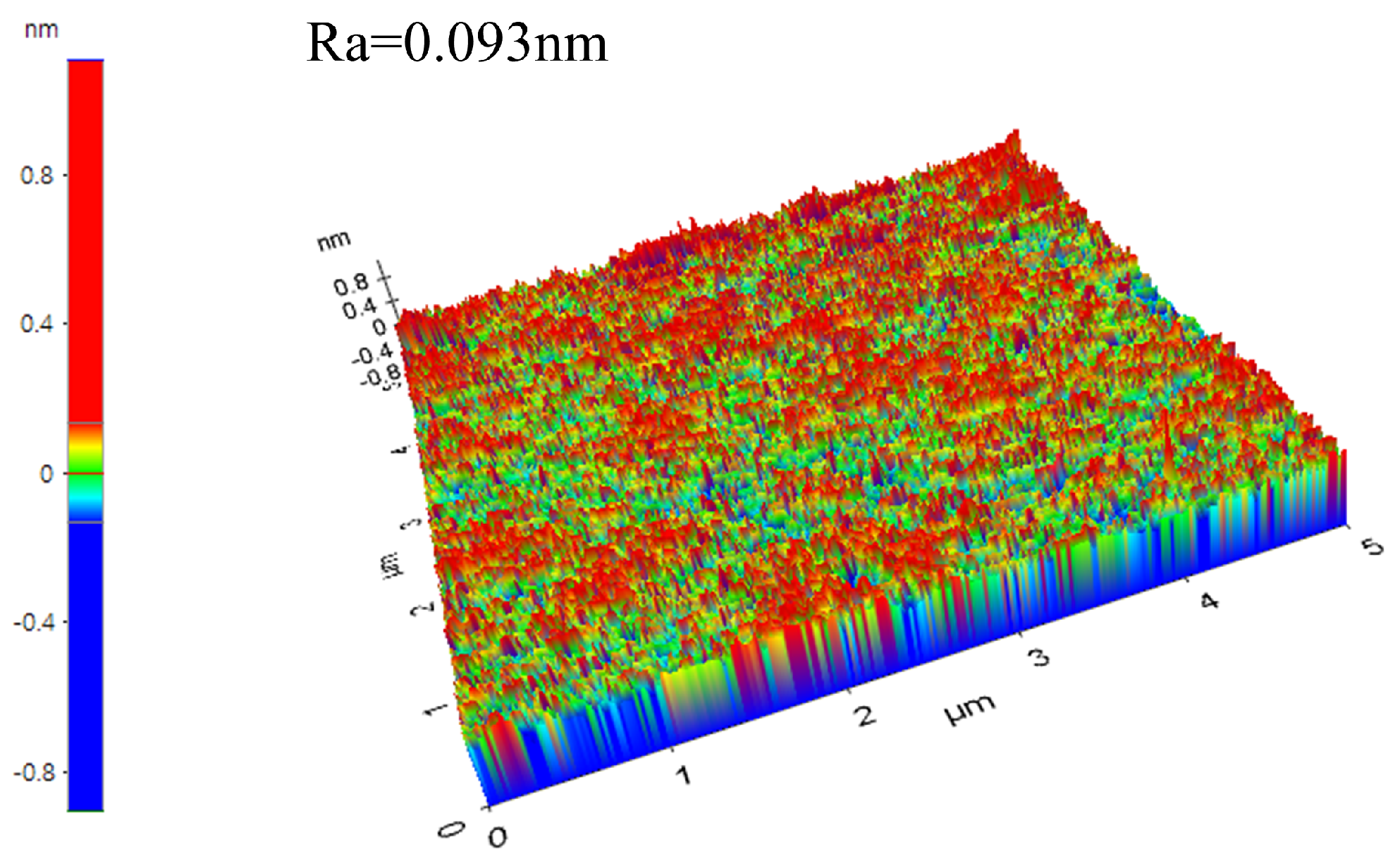
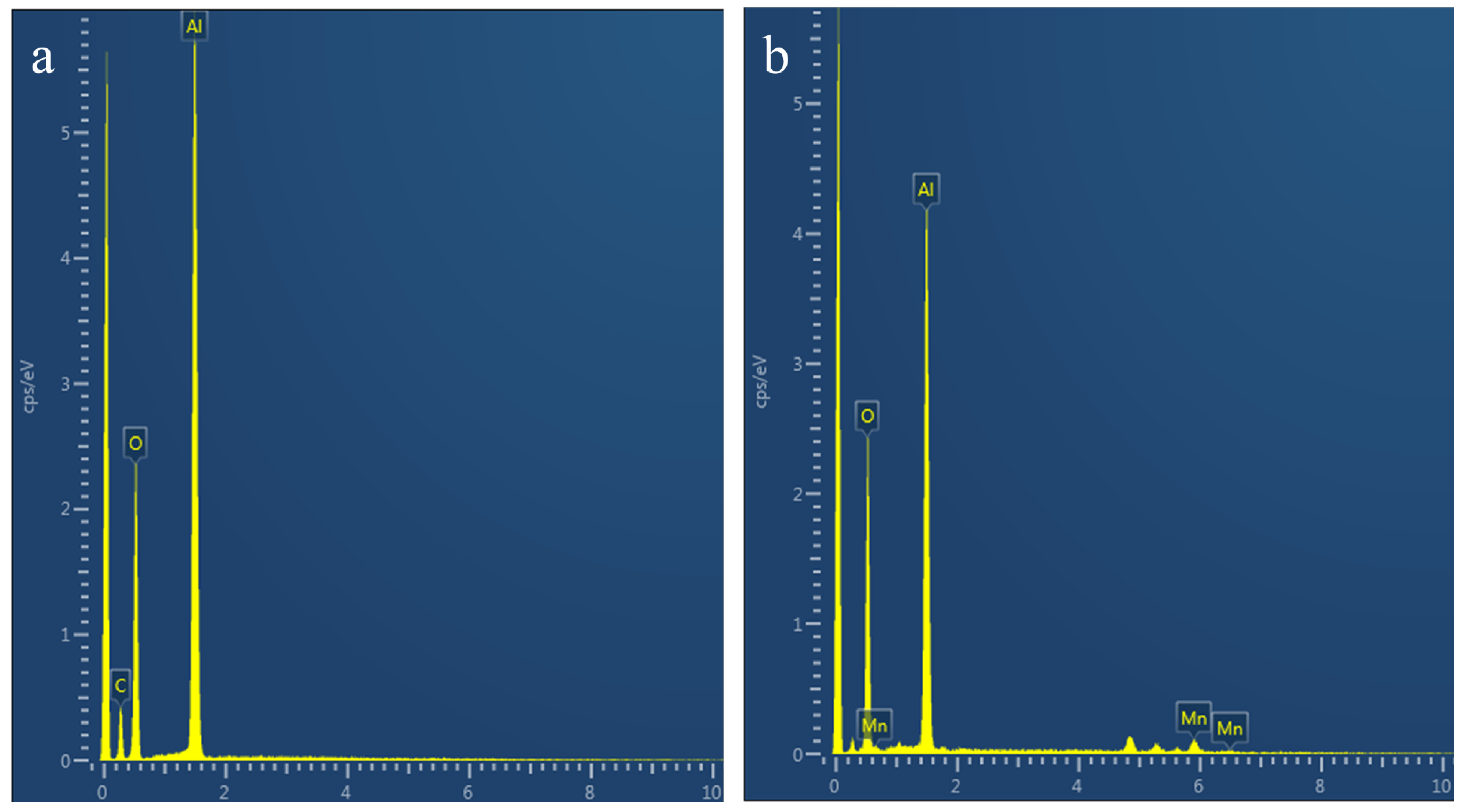
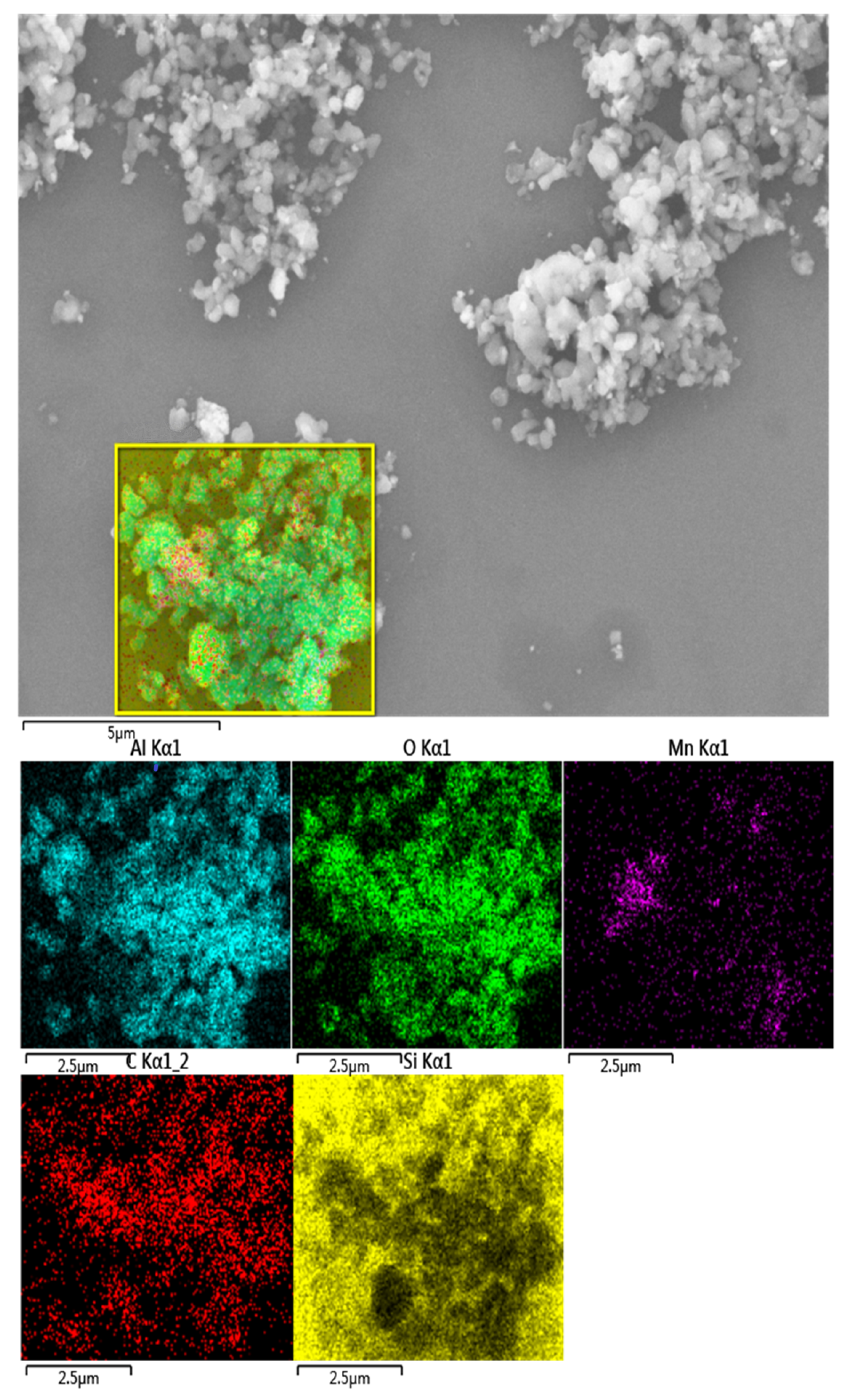
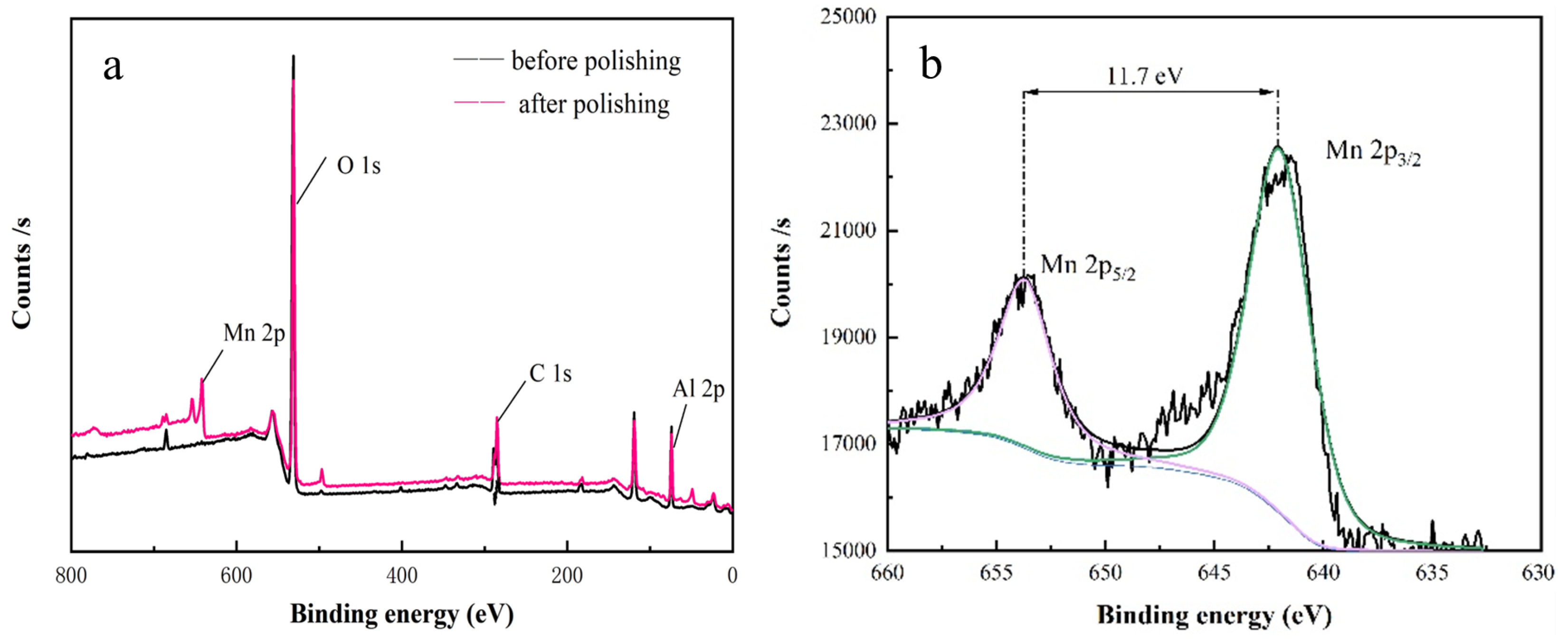
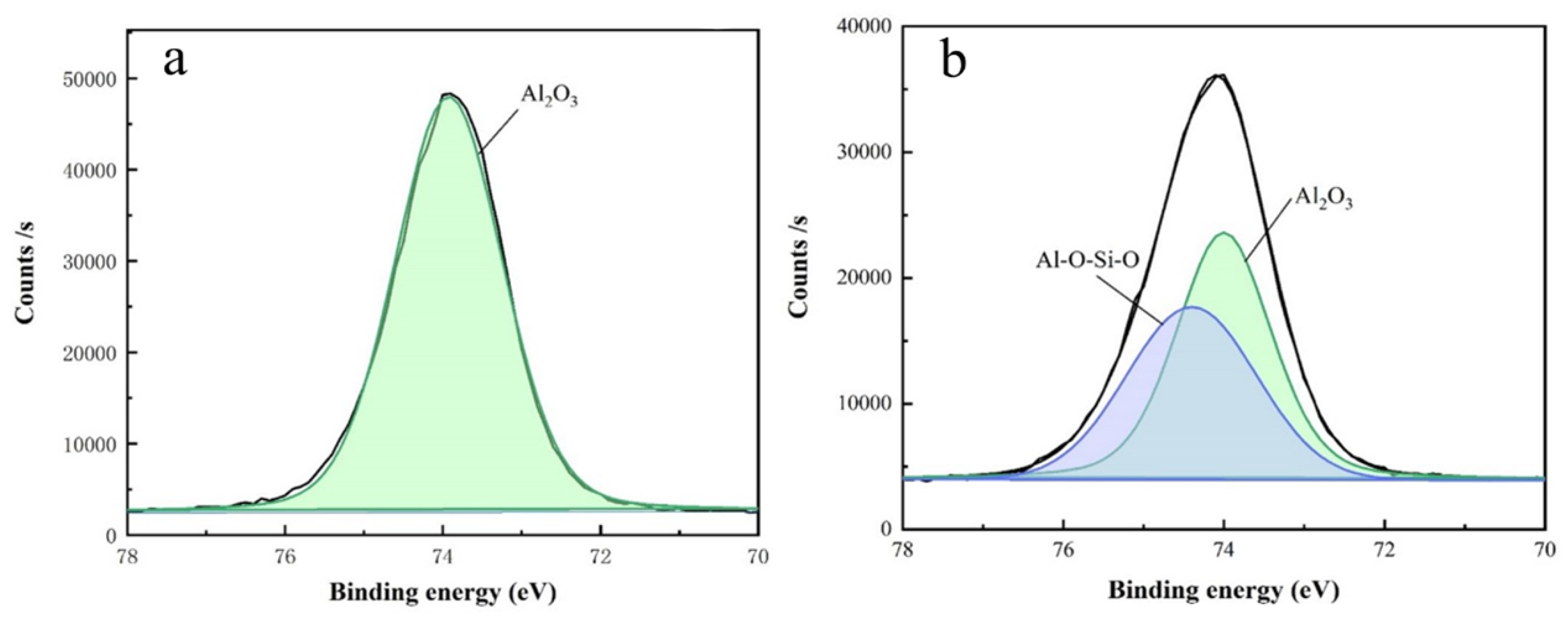
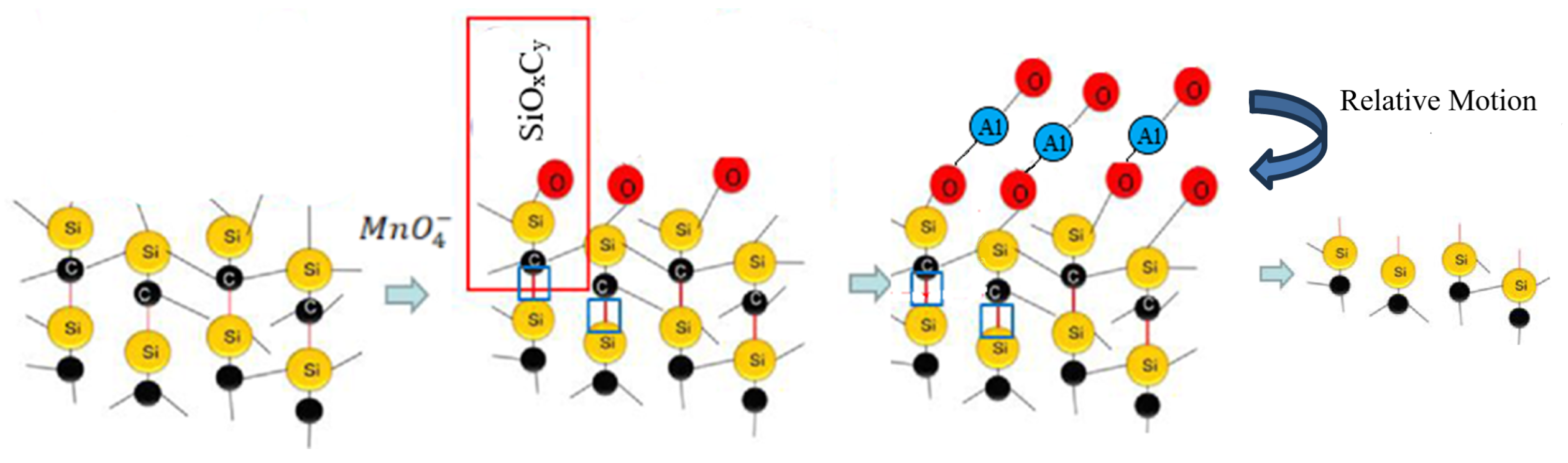
3.2. Analysis of Polishing Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rafin, S.M.S.H.; Ahmed, R.; Haque, M.A.; Hossain, M.K.; Haque, M.A.; Mohammed, O.A. Power Electronics Revolutionized: A Comprehensive Analysis of Emerging Wide and Ultrawide Bandgap Devices. Micromachines 2023, 14, 2045. [Google Scholar] [CrossRef]
- Motohiro, I. Silicon carbide: Fundamentals. Nucl. Instrum. Methods Phys. Res. Sect. A 2001, 466, 406–411. [Google Scholar] [CrossRef]
- Chaudhary, O.S.; Denaï, M.; Refaat, S.S.; Pissanidis, G. Technology and Applications of Wide Bandgap Semiconductor Materials: Current State and Future Trends. Energies 2023, 16, 6689. [Google Scholar] [CrossRef]
- Ranjbar, A. Applications of Wide Bandgap (WBG) Devices in the Transportation Sector. Recent Advances in (WBG) Semiconductor Material (e.g., SiC and GaN) and Circuit Topologies. In Transportation Electrification: Breakthroughs in Electrified Vehicles, Aircraft, Rolling Stock, and Watercraft; Mohamed, A.A., Khan, A.A., Elsayed, A.T., Elshaer, M.A., Eds.; IEEE: Piscataway, NJ, USA, 2022; pp. 47–72. [Google Scholar]
- Lee, H.; Kim, H.; Jeong, H. Approaches to Sustainability in Chemical Mechanical Polishing (CMP): A Review. Int. J. Precis. Eng. Manuf.-Green Technol. 2022, 9, 349–367. [Google Scholar] [CrossRef]
- Akbar, W.; Ertunç, Ö. A Coupled Material Removal Model for Chemical Mechanical Polishing Processes. ECS J. Solid State Sci. 2021, 10, 104003. [Google Scholar] [CrossRef]
- Aida, H.; Doi, T.; Takeda, H.; Katakura, H.; Kim, S.W.; Koyama, K.; Yamazaki, T.; Uneda, M. Ultraprecision CMP for sap phire, GaN, and SiC for advanced optoelectronics materials. Curr. Appl. Phys. 2012, 12, 41–46. [Google Scholar] [CrossRef]
- Lagudu, U.R.K.; Isono, S.; Krishnan, S.; Babu, S.V. Role of ionic strength in chemical mechanical polishing of silicon carbide using silica slurries. Colloids Surf. A Physicochem. Eng. Asp. 2014, 445, 119–127. [Google Scholar] [CrossRef]
- Pan, G.; Zhou, Y.; Luo, G.; Shi, X.; Zou, C.; Gong, H. Chemical mechanical polishing (CMP) of on-axis Si-face 6H-SiC wafer for obtaining atomically flat defect-free surface. J. Mater. Sci. Mater. Electron. 2013, 24, 5040–5047. [Google Scholar] [CrossRef]
- Heydemann, V.; Everson, W.J.; Gamble, R.D.; Snyder, D.W.; Skowronski, M. Chemi-Mechanical Polishing of On-Axis Semi-Insulating SiC Substrates. Mater. Sci. Forum 2004, 457, 805–808. [Google Scholar] [CrossRef]
- Su, J.X.; Du, J.X.; Liu, H.N.; Liu, X.L. Research on Material Removal Rate of CMP 6H-SiC Crystal Substrate (0001) Si Surface Based on Abrasive Alumina (Al2O3). Procedia Eng. 2011, 24, 441–446. [Google Scholar] [CrossRef]
- Chen, G.P.; Li, J.G.; Long, J.Y.; Luo, H.M.; Zhou, Y.; Xie, X.Z.; Pan, G.S. Surface modulation to enhance chemical mechanical polishing performance of sliced silicon carbide Si-face. Appl. Surf. Sci. 2021, 536, 147963. [Google Scholar] [CrossRef]
- Wang, W.T.; Zhang, B.G.; Shi, Y.H.; Zhou, J.K.; Wang, R.; Zeng, N.Y. Improved chemical mechanical polishing performance in 4H-SiC substrate by combining novel mixed abrasive slurry and photocatalytic effect. Appl. Surf. Sci. 2022, 575, 151676. [Google Scholar] [CrossRef]
- Poltavets, V.; Krawczyk, M.; Maslak, G.; Abraimova, O.; Jönsson-Niedziółka, M. Formation of MnO2-coated ITO electrodes with high catalytic activity for enzymatic glucose detection. Dalton Trans. 2023, 52, 13769–13780. [Google Scholar] [CrossRef] [PubMed]
- Keal, M.E.; Courtney, J.M.; Rees, N.V. Redox Electrochemistry of Mn(II) via Carbon Black Nanoparticle Impacts. J. Phys. Chem. C 2023, 127, 13380–13388. [Google Scholar] [CrossRef]
- Kurokawa, S.; Doi, T.; Ohnishi, O.; Yamazaki, T.; Tan, Z.; Yin, T. Characteristics in SiC-CMP using MnO2 slurry with Strong Oxidant under Different Atmospheric Conditions. MRS Online Proc. Libr. 2013, 1560, 301. [Google Scholar] [CrossRef]
- Wang, W.T.; Zhang, B.G.; Shi, Y.H.; Ma, T.D.; Zhou, J.K.; Wang, R.; Wang, H.X.; Zeng, N.Y. Improvement in chemical mechanical polishing of 4H-SiC wafer by activating persulfate through the synergistic effect of UV and TiO2. J. Mater. Process. Technol. 2021, 295, 117150. [Google Scholar] [CrossRef]
- Lagudu, U.R.K. Advances in Chemical Mechanical Planarization (CMP), 2nd ed.; Woodhead Publishing: Cambridge, UK, 2022; pp. 175–194. [Google Scholar]
- Hometz, B.; Michel, H.-J.; Halbritter, J. ARXPS studies of SiO2-SiC interfaces and oxidation of 6H SiC single crystal Si-(001) and C-(00) surfaces. J. Mater. Process. Technol. 1994, 9, 3088–3094. [Google Scholar] [CrossRef]
- Rowson, D.M.; Quinn, T.F.J. Frictional heating and the oxidational theory of wear. J. Phys. D Appl. Phys 1980, 13, 209. [Google Scholar] [CrossRef]
- Quinn, T.F.J.; Winer, W.O. The Thermal Aspects of Oxidational Wear. Wear 1985, 102, 67–68. [Google Scholar] [CrossRef]
- Bowden, F.P.; Ridler, K.E.W. Physical properties of surfaces-III—The surface temperature of sliding metals-The temperature of lubricated surfaces. Proc. R. Soc. Lond. A 1936, 154, 640–656. [Google Scholar] [CrossRef]




| Items | Value |
|---|---|
| Wafer | 2-inch 4H-SiC |
| Polishing machine | Bruker CP-4 |
| Polishing pad | Suba 800 |
| Load (psi) | 4 |
| Polishing head rotation speed (r/min) | 100 |
| Polishing platen speed (r/min) | 90 |
| Polishing fluid flow rate (mL/min) | 90 |
| Polishing time (min) | 60 |
| Test Number | pH Value | Oxidant Content (wt%) | Abrasive Content (wt%) | MRR (μm/h) | Ra (nm) |
|---|---|---|---|---|---|
| 1 | 2 | 1 | 1 | 0.5212 | 0.348 |
| 2 | 2 | 2 | 3 | 0.7665 | 1.277 |
| 3 | 2 | 3 | 5 | 0.7972 | 0.365 |
| 4 | 2 | 4 | 2 | 0.4906 | 0.122 |
| 5 | 2 | 5 | 4 | 0.6439 | 0.113 |
| 6 | 3 | 1 | 5 | 0.5519 | 0.248 |
| 7 | 3 | 2 | 2 | 0.4599 | 0.311 |
| 8 | 3 | 3 | 4 | 0.7052 | 0.355 |
| 9 | 3 | 4 | 1 | 0.6439 | 0.123 |
| 10 | 3 | 5 | 3 | 0.6439 | 1.358 |
| 11 | 4 | 1 | 4 | 0.4906 | 0.176 |
| 12 | 4 | 2 | 1 | 0.7052 | 0.809 |
| 13 | 4 | 3 | 3 | 0.5212 | 1.828 |
| 14 | 4 | 4 | 5 | 0.7052 | 0.129 |
| 15 | 4 | 5 | 2 | 0.7358 | 2.468 |
| 16 | 5 | 1 | 3 | 0.5212 | 0.225 |
| 17 | 5 | 2 | 5 | 0.7052 | 0.766 |
| 18 | 5 | 3 | 2 | 0.7052 | 0.408 |
| 19 | 5 | 4 | 4 | 0.8278 | 0.149 |
| 20 | 5 | 5 | 1 | 0.7972 | 1.970 |
| 21 | 6 | 1 | 2 | 0.5825 | 0.268 |
| 22 | 6 | 2 | 4 | 0.7358 | 0.264 |
| 23 | 6 | 3 | 1 | 0.6745 | 0.210 |
| 24 | 6 | 4 | 3 | 0.7665 | 0.110 |
| 25 | 6 | 5 | 5 | 0.7052 | 0.116 |
| Parameter | pH Value | Oxidant Content (wt%) | Abrasive Content (wt%) |
|---|---|---|---|
| 3.2193 | 2.66742 | 3.34194 | |
| 3.00468 | 3.3726 | 2.97402 | |
| 3.15798 | 3.40326 | 3.2193 | |
| 3.55656 | 3.43392 | 3.40326 | |
| 3.46458 | 3.5259 | 3.46458 | |
| 0.64386 | 0.533484 | 0.668388 | |
| 0.60094 | 0.67452 | 0.594804 | |
| 0.631596 | 0.680652 | 0.64386 | |
| 0.711312 | 0.686784 | 0.680652 | |
| 0.692916 | 0.70518 | 0.692916 | |
| 0.110376 | 0.171696 | 0.097812 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, J.; Wang, W.; Liu, W.; Song, Z. Polishing Mechanism of CMP 4H-SiC Crystal Substrate (0001) Si Surface Based on an Alumina (Al2O3) Abrasive. Materials 2024, 17, 679. https://doi.org/10.3390/ma17030679
Gong J, Wang W, Liu W, Song Z. Polishing Mechanism of CMP 4H-SiC Crystal Substrate (0001) Si Surface Based on an Alumina (Al2O3) Abrasive. Materials. 2024; 17(3):679. https://doi.org/10.3390/ma17030679
Chicago/Turabian StyleGong, Juntao, Weilei Wang, Weili Liu, and Zhitang Song. 2024. "Polishing Mechanism of CMP 4H-SiC Crystal Substrate (0001) Si Surface Based on an Alumina (Al2O3) Abrasive" Materials 17, no. 3: 679. https://doi.org/10.3390/ma17030679





