Effect of Sintering Temperature on Microstructure Characteristics of Porous NiTi Alloy Fabricated via Elemental Powder Sintering
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
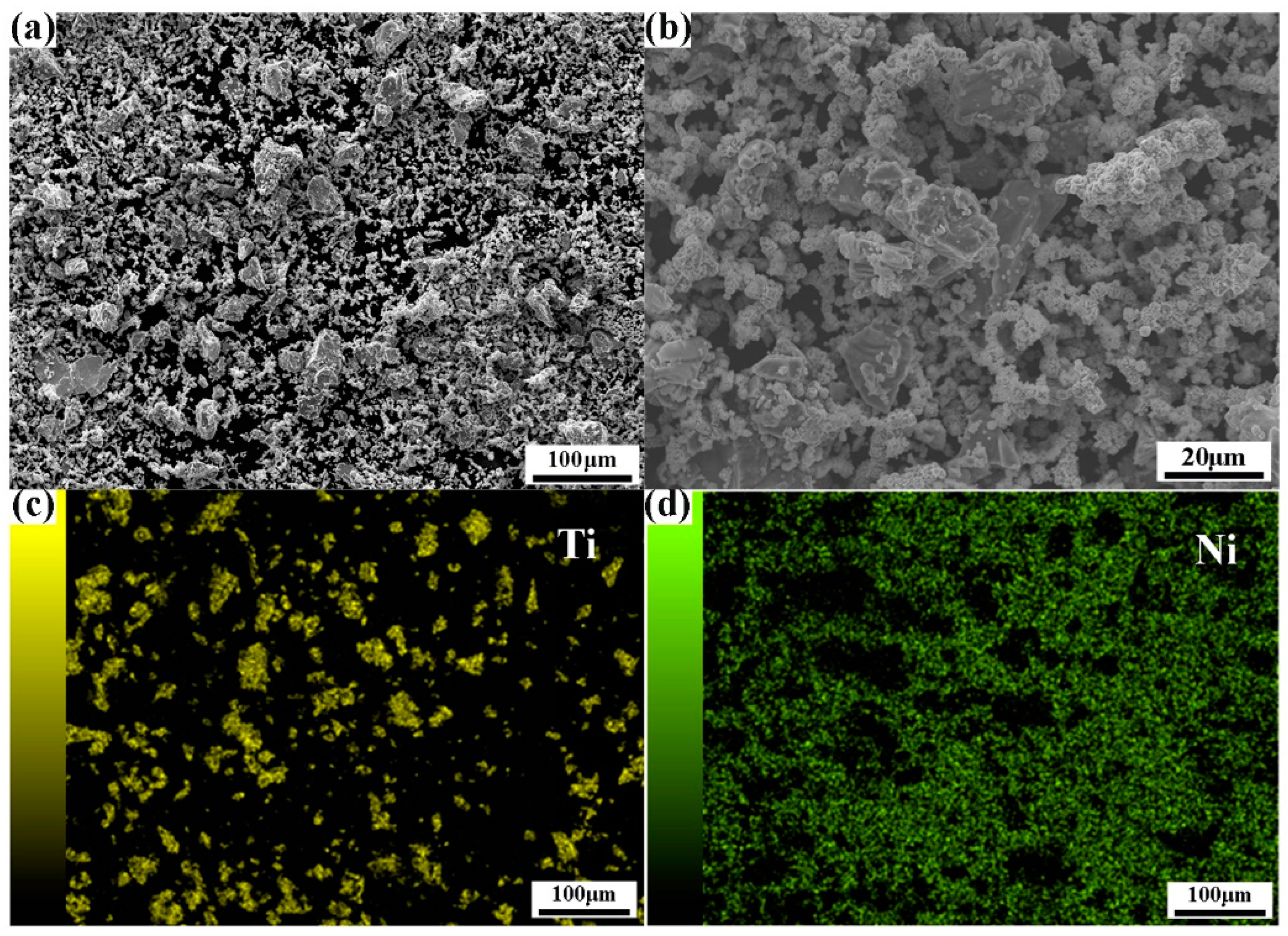
3.1. Characterization of Initial Powders and Mixed Powder
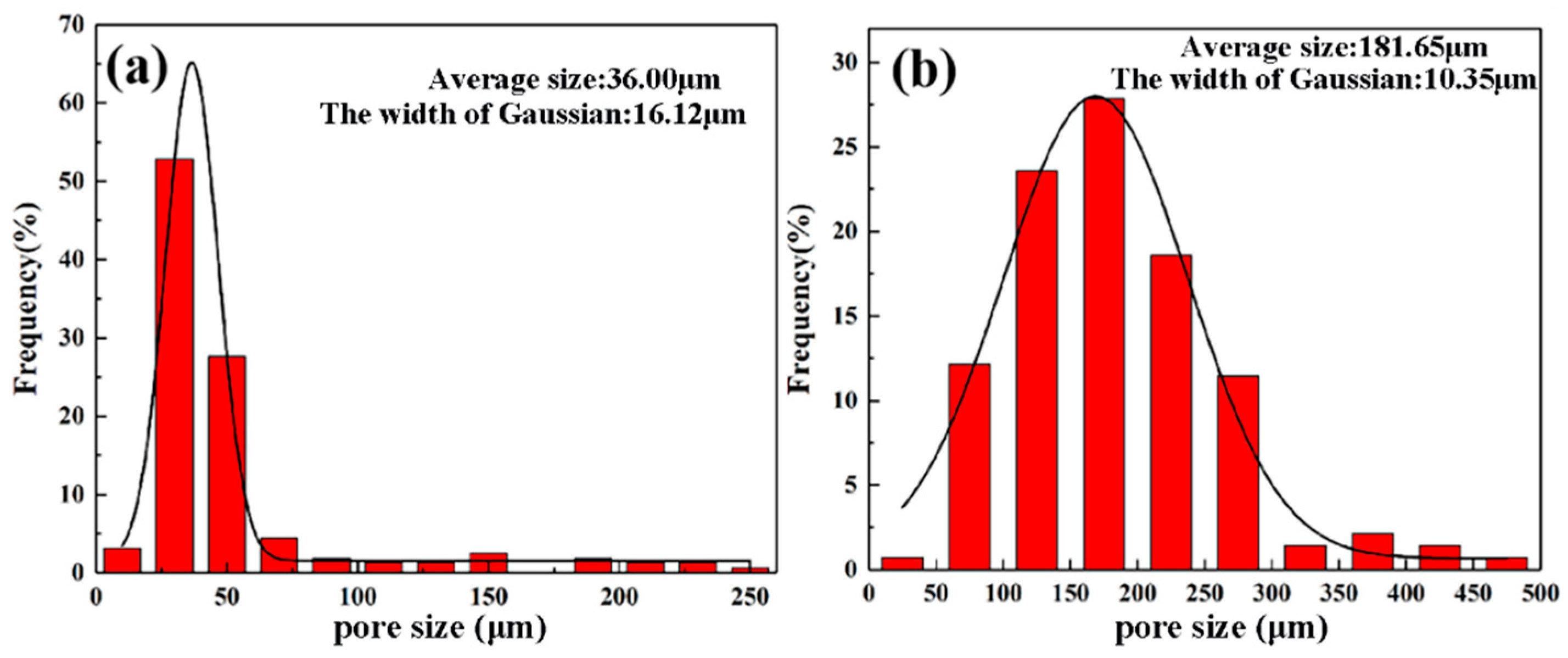
3.2. Effect of Sintering Temperature on Pore Characteristics
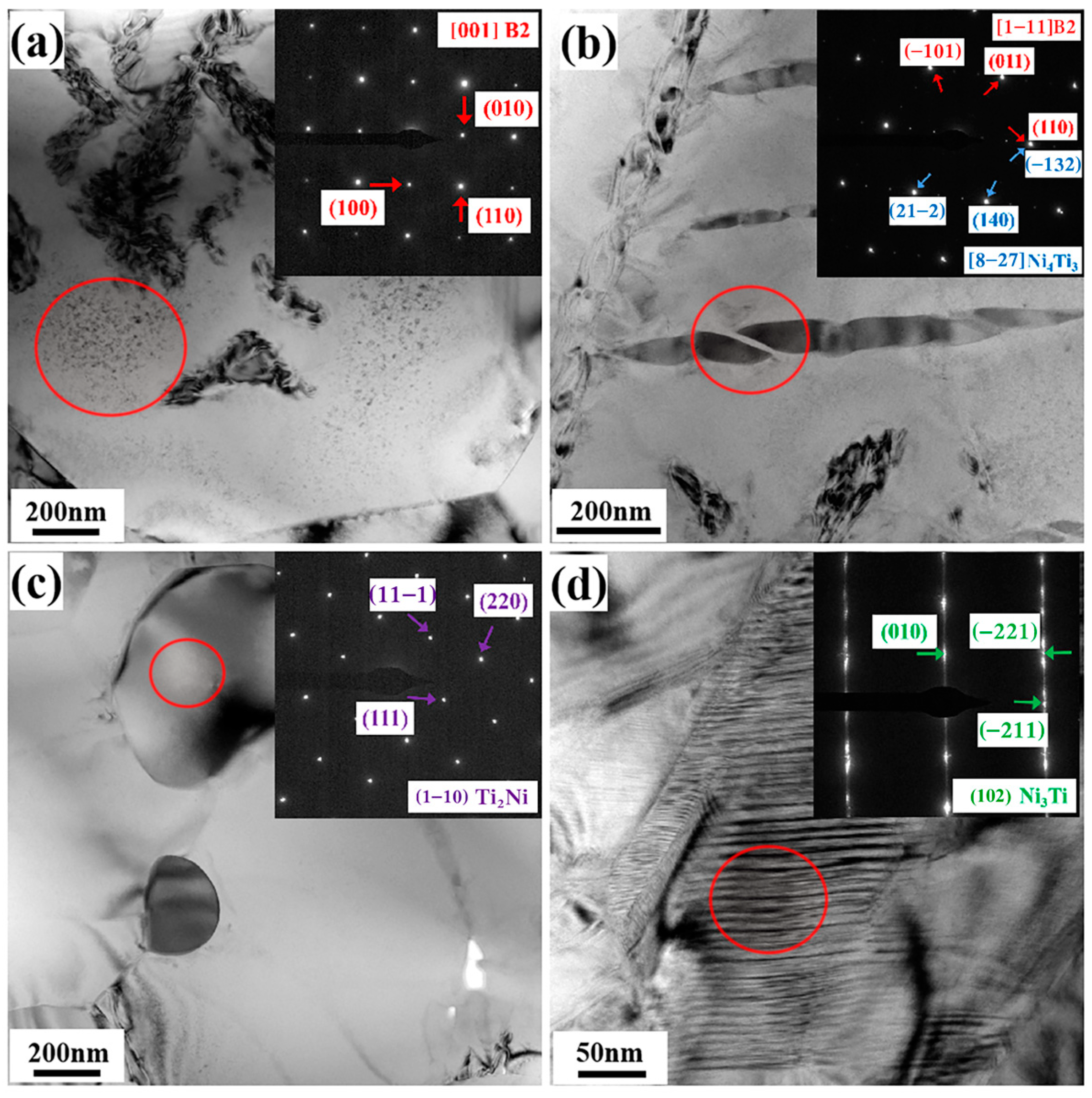
3.3. Effect of Sintering Temperature on Microstructure Characteristics
4. Conclusions
- (1)
- The porous NiTi alloys prepared at 950 °C and 1000 °C have densities of 2.556 g/cm3 and 3.030 g/cm3, porosities of 60.4% and 51.8% and average pore sizes of 36.00 μm and 181.65 μm, respectively. An increase in sintering temperature is useful to improve the densification of sintered porous NiTi alloys.
- (2)
- There is no difference in the phase types within the fabricated porous NiTi alloys prepared at 950 °C and 1000 °C; namely, there are a large number of Ni4Ti3 precipitates with a lens-like morphology, a small amount of Ni3Ti precipitates with a round-shaped morphology and a small number of Ti2Ni precipitates with a block morphology in the B2 NiTi matrix.
- (3)
- The phase distribution within the porous NiTi alloy sintered at 950 °C is obviously different from that within the porous NiTi alloy sintered at 1000 °C; that is, with an increase in the sintering temperature, the number of Ni4Ti3 precipitates decreases obviously. The formation of the Ni4Ti3 phase in the present study is closely related to the enrichment of Ni content in the matrix owing to the diffusion rate difference between Ni atoms and Ti atoms and the absence of TLP during the sintering process owing to the relatively low sintering temperature (lower than the eutectic temperature of 1118 °C). Compared to a sintering temperature of 950 °C, a sintering temperature of 1000 °C would speed up atomic diffusion, which contributes to a reduction in the enrichment of Ni as well as the number of formed Ni4Ti3 precipitates.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilberforce, T.; Alaswad, A.; Baroutaji, A.; Abdelkareem, M.A.; Ramadan, M.; Olabi, A.G.; Sayed, E.T.; Elsaid, K.; Maghrabie, H.M. Future Directions for Shape Memory Alloy Development. Encycl. Smart Mater. 2022, 1, 231–242. [Google Scholar] [CrossRef]
- Shi, G.; Li, L.; Yu, Z.; Sha, P.; Cao, Q.; Xu, Z.; Liu, Y.; Guo, Y.; Si, J.; Liu, J. Effect of Crystallographic Anisotropy on Phase Transformation and Tribological Properties of Ni-Rich NiTi Shape Memory Alloy Fabricated by LPBF. Opt. Laser Technol. 2023, 157, 108731. [Google Scholar] [CrossRef]
- Shukla, U.; Garg, K. Journey of Smart Material from Composite to Shape Memory Alloy (SMA), Characterization and Their Applications—A Review. Smart Mater. Med. 2023, 4, 227–242. [Google Scholar] [CrossRef]
- Zhou, S.; Xie, M.; Wu, C.; Yi, Y.; Chen, D.; Zhang, L.-C. Selective Laser Melting of Bulk Immiscible Alloy with Enhanced Strength: Heterogeneous Microstructure and Deformation Mechanisms. J. Mater. Sci. Technol. 2022, 104, 81–87. [Google Scholar] [CrossRef]
- Bansiddhi, A.; Dunand, D.C. 7-Titanium and NiTi foams for bone replacement. In Bone Substitute Biomaterials; Woodhead Publishing: Sawston, UK, 2014; pp. 142–179. [Google Scholar] [CrossRef]
- Yao, X.; Cao, S.; Zhang, X.P.; Schryvers, D. Microstructural Characterization and Transformation Behavior of Porous Ni50.8Ti49.2. Mater. Today Proc. 2015, 2, S833–S836. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Liu, Z.Q.; Zhang, Q.L.; Zhao, J.F.; Zhang, H.M. Microstructure and mechanical properties of NiTi na-noporous structures fabricated with dealloying process. J. Alloys Compd. 2023, 933, 167804. [Google Scholar] [CrossRef]
- Chen, F.; Yan, Z.Q.; Shi, Q.; Liu, Y.; Liu, B. Preparation and perforbmance characterization of porous NiTi alloys by space-controlled self-propagating method. Rare Metal Mat. Eng. 2020, 3, 1009–1014. [Google Scholar]
- Yang, Q.; Sun, K.H.; Yang, C.; Sun, M.Y.; Peng, H.B.; Shen, X.F.; Huang, S.K.; Chen, J. Compression and super elasticity be-haviors of NiTi porous structures with tiny strut fabricated by selective laser melting. J. Alloys Compd. 2021, 858, 157674. [Google Scholar] [CrossRef]
- Lai, T.; Xu, J.L.; Xiao, Q.F.; Tong, Y.X.; Huang, J.; Zhang, J.P.; Luo, J.M.; Liu, Y. Preparation and characterization of porous NiTi alloys synthesized by microwave sintering using Mg space holder. Trans. Nonferrous Met. Soc. China 2021, 31, 485–498. [Google Scholar] [CrossRef]
- Ismail, M.H.; Goodall, R.; Davies, H.A.; Todd, T. Formation of microporous NiTi by transient liquid phase sintering of elemental. Powders. Mater. Sci. Eng. C 2012, 32, 1480–1485. [Google Scholar] [CrossRef]
- Zhu, S.L.; Yang, X.J.; Fu, D.H.; Zhang, Z.Y.; Li, C.Y.; Cui, Z.D. Stress–strain behavior of porous NiTi alloys prepared by powders sintering. Mater. Sci. Eng. A 2005, 408, 264–268. [Google Scholar] [CrossRef]
- Bansiddhi, A.; Dunand, D. Shape-memory NiTi foams produced by replication of NaCl space-holders. Acta Biomater. 2008, 4, 1996–2007. [Google Scholar] [CrossRef]
- Hu, L.; Shen, Z.Y.; Chen, X.Y.; Hu, K.Y.; Tang, M.; Wang, L. Microstructure Characteristics of Porous NiTi Shape Memory Alloy Synthesized by Powder Metallurgy during Compressive Deformation at Room Temperature. Metals 2023, 13, 1806. [Google Scholar] [CrossRef]
- Deng, X.; He, Y.H.; Jiao, M.Q.; Zhang, Y.Q.; Jiang, Y.H. Effects of sintering temperatures on microstructure and properties of NiTi/surface porous Ti graded alloy by spark plasma sintering. Chin. J. Nonferrous Met. 2019, 29, 2312–2320. [Google Scholar] [CrossRef]
- Li, B.Y.; Rong, L.J.; Li, Y.Y. The influence of addition of TiH2 in elemental powder sintering porous Ni–Ti alloys. Mater. Sci. Eng. A 2000, 281, 169–175. [Google Scholar] [CrossRef]
- Zeng, Z.; Oliveira, J.P.; Ao, S.; Zhang, W.; Cui, J.; Yan, S.; Peng, B. Fabrication and characterization of a novel bionic manipulator using a laser processed NiTi shape memory alloy. Opt. Laser Technol. 2020, 122, 105876. [Google Scholar] [CrossRef]
- Zhu, S.L.; Yang, X.J.; Deng, S.H.; Cui, Z.D. Processing of porous TiNi shape memory alloy from elemental powders by Ar-sintering. Mater. Lett. 2004, 58, 2369–2373. [Google Scholar] [CrossRef]
- Lu, H.Z.; Liu, L.H.; Yang, C.; Luo, X.; Song, C.H.; Wang, Z.; Wang, J.; Su, Y.D.; Ding, Y.F.; Zhang, L.C.; et al. Simultaneous enhancement of mechanical and shape memory properties by heat-treatment homogenization of Ti2Ni precipitates in TiNi shape memory alloy fabricated by selective laser melting. J. Mater. Sci. Technol. 2022, 101, 205–216. [Google Scholar] [CrossRef]
- Yan, B.Y.; Zhang, Y.Q.; Jiang, S.Y.; Yu, J.B.; Sun, D.; Tang, M. Mechanical properties and fracture mechanisms of martensitic NiTi shape memory alloy based on various thermomechanical-processing microstructures. J. Alloys Compd. 2021, 833, 160797. [Google Scholar] [CrossRef]
- Barrabés, M.; Sevilla, P.; Planell, J.A.; Gil, F.J. Mechanical properties of nickel–titanium foams for reconstructive ortho-paedics. Mater. Sci. Eng. C 2008, 28, 23–27. [Google Scholar] [CrossRef]
- Hagihara, K.; Tanaka, T.; Fujimoto, H.; Nakano, T.; Umakoshi, Y. Microstructure and plastic deformation behavior of Ni3(Ti,X) (X = Nb,Al) single crystals with long-period geometrically closely packed crystal structures. Intermetallics 2006, 14, 1332–1338. [Google Scholar] [CrossRef]
- Jafar, K.A.; Antonin, D.; Gunther, E. Ni4Ti3-precipitation during aging of NiTi shape memory alloys and its influence on martensitic phase transformations. Acta Mater. 2002, 50, 4255–4274. [Google Scholar] [CrossRef]
- Li, D.Y.; He, H.; Lou, J.; He, H.; He, Z.Y.; Luo, F.H.; Li, Y.M.; Shu, C. Local formation of Ni4Ti3 in non-equilibrium state and its influence on the transformation temperature of Ti-rich NiTi alloys. J. Alloys Compd. 2021, 852, 157065. [Google Scholar] [CrossRef]



| Temperature | Measured Data | Location 1 | Location 2 | Location 3 | Location 4 | Location 5 | Location 6 | Location 7 | Location 8 | Average Value | Standard Error |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 950 °C | Density (g/cm3) | 2.453 | 2.993 | 2.510 | 2.352 | 2.224 | 2.949 | 2.497 | 2.477 | 2.556 | 0.27 |
| Porosity (%) | 62.0 | 53.6 | 61.2 | 63.5 | 65.5 | 54.4 | 61.2 | 61.7 | 60.4 | 4.19 | |
| 1000 °C | Density (g/cm3) | 2.550 | 3.080 | 2.896 | 3.061 | 3.190 | 3.082 | 3.201 | 3.202 | 3.030 | 0.30 |
| Porosity (%) | 59.8 | 50.8 | 54.2 | 51.4 | 49.3 | 51.0 | 49.1 | 48.9 | 51.8 | 3.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, T.; Zhan, S.; Chen, X.; Hu, L. Effect of Sintering Temperature on Microstructure Characteristics of Porous NiTi Alloy Fabricated via Elemental Powder Sintering. Materials 2024, 17, 743. https://doi.org/10.3390/ma17030743
Miao T, Zhan S, Chen X, Hu L. Effect of Sintering Temperature on Microstructure Characteristics of Porous NiTi Alloy Fabricated via Elemental Powder Sintering. Materials. 2024; 17(3):743. https://doi.org/10.3390/ma17030743
Chicago/Turabian StyleMiao, Tianhu, Sha Zhan, Xiaojuan Chen, and Li Hu. 2024. "Effect of Sintering Temperature on Microstructure Characteristics of Porous NiTi Alloy Fabricated via Elemental Powder Sintering" Materials 17, no. 3: 743. https://doi.org/10.3390/ma17030743
APA StyleMiao, T., Zhan, S., Chen, X., & Hu, L. (2024). Effect of Sintering Temperature on Microstructure Characteristics of Porous NiTi Alloy Fabricated via Elemental Powder Sintering. Materials, 17(3), 743. https://doi.org/10.3390/ma17030743






