Preparation and Performance Study of High-Strength and Corrosion-Resistant Cement-Based Materials Applied in Coastal Acid Rain Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Methods
2.2.1. Mix Proportion Optimization Design
2.2.2. Mix Proportion Optimization Design
2.2.3. Durability Test
- Acid corrosion resistance test
- 2.
- Chloride ion permeability coefficient test
2.2.4. X-ray Diffraction (XRD) Analysis
2.2.5. Microscopic Morphology Observation
2.2.6. Pore Structure Test
2.2.7. Statistical Analysis
- 1
- Range analysis
- 2
- Variance analysis
- 3.
- Comprehensive scoring evaluation
3. Results and Analysis
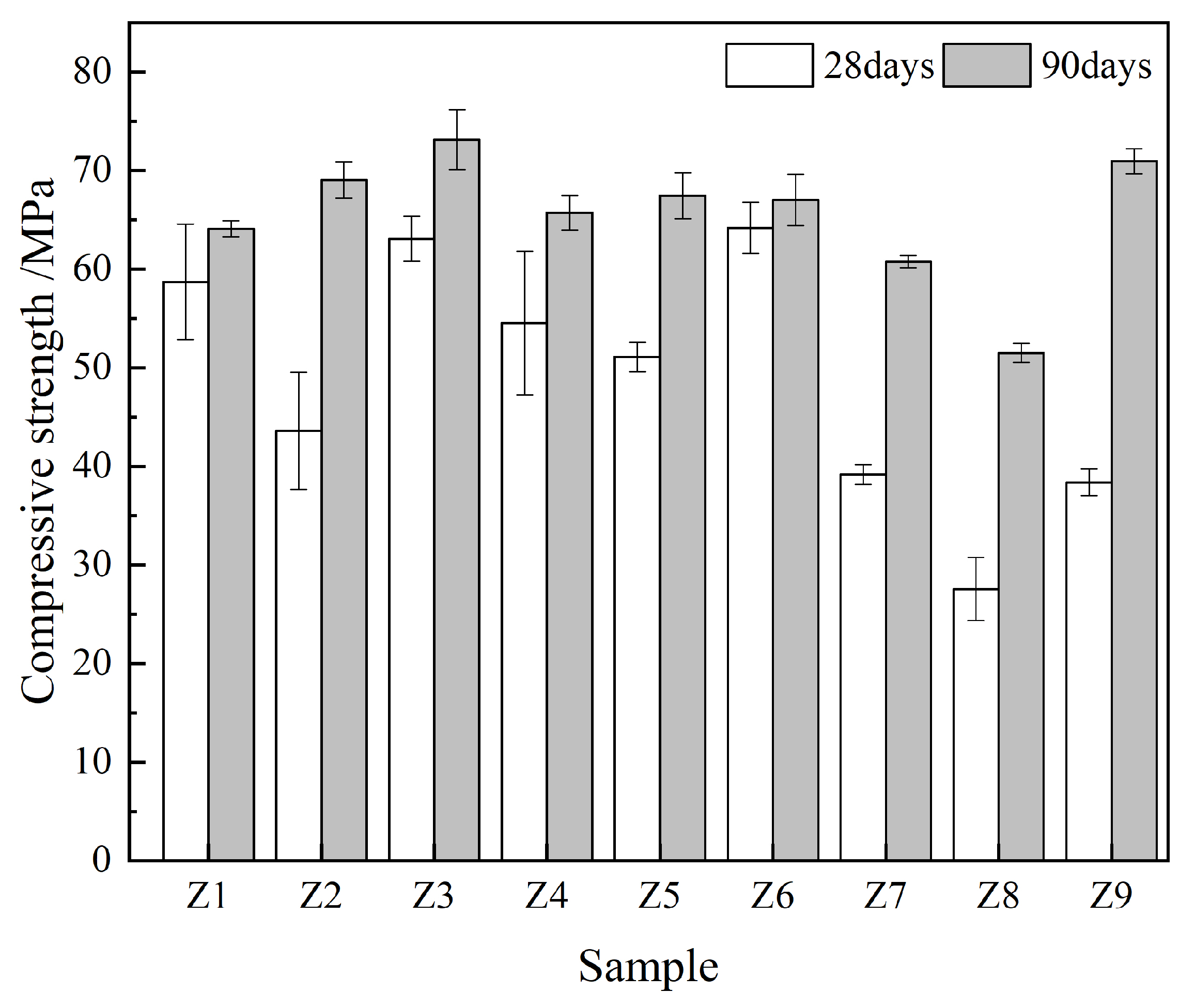
3.1. Compressive Strength
3.2. Durability
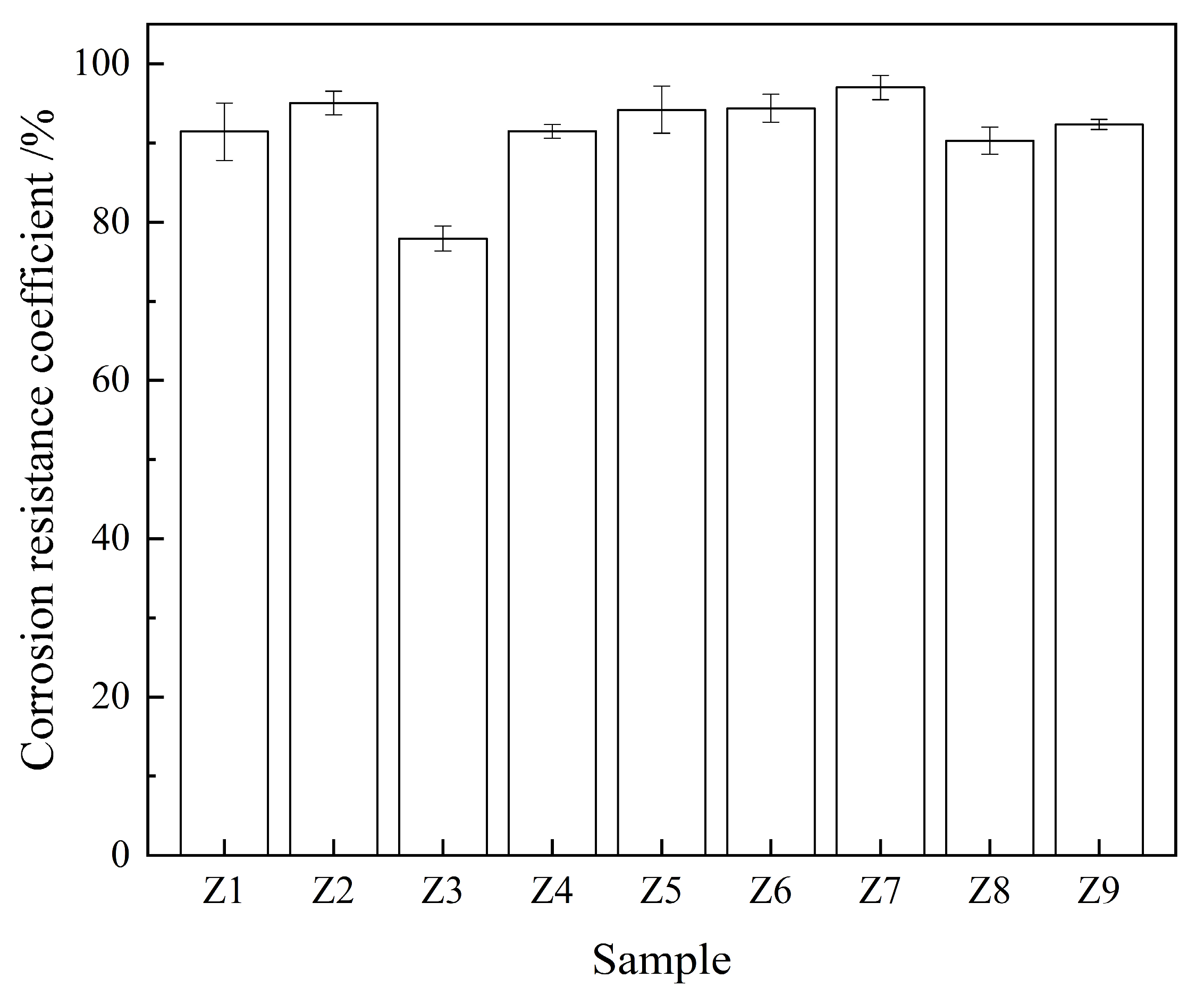
3.2.1. Acid Corrosion Resistance
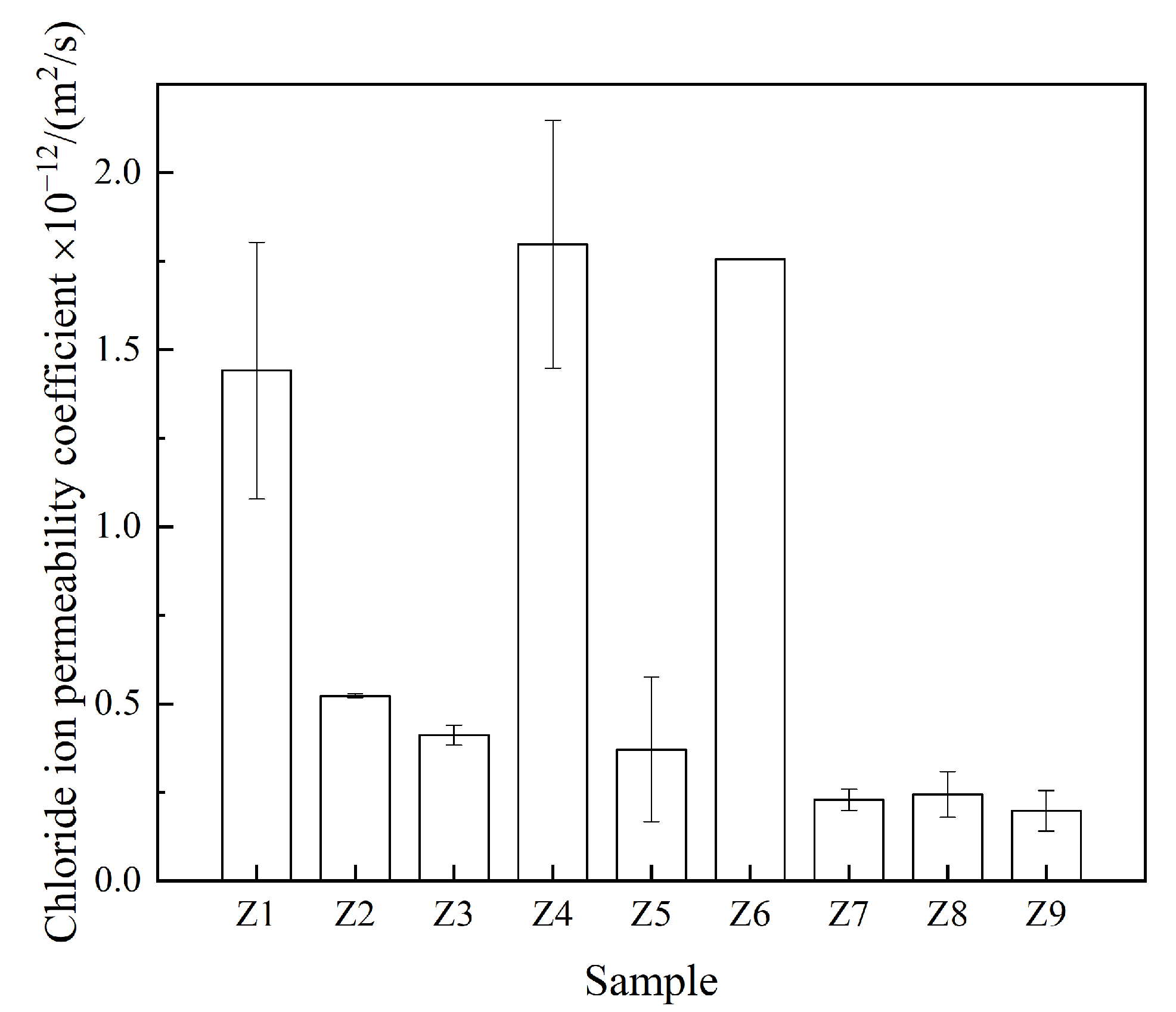
3.2.2. Resistance to Chloride Ion Penetration
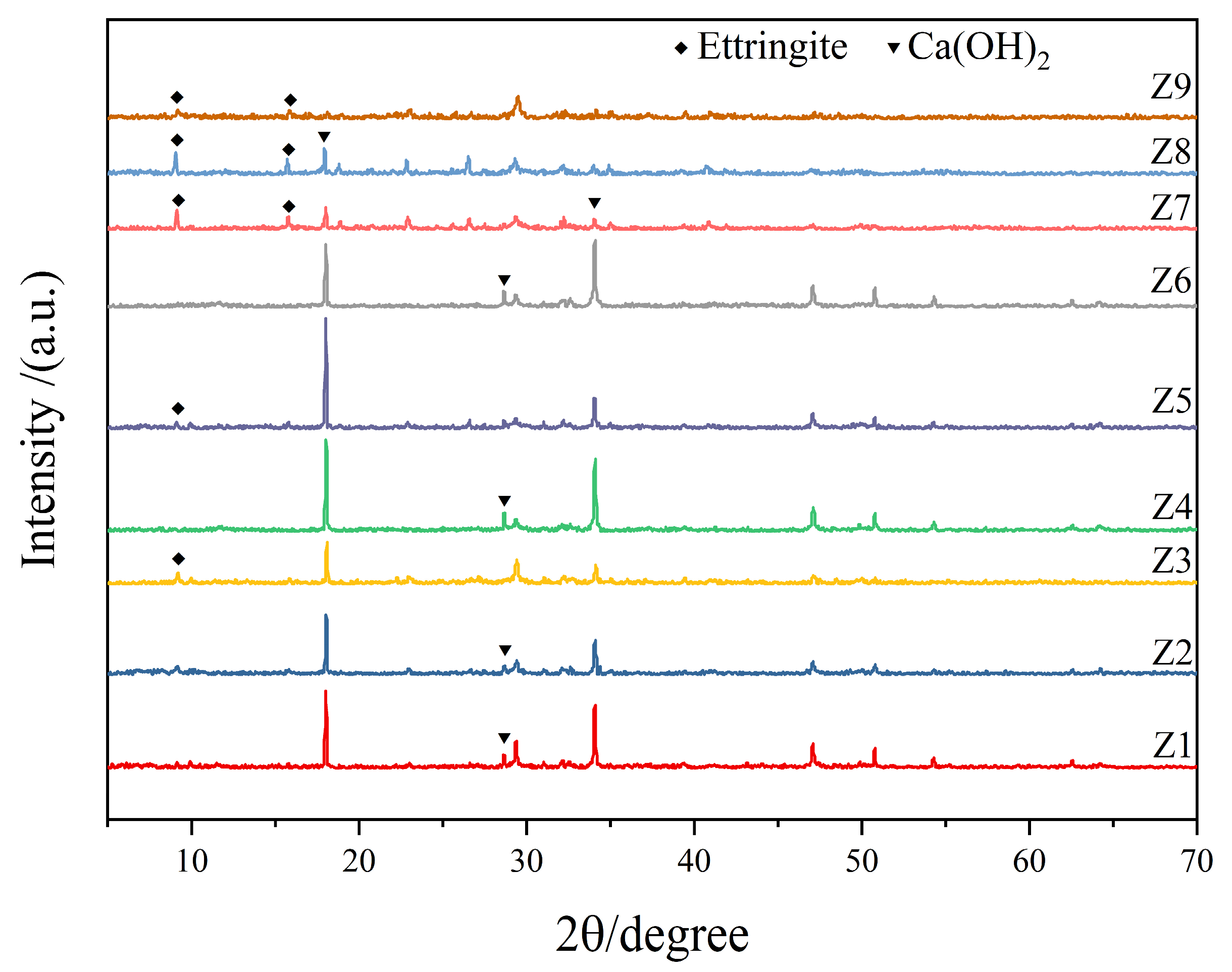
3.3. XRD
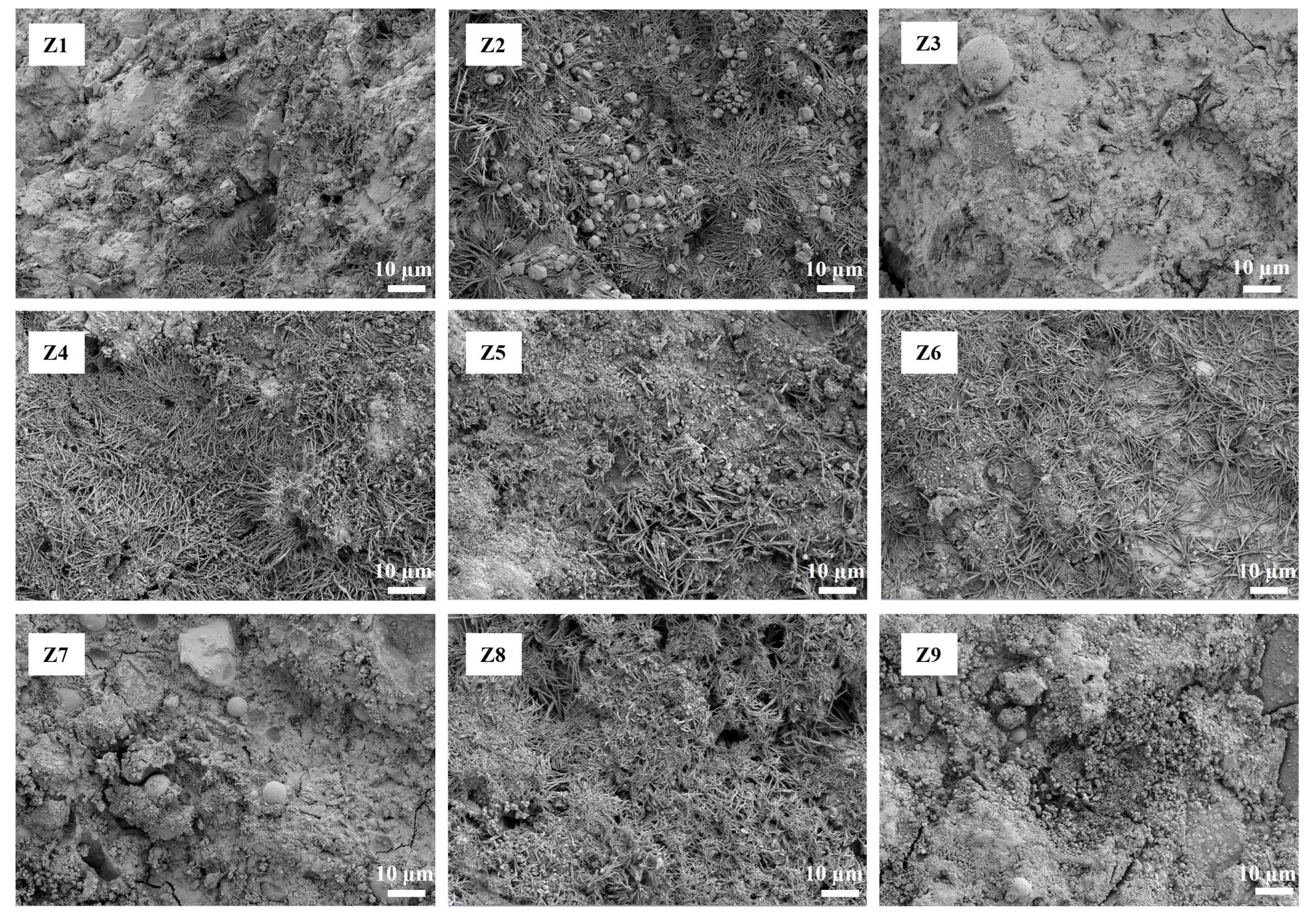
3.4. Microscopic Morphology Observation
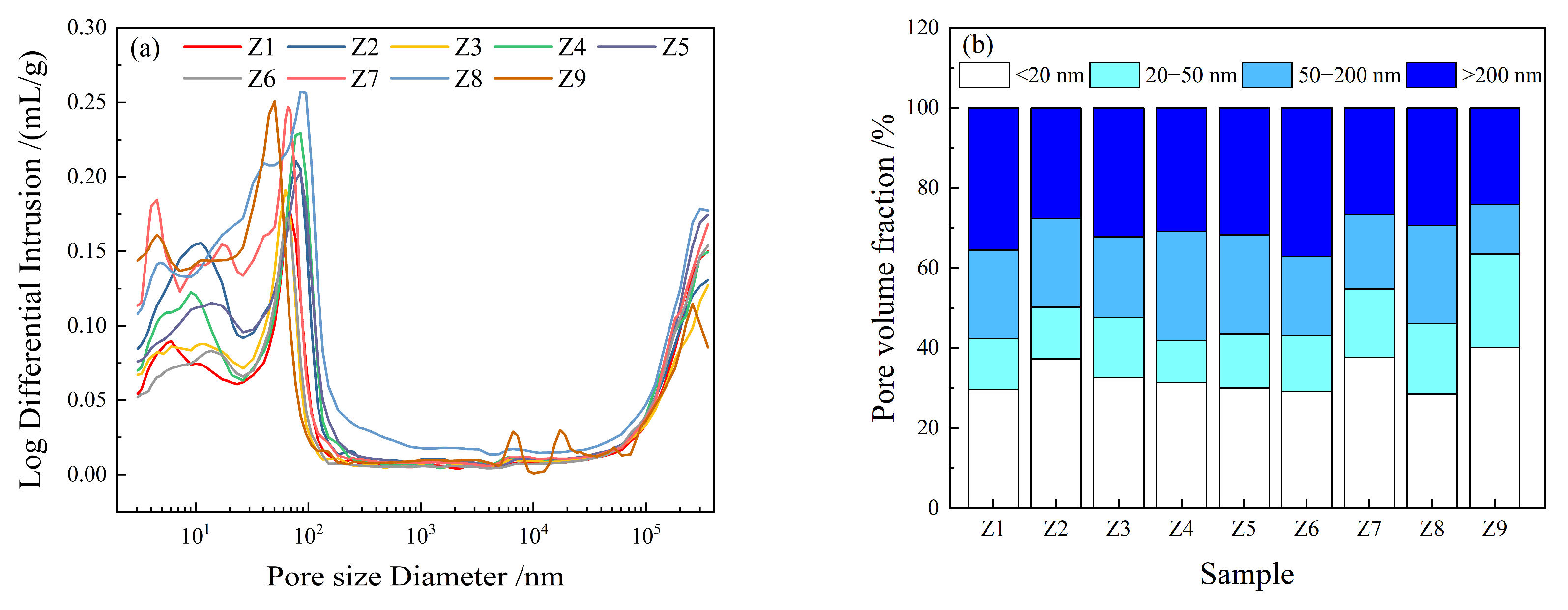
3.5. Pore Structure
3.6. Statistical Analysis
3.6.1. Range Analysis
3.6.2. Variance Analysis
3.6.3. Comprehensive Scoring Evaluation
4. Discussion
5. Conclusions
- (1)
- The addition of the FA–GBFS–SF–DG cementitious system to cement can increase the compressive strength of the cementitious material by up to 9.1% and the acid corrosion resistance coefficient by 6%, and the chloride ion permeability coefficient is reduced by 89% compared to ordinary cement materials.
- (2)
- The addition of the FA–GBFS–SF–DG cementitious system to cement consumed CH crystals produced via cement hydration, promoted the formation of AFt, and optimized the pore structure of the cementitious materials.
- (3)
- Compared with the water–binder and cementitious material ratios, the cement replacement ratio significantly reduced the chloride ion permeability coefficient of the cementitious materials.
- (4)
- When a FA:GBFS:SF:DG ratio of 2:6:1:1 was implemented to replace 60% of cement, the compressive strength, acid corrosion resistance, and resistance to chloride ion permeability improved. This ratio is expected to benefit concrete structures in coastal acid rain environments.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- James, A.; Bazarchi, E.; Chiniforush, A.A.; Aghdam, P.P.; Hosseini, M.R.; Akbarnezhad, A.; Martek, I.; Ghodoosi, F. Rebar corrosion detection, protection, and rehabilitation of reinforced concrete structures in coastal environments: A review. Constr. Build. Mater. 2019, 224, 1026–1039. [Google Scholar] [CrossRef]
- Liu, J.; Ou, G.F.; Qiu, Q.W.; Xing, F.; Tang, K.F.; Zeng, J.X. Atmospheric chloride deposition in field concrete at coastal region. Constr. Build. Mater. 2018, 190, 1015–1022. [Google Scholar] [CrossRef]
- Zhang, W.M.; Ba, H.J. Accelerated Life Test of Concrete in Chloride Environment. J. Mater. Civ. Eng. 2011, 23, 330–334. [Google Scholar] [CrossRef]
- Xie, S.D.; Qi, L.; Zhou, D. Investigation of the effects of acid rain on the deterioration of cement concrete using accelerated tests established in laboratory. Atmos. Environ. 2004, 38, 4457–4466. [Google Scholar] [CrossRef]
- Du, J.M.; Tang, Z.Y.; Li, G.; Yang, H.; Li, L.L. Key inhibitory mechanism of external chloride ions on concrete sulfate attack. Constr. Build. Mater. 2019, 225, 611–619. [Google Scholar] [CrossRef]
- Jin, Z.Q.; Zhao, X.; Zhao, T.J.; Li, J.Q. Chloride ions transportation behavior and binding capacity of concrete exposed to different marine corrosion zones. Constr. Build. Mater. 2018, 177, 170–183. [Google Scholar]
- Muralidharan, S.; Vedalakshmi, R.; Saraswathi, V.; Joseph, J.; Palaniswamy, N. Studies on the aspects of chloride ion determination in different types of concrete under macro-cell corrosion conditions. Build. Environ. 2005, 40, 1275–1281. [Google Scholar] [CrossRef]
- Masuda, Y.; Wu, F.R.; Nakamura, S.; Sato, S. Penetration behavior of chloride ions into concrete in coastal areas. In Environmental Ecology and Technology of Concrete; Feng, N.Q., Peng, G.F., Eds.; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2006; pp. 584–590. Available online: https://www.google.com/search?q=Penetration+Behavior+of+Chloride+Ions+into+Concrete+in+Coastal+Areas+%7C+Scientific.Net&rlz=1C1CHBF_enRS855RS855&oq=Penetration+Behavior+of+Chloride+Ions+into+Concrete+in+Coastal+Areas+%7C+Scientific.Net&gs_lcrp=EgZjaHJvbWUqBggAEEUYOzIGCAAQRRg70gEHNTAyajBqOagCALACAA&sourceid=chrome&ie=UTF-8 (accessed on 18 March 2023).
- Fan, Y.; Hu, Z.; Zhang, Y.; Liu, J. Deterioration of compressive property of concrete under simulated acid rain environment. Constr. Build. Mater. 2010, 24, 1975–1983. [Google Scholar] [CrossRef]
- Zhou, C.L.; Zhu, Z.M.; Zhu, A.J.; Zhou, L.; Fan, Y.; Lang, L. Deterioration of mode II fracture toughness, compressive strength and elastic modulus of concrete under the environment of acid rain and cyclic wetting-drying. Constr. Build. Mater. 2019, 228, 116809. [Google Scholar] [CrossRef]
- Fan, Y.F.; Luan, H.Y. Pore structure in concrete exposed to acid deposit. Constr. Build. Mater. 2013, 49, 407–416. [Google Scholar] [CrossRef]
- Liu, Z.; Takasu, K.; Koyamada, H.; Suyama, H. A study on engineering properties and environmental impact of sustainable concrete with fly ash or GGBS. Constr. Build. Mater. 2022, 316, 125776. [Google Scholar] [CrossRef]
- Behl, V.; Singh, V.; Dahiya, V.; Kumar, A. Characterization of physico-chemical and functional properties of fly ash concrete mix. Mater. Today Proc. 2022, 50, 941–945. [Google Scholar] [CrossRef]
- Karumanchi, M.; Bellum, R.R.; Chennupati, M.; Kunchala, V.; Regulagunta, M. Influence on mechanical properties of concrete of cement replacement with fly ash and river sand replacement with foundry sand. Mater. Today Proc. 2022, 65, 3547–3551. [Google Scholar] [CrossRef]
- Kumar, B.G.; Muthu, M.; Chajec, A.; Sadowski, Ł.; Govindaraj, V. The effect of silica fume on the washout resistance of environmentally friendly underwater concrete with a high-volume of siliceous fly ash. Constr. Build. Mater. 2022, 327, 127058. [Google Scholar] [CrossRef]
- Huseien, G.F.; Khamehchi, M.; Kubba, Z.; Benjeddou, O.; Mahmoodi, M.J. Freeze-thaw cycle and abrasion resistance of alkali-activated FA and POFA-based mortars: Role of high volume GBFS incorporation. Heliyon 2023, 9, e17672. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Mirza, J.; Tahir, M.M. Evaluation of alkali-activated mortars containing high volume waste ceramic powder and fly ash replacing GBFS. Constr. Build. Mater. 2019, 210, 78–92. [Google Scholar] [CrossRef]
- Kaplan, G.; Bayraktar, O.Y.; Li, Z.; Bodur, B.; Yılmazoglu, M.U.; Alcan, B.A. Improving the eco-efficiency of fiber reinforced composite by ultra-low cement content/high FA-GBFS addition for structural applications: Minimization of cost, CO2 emissions and embodied energy. J. Build. Eng. 2023, 76, 107280. [Google Scholar] [CrossRef]
- Zhao, J.; Song, K.; Wang, Z.; Wu, D. Effect of Nano-SiO2/Steel Fiber on the Mechanical Properties and Sulfate Resistance of High-Volume Fly Ash Cement Materials. Constr. Build. Mater. 2023, 409, 133737. [Google Scholar] [CrossRef]
- Padavala, A.B.; Potharaju, M.; Kode, V.R. Mechanical properties of ternary blended mix concrete of fly ash and silica fume. Mater. Today Proc. 2021, 43, 2198–2202. [Google Scholar] [CrossRef]
- Li, Y.; Kwan, A. Ternary blending of cement with fly ash microsphere and condensed silica fume to improve the performance of mortar. Cem. Concr. Compos. 2014, 49, 26–35. [Google Scholar] [CrossRef]
- Anwar, M.; Emarah, D.A. Pore structure of concrete containing ternary cementitious blends. Results Mater. 2019, 1, 100019. [Google Scholar] [CrossRef]
- Anwar, M.; Emarah, D.A. Resistance of concrete containing ternary cementitious blends to chloride attack and carbonation. J. Mater. Res. Technol. 2020, 9, 3198–3207. [Google Scholar] [CrossRef]
- Jiang, L.; Li, C.; Wang, C.; Xu, N.; Chu, H. Utilization of flue gas desulfurization gypsum as an activation agent for high-volume slag concrete. J. Clean. Prod. 2018, 205, 589–598. [Google Scholar] [CrossRef]
- Nochaiya, T.; Suriwong, T.; Julphunthong, P. Acidic corrosion-abrasion resistance of concrete containing fly ash and silica fume for use as concrete floors in pig farm. Case Stud. Constr. Mater. 2022, 16, e01010. [Google Scholar] [CrossRef]
- Dong, C.R.; Zhang, Q.Y.; Chen, C.; Jiang, T.X.; Guo, Z.G.; Liu, Y.; Lin, S.L. Fresh and hardened properties of recycled plastic fiber reinforced self-compacting concrete made with recycled concrete aggregate and fly ash, slag, silica fume. J. Build. Eng. 2022, 62, 105384. [Google Scholar] [CrossRef]
- Yan, J.; Zhu, Z.; Liu, R.; Chen, M.; Shao, C.; Zhang, C.; Li, X. A multi-perspective study on the influence of physical and chemical properties of 5 types of fly ash on the performance of high-volume blended fly ash cementitious slurry. Constr. Build. Mater. 2024, 411, 134301. [Google Scholar] [CrossRef]
- Lu, S.; Liu, J.; Peng, C.; Zhang, J.; Sun, H. Multi-objective Optimization of Mix Proportions for Mass Concrete with Enhanced Resistance to Cracking and Reduced Temperature Rise. Case Stud. Constr. Mater. 2023, 20, e02810. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, C.; Song, F.; He, B.; Li, W.; Jiang, Z. Autogenous self-healing of ultra-high-performance fiber-reinforced concrete with varying silica fume dosages: Secondary hydration and structural regeneration. Cem. Concr. Compos. 2023, 137, 104905. [Google Scholar] [CrossRef]
- Baran, E.; Hynowski, M.; Kotwica, L.; Rogowski, J. Effect of Portland Cement on the Selected Properties of Flue Gas Desulfurization Gypsum-Based Plasters. Materials 2023, 16, 5058. [Google Scholar] [CrossRef] [PubMed]
- Kutchko, B.G.; Kim, A.G. Fly ash characterization by SEM-EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Diamond, S.; Sahu, S. Thaulow, Reaction products of densifted silica fume agglomerates in concrete. Cem. Concr. Res. 2004, 34, 1625–1632. [Google Scholar] [CrossRef]
- Wan, H.W.; Shui, Z.H.; Lin, Z.S. Analysis of geometric characteristics of GGBS particles and their influences on cement properties. Cem. Concr. Res. 2004, 34, 133–137. [Google Scholar] [CrossRef]
- Yang, B.J.; Dong, Y.Z.; Wang, B.N.; Yang, M.M.; Yang, C.J. A mild alcohol-salt route to synthesize alpha-hemihydrate gypsum microrods from flue gas desulfurization gypsum in large scale. Mater. Res. Express 2019, 6, 045507. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method for Strength of Hydraulic Cement Mortar. National Standard of the P.R. China: Beijing, China, 2021. (In Chinese)
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. National Standard of the P.R. China: Beijing, China, 2009. (In Chinese)
- Lu, X.Y. Application of the Nernst-Einstein equation to concrete. Cem. Concr. Res. 1997, 27, 293–302. [Google Scholar] [CrossRef]
- Cong, M.L.; Zhang, S.S.; Sun, D.D.; Zhou, K.P. Optimization of Preparation of Foamed Concrete Based on Orthogonal Experiment and Range Analysis. Front. Mater. 2021, 8, 778173. [Google Scholar] [CrossRef]
- Fu, Q.; Xu, W.; Bu, M.; Guo, B.; Niu, D. Orthogonal experimental study on hybrid-fiber high-durability concrete for marine environment. J. Mater. Res. Technol. 2021, 13, 1790–1804. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, S.; Hang, M.; Wang, J.; Yang, T. Optimization design of ultrahigh-performance concrete based on interaction analysis of multiple factors. Case Stud. Constr. Mater. 2022, 16, e00858. [Google Scholar] [CrossRef]
- Casella, G.; Berger, R.L. Statistical Inference; Duxbury Press: Belmont, CA, USA, 2001. [Google Scholar]
- Li, X.; Wang, Z.; Zhang, M.; Liu, R.; Wang, Z.; Zhang, C.; Yan, J.; Liu, Y.; Zhang, Z.; Wu, W. Properties of the cement containing hybrid micro-fibers and polymer latex using the orthogonal test: A comparative study of freshwater and seawater curing conditions. Constr. Build. Mater. 2024, 411, 134661. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, H.B.; Zhang, L.; Guo, D.Q.; Chen, S.H.Z.; Qiu, X.Y.; Hou, X.J. The effect of superfine tea powder addition on the acrylamide content of innovative Xinjiang nang products (tea nang). Food Addit. Contam. Part A-Chem. Anal. Control. Expo. Risk Assess. 2020, 37, 1–18. [Google Scholar] [CrossRef]
- Zhang, T.S.; Yu, Q.J.; Wei, J.X.; Gao, P.; Chen, P.X.; Hu, J. Micro-structural development of gap-graded blended cement pastes containing a high amount of supplementary cementitious materials. Cem. Concr. Compos. 2012, 34, 1024–1032. [Google Scholar] [CrossRef]
- Jiang, D.B.; Li, X.G.; Lv, Y.; Zhou, M.K.; He, C.H.; Jiang, W.G.; Liu, Z.L.; Li, C.J. Utilization of limestone powder and fly ash in blended cement: Rheology, strength and hydration characteristics. Constr. Build. Mater. 2020, 232, 117228. [Google Scholar] [CrossRef]
- Rao, K.J.; Keerthi, K.; Vasam, S. Acid resistance of quaternary blended recycled aggregate concrete. Case Stud. Constr. Mater. 2018, 8, 423–433. [Google Scholar] [CrossRef]
- Aydin, S.; Yazici, H.; Yigiter, H.; Baradan, B. Sulfuric acid resistance of high-volume fly ash concrete. Build. Environ. 2007, 42, 717–721. [Google Scholar] [CrossRef]
- Bjegovic, D.; Stirmer, N.; Serdar, M. Durability properties of concrete with blended cements. Mater. Corros.-Werkst. Korros. 2012, 63, 1087–1096. [Google Scholar] [CrossRef]
- Yang, R.H.; He, T.S. Influence of liquid accelerators combined with mineral admixtures on early hydration of cement pastes. Constr. Build. Mater. 2021, 295, 123659. [Google Scholar] [CrossRef]
- Tangpagasit, J.; Cheerarot, R.; Jaturapitakkul, C.; Kiattikomol, K. Packing effect and pozzolanic reaction of fly ash in mortar. Cem. Concr. Res. 2005, 35, 1145–1151. [Google Scholar] [CrossRef]
- Wu, Z.W. An approach to the recent trends of concrete science and technology. J. Chin. Ceram. Soc. 1979, 7, 262–270. (In Chinese) [Google Scholar]
- Elsharief, A.; Cohen, M.D.; Olek, J. Influence of aggregate size, water cement ratio and age on the microstructure of the interfacial transition zone. Cem. Concr. Res. 2003, 33, 1837–1849. [Google Scholar] [CrossRef]
- Piasta, W.; Zarzycki, B. The effect of cement paste volume and w/c ratio on shrinkage strain, water absorption and compressive strength of high performance concrete. Constr. Build. Mater. 2017, 140, 395–402. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Ni, C.X.; Tang, M.L.; Shen, X.D. Relationship between water permeability and pore structure of Portland cement paste blended with fly ash. Constr. Build. Mater. 2018, 175, 458–466. [Google Scholar] [CrossRef]
- Li, Y.; Bao, J.L.; Guo, Y.L. The relationship between autogenous shrinkage and pore structure of cement paste with mineral admixtures. Constr. Build. Mater. 2010, 24, 1855–1860. [Google Scholar] [CrossRef]
- Richardson, I.G. The nature of the hydration products in hardened cement pastes. Cem. Concr. Compos. 2000, 22, 97–113. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, K.; Fen-chong, T.; Dangla, P. Determination of cement hydration and pozzolanic reaction extents for fly-ash cement pastes. Constr. Build. Mater. 2012, 27, 560–569. [Google Scholar] [CrossRef]
- Li, S. Microstructure and composition characterisation of three 20-year-old GGBS-OPC blended pastes. Constr. Build. Mater. 2016, 123, 226–234. [Google Scholar] [CrossRef]
- Teng, S.; Lim, T.Y.D.; Divsholi, B.S. Durability and Mechanical Properties of High Strength Concrete Incorporating Ultra Fine Ground Granulated Blast-Furnace Slag. Constr. Build. Mater. 2013, 40, 875–881. [Google Scholar] [CrossRef]
- Wu, L.P.; Huang, G.P.; Liu, W.V. Performance evaluation of nano-silica and silica fume on enhancing acid resistance of cement-based composites for underground structures. J. Cent. South Univ. 2020, 27, 3821–3838. [Google Scholar] [CrossRef]
- Wansom, S.; Chintasongkro, P.; Srijampan, W. Water resistant blended cements containing flue-gas desulfurization gypsum, Portland cement and fly ash for structural applications. Cem. Concr. Compos. 2019, 103, 134–148. [Google Scholar] [CrossRef]
- Duan, S.; Liao, H.; Cheng, F.; Song, H.; Yang, H. Investigation into the synergistic effects in hydrated gelling systems containing fly ash, desulfurization gypsum and steel slag. Constr. Build. Mater. 2018, 187, 1113–1120. [Google Scholar] [CrossRef]
- Ma, B.; Liu, X.; Tan, H.; Zhang, T.; Mei, J.; Qi, H.; Jiang, W.; Zou, F. Utilization of pretreated fly ash to enhance the chloride binding capacity of cement-based material. Constr. Build. Mater. 2018, 175, 726–734. [Google Scholar] [CrossRef]
- Qiao, C.; Suraneni, P.; Ying, T.N.W.; Choudhary, A.; Weiss, J. Chloride binding of cement pastes with fly ash exposed to CaCl2 solutions at 5 and 23 °C. Cem. Concr. Compos. 2019, 97, 43–53. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, W.; Guo, Y.; Bogush, A.; Khayrulina, E.; Wei, J.; Yu, Q. The volumetric stability, chloride binding capacity and stability of the Portland cement-GBFS pastes: An approach from the viewpoint of hydration products. Constr. Build. Mater. 2019, 205, 357–367. [Google Scholar] [CrossRef]
- Ming, X.; Li, Y.; Liu, Q.; Wang, M.; Cai, Y.; Chen, B.; Li, Z. Chloride binding behaviors and early age hydration of tricalcium aluminate in chloride-containing solutions. Cem. Concr. Compos. 2023, 137, 104928. [Google Scholar] [CrossRef]
- Orozco, C.; Babel, S.; Tangtermsirikul, S.; Sugiyama, T. Comparison of environmental impacts of fly ash and slag as cement replacement materials for mass concrete and the impact of transportation. Sustain. Mater. Technol. 2024, 39, e00796. [Google Scholar] [CrossRef]
- Nie, S.; Zhou, J.; Yang, F.; Lan, M.; Li, J.; Zhang, Z.; Chen, Z.; Xu, M.; Li, H.; Sanjayan, J.G. Analysis of theoretical carbon dioxide emissions from cement production: Methodology and application. J. Clean. Prod. 2022, 334, 130270. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Wojtacha-Rychter, K.; Kucharski, P.; Smolinski, A. Conventional and Alternative Sources of Thermal Energy in the Production of Cement-An Impact on CO2 Emission. Energies 2021, 14, 1539. [Google Scholar] [CrossRef]
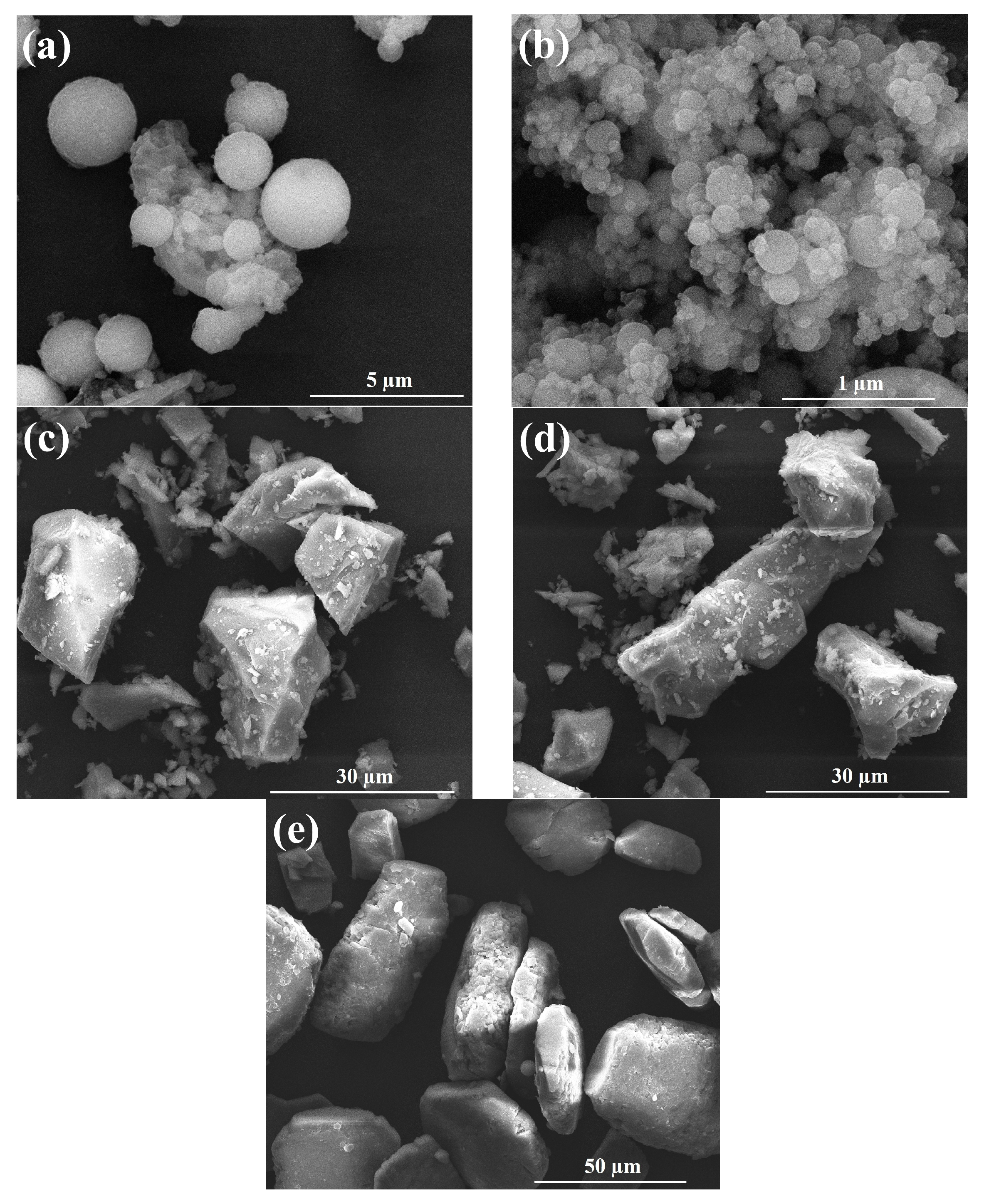
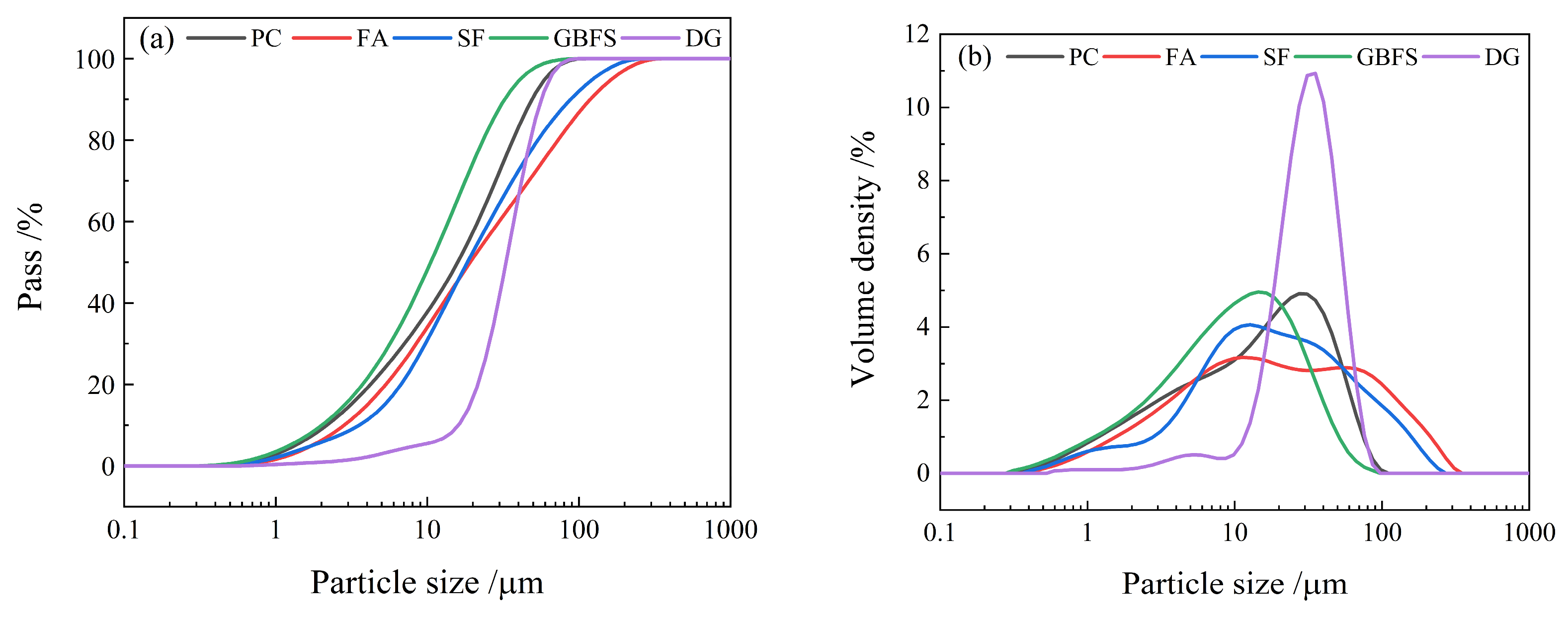

| Materials | CaO | SiO2 | Fe2O3 | Al2O3 | SO3 | K2O | TiO2 | MgO | N |
|---|---|---|---|---|---|---|---|---|---|
| Cement | 72.0 | 15.3 | 3.56 | 3.45 | 2.93 | 0.79 | 0.19 | 0.55 | 0.76 |
| FA | 11.2 | 41.8 | 4.01 | 36.6 | 1.60 | 0.76 | 1.28 | 0.72 | 0.52 |
| GBFS | 48.6 | 28.0 | 0.48 | 14.2 | 1.98 | 0.28 | 0.74 | 4.91 | 0.00 |
| SF | 1.44 | 96.6 | 0.14 | 0.49 | 0.09 | 0.39 | 0.00 | 0.18 | 0.26 |
| DG | 47.4 | 2.48 | 0.52 | 1.38 | 47.5 | 0.12 | 0.08 | 0.22 | 0.00 |
| Levels | Experimental Factors | ||
|---|---|---|---|
| A (Water–Binder Ratio) | B (Cementitious Ratio) | C (Replacement Cement Ratio) | |
| 1 | 0.55 | 4:4:1:1 | 0% |
| 2 | 0.45 | 6:2:1:1 | 30% |
| 3 | 0.50 | 2:6:1:1 | 60% |
| Mix NO. | Factors | ||
|---|---|---|---|
| A | B | C | |
| Z1 | 3 (0.50) | 2(6:2:1:1) | 1 (0.0%) |
| Z2 | 1 (0.55) | 2 | 2 (30%) |
| Z3 | 2 (0.45) | 1(4:4:1:1) | 2 |
| Z4 | 1 | 1 | 1 |
| Z5 | 3 | 3(2:6:1:1) | 2 |
| Z6 | 2 | 3 | 1 |
| Z7 | 3 | 1 | 3 (60%) |
| Z8 | 1 | 3 | 3 |
| Z9 | 2 | 2 | 3 |
| Mix NO. | Quality (g) | ||||||
|---|---|---|---|---|---|---|---|
| GBFS | FA | SF | DG | Cement | Sand | Water | |
| Z1 | 0 | 0 | 0 | 0 | 450 | 1350 | 225.0 |
| Z2 | 81 | 27 | 13.5 | 13.5 | 315 | 1350 | 247.5 |
| Z3 | 54 | 54 | 13.5 | 13.5 | 315 | 1350 | 202.5 |
| Z4 | 0 | 0 | 0 | 0 | 450 | 1350 | 247.5 |
| Z5 | 27 | 81 | 13.5 | 13.5 | 315 | 1350 | 225.0 |
| Z6 | 0 | 0 | 0 | 0 | 450 | 1350 | 202.5 |
| Z7 | 108 | 108 | 27 | 27 | 180 | 1350 | 225.0 |
| Z8 | 54 | 162 | 27 | 27 | 180 | 1350 | 247.5 |
| Z9 | 162 | 54 | 27 | 27 | 180 | 1350 | 202.5 |
| Indicator Value | Compressive Strength/MPa | Corrosion Resistance Coefficient/% | Chloride Ion Permeability Coefficient /(m2/s) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | A | B | C | |
| Kj1 | 186 | 200 | 197 | 277 | 266 | 277 | 2.57 × 10−12 | 2.44 × 10−12 | 4.99 × 10−12 |
| Kj2 | 190 | 204 | 210 | 264 | 279 | 268 | 2.37 × 10−12 | 2.16 × 10−12 | 1.40 × 10−12 |
| Kj3 | 192 | 186 | 183 | 284 | 280 | 280 | 2.14 × 10−12 | 2.47 × 10−12 | 0.67 × 10−12 |
| kj1 | 62.0 | 66.5 | 65.6 | 92.4 | 88.8 | 92.3 | 0.86 × 10−12 | 0.81 × 10−12 | 1.67 × 10−12 |
| kj2 | 63.4 | 68.0 | 69.8 | 88.1 | 93.0 | 89.4 | 0.79 × 10−12 | 0.72 × 10−12 | 0.47 × 10−12 |
| kj3 | 64.1 | 62.0 | 61.1 | 94.5 | 93.3 | 93.4 | 0.71 × 10−12 | 0.82 × 10−12 | 0.22 × 10−12 |
| R | 2.10 | 6.00 | 8.70 | 6.40 | 4.50 | 4.00 | 0.15 × 10−12 | 0.10 × 10−12 | 1.45 × 10−12 |
| Ranking of impact | C > B > A | A > B > C | C > A > B | ||||||
| Dependent Variable | Independent Variable (Factor) | Type III Sum of Squares | Df (Free Degree) | F Value | p Value |
|---|---|---|---|---|---|
| Compressive strength/MPa | B | 59.4 | 2 | 0.79 | 0.51 |
| C | 115 | 2 | 1.53 | 0.32 | |
| Error | 150 | 4 | |||
| R2 = 0.54 (Adjust R2 = 0.08) | |||||
| Corrosion resistance coefficient/% | A | 63.3 | 2 | 0.869 | 0.49 |
| B | 36.8 | 2 | 0.506 | 0.64 | |
| Error | 145 | 4 | |||
| R2 = 0.41 (Adjust R2 = 0.19) | |||||
| Chloride ion permeability coefficient/(m2/s) | A | 0.03 × 10−12 | 2 | 1.01 | 0.44 |
| C | 3.57 × 10−12 | 2 | 128 | 0.00 | |
| Error | 0.06 × 10−12 | 4 | |||
| R2 = 0.99 (Adjust R2 = 0.97) | |||||
| Mix NO. | Compressive Strength/MPa | Corrosion Resistance Coefficient/% | Chloride Ion Permeability Coefficient/(m2/s) | CI Values |
|---|---|---|---|---|
| Z1 | 64.1 | 91.4 | 1.44 × 10−12 | 0.45 |
| Z2 | 69.0 | 95.1 | 0.52 × 10−12 | 0.83 |
| Z3 | 73.1 | 78.0 | 0.42 × 10−12 | 0.00 |
| Z4 | 65.7 | 91.5 | 1.80 × 10−12 | 0.00 |
| Z5 | 67.4 | 95.1 | 0.48 × 10−12 | 0.82 |
| Z6 | 67.0 | 94.0 | 1.76 × 10−12 | 0.25 |
| Z7 | 60.8 | 97.0 | 0.23 × 10−12 | 0.75 |
| Z8 | 51.5 | 90.7 | 0.24 × 10−12 | 0.00 |
| Z9 | 70.9 | 92.4 | 0.20 × 10−12 | 0.87 |
| Materials | Cement | FA | GBFS | SF | DG |
|---|---|---|---|---|---|
| Price per ton/RMB | 515 | 130.2 | 212.5 | 800 | 46.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, S.; Fu, Q.; Hu, Y.; Lu, L.; Wang, Z. Preparation and Performance Study of High-Strength and Corrosion-Resistant Cement-Based Materials Applied in Coastal Acid Rain Areas. Materials 2024, 17, 752. https://doi.org/10.3390/ma17030752
Wang J, Zhang S, Fu Q, Hu Y, Lu L, Wang Z. Preparation and Performance Study of High-Strength and Corrosion-Resistant Cement-Based Materials Applied in Coastal Acid Rain Areas. Materials. 2024; 17(3):752. https://doi.org/10.3390/ma17030752
Chicago/Turabian StyleWang, Junfeng, Shaoxuan Zhang, Qionglin Fu, Yang Hu, Liulei Lu, and Zhihao Wang. 2024. "Preparation and Performance Study of High-Strength and Corrosion-Resistant Cement-Based Materials Applied in Coastal Acid Rain Areas" Materials 17, no. 3: 752. https://doi.org/10.3390/ma17030752




