Temperature-Controlled Chain Dynamics in Polyimide Doped with CoCl2 Probed Using Dynamic Mechanical Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the CoCl2/Polyimide Film Materials
2.3. Characterization
3. Results and Discussion
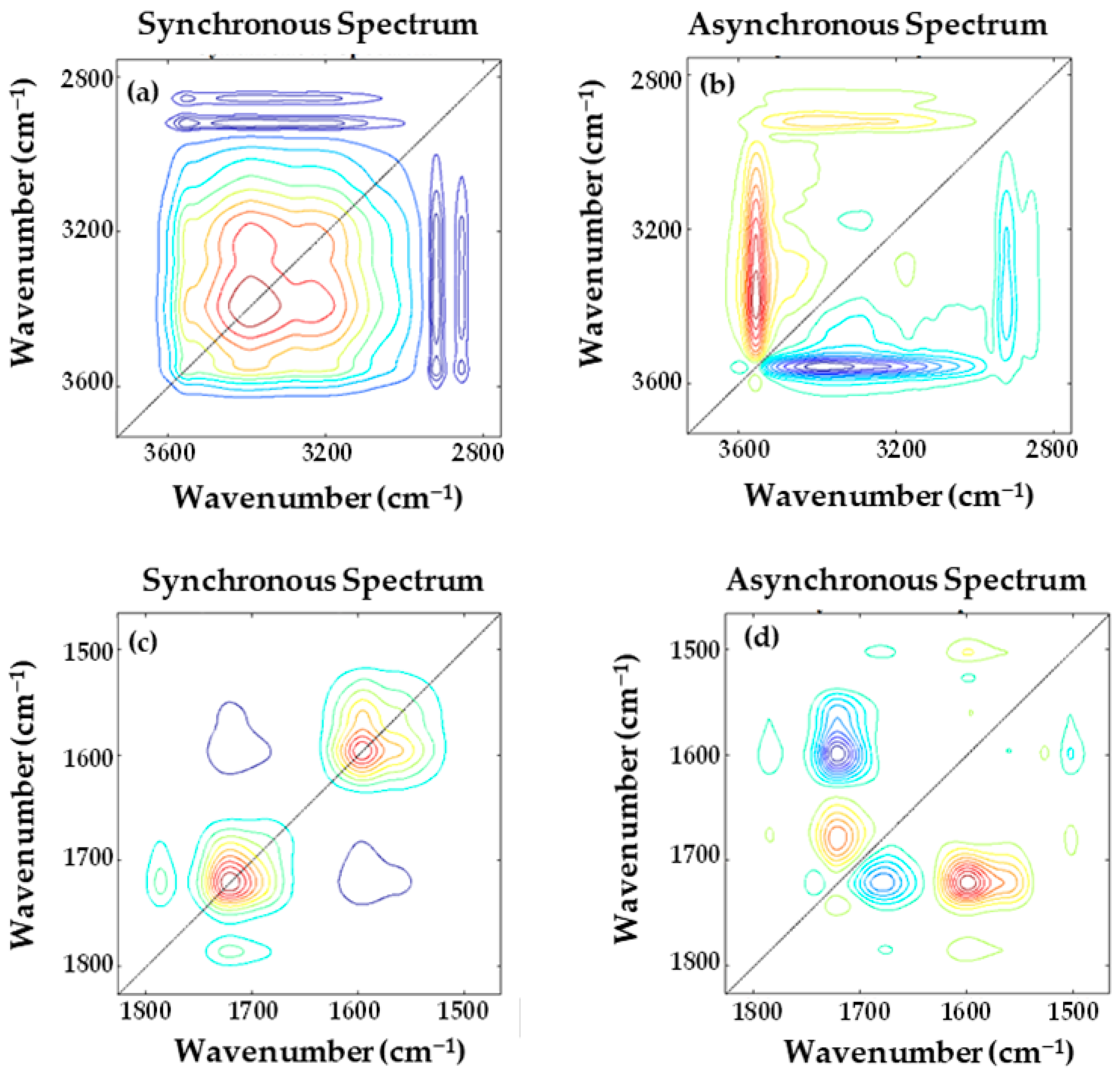
3.1. Structural Characterization of the Polyimide Films Using FTIR
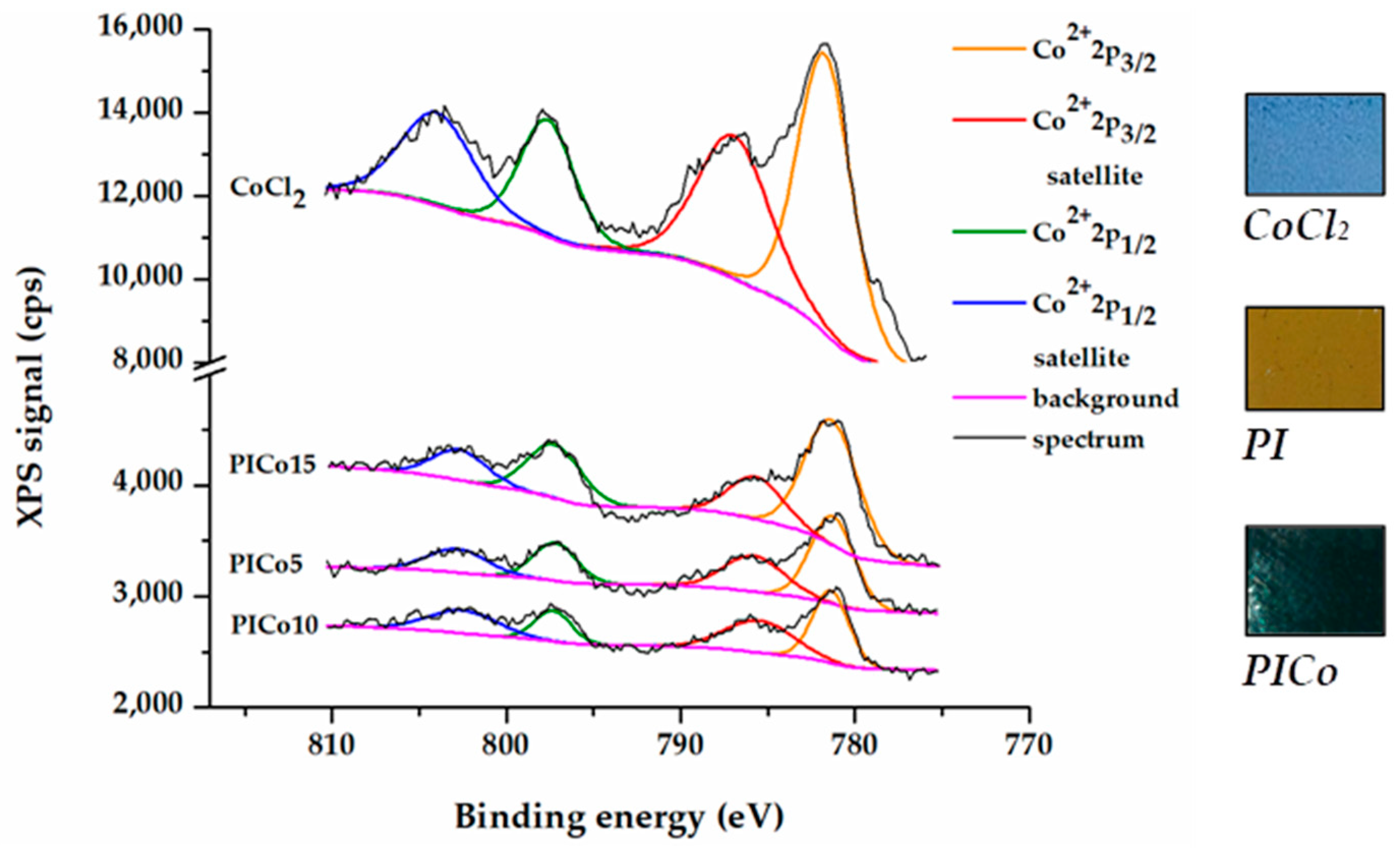
3.2. X-ray Photoelectron Spectroscopy
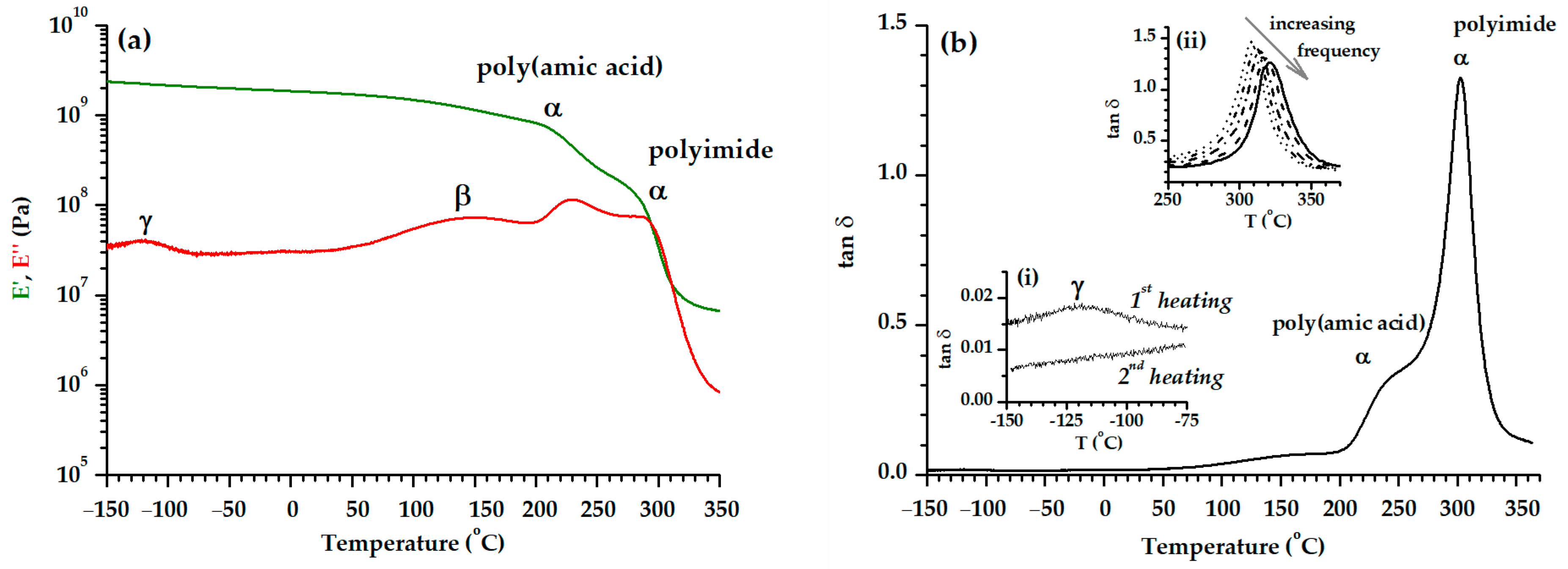
3.3. Dynamic Mechanical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sroog, C.E. History in the invention and development of the polyimides. In Polyimides. Fundamentals and Applications, 1st ed.; Ghosh, M.K., Mittal, K.L., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 1–6. [Google Scholar] [CrossRef]
- Liaw, D.J.; Wang, K.L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Synthesis, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Damaceanu, M.-D. Progress on polymers containing imide rings for advanced technologies: A contribution from ICMPP of the Romania Academy. Chemistry 2022, 4, 1339–1359. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Wang, Y.; Chung, T.-S.; Qiao, X.Y.; Lai, J.-Y. Polyimides membranes for pervaporation and biofuels separation. Prog. Polym. Sci. 2009, 34, 1135–1160. [Google Scholar] [CrossRef]
- Verbiest, T.; Burland, D.M.; Jurich, M.C.; Lee, V.Y.; Miller, R.D.; Volksen, W. Exceptionally thermally stable polyimides for second-order nonlinear optical applications. Science 1995, 268, 1604–1606. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Sanip, S.M.; Ng, B.C.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Li, J.; Qi, S.; Li, J.; Zhang, M.; Wang, Z. A highly thermostable and transparent lateral heat spreader based on silver nanowire/polyimide composite. RSC Adv. 2015, 5, 59398. [Google Scholar] [CrossRef]
- Rancourt, J.D.; Taylor, L.T. Preparation and properties of surface-conductive polyimide films via in situ codeposition of metal salts. Macromolecules 1987, 20, 790–795. [Google Scholar] [CrossRef]
- Ding, D.; Yan, X.; Zhang, X.; He, Q.; Qiu, B.; Jiang, D.; Wei, H.; Guo, J.; Umar, A.; Sun, L.; et al. Preparation and enhanced properties of Fe3O4 nanoparticles reinforced polyimide nanocomposites. Superlattices Microstruct. 2015, 85, 305–320. [Google Scholar] [CrossRef]
- Kaburagi, Y.; Hataori, H.; Yoshida, A.; Hishiyama, Y.; Inagaki, M. Carbon films containing transition metal particles of nano and submicron sizes. Synth. Met. 2001, 125, 171–182. [Google Scholar] [CrossRef]
- Khor, E.; Taylor, L.T. A study of cobalt containing polyimide films. In Metal-Containing Polymeric Systems; Sheats, J.E., Carraher, C.E., Pittman, C.E., Eds.; Springer: Boston, MA, USA, 1985; pp. 367–384. [Google Scholar] [CrossRef]
- Sawada, T.; Ando, S. Synthesis, characterization and optical properties of metal-containing fluorinated polyimide films. Chem. Mater. 1998, 10, 3368–3378. [Google Scholar] [CrossRef]
- Mu, S.; Wu, Z.; Wang, Y.; Qi, S.; Yang, X.; Wu, D. Formation and characterization of cobalt oxide layers on polyimide films via surface modification and ion-exchange technique. Thin Solid Films 2010, 518, 4175–4182. [Google Scholar] [CrossRef]
- Maya, E.M.; de la Torre, G.; Lozano, A.E.; Torres, T.; de la Campa, J.G.; de Abajo, J. Novel cobalt (II) phthalocyanine-containing polyimides: Synthesis, characterization, thermal and optical properties. Macromol. Rapid Commun. 2006, 27, 1852–1858. [Google Scholar] [CrossRef]
- Rancourt, J.D.; Taylor, L.T. Characterization of cobalt (II) chloride-modified condensation polyimide films—Properties before and after solvent extraction. In Cross-Linked Polymers: Chemistry, Properties and Applications, ACS Symposium Series; Dickie, R.A., Labana, S.S., Bauer, R.S., Eds.; American Chemical Society: Washington, DC, USA, 1988; Volume 367, pp. 395–408. [Google Scholar]
- Bushkova, O.V.; Koryakova, I.P.; Skorik, Y.A.; Lirova, B.I.; Pestov, A.V.; Zhukovsky, V.M. Influence of metal coordination on conductivity behavior in poly(butadiene-acrylonitrile)-CoCl2 system. Electrochim. Acta 2008, 53, 5322–5333. [Google Scholar] [CrossRef]
- Gao, R.; Wang, K.; Li, Y.; Wang, F.; Sun, W.-H.; Redshaw, C.; Bochmann, M. 2-Benzoxazolyl-6-(1-(arylimino)ethyl)pyridyl cobalt (II) chlorides: A temperature switch catalyst in oligomerization and polymerization of ethylene. J. Mol. Catal. A-Chem. 2009, 309, 166–171. [Google Scholar] [CrossRef]
- Sister, V.G.; Kosivtsov, Y.Y.; Sul’man, E.M.; Lugovoi, Y.V.; Ivannikova, E.M.; Yamchuk, A.I. Effect of cobalt chloride on the physical and chemical properties of the solid residue from the pyrolysis of polymer tire cord. Theor. Found. Chem. Eng. 2011, 45, 744–746. [Google Scholar] [CrossRef]
- Li, F.; Arthur, E.E.; La, D.; Li, Q.; Kim, H. Immobilization of CoCl2 (cobalt chloride) on PAN (polyacrylonitrile) composite nanofiber mesh filled with carbon nanotubes for hydrogen production from hydrolysis of NaBH4 (sodium borohydride). Energy 2014, 71, 32–39. [Google Scholar] [CrossRef]
- Naboka, O.; Sanz-Velasco, A.; Lundgren, P.; Enoksson, P.; Gatenholm, P. Cobalt (II) chloride promoted formation of honeycomb patterned cellulose acetate films. J. Colloid Interf. Sci. 2012, 367, 485–493. [Google Scholar] [CrossRef]
- Young, J.A. Cobalt(II) Chloride Hexahydrate. J. Chem. Educ. 2003, 80, 610. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kanungo, S.B. Thermal dehydration and decomposition of cobalt chloride hydrate (CoCl2·xH2O). J. Therm. Anal. 1992, 38, 2437–2457. [Google Scholar] [CrossRef]
- Tay, C.M.; Tan, K.M.; Tjin, S.C.; Chan, C.C.; Rahardjo, H. Humidity sensing using plastic optical fibers. Microw. Opt. Technol. Lett. 2004, 43, 387–390. [Google Scholar] [CrossRef]
- Zidan, H.M.; Abu-Elnader, M. Structural and optical properties of pure PMMA and metal chloride-doped PMMA films. Physica B 2005, 355, 308–317. [Google Scholar] [CrossRef]
- Ahmed, R.M. Optical properties and structure of cobalt chloride doped PVA and its blend with PVP. Int. J. Mod. Phys. B 2014, 28, 1450036. [Google Scholar] [CrossRef]
- Abiona, A.A.; Ajao, J.A.; Chigome, S.; Kana, J.B.K.; Osinkolu, G.A.; Maaza, M. Synthesis and characterization of cobalt chloride/poly(ethylene oxide) electrospun hybrid nanofibers. J. Sol-Gel Technol. 2010, 55, 235–241. [Google Scholar] [CrossRef]
- Shao, C.; Wang, Q.; Mao, Y.; Li, Q.; Wu, C. Influence of carbon nanotubes content on the properties of acrylonitrile-butadiene rubber/cobalt chloride composites. Mater. Res. Express 2019, 6, 075323. [Google Scholar] [CrossRef]
- Zia, M.A.; Khosa, M.K.; Noor, A.; Qayyum, S.; Shakir, M.S. PMMA/ABS/CoCl2 composites for pharmaceutical applications: Thermal, antimicrobial, antibiofilm, and antioxidant studies. Molecules 2022, 27, 7669. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Abou-Laila, M.T.; Taha, E.O. Investigating into physical properties of composites of polymer blends and cobalt chloride irradiated by gamma ray for optical devices development. Mater. Today Commun. 2023, 35, 105752. [Google Scholar] [CrossRef]
- Belfiore, L.A.; McCurdie, M.P.; Ueda, E. Polymeric coordination complexes based on cobalt, nickel, and ruthenium that exhibit synergistic thermal properties. Macromolecules 1993, 26, 6908–6917. [Google Scholar] [CrossRef]
- Guan, H.-M.; Cheng, X.-S. Study of cobalt(II)-chitosan coordination polymer and its catalytic activity and selectivity for vinyl monomer polymerization. Polym. Adv. Technol. 2004, 15, 89–92. [Google Scholar] [CrossRef]
- Shen, Q.-D.; Yang, C.-Z. Transition metal complexes of N-picolyl polyurethane. J. Polym. Sci. B Polym. Phys. 1998, 36, 1539–1546. [Google Scholar] [CrossRef]
- Huang, J.; Qian, S.; Shen, F.; Wu, C. Investigation on the coordination interfacial reaction by dynamical mechanical analysis. J. Macromol. Sci. B 2007, 47, 52–64. [Google Scholar] [CrossRef]
- Sava, I.; Chisca, S.; Bruma, M.; Lisa, G. Comparative study of aromatic polyimides containing methylene units. Polym. Bull. 2010, 65, 363–375. [Google Scholar] [CrossRef]
- Bogges, R.K.; Taylor, L.T. Characterization of cobalt-modified polyimides. J. Polym. Sci. A Polym. Chem. 1987, 25, 685–702. [Google Scholar] [CrossRef]
- Varma, I.K.; Saxena, S.; Tripathi, A.; Goel, T.C.; Varma, D.S. Effect of metal halides on thermal, mechanical, and electrical properties of polypyromellitimide films. J. Appl. Polym. Sci. 1986, 32, 3987–4000. [Google Scholar] [CrossRef]
- Halper, S.R.; Villahermosa, R.M. Cobalt-containing polyimides for moisture sensing and absorption. ACS Appl. Mater. Inter. 2009, 1, 1041–1044. [Google Scholar] [CrossRef]
- Thomson, B.; Bartges, D.; Czerniawski, D.; Painter, P.C. FTIR studies of polyimides: Thermal curing. Macromolecules 1989, 22, 4166–4172. [Google Scholar] [CrossRef]
- Diaham, S.; Locatelli, M.L.; Lebey, T.; Malec, D. Thermal imidization optimization of polyimide thin films using Fourier transform infrared spectroscopy and electrical measurements. Thin Solid Films 2011, 519, 1851–1856. [Google Scholar] [CrossRef]
- Kumar, A.; Tateyama, S.; Yasaki, K.; Ali, M.A.; Takaya, N.; Singh, R.; Kaneko, T. 1H NMR and FT-IR dataset based structural investigation of poly(amic acid)s and polyimides from 4,4′-diaminostilbene. Data Brief 2016, 7, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.J.; Ree, M. Thermal imidization and structural evolution of thin films of poly(4,4′-oxyduphenylene p-pyromellitamic diethyl ester). J. Phys. Chem. B 2007, 111, 13894–13900. [Google Scholar] [CrossRef] [PubMed]
- Thomson, B.; Park, Y.; Painter, P.C.; Snyder, R.W. Hydrogen bonding in poly(amic acid)s. Macromolecules 1989, 22, 4159–4166. [Google Scholar] [CrossRef]
- Guerra, G.; Choe, S.; Williams, D.J.; Karasz, F.E.; MacKnight, W.J. Fourier transform infrared spectroscopy of some miscible polybenzimidazole/polyimide blends. Macromolecules 1988, 21, 231–234. [Google Scholar] [CrossRef]
- Shin, T.J.; Ree, M. Poly(3,4′-oxydiphenylene pyromellitamic acid) 1. Time-resolved infrared spectroscopic study of thermal imidization in thin films. Macromol. Chem. Phys. 2002, 203, 791–800. [Google Scholar] [CrossRef]
- Shin, T.J.; Lee, B.; Youn, H.S.; Lee, K.-B.; Ree, M. Time-resolved synchrotron X-ray diffraction and infrared spectroscopic studies of imidization and structural evolution in a microscaled film of PMDA-3,4′-ODA poly(amic acid). Langmuir 2001, 17, 7842–7850. [Google Scholar] [CrossRef]
- Lockwood, D.J. Lattice vibrations of CdCl2, CdBr2, MnCl2, and CoCl2: Infrared and Raman spectra. J. Opt. Soc. Am. 1973, 63, 374–382. Available online: https://opg.optica.org/josa/abstract.cfm?URI=josa-63-3-374 (accessed on 1 November 2023). [CrossRef]
- Cho, D.; Choi, Y.; Drzal, L.T. Simultaneous monitoring of the imidization and cure of LaRC PETI-5 sized on a braided glass fabric substrate by dynamic mechanical analysis. Polymer 2001, 42, 4611–4618. [Google Scholar] [CrossRef]
- Cristea, M.; Ionita, D.; Garbea, M.; Damaceanu, M.-D. Dynamic molecular phenomena in polyimides investigated by dynamic mechanical analysis. In Imidic Polymers and Green Polymer Chemistry; Barzic, A.I., Rawat, N.K., Haghi, A.K., Eds.; Apple Academic Press: Palm Bay, FL, USA; Burlington, ON, Canada; CRC Press: Boca Raton, FL, USA; Abington, UK, 2021; pp. 325–348. [Google Scholar]
- Chen, W.J.; Chen, W.; Zhang, B.; Yang, S.; Liu, C.-Y. Thermal imidization process of polyimide film: Interplay between solvent evaporation and imidization. Polymer 2017, 109, 205–215. [Google Scholar] [CrossRef]
- Sava, I.; Chisca, S.; Bruma, M.; Lisa, G. Effect of thermal curing on the properties of thin films based on benzophenonetetracarboxylic dianhydride and 4,4′-diamino-3,3′-dimethyldiphenylmethane. J. Therm. Anal. Calorim. 2011, 104, 1135–1143. [Google Scholar] [CrossRef]
- Lee, C.Y.-C.; Goldfarb, I.J. The glass transition temperature of partially cured polymers as a non-equilibrium parameter and its effect on observed dynamic mechanical behavior. Polym. Eng. Sci. 1981, 21, 951–957. [Google Scholar] [CrossRef]
- Feger, C. Curing of polyimides. Polym. Eng. Sci. 1989, 29, 347–351. [Google Scholar] [CrossRef]
- Tyan, H.-L.; Liu, Y.-C.; Wei, K.-H. Enhancement of imidization of poly(amic acid) through forming poly(amic acid)/organoclay nanocomposites. Polymer 1999, 40, 4877–4886. [Google Scholar] [CrossRef]
- Tang, Q.-Y.; Chen, J.; Chan, Y.C.; Chung, C.Y. Effect of carbon nanotubes and their dispersion on thermal curing of polyimide precursors. Polym. Degrad. Stab. 2010, 95, 1672–1678. [Google Scholar] [CrossRef]
- Ikeda, S.; Akamatsu, K.; Nawafune, H.; Nishino, T.; Deki, S. Formation and growth of copper nanoparticles from ion-doped precursor polyimide layers. J. Phys. Chem. B 2004, 108, 15599–15607. [Google Scholar] [CrossRef]
- Noda, I. 2DCOS and I. Three decades of two-dimensional correlation spectroscopy. J. Mol. Struct. 2016, 1124, 3–7. [Google Scholar] [CrossRef]
- Noda, I. Two-dimensional correlation analysis useful for spectroscopy, chromatography, and other analytical measurements. Anal. Sci. 2007, 23, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Wu, P. A infrared spectroscopic study on the mechanism of temperature-induced phase transition of concentrated aqueous solutions of poly(N-isopropylacrylamide) and N-isopropylpropionamide. Polymer 2010, 51, 1404–1412. [Google Scholar] [CrossRef]
- Popescu, M.-C.; Filip, D.; Vasile, C.; Cruz, C.; Rueff, J.M.; Marcos, M.; Serrano, J.L.; Singurel, G. Characterization by Fourier Transform Infrared Spectroscopy (FT-IR) and 2D IR Correlation Spectroscopy of PAMAM dendrimer. J. Phys. Chem. B 2006, 110, 14198–14211. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.H.; Yoo, Y.H.; Rhee, J.M.; Lee, M.-H.; Yu, S.-C. Two-dimensional Raman correlation spectroscopy study of the pathway for the thermal imidization of poly(amic acid). Bull. Korean Soc. 2003, 24, 357–362. [Google Scholar] [CrossRef]
- Park, Y.; Noda, I.; Jung, Y.M. Novel developments and applications of two-dimensional correlation spectroscopy. J. Mol. Struct. 2016, 1124, 11–28. [Google Scholar] [CrossRef]
- Seo, H.; Chae, B.; Im, J.H.; Jung, Y.M.; Lee, S.W. Imidization induced structural changes of 6FDA-ODA poly(amic acid) by two-dimensional (2D) infrared correlation spectroscopy. J. Mol. Struct. 2014, 1069, 196–199. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Nakano, T.; Noda, I. Molecular interaction of polyimide films probed by using soft-pulse dynamic compression ATR time-resolved infrared and double Fourier-transform based 2D-IR spectroscopy. Vib. Spectrosc. 2014, 72, 79–89. [Google Scholar] [CrossRef]
- Lee, Y.J.; Chae, B.; Park, Y.; Jung, Y.M.; Lee, S.W. 2D infrared correlation study of the effect of base catalyst on thermal imidization of polyamic acid. J. Mol. Struct. 2020, 1217, 128383. [Google Scholar] [CrossRef]
- Damaceanu, M.-D.; Sava, I.; Constantin, C.-P. The chromic and electrochemical response of CoCl2—Filled polyimide materials for sensing applications. Sens. Actuat. B-Chem. 2016, 234, 549–561. [Google Scholar] [CrossRef]
- Sava, I.; Damaceanu, M.-D.; Lisa, G. Insights into the physico-chemical behavior of CoCl2/polyimide hybrid materials. J. Polym. Res. 2016, 23, 130. [Google Scholar] [CrossRef]
- Noda, I. Generalized two-dimensional correlation method applicable to Infrared, Raman, and other types of spectroscopy. Appl. Spectrosc. 1993, 47, 1329–1336. [Google Scholar] [CrossRef]
- Zhuang, Y.; Gu, Y. Poly(benzoxazole-amide-imide) copolymers for interlevel dielectrics: Interchain hydrogen bonding, molecular arrangement and properties. J. Polym. Res. 2013, 20, 168. [Google Scholar] [CrossRef]
- Sava, I.; Iosip, M.D.; Bruma, M.; Hamciuc, C.; Robison, J.; Okrasa, L.; Pakula, T. Aromatic polyamides with pendent acetoxybenzamide groups and thin films made therefrom. Eur. Polym. J. 2003, 39, 725–738. [Google Scholar] [CrossRef]
- Niu, H.; Huang, M.; Qi, S.; Han, E.; Tian, G.; Wang, X.; Wu, D. High-performance copolyimide fibers containing quinazolinone moiety: Preparation, structure and properties. Polymer 2013, 54, 1700–1708. [Google Scholar] [CrossRef]
- Frost, D.C.; McDowell, I.S. π for multiple splitting of 2p photoelectron lines of transition metal complexes. Chem. Phys. Lett. 1972, 17, 320–323. [Google Scholar] [CrossRef]
- Brown, D.G.; Weser, U. XPS spectra of spin-triplet cobalt(III) complexes. Z. Naturforsch. B 1979, 34, 1468–1470. [Google Scholar] [CrossRef]
- Burness, J.H.; Dillard, J.G.; Taylor, L.T. An X-ray photoelectron spectroscopic study of cobalt(II) Schiff base complexes and their oxygenation products. J. Am. Chem. Soc. 1975, 97, 6080–6087. [Google Scholar] [CrossRef]
- Rebenfeld, L.; Makarewicz, P.J.; Weigmann, H.-D.; Wilkes, G.L. Interactions between solvents and polymers in the solid state. J. Macromol. Sci. Polym. Rev. 1976, 15, 279–393. [Google Scholar] [CrossRef]
- Bernier, G.A.; Kline, D.E. Dynamic mechanical behavior of a polyimide. J. Appl. Polym. Sci. 1968, 12, 593–604. [Google Scholar] [CrossRef]
- Bas, C.; Tamagna, C.; Pascal, T.; Alberola, N.D. On the dynamic mechanical behavior of polyimides based on aromatic and alicyclic dianhydrides. Polym. Eng. Sci. 2003, 43, 344–355. [Google Scholar] [CrossRef]
- Tang, H.; Dong, L.; Zhang, J.; Ding, M.; Feng, Z. Study on the β relaxation mechanism of thermosetting polyimide/thermoplastic polyimide blends. Eur. Polym. J. 1996, 32, 1221–1227. [Google Scholar] [CrossRef]
- Li, F.; Fang, S.; Ge, J.J.; Honigfort, P.S.; Chen, J.-C.; Harris, F.W.; Cheng, S.Z.D. Diamine architecture effects on glass transitions, relaxation processes and other material properties in organo-soluble aromatic polyimide films. Polymer 1999, 40, 4571–4583. [Google Scholar] [CrossRef]
- Cristea, M.; Ionita, D.; Hulubei, C.; Timpu, D.; Popovici, D. Chain packing versus chain mobility in semialiphatic BTDA-based copolyimides. Polymer 2011, 52, 1820–1828. [Google Scholar] [CrossRef]
- Kim, K.; Ryou, J.H.; Kim, Y.; Ree, M.; Chang, T. Thermal imidization behavior of aromatic poly(amic dialkyl ester) precursors derived from biphenyltetracarboxylic dianhydride. Polym. Bull. 1995, 34, 219–226. [Google Scholar] [CrossRef]
- Lee, C.Y.C. Torsion impregnated cloth analysis for resin rheological studies. Polym. Eng. Sci. 1988, 28, 578–582. [Google Scholar] [CrossRef]
- Cristea, M.; Ionita, D.; Simionescu, B.C. A new insight in the dynamo-mechanical behavior of poly(ethylene terephtalate). Eur. Polym. J. 2010, 46, 2005–2010. [Google Scholar] [CrossRef]
- Stark, W. Investigation of the curing behavior of carbon fibre epoxy prepreg by dynamic mechanical analysis DMA. Polym. Test. 2013, 32, 231–239. [Google Scholar] [CrossRef]
- Cristea, M.; Ionita, D.; Iftime, M.M. Dynamic mechanical analysis investigations of PLA-based renewable materials: How are they useful? Materials 2020, 13, 5302. [Google Scholar] [CrossRef]


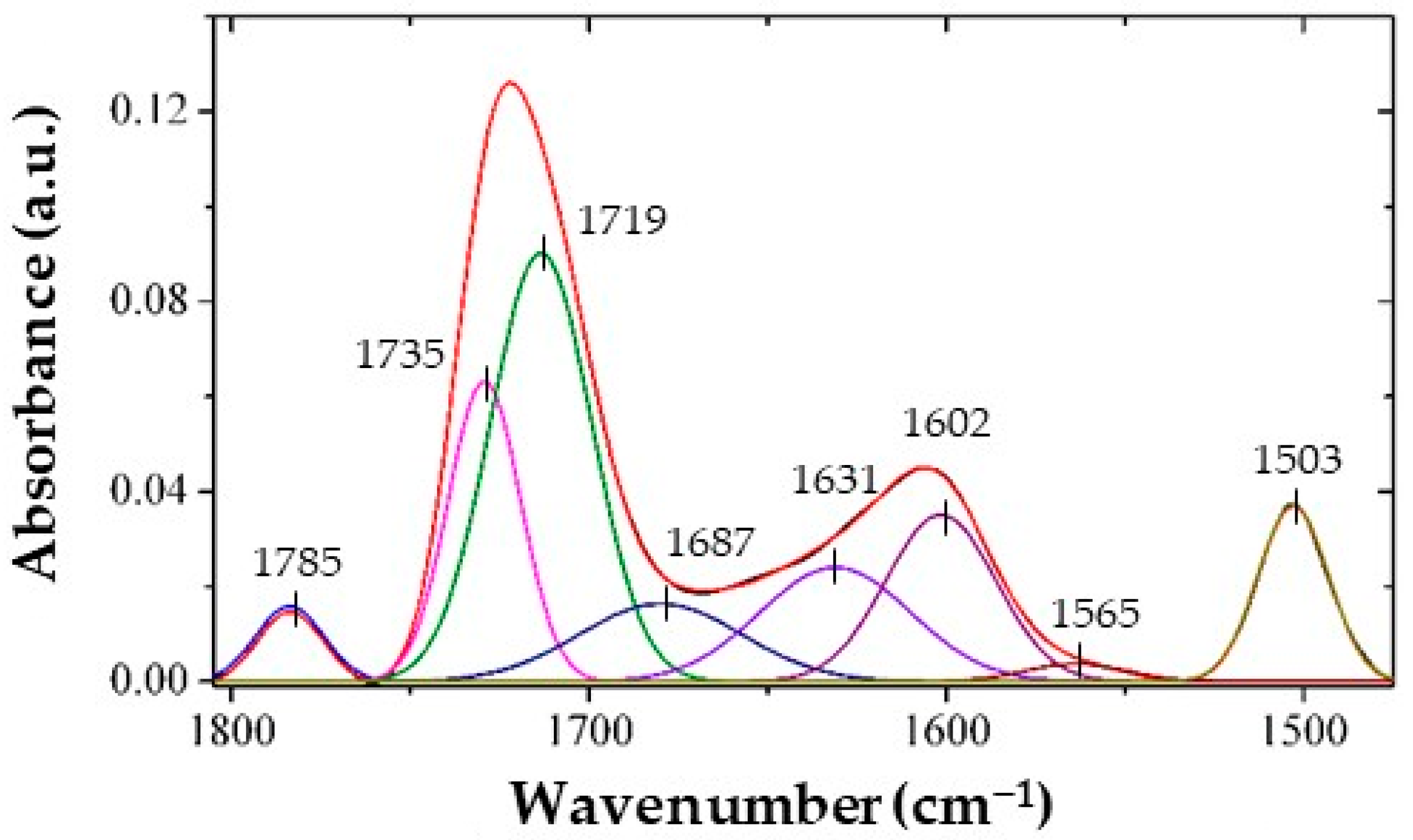
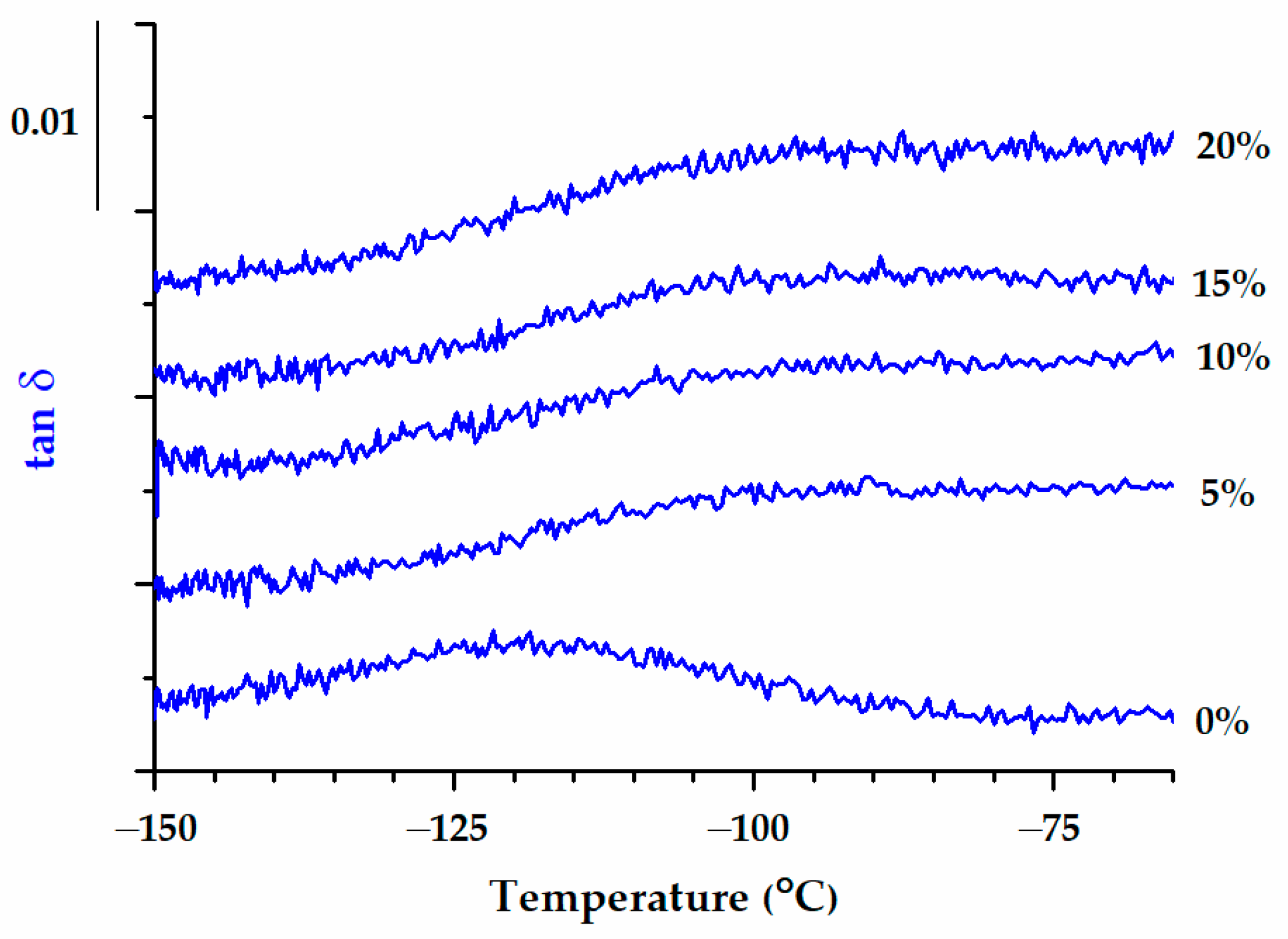
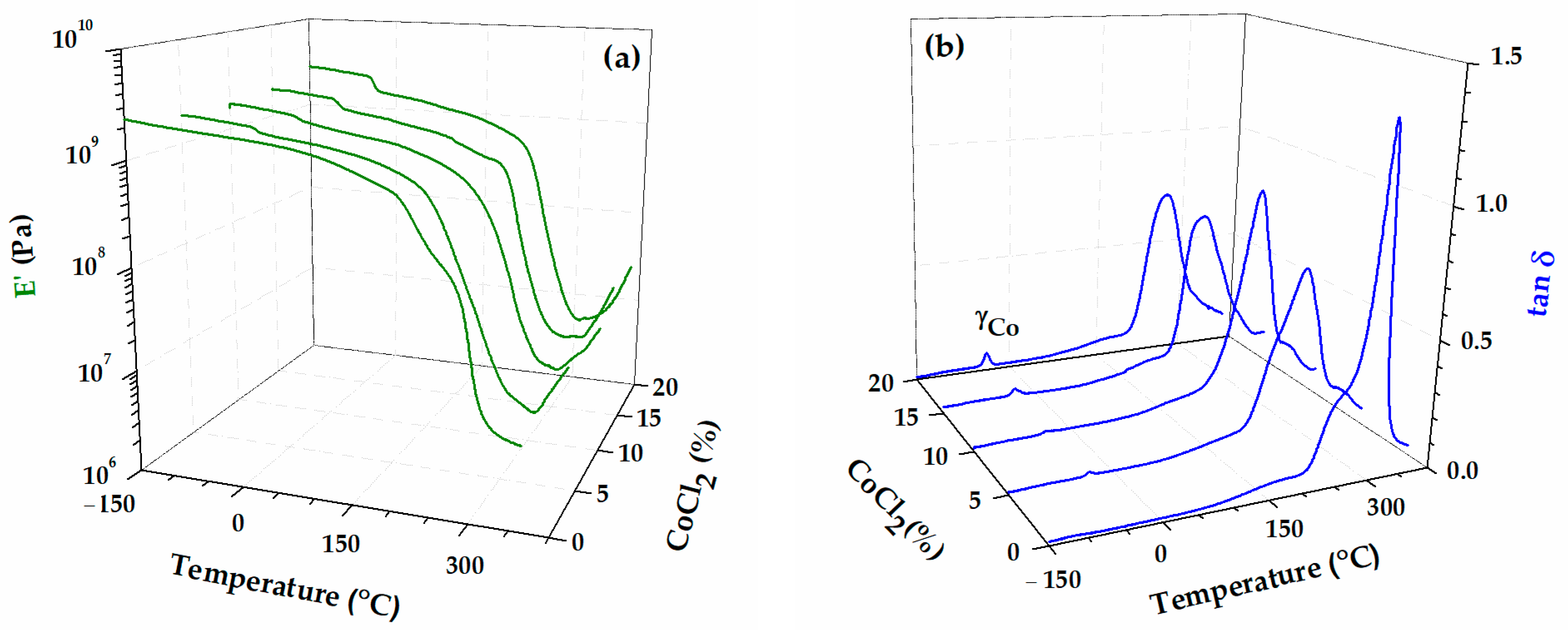
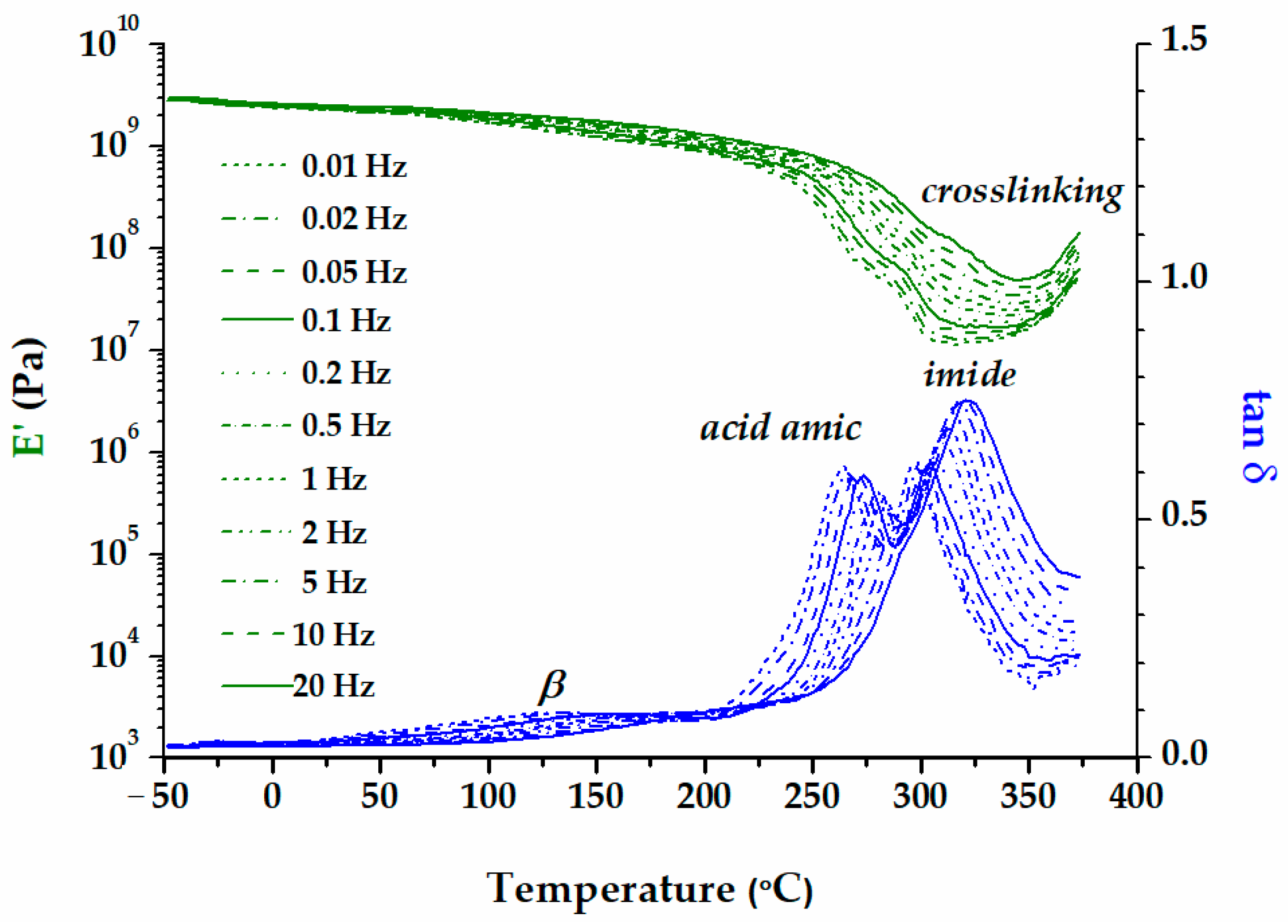
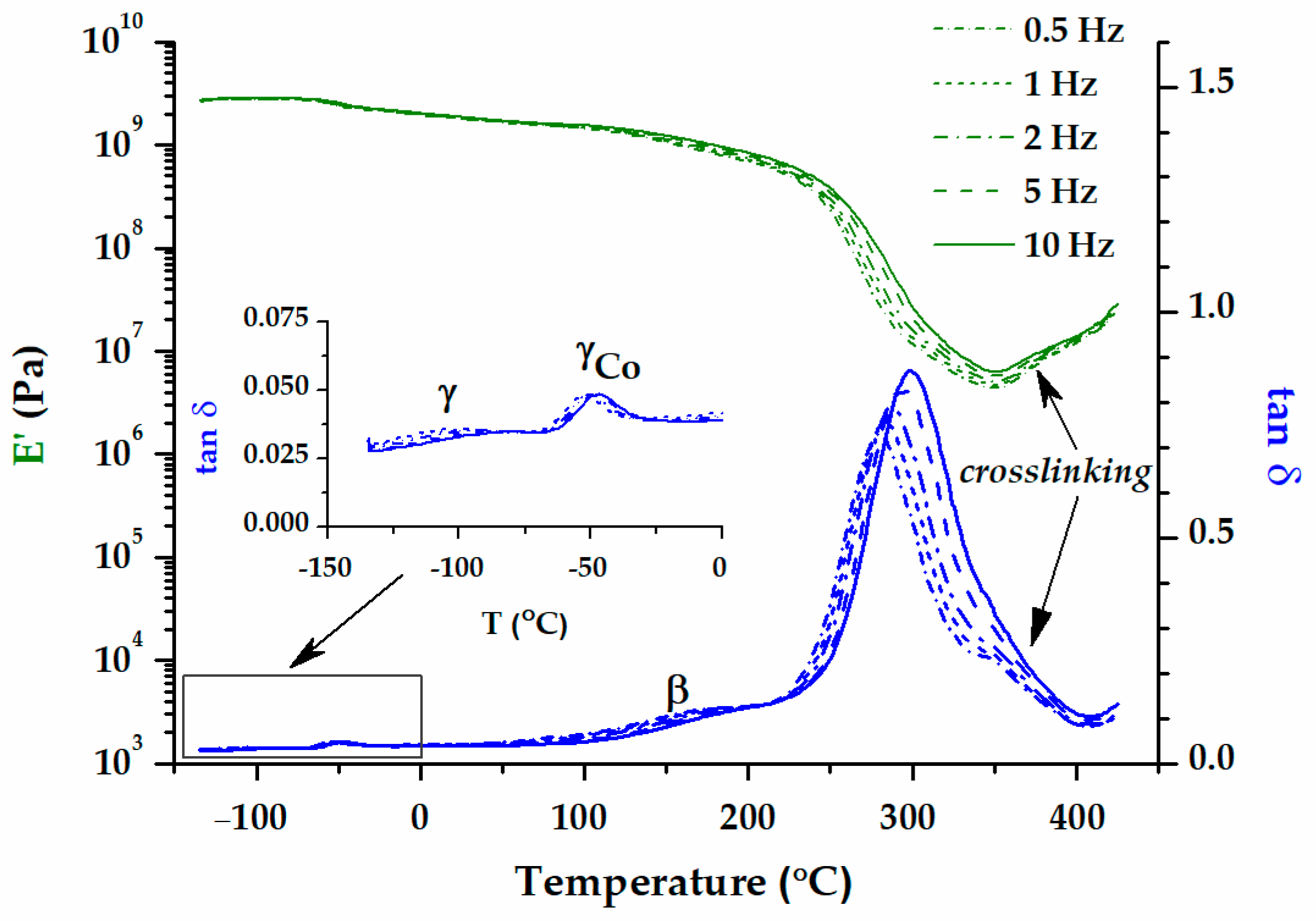
| Sample | Band Area | ||||
|---|---|---|---|---|---|
| 1784 cm−1 | 1719 cm−1 | 1630 cm−1 | 1604 cm−1 | 1555 cm−1 | |
| PI | 4.7 | 41.2 | 12.8 | 5.9 | - |
| PICo5 | 3.8 | 32.7 | 10.6 | 17.9 | 0.3 |
| PICo10 | 5.4 | 42.9 | 8.3 | 16.8 | 2.0 |
| PICo15 | 4.7 | 35.3 | 8.4 | 25.3 | 4.1 |
| Sample | N 1s | O 1s | Co 2p | ||||
|---|---|---|---|---|---|---|---|
| Ether | Imide | Satellite 1 | Co 2p1/2 | Satellite 2 | Co 2p3/2 | ||
| CoCl2 | - | - | - | 803.5 | 797.5 | 787.0 | 781.5 |
| PI | 399.7 | 533.5 | 531.8 | - | - | - | - |
| PICo5 | 400.0 | 533.4 | 531.8 | 802.9 | 797.3 | 785.8 | 781.3 |
| PICo10 | 399.8 | 533.4 | 531.8 | 802.6 | 797.3 | 785.3 | 781.5 |
| PICo15 | 399.7 | 533.2 | 531.6 | 802.8 | 797.4 | 785.5 | 781.5 |
| Sample | E′ (GPa) Glassy Region | α-Relaxation Onset | tan δ α-Relaxations | E′ (MPa) Rubbery Plateau | R 6 | ||||
|---|---|---|---|---|---|---|---|---|---|
| –140 °C 1 | 20 °C 2 | E′ (GPa) | T (°C) | T (°C) 3 | T (°C) 4 | htanδ 5 | |||
| PI | 2.33 | 1.80 | 0.72 | 210 | 238 | 305 | 1.30 | 6.7 (350 °C) | 108.0 |
| PICo5 | 1.88 | 1.25 | 0.50 | 195 | 225 | 279 | 0.69 | 5.2 (340 °C) | 96.0 |
| PICo10 | 1.80 | 1.10 | 0.42 | 205 | - | 285 | 0.89 | 6.3 (340 °C) | 66.5 |
| PICo15 | 1.95 | 1.14 | 0.46 | 202 | - | 266 | 0.70 | 6.6 (325 °C) | 70.0 |
| PICo20 | 2.76 | 1.37 | 0.40 | 217 | - | 272 | 0.70 | 5.0 (310 °C) | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionita, D.; Cristea, M.; Sava, I.; Popescu, M.-C.; Dobromir, M.; Simionescu, B.C. Temperature-Controlled Chain Dynamics in Polyimide Doped with CoCl2 Probed Using Dynamic Mechanical Analysis. Materials 2024, 17, 753. https://doi.org/10.3390/ma17030753
Ionita D, Cristea M, Sava I, Popescu M-C, Dobromir M, Simionescu BC. Temperature-Controlled Chain Dynamics in Polyimide Doped with CoCl2 Probed Using Dynamic Mechanical Analysis. Materials. 2024; 17(3):753. https://doi.org/10.3390/ma17030753
Chicago/Turabian StyleIonita, Daniela, Mariana Cristea, Ion Sava, Maria-Cristina Popescu, Marius Dobromir, and Bogdan C. Simionescu. 2024. "Temperature-Controlled Chain Dynamics in Polyimide Doped with CoCl2 Probed Using Dynamic Mechanical Analysis" Materials 17, no. 3: 753. https://doi.org/10.3390/ma17030753







