Advancements and Challenges in High-Capacity Ni-Rich Cathode Materials for Lithium-Ion Batteries
Abstract
1. Introduction
2. Origin of Problems
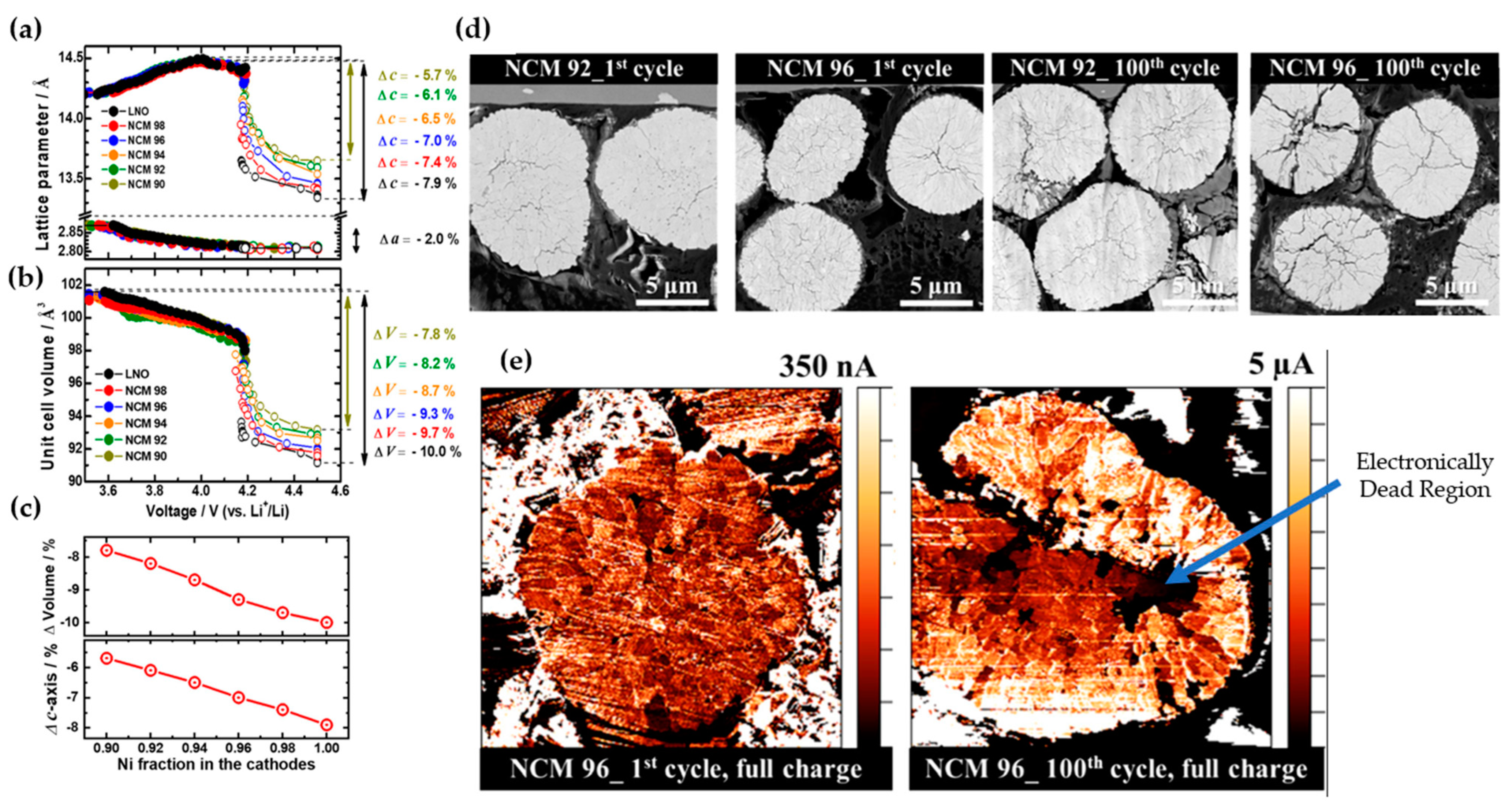
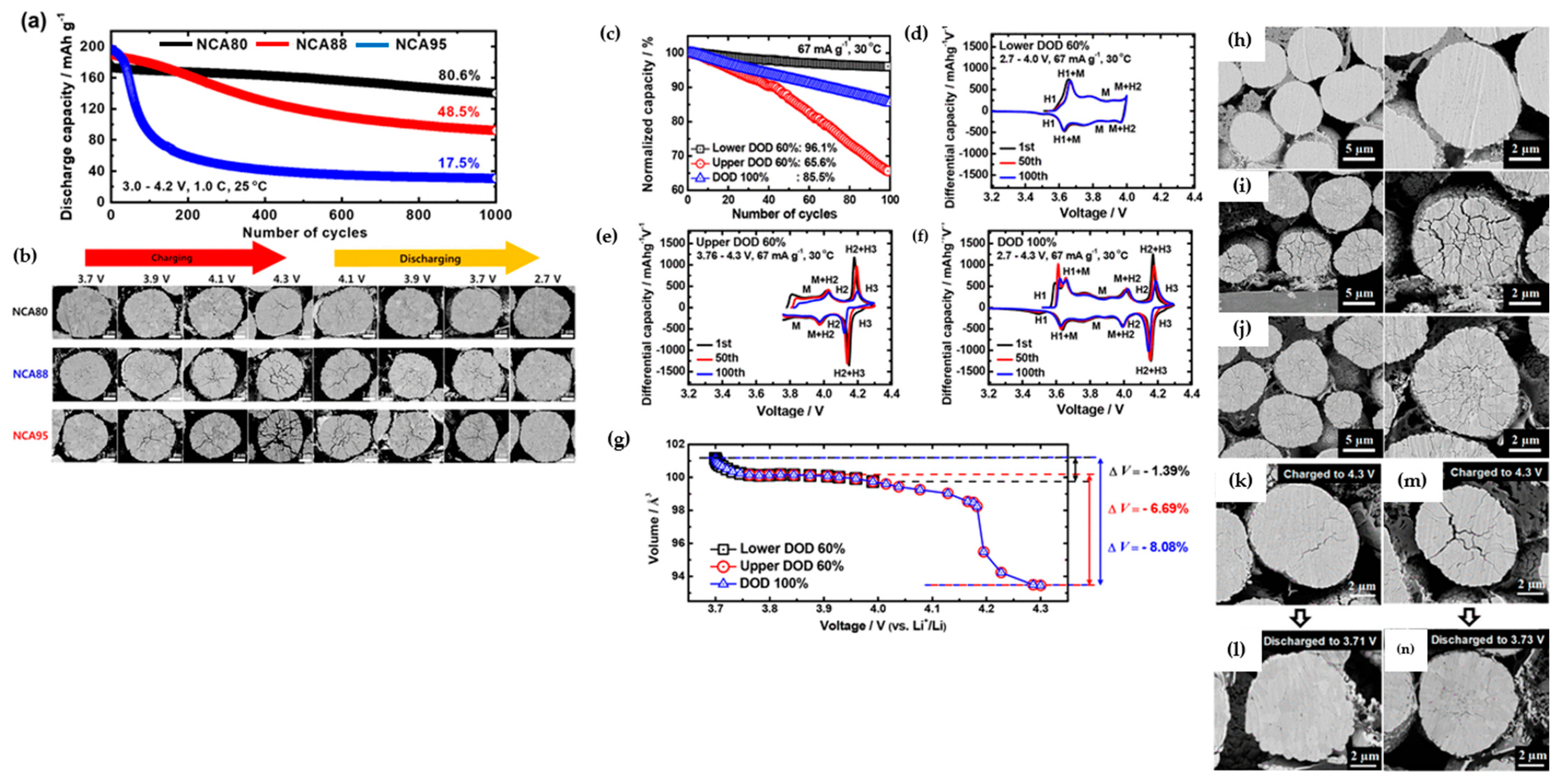
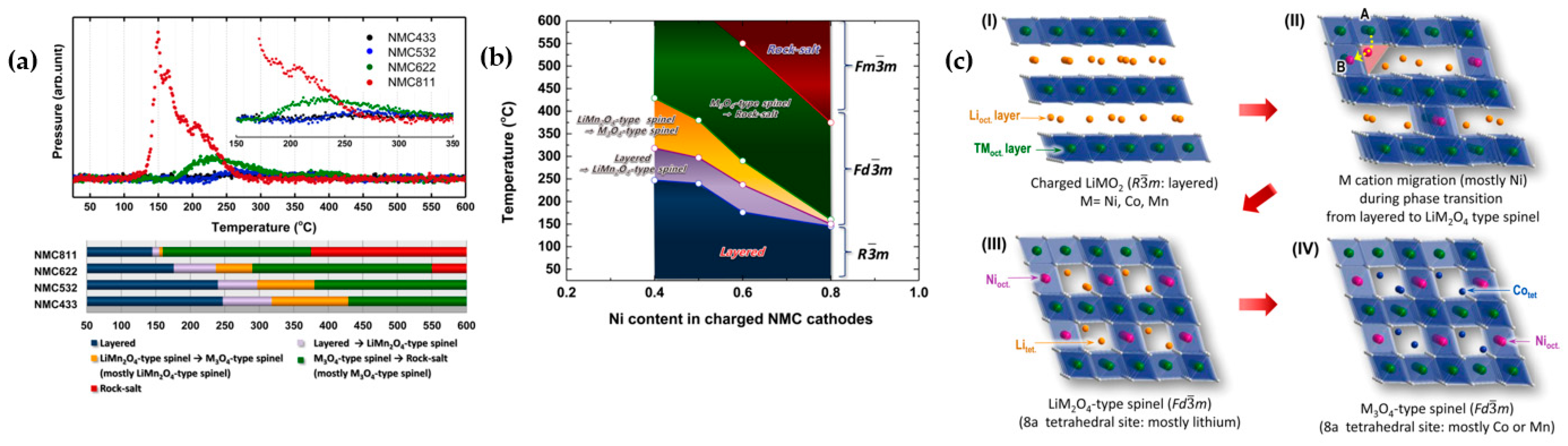
2.1. Understanding the Structural Instability
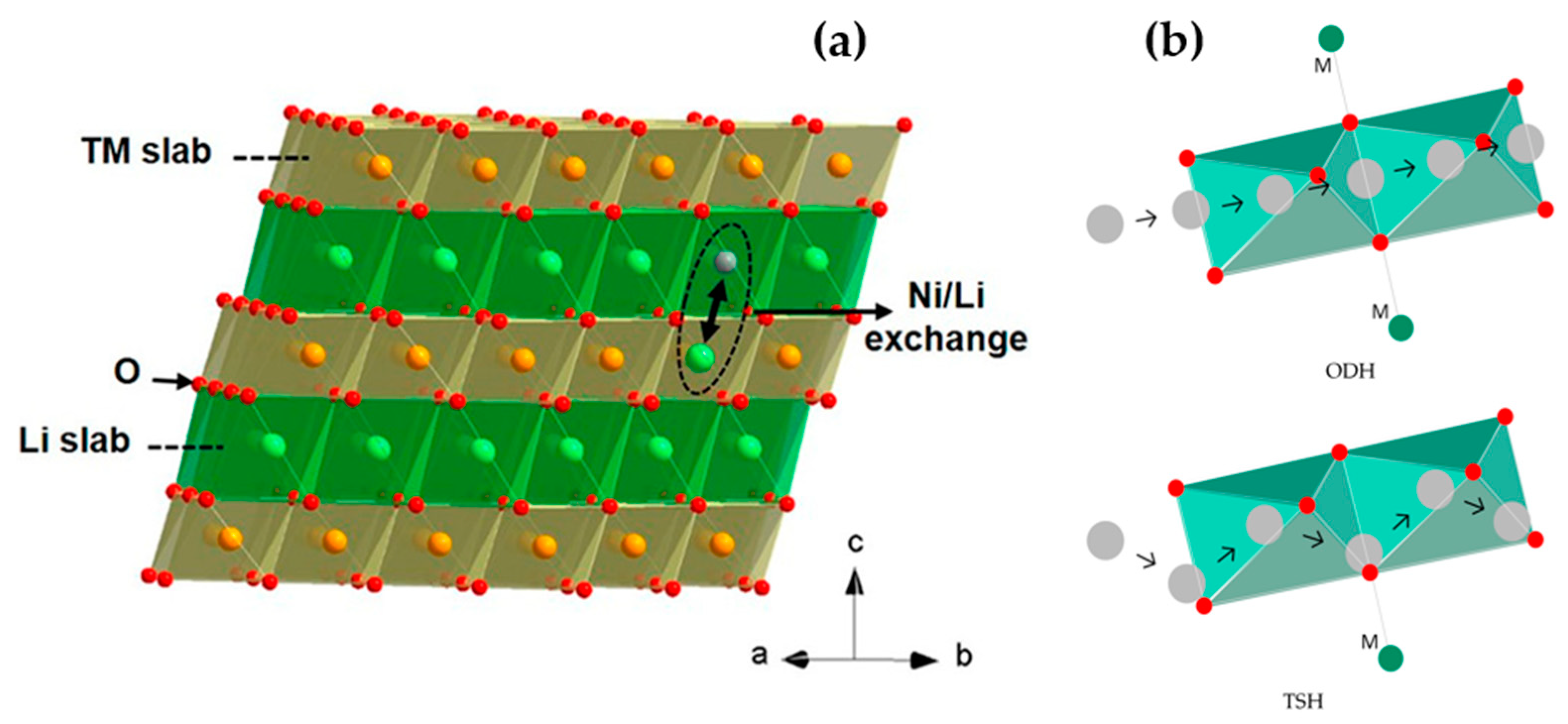
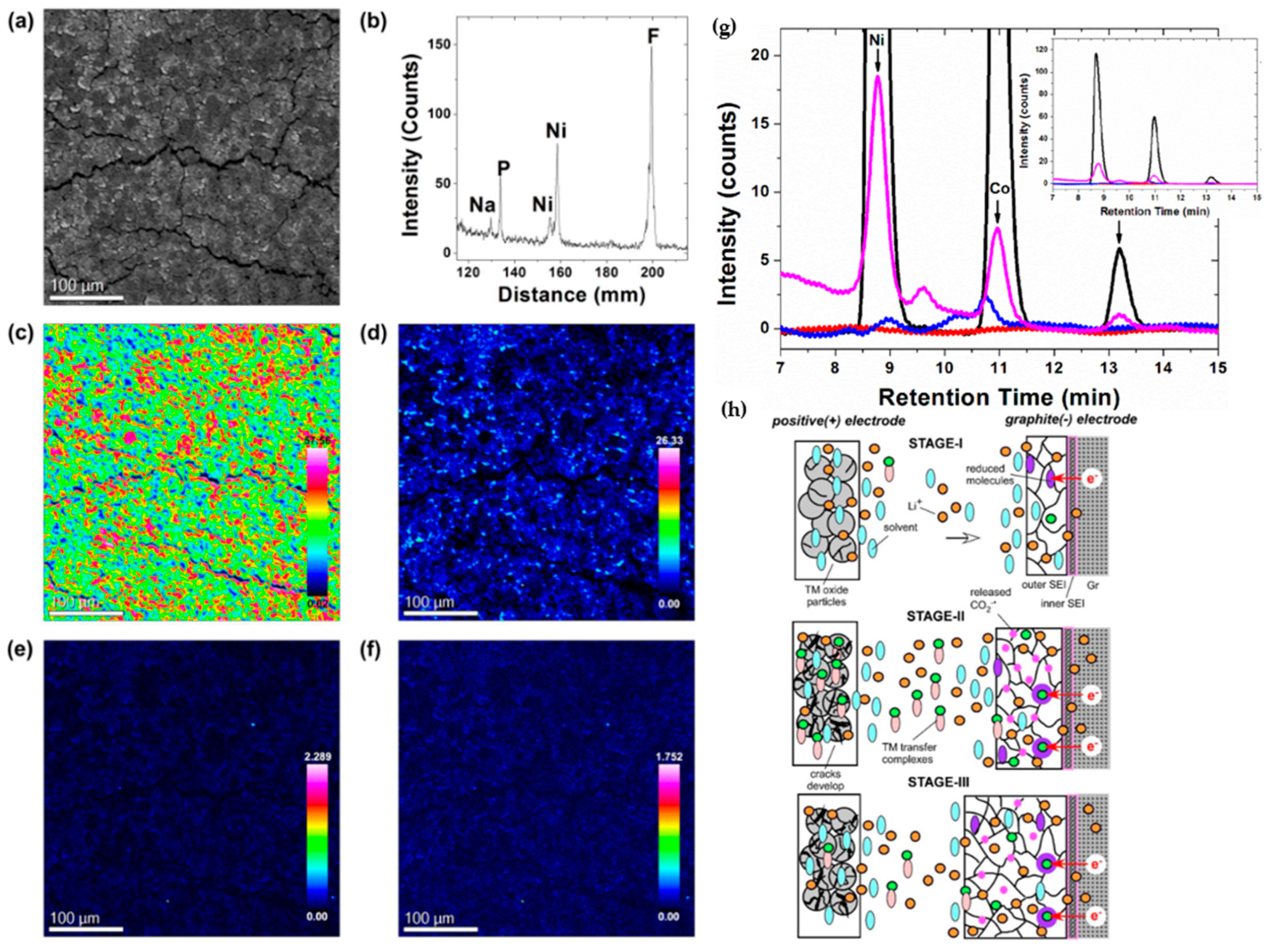
2.2. Electrode-Electrolyte Interface Instability
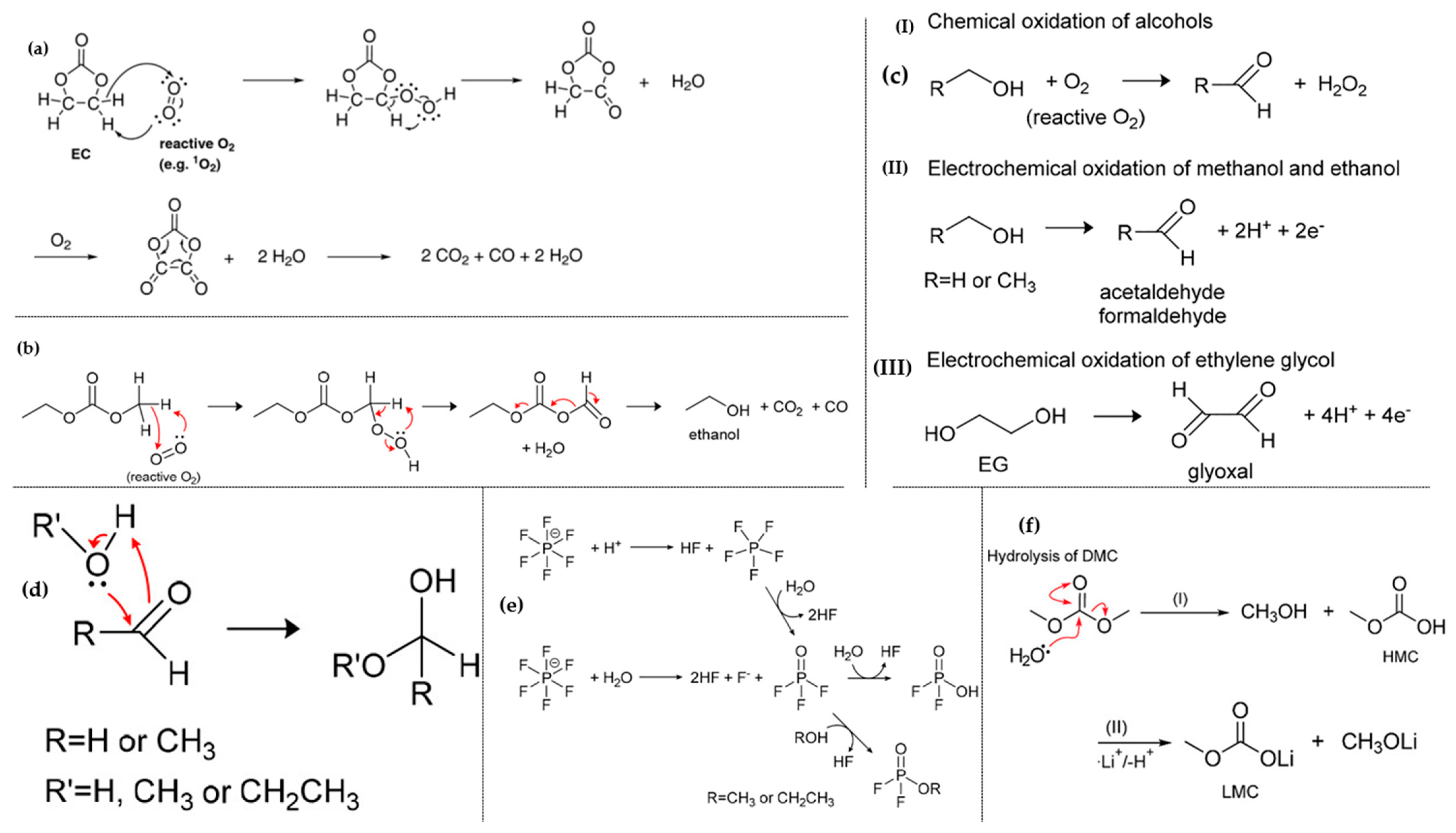
3. Strategies and Countermeasures
3.1. Elemental Doping
3.2. Surface Coating
3.3. Structural Engineering
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koo, J.K.; Choi, H.; Seo, J.K.; Hwang, S.M.; Lee, J.; Kim, Y.J. Microstructure engineering of nickel-rich oxide/carbon composite cathodes for fast charging of lithium-ion batteries. Ceram. Int. 2022, 48, 31859–31865. [Google Scholar] [CrossRef]
- Rudnicka, E.; Jakobczyk, P.; Lewandowski, A. Thermodynamic and kinetic limits of Li-ion battery operation. J. Energy Storage 2022, 55, 105747. [Google Scholar] [CrossRef]
- Entwistle, T.; Sanchez-Perez, E.; Murray, G.J. Co-precipitation synthesis of nickel-rich cathodes for Li-ion batteries. Energy Rep. 2022, 8, 67–73. [Google Scholar] [CrossRef]
- Chen, Z.; Danilov, D.L.; Rajimakers, L.H.J. Overpotential analysis of graphite-based Li-ion batteries seen from a porous electrode modeling perspective. J. Power Sources 2021, 509, 230345. [Google Scholar] [CrossRef]
- Zheng, H.; Li, J.; Song, X.; Liu, L.; Battaglia, V.S. A comprehensive understanding of electrode thickness effects on the electrochemical performances of Li-ion battery cathodes. Electrochim. Acta 2012, 71, 258–265. [Google Scholar] [CrossRef]
- Xie, Y.; Jin, Y.; Xiang, L. Li-rich layered oxides: Structure, capacity and voltage fading mechanisms and solving strategies. Particuology 2022, 61, 1–10. [Google Scholar] [CrossRef]
- Gamble, F.; Osiecki, J.H.; Cais, M.; Pisharody, R.; Disalvo, F.J.; Geballe, T.H. Intercalation complexes of Lewis bases and layered sulfides: A large class of new superconductors. J. Sci. 1971, 174, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wang, S.; Liao, J.; Wang, S.; He, X.; Pan, B.; He, H.; Chen, C. Facilitating lithium-ion diffusion in layered cathode materials by introducing Li+/Ni2+ antisite defects for high-rate Li-ion batteries. Res. J. 2019, 2019, 2198906. [Google Scholar] [CrossRef]
- Huang, D.; Engtrakul, C.; Nanayakkara, S.; Mulder, D.W.; Han, S.D.; Zhou, M.; Luo, H.; Tenent, R.C. Understanding degradation at the lithium-ion battery cathode/electrolyte interface: Connecting transition-metal dissolution mechanisms to electrolyte composition. ACS Appl. Mater. Interfaces 2021, 13, 11930–11939. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Bae, C.; Denlinger, A.; Miller, T. Electric vehicles batteries: Requirements and challenges. Joule 2020, 4, 511–515. [Google Scholar] [CrossRef]
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and challenges of lithium ion batteries in automotive applications. ACS Energy Lett. 2021, 6, 621–630. [Google Scholar] [CrossRef]
- Jiang, M.; Qian Zhang, Q.; Wu, X.; Chen, Z.; Danilov, D.L.; Eichel, R.; Notte, P.H.L. Synthesis of Ni-rich layered-oxide nanomaterials with enhanced Li-ion diffusion pathways as high-rate cathodes for Li-ion batteries. ACS Appl. Energy Mater. 2020, 3, 6583–6590. [Google Scholar] [CrossRef]
- Manthiram, A. An outlook on lithium ion battery technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar]
- Zaghib, K.; Mauger, A.; Julien, C.M. Olivine-based cathode materials In Rechargeable Batteries. Green Energy Technol. 2015, 25–65. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Zaghib, K.; Groult, H. Comparative issues of cathode materials for Li-ion batteries. Inorganics 2014, 2, 132–154. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, L.; Jizhou Jiang, J.; Duo Wang, D.; Zhang, D.; Yin, D.; Wang, D.L.; Zhang, X.; Huang, G.; Cheng, Y. Stabilization of high-voltage layered oxide cathode by multi-electron rare earth oxide. Chem. Eng. J. 2023, 454, 140249. [Google Scholar] [CrossRef]
- Yang, W. Oxygen release and oxygen redox. Nat. Energy. 2018, 3, 619–620. [Google Scholar] [CrossRef]
- Garcia, J.C.; Bareño, J.; Yan, J.; Chen, G.; Hauser, A.; Croy, J.R.; Iddir, H. Surface structure, morphology, and stability of Li(Ni1/3Mn1/3Co1/3)O2 cathode material. J. Phys. Chem. C 2017, 121, 8290–8299. [Google Scholar] [CrossRef]
- Ryu, H.H.; Sun, H.H.; Myung, S.T.; Chong, S.; Yoon, C.S.; Sun, Y.K. Reducing cobalt from lithium-ion batteries for the electric vehicle era. Energy Environ. Sci. 2021, 14, 844–852. [Google Scholar] [CrossRef]
- Kim, M.H.; Shin, H.S.; Shin, D.; Sun, Y.K. Synthesis and electrochemical properties of Li[Ni0.8Co0.1Mn0.1]O2 and Li[Ni0.8Co0.2]O2 via co-precipitation. J. Power Sources 2006, 159, 1328–1333. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Li, H.; Liu, A.; Huang, Q.; Ma, L.; Li, Y.; Dahn, J.R. Structural, Electrochemical, and Thermal Properties of Nickel-Rich LiNixMnyCozO2 Materials. Chem. Mater. 2018, 30, 8852–8860. [Google Scholar] [CrossRef]
- Xiao, J.; Chernova, N.A.; Whittingham, M.S. Layered mixed transition metal oxide cathodes with reduced cobalt content for lithium ion batteries. Chem. Mater. 2008, 20, 7454–7464. [Google Scholar] [CrossRef]
- Tian, C.; Lin, F.; Doeff, M.M. Electrochemical characteristics of layered transition metal oxide cathode materials for lithium ion batteries: Surface, bulk behavior, and thermal properties. Acc. Chem. Res. 2017, 51, 89–96. [Google Scholar] [CrossRef]
- Aryal, S.; Durham, J.L.; Lipson, A.L.; Pupek, K.Z.; Kahvecioglu, O. Roles of Mn and Co in Ni-rich layered oxide cathodes synthesized utilizing a Taylor Vortex Reactor. Electrochim. Acta. 2021, 391, 138929. [Google Scholar] [CrossRef]
- Liang, C.; Kong, F.; Longo, R.C.; Santosh, K.C.; Kim, J.S.; Jeon, S.H.; Choi, S.A.; Cho, K. Unraveling the origin of instability in Ni-rich LiNi1-2xCoxMnxO2 (NCM) cathode materials. J. Phys. Chem. C 2016, 120, 6383–6393. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef]
- Belharouak, I.; Sun, Y.K.; Liu, J.; Amine, K. Li(Ni1/3Co1/3Mn1/3)O2 as a suitable cathode for high power applications. J. Power Sources 2003, 123, 247–252. [Google Scholar] [CrossRef]
- Choi, J.; Manthiram, A. Role of chemical and structural stabilities on the electrochemical properties of layered LiNi1/3Mn1/3Co1/3O2 cathodes. J. Electrochem. Soc. 2005, 152, A1714. [Google Scholar] [CrossRef]
- Li, F.; Liu, Z.; Shen, J.; Xu, X.; Zeng, L.; Zhang, B.; Zhu, H.; Liu, Q.; Liu, J.; Zhu, M. A nanorod-like Ni-rich layered cathode with enhanced Li+ diffusion pathways for high-performance lithium-ion batteries. J. Mater. Chem. A 2021, 9, 2830–2839. [Google Scholar] [CrossRef]
- Zhuang, D.; Bazant, M.Z. Theory of layered-oxide cathode degradation in Li-ion batteries by oxidation-induced cation disorder. J. Electrochem. Soc. 2022, 169, 100536. [Google Scholar] [CrossRef]
- Evertz, M.; Horsthemke, F.; Kasnatscheew, J.; Börner, M.; Winter, M.; Nowak, S. Unraveling transition metal dissolution of Li1.04Ni1/3Co1/3Mn1/3O2 (NCM 111) in lithium ion full cells by using the total reflection X-ray fluorescence technique. J. Power Sources 2016, 329, 364–371. [Google Scholar] [CrossRef]
- Kim, T.; Ono, L.K.; Fleck, N.; Raga, S.R.; Qi, Y. Transition metal speciation as a degradation mechanism with the formation of a solid-electrolyte interphase (SEI) in Ni-rich transition metal oxide cathodes. J. Mater. Chem. A. 2018, 6, 14449–14463. [Google Scholar] [CrossRef]
- Kim, H.R.; Woo, S.W.; Kim, J.H.; Cho, W.; Kim, Y.K. Capacity fading behavior of Ni-rich layered cathode materials in Li-ion full cells. J. Electroanal. Chem. 2016, 782, 168–173. [Google Scholar] [CrossRef]
- Cui, J.; Ding, X.; Luo, D.; Xie, H.; Zhang, Z.; Zhang, B.; Tan, F.; Liu, C.; Lin, Z. Effect of cationic uniformity in precursors on Li/Ni mixing of Ni-rich layered cathodes. Energy Fuels 2021, 35, 1842–1850. [Google Scholar] [CrossRef]
- Yoon, C.S.; Park, K.P.; Kim, U.H.; Kang, K.H.; Ryu, H.H.; Sun, Y.K. High-Energy Ni-Rich Li[NixCoyMn1–x–y]O2 Cathodes via Compositional Partitioning for Next-Generation Electric Vehicles. Chem. Mater. 2017, 29, 10436–10445. [Google Scholar] [CrossRef]
- Nam, G.W.; Park, N.Y.; Park, K.J.; Yang, J.; Liu, J.; Yoon, C.S.; Sun, Y.K. Capacity fading of Ni-rich NCA cathodes: Effect of microcracking extent. ACS Energy Lett. 2019, 4, 2995–3001. [Google Scholar] [CrossRef]
- Arai, H.; Okada, S.; Ohtsuka, H.; Ichimura, M.; Yamaki, J. Characterization and cathode performance of Li1−xNi1+xO2 prepared with the excess lithium method. Solid. State Ion. 1995, 80, 261–269. [Google Scholar] [CrossRef]
- Zheng, J.; Ye, Y.; Liu, T.; Xiao, Y.; Wang, C.; Wang, F.; Pan, F. Ni/Li disordering in layered transition metal oxide: Electrochemical impact, origin, and control. Acc. Chem. Res. 2019, 52, 2201–2209. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, J.; Cui, S.; Song, X.; Su, Y.; Deng, W.; Wu, Z.; Wang, X.; Wang, W.; Rao, M.; et al. Kinetics tuning of Li-ion diffusion in layered Li(NixMnyCoz)O2. J. Am. Chem. Soc. 2015, 137, 8364–8367. [Google Scholar] [CrossRef]
- Salgado, R.M.; Danzi, F.; Oliveira, J.E.; El-Azab, A.; Camanho, P.P.; Braga, M.H. The Latest Trends in Electric Vehicles Batteries. Molecules 2021, 26, 3188. [Google Scholar] [CrossRef]
- Yu, H.; Qian, Y.; Otani, M.; Tang, D.; Guo, S.; Zhue, Y.; Zhou, H. Study of the lithium/nickel ions exchange in the layered LiNi0.42Mn0.42Co0.16O2 cathode material for lithium ion batteries: Experimental and first-principles calculations. Energy Environ. Sci. 2014, 7, 1068–1078. [Google Scholar] [CrossRef]
- Zheng, J.; Teng, G.; Chao Xin, C.; Zhuo, Z.; Liu, J.; Li, Q.; Hu, Z.; Xu, M.; Yan, S.; Yang, W.; et al. Role of superexchange interaction on tuning of Ni/Li disordering in layered Li(NixMnyCoz)O2. J. Phys. Chem. Lett. 2017, 8, 5537–5542. [Google Scholar] [CrossRef]
- Kanamori, J. Superexchange interaction and symmetry properties of electron orbitals. J. Phys. Chem. Solids 1959, 10, 87–98. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Lv, D.; Wei, Y.; Zheng, J.; Wang, Z.; Kuppan, S.; Yu, J.; Luo, L.; Edwards, D.; et al. Atomic-resolution visualization of distinctive chemical mixing behavior of Ni, Co, and Mn with Li in layered lithium transition-metal oxide cathode materials. Chem. Mater. 2015, 27, 5393–5401. [Google Scholar] [CrossRef]
- Lin, Q.; Guan, W.; Zhou, J.; Meng, J.; Huang, W.; Chen, T.; Gao, Q.; Wei, X.; Zeng, Y.; Li, J.; et al. Ni–Li anti-site defect induced intragranular cracking in Ni-rich layer-structured cathode. Nano Energy 2020, 76, 105021. [Google Scholar] [CrossRef]
- Zheng, J.; Gu, M.; Xiao, J.; Zuo, P.; Wang, C.; Zhang, J.G. Corrosion/fragmentation of layered composite cathode and related capacity/voltage fading during cycling process. Nano Lett. 2013, 13, 3824–3830. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shao, R.; Wang, Q.; Zhou, T.; Lu, J.; Jiang, N.; Gao, P.; Liu, W.; Yu, Y.; Zhou, H. Bulk and surface degradation in layered Ni-rich cathode for Li ions batteries: Defect proliferation via chain reaction mechanism. En. Stor. Mater. 2021, 35, 62–69. [Google Scholar] [CrossRef]
- Noh, H.J.; Youn, S.; Yoon, C.S.; Sun, Y.K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x= 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Zeng, D.; Cabana, J.; Bréger, J.; Yoon, W.S.; Grey, C.P. Cation Ordering in Li[NixMnxCo(1–2x)]O2 Layered Cathode Materials: A Nuclear Magnetic Resonance (NMR), Pair Distribution Function, X-ray Absorption Spectroscopy, and Electrochemical Study. Chem. Mater. 2007, 19, 6277–6289. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, S.M.; Bak, S.M.; Cho, B.W.; Chung, K.Y.; Lee, J.Y.; Chang, W.; Eric, A. Stac. Investigating local degradation and thermal stability of charged nickel-based cathode materials through real-time electron microscopy. ACS Appl. Mater. Interfaces 2014, 6, 15140–15147. [Google Scholar] [CrossRef]
- Yoon, C.S.; Jun, D.W.; Myung, S.T.; Sun, Y.K. Structural stability of LiNiO2 cycled above 4.2 V. ACS Energy Lett. 2017, 2, 1150–1155. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Nagayama, M. Electrochemistry and structural chemistry of LiNiO2 (R3m) for 4 volt secondary lithium cells. J. Electrochem. Soc. 1993, 140, 1862. [Google Scholar] [CrossRef]
- Kim, J.H.; Ryu, H.H.; Kim, S.J.; Yoon, C.S.; Sun, Y.K. Degradation mechanism of highly Ni-rich Li[NixCoyMn1–x–y]O2 cathodes with x> 0.9. ACS Appl. Mater. Interfaces 2019, 11, 30936–30942. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen release and its effect on the cycling stability of LiNixMnyCozO2 (NMC) cathode materials for Li-ion batteries. J. Electrochem. Soc. 2017, 164, A1361. [Google Scholar] [CrossRef]
- Ceder, G.; Van der Ven, A. Phase diagrams of lithium transition metal oxides: Investigations from first principles. Electrochim. Acta 1999, 45, 131–150. [Google Scholar] [CrossRef]
- Kuo, L.Y.; Guillon, O.; Kaghazchi, P. Origin of Structural Phase Transitions in Ni-Rich LixNi0.8Co0.1Mn0.1O2 with Lithiation/Delithiation: A First-Principles Study. ACS Sustain. Chem. Eng. 2021, 9, 7437–7446. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Cheng, F. Concentration-Gradient LiNi0.85Co0.12Al0.03O2 Cathode Assembled with Primary Particles for Rechargeable Lithium-Ion Batteries. Energy Fuels 2021, 35, 13474–13482. [Google Scholar] [CrossRef]
- Park, K.J.; Hwang, J.Y.; Ryu, H.H.; Maglia, F.; Kim, S.J.; Lamp, P.; Yoon, C.S.; Sun, Y.K. Degradation mechanism of Ni-enriched NCA cathode for lithium batteries: Are microcracks really critical? ACS Energy Lett. 2019, 4, 1394–1400. [Google Scholar] [CrossRef]
- Park, N.Y.; Park, G.T.; Kim, S.B.; Jung, W.; Park, B.C.; Sun, Y.K. Degradation mechanism of Ni-rich cathode materials: Focusing on particle interior. ACS Energy Lett. 2022, 7, 2362–2369. [Google Scholar] [CrossRef]
- Terada, Y.; Nishiwaki, Y.; Nakai, I.; Nishikaw, F. Study of Mn dissolution from LiMn2O4 spinel electrodes using in situ total reflection X-ray fluorescence analysis and fluorescence XAFS technique. J. Power Sources 2001, 97, 420–422. [Google Scholar] [CrossRef]
- Tsunekawa, H.; Tanimoto, S.; Marubayashi, R.; Fujita, M.; Kifune, K.; Sano, M. Capacity fading of graphite electrodes due to the deposition of manganese ions on them in Li-ion batteries. J. Electrochem. Soc. 2002, 149, A1326. [Google Scholar] [CrossRef]
- Jang, D.H.; Shin, Y.J.; Oh, S.M. Dissolution of spinel oxides and capacity losses in 4 V li/lixMn2O4 cells. J. Electrochem. Soc. 1996, 143, 2204. [Google Scholar] [CrossRef]
- Solchenbach, S.; Hong, G.; Freiberg, A.T.S.; Jung, R.; Gasteiger, H.A. Electrolyte and SEI decomposition reactions of transition metal ions investigated by on-line electrochemical mass spectrometry. J. Electrochem. Soc. 2018, 165, A3304–A3312. [Google Scholar] [CrossRef]
- Wandt, J.; Freiberg, A.; Thomas, R.; Gorlin, Y.; Siebel, A.; Jung, R.; Gasteigera, H.A.; Tromp, M. Transition metal dissolution and deposition in Li-ion batteries investigated by operando X-ray absorption spectroscopy. J. Mater. Chem. A. 2016, 4, 18300–18305. [Google Scholar] [CrossRef]
- Wachs, S.J.; Behling, C.; Ranninger, J.; Möller, J.; Mayrhofer, K.J.J.; Berkes, B.B. Online monitoring of transition-metal dissolution from a high-Ni-content cathode material. ACS Appl. Mater. Interfaces 2021, 13, 33075–33082. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Sun, Q.; Liu, G.; Song, X.; Battaglia, V.S. Correlation between dissolution behavior and electrochemical cycling performance for LiNi1/3Co1/3Mn1/3O2-based cells. J. Power Sources 2012, 207, 134–140. [Google Scholar] [CrossRef]
- Ko, D.S.; Park, J.H.; Park, S.; Ham, Y.N.; Ahn, S.J.; Park, J.H.; Han, H.N.; Lee, E.; Jeon, W.S.; Jung, C. Microstructural visualization of compositional changes induced by transition metal dissolution in Ni-rich layered cathode materials by high-resolution particle analysis. Nano Energy 2019, 56, 434–442. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Shkrob, I.A.; Abraham, D.P. Transition metal dissolution, ion migration, electrocatalytic reduction and capacity loss in lithium-ion full cells. J. Electrochem. Soc. 2017, 164, A389. [Google Scholar] [CrossRef]
- Konishi, H.; Yuasa, T.; Yoshikawa, M. Thermal stability of Li1−yNixMn(1−x)/2Co(1− x)/2O2 layer-structured cathode materials used in Li-Ion batteries. J. Power Sources 2011, 196, 6884–6888. [Google Scholar] [CrossRef]
- Lee, K.K.; Yoon, W.S.; Kim, K.B.; Lee, K.Y.; Hong, S.T. Thermal behavior and the decomposition mechanism of electrochemically delithiated Li1−xNiO2. J. Power Sources 2001, 97, 321–325. [Google Scholar] [CrossRef]
- Bak, S.M.; Hu, E.; Zhou, Y.; Yu, X.; Senanayake, S.D.; Cho, S.J.; Kim, K.B.; Chung, K.Y.; Yang, X.Q.; Nam, K.W. Structural changes and thermal stability of charged LiNixMnyCozO2 cathode materials studied by combined in situ time-resolved XRD and mass spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef]
- Nam, K.W.; Bak, S.M.; Hu, E.; Yu, X.; Zhou, Y.; Wang, X.; Wu, L.; Zhu, Y.; Chung, K.Y.; Yang, X.Q. Combining in situ synchrotron X-ray diffraction and absorption techniques with transmission electron microscopy to study the origin of thermal instability in overcharged cathode materials for lithium-ion batteries. Adv. Funct. Mater. 2013, 23, 1047–1063. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kim, Y.T.; Li, H.H.; Shao-Hor, S. Thermal instability of cycled LixNi0.5Mn0.5O2 electrodes: An in situ synchrotron X-ray powder diffraction study. Chem. Mater. 2008, 20, 4936–4951. [Google Scholar] [CrossRef]
- Reed, J.; Ceder, G. Role of electronic structure in the susceptibility of metastable transition-metal oxide structures to transformation. Chem. Rev. 2004, 104, 4513–4534. [Google Scholar] [CrossRef] [PubMed]
- Bak, S.M.; Nam, K.W.; Chang, W.; Yu, X.; Hu, E.; Hwang, S.; Stach, E.A.; Kim, K.B.; Chung, K.Y.; Yang, X.Q. Correlating structural changes and gas evolution during the thermal decomposition of charged LixNi0.8Co0.15Al0.05O2 cathode materials. Chem. Mater. 2013, 25, 337–351. [Google Scholar] [CrossRef]
- Wu, L.; Nam, K.W.; Wang, X.; Zhou, Y.; Zheng, J.C.; Yang, X.Q.; Zhu, Y. Structural origin of overcharge-induced thermal instability of Ni-containing layered-cathodes for high-energy-density lithium batteries. Chem. Mater. 2011, 23, 3953–3960. [Google Scholar] [CrossRef]
- Belharouak, I.; Lu, W.; Vissers, D.; Amine, K. Safety characteristics of Li(Ni0.8Co0.15Al0.05)O2 and Li(Ni1/3Co1/3Mn1/3)O2. Electrochem. Commun. 2006, 8, 329–335. [Google Scholar] [CrossRef]
- Belharouak, I.; Lu, W.; Liu, J.; Vissers, D.; Amine, K. Thermal behavior of delithiated Li(Ni0.8Co0.15Al0.05)O2 and Li1.1(Ni1/3Co1/3Mn1/3)0.9O2 powders. J. Power Sources 2007, 174, 905–909. [Google Scholar] [CrossRef]
- Seo, D.M.; Chalasani, D.; Parimalam, B.S.; Kadam, R.; Nie, M.; Lucht, B.L. Reduction reactions of carbonate solvents for lithium ion batteries. ECS Electrochem. Lett. 2014, 3, A91. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, S.M.; Bak, S.M.; Kim, S.Y.; Cho, B.W.; Chung, K.Y.; Lee, J.Y.; Stach, E.A.; Chang, W.U. sing real-time electron microscopy to explore the effects of transition-metal composition on the local thermal stability in charged LixNiyMnzCo1–y–zO2 cathode materials. Chem. Mater. 2015, 27, 3927–3935. [Google Scholar] [CrossRef]
- Tan, K.; Reddy, M.V.; Rao, G.V.S.; Chowdari, B.V.R. Effect of AlPO4-coating on cathodic behavior of Li(Ni0.8Co0.2)O2. J. Power Sources 2005, 141, 129–142. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.; Yang, L.; Shen, X.; Liu, Q.; Hu, Z.; Kong, Q.; Ma, J.; Li, J.; Lin, H.J.; et al. Anionic redox reaction and structural evolution of Ni-rich layered oxide cathode material. Nano Energy 2022, 98, 107335. [Google Scholar] [CrossRef]
- Seo, J.H.; Park, J.; Plett, G.; Sastry, A.M. Gas-evolution induced volume fraction changes and their effect on the performance degradation of Li-ion batteries. Electrochem. Solid-State Lett. 2010, 13, A135. [Google Scholar] [CrossRef]
- Kaufman, L.A.; Huang, T.-Y.; Lee, D.; McCloskey, B.D. Particle Surface Cracking Is Correlated with Gas Evolution in High-Ni Li-Ion Cathode Materials. ACS Appl. Mater. Interfaces 2022, 14, 39959–39964. [Google Scholar] [CrossRef] [PubMed]
- Heenan, T.M.; Wade, A.; Tan, C.; Parker, J.E.; Matras, D.; Leach, A.S.; Robinson, J.B.; Llewellyn, A.; Dimitrijevic, A.; Jervis, R.; et al. Identifying the origins of microstructural defects such as cracking within Ni-rich NMC811 cathode particles for lithium-ion batteries. Adv. Energy Mater. 2020, 10, 2002655. [Google Scholar] [CrossRef]
- Wandt, J.; Freiberg, A.T.S.; Ogrodnik, A.; Gasteiger, H.A. Singlet oxygen evolution from layered transition metal oxide cathode materials and its implications for lithium-ion batteries. Mater. Today 2018, 21, 825–833. [Google Scholar] [CrossRef]
- Dose, W.M.; Temprano, I.; Allen, J.P.; Björklund, E.; O’Keefe, C.A.; Li, W.; Mehdi, B.L.; Weatherup, R.S.; Volder, M.F.L.D.; Grey, C.P. Electrolyte reactivity at the charged Ni-rich cathode interface and degradation in Li-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 13206–13222. [Google Scholar] [CrossRef]
- Heiskanen, S.K.; Laszczynski, N.; Lucht, B.L. Perspective—Surface reactions of electrolyte with LiNixCoyMnzO2 cathodes for lithium ion batteries. J. Electrochem. Soc. 2020, 167, 100519. [Google Scholar] [CrossRef]
- Rinkel, B.L.; Vivek, J.P.; Garcia-Araez, N.; Grey, C.P. Two electrolyte decomposition pathways at nickel-rich cathode surfaces in lithium-ion batteries. Energy Environ. Sci. 2022, 15, 3416–3438. [Google Scholar] [CrossRef]
- Rinkel, B.L.; Hall, D.S.; Temprano, I.; Grey, C.P. Electrolyte oxidation pathways in lithium-ion batteries. J. Am. Chem. Soc. 2020, 142, 15058–15074. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Jo, C.H.; Cho, W.; Kim, Y.J.; Yashiro, H.; Sun, Y.K.; Myung, S.T. Effect of residual lithium compounds on layer Ni-rich Li[Ni0.7Mn0.3]O2. J. Electrochem. Soc. 2014, 161, A920. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, B.; Kang, Y.-S.; Park, S.Y.; Yun, D.J.; Park, I.; Shim, J.H.; Park, J.H.; Han, H.N.; Park, K. Effect of residual lithium rearrangement on Ni-rich layered oxide cathodes for lithium-ion batteries. Energy Technol. 2018, 6, 1361–1369. [Google Scholar] [CrossRef]
- Eom, J.; Kim, M.G.; Cho, J. Storage characteristics of LiNi0.8Co0.1+xMn0.1−xO2 (x = 0, 0.03, and 0.06) cathode materials for lithium batteries. J. Electrochem. Soc. 2008, 155, A239. [Google Scholar] [CrossRef]
- Zhuang, G.V.; Chen, G.; Shim, J.; Song, X.; Ross, P.N.; Richardson, T.J. Li2CO3 in LiNi0.8Co0.15Al0.05O2 cathodes and its effects on capacity and power. J. Power Sources 2004, 134, 293–297. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kuzuo, R.; Takeya, K.; Yamanaka, A. Effects of CO2 in air on Li deintercalation from LiNi1−x−yCoxAlyO2. J. Power Sources 1999, 81, 558–561. [Google Scholar] [CrossRef]
- Li, W.; Lucht, B.L. Lithium-ion batteries: Thermal reactions of electrolyte with the surface of metal oxide cathode particles. J. Electrochem. Soc. 2006, 153, A1617. [Google Scholar] [CrossRef]
- Bai, X.; Li, W.; Zhuang, W.; Lu, S.; Su, Z. Synthesis of Ni–rich LiNi0.83Co0.12Mn0.05O2 cathode materials with low residual lithium content without washing. Solid. State Ion. 2020, 355, 115418. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, Z.; Yue, P.; Guo, H.; Wu, F.; Wang, J.; Li, X. Washing effects on electrochemical performance and storage characteristics of LiNi0.8Co0.1Mn0.1O2 as cathode material for lithium-ion batteries. J. Power Sources 2013, 222, 318–325. [Google Scholar] [CrossRef]
- Xu, S.; Du, C.; Xu, X.; Han, G.; Zuo, P.; Cheng, X.; Ma, Y.; Yin, G. A mild surface washing method using protonated polyaniline for Ni-rich LiNi0.8Co0.1Mn0.1O2 material of lithium-ion batteries. Electrochim. Acta 2017, 248, 534–540. [Google Scholar] [CrossRef]
- Kim, Y. Mechanism of gas evolution from the cathode of lithium-ion batteries at the initial stage of high-temperature storage. J. Mater. Sci. 2013, 48, 8547–8551. [Google Scholar] [CrossRef]
- Kaufman, L.A.; McCloskey, B.D. Surface lithium carbonate influences electrolyte degradation via reactive oxygen attack in lithium-excess cathode materials. Chem. Mater. 2021, 33, 4170–4176. [Google Scholar] [CrossRef]
- Mahne, N.; Renfrew, S.E.; McCloskey, B.D.; Freunberger, S.A. Electrochemical oxidation of lithium carbonate generates singlet oxygen. Angew. Chem. Int. Ed. 2018, 57, 5529–5533. [Google Scholar] [CrossRef] [PubMed]
- Hatsukade, T.; Schiele, A.; Hartmann, P.; Brezesinski, T.; Janek, J. Origin of carbon dioxide evolved during cycling of nickel-rich layered NCM cathodes. ACS Appl. Mater. Interfaces 2018, 10, 38892–38899. [Google Scholar] [CrossRef]
- Song, M.; Lee, D.; Kim, J.; Heo, Y.; Nam, C.; Ko, D.; Lim, J. Chemical decomposition pathway of residual lithium carbonate of Li-ion battery cathodes. J. Power Sources 2023, 560, 232699. [Google Scholar] [CrossRef]
- Levartovsky, Y.; Wu, X.; Erk, C.; Maiti, S.; Grinblat, J.; Talianker, M.; Aurbach, D. Enhancement of structural, electrochemical, and thermal properties of Ni-rich LiNi0.85Co0.1Mn0.05O2 cathode materials for Li-ion batteries by Al and Ti doping. Batter. Supercaps 2021, 4, 221–231. [Google Scholar] [CrossRef]
- Hua, W.; Zhang, J.; Zheng, Z.; Liu, W.; Peng, X.; Guo, X.D.; Zhong, B.; Wang, Y.J.; Wang, X. Na-doped Ni-rich LiNi0.5Co0.2Mn0.3O2 cathode material with both high rate capability and high tap density for lithium-ion batteries. Dalton Trans. 2014, 43, 14824–14832. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Meng, Y.S.; Bréger, J.; Grey, C.P.; Ceder, G. Electrodes with high power and high capacity for rechargeable lithium batteries. Science 2006, 311, 977–980. [Google Scholar] [CrossRef]
- Rajkamal, A.; Kim, H. Formation of pillar-ions in the Li layer decreasing the Li/Ni disorder and improving the structural stability of cation-doped Ni-rich LiNi0.8Co0.1Mn0.1O2: A first-principles verification. ACS Appl. Energy Mater. 2021, 4, 14068–14079. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Z.; Zheng, X.; Guo, H.; Li, X.; Jing, Q.; Yang, Z. Effect of Mg doping on the structural and electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode materials. Electrochim. Acta 2015, 182, 795–802. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Q.; Dai, S.; Li, W.; Liu, X.; Ding, F.; Zhang, J. Synergistic effect of F–doping and LiF coating on improving the high-voltage cycling stability and rate capacity of LiNi0.5Co0.2Mn0.3O2 cathode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 34153–34162. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, G.; Tang, W.; Xiao, Y.; Yan, K. Enhanced rate performance and high potential as well as decreased strain of LiNi0.6Co0.2Mn0.2O2 by facile fluorine modification. Electrochim. Acta 2018, 261, 565–577. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, M.; Zhu, H.; Xie, T.; Wang, W.; Zhao, Q. Enhanced electrochemical performance of polyacene coated LiMn2O3.95F0.05 for lithium-ion batteries. Appl. Surf. Sci. 2013, 286, 177–183. [Google Scholar] [CrossRef]
- Kim, S.B.; Kim, H.; Park, D.H.; Kim, J.H.; Shin, J.H.; Jang, J.S.; Moon, S.H.; Choi, J.H.; Park, K.W. Li-ion diffusivity and electrochemical performance of Ni-rich cathode material doped with fluoride ions. J. Power Sources 2021, 506, 230219. [Google Scholar] [CrossRef]
- Su, Y.; Yang, Y.; Chen, L.; Lu, Y.; Bao, L.; Chen, G.; Yang, Z.; Zhang, Q.; Wang, J.; Chen, R.; et al. Improving the cycling stability of Ni-rich cathode materials by fabricating surface rock salt phase. Electrochim. Acta 2018, 292, 217–226. [Google Scholar] [CrossRef]
- Susai, F.A.; Bano, A.; Maiti, S.; Grinblat, J.; Chakraborty, A.; Sclar, H.; Kravchuk, T.; Kondrakov, A.; Tkachev, M.; Talianker, M.; et al. Stabilizing Ni-rich high energy cathodes for advanced lithium-ion batteries: The case of LiNi0.9Co0.1O2. J. Mater. Chem. A 2023, 11, 12958–12972. [Google Scholar] [CrossRef]
- Zhao, W.; Zou, L.; Jia, H.; Zheng, J.; Wang, D.; Song, J.; Hong, C.; Liu, R.; Xu, W.; Yang, Y.; et al. Optimized Al doping improves both interphase stability and bulk structural integrity of Ni-rich NMC cathode materials. ACS Appl. Energy Mater. 2020, 3, 3369–3377. [Google Scholar] [CrossRef]
- Jeong, M.; Kim, H.; Lee, W.; Ahn, S.J.; Lee, E.; Yoon, W.S. Stabilizing effects of Al-doping on Ni-rich LiNi0.80Co0.15Mn0.05O2 cathode for Li rechargeable batteries. J. Power Sources 2020, 474, 228592. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, X.; Luo, B.; Zhao, Z.; Shen, J.; Liu, Z.; Xiao, Z.; Zhang, B.; Zhang, J.; Ming, L.; et al. Understanding the enhancement effect of boron doping on the electrochemical performance of single-crystalline Ni-rich cathode materials. J. Colloid. Interface Sci. 2021, 604, 776–784. [Google Scholar] [CrossRef]
- Chu, M.; Huang, Z.; Zhang, T.; Wang, R.; Shao, T.; Wang, C.; Zhu, W.; He, L.; Chen, J.; Zhao, W.; et al. Enhancing the electrochemical performance and structural stability of Ni-rich layered cathode materials via dual-site doping. ACS Appl. Mater. Interfaces 2021, 13, 19950–19958. [Google Scholar] [CrossRef]
- Xin, F.; Goel, A.; Chen, X.; Zhou, H.; Bai, J.; Liu, S.; Wang, F.; Zhou, G.; Whittingham, M.S. Electrochemical characterization and microstructure evolution of Ni-rich layered cathode materials by niobium coating/substitution. Chem. Mater. 2022, 34, 7858–7866. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Zhang, D.; Yan, Y.; Li, Z. An effective doping strategy to improve the cyclic stability and rate capability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode. Chem. Eng. J. 2020, 402, 126195. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Naveed, A.; Zeng, T.; Zhang, X.; Shi, H.; Su, M.; Dou, A.; Zhou, Y.; Liu, Y. Comparison of structural and electrochemical properties of LiNi0.8Co0.15Al0.05O2 with Li site doping by different cations. Appl. Surf. Sci. 2022, 599, 153933. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Z.; Jing, Q.; Guo, H.; Li, X.; Yang, Z. Investigation on the effect of Na doping on structure and Li-ion kinetics of layered LiNi0.6Co0.2Mn0.2O2 cathode material. Electrochim. Acta 2016, 192, 120–126. [Google Scholar] [CrossRef]
- Liu, D.; Liu, S.; Zhang, C.; You, L.; Huang, T.; Yu, A. Revealing the effect of Ti doping on significantly enhancing cyclic performance at a high cutoff voltage for Ni-rich LiNi0.8Co0.15Al0.05O2 cathode. ACS Sustain. Chem. Eng. 2019, 7, 10661–10669. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, K.; Li, N.; Deng, X.; Jiao, J.; Zhao, E.; Yin, W.; Wang, B.; Zhao, J.; Xiao, X. Enhancing Thermal and High-Voltage Cycling Stability of Ni-Rich Layered Cathodes through a Ti-Doping-Induced Surface-Disordered Structure. ACS Appl. Energy Mater. 2022, 5, 12673–12681. [Google Scholar] [CrossRef]
- Sun, H.; Cao, Z.; Wang, T.; Lin, R.; Li, Y.; Liu, X.; Zhang, L.; Lin, F.; Huang, Y.; Luo, W. Enabling high rate performance of Ni-rich layered oxide cathode by uniform titanium doping. Mater. Today Energy 2019, 13, 145–151. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.; Kim, W.J.; Kim, Y.C.; Jung, J.Y.; Rhee, D.Y.; Song, J.H.; Cho, W.; Park, M.S. Incorporation of titanium into Ni-rich layered cathode materials for lithium-ion batteries. ACS Appl. Energy Mater. 2020, 3, 12204–12211. [Google Scholar] [CrossRef]
- Wu, F.; Liu, N.; Chen, L.; Su, Y.; Tan, G.; Bao, L.; Zhang, Q.; Lu, Y.; Wang, J.; Chen, S.; et al. Improving the reversibility of the H2-H3 phase transitions for layered Ni-rich oxide cathode towards retarded structural transition and enhanced cycle stability. Nano Energy 2019, 59, 50–57. [Google Scholar] [CrossRef]
- Steiner, J.D.; Cheng, H.; Walsh, J.; Zhang, Y.; Zydlewski, B.; Mu, L.; Xu, Z.; Rahman, M.M.; Sun, H.; Michel, F.M.; et al. Targeted surface doping with reversible local environment improves oxygen stability at the electrochemical interfaces of nickel-rich cathode materials. ACS Appl. Mater. Interfaces 2019, 11, 37885–37891. [Google Scholar] [CrossRef]
- Kasim, M.F.; Wan Azizan, W.A.H.; Elong, K.A.; Kamarudin, N.; Yaakob, M.K.; Badar, N. Enhancing the structural stability and capacity retention of Ni-rich LiNi0.7Co0.3O2 cathode materials via Ti doping for rechargeable Li-ion batteries: Experimental and computational approaches. J. Alloys Compd. 2021, 888, 161559. [Google Scholar] [CrossRef]
- Qiu, L.; Xiang, W.; Tian, W.; Xu, C.L.; Li, Y.C.; Wu, Z.G.; Chen, T.R.; Jia, K.; Wang, D.; He, F.R.; et al. Polyanion and cation co-doping stabilized Ni-rich Ni–Co–Al material as cathode with enhanced electrochemical performance for Li-ion battery. Nano Energy 2019, 63, 103818. [Google Scholar] [CrossRef]
- Li, C.; Kan, W.H.; Xie, H.; Jiang, Y.; Zhao, Z.; Zhu, C.; Xia, Y.; Zhang, J.; Xu, K.; Mu, D.; et al. Inducing Favorable Cation Antisite by Doping Halogen in Ni-Rich Layered Cathode with Ultrahigh Stability. Adv. Sci. 2019, 6, 1801406. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.H.; Park, G.T.; Conlin, P.; Ashburn, N.; Cho, K.; Yu, Y.S.; Shapiro, D.A.; Maglia, F.; Kim, S.J.; Lamp, P.; et al. Cation ordered Ni-rich layered cathode for ultra-long battery life. Energy Environ. Sci. 2021, 14, 1573–1583. [Google Scholar] [CrossRef]
- Sun, H.H.; Kim, U.H.; Park, J.H.; Park, S.W.; Seo, D.H.; Heller, A.; Mullins, C.B.; Yoon, C.S.; Sun, Y.K. Transition metal-doped Ni-rich layered cathode materials for durable Li-ion batteries. Nat. Commun. 2021, 12, 6552. [Google Scholar] [CrossRef] [PubMed]
- Park, N.Y.; Kim, S.B.; Kim, M.C.; Han, S.M.; Kim, D.H.; Kim, M.S.; Sun, Y.K. Mechanism of Doping with High-Valence Elements for Developing Ni-Rich Cathode Materials. Adv. Energy Mater. 2023, 13, 2301530. [Google Scholar] [CrossRef]
- Hüger, E.; Riedel, L.; Zhu, J.; Stahn, J.; Heitjans, P.; Schmidt, H. Lithium Niobate for Fast Cycling in Li-ion Batteries: Review and New Experimental Results. Batteries 2023, 9, 244. [Google Scholar] [CrossRef]
- Kim, U.H.; Lee, S.B.; Ryu, J.H.; Yoon, C.S.; Sun, Y.K. Optimization of Ni-rich Li[Ni0.92−xCo0.04Mn0.04Alx]O2 cathodes for high energy density lithium-ion batteries. J. Power Sources 2023, 564, 232850. [Google Scholar] [CrossRef]
- Do, S.J.; Santhoshkumar, P.; Kang, S.H.; Prasanna, K.; Jo, Y.N.; Lee, C.W. Al-doped Li[Ni0.78Co0.1Mn0.1Al0.02]O2 for high performance of lithium ion batteries. Ceram. Int. 2019, 45, 6972–6977. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Wu, L.; Feng, L.; Jin, S.; Zhang, R.; Jin, M. Effect of Ti ion doping on electrochemical performance of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode material. Electrochim. Acta 2019, 328, 135086. [Google Scholar] [CrossRef]
- Wu, L.; Tang, X.; Chen, X.; Rong, Z.; Dang, W.; Wang, Y.; Li, X.; Huang, L.; Zhang, Y. Improvement of electrochemical reversibility of the Ni-Rich cathode material by gallium doping. J. Power Sources 2020, 445, 227337. [Google Scholar] [CrossRef]
- Lu, Y.; Jin, H.; Mo, Y.; Qu, Y.; Du, B.; Chen, Y. Synthesis and characterization of Cu-doped LiNi0.6Co0.2Mn0.2O2 materials for Li-ion batteries. J. Alloys Compd. 2020, 844, 156180. [Google Scholar] [CrossRef]
- Wu, T.; Wang, G.; Liu, B.; Huang, Q.; Su, Y.; Wu, F.; Kelly, R.M. The role of Cu impurity on the structure and electrochemical performance of Ni-rich cathode material for lithium-ion batteries. J. Power Sources 2021, 494, 229774. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, E.; Chen, D.; Wu, M.; Han, S.; Huang, Q.; Yang, L.; Xiao, X.; Hu, Z. Decreasing Li/Ni disorder and improving the electrochemical performances of Ni-rich LiNi0.8Co0.1Mn0.1O2 by Ca doping. Inorg. Chem. 2017, 56, 8355–8362. [Google Scholar] [CrossRef]
- Jung, C.H.; Li, Q.; Kim, D.H.; Eum, D.; Ko, D.; Choi, J.; Lee, J.; Kim, K.H.; Kang, K.; Yang, W.; et al. Revisiting the role of Zr doping in Ni-rich layered cathodes for lithium-ion batteries. J. Mater. Chem. A 2021, 9, 17415–17424. [Google Scholar] [CrossRef]
- Gomez-Martin, A.; Reissig, F.; Frankenstein, L.; Heidbüchel, M.; Winter, M.; Placke, T.; Schmuch, R. Magnesium substitution in Ni-rich NMC layered cathodes for high-energy lithium-ion batteries. Adv. Energy Mater. 2022, 12, 2103045. [Google Scholar] [CrossRef]
- Chu, B.; Liu, S.; You, L.; Liu, D.; Huang, T.; Li, Y.; Yu, A. Enhancing the cycling stability of Ni-rich LiNi0.6Co0.2Mn0.2O2 cathode at a high cutoff voltage with Ta doping. ACS Sustain. Chem. Eng. 2020, 8, 3082–3090. [Google Scholar] [CrossRef]
- Sattar, T.; Lee, S.H.; Jin, B.S.; Kim, H.S. Influence of Mo addition on the structural and electrochemical performance of Ni-rich cathode material for lithium-ion batteries. Sci. Rep. 2020, 10, 8562. [Google Scholar] [CrossRef]
- He, T.; Chen, L.; Su, Y.; Lu, Y.; Bao, L.; Chen, G.; Zhang, Q.; Chen, S.; Wu, F. The effects of alkali metal ions with different ionic radii substituting in Li sites on the electrochemical properties of Ni-Rich cathode materials. J. Power Sources 2019, 441, 227195. [Google Scholar] [CrossRef]
- Yue, P.; Wang, Z.; Wang, J.; Guo, H.; Xiong, X.; Li, X. Effect of fluorine on the electrochemical performance of spherical LiNi0.8Co0.1Mn0.1O2 cathode materials via a low temperature method. Powder Technol. 2013, 237, 623–626. [Google Scholar] [CrossRef]
- Yao, W.; Liu, Y.; Li, D.; Zhang, Q.; Zhong, S.; Cheng, H.; Yan, Z. Synergistically enhanced electrochemical performance of Ni-rich cathode materials for lithium-ion batteries by K and Ti Co-modification. J. Phys. Chem. C 2020, 124, 2346–2356. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhang, Y.; Liu, Z.; Gao, Y.; Liu, J.; Zeng, Q. Stabilizing Ni-Rich LiNi0.92Co0.06Al0.02O2 Cathodes by Boracic Polyanion and Tungsten Cation Co-Doping for High-Energy Lithium-Ion Batteries. ChemElectroChem 2020, 7, 3811–3817. [Google Scholar] [CrossRef]
- Chen, T.; Li, X.; Wang, H.; Yan, X.; Wang, L.; Deng, B.; Ge, W.; Qu, M. The effect of gradient boracic polyanion-doping on structure, morphology, and cycling performance of Ni-rich LiNi0.8Co0.15Al0.05O2 cathode material. J. Power Sources 2018, 374, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, T.; Zhang, J.; Duan, J.; Li, X.; Zhou, Z.; Dong, P.; Wang, D. The role of boracic polyanion substitution on structure and high voltage electrochemical performance of Ni-Rich cathode materials for lithium ion batteries. J. Alloys Compd. 2019, 805, 1288–1296. [Google Scholar] [CrossRef]
- Wu, Y.; Manthiram, A. Impact of surface modifications on the layered solid solution cathodes in (1− z) Li[Li1/3Mn2/3]O2−(z) Li[Mn0.5−yNi0.5−yCo2y]O2. Solid. State Ion. 2009, 180, 50–56. [Google Scholar] [CrossRef]
- Ying, J.; Wan, C.; Jiang, C. Surface treatment of LiNi0.8Co0.2O2 cathode material for lithium secondary batteries. J. Power Sources 2001, 102, 162–166. [Google Scholar] [CrossRef]
- Liu, J.; Manthiram, A. Improved electrochemical performance of the 5 V spinel cathode LiMn1.5Ni0.42Zn0.08O4 by surface modification. J. Electrochem. Soc. 2008, 156, A66. [Google Scholar] [CrossRef]
- Kannan, A.; Manthiram, A. Surface/chemically modified LiMn2O4 cathodes for lithium-ion batteries. Electrochem. Solid-State Lett. 2002, 5, A167. [Google Scholar] [CrossRef]
- Sun, Y.K.; Hong, K.J.; Prakash, J.; Amine, K. Electrochemical performance of nano-sized ZnO-coated LiNi0.5Mn1.5O4 spinel as 5 V materials at elevated temperatures. Electrochem. Commun. 2002, 4, 344–348. [Google Scholar] [CrossRef]
- Karayaylali, P.; Tatara, R.; Zhang, Y.; Chan, K.L.; Yu, Y.; Giordano, L.; Maglia, F.; Jung, R.; Lund, I.; Shao-Horn, Y. Coating-dependent electrode-electrolyte interface for Ni-rich positive electrodes in Li-Ion batteries. J. Electrochem. Soc. 2019, 166, A1022. [Google Scholar] [CrossRef]
- Xu, Y.D.; Xiang, W.; Wu, Z.G.; Xu, C.L.; Li, Y.C.; Guo, X.D.; Lv, G.P.; Peng, X.; Zhong, B.H. Improving cycling performance and rate capability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode materials by Li4Ti5O12 coating. Electrochim. Acta 2018, 268, 358–365. [Google Scholar] [CrossRef]
- Ho, V.C.; Jeong, S.; Yim, T.; Mun, J. Crucial role of thioacetamide for ZrO2 coating on the fragile surface of Ni-rich layered cathode in lithium ion batteries. J. Power Sources 2020, 450, 227625. [Google Scholar] [CrossRef]
- Schipper, F.; Bouzaglo, H.; Dixit, M.; Erickson, E.M.; Weigel, T.; Talianker, M.; Grinblat, J.; Burstein, L.; Schmidt, M.; Lampert, J.; et al. From surface ZrO2 coating to bulk Zr doping by high temperature annealing of nickel-rich lithiated oxides and their enhanced electrochemical performance in lithium ion batteries. Adv. Energy Mater. 2018, 8, 1701682. [Google Scholar] [CrossRef]
- Sim, S.-J.; Lee, S.-H.; Jin, B.-S.; Kim, H.-S. Effects of lithium tungsten oxide coating on LiNi0.90Co0.05Mn0.05O2 cathode material for lithium-ion batteries. J. Power Sources 2021, 481, 229037. [Google Scholar] [CrossRef]
- Song, B.; Li, W.; Oh, S.-M.; Manthiram, A. Long-life nickel-rich layered oxide cathodes with a uniform Li2ZrO3 surface coating for lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 9718–9725. [Google Scholar] [CrossRef] [PubMed]
- Shim, T.Y.; Yoo, Y.W.; Hwang, D.Y.; Lee, S.H. Highly improved structural stability and electrochemical properties of Ni-rich NCM cathode materials. Ceram. Int. 2023, 49, 12138–12143. [Google Scholar] [CrossRef]
- Sattar, T.; Sim, S.J.; Jin, B.S.; Kim, H.S. Improving the cycle stability and rate performance of LiNi0.91Co0.06Mn0.03O2 Ni-rich cathode material by La2O3 coating for lithium-ion batteries. Curr. Appl. Phys. 2022, 36, 176–182. [Google Scholar] [CrossRef]
- Fan, Q.; Lin, K.; Yang, S.; Guan, S.; Chen, J.; Feng, S.; Liu, J.; Liu, L.; Li, J.; Shi, Z. Constructing effective TiO2 nano-coating for high-voltage Ni-rich cathode materials for lithium-ion batteries by precise kinetic control. J. Power Sources 2020, 477, 228745. [Google Scholar] [CrossRef]
- Cho, J.; Kim, Y.W.; Kim, B.; Lee, J.G.; Park, B. A breakthrough in the safety of lithium secondary batteries by coating the cathode material with AlPO4 nanoparticles. Angew. Chem. 2003, 115, 1656–1659. [Google Scholar] [CrossRef]
- Rastogi, P.K.; Kim, Y.W. Effects of an inorganic coating on the structure and magnetic properties of a 1 wt.% silicon steel. J. Appl. Phys. 1985, 57, 4223–4225. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Li, S.; Tong, Z.; Wang, D.; Zhuang, H.L.; Wang, X.; Lu, Y. Lithium-Aluminum-Phosphate coating enables stable 4.6 V cycling performance of LiCoO2 at room temperature and beyond. Energy Storage Mater. 2021, 37, 67–76. [Google Scholar] [CrossRef]
- Cho, S.W.; Kim, G.O.; Ryu, K.S. Sulfur anion doping and surface modification with LiNiPO4 of a Li[Co0.1Ni0.15Li0.2Mn0.55]O2 cathode material for Li-ion batteries. Solid. State Ion. 2012, 206, 84–90. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, L.; Zhao, F.; Liu, Y.; Hou, L.; Yuan, C. Ni-rich LiNi0.8Co0.1Mn0.1O2 coated with Li-ion conductive Li3PO4 as competitive cathodes for high-energy-density lithium-ion batteries. Electrochim. Acta 2020, 340, 135871. [Google Scholar] [CrossRef]
- Jo, C.H.; Cho, D.H.; Noh, H.J.; Yashiro, H.; Sun, Y.K.; Myung, S.T. An effective method to reduce residual lithium compounds on Ni-rich Li[Ni0.6Co0.2Mn0.2]O2 active material using a phosphoric acid-derived Li3PO4 nanolayer. Nano Res. 2015, 8, 1464–1479. [Google Scholar] [CrossRef]
- Park, K.; Park, J.H.; Choi, B.; Kim, J.H.; Hong, S.G.; Han, H.N. Metal phosphate-coated Ni-rich layered oxide positive electrode materials for Li-ion batteries: Improved electrochemical performance and decreased Li residuals content. Electrochim. Acta 2017, 257, 217–223. [Google Scholar] [CrossRef]
- Sattar, T.; Sim, S.J.; Jin, B.S.; Kim, H.S. Dual function Li-reactive coating from residual lithium on Ni-rich NCM cathode material for Lithium-ion batteries. Sci. Rep. 2021, 11, 18590. [Google Scholar] [CrossRef]
- Hua, W.; Zhang, J.; Wang, S.; Cheng, Y.; Li, H.; Tseng, J.; Wu, Z.; Shen, C.H.; Dolotko, O.; Liu, H.; et al. Long-Range Cationic Disordering Induces two Distinct Degradation Pathways in Co-Free Ni-Rich Layered Cathodes. Angew. Chem. Int. Ed. 2023, 62, e202214880. [Google Scholar] [CrossRef]
- Tang, Z.F.; Wu, R.; Huang, P.F.; Wang, Q.S.; Chen, C.H. Improving the electrochemical performance of Ni-rich cathode material LiNi0.815Co0.15Al0.035O2 by removing the lithium residues and forming Li3PO4 coating layer. J. Alloys Compd. 2017, 693, 1157–1163. [Google Scholar] [CrossRef]
- Xiong, X.; Ding, D.; Bu, Y.; Wang, Z.; Huang, B.; Guo, H.; Li, X. Enhanced electrochemical properties of a LiNiO2-based cathode material by removing lithium residues with (NH4)2HPO4. J. Mater. Chem. A 2014, 2, 11691–11696. [Google Scholar] [CrossRef]
- Zou, P.; Lin, Z.; Fan, M.; Wang, F.; Liu, Y.; Xiong, X. Facile and efficient fabrication of Li3PO4-coated Ni-rich cathode for high-performance lithium-ion battery. Appl. Surf. Sci. 2020, 504, 144506. [Google Scholar] [CrossRef]
- Huang, J.; Du, K.; Peng, Z.; Cao, Y.; Xue, Z.; Duan, J.; Wang, F.; Liu, Y.; Hu, G. Enhanced High-Temperature Electrochemical Performance of Layered Nickel-Rich Cathodes for Lithium-Ion Batteries after LiF Surface Modification. ChemElectroChem 2019, 6, 5428–5432. [Google Scholar] [CrossRef]
- Sun, X.G.; Jafta, C.J.; Tan, S.; Borisevich, A.; Gupta, R.B.; Paranthaman, M.P. Facile surface coatings for performance improvement of NMC811 battery cathode material. J. Electrochem. Soc. 2022, 169, 020565. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Kim, S.; Kim, Y. Fluorination of free lithium residues on the surface of lithium nickel cobalt aluminum oxide cathode materials for lithium-ion batteries. Mater. Des. 2016, 100, 175–179. [Google Scholar] [CrossRef]
- Yang, G.; Pan, K.; Yan, Z.; Yang, S.; Peng, F.; Liang, J.; Lai, F.; Wang, H.; Zhang, X.; Li, Q. Fully coating of Mg3B2O6 in nonaqueous solution on Ni-rich LiNi0.8Co0.1Mn0.1O2 secondary particles to improve cycling stability of lithium-ion batteries. Chem. Eng. J. 2023, 452, 139405. [Google Scholar] [CrossRef]
- Huang, Y.; Yao, X.; Hu, X.; Han, Q.; Wang, S.; Ding, L.X.; Wang, H. Surface coating with Li-Ti-O to improve the electrochemical performance of Ni-rich cathode material. Appl. Surf. Sci. 2019, 489, 913–921. [Google Scholar] [CrossRef]
- Kong, J.Z.; Chen, Y.; Cao, Y.Q.; Wang, Q.Z.; Li, A.D.; Li, H.; Zhou, F. Enhanced electrochemical performance of Ni-rich LiNi0.6Co0.2Mn0.2O2 coated by molecular layer deposition-derived dual-functional C-Al2O3 composite coating. J. Alloys Compd. 2019, 799, 89–98. [Google Scholar] [CrossRef]
- Qian, R.; Liu, Y.; Cheng, T.; Li, P.; Chen, R.; Lyu, Y.; Guo, B. Enhanced surface chemical and structural stability of Ni-rich cathode materials by synchronous lithium-ion conductor coating for lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 13813–13823. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Yang, P.; He, W.; Bie, S.; Zhao, H.; Yin, J.; Zou, Z.; Liu, J. Enhanced high-voltage cycling stability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode coated with Li2O–2B2O3. J. Alloys Compd. 2019, 805, 991–998. [Google Scholar] [CrossRef]
- Liu, S.; Su, J.; Zhang, C.; Chen, X.; Zhao, J.; Huang, T.; Wu, J.; Yu, A. Understanding the effects of surface modification on improving the high-voltage performance of Ni-rich cathode materials. Mater. Today Energy 2018, 10, 40–47. [Google Scholar] [CrossRef]
- Wang, F.; Luo, Y.; Liu, P.; Balogun, M.-S.; Deng, J.; Wang, Z. Improved cycling performance and high rate capacity of LiNi0.8Co0.1Mn0.1O2 cathode achieved by Al(PO3)3 modification via dry coating ball milling. Coatings 2022, 12, 319. [Google Scholar] [CrossRef]
- Zhong, Z.; Chen, L.; Zhu, C.; Ren, W.; Kong, L.; Wan, Y. Nano LiFePO4 coated Ni-rich composite as cathode for lithium-ion batteries with high thermal stability and excellent cycling performance. J. Power Sources 2020, 464, 228235. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Xue, L.; Chen, Y.; Lei, T.; Deng, S.; Cao, G. Enhanced electrochemical performance of Li3PO4 modified Li[Ni0.8Co0.1Mn0.1]O2 cathode material via lithium-reactive coating. J. Alloys Compd. 2019, 773, 112–120. [Google Scholar] [CrossRef]
- Song, H.J.; Oh, S.H.; Lee, Y.; Kim, J.; Yim, T. Dually modified cathode-electrolyte interphases layers by calcium phosphate on the surface of nickel-rich layered oxide cathode for lithium-ion batteries. J. Power Sources 2021, 483, 229218. [Google Scholar] [CrossRef]
- Wang, D.; Yan, Q.; Li, M.; Gao, H.; Tian, J.; Shan, Z.; Wang, N.; Luo, J.; Zhou, M.; Chen, Z. Boosting the cycling stability of Ni-rich layered oxide cathode by dry coating of ultrastable Li3V2(PO4)3 nanoparticles. Nanoscale 2021, 13, 2811–2819. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, Z.; Yin, X.; Guo, H.; Li, X. A modified LiF coating process to enhance the electrochemical performance characteristics of LiNi0.8Co0.1Mn0.1O2 cathode materials. Mater. Lett. 2013, 110, 4–9. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoon, C.S.; Amine, K.; Sun, Y.K. Improvement of long-term cycling performance of Li[Ni0.8Co0.15Al0.05]O2 by AlF3 coating. J. Power Sources 2013, 234, 201–207. [Google Scholar] [CrossRef]
- Dai, S.; Yan, G.; Wang, L.; Luo, L.; Li, Y.; Yang, Y.; Liu, H.; Liu, Y.; Yuan, M. Enhanced electrochemical performance and thermal properties of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode material via CaF2 coating. J. Electroanal. Chem. 2019, 847, 113197. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Lu, X.; Jiang, H.; Zhang, Q.; Wang, B.; Lai, C. Enhancing high-potential stability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode with PrF3 coating. Ceram. Int. 2021, 47, 6341–6351. [Google Scholar] [CrossRef]
- Lim, B.B.; Yoon, S.J.; Park, K.J.; Yoon, C.S.; Kim, S.J.; Lee, J.J.; Sun, Y.K. Advanced concentration gradient cathode material with two-slope for high-energy and safe lithium batteries. Adv. Funct. Mater. 2015, 25, 4673–4680. [Google Scholar] [CrossRef]
- Hou, P.; Zhang, L.; Gao, X. A high-energy, full concentration-gradient cathode material with excellent cycle and thermal stability for lithium ion batteries. J. Mater. Chem. A 2014, 2, 17130–17138. [Google Scholar] [CrossRef]
- Mohanty, D.; Dahlberg, K.; King, D.M.; David, L.A.; Sefat, A.S.; Wood, D.L.; Daniel, C.; Dhar, S.; Mahajan, V.; Lee, M.; et al. Modification of Ni-rich FCG NMC and NCA cathodes by atomic layer deposition: Preventing surface phase transitions for high-voltage lithium-ion batteries. Sci. Rep. 2016, 6, 26532. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.H.; Myung, S.T.; Yoon, C.S.; Sun, Y.K. Extending the battery life using an Al-doped Li[Ni0.76Co0.09Mn0.15]O2 cathode with concentration gradients for lithium-ion batteries. ACS Energy Lett. 2017, 2, 1848–1854. [Google Scholar] [CrossRef]
- Liao, J.Y.; Manthiram, A. Surface-modified concentration-gradient Ni-rich layered oxide cathodes for high-energy lithium-ion batteries. J. Power Sources 2015, 282, 429–436. [Google Scholar] [CrossRef]
- Park, K.J.; Choi, M.J.; Maglia, F.; Kim, S.J.; Kim, K.H.; Yoon, C.S.; Sun, Y.K. High-Capacity Concentration Gradient Li[Ni0.865Co0.120Al0.015]O2 Cathode for Lithium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703612. [Google Scholar] [CrossRef]
- Xu, X.; Xiang, L.; Wang, L.; Jian, J.; Du, C.; He, X.; Huo, H.; Cheng, X.; Yin, G. Progressive concentration gradient nickel-rich oxide cathode material for high-energy and long-life lithium-ion batteries. J. Mater. Chem. A 2019, 7, 7728–7735. [Google Scholar] [CrossRef]

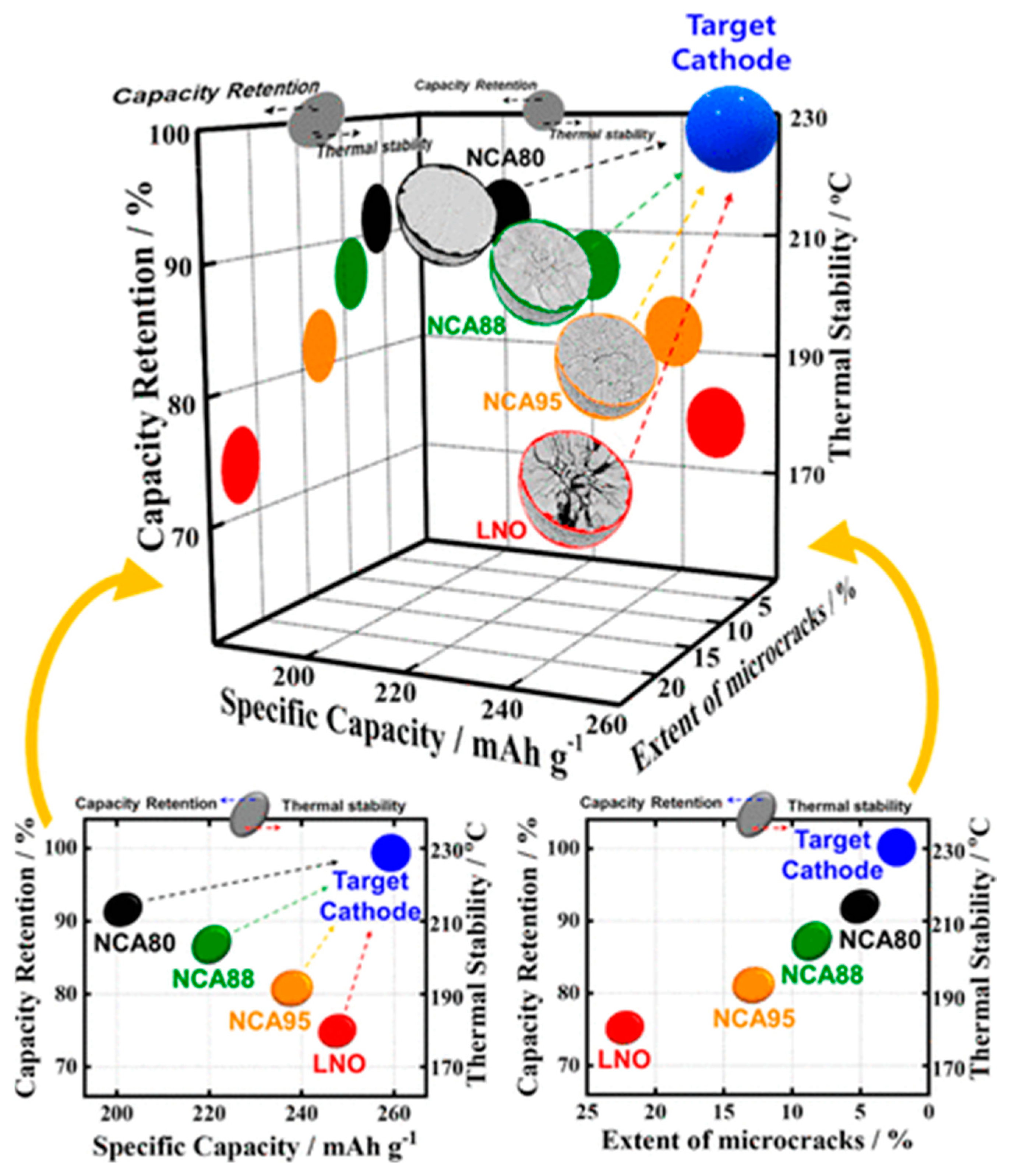
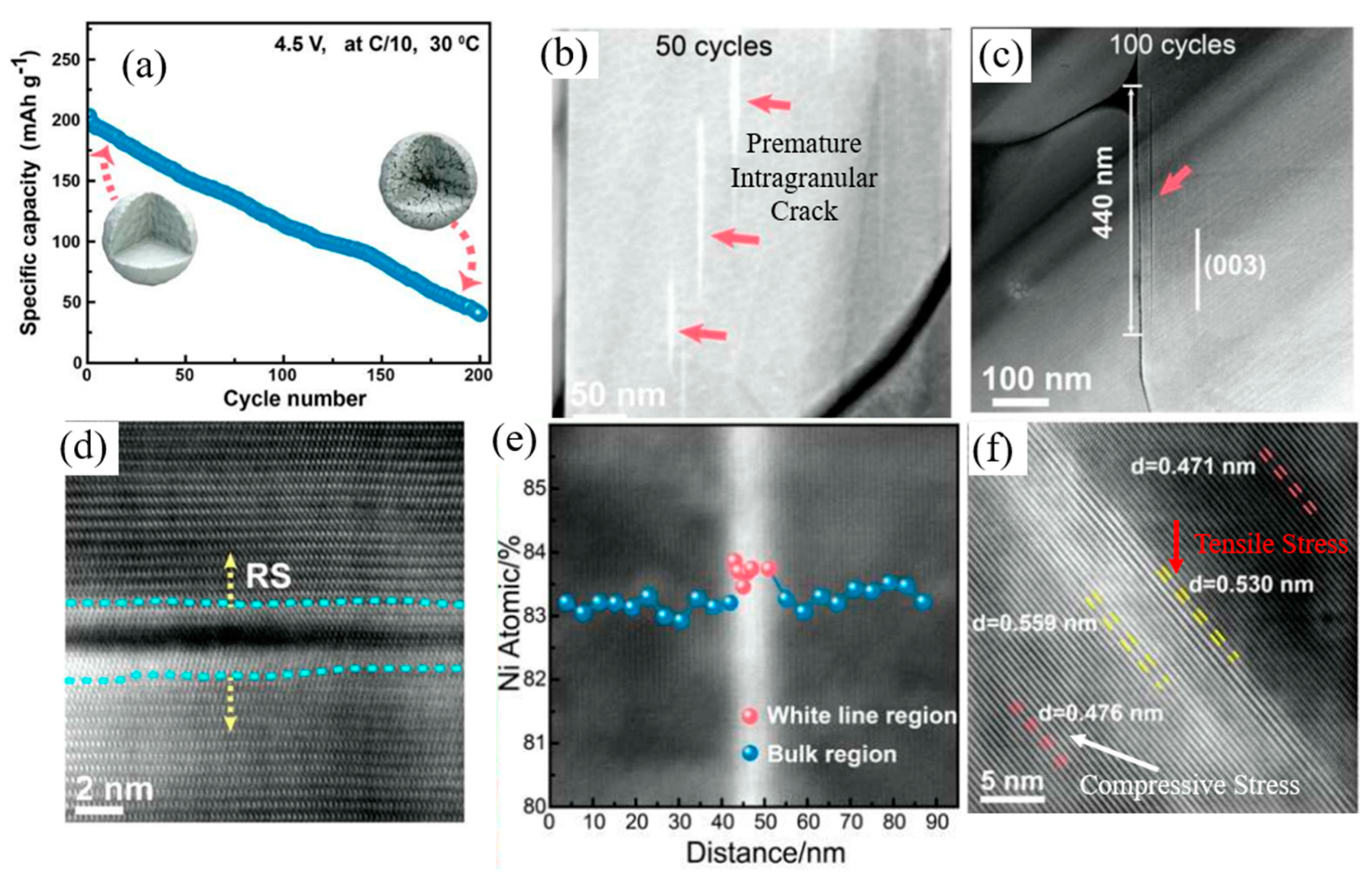


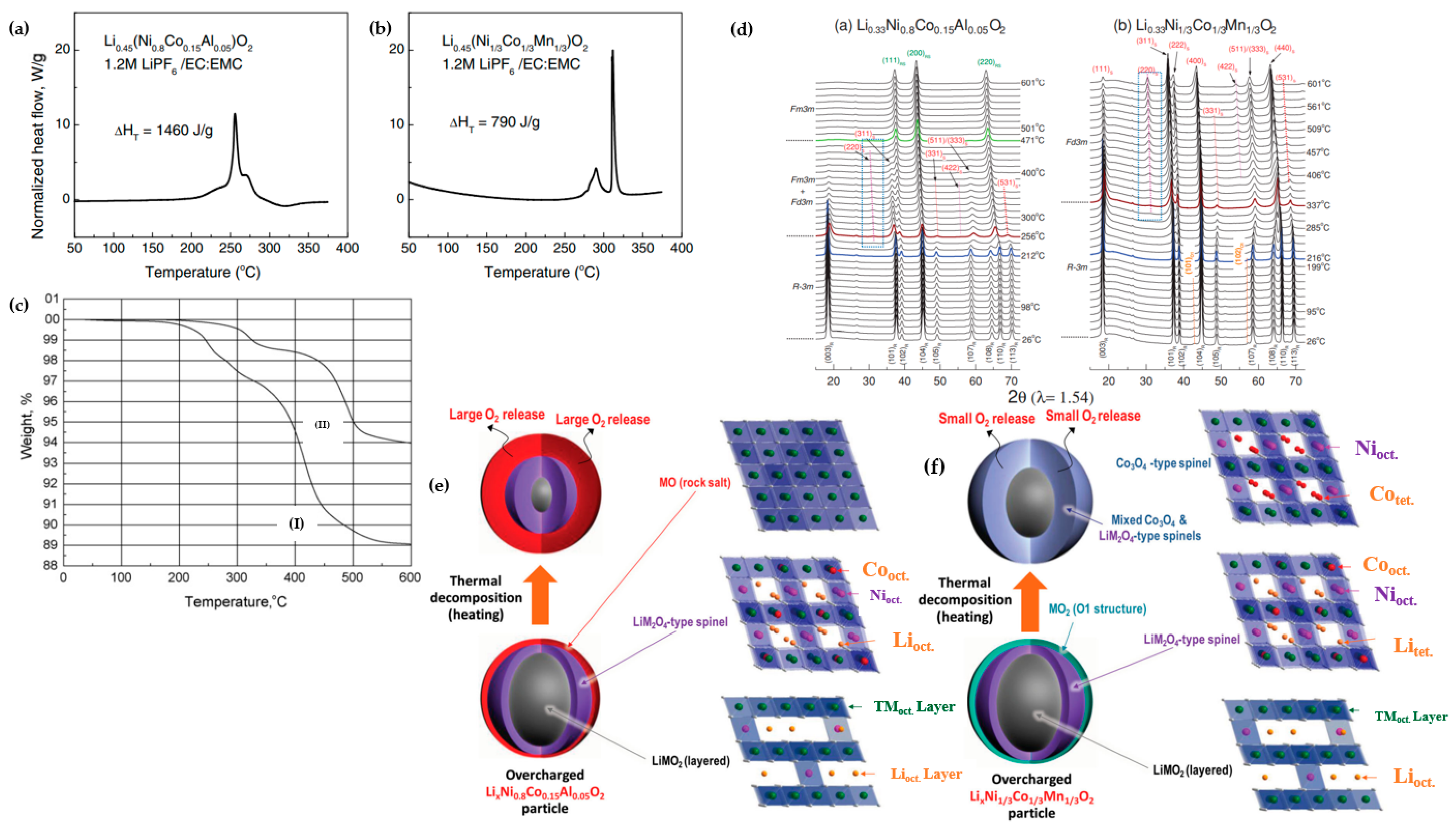
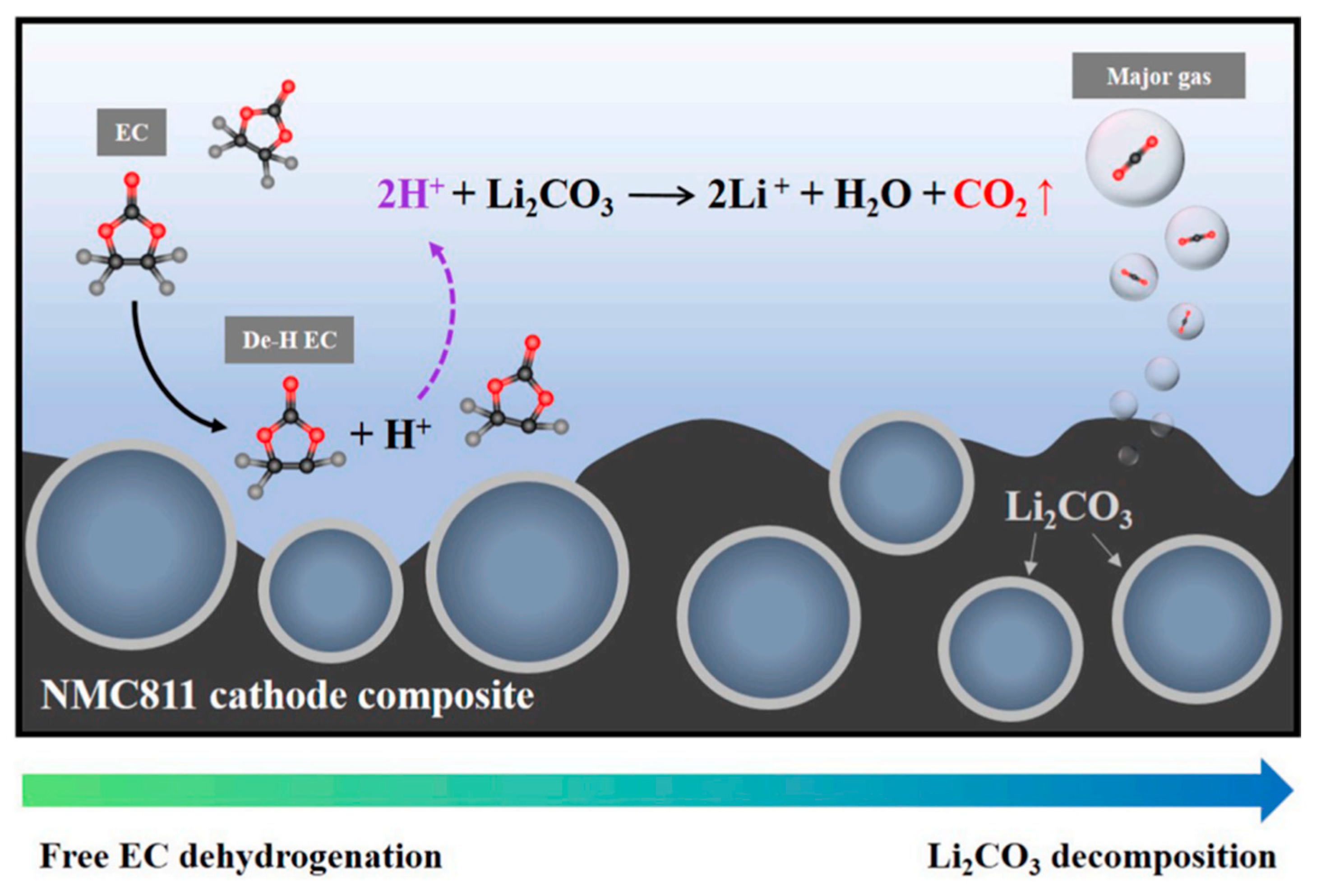
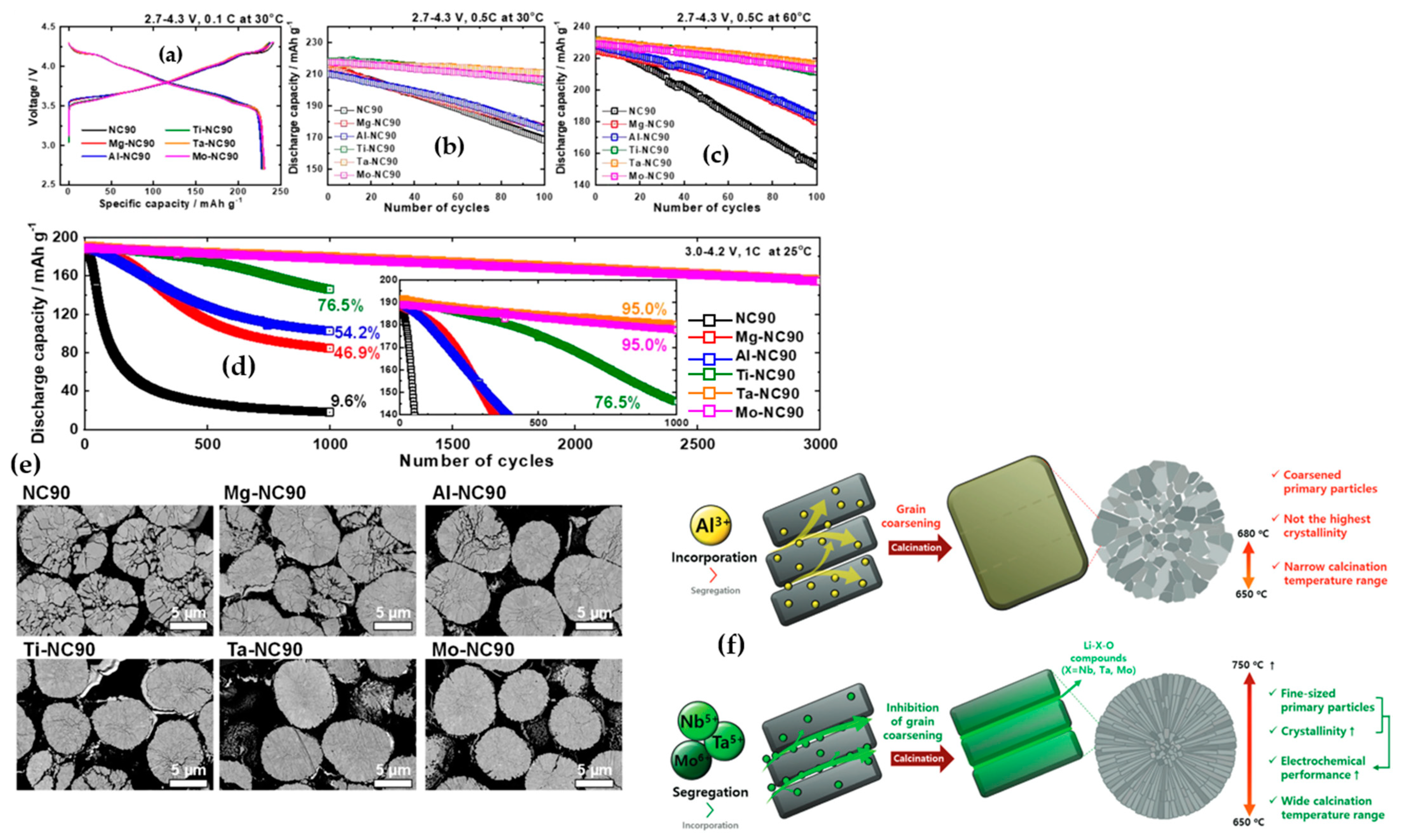
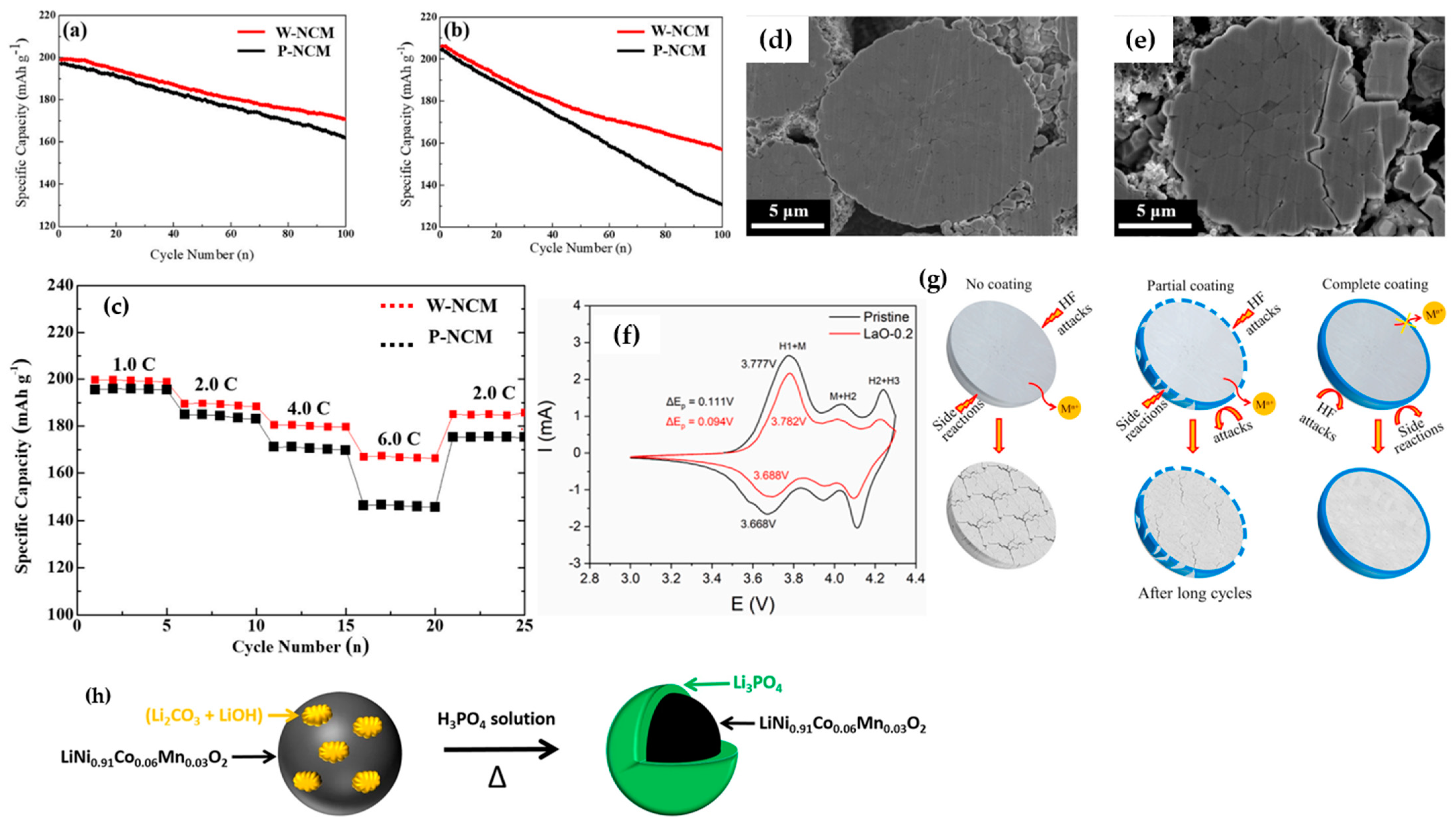
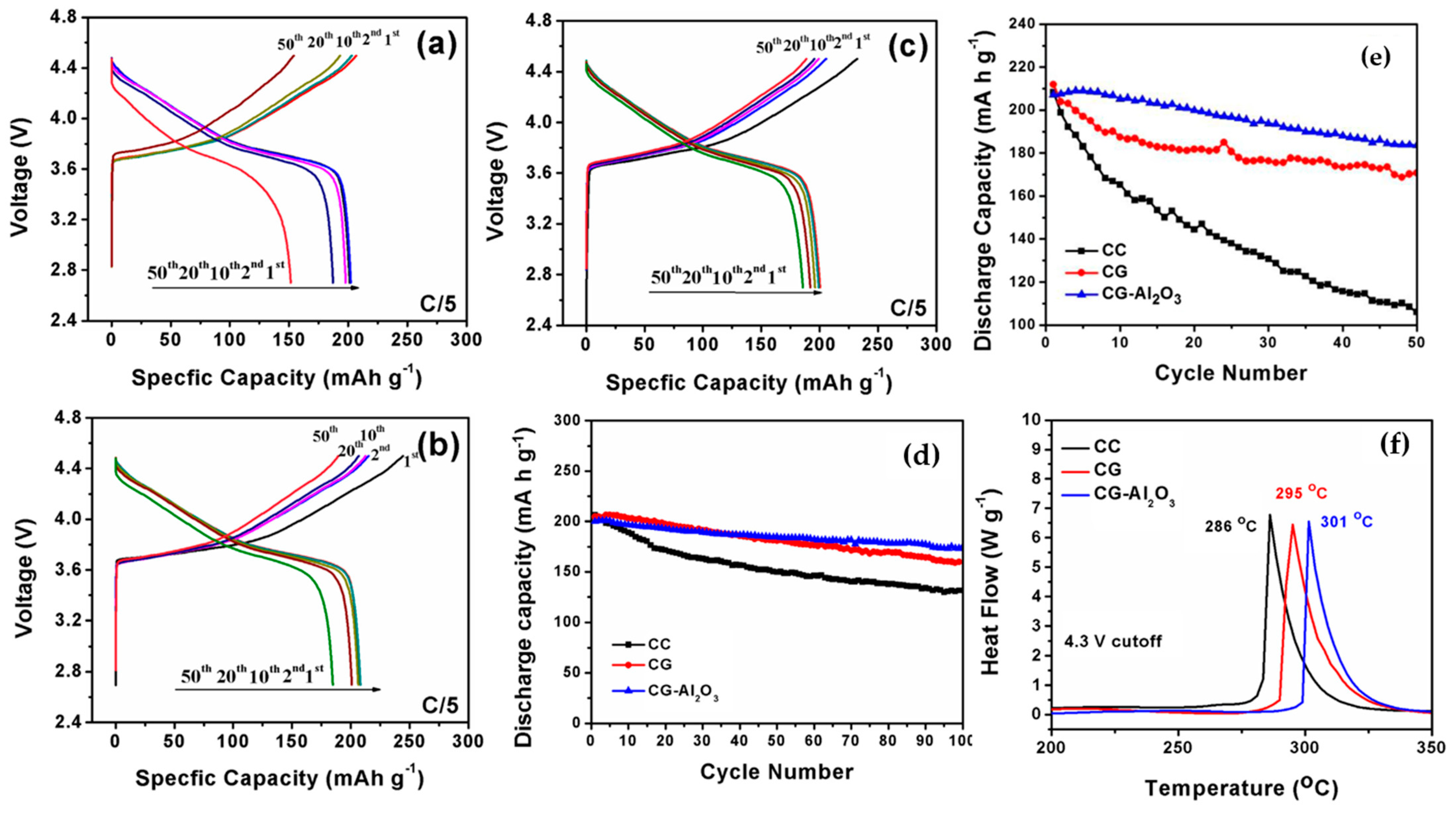
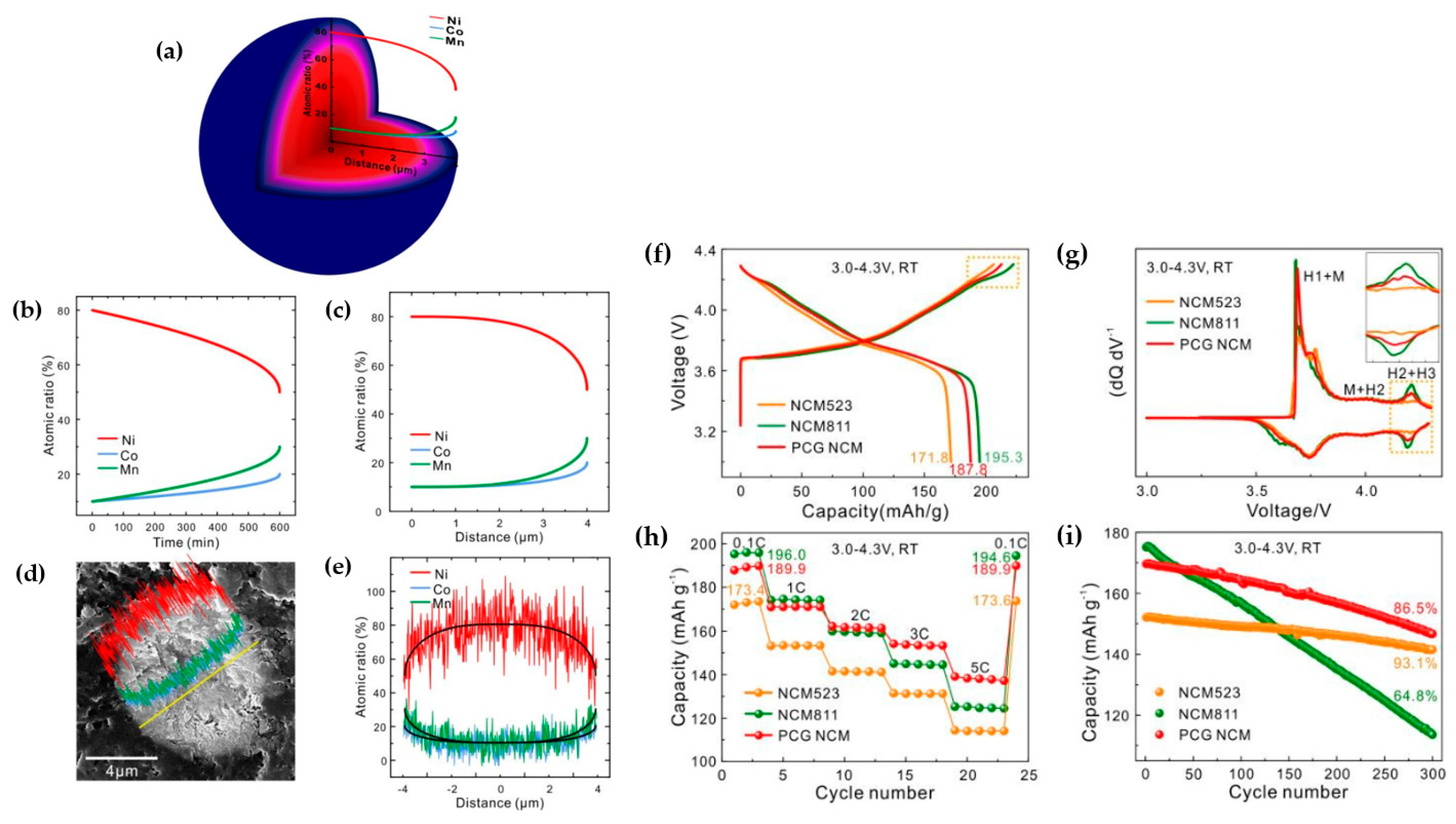
| Battery Attributes | Main Requirements | Main Challenges |
|---|---|---|
| Energy density | >750 Wh L−1 and >350 Wh kg−1 for cells | Difficult to find one battery technology that meets all aspects; Tradeoffs must be managed effectively. |
| Cost | <$100 per kWh for cells | |
| Fast charge and power | 80% ∆SOC * in 15 min | |
| Life | 15 years | |
| Performance | Minimum impact by environment | |
| Safety | No fire/flame/rupture/explosion | |
| Temperature range | −30 to 52 °C [11] |
| Layered Oxide Cathode Material Composition | I(003)/I(104) |
|---|---|
| Li[Ni1/3Co1/3Mn1/3]O2 | 1.35 |
| Li[Ni0.5Co0.2Mn0.3]O2 | 1.32 |
| Li[Ni0.6Co0.2Mn0.2]O2 | 1.26 |
| Li[Ni0.7Co0.15Mn0.15]O2 | 1.20 |
| Li[Ni0.8Co0.1Mn0.1]O2 | 1.19 |
| Li[Ni0.85Co0.075Mn0.075]O2 | 1.18 |
| Researchers | Doping Element | Composition | Testing Conditions | Pristine Material [mAh g−1] | Doped Material [mAh g−1] |
|---|---|---|---|---|---|
| Kim et al. [137] | Al | Ni0.92Co0.04Mn0.04 | 2.7–4.3 V RT * 0.5C | 215 (initial) 174 (100 cycles) | 202 (initial) 187 (100 cycles) |
| Do et al. [138] | Al | Ni0.8Co0.1Mn0.1 | 3.0–4.3 V RT 1C | 172.4 (initial) 120 (100 cycles) | 171.71 (initial) 163 (100 cycles) |
| Chu et al. [119] | Nb | Ni0.8Co0.1Mn0.1 | 2.7–4.5 V RT 2C | 163.5 (initial) 97.3 (200 cycles) | 202.8 (initial) 164.1 (200 cycles) |
| Zhang et al. [139] | Ti | Ni0.8Co0.1Mn0.1 | 2.8–4.3 V RT 1C | 147.41 (initial) 96.23 (150 cycles) | 165.6 (initial) 127.53 (150 cycles) |
| Wu et al. [140] | Ga | Ni0.8Co0.1Mn0.1 | 2.8–4.5 V RT 1C | 205 (initial) 130 (100 cycles) | 183 (initial) 174 (100 cycles) |
| Lu et al. [141] | Cu | Ni0.6Co0.2Mn0.2 | 2.7–4.4 V RT 5C | 130.5 (initial) 80.91 (350 cycles) | 140.8 (initial) 108.7 (350 cycles) |
| Wu et al. [142] | Cu | Ni0.8Co0.1Mn0.1 | 2.8–4.3 V RT 1C | 145.8 (initial) 127 (100 cycles) | 187.9 (initial) 169.4 (100 cycles) |
| Chen et al. [143] | Ca | Ni0.8Co0.1Mn0.1 | 3.0–4.3 V RT 0.2C | 130 (initial) 86 (50 cycles) | 150 (initial) 122 (50 cycles) |
| Jung et al. [144] | Zr | Ni0.92Co0.04Mn0.04 | 2.5–4.4 V RT 0.3C | 230.1 (initial at 0.1C) 140 (100 cycles) | 225.2 (initial) 178 (100 cycles) |
| Gomez-Martin et al. [145] | Mg | Ni0.8Co0.1Mn0.1 | 2.8–4.2 V RT 0.33C | 195 (initial) 157 (200 cycles) | 175 (initial) 140 (600 cycles) |
| Chu et al. [146] | Ta | Ni0.6Co0.2Mn0.2 | 3.0–4.5 V RT 1C | 180 (initial) 144 (100 cycles) | 177 (initial) 152 (100 cycles) |
| Sattar et al. [147] | Mo | Ni0.84Co0.11Mn0.05 | 3.0–4.5 V RT 0.5C | 209 (initial) 87 (80 cycles) | 222 (initial) 172.72 (100 cycles) |
| He et al. [148] | Na | Ni0.8Co0.1Mn0.1 | 2.8–4.3 V RT 1C | 173.9 (initial) 162.07 (200 cycles) | 173.6 (initial) 168.04 (200 cycles) |
| Yue et al. [149] | F | Ni0.8Co0.1Mn0.1 | 2.8–4.3 V RT 2C | 178 (initial) 140 (100 cycles) | 164 (initial) 158 (100 cycles) |
| Yao et al. [150] | K and Ti | Ni0.8Co0.1Mn0.1 | 2.75–4.2 V RT 0.2C | 195.91 (initial) 166.14 (100 cycles) | 193 (initial) 184.72 (100 cycles) |
| Qiu et al. [151] | W and BO3−3 | Ni0.92Co0.06Al0.02 | 2.8–4.3 V RT 1C | 202.3 (initial) 107.2 (100 cycles) | 199.3 (initial) 170.6 (100 cycles) |
| Chen et al. [152] | BO3−3 and BO5−4 | Ni0.8Co0.15Al0.05 | 2.8–4.3 V RT 2C | 168.8 (initial) 125.8 (200 cycles) | 160.4 (initial) 155.1 (200 cycles) |
| Zhang et al. [153] | BO3−3 and BO5−4 | Ni0.6Co0.2Mn0.2 | 2.8–4.5 V RT 1C | 178.2 (initial) 105.4 (100 cycles) | 182.2 (initial) 138.6 (100 cycles) |
| Researchers | Coating | Composition | Testing Conditions | Pristine Material [mAh g−1] | Coated Material [mAh g−1] |
|---|---|---|---|---|---|
| Yang et al. [183] | Mg3B2O6 | Ni0.8Co0.1Mn0.1 | 2.7–4.5 V RT * 1C | 204.3 (initial) 108.8 (400 cycles) | 200 (initial) 160.6 (400 cycles) |
| Huang et al. [184] | LiTiO2 | Ni0.8Co0.1Mn0.1 | 2.8–4.4 V RT 0.5C | 182.6 (initial) 144.26 (100 cycles) | 184.5 (initial) 163.47 (100 cycles) |
| Kong et al. [185] | C-Al2O3 | Ni0.6Co0.2Mn0.2 | 3.0–4.5 V RT 1C | 163 (initial) 133 (100 cycles) | 199.58 (initial) 186.6 (100 cycles) |
| Qian et al. [186] | Li2SiO3 | Ni0.9Co0.05Mn0.05 | 2.7–4.3 V RT 2C | 169.27 (initial) 134.4 (100 cycles) | 177.9 (initial) 156.9 (100 cycles) |
| Du et al. [187] | Li2O-2B2O3 | Ni0.8Co0.1Mn0.1 | 2.75–4.5 V RT 1C | 189.1 (initial) 96.0 (100 cycles) | 192.0 (initial) 157.7 (100 cycles) |
| Liu et al. [188] | La2Zr2O3 | Ni0.6Co0.2Mn0.2 | 3.0–4.5 V RT 1C | 177.17 (initial) 131.1 (200 cycles) | 176.63 (initial) 146.6 (200 cycles) |
| Wang et al. [189] | AlPO4 | Ni0.8Co0.1Mn0.1 | 2.8–4.5 V RT 1C | 178.3 (initial) 122.5 (200 cycles) | 178.1 (initial) 167.2 (200 cycles) |
| Zhong et al. [190] | LiFePO4 | Ni0.82Co0.12Mn0.06 | 3.0–4.2 V RT 1C | 198.25 (initial) 132 (100 cycles) | 193.98 (initial) 171 (100 cycles) |
| Zhu et al. [191] | Li3PO4 | Ni0.8Co0.1Mn0.1 | 3.0–4.4 V RT 1C | 194.89 (initial) 167.8 (100 cycles) | 193.85 (initial) 179.5 (100 cycles) |
| Song et al. [192] | Ca3(PO4)2 | Ni0.8Co0.1Mn0.1 | 3.0–4.3 V 45 °C 1C | 210.28 (initial) 83.9 (150 cycles) | 213.8 (initial) 155 (150 cycles) |
| Wang et al. [193] | Li3V2(PO4)3 | Ni0.6Co0.2Mn0.2 | 3.0–4.3 V RT 2C | 153.7 (initial) 103.9 (200 cycles) | 149.6 (initial) 127.1 (200 cycles) |
| Xiong et al. [194] | LiF | Ni0.8Co0.1Mn0.1 | 2.8–4.3 V RT 2C | 171 (initial) 122.78 (200 cycles) | 169 (initial) 138.92 (200 cycles) |
| Lee et al. [195] | AlF3 | Ni0.8Co0.15Al0.05 | 2.7–4.3 V 55 °C 0.5C | 197 (initial) 155.827 (200 cycles) | 200 (initial) 174.8 (200 cycles) |
| Dai et al. [196] | CaF2 | Ni0.8Co0.1Mn0.1 | 2.7–4.3 V RT 1C | 150.4 (initial) 119.0 (200 cycles) | 148.2 (initial) 126.5 (200 cycles) |
| Li et al. [197] | PrF3 | Ni0.8Co0.1Mn0.1 | 2.8–4.3 V RT 1C | 197.4 (initial) 138.7 (100 cycles) | 187.2 (initial) 161.5 (100 cycles) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahangari, M.; Szalai, B.; Lujan, J.; Zhou, M.; Luo, H. Advancements and Challenges in High-Capacity Ni-Rich Cathode Materials for Lithium-Ion Batteries. Materials 2024, 17, 801. https://doi.org/10.3390/ma17040801
Ahangari M, Szalai B, Lujan J, Zhou M, Luo H. Advancements and Challenges in High-Capacity Ni-Rich Cathode Materials for Lithium-Ion Batteries. Materials. 2024; 17(4):801. https://doi.org/10.3390/ma17040801
Chicago/Turabian StyleAhangari, Mehdi, Benedek Szalai, Josue Lujan, Meng Zhou, and Hongmei Luo. 2024. "Advancements and Challenges in High-Capacity Ni-Rich Cathode Materials for Lithium-Ion Batteries" Materials 17, no. 4: 801. https://doi.org/10.3390/ma17040801
APA StyleAhangari, M., Szalai, B., Lujan, J., Zhou, M., & Luo, H. (2024). Advancements and Challenges in High-Capacity Ni-Rich Cathode Materials for Lithium-Ion Batteries. Materials, 17(4), 801. https://doi.org/10.3390/ma17040801






