Mechanical and Computational Fluid Dynamic Models for Magnesium-Based Implants
Abstract
1. Introduction
2. Modeling and Simulation
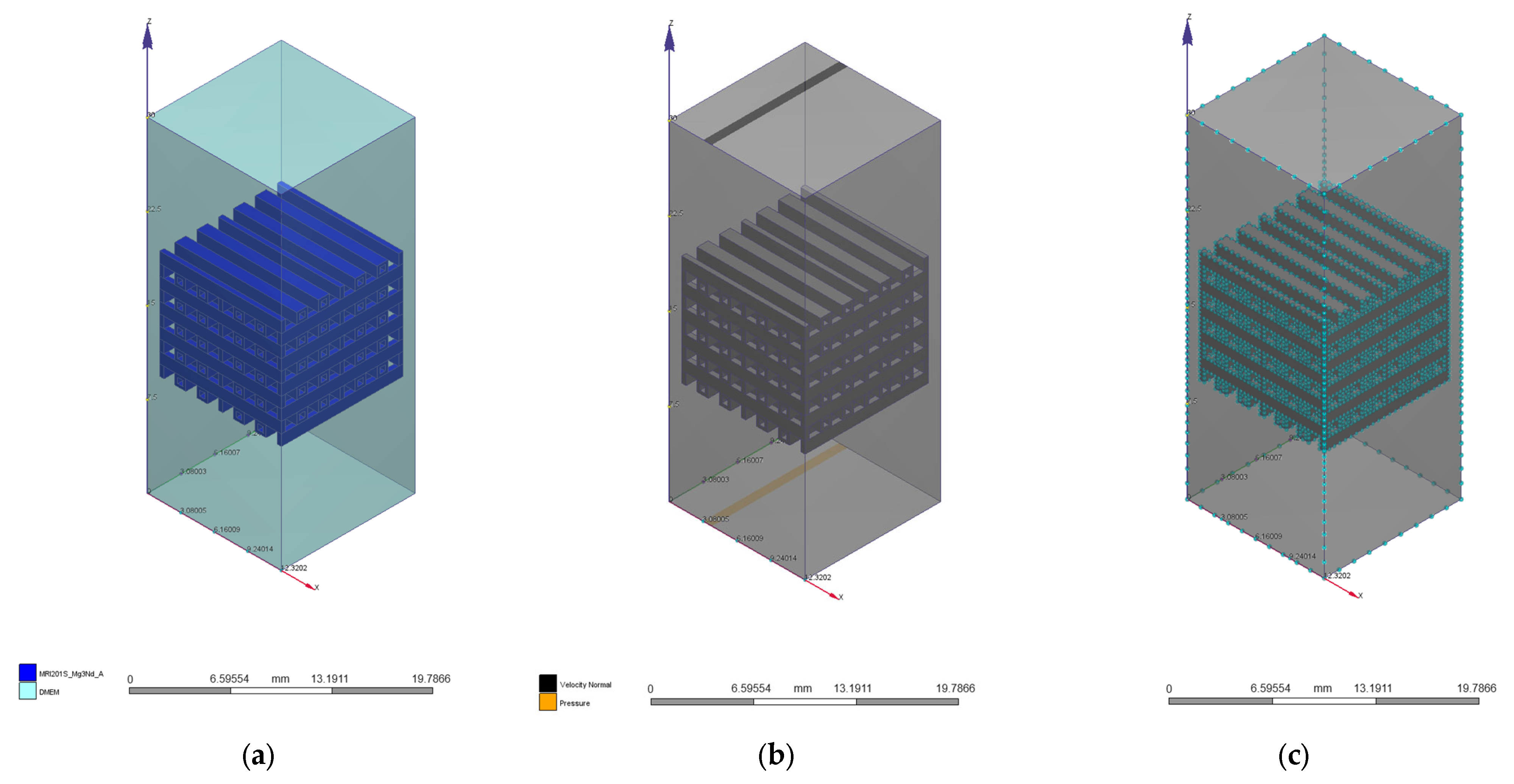
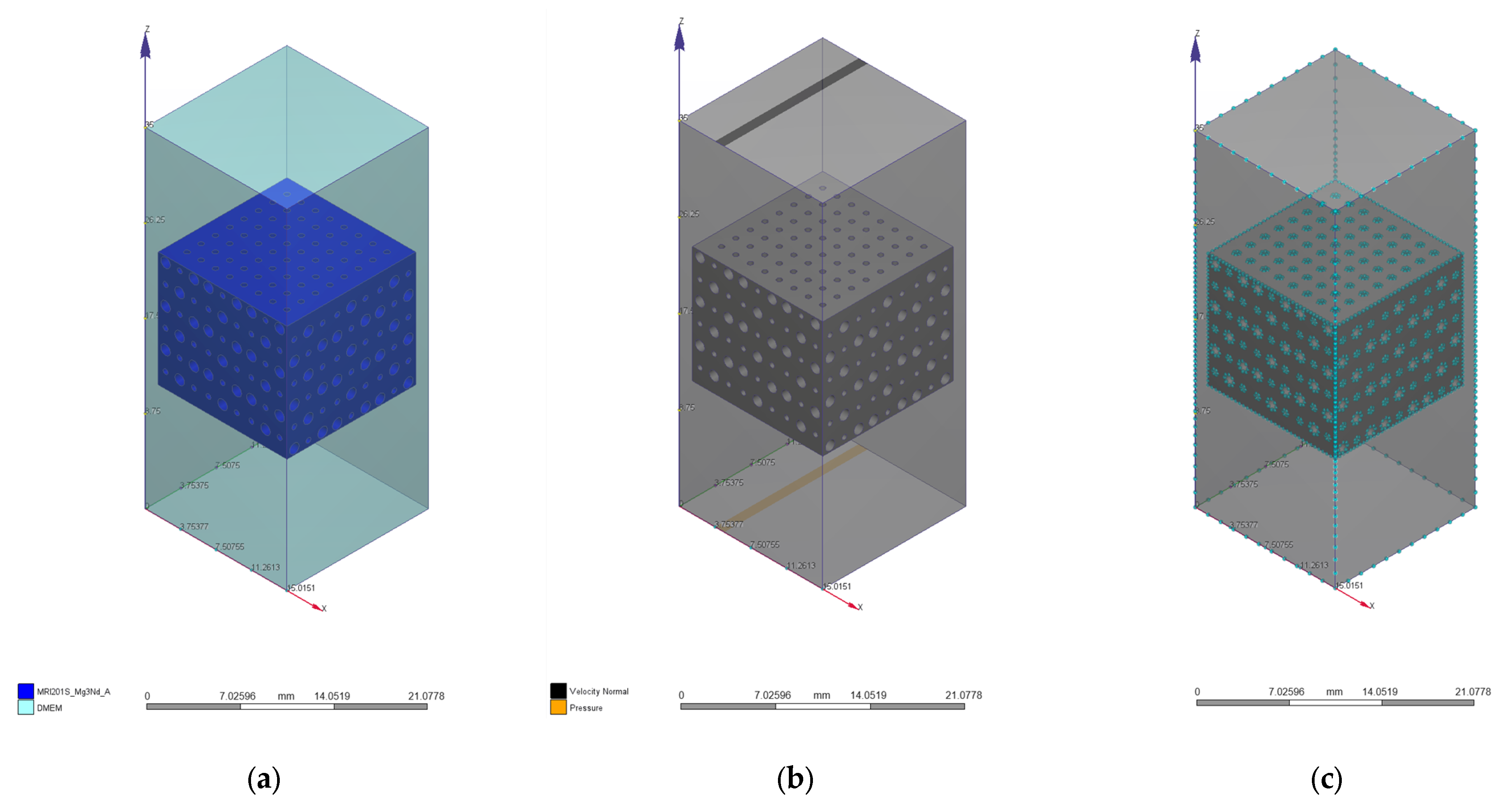
2.1. Scaffold Design
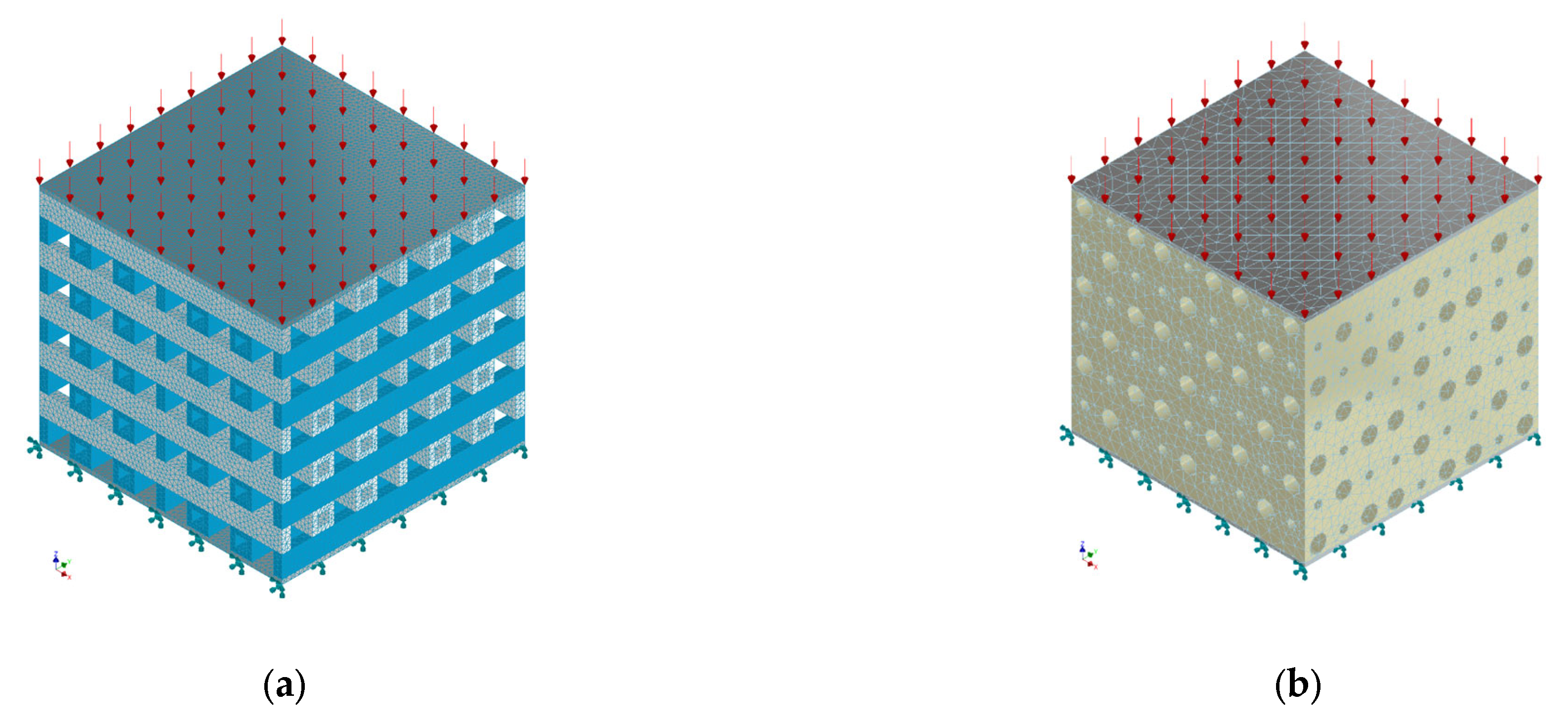
2.2. Mechanical Simulation
2.3. Computational Fluid Dynamics Simulation
3. Results and Discussion
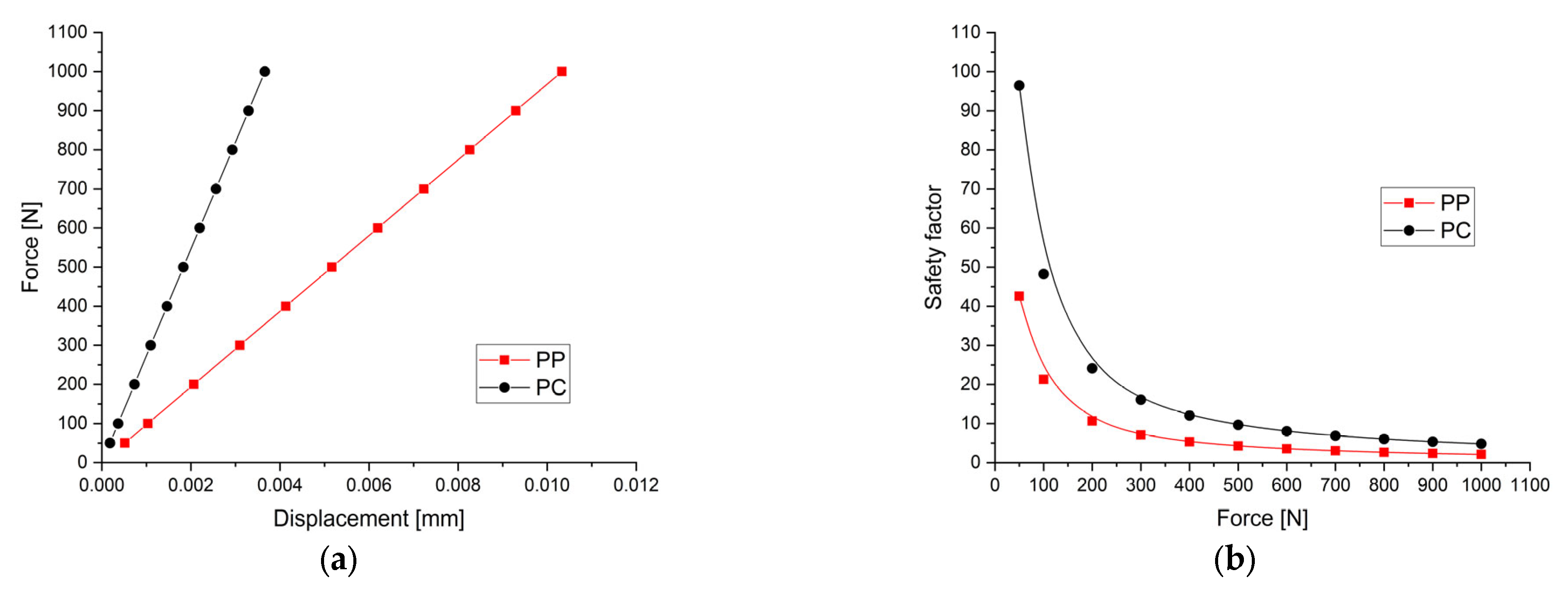
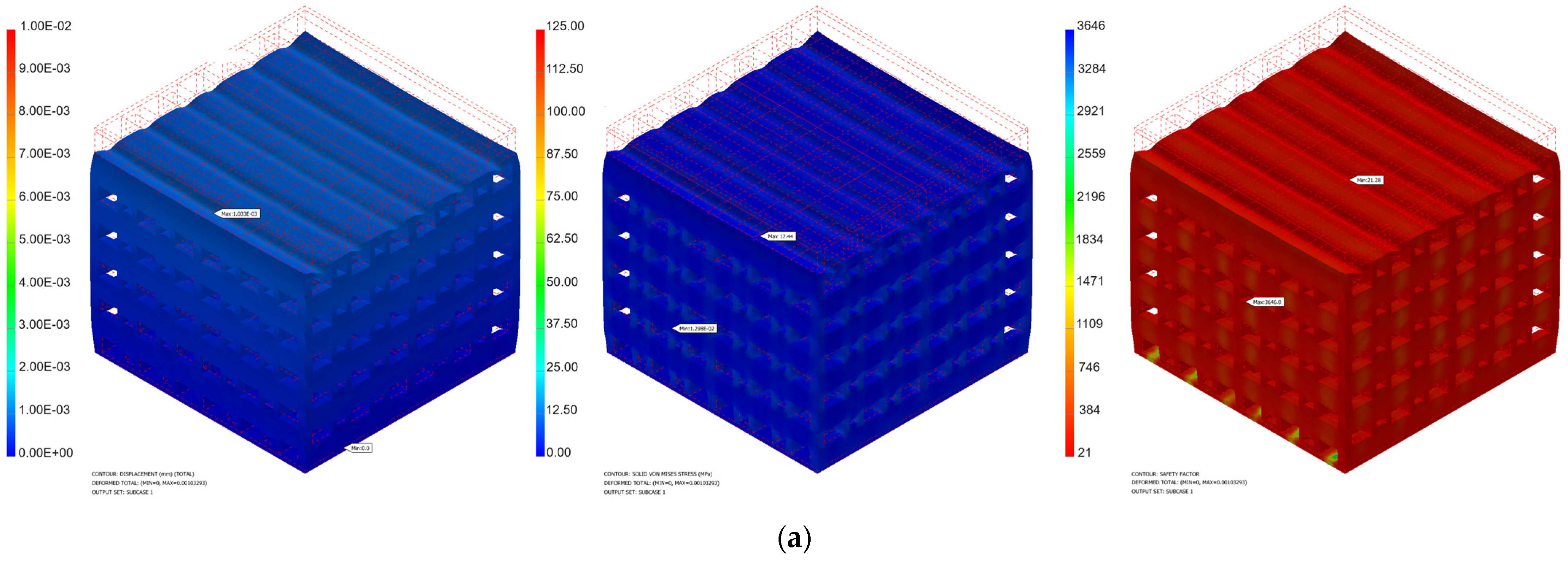
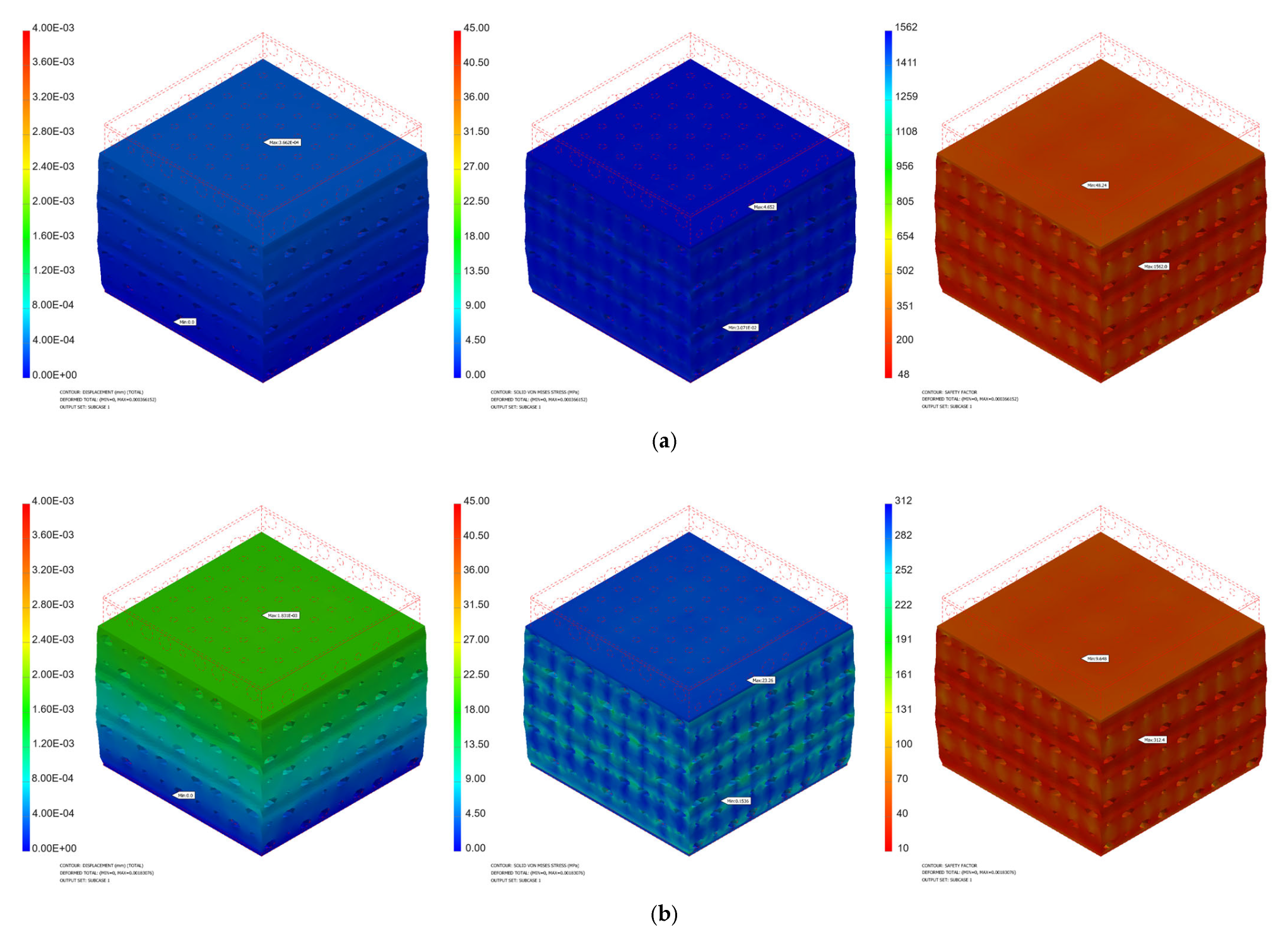
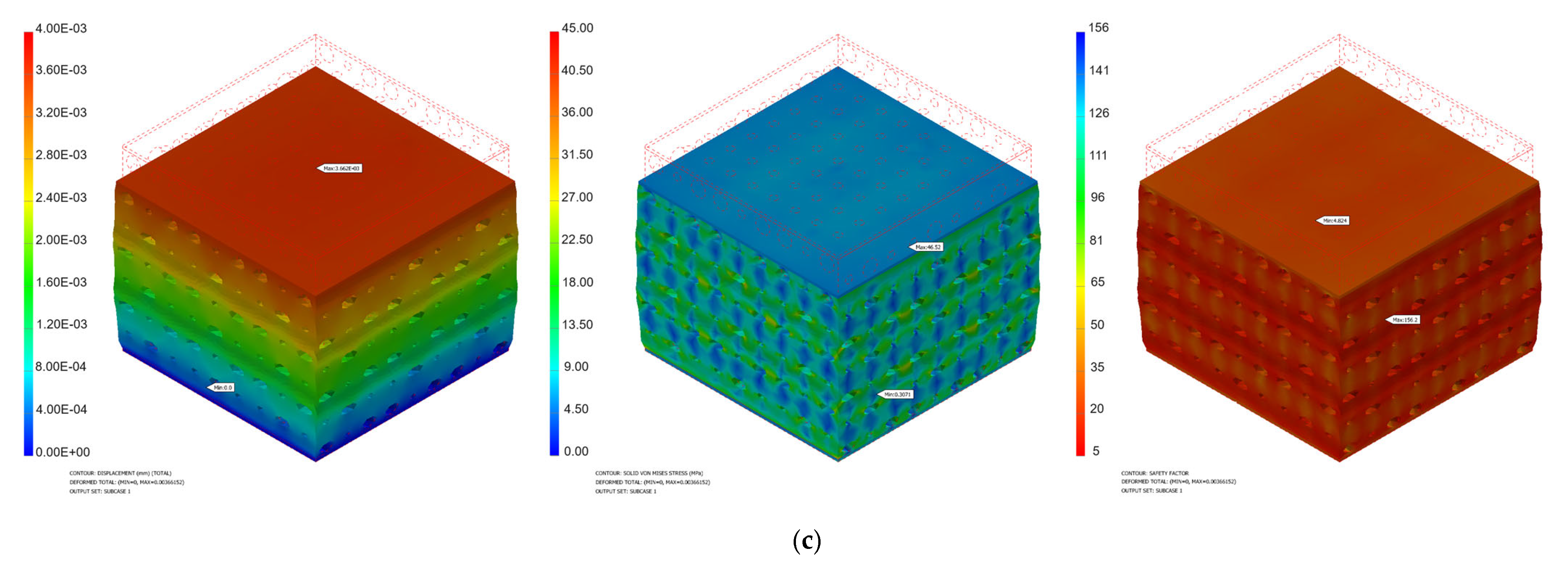
3.1. Mechanical Simulation Results
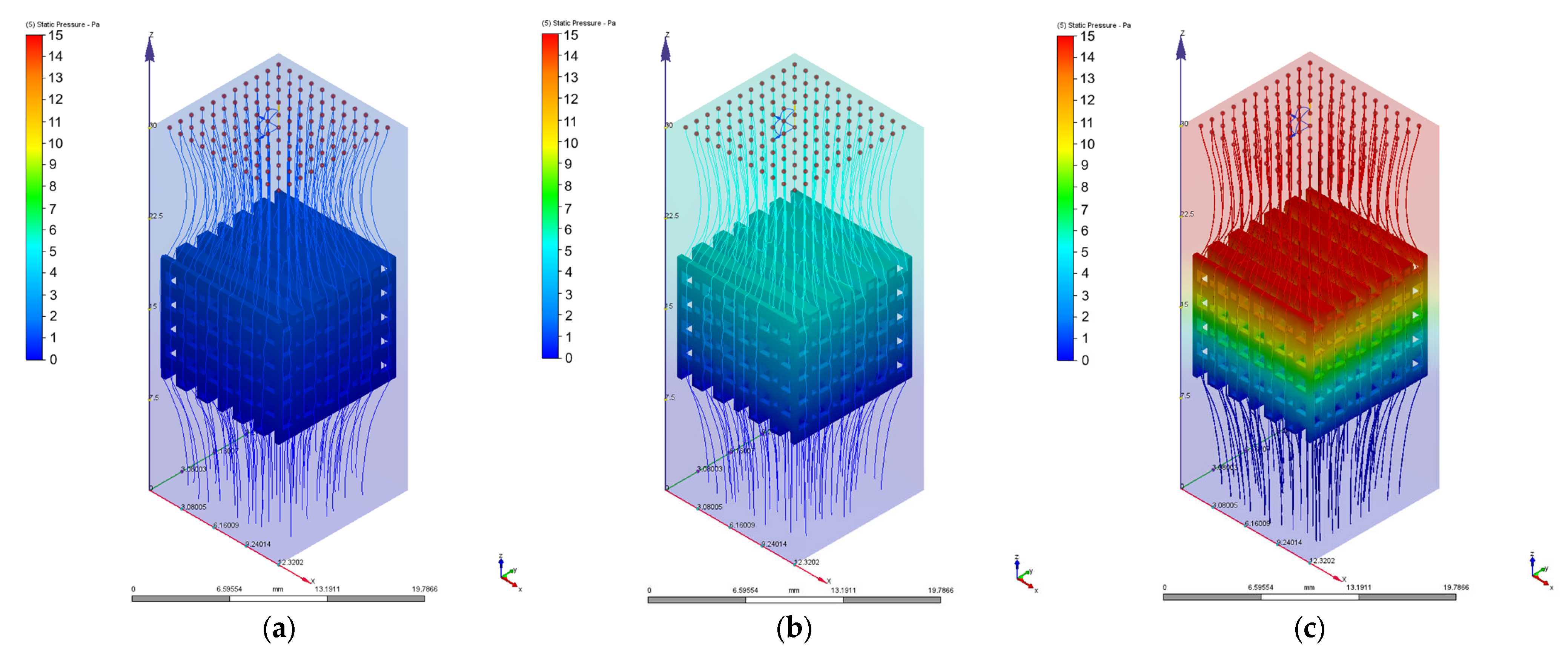
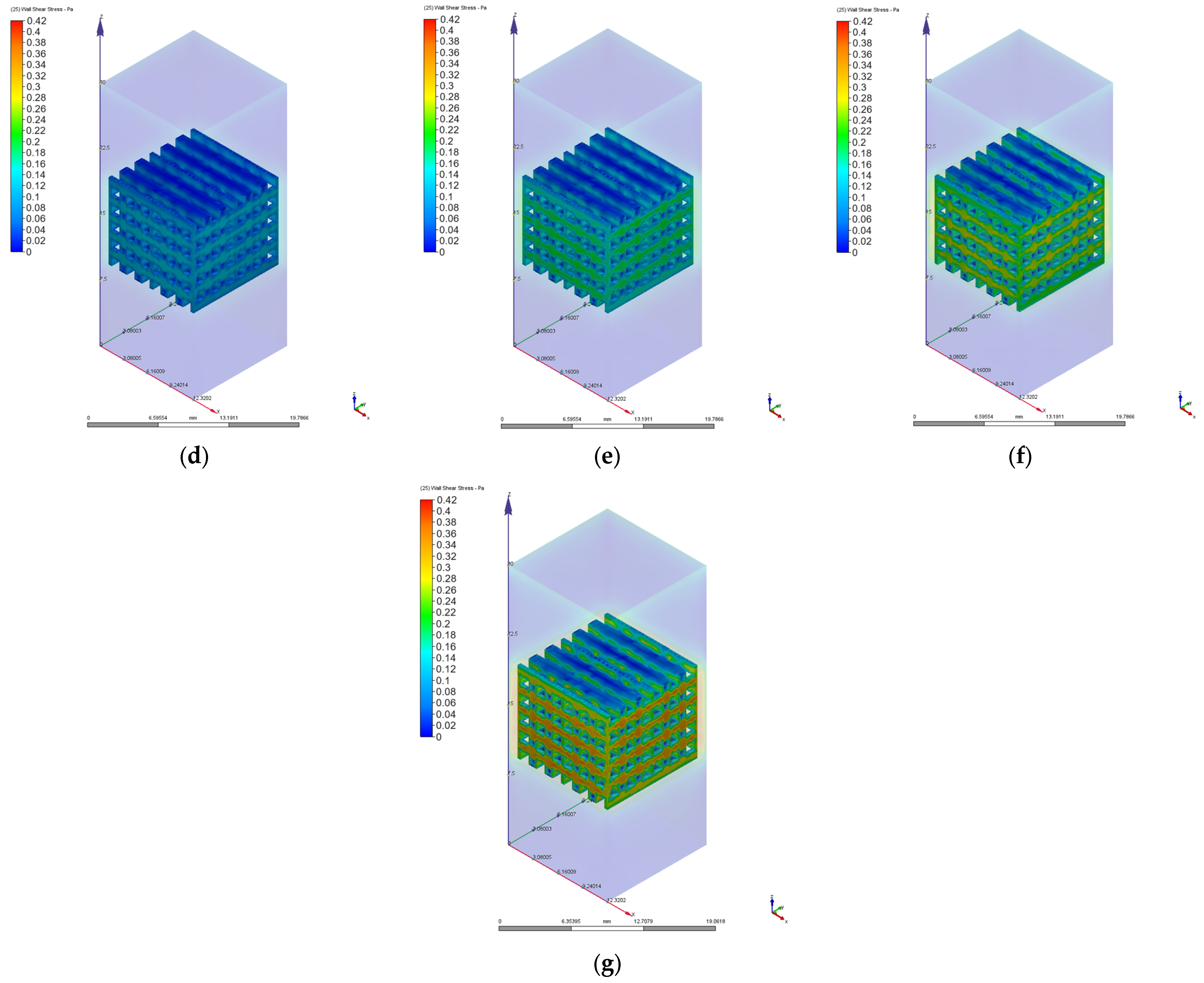
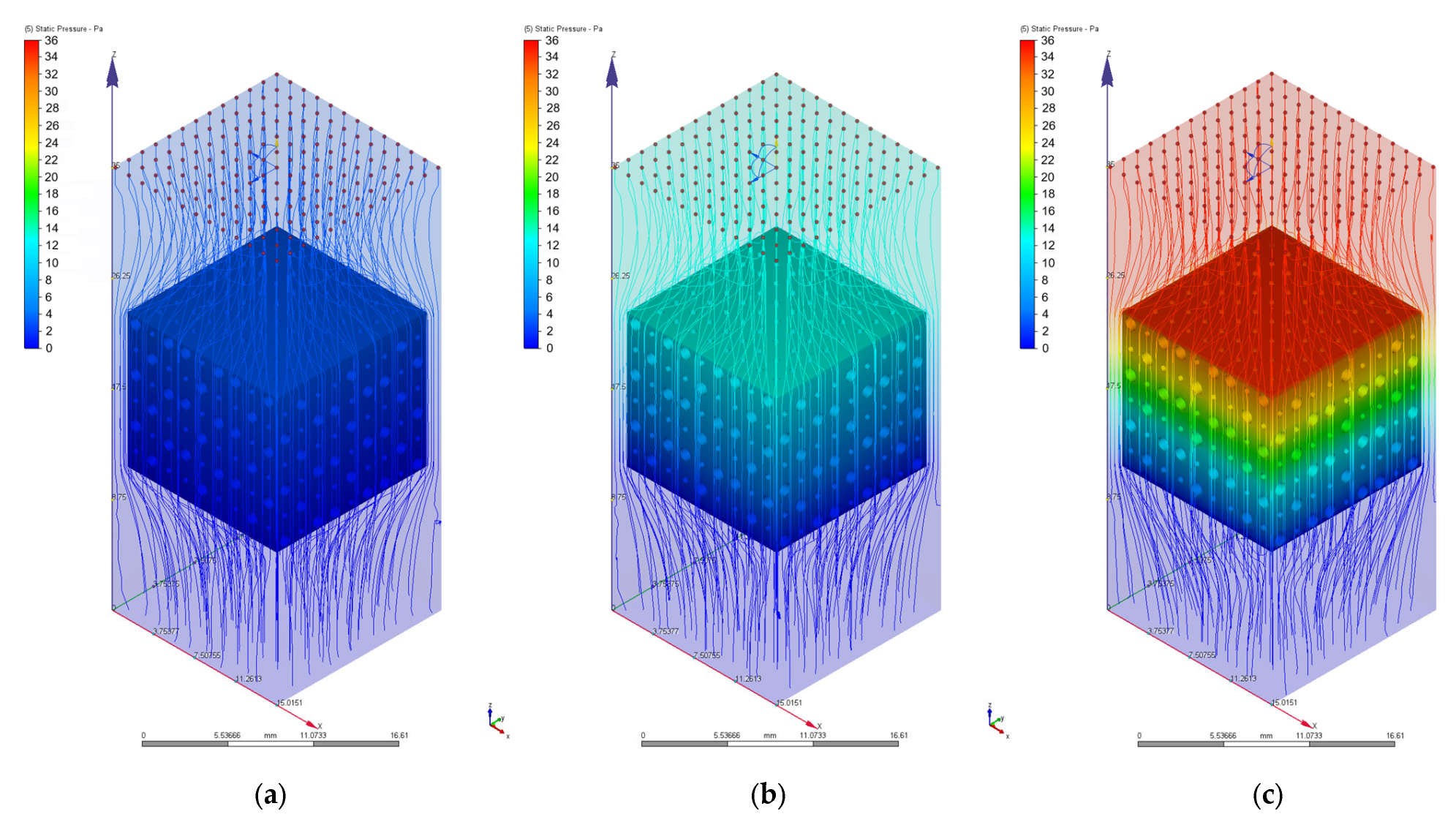
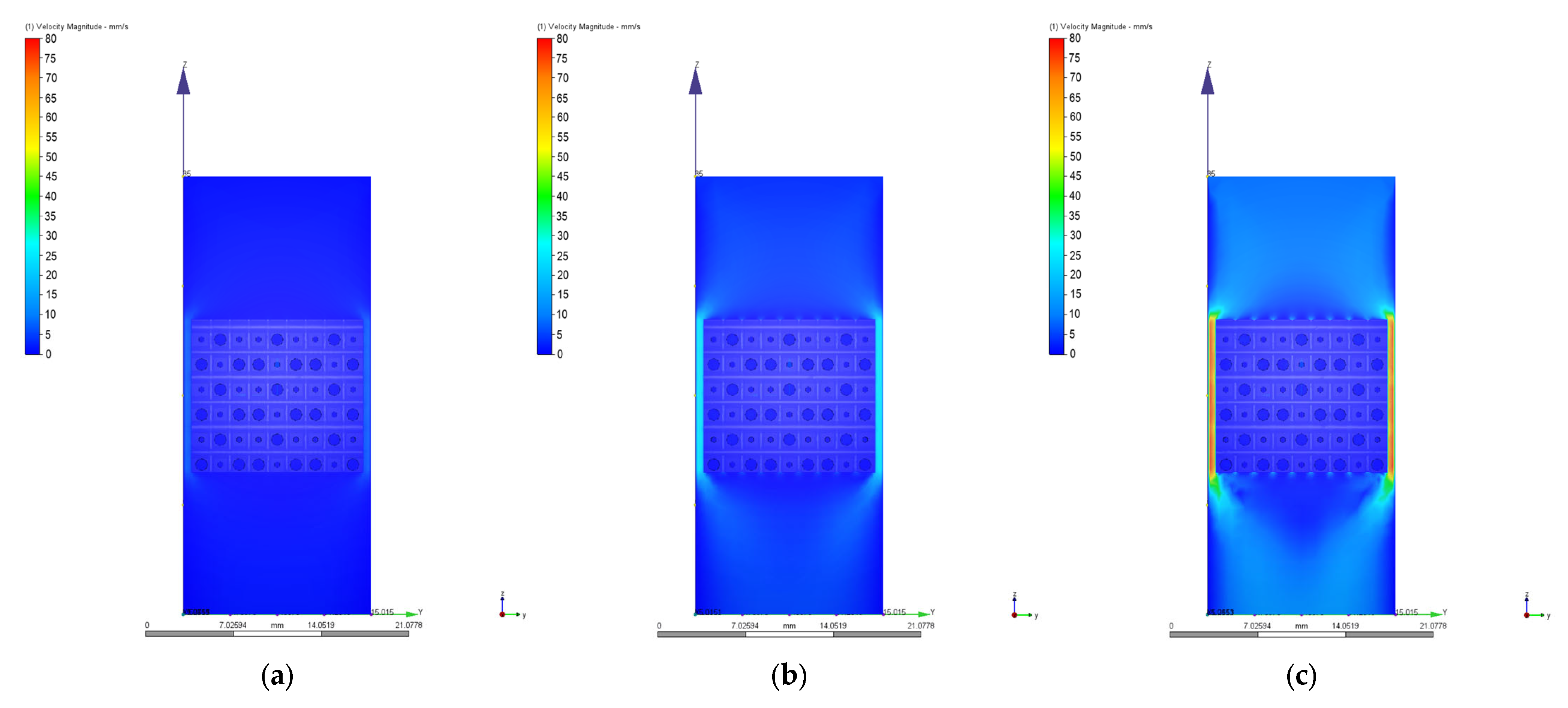
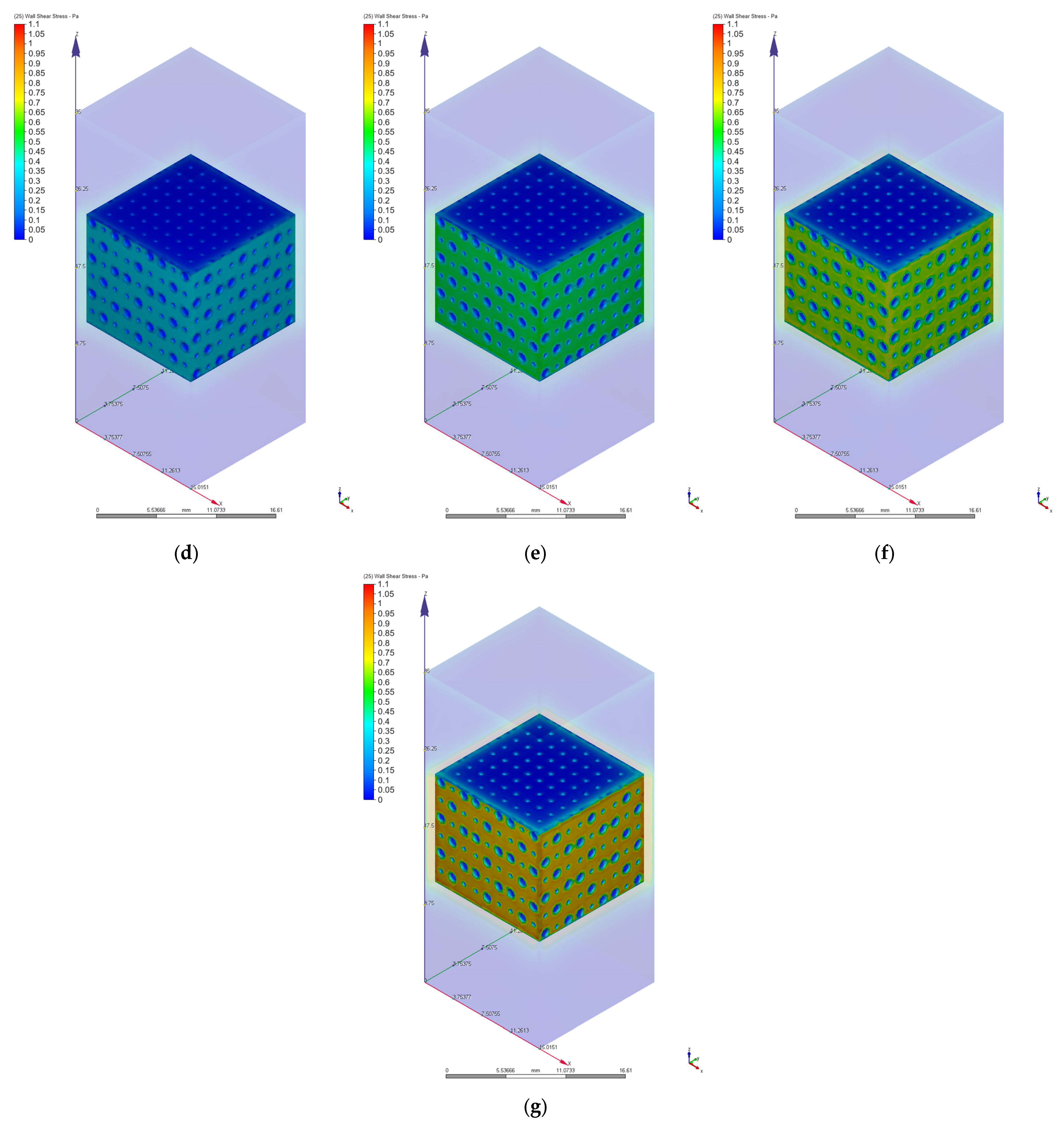
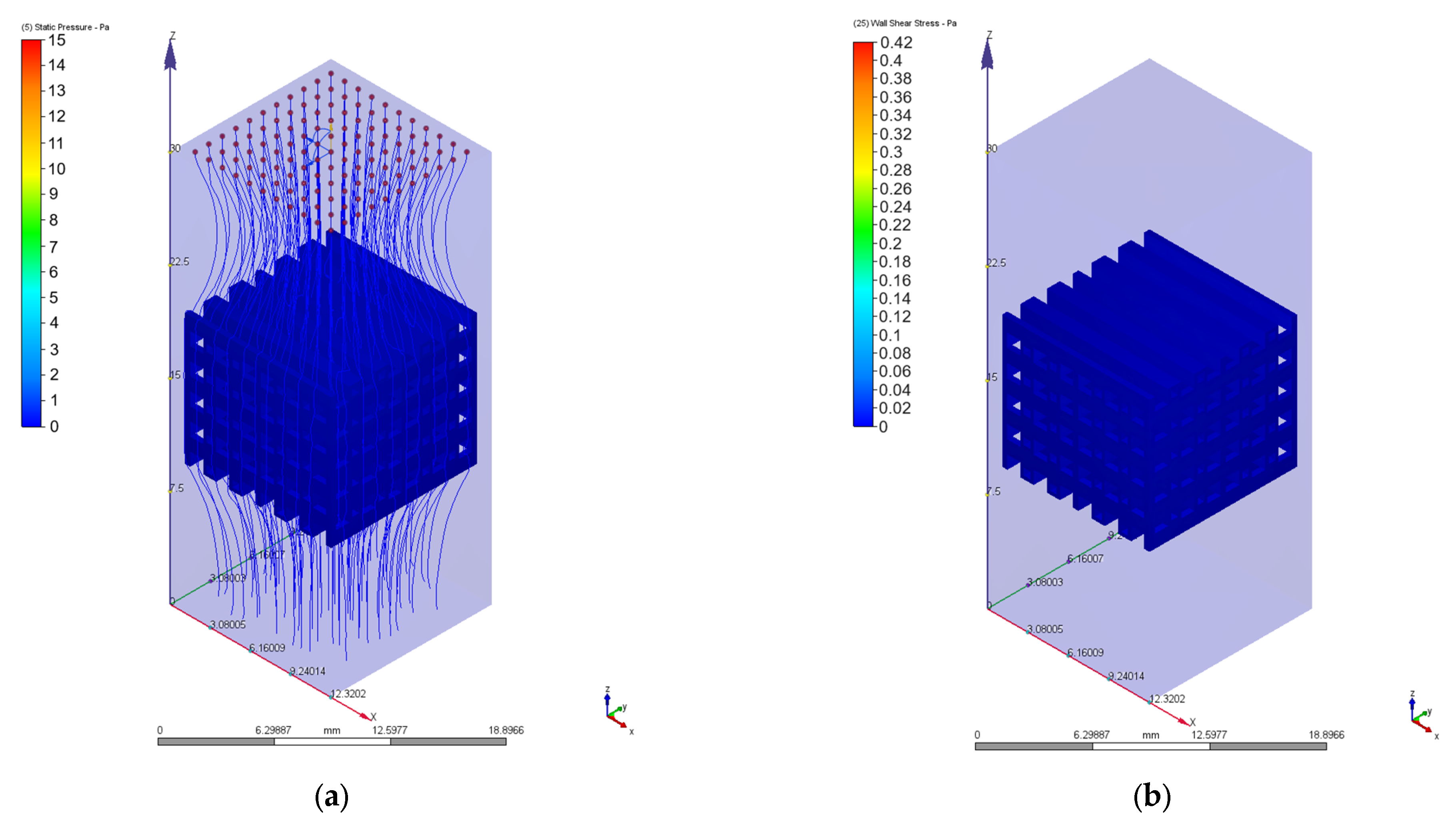
3.2. Computational Fluid Dynamics Simulation Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sohn, H.-S.; Oh, J.-K. Review of Bone Graft and Bone Substitutes with an Emphasis on Fracture Surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone Substitutes: A Review of Their Characteristics, Clinical Use, and Perspectives for Large Bone Defects Management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef]
- Antoniac, I.; Manescu (Paltanea), V.; Antoniac, A.; Paltanea, G. Magnesium-Based Alloys with Adapted Interfaces for Bone Implants and Tissue Engineering. Regen. Biomater. 2023, 10, rbad095. [Google Scholar] [CrossRef]
- Paltanea, G.; Manescu (Paltanea), V.; Antoniac, I.; Antoniac, A.; Nemoianu, I.V.; Robu, A.; Dura, H. A Review of Biomimetic and Biodegradable Magnetic Scaffolds for Bone Tissue Engineering and Oncology. Int. J. Mol. Sci. 2023, 24, 4312. [Google Scholar] [CrossRef]
- Kuzyk, P.R.; Schemitsch, E.H. The Basic Science of Peri-Implant Bone Healing. Indian J. Orthop. 2011, 45, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Müller, R.; Ruffoni, D. Bone Remodeling and Mechanobiology around Implants: Insights from Small Animal Imaging. J. Orthop. Res. 2018, 36, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.M.; Yavari, S.A.; Wauthle, R.; Pouran, B.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Additively Manufactured Open-Cell Porous Biomaterials Made from Six Different Space-Filling Unit Cells: The Mechanical and Morphological Properties. Materials 2015, 8, 1871–1896. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, S.F.S.; Gharehkhani, S.; Mehrali, M.; Yarmand, H.; Metselaar, H.S.C.; Adib Kadri, N.; Osman, N.A.A. A Review on Powder-Based Additive Manufacturing for Tissue Engineering: Selective Laser Sintering and Inkjet 3D Printing. Sci. Technol. Adv. Mater. 2015, 16, 033502. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.F.; Tan, K.C.; Lim, T.C. State of the Art and Future Directions of Scaffold-Based Bone Engineering from a Biomaterials Perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, B.; Yang, L.; Shi, Y. Tailored Mechanical Response and Mass Transport Characteristic of Selective Laser Melted Porous Metallic Biomaterials for Bone Scaffolds. Acta Biomater. 2020, 112, 298–315. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, Z.; Yan, R.; Yang, Y. Mechanical Properties of Graded Scaffolds Developed by Curve Interference Coupled with Selective Laser Sintering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111181. [Google Scholar] [CrossRef]
- Pałka, K.; Pokrowiecki, R. Porous Titanium Implants: A Review. Adv. Eng. Mater. 2018, 20, 1700648. [Google Scholar] [CrossRef]
- Boccaccio, A.; Uva, A.E.; Fiorentino, M.; Lamberti, L.; Monno, G. A Mechanobiology-Based Algorithm to Optimize the Microstructure Geometry of Bone Tissue Scaffolds. Int. J. Biol. Sci. 2016, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Čapek, J.; Machová, M.; Fousová, M.; Kubásek, J.; Vojtěch, D.; Fojt, J.; Jablonská, E.; Lipov, J.; Ruml, T. Highly Porous, Low Elastic Modulus 316L Stainless Steel Scaffold Prepared by Selective Laser Melting. Mater. Sci. Eng. C 2016, 69, 631–639. [Google Scholar] [CrossRef]
- Nune, K.C.; Misra, R.D.K.; Li, S.J.; Hao, Y.L.; Yang, R. Cellular Response of Osteoblasts to Low Modulus Ti-24Nb-4Zr-8Sn Alloy Mesh Structure. J. Biomed. Mater. Res. Part A 2017, 105, 859–870. [Google Scholar] [CrossRef]
- Li, G.; Wu, Y.; Zhang, Y.; Zhi, R.; Wang, J.; Ma, C. Performance Study on a Single-Screw Expander for a Small-Scale Pressure Recovery System. Energies 2017, 10, 6. [Google Scholar] [CrossRef]
- Goldstein, S.A. The Mechanical Properties of Trabecular Bone: Dependence on Anatomic Location and Function. J. Biomech. 1987, 20, 1055–1061. [Google Scholar] [CrossRef]
- Parthasarathy, J.; Starly, B.; Raman, S.; Christensen, A. Mechanical Evaluation of Porous Titanium (Ti6Al4V) Structures with Electron Beam Melting (EBM). J. Mech. Behav. Biomed. Mater. 2010, 3, 249–259. [Google Scholar] [CrossRef]
- Pennings, S.; Liu, K.J.; Qian, H. The Stem Cell Niche: Interactions between Stem Cells and Their Environment. Stem Cells Int. 2018, 2018, e4879379. [Google Scholar] [CrossRef]
- Chen, J.; Hendriks, M.; Chatzis, A.; Ramasamy, S.K.; Kusumbe, A.P. Bone Vasculature and Bone Marrow Vascular Niches in Health and Disease. J. Bone Miner. Res. 2020, 35, 2103–2120. [Google Scholar] [CrossRef]
- Daskalakis, E.; Liu, F.; Huang, B.; Acar, A.A.; Cooper, G.; Weightman, A.; Blunn, G.; Koç, B.; Bartolo, P. Investigating the Influence of Architecture and Material Composition of 3D Printed Anatomical Design Scaffolds for Large Bone Defects. Int. J. Bioprinting 2021, 7, 43–52. [Google Scholar] [CrossRef]
- Hendrikson, W.; van Blitterswijk, C.; Rouwkema, J.; Moroni, L. The Use of Finite Element Analyses to Design and Fabricate Three-Dimensional Scaffolds for Skeletal Tissue Engineering. Front. Bioeng. Biotechnol. 2017, 5, 30. [Google Scholar] [CrossRef]
- Schipani, R.; Nolan, D.R.; Lally, C.; Kelly, D.J. Integrating Finite Element Modelling and 3D Printing to Engineer Biomimetic Polymeric Scaffolds for Tissue Engineering. Connect. Tissue Res. 2020, 61, 174–189. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, C.; Wang, J.; Wang, P.; Bi, S.; Abbas, C.A. Design and Simulation of Flow Field for Bone Tissue Engineering Scaffold Based on Triply Periodic Minimal Surface. Chin. J. Mech. Eng. 2019, 32, 19. [Google Scholar] [CrossRef]
- Chabanon, M.; Duval, H.; Grenier, J.; Beauchesne, C.; Goyeau, B.; David, B. Histological Method to Study the Effect of Shear Stress on Cell Proliferation and Tissue Morphology in a Bioreactor. Tissue Eng. Regen. Med. 2019, 16, 225–235. [Google Scholar] [CrossRef]
- Melke, J.; Zhao, F.; Van Rietbergen, B.; Ito, K.; Hofmann, S. Localisation of Mineralised Tissue in a Complex Spinner Flask Environment Correlates with Predicted Wall Shear Stress Level Localisation. eCM 2018, 36, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; van Rietbergen, B.; Ito, K.; Hofmann, S. Flow Rates in Perfusion Bioreactors to Maximise Mineralisation in Bone Tissue Engineering in Vitro. J. Biomech. 2018, 79, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Salinas, E.Y.; Aryaei, A.; Paschos, N.; Berson, E.; Kwon, H.; Hu, J.C.; Athanasiou, K.A. Shear Stress Induced by Fluid Flow Produces Improvements in Tissue-Engineered Cartilage. Biofabrication 2020, 12, 045010. [Google Scholar] [CrossRef] [PubMed]
- Seddiqi, H.; Saatchi, A.; Amoabediny, G.; Helder, M.N.; Abbasi Ravasjani, S.; Safari Hajat Aghaei, M.; Jin, J.; Zandieh-Doulabi, B.; Klein-Nulend, J. Inlet Flow Rate of Perfusion Bioreactors Affects Fluid Flow Dynamics, but Not Oxygen Concentration in 3D-Printed Scaffolds for Bone Tissue Engineering: Computational Analysis and Experimental Validation. Comput. Biol. Med. 2020, 124, 103826. [Google Scholar] [CrossRef] [PubMed]
- Moradkhani, M.; Vahidi, B.; Ahmadian, B. Finite Element Study of Stem Cells under Fluid Flow for Mechanoregulation toward Osteochondral Cells. J. Mater. Sci. Mater. Med. 2021, 32, 84. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liang, H.; Xie, D.; Mao, N.; Zhao, J.; Tian, Z.; Wang, C.; Shen, L. Finite Element Analysis of Mechanical Behavior, Permeability of Irregular Porous Scaffolds and Lattice-Based Porous Scaffolds. Mater. Res. Express 2019, 6, 105407. [Google Scholar] [CrossRef]
- Zhao, F.; Melke, J.; Ito, K.; van Rietbergen, B.; Hofmann, S. A Multiscale Computational Fluid Dynamics Approach to Simulate the Micro-Fluidic Environment within a Tissue Engineering Scaffold with Highly Irregular Pore Geometry. Biomech. Model. Mechanobiol. 2019, 18, 1965–1977. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Lacroix, D.; Ito, K.; van Rietbergen, B.; Hofmann, S. Changes in Scaffold Porosity during Bone Tissue Engineering in Perfusion Bioreactors Considerably Affect Cellular Mechanical Stimulation for Mineralization. Bone Rep. 2020, 12, 100265. [Google Scholar] [CrossRef]
- Abdelaziz, A.G.; Nageh, H.; Abdo, S.M.; Abdalla, M.S.; Amer, A.A.; Abdal-hay, A.; Barhoum, A. A Review of 3D Polymeric Scaffolds for Bone Tissue Engineering: Principles, Fabrication Techniques, Immunomodulatory Roles, and Challenges. Bioengineering 2023, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A. State of Art Review on Bioabsorbable Polymeric Scaffolds for Bone Tissue Engineering. Mater. Today Proc. 2021, 44, 1391–1400. [Google Scholar] [CrossRef]
- Hu, X.; Lin, Z.; He, J.; Zhou, M.; Yang, S.; Wang, Y.; Li, K. Recent Progress in 3D Printing Degradable Polylactic Acid-Based Bone Repair Scaffold for the Application of Cancellous Bone Defect. MedComm–Biomater. Appl. 2022, 1, e14. [Google Scholar] [CrossRef]
- Panseri, S.; Montesi, M.; Hautcoeur, D.; Dozio, S.M.; Chamary, S.; De Barra, E.; Tampieri, A.; Leriche, A. Bone-like Ceramic Scaffolds Designed with Bioinspired Porosity Induce a Different Stem Cell Response. J. Mater. Sci. Mater. Med. 2021, 32, 3. [Google Scholar] [CrossRef]
- Suárez, M.; Fernández-García, E.; Fernández, A.; López-Píriz, R.; Díaz, R.; Torrecillas, R. Novel Antimicrobial Phosphate-Free Glass–Ceramic Scaffolds for Bone Tissue Regeneration. Sci. Rep. 2020, 10, 13171. [Google Scholar] [CrossRef]
- Ribas, R.G.; Schatkoski, V.M.; do Amaral Montanheiro, T.L.; de Menezes, B.R.C.; Stegemann, C.; Leite, D.M.G.; Thim, G.P. Current Advances in Bone Tissue Engineering Concerning Ceramic and Bioglass Scaffolds: A Review. Ceram. Int. 2019, 45, 21051–21061. [Google Scholar] [CrossRef]
- Antoniac, I.; Manescu (Paltanea), V.; Paltanea, G.; Antoniac, A.; Nemoianu, I.V.; Petrescu, M.I.; Dura, H.; Bodog, A.D. Additive Manufactured Magnesium-Based Scaffolds for Tissue Engineering. Materials 2022, 15, 8693. [Google Scholar] [CrossRef]
- Antoniac, I.; Miculescu, M.; Mănescu (Păltânea), V.; Stere, A.; Quan, P.H.; Păltânea, G.; Robu, A.; Earar, K. Magnesium-Based Alloys Used in Orthopedic Surgery. Materials 2022, 15, 1148. [Google Scholar] [CrossRef]
- Cockerill, I.; Su, Y.; Sinha, S.; Qin, Y.-X.; Zheng, Y.; Young, M.L.; Zhu, D. Porous Zinc Scaffolds for Bone Tissue Engineering Applications: A Novel Additive Manufacturing and Casting Approach. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110738. [Google Scholar] [CrossRef]
- Wu, H.; Xie, X.; Wang, J.; Ke, G.; Huang, H.; Liao, Y.; Kong, Q. Biological Properties of Zn–0.04Mg–2Ag: A New Degradable Zinc Alloy Scaffold for Repairing Large-Scale Bone Defects. J. Mater. Res. Technol. 2021, 13, 1779–1789. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, Q.; Jia, G.; Li, H.; Yuan, G.; Yu, H. A Biomimetic Zinc Alloy Scaffold Coated with Brushite for Enhanced Cranial Bone Regeneration. ACS Biomater. Sci. Eng. 2021, 7, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Wahafu, T.; Jiang, G.; Liu, W.; Qiao, Y.; Peng, X.; Cheng, T.; Zhang, X.; He, G.; Liu, X. A Novel Open-Porous Magnesium Scaffold with Controllable Microstructures and Properties for Bone Regeneration. Sci. Rep. 2016, 6, 24134. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Sen, S. Finite Element Analysis of Mechanical Behavior, Permeability and Fluid Induced Wall Shear Stress of High Porosity Scaffolds with Gyroid and Lattice-Based Architectures. J. Mech. Behav. Biomed. Mater. 2017, 75, 262–270. [Google Scholar] [CrossRef]
- Zhao, F.; Vaughan, T.J.; McNamara, L.M. Quantification of Fluid Shear Stress in Bone Tissue Engineering Scaffolds with Spherical and Cubical Pore Architectures. Biomech. Model. Mechanobiol. 2016, 15, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Zhang, B.; Yang, H.; Mushtaq, R.T.; Liu, M.; Bao, C.; Shi, Y.; Luo, Z.; Zhang, W. The Design and Evaluation of Bionic Porous Bone Scaffolds in Fluid Flow Characteristics and Mechanical Properties. Comput. Methods Programs Biomed. 2022, 225, 107059. [Google Scholar] [CrossRef]
- Fallah, A.; Altunbek, M.; Bartolo, P.; Cooper, G.; Weightman, A.; Blunn, G.; Koc, B. 3D Printed Scaffold Design for Bone Defects with Improved Mechanical and Biological Properties. J. Mech. Behav. Biomed. Mater. 2022, 134, 105418. [Google Scholar] [CrossRef]
- Gómez, S.; Vlad, M.D.; López, J.; Fernández, E. Design and Properties of 3D Scaffolds for Bone Tissue Engineering. Acta Biomater. 2016, 42, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Jayasathyakawin, S.; Ravichandran, M.; Baskar, N.; Anand Chairman, C.; Balasundaram, R. Mechanical Properties and Applications of Magnesium Alloy—Review. Mater. Today Proc. 2020, 27, 909–913. [Google Scholar] [CrossRef]
- Arjunan, A.; Demetriou, M.; Baroutaji, A.; Wang, C. Mechanical Performance of Highly Permeable Laser Melted Ti6Al4V Bone Scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 102, 103517. [Google Scholar] [CrossRef] [PubMed]
- Daroonparvar, M.; Yajid, M.A.M.; Gupta, R.K.; Yusof, N.M.; Bakhsheshi-Rad, H.R.; Ghandvar, H.; Ghasemi, E. Antibacterial Activities and Corrosion Behavior of Novel PEO/Nanostructured ZrO2 Coating on Mg Alloy. Trans. Nonferrous Met. Soc. China 2018, 28, 1571–1581. [Google Scholar] [CrossRef]
- Hussein, N.A.K.; Mohtar, N.A.J.; Neza, L.H.; Noordin, M.A.; Saad, A.P. Pore Size Effect on Mechanical Response and Fluid Permeability for Bone Scaffold Regeneration. Malays. J. Med. Health Sci. 2021, 17, 34–39. [Google Scholar]
- Perevoshchikova, N.; Moerman, K.M.; Akhbari, B.; Bindra, R.; Maharaj, J.N.; Lloyd, D.G.; Gomez Cerezo, M.; Carr, A.; Vaquette, C.; Saxby, D.J. Finite Element Analysis of the Performance of Additively Manufactured Scaffolds for Scapholunate Ligament Reconstruction. PLoS ONE 2021, 16, e0256528. [Google Scholar] [CrossRef]
- Bronfin, B.; Aghion, A.; Fantetti, N.; Von Buch, F.; Schumann, S.; Friedrich, H. High Temperature Mg Alloys for Sand and Permanent Mold Casting Applications. SAE Trans. 2004, 113, 266–270. [Google Scholar]
- Bronfin, B.; Moscovitch, N. New Magnesium Alloys for Transmission Parts. Met. Sci. Heat Treat. 2006, 48, 479–486. [Google Scholar] [CrossRef]
- Zengin, H.; Turen, Y. Effect of Y Addition on Microstructure and Corrosion Behavior of Extruded Mg–Zn–Nd–Zr Alloy. J. Magnes. Alloys 2020, 8, 640–653. [Google Scholar] [CrossRef]
- Aghion, E.; Moscovitch, N.; Arnon, A. Mechanical Properties of Die-Cast Magnesium Alloy MRI 230D. J. Mater. Eng. Perform. 2009, 18, 912–916. [Google Scholar] [CrossRef]
- Wang, P.; Gammer, C.; Brenne, F.; Prashanth, K.G.; Mendes, R.G.; Rümmeli, M.H.; Gemming, T.; Eckert, J.; Scudino, S. Microstructure and Mechanical Properties of a Heat-Treatable Al-3.5Cu-1.5Mg-1Si Alloy Produced by Selective Laser Melting. Mater. Sci. Eng. A 2018, 711, 562–570. [Google Scholar] [CrossRef]
- Gaon, O.; Dror, G.; Davidi, O.; Lugovskoy, A. The Effect of the Local Microstructure of MRI 201S Magnesium Alloy on Its Corrosion Rate. Corros. Sci. 2015, 93, 167–171. [Google Scholar] [CrossRef]
- Streza, A.; Antoniac, A.; Manescu (Paltanea), V.M.; Ciocoiu, R.; Cotrut, C.-M.; Miculescu, M.; Miculescu, F.; Antoniac, I.; Fosca, M.; Rau, J.V.; et al. In Vitro Studies Regarding the Effect of Cellulose Acetate-Based Composite Coatings on the Functional Properties of the Biodegradable Mg3Nd Alloys. Biomimetics 2023, 8, 526. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nelson, T.; Chakroff, J.; Cromeens, B.; Johnson, J.; Lannutti, J.; Besner, G.E. Comparison of Polyglycolic Acid, Polycaprolactone, and Collagen as Scaffolds for the Production of Tissue Engineered Intestine. J. Biomed. Mater. Res. 2019, 107, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Sen, S. Permeability and Fluid Flow-Induced Wall Shear Stress of Bone Tissue Scaffolds: Computational Fluid Dynamic Analysis Using Newtonian and Non-Newtonian Blood Flow Models. Comput. Biol. Med. 2018, 99, 201–208. [Google Scholar] [CrossRef]
- Matos, S.; Guerra, F.; Krauser, J.T.; Figueiredo, H.; Marcelino, J.P.; Sanz, M. Evaluation of an Anorganic Bovine-Derived Mineral with P-15 Hydrogel Bone Graft: Preliminary Study in a Rabbit Cranial Bone Model. Clin. Oral Implant. Res. 2012, 23, 698–705. [Google Scholar] [CrossRef]
- Falacho, R.I.; Palma, P.J.; Marques, J.A.; Figueiredo, M.H.; Caramelo, F.; Dias, I.; Viegas, C.; Guerra, F. Collagenated Porcine Heterologous Bone Grafts: Histomorphometric Evaluation of Bone Formation Using Different Physical Forms in a Rabbit Cancellous Bone Model. Molecules 2021, 26, 1339. [Google Scholar] [CrossRef]
- Ali, D.; Sen, S. Computational Fluid Dynamics Study of the Effects of Surface Roughness on Permeability and Fluid Flow-Induced Wall Shear Stress in Scaffolds. Ann. Biomed. Eng. 2018, 46, 2023–2035. [Google Scholar] [CrossRef]
- Olivares, A.L.; Marsal, È.; Planell, J.A.; Lacroix, D. Finite Element Study of Scaffold Architecture Design and Culture Conditions for Tissue Engineering. Biomaterials 2009, 30, 6142–6149. [Google Scholar] [CrossRef]
- Vossenberg, P.; Higuera, G.A.; van Straten, G.; van Blitterswijk, C.A.; van Boxtel, A.J.B. Darcian Permeability Constant as Indicator for Shear Stresses in Regular Scaffold Systems for Tissue Engineering. Biomech. Model. Mechanobiol. 2009, 8, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Patel, M.K.; Kersaudy-Kerhoas, M.; Desmulliez, M.P.Y.; Bailey, C.; Topham, D. Analysis of Fluid Separation in Microfluidic T-Channels. Appl. Math. Model. 2012, 36, 743–755. [Google Scholar] [CrossRef]
- Truscello, S.; Kerckhofs, G.; Van Bael, S.; Pyka, G.; Schrooten, J.; Van Oosterwyck, H. Prediction of Permeability of Regular Scaffolds for Skeletal Tissue Engineering: A Combined Computational and Experimental Study. Acta Biomater. 2012, 8, 1648–1658. [Google Scholar] [CrossRef]
- Liu, H.; Lan, L.; Abrigo, J.; Ip, H.L.; Soo, Y.; Zheng, D.; Wong, K.S.; Wang, D.; Shi, L.; Leung, T.W.; et al. Comparison of Newtonian and Non-Newtonian Fluid Models in Blood Flow Simulation in Patients With Intracranial Arterial Stenosis. Front. Physiol. 2021, 12, 718540. [Google Scholar] [CrossRef]
- Egger, D.; Fischer, M.; Clementi, A.; Ribitsch, V.; Hansmann, J.; Kasper, C. Development and Characterization of a Parallelizable Perfusion Bioreactor for 3D Cell Culture. Bioengineering 2017, 4, 51. [Google Scholar] [CrossRef]
- Lesman, A.; Blinder, Y.; Levenberg, S. Modeling of Flow-Induced Shear Stress Applied on 3D Cellular Scaffolds: Implications for Vascular Tissue Engineering. Biotechnol. Bioeng. 2010, 105, 645–654. [Google Scholar] [CrossRef]
- Marín, F.; Roldán, V. GDF-15 and Risk Stratification in Atrial Fibrillation. Nat. Rev. Cardiol. 2015, 12, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, S.; Dominissini, D.; Redaelli, A.; Passoni, G. The Effect of Turbulence Modelling on the Assessment of Platelet Activation. J. Biomech. 2021, 128, 110704. [Google Scholar] [CrossRef]
- Saqr, K.M.; Tupin, S.; Rashad, S.; Endo, T.; Niizuma, K.; Tominaga, T.; Ohta, M. Physiologic Blood Flow Is Turbulent. Sci. Rep. 2020, 10, 15492. [Google Scholar] [CrossRef]
- Andersson, M. Turbulence Descriptors in Arterial Flows Patient-Specific Computational Hemodynamics; Linköping Studies in Science and Technology; Linköping University Electronic Press: Linköping, Sweden, 2021; Volume 2129, ISBN 978-91-7929-686-5. [Google Scholar]
- Salles, S.; Shepherd, J.; Vos, H.J.; Renaud, G. Revealing Intraosseous Blood Flow in the Human Tibia with Ultrasound. JBMR Plus 2021, 5, e10543. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, I.; Sanz-Herrera, J.A.; García-Aznar, J.M.; Doblaré, M.; Yunos, D.M.; Boccaccini, A.R. Permeability Evaluation of 45S5 Bioglass®-Based Scaffolds for Bone Tissue Engineering. J. Biomech. 2009, 42, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.J.; Williams, J.L. Measurements of Permeability in Human Calcaneal Trabecular Bone. J. Biomech. 1997, 30, 743–745. [Google Scholar] [CrossRef] [PubMed]
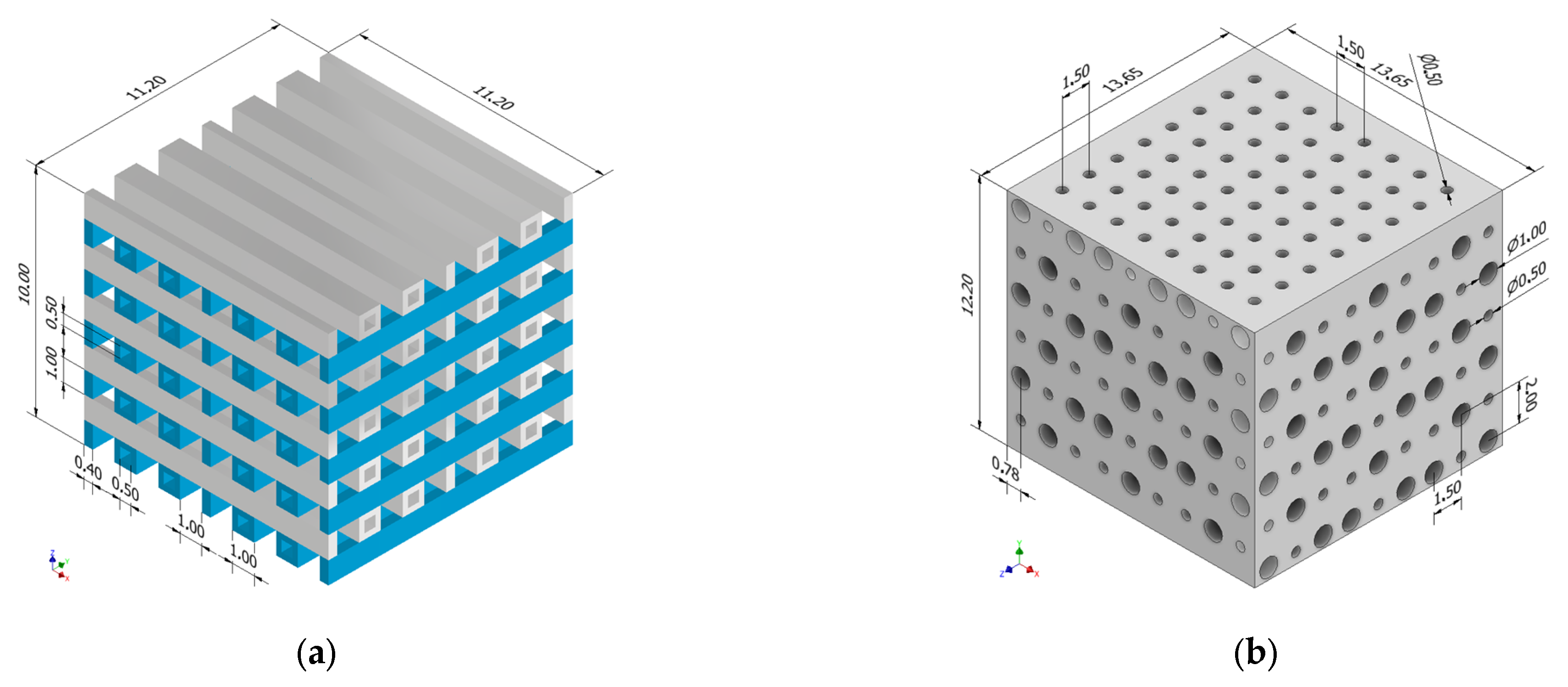
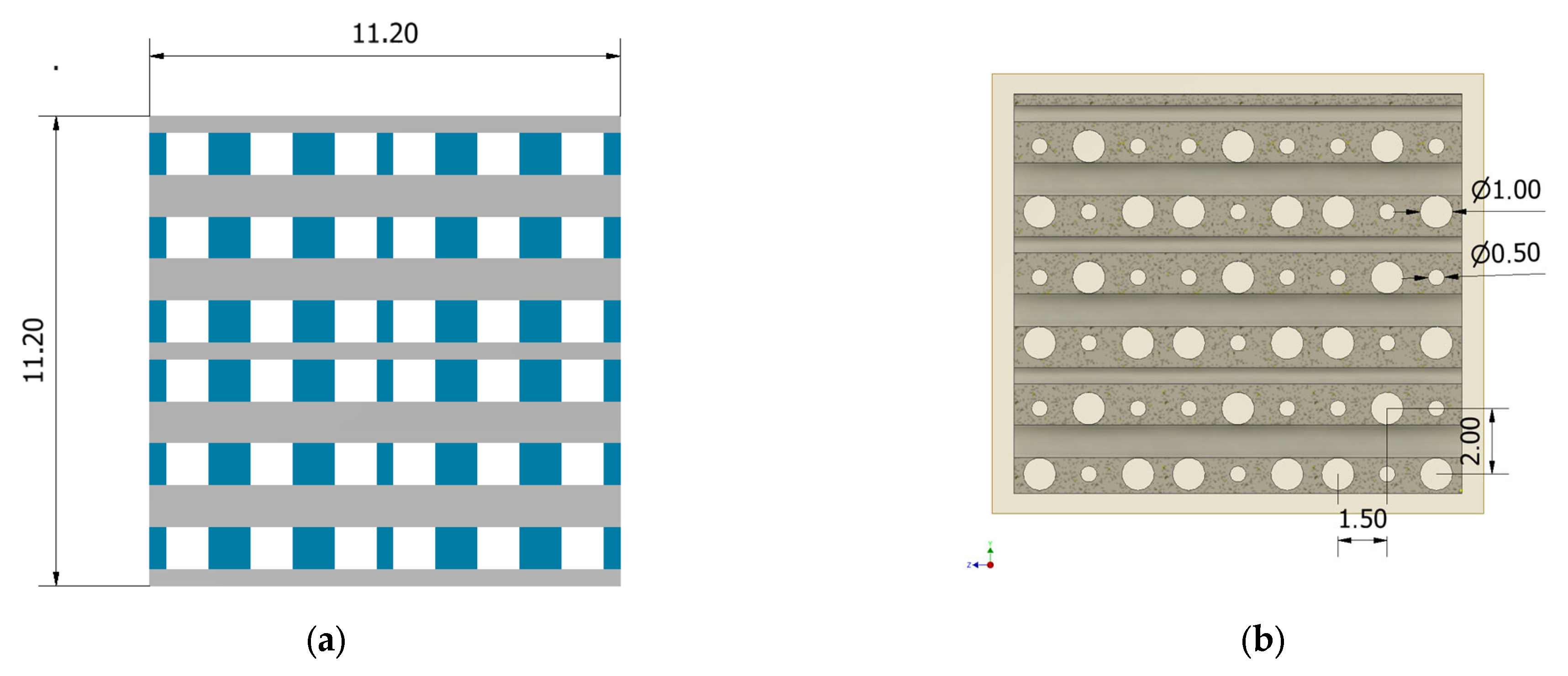






| Geometrical Configuration | VS [mm3] | Vt [mm3] | P [%] |
|---|---|---|---|
| PP | 1254.40 | 470.40 | 62 |
| PC | 2273.13 | 1396.18 | 38 |
| Force [N] | Pressure [MPa] | Minimum von Mises Stress [MPa] | Maximum von Mises Stress [MPa] | Maximum Displacement [mm] | Safety Factor | ||||
|---|---|---|---|---|---|---|---|---|---|
| PP | PC | PP | PC | PP | PC | PP | PC | ||
| 50 | 0.39 | 6.49 × 10−3 | 1.54 × 10−2 | 6.222 | 2.326 | 5.16 × 10−4 | 1.83 × 10−4 | 42.57 | 96.46 |
| 100 | 0.79 | 1.30 × 10−2 | 3.07 × 10−2 | 12.44 | 4.652 | 1.03 × 10−3 | 3.66 × 10−4 | 21.28 | 48.24 |
| 200 | 1.59 | 2.60 × 10−2 | 6.14 × 10−2 | 24.89 | 9.303 | 2.07 × 10−3 | 7.32 × 10−4 | 10.64 | 24.12 |
| 300 | 2.39 | 3.89 × 10−2 | 9.21 × 10−2 | 37.33 | 13.95 | 3.10 × 10−3 | 1.10 × 10−3 | 7.09 | 16.08 |
| 400 | 3.18 | 5.19 × 10−2 | 1.23 × 10−1 | 49.78 | 18.61 | 4.13 × 10−3 | 1.47 × 10−3 | 5.32 | 12.06 |
| 500 | 3.98 | 6.49 × 10−2 | 1.54 × 10−1 | 62.22 | 23.26 | 5.17 × 10−3 | 1.83 × 10−3 | 4.25 | 9.64 |
| 600 | 4.78 | 7.79 × 10−2 | 1.84 × 10−1 | 74.66 | 27.91 | 6.20 × 10−3 | 2.20 × 10−3 | 3.54 | 8.04 |
| 700 | 5.58 | 9.09 × 10−2 | 2.15 × 10−1 | 87.11 | 32.56 | 7.23 × 10−3 | 2.56 × 10−3 | 3.04 | 6.89 |
| 800 | 6.37 | 1.04 × 10−2 | 2.46 × 10−1 | 99.55 | 37.21 | 8.26 × 10−3 | 2.93 × 10−3 | 2.66 | 6.03 |
| 900 | 7.17 | 1.17 × 10−1 | 2.76 × 10−1 | 112 | 41.86 | 9.30 × 10−3 | 3.30 × 10−3 | 2.36 | 5.36 |
| 1000 | 7.97 | 1.30 × 10−1 | 3.07 × 10−1 | 124.4 | 46.52 | 1.03 × 10−2 | 3.66 × 10−2 | 2.12 | 4.82 |
| Velocity [mm/s] | Pressure Drops [Pa] | Permeability [m2] | Reynolds Number | Maximum Wall Shear Stress [Pa] | |||
|---|---|---|---|---|---|---|---|
| PP | PC | PP | PC | PP/PC | PP | PC | |
| 1 | 1.35 | 3.29 | 1.06 × 10−8 | 5.36 × 10−9 | 0.68 | 0.04 | 0.10 |
| 2 | 2.73 | 6.63 | 1.06 × 10−8 | 5.33 × 10−9 | 1.37 | 0.08 | 0.20 |
| 3 | 4.15 | 10.02 | 1.05 × 10−8 | 5.29 × 10−9 | 2.07 | 0.12 | 0.31 |
| 4 | 5.61 | 13.49 | 1.03 × 10−8 | 5.24 × 10−9 | 2.75 | 0.16 | 0.42 |
| 6 | 8.77 | 20.57 | 9.91 × 10−9 | 5.16 × 10−9 | 4.14 | 0.25 | 0.64 |
| 8 | 12.10 | 27.78 | 9.58 × 10−9 | 5.09 × 10−9 | 5.52 | 0.34 | 0.87 |
| 10 | 15.62 | 35.39 | 9.28 × 10−9 | 4.99 × 10−9 | 6.89 | 0.43 | 1.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manescu, V.; Paltanea, G.; Antoniac, A.; Gruionu, L.G.; Robu, A.; Vasilescu, M.; Laptoiu, S.A.; Bita, A.I.; Popa, G.M.; Cocosila, A.L.; et al. Mechanical and Computational Fluid Dynamic Models for Magnesium-Based Implants. Materials 2024, 17, 830. https://doi.org/10.3390/ma17040830
Manescu V, Paltanea G, Antoniac A, Gruionu LG, Robu A, Vasilescu M, Laptoiu SA, Bita AI, Popa GM, Cocosila AL, et al. Mechanical and Computational Fluid Dynamic Models for Magnesium-Based Implants. Materials. 2024; 17(4):830. https://doi.org/10.3390/ma17040830
Chicago/Turabian StyleManescu (Paltanea), Veronica, Gheorghe Paltanea, Aurora Antoniac, Lucian Gheorghe Gruionu, Alina Robu, Marius Vasilescu, Stefan Alexandru Laptoiu, Ana Iulia Bita, Georgiana Maria Popa, Andreea Liliana Cocosila, and et al. 2024. "Mechanical and Computational Fluid Dynamic Models for Magnesium-Based Implants" Materials 17, no. 4: 830. https://doi.org/10.3390/ma17040830
APA StyleManescu, V., Paltanea, G., Antoniac, A., Gruionu, L. G., Robu, A., Vasilescu, M., Laptoiu, S. A., Bita, A. I., Popa, G. M., Cocosila, A. L., Silviu, V., & Porumb, A. (2024). Mechanical and Computational Fluid Dynamic Models for Magnesium-Based Implants. Materials, 17(4), 830. https://doi.org/10.3390/ma17040830



.png)






