Zinc Ferrite Nanoparticle Coatings on Austenitic Alloy Steel
Abstract
:1. Introduction
2. Materials and Methods
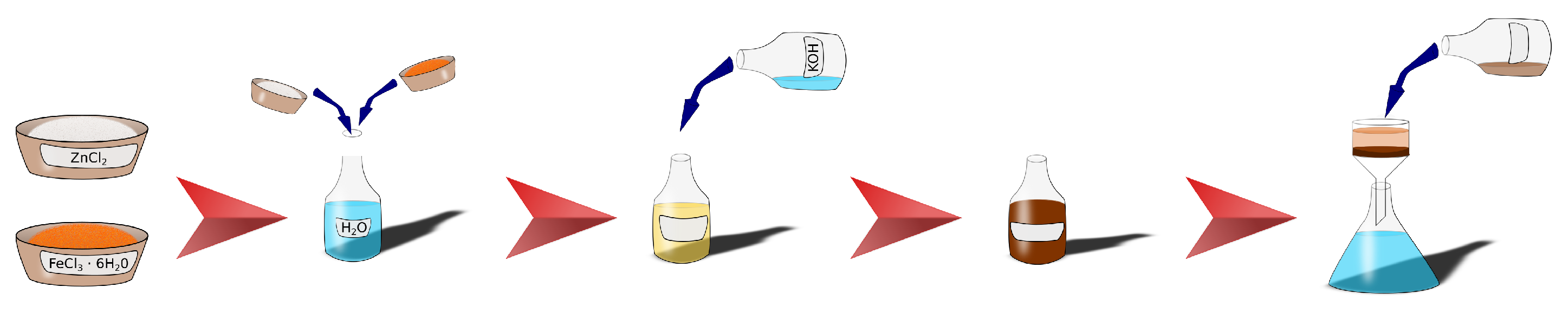
2.1. Zinc Ferrite Nanoparticles
2.2. Austenitic Stainless Steel CL20ES
2.3. CL20ES and ZnFe2O4 Mixture
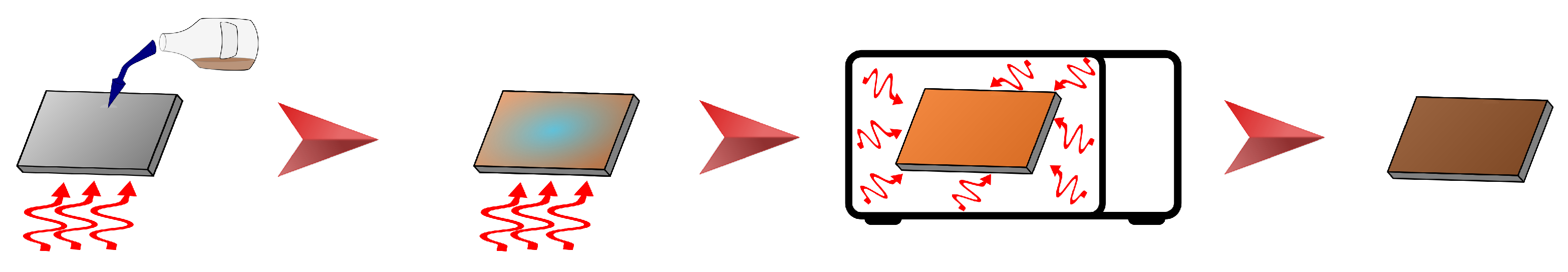
2.4. Fabrication of ZnFe2O4 Covering Layer
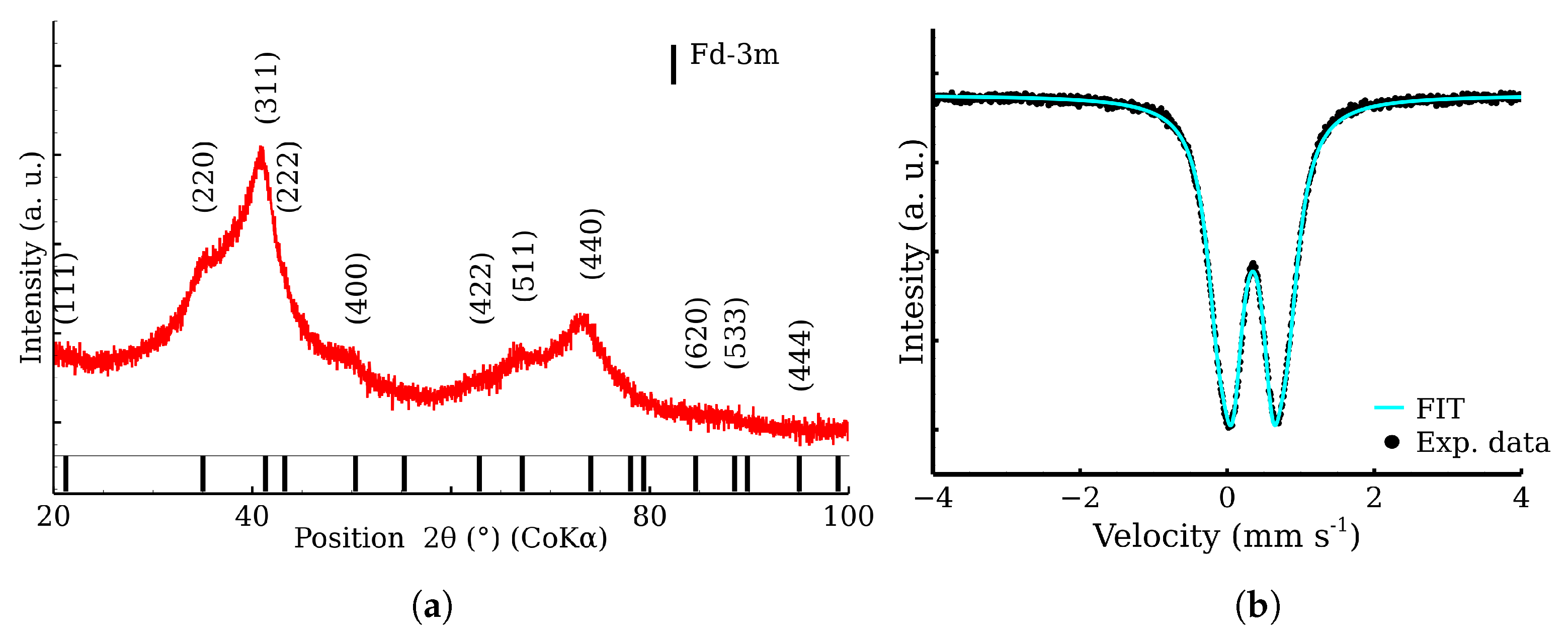
2.5. X-ray Powder Diffraction
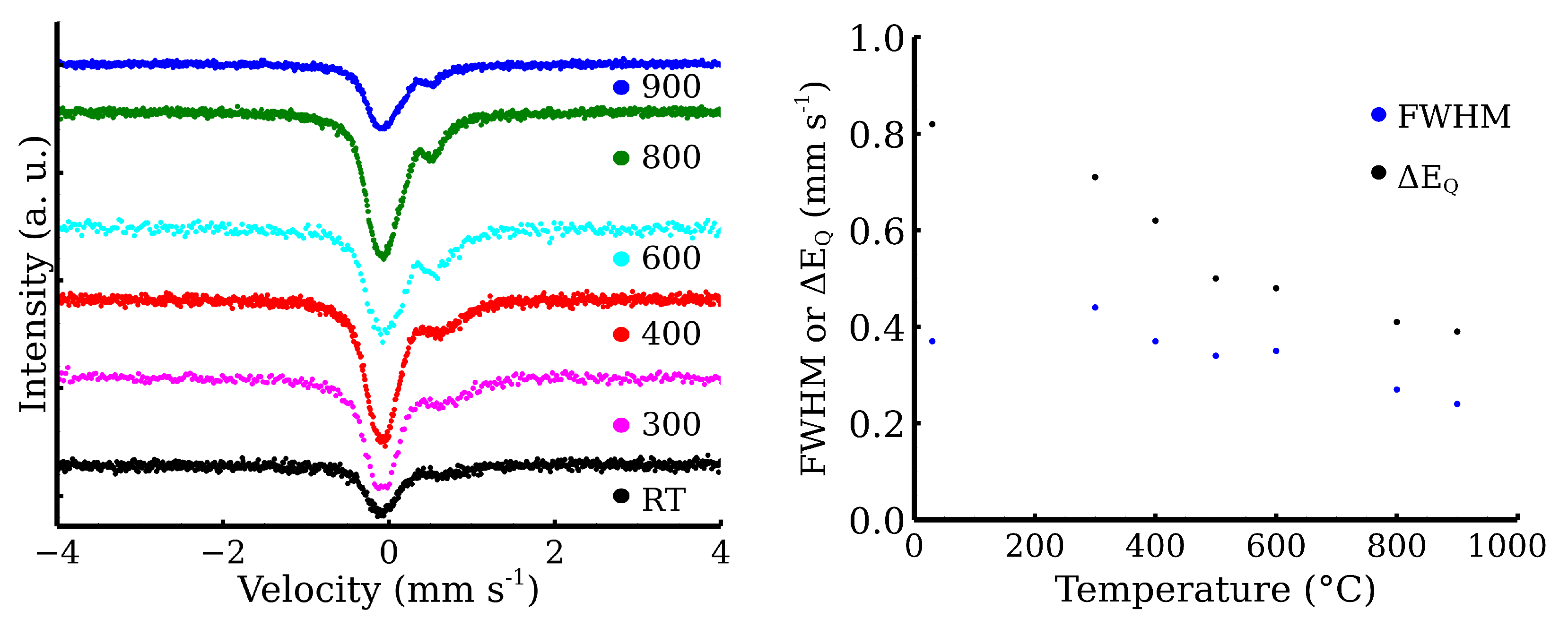
2.6. 57Fe Mössbauer Spectroscopy
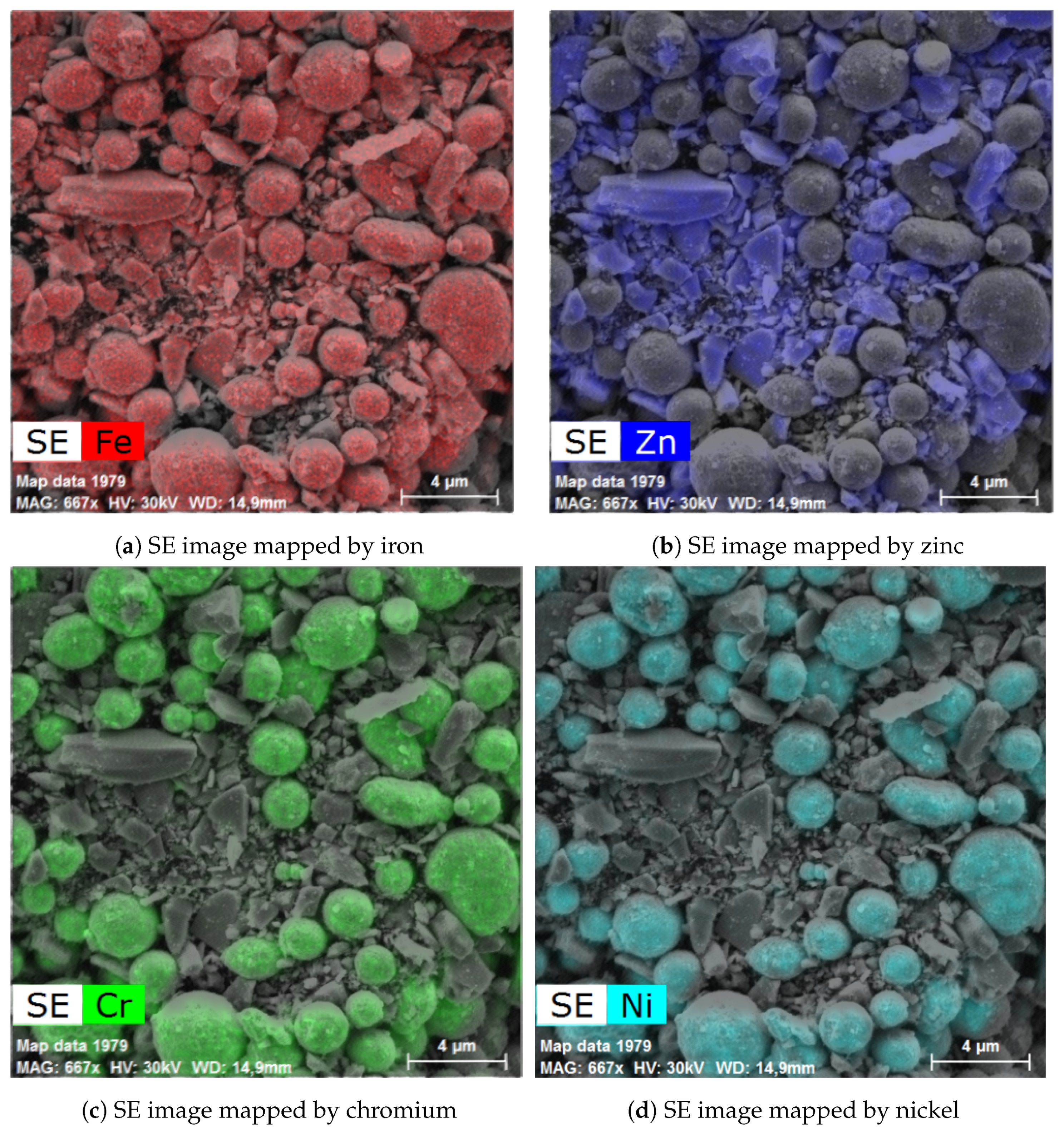
2.7. Scanning Electron Microscopy
2.8. Ultrasonic Hardness Tester
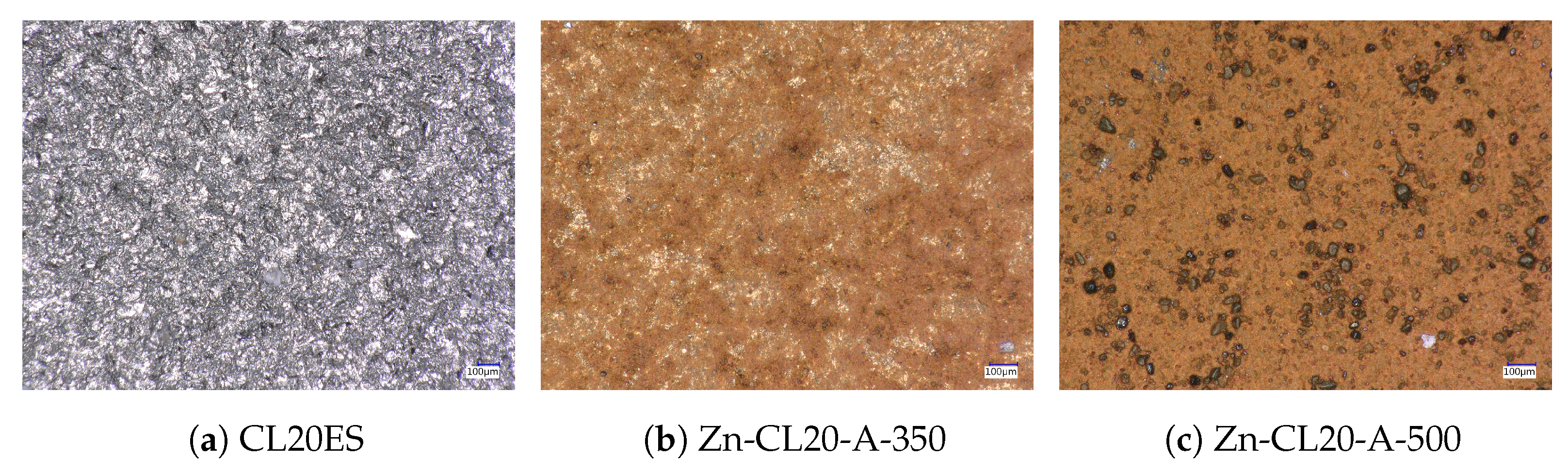
2.9. Digital Optical Microscope
3. Results
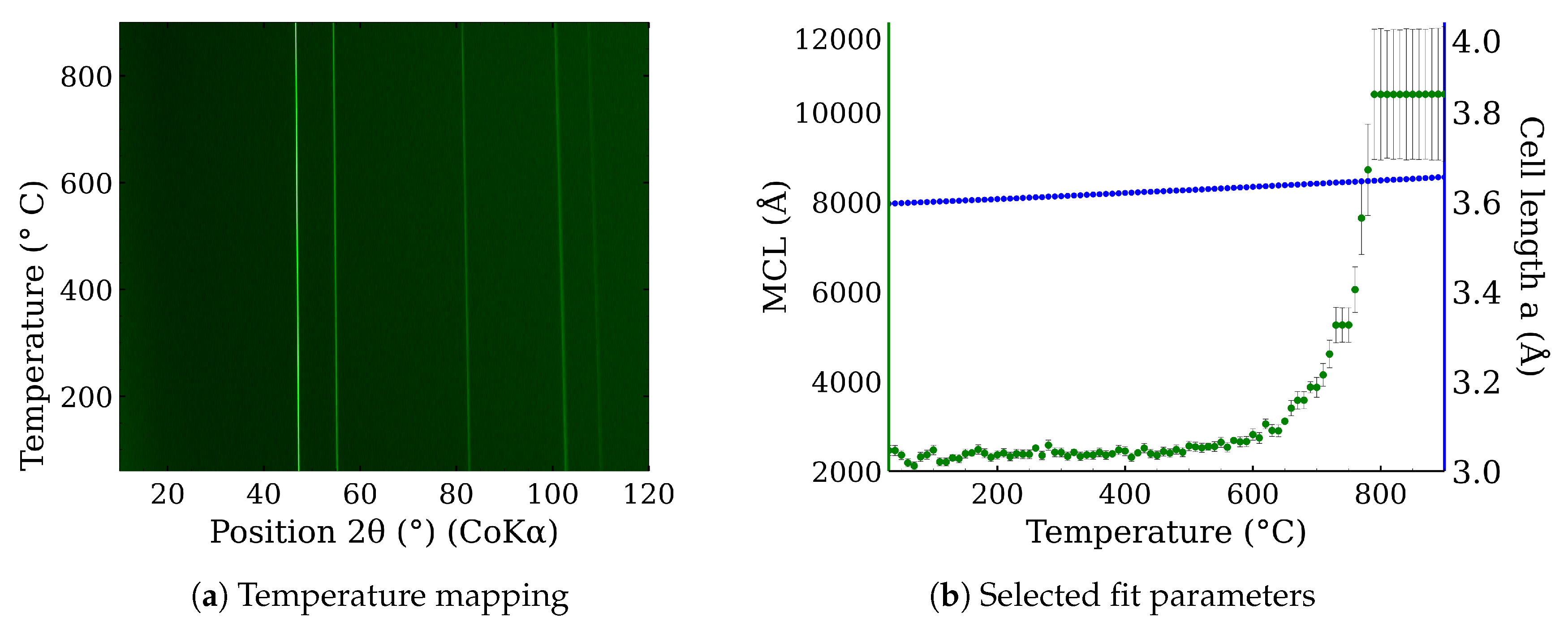
3.1. The High-Temperature Annealing of CL20ES, ZnFe2O4 Powders, and Their Mixture
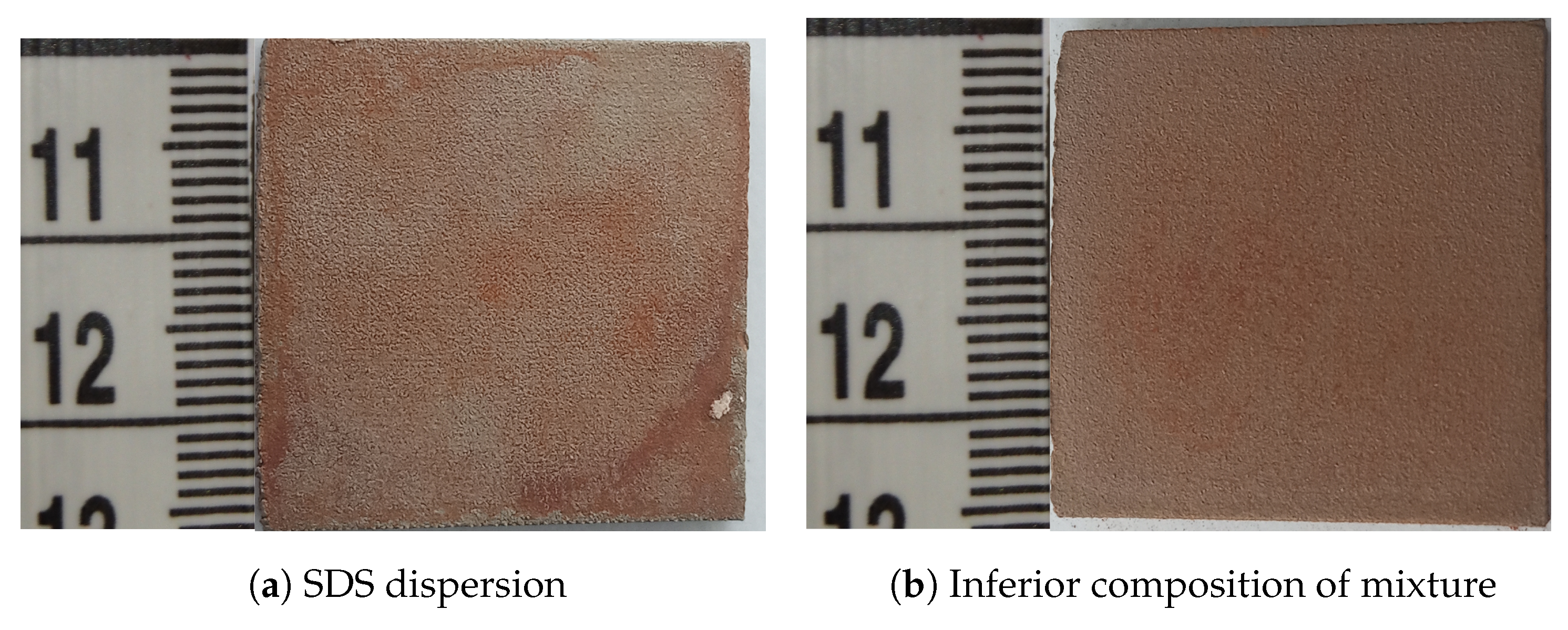
3.2. ZnFe2O4 Layers on CL2OES 3D-Printed Parts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasegawa, S.; Kim, S.-Y.; Ebina, T.; Tokuda, H.; Ito, T.; Nagano, N.; Hitomi, K.; Ishii, K. Effect of Nitrate on Corrosion of Austenitic Stainless Steel in Boiling Nitric Acid Solution Containing Chromium Ions. J. Nucl. Sci. Technol. 2016, 53, 1332–1341. [Google Scholar] [CrossRef]
- Samusawa, I.; Shiotani, K. Influence and Role of Ethanol Minor Constituents of Fuel Grade Ethanol on Corrosion Behavior of Carbon Steel. Corros. Sci. 2015, 90, 266–275. [Google Scholar] [CrossRef]
- Bystrov, S.G.; Reshetnikov, S.M.; Kolotov, A.A.; Drozdov, A.Y.; Bayankin, V.Y. Effect of Oxygen Ion Implantation on Physicochemical Structure and Corrosion-Electrochemical Behavior of High-Chromium Steel. Inorg. Mater. Appl. Res. 2021, 12, 625–632. [Google Scholar] [CrossRef]
- Santambrogio, M.; Perrucci, G.; Trueba, M.; Trasatti, S.P.; Casaletto, M.P. Effect of Major Degradation Products of Ethylene Glycol Aqueous Solutions on Steel Corrosion. Electrochim. Acta 2016, 203, 439–450. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P. Corrosion-Resistant High-Entropy Alloys: A Review. J. Met. 2017, 7, 43. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.; Gao, K. Corrosion of Low Alloy Steel and Stainless Steel in Supercritical CO2/H2O/H2S Systems. Corros. Sci. 2016, 111, 637–648. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J. Advanced Lightweight Materials for Automobiles: A Review. Mater. Des. 2022, 221, 110994. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Huang, S.; An, H.; Santagata, R.; Ulgiati, S. Environmental and Economic-Related Impact Assessment of Iron and Steel Production. A Call for Shared Responsibility in Global Trade. J. Clean. Prod. 2020, 269, 122239. [Google Scholar] [CrossRef]
- Dennis, J.K.; Such, T.E. Control and Purification of Nickel Electroplating Solutions. In Nickel and Chromium Plating; Elsevier: Amsterdam, The Netherlands, 1993; pp. 132–161. [Google Scholar]
- Linderhof, F.; Mashlan, M.; Doláková, H.; Ingr, T.; Ivanova, T. Surface Micromorphology and Structure of Stainless and Maraging Steel Obtained via Selective Laser Melting: A Mössbauer Spectroscopy Study. J. Met. 2021, 11, 1028. [Google Scholar] [CrossRef]
- Ivanova, T.; Kořenek, M.; Mashlan, M.; Svačinová, V. Mössbauer Study of Thermal Behavior of CL20ES and CL50WS Steel Powders Used in Selective Laser Melting. Chem. Pap. 2023, 77, 7289–7302. [Google Scholar] [CrossRef]
- Zhong, J.-Y.; Sun, J.-Y.; Liu, D.-B.; Li, X.-G.; Liu, T.-Q. Effects of Chromium on the Corrosion and Electrochemical Behaviors of Ultra High Strength Steels. Int. J. Miner. Metall. Mater. 2010, 17, 282–289. [Google Scholar] [CrossRef]
- Kashima, K.; Sugae, K.; Kamimura, T.; Miyuki, H.; Kudo, T. Effect of Chromium Contents on Atmospheric Corrosion of Steel in Chloride Environment. J. Jpn. Inst. Met. 2013, 77, 107–113. [Google Scholar] [CrossRef]
- Wint, N.; de Vooys, A.C.A.; McMurray, H.N. The Corrosion of Chromium Based Coatings for Packaging Steel. Electrochim. Acta 2016, 203, 326–336. [Google Scholar] [CrossRef]
- Kamimura, T.; Stratmann, M. The Influence of Chromium on the Atmospheric Corrosion of Steel. Corros. Sci. 2001, 43, 429–447. [Google Scholar] [CrossRef]
- Rezaee, N.; Attar, M.M.; Ramezanzadeh, B. Studying Corrosion Performance, Microstructure and Adhesion Properties of a Room Temperature Zinc Phosphate Conversion Coating Containing Mn2+ on Mild Steel. Surf. Coat. Technol. 2013, 236, 361–367. [Google Scholar] [CrossRef]
- Maurice, V.; Marcus, P. Current Developments of Nanoscale Insight into Corrosion Protection by Passive Oxide Films. Curr. Opin. Solid State Mater. Sci. 2018, 22, 156–167. [Google Scholar] [CrossRef]
- Jiang, C.; Gao, Z.; Pan, H.; Cheng, X. The Initiation and Formation of a Double-Layer Phosphate Conversion Coating on Steel. Electrochem. Commun. 2020, 114, 106676. [Google Scholar] [CrossRef]
- Ujiro, T.; Yoshioka, K.; Staehle, R.W. Differences in Corrosion Behavior of Ferritic and Austenitic Stainless Steels. Corrosion 1994, 50, 953–962. [Google Scholar] [CrossRef]
- Klapper, H.S.; Burkert, A.; Burkert, A.; Lehmann, J.; Villalba, A.L. Influence of Surface Treatments on the Pitting Corrosion of Type 304 Stainless Steel by Electrochemical Noise Measurements. Corrosion 2011, 67, 075004-1–075004-13. [Google Scholar] [CrossRef]
- Hoshino, K.; Furuya, S.; Buchheit, R.G. Effect of Solution Ph on Layered Double Hydroxide Formation on Electrogalvanized Steel Sheets. J. Mater. Eng. Perform. 2019, 28, 2237–2244. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Vakili, H.; Amini, R. The Effects of Addition of Poly(Vinyl) Alcohol (PVA) as a Green Corrosion Inhibitor to the Phosphate Conversion Coating on the Anticorrosion and Adhesion Properties of the Epoxy Coating on the Steel Substrate. Appl. Surf. Sci. 2015, 327, 174–181. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Guan, H.; Mahajanam, S.; Wong, F. Active Corrosion Protection and Corrosion Sensing in Chromate-Free Organic Coatings. Prog. Org. Coat. 2003, 47, 174–182. [Google Scholar] [CrossRef]
- Winn, D.; Dalton, W. Chromium-Free Corrosion Solutions. Met. Finish. 2008, 106, 70–74. [Google Scholar] [CrossRef]
- Jing, C.; Dong, B.; Raza, A.; Zhang, T.; Zhang, Y. Corrosion Inhibition of Layered Double Hydroxides for Metal-Based Systems. Nano Mater. Sci. 2021, 3, 47–67. [Google Scholar] [CrossRef]
- Li, X.; Sun, W.; Zheng, Y.; Long, C.; Wang, Q. New Strategy for the Design of Anti-Corrosion Coatings in Bipolar Plates Based on Hybrid Organic–Inorganic Layers. Molecules 2023, 28, 3279. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Tran, B.A.; Vu, K.O.; Nguyen, A.S.; Trinh, A.T.; Pham, G.V.; To, T.X.; Phan, M.V.; Phan, T.T. Corrosion Protection of Carbon Steel Using Hydrotalcite/Graphene Oxide Nanohybrid. J. Coat. Technol. Res. 2018, 16, 585–595. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Gnedenkov, S.V.; Alpysbaeva, D.A.; Egorkin, V.S.; Emelyanenko, A.M.; Sinebryukhov, S.L.; Zaretskaya, A.K. Corrosion Resistance of Composite Coatings on Low-Carbon Steel Containing Hydrophobic and Superhydrophobic Layers in Combination with Oxide Sublayers. Corros. Sci. 2012, 55, 238–245. [Google Scholar] [CrossRef]
- Imran Din, M.; Rafique, F.; Hussain, M.S.; Arslan Mehmood, H.; Waseem, S. Recent Developments in the Synthesis and Stability of Metal Ferrite Nanoparticles. Sci. Prog. 2019, 102, 61–72. [Google Scholar] [CrossRef]
- Hasirci, C.; Karaagac, O.; Köçkar, H. Superparamagnetic Zinc Ferrite: A Correlation between High Magnetizations and Nanoparticle Sizes as a Function of Reaction Time via Hydrothermal Process. J. Magn. Magn. Mater. 2019, 474, 282–286. [Google Scholar] [CrossRef]
- Ochmann, M.; Vrba, V.; Kopp, J.; Ingr, T.; Malina, O.; Machala, L. Microwave-Enhanced Crystalline Properties of Zinc Ferrite Nanoparticles. J. Nanomater. 2022, 12, 2987. [Google Scholar] [CrossRef]
- Ochmann, M.; Linderhof, F.M.; Machala, L. Spinel Ferrites Nanoparticles for Alloy Steel Protective Layers. In Proceedings of the 12th International Conference on Nanomaterials-Research & Application, Brno, Czech Republic, 21–23 October 2020. [Google Scholar]
- Pitrmuc, Z.; Šimota, J.; Beránek, L.; Mikeš, P.; Andronov, V.; Sommer, J.; Holešovský, F. Mechanical and Microstructural Anisotropy of Laser Powder Bed Fusion 316L Stainless Steel. Materials 2022, 15, 551. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, D.; Li, X.; Jiang, S.; Zhang, Q. Current Status, Opportunities and Challenges in Chemical Conversion Coatings for Zinc. Colloids Surf. 2018, 546, 221–236. [Google Scholar] [CrossRef]
- Saei, E.; Ramezanzadeh, B.; Amini, R.; Kalajahi, M.S. Effects of Combined Organic and Inorganic Corrosion Inhibitors on the Nanostructure Cerium Based Conversion Coating Performance on az31 Magnesium Alloy: Morphological and Corrosion Studies. Corros. Sci. 2017, 127, 186–200. [Google Scholar] [CrossRef]
- Holzner, T.; Luckeneder, G.; Strauss, B.; Valtiner, M. Environmentally Friendly Layered Double Hydroxide Conversion Layers: Formation Kinetics on Zn-Al-Mg-Coated Steel. ACS Appl. Mater. Interfaces 2022, 14, 6109–6119. [Google Scholar] [CrossRef]
- Gražulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An Open-Access Collection of Crystal Structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Lutterotti, L. Total Pattern Fitting for the Combined Size–Strain–Stress–Texture Determination in Thin Film Diffraction. Nucl. Instrum. Methods Phys. Res. B 2010, 268, 334–340. [Google Scholar] [CrossRef]
- Stejskal, A.; Procházka, V.; Dudka, M.; Vrba, V.; Kočiščák, J.; Šretrová, P.; Novák, P. A Dual Mössbauer Spectrometer for Material Research, Coincidence Experiments and Nuclear Quantum Optics. Measurement 2023, 215, 112850. [Google Scholar] [CrossRef]
- Pechoušek, J.; Jančík, D.; Frydrych, J.; Navařík, J.; Novák, P. Setup of Mössbauer Spectrometers at RCPTM. AIP Conf. Proc. 2012, 1489, 186–193. [Google Scholar]
- Klencsár, Z. Mössbauer Spectrum Analysis by Evolution Algorithm. Nucl. Instrum. Methods Phys. Res. B 1997, 129, 527–533. [Google Scholar] [CrossRef]
- Standard Test Method for Portable Hardness Testing by the Ultrasonic Contact Impedance Method. Available online: https://www.astm.org/a1038-19.html (accessed on 9 February 2024).
- DIN 50159-1; Metallic Materials—Hardness Testing with the UCI Method—Part 1: Test Method. European Standards: Brussels, Belgium, 2022. Available online: https://www.en-standard.eu/din-50159-1-metallic-materials-hardness-testing-with-the-uci-method-part-1-test-method/ (accessed on 9 February 2024).







| Element | Cr | Ni | Mo | Mn | Si | P | C, S |
|---|---|---|---|---|---|---|---|
| Min | |||||||
| Max |
| Sample | FWHM | RA | Component | ||
|---|---|---|---|---|---|
| ±0.02 | ±0.02 | ±0.02 | ±3 | ||
| (mm s−1) | (mm s−1) | (mm s−1) | (%) | ||
| Zn-CL20-RT | 0.34 | 0.82 | 0.45 | 100 | Fe3+ |
| Zn-CL20-300 | 0.34 * | 0.71 | 0.44 | 29 | Fe3+ |
| −0.10 | — | 0.44 | 71 | γ-Fe | |
| Zn-CL20-400 | 0.34 * | 0.61 | 0.33 | 26 | Fe3+ |
| −0.11 | — | 0.41 | 74 | γ-Fe | |
| Zn-CL20-500 | 0.34 * | 0.50 | 0.34 | 34 | Fe3+ |
| −0.10 | — | 0.39 | 66 | γ-Fe | |
| Zn-CL20-600 | 0.34 * | 0.48 | 0.35 | 41 | Fe3+ |
| −0.11 | — | 0.41 | 59 | γ-Fe | |
| Zn-CL20-800 | 0.34 * | 0.41 | 0.27 | 24 | Fe3+ |
| −0.10 | — | 0.40 | 76 | γ-Fe | |
| Zn-CL20-900 | 0.34 * | 0.39 | 0.24 | 22 | Fe3+ |
| −0.09 | — | 0.40 | 78 | γ-Fe |
| Sample | FWHM | RA | Component | ||
|---|---|---|---|---|---|
| (mm s−1) | (mm s−1) | (mm s−1) | (%) | ||
| CL20ES | −0.10 | 0.15 | 0.29 | 100 | γ-Fe |
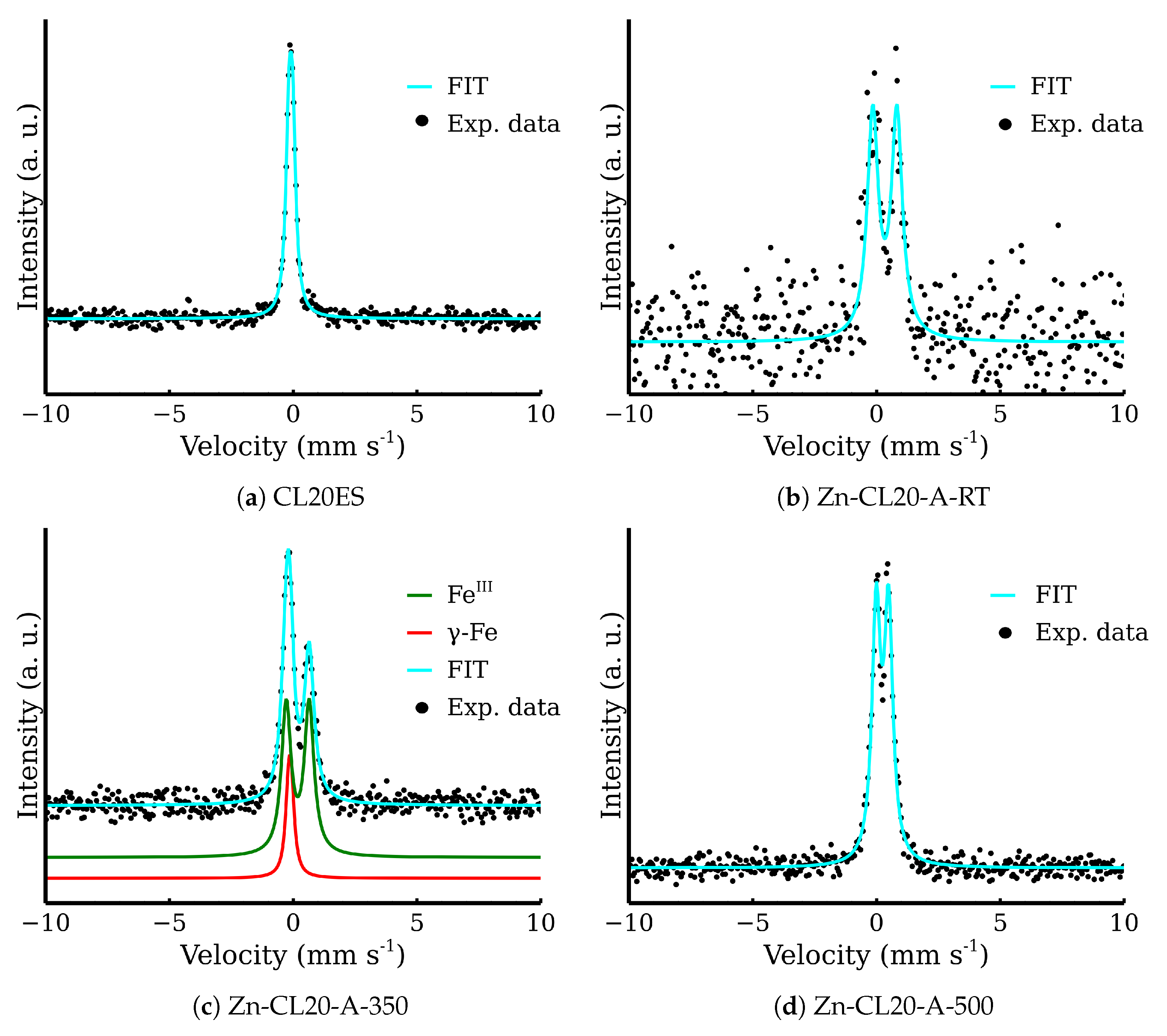
| Zn-CL20-A-RT | 0.34 | 0.97 | 0.53 | 100 | Fe3+ |
| Zn-CL20-A-350 | −0.10 | 0.09 | 0.30 | 18 | γ-Fe |
| 0.18 | 0.93 | 0.49 | 82 | Fe3+ | |
| Zn-CL20-A-500 | 0.24 | 0.50 | 0.41 | 100 | Fe3+ |
| Sample | Hardness | Improvement |
|---|---|---|
| (HRC) | (%) | |
| CL20ES | 42 | — |
| Zn-CL20-A-350 | 47 | 12 |
| Zn-CL20-A-500 | 52 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochmann, M.; Machala, L.; Mašláň, M.; Heger, V.; Krátký, T. Zinc Ferrite Nanoparticle Coatings on Austenitic Alloy Steel. Materials 2024, 17, 857. https://doi.org/10.3390/ma17040857
Ochmann M, Machala L, Mašláň M, Heger V, Krátký T. Zinc Ferrite Nanoparticle Coatings on Austenitic Alloy Steel. Materials. 2024; 17(4):857. https://doi.org/10.3390/ma17040857
Chicago/Turabian StyleOchmann, Martin, Libor Machala, Miroslav Mašláň, Vítězslav Heger, and Tomáš Krátký. 2024. "Zinc Ferrite Nanoparticle Coatings on Austenitic Alloy Steel" Materials 17, no. 4: 857. https://doi.org/10.3390/ma17040857





