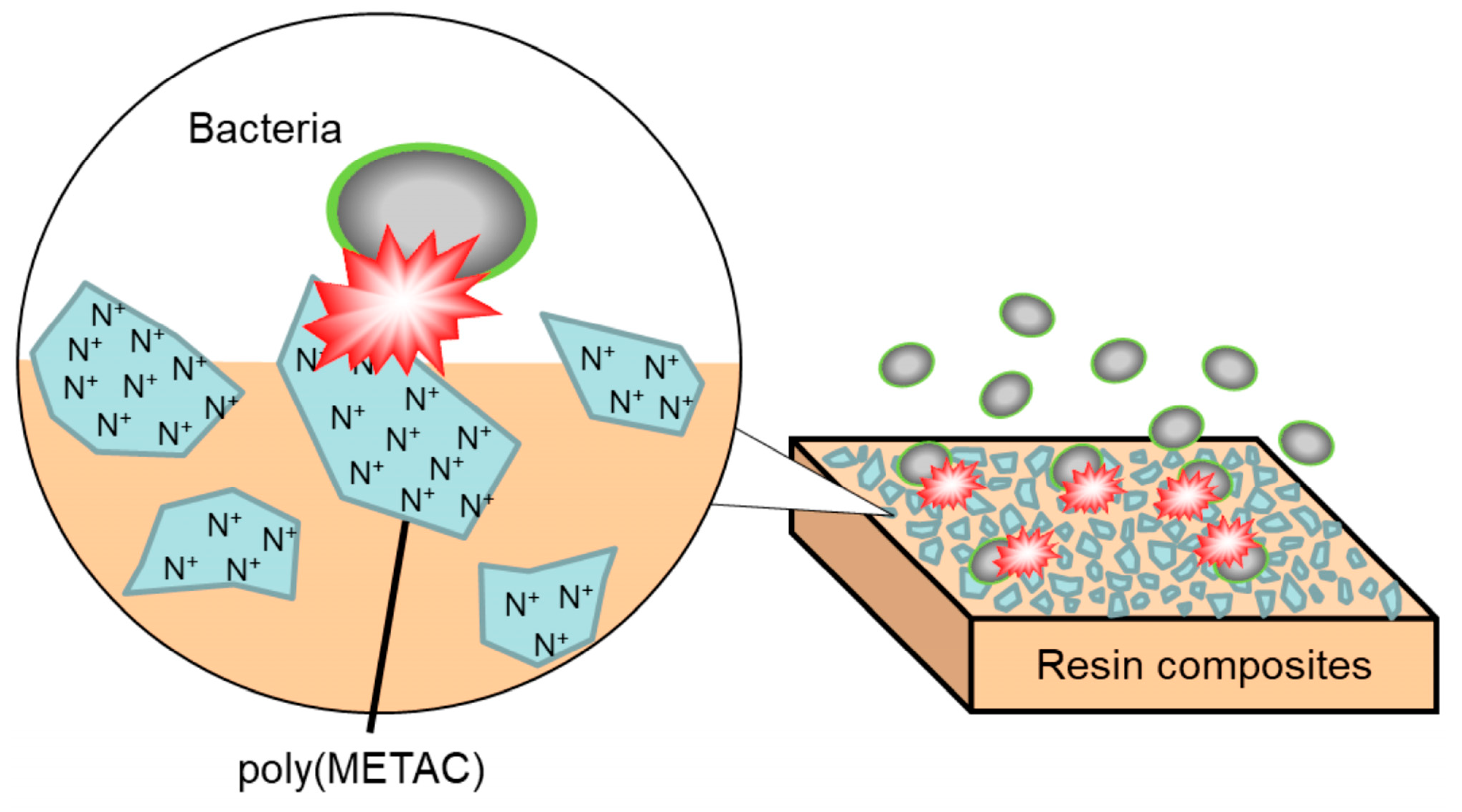
Development of Antibacterial Resin Composites Incorporating Poly(METAC) Clusters
Abstract
:1. Introduction
2. Methods
2.1. Fabrication and Characterization of Resin Composites Incorporating Poly(METAC)
2.2. Antibacterial Activity of Resin Composites Incorporating Poly(METAC)
2.3. Physical Properties of Resin Composites Incorporating Poly(METAC)
2.4. Statistical Analysis
3. Results
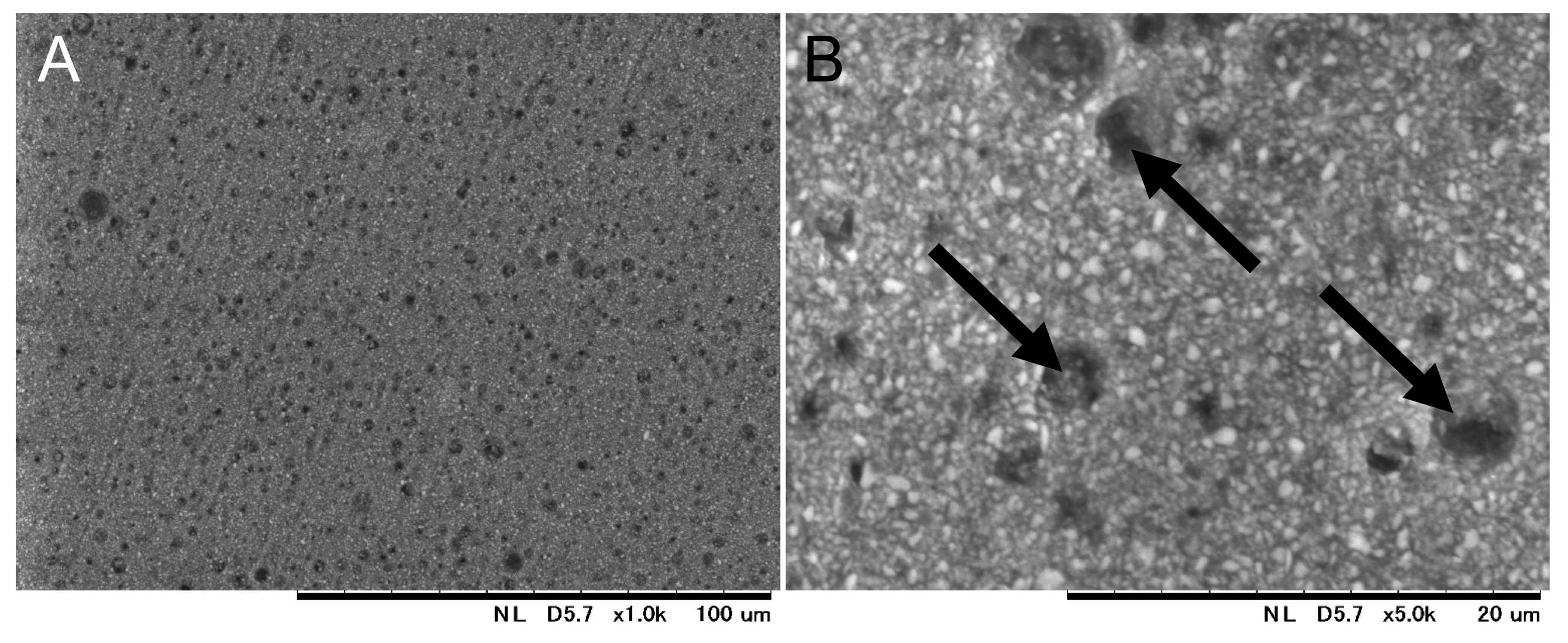
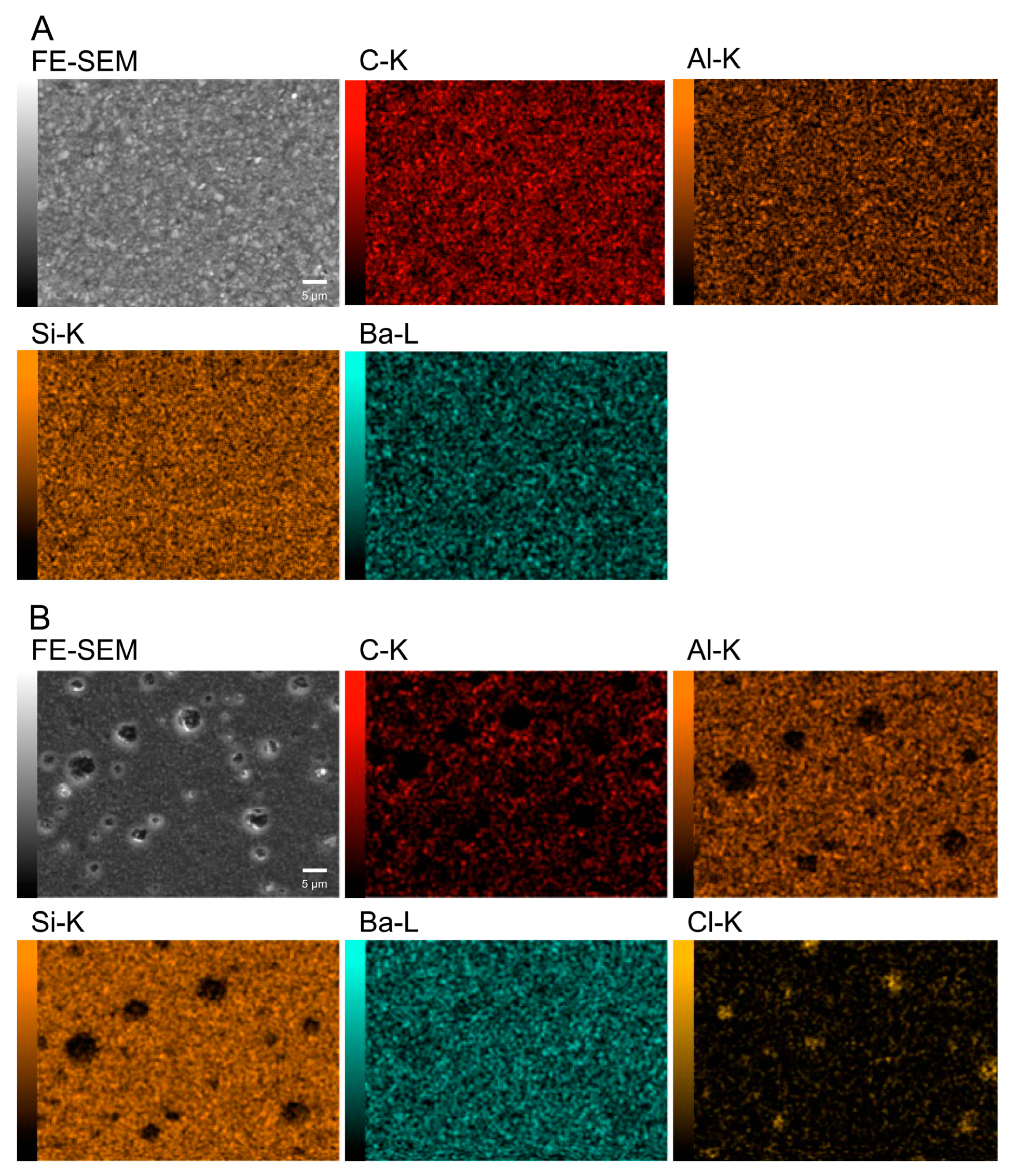
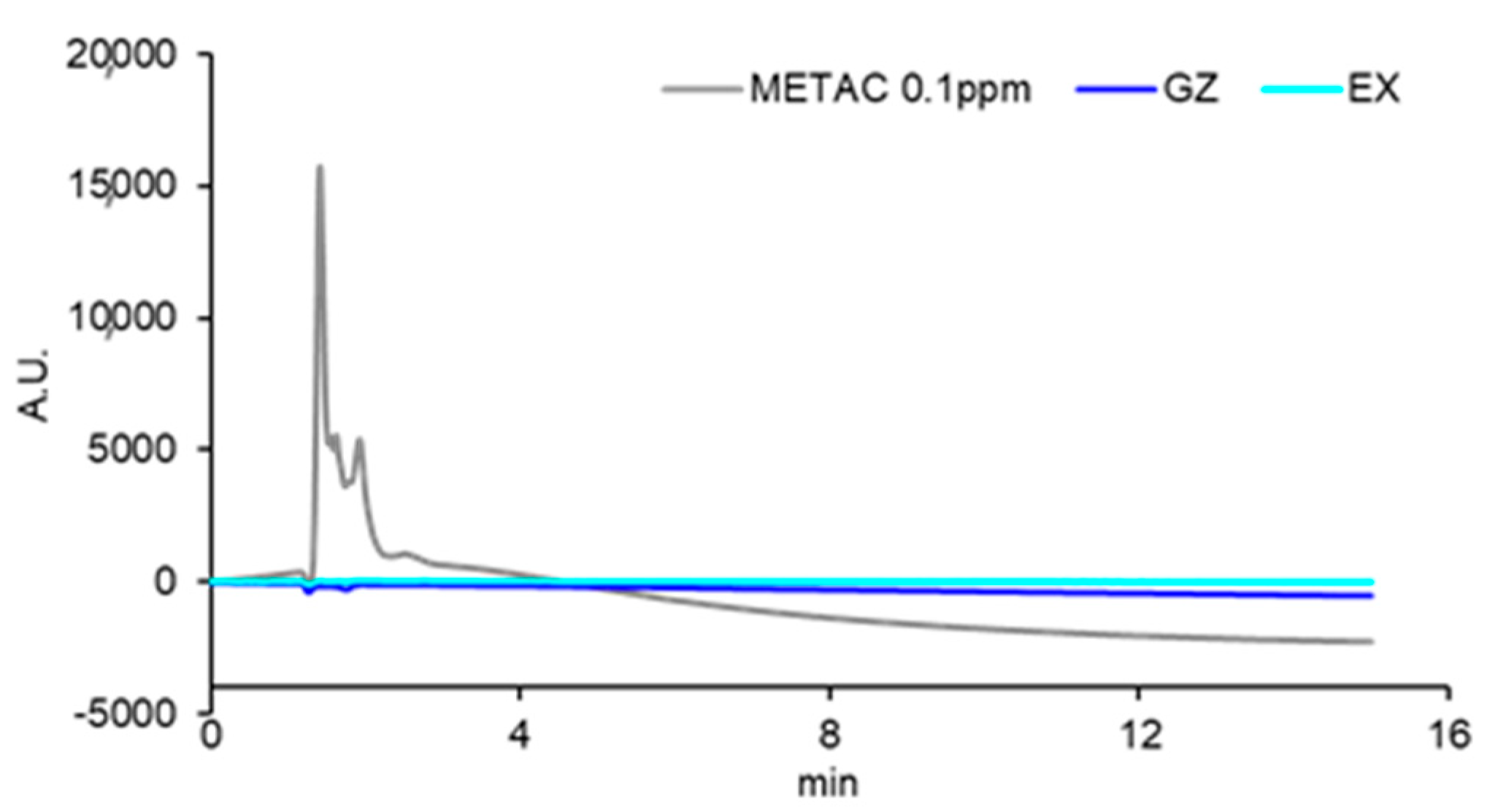
3.1. Fabrication and Characterization of Resin Composites Incorporating Poly(METAC)
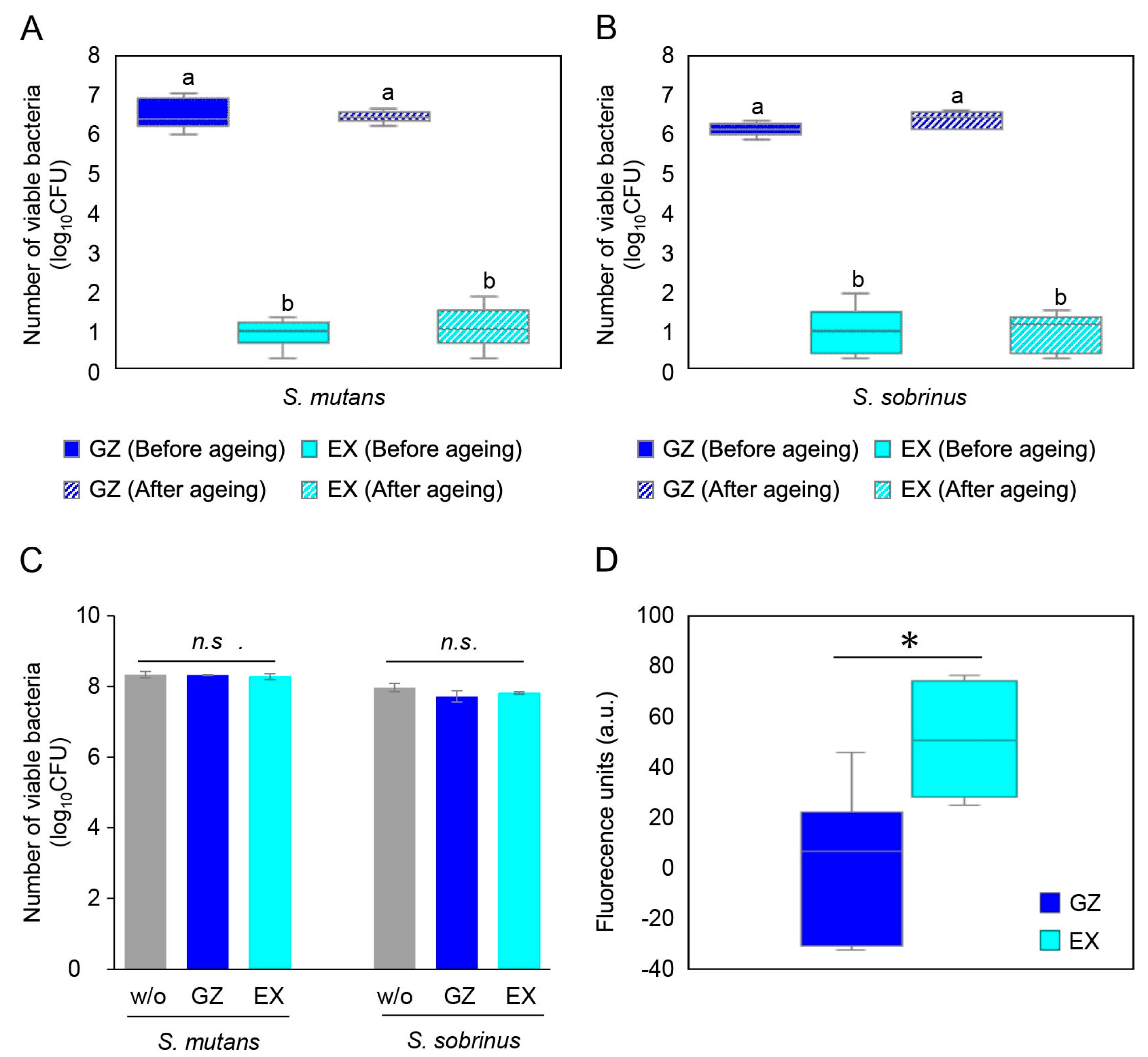
3.2. Antibacterial Activity of Resin Composites Incorporating Poly(METAC)
3.3. Physical Properties of Resin Composites Incorporating Poly(METAC)
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Imazato, S.; Kitagawa, H. Dental Resin-Based Materials with Antibacterial Properties: Contact Inhibition and Controlled Release. In Oral Biofilms and Modern Dental Materials. Advances Toward Bioactivity; Ionescu, A.C., Hahnel, S., Eds.; Springer: Cham, Switzerland, 2021; pp. 127–140. [Google Scholar]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S. Antibacterial properties of resin composites and dentin bonding systems. Dent. Mater. 2003, 19, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Ebi, N.; Imazato, S.; Noiri, Y.; Ebisu, S. Inhibitory effects of resin composite containing bactericide-immobilized filler on plaque accumulation. Dent. Mater. 2001, 17, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Yudovin-Farber, I.; Perez-Davidi, M.; Domb, A.J.; Weiss, E.I. Polyethyleneimine nanoparticles incorporated into resin composite cause cell death and trigger biofilm stress in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 22038–22043. [Google Scholar] [CrossRef]
- Thongthai, P.; Kitagawa, H.; Iwasaki, Y.; Noree, S.; Kitagawa, R.; Imazato, S. Immobilizing bactericides on dental resins via electron beam irradiation. J. Dent. Res. 2021, 100, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Shiga, T.; Mori, H.; Uemura, K.; Moriuchi, R.; Dohra, H.; Yamawaki-Ogata, A.; Narita, Y.; Saito, A.; Kotsuchibashi, Y. Evaluation of the bactericidal and fungicidal activities of poly([2-(methacryloyloxy)ethyl ]trimethyl ammonium chloride)(poly (METAC))-based materials. Polymers 2018, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Collares, F.M.; Leitune, V.C.B.; Franken, P.; Parollo, C.F.; Ogliari, F.A.; Samuel, S.M.W. Influence of addition of [2-(methacryloyloxy)ethyl]trimethylammonium chloride to an experimental adhesive. Braz. Oral. Res. 2017, 31, e31. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Rodrigues, S.B.; de Souza Balbinot, G.; Visioli, F.; Leitune, V.C.B.; Collares, F.M. Quaternary ammonium compound as antimicrobial agent in resin-based sealants. Clin. Oral. Investig. 2020, 24, 777–784. [Google Scholar] [CrossRef]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef]
- ISO 4049:2019; Dentistry—Polymer-Based Restorative Materials. International Organization for Standardisation: Geneva, Switzerland, 2019.
- Melo, M.A.S.; Garcia, I.M.; Mokeem, L.; Weir, M.D.; Xu, H.H.K.; Montoya, C.; Orrego, S. Developing bioactive dental resins for restorative dentistry. J. Dent. Res. 2023, 102, 1180–1190. [Google Scholar] [CrossRef]
- Xiao, Y.H.; Ma, S.; Chen, J.H.; Chai, Z.G.; Li, F.; Wang, Y.J. Antibacterial activity and bonding ability of an adhesive incorporating an antibacterial monomer DMAE-CB. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Niu, L.N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, J.M.; Zeiger, D.N.; Tang, K.; Lin-Gibson, S.; Fowler, B.O.; Lin, N.J. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent. Mater. 2012, 28, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liang, J.; Huang, X.; Weir, M.D.; Masri, R.; Oates, T.W.; Xu, H.H.K.; Cheng, L. Novel antibacterial titanium implant healing abutment with dimethylaminohexadecyl methacrylate to combat implant-related infections. Dent. Mater. 2024, 40, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Huang, Q.; Liu, F.; He, J.; Lin, Z. Synthesis of novel antibacterial monomers (UDMQA) and their potential application in dental resin. J. Appl. Polym. Sci. 2013, 129, 3373–3381. [Google Scholar] [CrossRef]
- Makvandi, P.; Ghaemy, M.; Mohseni, M. Synthesis and characterization of photo-curable bis-quaternary ammonium dimethacrylate with antimicrobial activity for dental restoration materials. Eur. Polym. J. 2016, 74, 81–90. [Google Scholar] [CrossRef]
- Farah, S.; Aviv, O.; Laout, N.; Ratner, S.; Beyth, N.; Domb, A.J. Quaternary ammonium polyethylenimine nanoparticles for treating bacterial contaminated water. Colloids Surf. B Biointerfaces 2015, 128, 614–619. [Google Scholar] [CrossRef]
- Yudovin-Farber, I.; Beyth, N.; Nyska, A.; Weiss, E.I.; Golenser, J.; Domb, A.J. Surface characterization and biocompatibility of restorative resin containing nanoparticles. Biomacromolecules 2008, 9, 3044–3050. [Google Scholar] [CrossRef]
- Shvero, D.K.; Davidi, M.P.; Weiss, E.I.; Srerer, N.; Beyth, N. Antibacterial effect of polyethyleneimine nanoparticles incorporated in provisional cements against Streptococcus mutans. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 367–371. [Google Scholar] [CrossRef]
- Kesler Shvero, D.K.; Abramovitz, I.; Zaltsman, N.; Perez Davidi, M.; Weiss, E.I.; Beyth, N. Towards antibacterial endodontic sealers using quaternary ammonium nanoparticles. Int. Endod. J. 2013, 46, 747–754. [Google Scholar] [CrossRef]
- Zaltsman, N.; Ionescu, A.C.; Weiss, E.I.; Brambilla, E.; Beyth, S.; Beyth, N. Surface-modified nanoparticles as anti-biofilm filler for dental polymers. PLoS ONE 2017, 12, e0189397. [Google Scholar] [CrossRef] [PubMed]
- Dekel-Steinkeller, M.; Weiss, E.I.; Samovici, T.L.; Abramovitz, I. Antibacterial performance of composite containing quaternary ammonium silica (QASi) filler—A preliminary study. J. Dent. 2022, 123, 104209. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhang, M.; He, J.; Ni, P. Synthesis of amphiphilic cationic copolymers poly[2-(methacryloyloxy)ethyl trimethylammonium chloride-co-stearyl methacrylate] and their self-assembly behavior in water and water-ethanol mixtures. J. Polym. Sci. A Polym. Chem. 2009, 47, 4670–4684. [Google Scholar] [CrossRef]
- Ye, G.; Lee, J.; Perreault, F.; Elimelech, M. Controlled architecture of dual-functional block copolymer brushes on thin-film composite membranes for integrated “Defending” and “Attacking” strategies against biofouling. ACS Appl. Mater. Interfaces 2015, 7, 23069–23079. [Google Scholar] [CrossRef]
- Imazato, S.; Imai, T.; Russell, R.R.; Torii, M.; Ebisu, S. Antibacterial activity of cured dental resin incorporating the antibacterial monomer MDPB and an adhesion-promoting monomer. J. Biomed. Mater. Res. 1998, 39, 511–515. [Google Scholar] [CrossRef]
- Yang, X.; Sha, D.; Xu, J.; Niu, N.; Shi, K.; Pan, Y.; Yu, C.; Wei, H.; Wang, B.; Ji, X. Preparation of cationic polyelectrolyte grafted polyvinyl alcohol-formaldehyde macroporous hydrogels and their antibacterial properties. New J. Chem. 2019, 43, 14961–14971. [Google Scholar] [CrossRef]
- Vilela, C.; Oliveira, H.; Almeida, A.; Silvestre, A.J.D.; Freire, C.S.R. Nanocellulose-based antifungal nanocomposites against the polymorphic fungus Candida albicans. Carbohydr. Polym. 2019, 217, 207–216. [Google Scholar] [CrossRef]
- Shapiro, H.M. Cell membrane potential analysis. Methods Cell Biol. 1990, 33, 25–35. [Google Scholar] [CrossRef]
- Trevors, J.T. Fluorescent probes for bacterial cytoplasmic membrane research. J. Biochem. Biophys. Methods 2003, 57, 87–103. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Mode of action of the antimicrobial peptide Mel4 is independent of Staphylococcus aureus cell membrane permeability. PLoS ONE 2019, 14, e0215703. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, G.B.; Alto, R.V.; Filho, H.R.; da Silva, E.M.; Fellows, C.E. Light transmission on dental resin composites. Dent. Mater. 2008, 24, 571–576. [Google Scholar] [CrossRef] [PubMed]



| Material | Code | Composition |
|---|---|---|
| GRACEFIL ZeroFlo (GC corporation) | GZ | Dimethacrylate, Silicon dioxide, Barium glass, Pigment, Photo initiator |
| Experimental resin composites | EX | GRACEFIL ZeloFlo + 6 wt.% METAC |
| Property | GZ | EX | ISO * range |
| Flexural strength (MPa) | 169 ± 3 | 91 ± 4 | ≥80 |
| Curing depth (mm) | 3.0 | 2.0 | ≥1.5 |
| Water absorption (μg/mm3) | 13.8 ± 0.2 | 26.4 ± 1.3 | ≤40 |
| Water dissolution (μg/mm3) | 0.0 ± 0.0 | 4.0 ± 0.5 | ≤7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohno, T.; Kitagawa, H.; Tsuboi, R.; Deng, F.; Sakai, H.; Wu, T.; Fan, Y.-S.; Xiao, L.; Imazato, S. Development of Antibacterial Resin Composites Incorporating Poly(METAC) Clusters. Materials 2024, 17, 896. https://doi.org/10.3390/ma17040896
Kohno T, Kitagawa H, Tsuboi R, Deng F, Sakai H, Wu T, Fan Y-S, Xiao L, Imazato S. Development of Antibacterial Resin Composites Incorporating Poly(METAC) Clusters. Materials. 2024; 17(4):896. https://doi.org/10.3390/ma17040896
Chicago/Turabian StyleKohno, Tomoki, Haruaki Kitagawa, Ririko Tsuboi, Fan Deng, Hirohiko Sakai, Tingyi Wu, Yo-Shiuan Fan, Linghao Xiao, and Satoshi Imazato. 2024. "Development of Antibacterial Resin Composites Incorporating Poly(METAC) Clusters" Materials 17, no. 4: 896. https://doi.org/10.3390/ma17040896
APA StyleKohno, T., Kitagawa, H., Tsuboi, R., Deng, F., Sakai, H., Wu, T., Fan, Y.-S., Xiao, L., & Imazato, S. (2024). Development of Antibacterial Resin Composites Incorporating Poly(METAC) Clusters. Materials, 17(4), 896. https://doi.org/10.3390/ma17040896






