Transformation and Detoxification of Typical Metallurgical Hazardous Waste into a Resource: A Review of the Development of Harmless Treatment and Utilization in China
Abstract
1. Introduction
2. Toxicity Detection Methods and Identification Standards of Hazardous Waste
2.1. Toxicity Detection Methods
2.2. The Identification Standards of Hazardous Waste
2.2.1. Identification Standards for Hazardous Wastes—Identification for Extraction Toxicity (GB 5058.3-2007)
2.2.2. Resource Conservation and Recovery Act 40 CFR 261.24
2.2.3. European Environment Agency Directive 91/689/EEC
3. State of the Art—Typical Ferrous Metallurgical Hazardous Waste
3.1. Formation of SSD and SSPS
3.2. Chemical Composition and Hazards of SSD and SSPS
3.2.1. Chemical Composition of SSD and SSPS
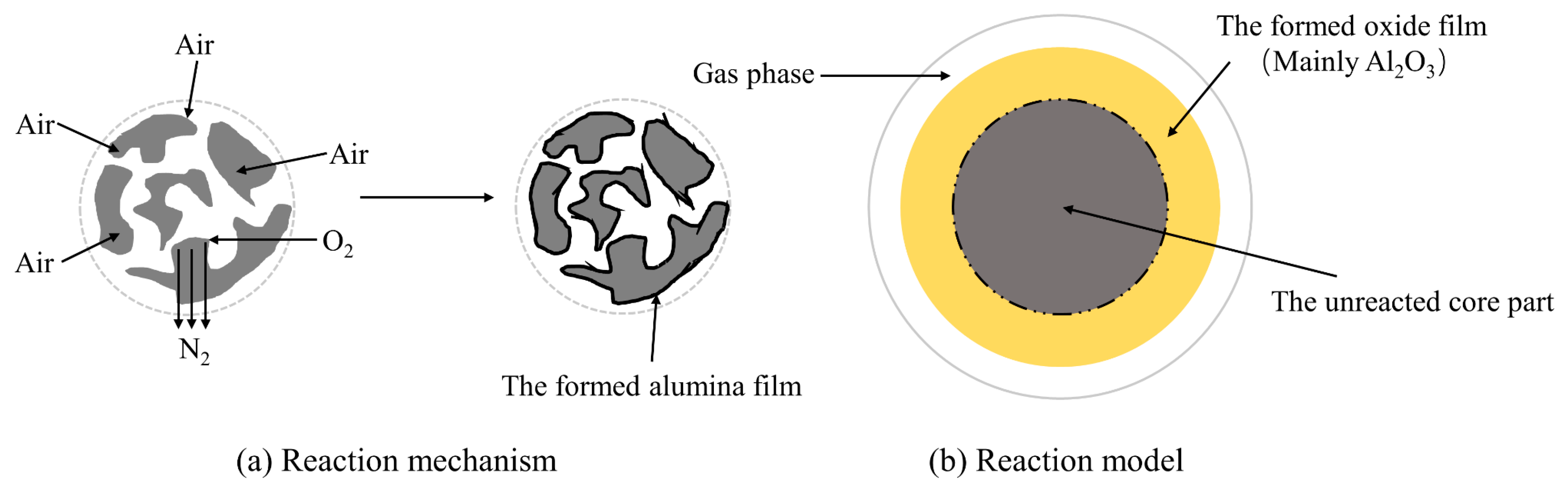
3.2.2. Hazards of SSD and SSPS
3.3. Toxicity Leaching of SSD and SSPS
3.3.1. Toxicity Extraction Test of SSD
3.3.2. Toxicity Extraction Test of SSPS
3.4. Harmless Treatment of SSD and SSPS
3.4.1. Solidification Process
3.4.2. Vitrification Process
3.5. Resource Utilization for SSD and SSPS
3.5.1. Recovery Treatment
- (1)
- Recovery of valuable metals from SSD
- (2)
- Recovery of valuable metals from SSPS
3.5.2. Preparation of Value-Added Materials
3.6. Development Trends of Harmless Treatment and Resource Utilization for SSD and SSPS
4. State of the Art—Typical Nonferrous Metallurgical Hazardous Waste
4.1. Formation of AA
4.2. Chemical Composition and Hazards of AA
- (1)
- Chemical composition of AA
- (2)
- Hazards of AA
4.3. Toxicity Extraction Test of AA
4.4. Harmless Treatment of AA
4.4.1. Removal of Aluminum Nitride
- (1)
- High-temperature roasting method
- (2)
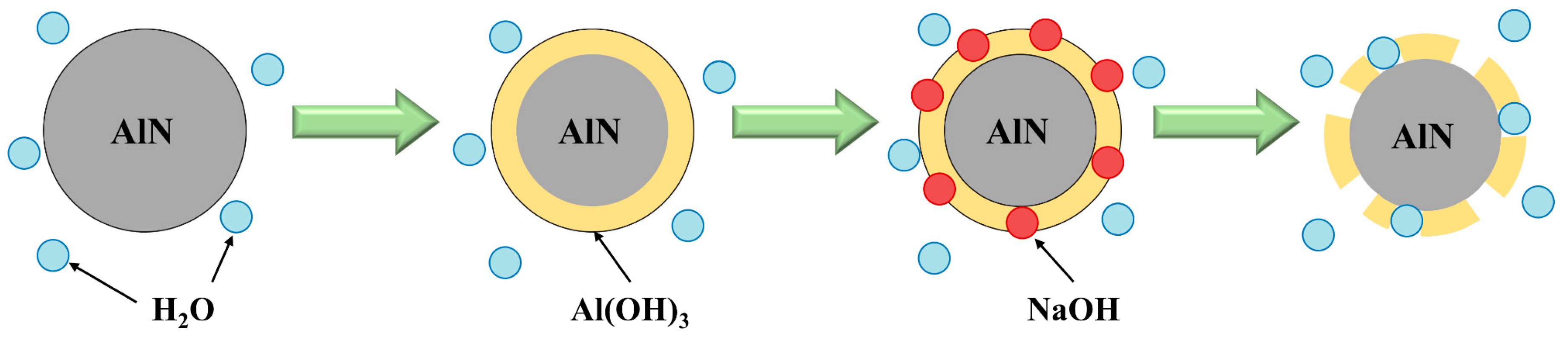
- Hydrolysis method
4.4.2. Removal of Fluoride and Chloride Salts
4.5. Research Status of Recovery Treatment and Resource Utilization of AA
4.5.1. Recovery Treatment of PAA
- (1)
- Salt-adding processes
- (2)
- Salt-free processes
4.5.2. Recovery Treatment of SAD
- (1)
- Acid leaching process
- (2)
- Alkali leaching process
4.5.3. Preparation of Value-Added Materials from SAD
- (1)
- Preparation of building materials
- (2)
- Preparation of refractory materials
- (3)
- Preparation of flocculants
4.6. Development Trends of Harmless Treatment and Resource Utilization for AA
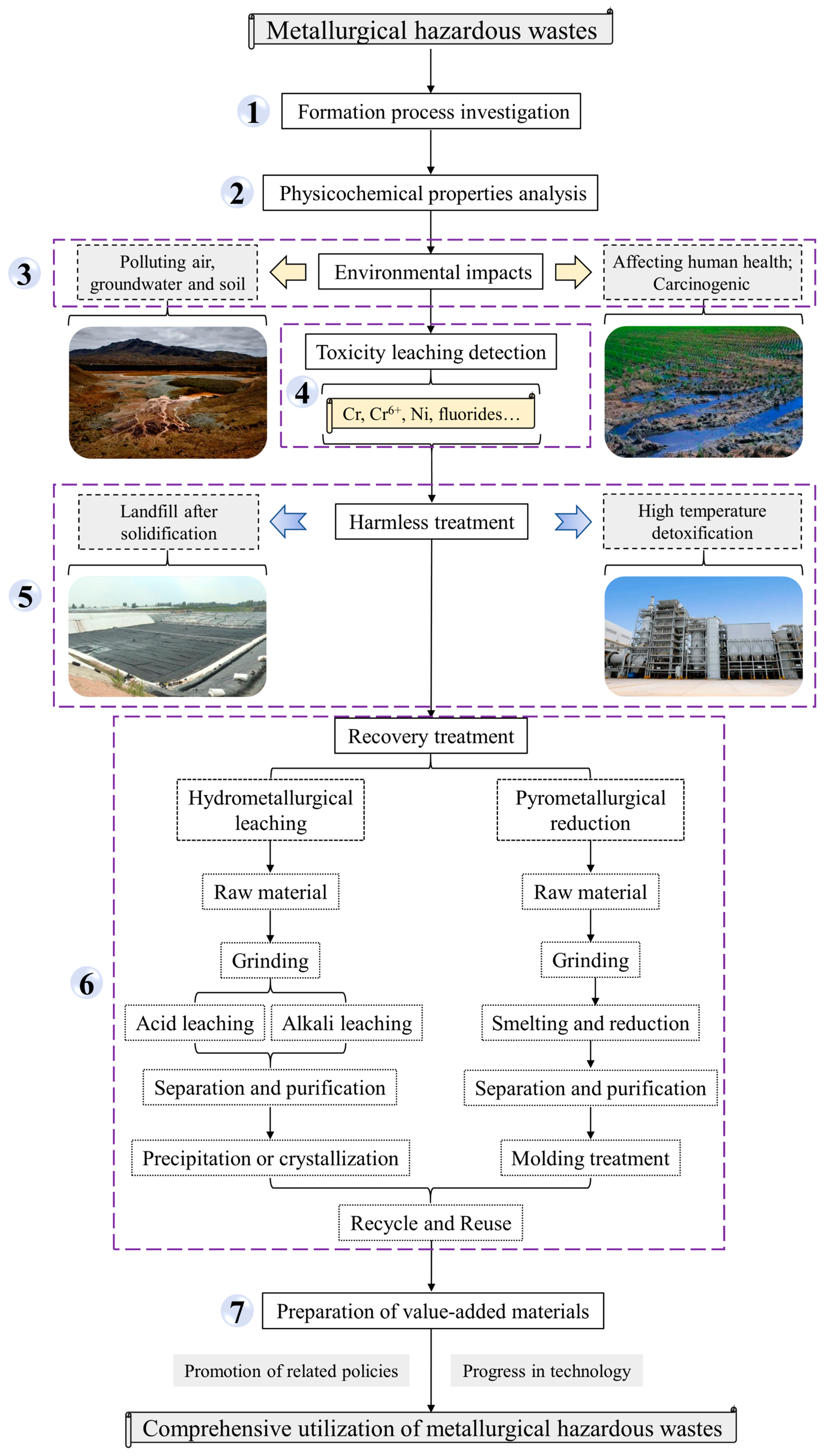
5. General Steps for the Comprehensive Utilization of Metallurgical Hazardous Wastes
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M. Present situation and development of utilization of solid metallurgical waste resources. World Nonferrous Met. 2019, 12, 14+16. [Google Scholar]
- Xi, C.; Zhang, C.H.; Liu, S.F. Research status of glass-ceramics prepared from metallurgical industry waste. Bull. Chin. Ceram. Soc. 2017, 36, 2642–2647. [Google Scholar]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Sadala, S.; Dutta, S.; Raghava, R.; Jyothsna, T.S.; Chakradhar, B.; Ghosh, S.K. Resource recovery as alternative fuel and raw material from hazardous waste. Waste Manag. Res. 2019, 37, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Peng, J. Study on the Direct Recycling of Stainless Steelmaking Dust. Ph.D. Thesis, Central South University, Changsha, China, 30 June 2007. [Google Scholar]
- Wang, Z. Development status of aluminum ash treatment and resource utilization technology. Biol. Chem. Eng. 2023, 9, 186–189+208. [Google Scholar]
- Wei, F.R.; Zhang, Y.L.; Wei, W.J.; Yang, X.G. Chemical composition of dust from stainless steel smelting and existing forms of Cr and Ni. Chin. J. Process Eng. 2011, 11, 786–793. [Google Scholar]
- Li, X.M.; Jia, L.F.; Zou, C.; Cui, Y.R. Progress and trend on comprehensive utilization of stainless steel pickling sludge. Iron Steel 2019, 54, 1–11. [Google Scholar]
- Wang, B.Q.; Wang, D.; Liao, Y.H.; Liu, Z.F.; Ren, B.F. Research progress of recovery technology for aluminum dross. Henan Chem. Ind. 2015, 32, 12–15. [Google Scholar]
- Wang, Z.J.; Li, Y.; Tang, J.Y.; Wang, J.S.; She, X.F.; Xue, Q.G.; Zuo, H.B. Status and progress of resource recovery and utilization of dust and sludge in the steel industry. Jiangxi Metall. 2023, 43, 87–94. [Google Scholar]
- Zhang, L.; Liu, X.D. Research on resource utilization and prospect of harmless treatment technology of aluminum ash. Shandong Chem. Ind. 2023, 52, 233–235. [Google Scholar]
- HJ/T 299-2007; Solid Waste-Extraction Procedure for Leaching Toxicity-Sulphuric Acid & Nitric Acid Method. Ministry of Ecology and Environment: Beijing, China, 2007. (In Chinese)
- HJ/T 300-2007; Solid Waste-Extraction Procedure for Leaching Toxicity-Acetic Acid Buffer Solution Method. Ministry of Ecology and Environment: Beijing, China, 2007. (In Chinese)
- HJ 557-2010; Solid Waste-Extraction Procedure for Leaching Toxicity-Horizontal Vibration Method. Ministry of Ecology and Environment: Beijing, China, 2010. (In Chinese)
- EN 12457-3; Characterization of Waste. Leaching. Compliance Test for Leaching of Granular Waste Materials and Sludges. Part 3: Two-Stage Batch Test at a Liquid to Solid Ratio of 2 L/kg and 8 L/kg for Materials with High Solid Content and with Particle Size below 4 mm (without or with Size Reduction). European Committee for Standardization: Madrid, Spain, 2003.
- SW-846; Test Method 1311 (TCLP): Toxicity Characteristic Leaching Procedure. Environmental Protection Agency: Washington, DC, USA, 1992.
- GB 5058.3-2007; Identification Standards for Hazardous Wastes-Identification for Extraction Toxicity. Standardization Administration of China: Beijing, China, 2007. (In Chinese)
- 40 CFR 261.24; Resource Conservation and Recovery Act. Environmental Protection Agency: Washington, DC, USA, 1976.
- 91/689/EEC; European Environment Agency Directive. European Environment Agency: Brussels, Belgium, 1991.
- Li, X.M.; Xie, G.; Hojamberdiev, M.; Cui, Y.R.; Zhao, J.X. Characterization and recycling of nickel- and chromium-contained pickling sludge generated in production of stainless steel. J. Cent. South Univ. 2014, 21, 3241–3246. [Google Scholar] [CrossRef]
- Su, P.D.; Zhang, J.K.; Li, Y.D. Chemical fixation of toxic metals in stainless steel pickling residue by Na2S∙xH2O, FeSO4∙6H2O and phosphoric acid for beneficial uses. J. Environ. Sci. 2020, 90, 364–374. [Google Scholar] [CrossRef]
- Yang, C.C.; Pan, J.; Zhu, D.Q.; Guo, Z.Q.; Li, X.M. Pyrometallurgical recycling of stainless steel pickling sludge: A review. J. Iron Steel Res. Int. 2019, 26, 547–557. [Google Scholar] [CrossRef]
- Zhao, H.Q.; Qi, Y.H.; Shi, Y.L. Analysis of physical and chemical characteristics of stainless steel dust. J. Iron Steel Res. 2017, 29, 105–110. [Google Scholar]
- Liu, P.J.; Liu, Z.G.; Chu, M.S.; Yan, R.J.; Li, F.; Tang, J. Silicate slag system in carbothermal reduction of stainless steel dust: Strengthening mechanism and stable regulation. Mater. Chem. Phys. 2023, 304, 127850. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Pan, J.; Zhu, D.Q.; Guo, Z.Q.; Yang, C.C.; Chou, J.L. Recovery of valuable metals from stainless steel dust and sludge pellets by pre-reduction-smelting. Chin. J. Nonferrous Met. 2022, 32, 2726–2740. [Google Scholar]
- Wang, M.H.; Lei, P.F.; Li, B.; Zhang, X.B.; Yu, H.M.; Zhang, H.J. Industrial test research on stainless steel dust with coal-based hydrogen metallurgy for recycling purpose. Multipurp. Util. Miner. Resour. 2023, 1–10. Available online: http://kns.cnki.net/kcms/detail/51.1251.TD.20231123.1344.028.html (accessed on 19 December 2023).
- Ma, G.J.; Garbers-Craig, A.M. Stabilisation of Cr(VI) in stainless steel plant dust through sintering using silica-rich clay. J. Hazard. Mater. 2009, 169, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Yin, W.D.; Shen, M.; Zou, C.; Cui, Y.R.; Zhao, J.X. Desulfurization dynamics on reduction pre-treatment of sludge from pickling of chromium nickel stainless steel. Iron Steel 2018, 53, 83–90. [Google Scholar]
- Lu, C.C. Transformation of Sulfur and Valuable Metal in Stainless Steel Pickling Sludge Recycling Utilization Process. Master’s Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 1 March 2017. [Google Scholar]
- Shen, H.L.; Liu, B.; Shi, Z.S.; Zhao, S.Z.; Zhang, J.J.; Zhang, S.G. Reduction for heavy metals in pickling sludge with aluminum nitride in secondary aluminum dross by pyrometallurgy, followed by glass ceramics manufacture. J. Hazard. Mater. 2021, 418, 126331. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.A.; Li, L.J.; Yang, J.; Bu, J.B.; Guo, B.; Liu, B.; Zhang, S.G.; Volinsky, A.A. Production of glass-ceramics from heavy metal gypsum and pickling sludge. Int. J. Environ. Sci. Technol. 2015, 12, 3047–3052. [Google Scholar] [CrossRef]
- Hou, P. An Integrated Technology for Recovery of Heavy Metals from Stainless Steel Pickling Sludge. Master’s Thesis, Nanjing University, Nanjing, China, 1 May 2012. [Google Scholar]
- Shi, C.H.; Zhang, Y.Q.; Zhou, S.; Jiang, J.C.; Huang, X.Y.; Hua, J. Status of research on the resource utilization of stainless steel pickling sludge in China: A review. Environ. Sci. Pollut. R. 2023, 30, 90223–90242. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Li, Q.J.; Yang, F.X.; Zhang, J.X.; Lu, X.G. Experimental study on stainless steel dust by reduction and enrichment for preparation raw material of powder metallurgy. Trans. Indian Inst. Met. 2021, 74, 119–127. [Google Scholar] [CrossRef]
- Li, G.H.; Wang, J.; Rao, M.J.; Luo, J.; Zhang, X.; You, J.X.; Peng, Z.W.; Jiang, T. Coprocessing of stainless-steel pickling sludge with laterite ore via rotary kiln-electric furnace route: Enhanced desulfurization and metal recovery. Process Saf. Environ. Prot. 2020, 142, 92–98. [Google Scholar] [CrossRef]
- Ma, G. Effect of nickel stress on germination and seedling growth of maize. J. Anhui Agric. Sci. 2010, 38, 18029–18030+18034. [Google Scholar]
- Song, W.F.; Cao, J.W.; Wang, Z.; Geng, X.H.; Lu, J.S. Glass-ceramics microstructure formation mechanism for simultaneous solidification of chromium and nickel from disassembled waste battery and chromium slag. J. Hazard. Mater. 2021, 403, 123598. [Google Scholar] [CrossRef]
- Li, Z.X.; Li, W.; Lei, J.J.; Li, Q.Y.; Liu, L.T.; Zhou, L. Effect and hazard of common metal elements on human body. Mater. China 2020, 39, 934–944. [Google Scholar]
- Ifthikar, J.; Shahib, I.I.; Jawad, A.; Gendy, E.A.; Wang, S.Q.; Wu, B.B.; Chen, Z.Q.; Chen, Z.L. The excursion covered for the elimination of chromate by exploring the coordination mechanisms between chromium species and various functional groups. Coord. Chem. Rev. 2021, 437, 213868. [Google Scholar] [CrossRef]
- Lin, Z.Z.; Chen, H.M. Research progress of the disposal technology of calcium fluoride sludge. Appl. Chem. Ind. 2019, 18, 2171–2174. [Google Scholar]
- Song, H.C.; Peng, B. Present situation of comprehensive utilization and research activity of stainless steel making dust. Multipurp. Util. Miner. Resour. 2004, 3, 18–22. [Google Scholar]
- Singhal, A.; Tewari, V.K.; Prakash, S. Characterization of stainless steel pickling bath sludge and its solidification/stabilization. Build. Environ. 2008, 43, 1010–1015. [Google Scholar] [CrossRef]
- Tang, M.T.; Peng, J.; Peng, B.; Yu, D.; Tang, C.B. Thermal solidification of stainless steelmaking dust. Trans. Nonferrous Met. Soc. China 2008, 18, 202–206. [Google Scholar] [CrossRef]
- Liao, C.Z.; Tang, Y.Y.; Liu, C.S.; Shih, K.M.; Li, F.B. Double-barrier mechanism for chromium immobilization: A quantitative study of crystallization and leachability. J. Hazard. Mater. 2016, 311, 246–253. [Google Scholar] [CrossRef]
- Pelino, M.; Karamanov, A.; Pisciella, P.; Crisucci, S.; Zonetti, D. Vitrification of electric arc furnace dusts. Waste Manag. 2002, 22, 945–949. [Google Scholar] [CrossRef]
- Ma, B.; Yan, X.F.; Zhao, Z.H.; Hu, J.H.; Zhang, H.H.; Jiao, S.J. Performance and hazardous characteristics of vitrification product of chromium dust from stainless steel. Res. Environ. Sci. 2021, 34, 1006–1014. [Google Scholar]
- Liu, W.D. Study and practice of reutilization of dust retracted from stainless steel refining. Steelmaking 2011, 27, 66–69. [Google Scholar]
- Ge, X.F.; Xu, A.J.; He, D.F.; Wang, H.B.; Tian, N.Y. Experimental research on the tunnel kiln process for processing stainless steel dust. J. Univ. Sci. Technol. Beijing 2012, 34, 859–866. [Google Scholar]
- Zhao, H.Q.; Qi, Y.H.; Shi, Y.L.; Feng, H.L.; Na, X.Z. The technical study on recycling of waste containing nickel and chromium. China Resour. Compr. Util. 2015, 33, 43–46. [Google Scholar]
- Qin, J.S.; Wang, Z.Q.; Wu, B.; Zhan, D.P. Utilization of dust from 400 series stainless steel for compound injection to pre-dephosphorization and recycle chrome. China Metall. 2011, 21, 47–50. [Google Scholar]
- Duan, J.P.; Zhang, Y.L.; Li, H. Experimental analysis on direct recycling Cr-Ni stainless steelmaking dust in EAF. Iron Steel 2009, 44, 76–80. [Google Scholar]
- Shen, Z.F.; Chi, H.B.; Zheng, H.Y. Study on application of stainless steel dust in the process of foaming EAF slag. Steelmaking 2016, 32, 64–68. [Google Scholar]
- Li, J.C. Recuperation of removable dust from stainless steel. J. Iron Steel Res. 2013, 25, 19–23. [Google Scholar]
- Zhang, J.S. Test of recycling stainless steel dedusted ash by direct reduction in submerged arc furnace. Gansu Metall. 2012, 34, 52–55+69. [Google Scholar]
- Hanewald, R.H.; Munson, W.A.; Schweyer, D.L. Processing EAF dusts and other nickel-chromium waste materials pyrometallurgically at Inmetco. Min. Metall. Explor. 1992, 9, 169–173. [Google Scholar] [CrossRef]
- Kohl, J. Recycling of steel mill wastes. Metal. Plant. Technol. Int. 1992, 15, 98–102. [Google Scholar]
- McClelland, J.M.; Metius, G.E. Recycling ferrous and nonferrous waste streams with FASTMET. Miner. Met. Mater. Soc. 2003, 55, 30–34. [Google Scholar] [CrossRef]
- Hara, Y.; Ishiwata, N.; Itaya, H.; Matsumoto, T. Smelting reduction process with a coke packed bed for steel-making dust recycling. ISIJ Int. 2000, 40, 231–237. [Google Scholar] [CrossRef]
- Eschenbach, R.C. Plasma arc systems for waste treatment and metal recovery. JOM 1996, 48, 49–52. [Google Scholar] [CrossRef]
- Shen, M. Migration Law of Sulfur in Pre-Treatment of Stainless Steel Acid-Pickled Sludge. Master’ Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 1 May 2017. [Google Scholar]
- Fang, J.L.; Yang, W.T. Present situation and prospect of treatment of stainless steel pickling. China Resour. Compr. Util. 2014, 32, 24–28. [Google Scholar]
- Liu, F.Q.; Yang, C.J.; Li, L.J.; Hou, P.; Wang, J.N.; Li, A.M.; Dai, J.J. A Method for Harmless and Resource Disposal of Stainless Steel Pickling Wastewater and Sludge. China Patent CN101982433B, 1 August 2012. [Google Scholar]
- Zhang, S.G.; Kuang, C.F.; Pan, D.A.; Tian, J.J.; Liu, B.; Li, B. A Green Method for Extracting Chromium and Nickel from Stainless Steel Pickling Sludge. China Patent CN102690956A, 26 September 2012. [Google Scholar]
- Zhang, L.H.; Liu, Y.Y.; Duan, F. Metal recovery and heavy metal migration characteristics of ferritic stainless steel pickling sludge reduced by municipal sludge. Waste Manag. 2022, 144, 57–66. [Google Scholar] [CrossRef]
- Wu, M.T.; Li, Y.L.; Guo, Q.; Shao, D.W.; He, M.M.; Qi, T. Harmless treatment and resource utilization of stainless steel pickling sludge via direct reduction and magnetic separation. J. Clean. Prod. 2019, 240, 118187. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zhang, G.H.; Chou, K.C. Preparation of low-carbon and low-sulfur Fe-Cr-Ni-Si alloy by using CaSO4-containing stainless steel pickling sludge. Metall. Mater. Trans. B 2020, 51, 2057–2067. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, G.J.; Jin, Y.B.; Cheng, P.H. Preparation of ceramic tiles with black pigments using stainless steel plant dust as a raw material. Ceram. Int. 2014, 40, 9693–9700. [Google Scholar] [CrossRef]
- Zeng, Y.C.; Zhang, J.Y.; Zhao, S.; Mei, Z.H. A Process for Disposing Stainless Steel Pickling Sludge. China Patent CN105084857A, 25 November 2015. [Google Scholar]
- Zhu, M.X.; Bai, H.; Liu, D.R. Preparation and properties of ceramsite from stainless steel pickling sludge and clay. J. Wuhan Univ. Sci. Technol. 2016, 39, 185–189. [Google Scholar]
- Cheng, T.W. Combined glassification of EAF dust and incinerator fly ash. Chemosphere 2003, 50, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, S.G.; Pan, D.A.; Liu, B.; Wu, C.L.; Volinsky, A.A. Treatment method of hazardous pickling sludge by reusing as glass-ceramics nucleation agent. Rare Met. 2016, 35, 269–274. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Z.W.; Yan, H.W.; Yang, W.Z.; Li, M.N.; Liu, S.X. Research and application progress of recycling and harmless treatment of aluminum ash. Bull. Chin. Ceram. Soc. 2022, 41, 2308–2320. [Google Scholar]
- Shen, H.L.; Liu, B.; Ekberg, C.; Zhang, S.G. Harmless disposal and resource utilization for secondary aluminum dross: A review. Sci. Total Environ. 2021, 760, 143968. [Google Scholar] [CrossRef] [PubMed]
- Li, L.S.; Zhang, Z.Y.; Wu, Y.S.; Wu, X.S. Research progress of aluminum dross reutilization. Light Met 2020, 505, 16–19. [Google Scholar]
- Chen, Z.K. Discussion on comprehensive utilization technology of aluminum ash. Leather Manu. Environ. Technol. 2021, 2, 152–153+156. [Google Scholar]
- Yu, X.Y.; Peng, J.; Zhang, F.; Chang, H.T.; Wang, Y.B. Comprehensive utilization of ash resources. China Foundry Mach. Technol. 2022, 57, 21–30. [Google Scholar]
- Wu, Z.; Zhang, X.Y.; Chen, Z.N.; Ke, J. Advances in reutilization technologies of hazardous secondary aluminum dross. Recycl. Resour. Circ. Econ. 2022, 15, 39–41. [Google Scholar]
- Hu, S.Y.; Wang, D.Y.; Hou, D.; Zhao, W.; Li, X.L.; Qu, T.P.; Zhu, Q.D. Research on the preparation parameters and basic properties of premelted calcium aluminate slag prepared from secondary aluminum dross. Materials 2021, 14, 5855. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.L. Analysis of aluminum ash reutilization treatment and disposal technology. Shanxi Chem. Ind. 2022, 42, 197–198. [Google Scholar]
- Dai, X.; Jiao, S.J.; Zheng, Y.; Zhang, H.H.; Zhao, Z.H.; Ye, F. Analysis on hazardous characteristics of quadratic aluminum dust generated by fluorine flux. Inorg. Chem. Ind. 2018, 51, 42–44. [Google Scholar]
- Kang, Z.S.; Liu, Z.K.; Tian, Y.; Yan, K.; Fan, Z.K.; He, X.Z. Research progress on characteristics of aluminum ash and practice of utilization and disposal technology in aluminum industry. Nonferrous Met. (Extr. Metall.) 2022, 9, 28–35. [Google Scholar]
- Tsakiridis, P.E.; Oustadakis, P.; Agatzini-Leonardou, S. Aluminium recovery during black dross hydrothermal treatment. J. Environ. Chem. Eng. 2013, 1, 23–32. [Google Scholar] [CrossRef]
- Wang, R.L.; Sun, J.L.; Bu, J.L.; Wang, Z.F. Dynamics research on non-isothermal oxidation reaction of AlN powder. J. Mater. Eng. 2011, 39, 29–32+37. [Google Scholar]
- Zhang, G.H.; Hou, X.M.; Chou, K.C. Kinetics of non-isothermal oxidation of AlN powder. J. Eur. Ceram. Soc. 2010, 30, 629–633. [Google Scholar] [CrossRef]
- Li, Y.; Peng, L.; Wang, H.B.; Qin, Z.Y.; Qu, Y.; Li, Y.G.; Li, C.L.; Wang, Y. Study on removal of fluoride and nitride in secondary aluminum dross by high temperature roasting. Conserv. Util. Miner. Resour. 2020, 40, 133–140. [Google Scholar]
- Wang, J.H.; Zhong, Y.Q.; Tong, Y.; Xu, X.L.; Lin, G.Y. Removal of AlN from secondary aluminum dross by pyrometallurgical treatment. J. Cent. South Univ. 2021, 28, 386–397. [Google Scholar] [CrossRef]
- Tang, L.H. Study on Transformation of Aluminum Nitride in Aluminum Dross in Roasting and Hydrolyzing Process. Master’s Thesis, Northeastern University, Shenyang, China, 1 June 2015. [Google Scholar]
- Li, S.; Liu, W.C.; Liu, Z.K.; He, X.Z. Experimental study on oxidizing roasting process of black dross. T. Indian I. Metals 2019, 72, 2293–2298. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Q.; Zhang, G.F.; Shi, Q. Investigations on the hydrolysis behavior of AlN in the leaching process of secondary aluminum dross. Hydrometallurgy 2018, 182, 121–127. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, H.L.; Zuo, Z.P.; Li, R.B.; Liu, F.Q. A thermodynamic and kinetic study of catalyzed hydrolysis of aluminum nitride in secondary aluminum dross. J. Mater. Res. Technol. 2020, 9, 9735–9745. [Google Scholar] [CrossRef]
- Abd Aziz, M.H.; Othman, M.H.D.; Hashim, N.A.B.; Rahman, M.A.; Jaafar, J.; Hubadillah, S.K.; Tai, Z.S. Pretreated aluminium dross waste as a source of inexpensive alumina-spinel composite ceramic hollow fibre membrane for pretreatment of oily saline produced water. Ceram. Int. 2018, 45, 2069–2078. [Google Scholar] [CrossRef]
- Bao, S.C.; Li, S.Q.; Zhang, C.Q.; Miao, X. Leaching and recycling analysis of fluorine and chlorine in secondary aluminum dross. Chin. Metall. 2018, 28, 24–28. [Google Scholar]
- Zhang, N.Y. Removal of fluorine, chlorine and recovery of aluminum in secondary aluminum dross by alkali roasting. Xinjiang Nonferrous Metall. 2019, 42, 47–49. [Google Scholar]
- Xie, M.Z.; Shan, D.; Han, J.S.; Wu, Z.G.; Liu, F.Q.; Zhao, H.L. Removal of harmful elements from aluminum dross and preparation of calcium aluminate by calcium conversion. Chin. Metall. 2023, 33, 115–121. [Google Scholar]
- Xing, X.J.; Wu, Y.D. Review on development on the utilization of aluminum dross. Environ. Eng. 2021, 39, 148–152. [Google Scholar]
- Li, S.; Liu, W.C.; Liu, Z.K.; Yan, K. Technical state and prospect on processing of aluminum dross. Nonferrous Met. (Extr. Metall.) 2018, 10, 25–30. [Google Scholar]
- Xie, M.Z. Synergistic and High-Efficient Resource Utilization of Multiple Al-Si-C Based Solid/Hazardous Wastes. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 28 May 2022. [Google Scholar]
- Mahinroosta, M.; Allahverdi, A. Hazardous aluminum dross characterization and recycling strategies: A critical review. J. Environ. Manag. 2018, 223, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Diao, W.Z.; Yang, D.J.; Liu, J.C. Research progress on comprehensive utilization of aluminum ash resources. Yunnan Metall. 2018, 47, 33–37. [Google Scholar]
- Gripenberg, H.; Falk, O.; Olausson, R.; Niedermair, F. Controlled melting of secondary aluminum rotary furnaces. In Proceedings of the LIGHT METALS 2003, San Diego, CA, USA, 2–6 March 2003; pp. 1083–1090. [Google Scholar]
- Shi, Z.P.; Jiang, L.; Yang, H.L.; Zhang, J.Z.; Fu, G.F. Research status of recycling and resource utilization of aluminum dross. Inorg. Chem. Ind. 2020, 52, 21–25. [Google Scholar]
- Watanabe, K.; Taki, Y. Method of Processing Hot Dross of Aluminum Resulting from an Aluminum Smelting Process and a Deoxidant Obtained from Said Method. European Patent EP060385B1, 24 July 2002. [Google Scholar]
- Gu, T.; Wang, X.X.; Lyu, S.S.; Ni, H.J.; Huang, M.Y.; Li, Z.Y. Current status and prospects of comprehensive recovery and utilization of aluminum ash. Hot Work. Technol. 2017, 46, 29–32. [Google Scholar]
- Meshram, A.; Singh, K.K. Recovery of valuable products from hazardous aluminum dross: A review. Resour. Conserv. Recycl. 2018, 130, 95–108. [Google Scholar] [CrossRef]
- Lavoie, S.; Dubé, G. A salt-free treatment of aluminum dross using plasma heating. JOM 1991, 43, 54–55. [Google Scholar] [CrossRef]
- Gripenberg, H.; Mullerthann, M.; Jager, N. Salt-free dross processing with ALUREC: Two years experience. Light Met. 1997, 1171–1175. [Google Scholar]
- Drouet, M.G.; Meunier, J.; Laflamme, C.B.; Handfield, M.D.; Biscaro, A.; Lemire, C. A rotary arc furnace for aluminium dross processing. Miner. Met. Mater. Soc. 1995, 803–812. [Google Scholar]
- Kos, B. Secondary aluminium and recycling-direct dross treatment by centrifuging of hot dross. Alum. Dusseld. 2000, 76, 35–36. [Google Scholar]
- Ünlü, N.; Drouet, M.G. Comparison of salt-free aluminum dross treatment processes. Resour. Conserv. Recycl. 2002, 36, 61–72. [Google Scholar] [CrossRef]
- Yang, C.; Feng, N.X. Current situation of recycling industrial secondary aluminum dross. Mod. Chem. Ind. 2022, 42, 73–77. [Google Scholar]
- Sarker, M.S.R.; Alam, M.Z.; Qadir, M.R.; Gafur, M.A.; Moniruzzaman, M. Extraction and characterization of alumina nanopowders from aluminum dross by acid dissolution process. Int. J. Min. Met. Mater. 2015, 22, 429–436. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Q.; Zhang, G.F.; Shi, Q.; Feng, H.G. Investigation of leaching kinetics of aluminum extraction from secondary aluminum dross with use of hydrochloric acid. Hydrometallurgy 2019, 187, 158–167. [Google Scholar] [CrossRef]
- Dash, B.; Das, B.R.; Tripathy, B.C.; Bhattacharya, I.N.; Das, S.C. Acid dissolution of alumina from waste aluminium dross. Hydrometallurgy 2008, 92, 48–53. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Allahverdi, A. A promising green process for synthesis of high purity activated-alumina nanopowder from secondary aluminum dross. J. Clean. Prod. 2018, 179, 93–102. [Google Scholar] [CrossRef]
- Shen, L.F.; Wang, L.; Xu, B.; Lv, F. Treatment technology, environmental evaluation and the corresponding management policies of aluminum ash. Met. Mine 2021, 7, 16–26. [Google Scholar]
- Tripathy, A.K.; Mahalik, S.; Sarangi, C.K.; Tripathy, B.C.; Sanjay, K.; Bhattacharya, I.N. A pyro-hydrometallurgical process for the recovery of alumina from waste aluminium dross. Miner. Eng. 2019, 137, 181–186. [Google Scholar] [CrossRef]
- Li, L.L.; Song, M.; Jin, Q. Recovery of alumina from secondary aluminum dross by alkali leaching. Inorg. Chem. Ind. 2019, 51, 53–57. [Google Scholar]
- Foo, C.T.; Salleh, M.A.M.; Ying, K.K.; Matori, K.A. Mineralogy and thermal expansion study of mullite-based ceramics synthesized from coal fly ash and aluminum dross industrial wastes. Ceram. Int. 2019, 45, 7488–7494. [Google Scholar] [CrossRef]
- Ewais, E.M.M.; Khalil, N.M.; Amin, M.S.; Ahmed, Y.M.Z.; Barakat, M.A. Utilization of aluminum sludge and aluminum slag (dross) for the manufacture of calcium aluminate cement. Ceram. Int. 2009, 35, 3381–3388. [Google Scholar] [CrossRef]
- Mailar, G.; Raghavendra, N.S.; Sreedhara, B.M.; Manu, D.S.; Hiremath, P.; Jayakesh, K. Investigation of concrete produced using recycled aluminium dross for hot weather concreting conditions. Resour.-Effic. Technol. 2016, 2, 68–80. [Google Scholar]
- Liu, Y.; Shen, H.L.; Zhang, J.J.; Li, W.H.; Liu, J.; Liu, B.; Zhang, S.G. High strength porous ceramics and its potential in adsorption and building materials: A short process to co-disposal secondary aluminum dross and quicklime. Constr. Build. Mater. 2023, 395, 132292. [Google Scholar] [CrossRef]
- Li, W.H.; Zhang, X.Y.; Shen, H.L.; Yang, J.J.; Liu, Y.; Liu, J.; Qu, Y.N.; Zhang, S.E.; Yang, J.L. Hydrolysis-induced simultaneous foaming and coagulation casting of secondary aluminum dross aqueous suspension. J. Am. Ceram. Soc. 2023, 106, 3273–3977. [Google Scholar] [CrossRef]
- Li, A.P.; Zhang, H.J.; Yang, H.M. Evaluation of aluminum dross as raw material for high-alumina refractory. Ceram. Int. 2014, 40, 12585–12590. [Google Scholar] [CrossRef]
- Adeosun, S.O.; Akpan, E.I.; Dada, M.O. Refractory characteristics of aluminum dross-kaolin composite. JOM 2014, 66, 2253–2261. [Google Scholar] [CrossRef]
- Liu, Z.K.; Kang, Z.S.; Tian, Y.; Yan, K.; He, X.H.; Fan, Z.K. Study on preparing of light refractory and insulation material from secondary aluminum dross and fly ash. Nonferrous Met. (Extr. Metall.) 2022, 9, 125–132. [Google Scholar]
- Yu, X.Y. Pretreatment of Secondary Aluminum Ash and Preparation of Aluminum-Magnesium Spinel Refractory Materials. Ph.D. Thesis, Inner Mongolia University of Science and Technology, Baotou, China, 11 June 2022. [Google Scholar]
- Dong, L.M.; Jiao, F.; Liu, W.; Wang, H.L.; Jiang, S.Q. Research progress of aluminum ash recovery and treatment. J. Cent. South Univ. (Sci. Technol.) 2022, 53, 3791–3801. [Google Scholar]
- Shi, J.L.; Huang, Z.L.; Qin, Q.W.; Yuan, C.G.; Chen, J.Z.; Zhou, Y. Experiment study on the preparation of polyaluminum chloride with secondary aluminum dross. Met. Mine 2021, 7, 206–210. [Google Scholar]
- GB/T 22627-2014; Water Treatment Chemical-Poly Aluminium Chloride. Standardization Administration of China: Beijing, China, 2014. (In Chinese)
- Du, X.W.; Zhao, Z.Q.; Lv, G.Z.; Chen, F.X.; Zhao, X.X.; Zhang, T.A. Study on preparation of polyaluminum chloride by nitrogen removal from secondary aluminum ash. Light Met. 2023, 3, 9–14+21. [Google Scholar]
- Dong, G.Q.; Wang, X.J.; Wang, X.L. Study on the process of preparing polyaluminum and high aluminum composite materials with aluminum ash. Tianjin Chem. Ind. 2021, 35, 57–60. [Google Scholar]
- Chen, F.X. Preparation of Nano Aluminum Nitride Powder by Recycling of Secondary Aluminum Dross Resources. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 1 June 2023. [Google Scholar]
- Shi, M.; Li, Y. Extraction of aluminum based on NH4HSO4 roasting and water leaching from secondary aluminum dross. JOM 2022, 74, 3239–3247. [Google Scholar] [CrossRef]

| Leaching Method | China | European Union | US | |||
|---|---|---|---|---|---|---|
| HJ/T 299-2007 | HJ/T 300-2007 | HJ 557-2010 | EN 12457-3 | TCLP | ||
| Leaching agent | 1#: m(H2SO4):m(HNO3) = 2:1 solution with pH of 3.2 | 1#: First, 5.7 mL glacial acetic acid is added to 500 mL deionized water, then 64.3 mL of 1 mol/L sodium hydroxide solution is added and diluted to 1 L. The pH value of the solution should be 4.93 ± 0.05 | Deionized water | Deionized water | 1#: First, 5.7 mL glacial acetic acid is added to 500 mL deionized water, then 64.3 mL of 1 mol/L sodium hydroxide solution is added and diluted to 1 L. The pH value of the solution should be 4.93 ± 0.05 | |
| 2#: Deionized water | 2#: Ice acetic acid solution with pH of 2.64 ± 0.05 | 2#: Ice acetic acid solution with pH of 2.88 ± 0.05 | ||||
| Applicability of different leaching agent | Leaching agent 1# was used to detect the leaching toxicity of heavy metals and semi-volatile organic compounds in samples Leaching agent 2# was used to determine the leaching toxicity of cyanide and volatile organic compounds in samples | If sample pH < 5.0, use leaching agent 1# If sample pH > 5.0, use leaching agent 2# | — | — | If sample pH < 5.0, use leaching agent 1# If sample pH > 5.0, use leaching agent 2# | |
| Liquid–solid ratio (L/kg) | 10:1 | 20:1 | 10:1 | 2:1 | 8:1 | 20:1 |
| Leaching time | 18 ± 2 h | 18 ± 2 h | 8 h | 6 ± 0.5 | 18 ± 0.5 | 6 h |
| Vibration mode | Vertical | Vertical | Horizontal | Vertical | Vertical | |
| Items | Cr | Cr6+ | Ni | Pb | Zn | Inorganic Fluorides (Excluding CaF2) |
|---|---|---|---|---|---|---|
| Concentration limit | 15 | 5 | 5 | 5 | 100 | 100 |
| Items | Cr | Cd | Hg | As | Pb | Zn |
|---|---|---|---|---|---|---|
| Concentration limit | 5.0 | 1.0 | 0.2 | 5.0 | 5.0 | — |
| Type of Waste | Cr | Cd | Hg | As | Pb | Zn |
|---|---|---|---|---|---|---|
| Inert waste | 0.5 | 0.04 | 0.01 | 0.5 | 0.5 | 4 |
| Non-hazardous waste | 10 | 1 | 0.2 | 2 | 10 | 50 |
| Hazardous waste | 70 | 5 | 2 | 25 | 50 | 200 |
| Ref. | CaO | SiO2 | MgO | Al2O3 | TFe | Fe2O3 | FeO | Cr2O3 | NiO | ZnO | Na2O | K2O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [23] | 16.70 | 4.52 | 3.34 | 0.36 | — | 51.54 | — | 15.19 | 3.48 | 0.12 | 0.47 | 0.86 |
| [24] | 15.01 | 4.15 | 2.87 | 1.13 | — | 38.09 | 18.67 | 13.20 | 2.73 | 0.35 | 0.22 | 1.00 |
| [25] | 14.40 | 4.80 | 1.27 | 0.52 | 33.82 | — | — | 9.38 | 0.44 | 4.00 | 0.72 | 1.17 |
| [26] | 11.40 | 4.10 | 2.90 | 0.88 | 39.00 | — | — | 13.70 | 1.50 | 0.31 | 0.87 | 0.63 |
| [27] | 12.90 | 4.81 | 5.44 | 0.40 | 31.60 | — | — | 14.60 | 2.79 | 4.49 | 0.60 | 0.97 |
| Ref. | CaO | SiO2 | MgO | Al2O3 | Cr2O3 | NiO | CaSO4 | CaF2 | Fe2O3 |
|---|---|---|---|---|---|---|---|---|---|
| [8] | 8.74 | 2.60 | 0.18 | 0.61 | 3.04 | 1.26 | 8.92 | 31.20 | 25.57 |
| [28] | 12.81 | 1.54 | — | 0.58 | 3.93 | 1.37 | 11.05 | 15.56 | 27.00 |
| [29] | 7.30 | 1.80 | 0.70 | — | 11.50 | 3.00 | 3.00 | 47.50 | 25.80 |
| [30] | 2.30 | 6.90 | 1.30 | 2.10 | 5.30 | 2.30 | — | 48.00 | 26.30 |
| [31] | 31.95 | 8.45 | — | — | 4.55 | 1.67 | — | 38.46 | 23.19 |
| Items | Cr | Cr6+ |
|---|---|---|
| SSD | 19.40 | 18.60 |
| Concentration limit (GB 5085.3-2007) | 15 | 5 |
| Items | Cr | Cu | Zn | Mn | Ni |
|---|---|---|---|---|---|
| SSPS | 15.2 | 0.14 | — | 0.044 | 0.27 |
| Concentration limit (GB 5085.3-2007) | 15 | 100 | 100 | — | 5 |
| Method | Feature | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|
| Rotary kiln process | A certain proportion of stainless steel billet grinding debris, reducing agent, and binder are added to SSD. After stirring evenly, it is pressed into pellets. Then, it is sintered at about 800 °C in a rotary kiln. The sintered ball is used as the raw material for electric furnace smelting. At 1600 °C, the oxides of Cr, Ni, and Fe in the dust are fully reduced by C and Si in the molten steel pool. | Low cost; effectively prolongs the furnace’s life | Slag overflow phenomenon | [47] |
| Tunnel kiln process | SSD is mixed with iron scale, water, and binder evenly. After drying, coke powder reducing agent is added, and then Ni-Cr sponge iron is formed by reduction at high temperature in the tunnel kiln. | Nickel and iron oxides can be fully reduced | Reduction rate of chromium is relatively low | [48] |
| Oxycup process | SSD, stainless steel oxide scale, coke powder and binder are mixed evenly to form blocks, and then the blocks are loaded into the Oxycup furnace for high-temperature and high-oxygen enrichment smelting, producing high nickel chromium alloy. | Short flow; efficient; environment-friendly | Chromium recovery rate is unstable | [49] |
| Direct return to production method | SSD is directly added to the hot metal pretreatment, electric arc furnace, converter, submerged arc furnace, and other smelting processes for recovery treatment after pelletizing. | Short flow; high recovery rate of iron; low slagging agent consumption; low cost | Unstable recovery rate of chromium | [50,51,52,53,54] |
| Method | Basic Equipment and Feature | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|
| Inmetco | The core equipment of the process is the annular rotary hearth furnace. Firstly, SSD is mixed with coal and water to make pellets. Then, the carbon-containing pellets are reduced to metal pellets at high temperature in a rotary hearth furnace. Finally, the metal pellets are melted and the chromium oxide is reduced by the residual carbon in the pellets to form metal chromium. | Fast heating rate; high reaction rate; high metal recovery rate | Complicated pretreatment; secondary waste and dust | [55,56] |
| Fastmet /Fastmelt | The core equipment of the process is also the annular rotary hearth furnace. First, SSD is mixed with coal and binder to make pellets. Then, the carbon-containing pellets are dried and put into a rotary hearth furnace for high-temperature reduction. | Short process; small occupation; short reaction time; no secondary pollution | The recovery rate of chromium is unstable, fluctuating between 70% and 90%; high energy consumption | [57] |
| STAR | The basic device of the process is a blast shaft furnace equipped with a fluidized bed. SSD is injected through an upper tuyere, and then the molten oxide in SSD is reduced to metallic elements in a high-temperature coke-packed bed. Elements with high vapor pressure, including zinc and lead, are evaporated and extracted from the top of the furnace. | High recovery rate of iron, nickel, and chromium | Complex process; small industrial scale | [58] |
| Plasmadust | The main device of the process is a shaft furnace with a coke-packed bed and a plasma generator. SSD is fed into the sealed and water-cooled primary chamber through an air-locked system, and then it is struck by the plasma beam to obtain an activated state. Subsequently, the activated SSD is reduced at high temperature for the recovery of valuable metals. | High recovery rate of iron, nickel, and chromium; pollution-free | High energy consumption; large electrode consumption; high noise | [59] |
| Types | Al | Al2O3 | AlN | MgO (MgAl2O4) | Others | Ref. |
|---|---|---|---|---|---|---|
| PAA | 30~70 | 10~40 | 15~30 | 1~5 | 5~15 | [74] |
| SAD | 2~5 | 40~50 | 15~25 | — | 8~20 | [75] |
| Samples | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Fluoride content | 510 | 621 | 515 | 1580 | 690 | 181 | 800 | 277 | 1910 | 669 |
| Concentration limit (GB 5083.3-2007) | Inorganic fluorides (excluding CaF2) ≤ 100 mg/L | |||||||||
| Technology | Features | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|
| Fried ash method | The PAA and salt flux are added to the inclined iron pan, and manually stir fried in it through the external heat source or its residual heat. After that, the aluminum melt is collected at the bottom of the iron pot. | Simple operation; low cost | Secondary pollution; poor operating environment | [99] |
| RSF | The PAA and salt flux in the rotary furnace are heated by oil or natural gas. With the rotation and rolling of the furnace body, PAA is fully mixed with the salt flux. The oxide film of alumina is destroyed by the salt flux, promoting the agglomeration of aluminum liquid and its effective separation from alumina. Furthermore, it prevents the further reoxidation of aluminum liquid, thereby improving the recovery rate of aluminum. | Simple operation; high aluminum recovery rate (about 80%) | Large smoke emission; high cost | [100,101] |
| MRM method | The heated PAA is added to special equipment with a stirring device, and the salt flux is added for continuous heating, so as to maintain the temperature of the PAA and to realize the complete recovery of liquid aluminum. Finally, the liquid aluminum is collected and deposited at the bottom of the container by mechanical stirring. | Fast processing speed; high aluminum recovery rate | High cost | [102] |
| Modified MRM method | In the modified MRM method, the whole process of stirring and aluminum recovery is carried out under argon protection. The aluminum burning loss rate is reduced to 4%, and the recovery rate is as high as 91%. | Low aluminum loss rate; high aluminum recovery rate (about 91%) | High cost | [103] |
| Technology | Features | Recovery Rate | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|---|
| Press | The hot aluminum dross is extruded by a slag extrusion machine and the molten aluminum is squeezed out under a certain pressure. | About 60% | Low cost; fewer impurities; simple | Low recovery rate | [104] |
| Alcan | Air and nitrogen are synchronously introduced into the slit between the two electrodes at the bottom of the rotary furnace. The electrode produces an arc to heat the gas to 700~800 °C and partially ionizes it. PAA is melted in a high-temperature atmosphere, and the rotary furnace rotates at the same time. The oxide film is broken under mechanical stirring, leading to the production of aluminum. | About 90% | High recovery rate; low energy consumption | High equipment failure rate; complex procedure | [105] |
| ALUREC | The rotary melting furnace is used, and the oxygen-rich combustion is carried out with natural gas as the fuel. The temperature required for melting aluminum is reached in a very short time. After melting, aluminum is enriched at the bottom of the rotary furnace, and non-metallic slag floats on the top of the aluminum melt. | About 70% | Good operating environment; easy to control | High cost; large dust emissions | [106] |
| DROSCAR | The PAA is heated by a DC arc between two graphite electrodes in a rotary furnace, facilitating the separation of molten aluminum through mechanical stirring. Simultaneously, the argon gas protection effectively prevents the reoxidation of the molten aluminum. | About 75% | High recovery rate; high efficiency | High energy consumption; low product purity | [107] |
| ECOCENT | The heated PAA is added to the centrifuge, and the relevant parameters, including temperature and centrifugal speed, are adjusted. Under the action of centrifugal force, the metal aluminum and alumina are separated. | About 85% | Easy control; low energy consumption | Narrow applicability | [108] |
| DROSRITE | The oxygen-contained fuel was blown into the rotary furnace, and the temperature in the furnace was maintained at about 973–1073 K. The metal aluminum was separated and extracted from PAA at high temperature with argon as the protective gas. Subsequently, the residue produced by the separation process was heated in an oxygen atmosphere to recover the residual metal aluminum. | About 90% | High recovery rate | Complex procedure | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, H.; Wang, X.; Chong, J.; Huo, X.; Guo, M.; Zhang, M. Transformation and Detoxification of Typical Metallurgical Hazardous Waste into a Resource: A Review of the Development of Harmless Treatment and Utilization in China. Materials 2024, 17, 931. https://doi.org/10.3390/ma17040931
Wang Y, Zhao H, Wang X, Chong J, Huo X, Guo M, Zhang M. Transformation and Detoxification of Typical Metallurgical Hazardous Waste into a Resource: A Review of the Development of Harmless Treatment and Utilization in China. Materials. 2024; 17(4):931. https://doi.org/10.3390/ma17040931
Chicago/Turabian StyleWang, Yuanhang, Haiquan Zhao, Xinyu Wang, Junkai Chong, Xiangtao Huo, Min Guo, and Mei Zhang. 2024. "Transformation and Detoxification of Typical Metallurgical Hazardous Waste into a Resource: A Review of the Development of Harmless Treatment and Utilization in China" Materials 17, no. 4: 931. https://doi.org/10.3390/ma17040931
APA StyleWang, Y., Zhao, H., Wang, X., Chong, J., Huo, X., Guo, M., & Zhang, M. (2024). Transformation and Detoxification of Typical Metallurgical Hazardous Waste into a Resource: A Review of the Development of Harmless Treatment and Utilization in China. Materials, 17(4), 931. https://doi.org/10.3390/ma17040931







