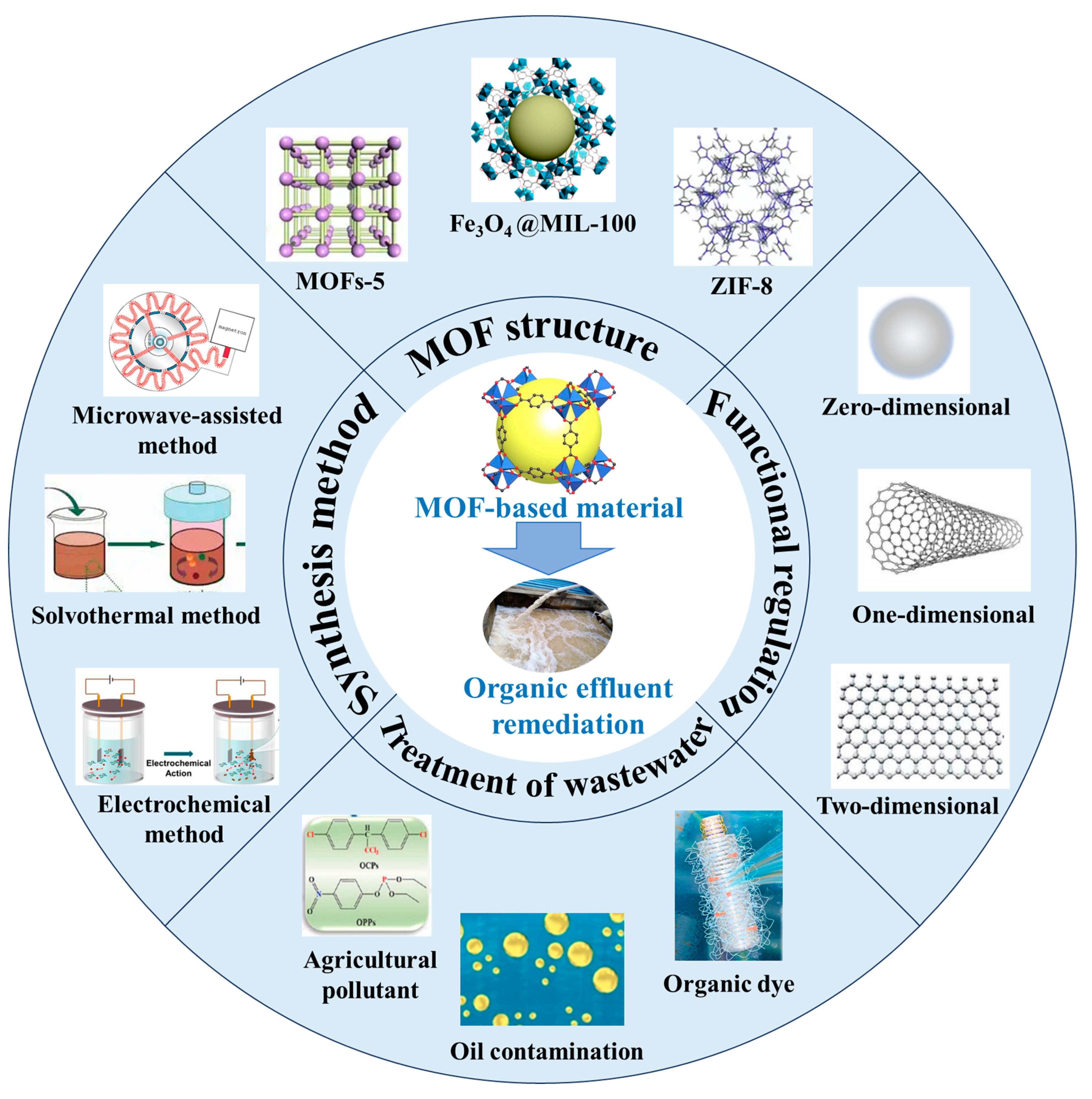
Recent Advances in Metal–Organic Framework (MOF)-Based Composites for Organic Effluent Remediation
Abstract
:1. Introduction
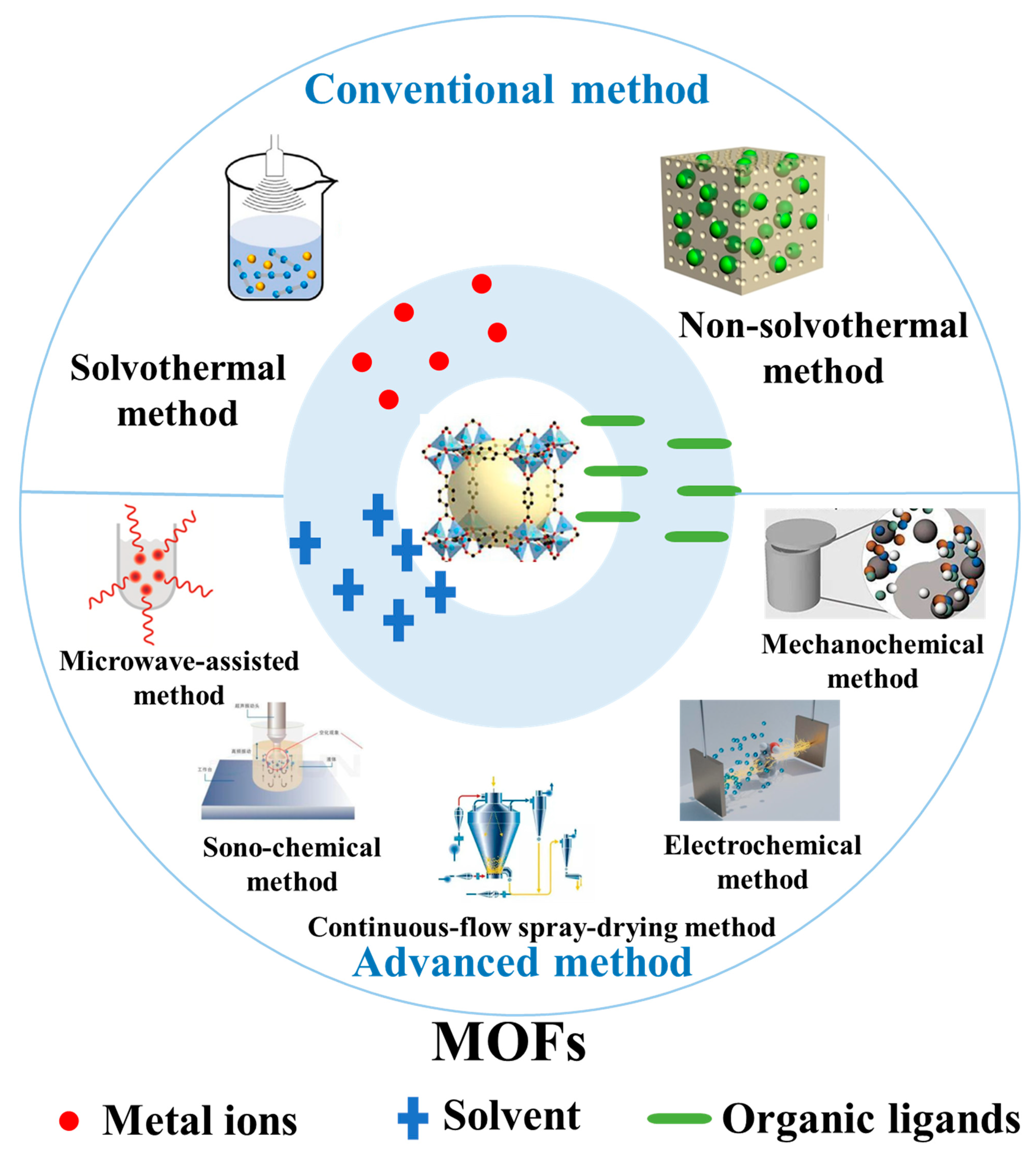
2. Synthesis Methods of MOF-Based Materials
2.1. Conventional Methods for the Synthesis of MOFs
2.2. Advanced Methods for the Synthesis of MOFs
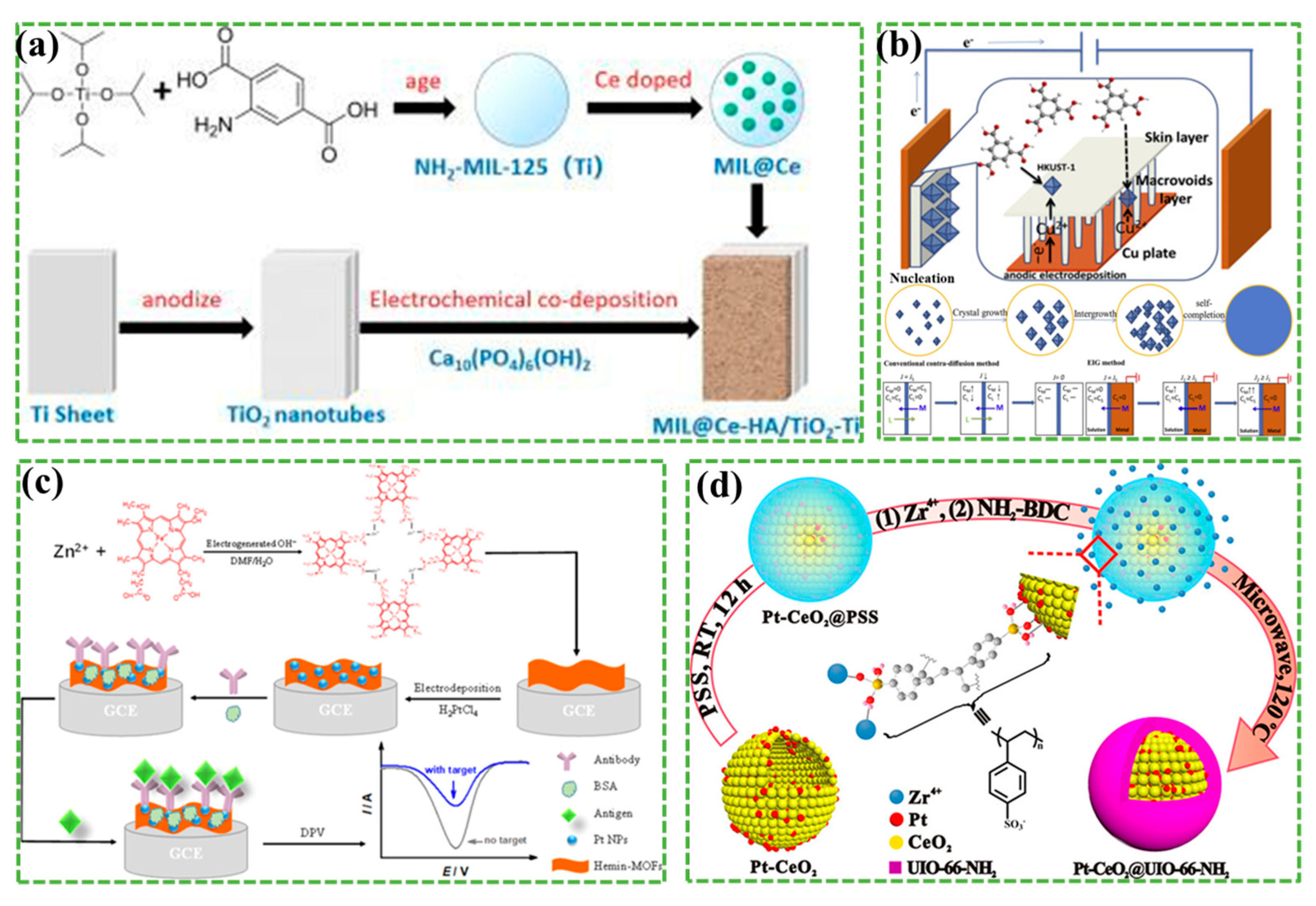
2.2.1. Electrochemical Synthesis
2.2.2. Microwave-Assisted Synthesis
3. Functional Regulations of MOF-Based Materials
3.1. Structural Regulation
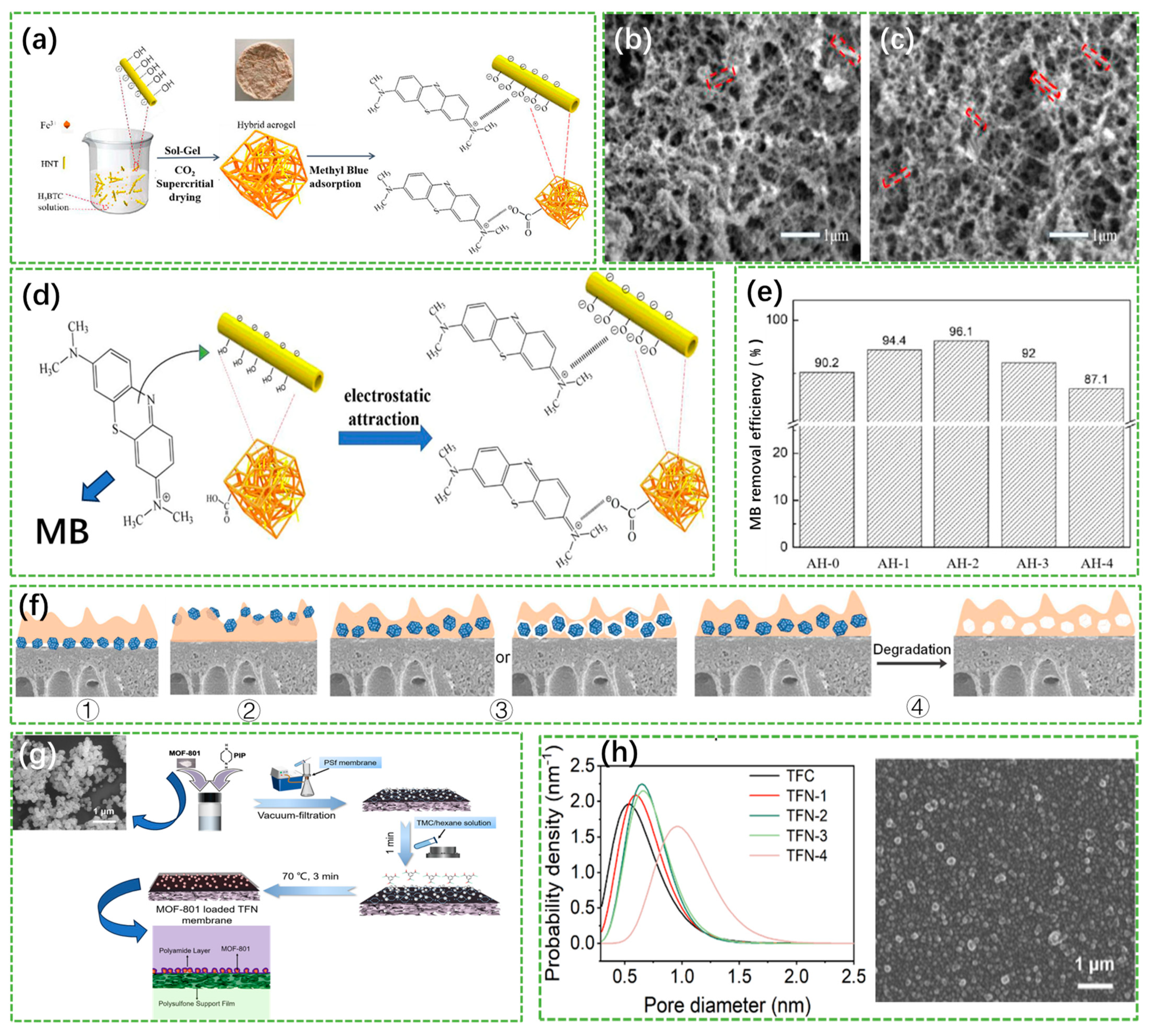
3.1.1. Aerogels and Hydrogels
3.1.2. Nanostructured Membranes
3.2. Functional Regulation
3.2.1. Composites of 0D Materials with MOF-Based Materials
3.2.2. Composites of 1D Materials with MOF-Based Materials
3.2.3. Composites of 2D Materials with MOF-Based Materials
4. Application of MOF-Based Materials for Treating Organic Effluents
4.1. Removal of Organic Dyes
4.2. Removal of Agricultural Pollutants

4.3. Removal of Oil Contaminants

5. Conclusions and Outlooks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mashuri, S.I.S.; Ibrahim, M.L.; Kasim, M.F.; Mastuli, M.S.; Rashid, U.; Abdullah, A.H.; Islam, A.; Mijan, N.A.; Tan, Y.H.; Mansir, N.; et al. Photocatalysis for organic wastewater treatment: From the basis to current challenges for society. Catalysts 2020, 10, 1260. [Google Scholar] [CrossRef]
- Zhang, M.H.; Dong, H.; Zhao, L.; Wang, D.X.; Meng, D. A review on fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of biochar for the adsorption of organic pollutants from wastewater: Modification strategies, mechanisms and challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Saxena, R.; Saxena, M.; Lochab, A. Recent progress in nanomaterials for adsorptive removal of organic contaminants from wastewater. ChemistrySelect 2020, 5, 335–353. [Google Scholar] [CrossRef]
- Li, Y.; Zou, G.; Yang, S.; Wang, Z.; Chen, T.; Yu, X.; Guo, Q.; He, R.; Duan, T.; Zhu, W. Integration of bio-inspired adsorption and photodegradation for the treatment of organics-containing radioactive wastewater. Chem. Eng. J. 2019, 364, 139–145. [Google Scholar] [CrossRef]
- Awad, A.M.; Jalab, R.; Benamor, A.; Nasser, M.S.; Ba-Abbad, M.M.; El-Naas, M.; Mohammad, A.W. Adsorption of organic pollutants by nanomaterial-based adsorbents: An overview. J. Mol. Liq. 2020, 301, 112335. [Google Scholar] [CrossRef]
- Guillossou, R.; Le Roux, J.; Mailler, R.; Pereira-Derome, C.S.; Varrault, G.; Bressy, A.; Vulliet, E.; Morlay, C.; Nauleau, F.; Rocher, V.; et al. Influence of dissolved organic matter on the removal of 12 organic micropollutants from wastewater effluent by powdered activated carbon adsorption. Water. Res. 2020, 172, 115487. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.; Fang, T.; Zhang, W.; Ren, G.; Tang, X.; Ding, Z.; Wang, Y.; Qi, P.; Liu, X. Enhanced antimony removal within lamellar nanoconfined interspaces through a self-cleaning MXene@CNF@FeOOH water purification membrane. Chem. Eng. J. 2023, 465, 143018. [Google Scholar] [CrossRef]
- Li, R.; Liu, D.; Zhang, Y.; Zhou, J.; Tsang, Y.F.; Liu, Z.; Duan, N.; Zhang, Y. Improved methane production and energy recovery of post-hydrothermal liquefaction waste water via integration of zeolite adsorption and anaerobic digestion. Sci. Total Environ. 2019, 651, 61–69. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Molavi, H.; Rezakazemi, M.; Tajahmadi, S.; Bahi, A.; Ko, F.; Aminabhavi, T.M.; Li, J.R.; Arjmand, M. UiO-66 metal-organic frameworks in water treatment: A critical review. Prog. Mater. Sci. 2022, 125, 100904. [Google Scholar] [CrossRef]
- Chakraborty, G.; Park, I.H.; Medishetty, R.; Vittal, J.J. Two-dimensional metal-organic framework materials: Synthesis, structures, properties and applications. Chem. Rev. 2021, 121, 3751–3891. [Google Scholar] [CrossRef]
- Sun, D.T.; Peng, L.; Reeder, W.S.; Moosavi, S.M.; Tiana, D.; Britt, D.K.; Oveisi, E.; Queen, W.L. Rapid, selective heavy metal removal from water by a metal–organic framework/polydopamine composite. ACS Cent. Sci. 2018, 4, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Li, P.Z.; Guo, W.H.; Zhao, Y.L.; Zou, R.Q. Titanium-based metal-organic frameworks for photocatalytic applications. Coordin. Chem. Rev. 2018, 359, 80–101. [Google Scholar] [CrossRef]
- Guo, R.; Cai, X.; Liu, H.; Yang, Z.; Meng, Y.; Chen, F.; Li, Y.; Wang, B. In situ growth of metal–organic frameworks in three-dimensional aligned lumen arrays of wood for rapid and highly efficient organic pollutant removal. Environ. Sci. Technol. 2019, 53, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Yang, Z.; Xiong, W.; Zhou, Y.; Cao, J.; Peng, Y.; Li, X.; Zhou, C.; Xu, R.; Zhang, Y. Construction of MIL-53(Fe) metal-organic framework modified by silver phosphate nanoparticles as a novel z-scheme photocatalyst: Visible-light photocatalytic performance and mechanism investigation. Appl. Surf. Sci. 2019, 465, 103–115. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Yang, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Graphene oxide/Fe(III)-based metal-organic framework membrane for enhanced water purification based on synergistic separation and photo-fenton processes. Appl. Catal. B 2020, 264, 118548. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Mohamed, A.K. Novel derived pectin hydrogel from mandarin peel based metal-organic frameworks composite for enhanced Cr(VI) and Pb(II) ions removal. Int. J. Biol. Macromol. 2020, 164, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Wang, B.; Cheng, J.; Xiao, D.; Xie, Z.; Zhao, J. 3d, eco-friendly metal-organic frameworks@carbon nanotube aerogels composite materials for removal of pesticides in water. J. Hazard. Mater. 2021, 401, 123718. [Google Scholar] [CrossRef]
- Jun, B.M.; Al-Hamadani, Y.A.J.; Son, A.; Park, C.M.; Jang, M.; Jang, A.; Kim, N.C.; Yoon, Y. Applications of metal-organic framework based membranes in water purification: A review. Sep. Purif. Technol. 2020, 247, 116947. [Google Scholar] [CrossRef]
- Tan, Z.K.; Gong, J.L.; Fang, S.Y.; Li, J.; Cao, W.C.; Chen, Z.P. Outstanding anti-bacterial thin-film composite membrane prepared by incorporating silver-based metal-organic framework (Ag-MOF) for water treatment. Appl. Surf. Sci. 2022, 590, 153059. [Google Scholar] [CrossRef]
- Ren, G.M.; Wan, K.M.; Kong, H.; Guo, L.; Wang, Y.; Liu, X.M.; Wei, G. Recent advance in biomass membranes: Fabrication, functional regulation, and antimicrobial applications. Carbohyd. Polym. 2023, 305, 120537. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.; Horcajada, P. Metal–organic frameworks for the removal of emerging organic contaminants in water. Chem. Rev. 2020, 120, 8378–8415. [Google Scholar] [CrossRef] [PubMed]
- Drout, R.J.; Robison, L.; Chen, Z.; Islamoglu, T.; Farha, O.K. Zirconium metal–organic frameworks for organic pollutant adsorption. Trends Chem. 2019, 1, 304–317. [Google Scholar] [CrossRef]
- Tchinsa, A.; Hossain, M.F.; Wang, T.; Zhou, Y. Removal of organic pollutants from aqueous solution using metal organic frameworks (MOFs)-based adsorbents: A review. Chemosphere 2021, 284, 131393. [Google Scholar] [CrossRef]
- Saeed, T.; Naeem, A.; Ud Din, I.; Alotaibi, M.A.; Alharthi, A.I.; Wali Khan, I.; Huma Khan, N.; Malik, T. Structure, nomenclature and viable synthesis of micro/nanoscale metal organic frameworks and their remarkable applications in adsorption of organic pollutants. Microchem. J. 2020, 159, 105579. [Google Scholar] [CrossRef]
- Butova, V.; Soldatov, M.; Guda, A.; Lomachenko, K.; Lamberti, C. Metal-organic frameworks: Structure, properties, methods of synthesis, and characterization. Russ. Chem. Rev. 2016, 85, 280–307. [Google Scholar] [CrossRef]
- Mahnke, M.; Mögel, H.J. Fractal analysis of physical adsorption on material surfaces. Colloids Surf. A 2003, 216, 215–228. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Huang, X.C.; Lin, Y.Y.; Zhang, J.P.; Chen, X.M. Ligand-directed strategy for zeolite-type metal-organic frameworks: Zinc(II) imidazolates with unusual zeolitic topologies. Angew. Chem. Int. Ed. 2006, 45, 1557–1559. [Google Scholar] [CrossRef]
- Volkringer, C.; Loiseau, T.; Guillou, N.; Férey, G.; Popov, D.; Burghammer, M.; Riekel, C. Synthesis and structural characterization of metal-organic frameworks with the mellitate linker m(OH)[COH]•2HO (m = Al, Ga, In) MIL-116. Solid State Sci. 2013, 26, 38–44. [Google Scholar] [CrossRef]
- Zorainy, M.Y.; Sheashea, M.; Kaliaguine, S.; Gobara, M.; Boffito, D.C. Facile solvothermal synthesis of a MIL-47(V) metal–organic framework for a high-performance epoxy/MOF coating with improved anticorrosion properties. RSC Adv. 2022, 12, 9008–9022. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, Q.Q.; Gao, Q.; Xu, H.; Zheng, T.F.; Zhu, Z.H.; Peng, Y.; Chen, J.L.; Liu, S.J.; Wen, H.R. Controllable synthesis of tbiii metal-organic frameworks with reversible luminescence sensing for benzaldehyde vapor. Ingor. Chem. 2023, 62, 3799–3807. [Google Scholar] [CrossRef]
- Du, Y.; Thompson, A.L.; Russell, N.; O’Hare, D. Resin-assisted solvothermal synthesis of transition metal–organic frameworks. Dalton Trans. 2010, 39, 3384–3395. [Google Scholar] [CrossRef]
- Ghosh, S.; Steinke, F.; Rana, A.; Alam, M.; Biswas, S. A metal-organic framework with allyloxy functionalization for aqueous-phase fluorescence recognition of Pd(II) ion. Eur. J. Inorg. Chem. 2021, 2021, 3846–3851. [Google Scholar] [CrossRef]
- Lee, Y.R.; Kim, J.; Ahn, W.S. Synthesis of metal-organic frameworks: A mini review. Korean J. Chem. Eng. 2013, 30, 1667–1680. [Google Scholar] [CrossRef]
- Huang, K.; Xu, Y.; Wang, L.; Wu, D. Heterogeneous catalytic wet peroxide oxidation of simulated phenol wastewater by copper metal–organic frameworks. RSC Adv. 2015, 5, 32795–32803. [Google Scholar] [CrossRef]
- Chen, Q.; Ying, Y.; Wang, L.; Guo, Z.; Zhou, Y.; Wang, D.; Li, C. A heterometallic MOF based on monofunctional linker by “one-pot” solvothermal method for highly selective gas adsorption. Z. Anorg. Allg. Chem. 2020, 646, 437–443. [Google Scholar] [CrossRef]
- Lang, X.; Dong, C.; Cai, K.; Li, L.; Zhang, Q. Highly active cluster structure manganese-based metal organic frameworks (mn-MOFs) like corals in the sea synthesized by facile solvothermal method as gas electrode catalyst for lithium-oxygen batteries. Int. J. Energy Res. 2020, 44, 1256–1263. [Google Scholar] [CrossRef]
- Kim, S.H.; Babu, R.; Kim, D.W.; Lee, W.; Park, D.W. Cycloaddition of coand propylene oxide by using M(HBTC)(4,4′-bipy)•3DMF (M = Ni, Co, Zn) metal-organic frameworks. Chin. J. Catal. 2018, 39, 1311–1319. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, Y.; Kanyuck, K.; Saenz, N.; Reed, K.; Zavalij, P.; Mowery, J.; Bauchan, G. Facile and template-free solvothermal synthesis of mesoporous/macroporous metal–organic framework nanosheets. RSC Adv. 2018, 8, 33059–33064. [Google Scholar] [CrossRef]
- Biemmi, E.; Christian, S.; Stock, N.; Bein, T. High-throughput screening of synthesis parameters in the formation of the metal-organic frameworks MOF-5 and HKUST-1. Microporous Mesoporous Mater. 2009, 117, 111–117. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, H.; Chen, J.; Wang, Z.; Sun, J.; Zhao, D.; Yan, Y. Synthesis, morphology control, and properties of porous metal–organic coordination polymers. Microporous Mesoporous Mater. 2003, 58, 105–114. [Google Scholar] [CrossRef]
- Li, J.; Cheng, S.; Zhao, Q.; Long, P.; Dong, J. Synthesis and hydrogen-storage behavior of metal–organic framework MOF-5. Int. J. Hydrogen Energy 2009, 34, 1377–1382. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Hunt, J.R.; Yaghi, O.M. Room temperature synthesis of metal-organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 2008, 64, 8553–8557. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, S.M.; Chen, S.; Feng, F.; Bai, J.Q.; Li, J.R. Ammoniated MOF-74(zn) derivatives as luminescent sensor for highly selective detection of tetrabromobisphenol A. Ecotoxicol. Environ. Saf. 2020, 187, 109821. [Google Scholar] [CrossRef] [PubMed]
- Chester, A.M.; Castillo-Blas, C.; Wondraczek, L.; Keen, D.A.; Bennett, T.D. Materials formed by combining inorganic glasses and metal-organic frameworks. Chem.-Eur. J. 2022, 28, e202200345. [Google Scholar] [CrossRef]
- Hou, J.W.; Ashling, C.W.; Collins, S.M.; Krajnc, A.; Zhou, C.; Longley, L.; Johnstone, D.N.; Chater, P.A.; Li, S.C.; Coulet, M.V.; et al. Metal-organic framework crystal-glass composites. Nat. Commun. 2019, 10, 2580. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Yu, S.W.; Collins, S.M.; Johnstone, D.N.; Ashling, C.W.; Sapnik, A.F.; Chater, P.A.; Keeble, D.S.; McHugh, L.N.; Midgley, P.A.; et al. A new route to porous metal-organic framework crystal-glass composites. Chem. Sci. 2020, 11, 9910–9918. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Zhang, S.; Yao, K.; Sun, Y.; Liu, Y.; Wang, X.; Huang, W. Synthesis of rare earth doped MOF base coating on TiO2 nanotubes arrays by electrochemicalmethod using as antibacterial implant material. Inorg. Chem. Commun. 2021, 127, 108484. [Google Scholar] [CrossRef]
- Zhang, Z.; Ni, X.; Yao, K.; Zhang, S.; Liu, Y.; Sun, Y.; Wang, X.; Huang, W.; Zhang, Y. An electrochemical synthesis of a rare-earth(La3+)-doped ZIF-8 hydroxyapatite composite coating for a Ti/TiO2 implant material. New J. Chem. 2021, 45, 6543–6549. [Google Scholar] [CrossRef]
- Zhan, F.; Zhao, Y.; Dai, X.; Zeng, J.; Wang, Q. Electrochemically synthesized polyanine@Cu-BTC MOF as a bifunctional matrix for aptasensing of tetracycline in aquatic products. Microchem. J. 2024, 196, 109512. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Van Goethem, C.; Wan, K.; Zhang, W.; Luo, J.; Vankelecom, I.F.J.; Fransaer, J. Electrochemically assisted interfacial growth of MOF membranes. Matter 2019, 1, 1285–1292. [Google Scholar] [CrossRef]
- Tang, D.; Yang, X.; Wang, B.; Ding, Y.; Xu, S.; Liu, J.; Peng, Y.; Yu, X.; Su, Z.; Qin, X. One-step electrochemical growth of 2D/3D Zn(II)-MOF hybrid nanocomposites on an electrode and utilization of a PtNPs@2D MOF nanocatalyst for electrochemical immunoassay. ACS Appl. Mater. Interfaces 2021, 13, 46225–46232. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Song, S.; Li, J.; Wu, L.; Wang, Q.; Liu, Y.; Jin, R.; Zhang, H. Pt/CeO2@MOF core@shell nanoreactor for selective hydrogenation of furfural via the channel screening effect. ACS Catal. 2018, 8, 8506–8512. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Zheng, Y.; Zheng, Z.; Li, C.; Li, Z. Anchoring NaYF4:Yb,Tm upconversion nanocrystals on concave MIL-53(Fe) octahedra for nir-light enhanced photocatalysis. Inorg. Chem. Front. 2017, 4, 1757–1764. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Lee, W.D. Self-assembled magnetic graphene supported ZIF-67 as a recoverable and efficient adsorbent for benzotriazole. Chem. Eng. J. 2016, 284, 1017–1027. [Google Scholar]
- Kanti Chattopadhyay, P.; Ranjan Singha, N. MOF and derived materials as aerogels: Structure, property, and performance relations. Coordin. Chem. Rev. 2021, 446, 214125. [Google Scholar] [CrossRef]
- Tran, V.V.; Park, D.; Lee, Y.C. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar] [CrossRef]
- Ma, S.; Yu, B.; Pei, X.; Zhou, F. Structural hydrogels. Polymer 2016, 98, 516–535. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Yuan, W.; Jiang, C.; Li, H.; Li, J.; Li, Y.; Zhang, B.; Chen, Z. Structure engineering of Fe-based MOF aerogel by halloysite nanotubes for efficient methylene blue adsorption. J. Sol-Gel Sci. Technol. 2021, 99, 55–62. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Q.; Zhu, S. Alginate hydrogel: A shapeable and versatile platform for in situ preparation of metal–organic framework–polymer composites. ACS Appl. Mater. Interfaces 2016, 8, 17395–17401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.L.; Yeung, W.S.; Zhao, Q.P.; Chung, T.S. Thin-film nanocomposite membranes incorporated with UiO-66-NH2 nanoparticles for brackish water and seawater desalination. J. Membr. Sci. 2020, 604, 118039. [Google Scholar] [CrossRef]
- Wang, D.; Su, H.; Han, S.; Tian, M.; Han, L. The role of microporous metal–organic frameworks in thin-film nanocomposite membranes for nanofiltration. Sep. Purif. Technol. 2024, 333, 125859. [Google Scholar] [CrossRef]
- Zhu, L.; Zong, L.; Wu, X.; Li, M.; Wang, H.; You, J.; Li, C. Shapeable fibrous aerogels of metal–organic-frameworks templated with nanocellulose for rapid and large-capacity adsorption. ACS Nano 2018, 12, 4462–4468. [Google Scholar] [CrossRef] [PubMed]
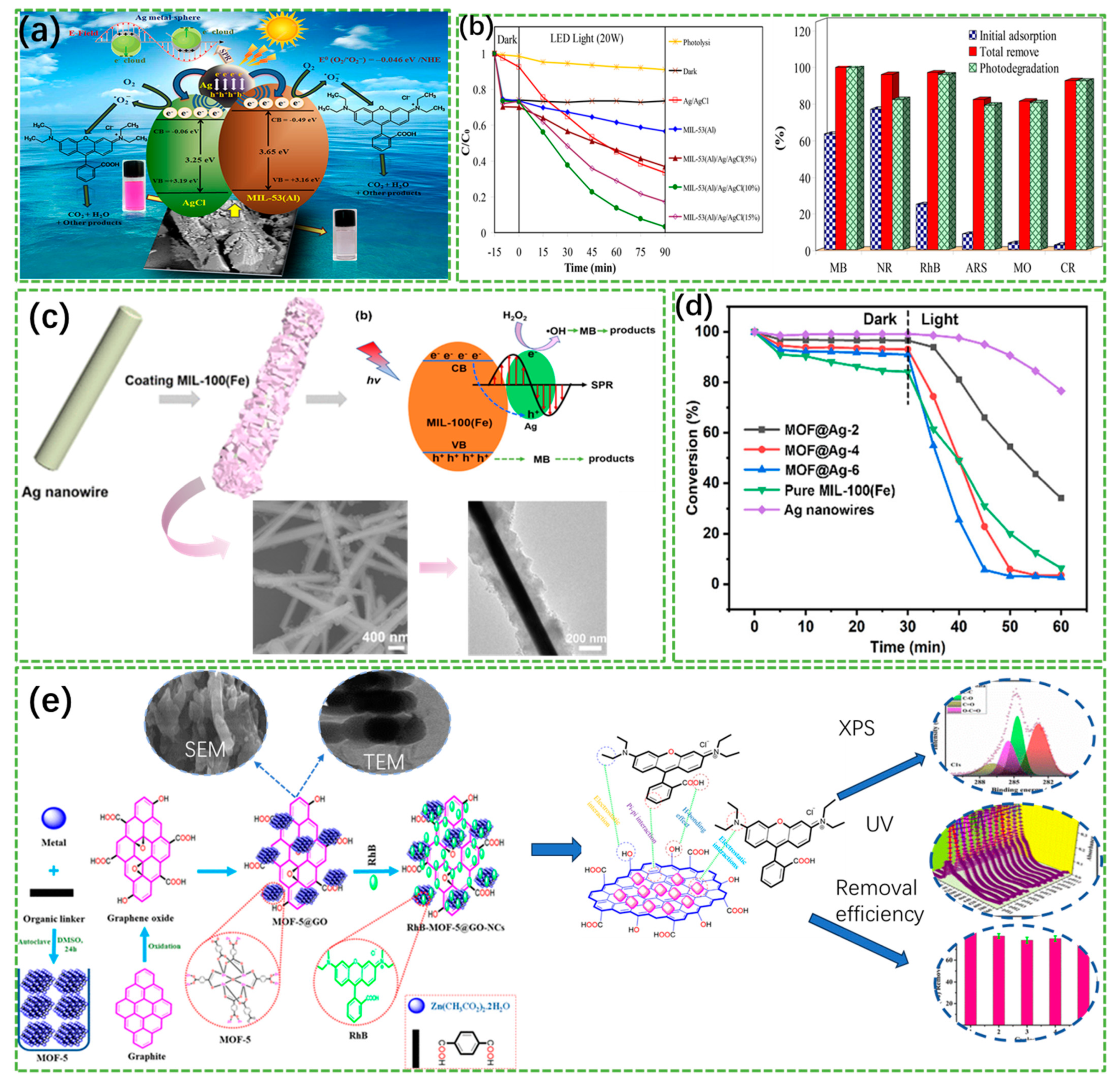
- Khodayari, A.; Sohrabnezhad, S. Fabrication of MIL-53(Al)/Ag/AgCl plasmonic nanocomposite: An improved metal organic framework based photocatalyst for degradation of some organic pollutants. J. Solid State Chem. 2021, 297, 122087. [Google Scholar] [CrossRef]
- Liu, L.; Xie, X.; Qi, S.; Li, R.; Zhang, X.; Song, X.; Gao, C. Thin film nanocomposite reverse osmosis membrane incorporated with UiO-66 nanoparticles for enhanced boron removal. J. Membr. Sci. 2019, 580, 101–109. [Google Scholar] [CrossRef]
- Zhao, Q.P.; Zhao, D.L.; Nai, M.H.; Chen, S.B.; Chung, T.S. Nanovoid-enhanced thin-film composite reverse osmosis membranes using ZIF-67 nanoparticles as a sacrificial template. ACS Appl. Mater. Interfaces 2021, 13, 33024–33033. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Sadam, H.; Zhang, Y.; Xia, J.; Yang, X.; Long, J.; Li, S.; Shao, L. A de novo sacrificial-MOF strategy to construct enhanced-flux nanofiltration membranes for efficient dye removal. Chem. Eng. Sci. 2020, 225, 115845. [Google Scholar] [CrossRef]
- Dai, R.; Guo, H.; Tang, C.Y.; Chen, M.; Li, J.; Wang, Z. Hydrophilic selective nanochannels created by metal organic frameworks in nanofiltration membranes enhance rejection of hydrophobic endocrine-disrupting compounds. Environ. Sci. Technol. 2019, 53, 13776–13783. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, H.J.; Ren, Q.; Luo, H.B.; Ren, X.M.; Tian, Z.F.; Lu, S.F. Extra water- and acid-stable MOF-801 with high proton conductivity and its composite membrane for proton-exchange membrane. ACS Appl. Mater. Interfaces 2018, 10, 28656–28663. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Cheng, L.; Song, Y.; Zeng, P.; Wen, X. Application of hard and soft acid base theory to uncover the destructiveness of lewis bases to UiO-66 type metal organic frameworks in aqueous solutions. J. Mater. Chem. 2021, 9, 14868–14876. [Google Scholar] [CrossRef]
- Bai, X.; Ma, W.; Liu, P.; Sun, Q.; Zhang, K.; Li, A.; Pan, J.; Lyu, Z. Catalytic TFN membranes containing MOF loaded Ag NPs prepared by interfacial polymerization. Microporous Mesoporous Mater. 2022, 335, 111811. [Google Scholar] [CrossRef]
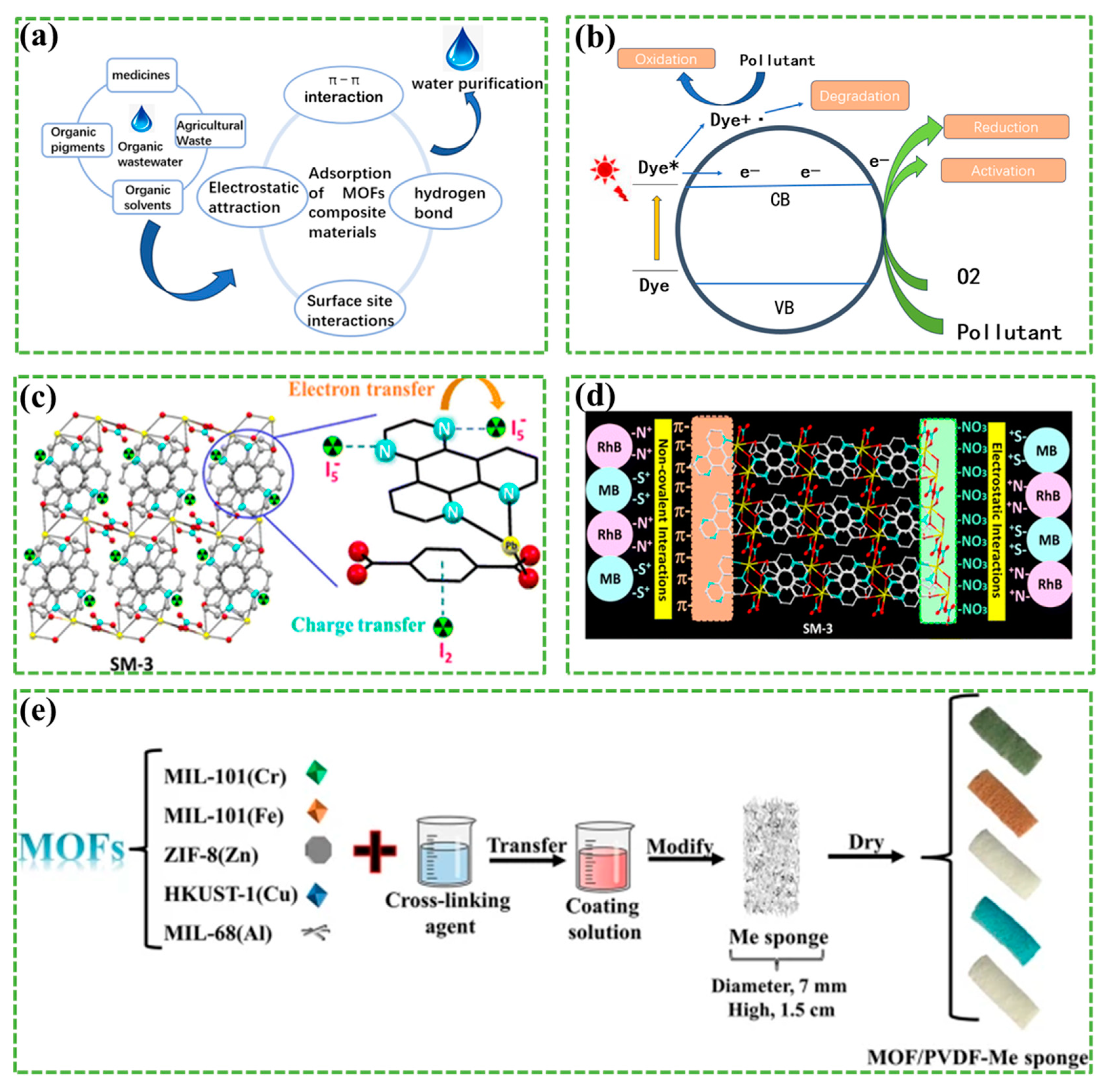
- Zurita-Méndez, N.N.; De la Torre, G.; Espinosa-Medina, M.A. MOF-based composite for methylene blue removal in wastewater. MRS Adv. 2024. [Google Scholar] [CrossRef]
- Troyano, J.; Carné-Sánchez, A.; Pérez-Carvajal, J.; León-Reina, L.; Imaz, I.; Cabeza, A.; Maspoch, D. A self-folding polymer film based on swelling metal-organic frameworks. Angew. Chem. Int. Ed. 2018, 57, 15420–15424. [Google Scholar] [CrossRef]
- Shen, S.S.; Li, H.L.; Shen, Y.; Bai, R.B.; Zhang, G.W. Modification of PVDF membrane by post-modified NH2-MIL-88b(Fe) showing improved permeability and oil/water separation performance. J. Environ. Chem. Eng. 2023, 11, 109621. [Google Scholar] [CrossRef]
- Xiang, W.L.; Zhang, Y.P.; Lin, H.F.; Liu, C.J. Nanoparticle/metal-organic framework composites for catalytic applications: Current status and perspective. Molecules 2017, 22, 2103. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Q. Metal-organic framework composites for catalysis. Matter 2019, 1, 57–89. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Pang, H.; Yang, C.; Ngai, T. Controlled synthesis of metal-organic frameworks coated with noble metal nanoparticles and conducting polymer for enhanced catalysis. J. Colloid Interface. Sci. 2019, 537, 262–268. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Kong, X.; Yao, K.; Liu, L.; Zhang, J.; Guo, Z.; Xu, W.; Fang, Z.; Liu, Y. Photocatalytic performance of the MOF-coating layer on spr-excited Ag nanowires. ACS Omega 2021, 6, 2882–2889. [Google Scholar] [CrossRef]
- Kumar, G.; Masram, D.T. Sustainable synthesis of MOF-5@Go nanocomposites for efficient removal of rhodamine B from water. ACS Omega 2021, 6, 9587–9599. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Lei, Q.; Xia, C.; Guan, Y.; Yang, Y.; He, J.; Yang, Y.; Zhang, Y.; Yan, M. Synthesis of Ag and AgCl co-doped ZIF-8 hybrid photocatalysts with enhanced photocatalytic activity through a synergistic effect. RSC Adv. 2020, 10, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, Y.; Dai, M.; Gong, Q.; Dang, Z. Ag/AgCl/MIL-101(Fe) catalyzed degradation of methylene blue under visible light irradation. Materials 2019, 12, 1453. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Yola, M.L.; Atar, N.; Orooji, Y.; Karimi, F.; Senthil Kumar, P.; Rouhi, J.; Baghayeri, M. A novel detection method for organophosphorus insecticide fenamiphos: Molecularly imprinted electrochemical sensor based on core-shell Co3O4@MOF-74 nanocomposite. J. Colloid Interface Sci. 2021, 592, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, V.; Veleta, J.M.; Zarei-Chaleshtori, M.; Gardea-Torresdey, J.; Villagrán, D. Green synthesis of magnetic MOF@Go and MOF@CNT hybrid nanocomposites with high adsorption capacity towards organic pollutants. Chem. Eng. J. 2016, 304, 774–783. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Zhang, Z.; Liu, X.; Zhang, W. Facile preparation of a novel SnO2@UiO-66/rGO hybrid with enhanced photocatalytic activity under visible light irradiation. RSC Adv. 2016, 6, 92011–92019. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, S.; Xue, H.; Pang, H. Metal-organic frameworks/graphene-based materials: Preparations and applications. Adv. Funct. Mater. 2018, 28, 1804950. [Google Scholar] [CrossRef]
- Rehman Shah, H.U.; Ahmad, K.; Naseem, H.A.; Parveen, S.; Ashfaq, M.; Rauf, A.; Aziz, T. Water stable graphene oxide metal-organic frameworks composite (ZIF-67@GO) for efficient removal of malachite green from water. Food Chem. Toxicol. 2021, 154, 112312. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Li, Z.; Huang, Y.F.; Gong, J.X.; Qiao, C.S.; Zhang, J.F. Preparation and application of MOF-based hydrogel materials. Prog. Chem. 2021, 33, 726–739. [Google Scholar]
- Tao, B.; Li, J.; Miao, F.; Zang, Y. Carbon cloth loaded NiCo2O4 nano-arrays to construct Co-MOF@Go nanocubes: A high-performance electrochemical sensor for non-enzymatic glucose. IEEE Sens. J. 2022, 22, 13898–13907. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, M.; Tang, B.; Li, S.; Jiang, L.; Sun, X.; Que, M.; Tao, C.; Wu, Z. Graphene modified Cu-BTC with high stability in water and controllable selective adsorption of various gases. J. Alloys Compd. 2019, 808, 151721. [Google Scholar] [CrossRef]
- Husain, S.; Verma, S.K.; Hemlata; Azam, M.; Sardar, M.; Haq, Q.M.R.; Fatma, T. Antibacterial efficacy of facile cyanobacterial silver nanoparticles inferred by antioxidant mechanism. Mater. Sci. Eng. C 2021, 122, 111888. [Google Scholar] [CrossRef] [PubMed]
- Abdi, S.; Nasiri, M. Enhanced hydrophilicity and water flux of poly(ether sulfone) membranes in the presence of aluminum fumarate metal–organic framework nanoparticles: Preparation and characterization. ACS Appl. Mater. Interfaces 2019, 11, 15060–15070. [Google Scholar] [CrossRef] [PubMed]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 19. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Abe, T.; Matsui, Y.; Shinno, K.; Kobayashi, S.; Shirasaki, N.; Matsushita, T. Differences in removal rates of virgin/decayed microplastics, viruses, activated carbon, and kaolin/montmorillonite clay particles by coagulation, flocculation, sedimentation, and rapid sand filtration during water treatment. Water Res. 2021, 203, 117550. [Google Scholar] [CrossRef]
- Qin, J.H.; Zhang, J.R.; Xiao, Z.; Wu, Y.P.; Xu, H.M.; Yang, X.G.; Ma, L.F.; Li, D.S. Topology- and guest-dependent photoelectric conversion of 2D anionic pyrene-based metal-organic framework. Cryst. Growth Des. 2022, 22, 4018–4024. [Google Scholar] [CrossRef]
- Kamal, S.; Khalid, M.; Khan, M.S.; Shahid, M.; Ahmad, M. Amine- and imine-functionalized Mn-based MOF as an unusual turn-on and turn-off sensor for d heavy metal ions and an efficient adsorbent to capture iodine. Cryst. Growth Des. 2022, 22, 3277–3294. [Google Scholar] [CrossRef]
- Vo, T.K.; Vu, P.V.; Nguyen, V.C.; Kim, J. Construction of OH sites within MIL-101(Cr)-NH2 framework for enhanced CO2 adsorption and CO2/N2 selectivity. Korean J. Chem. Eng. 2021, 38, 1676–1685. [Google Scholar] [CrossRef]
- Kamal, S.; Khalid, M.; Khan, M.S.; Shahid, M.; Ahmad, M. A bifunctionalised Pb-based MOF for iodine capture and dye removal. Dalton Trans. 2023, 52, 4501–4516. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Song, W.; Zhang, M.; Feng, Y.; Liu, B.; Zhang, H.; Kan, G.; Yu, K.; Jiang, J. MOFs bound to melamine sponge for dye removal. J. Mater. Sci. 2023, 58, 11486–11499. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Wen, J.; Fang, Y.; Zeng, G. Progress and prospect of adsorptive removal of heavy metal ions from aqueous solution using metal–organic frameworks: A review of studies from the last decade. Chemosphere 2018, 201, 627–643. [Google Scholar] [CrossRef]
- Jiang, M.; Ye, K.; Deng, J.; Lin, J.; Ye, W.; Zhao, S.; Van der Bruggen, B. Conventional ultrafiltration as effective strategy for dye/salt fractionation in textile wastewater treatment. Environ. Sci. Technol. 2018, 52, 10698–10708. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, M.; Ma, M.; Qi, M.; Lü, Z.; Gao, C. Impacts of membrane properties on reactive dye removal from dye/salt mixtures by asymmetric cellulose acetate and composite polyamide nanofiltration membranes. J. Membr. Sci. 2010, 350, 83–91. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, G.; Ji, S. Covalent crosslinked assembly of tubular ceramic-based multilayer nanofiltration membranes for dye desalination. AIChE J. 2013, 59, 3834–3842. [Google Scholar] [CrossRef]
- Zhu, J.; Tian, M.; Hou, J.; Wang, J.; Lin, J.; Zhang, Y.; Liu, J.; Van der Bruggen, B. Surface zwitterionic functionalized graphene oxide for a novel loose nanofiltration membrane. J. Mater. Chem. 2016, 4, 1980–1990. [Google Scholar] [CrossRef]
- Sağlam, S.; Türk, F.N.; Arslanoğlu, H. Use and applications of metal-organic frameworks (MOF) in dye adsorption: Review. J. Environ. Chem. Eng. 2023, 11, 110568. [Google Scholar] [CrossRef]
- Zhao, Z.; Shehzad, M.A.; Wu, B.; Wang, X.; Yasmin, A.; Zhu, Y.; Wang, X.; He, Y.; Ge, L.; Li, X.; et al. Spray-deposited thin-film composite MOFs membranes for dyes removal. J. Membr. Sci. 2021, 635, 119475. [Google Scholar] [CrossRef]
- Sun, H.; Wang, N.X.; Li, X.T.; An, Q.F. Fabrication of MOF derivatives composite membrane via in-situ sulfurization for dye/salt separation. J. Membr. Sci. 2022, 645, 120211. [Google Scholar] [CrossRef]
- Shahzad, K.; Hussain, S.; Altaf Nazir, M.; Jamshaid, M.; ur Rehman, A.; Alkorbi, A.S.; Alsaiari, R.; Alhemiary, N.A. Versatile Ag2O and ZnO nanomaterials fabricated via annealed Ag-PMOS and ZnO-PMOS: An efficient photocatalysis tool for azo dyes. J. Mol. Liq. 2022, 356, 119036. [Google Scholar] [CrossRef]
- Contreras, M.; Grande-Tovar, C.D.; Vallejo, W.; Chaves-López, C. Bio-removal of methylene blue from aqueous solution by galactomyces geotrichum kl20a. Water 2019, 11, 282. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Zhang, Q.; Zhang, L.; Ma, T.; Ma, X.; Liang, K.; Ying, Y.; Fu, Y. Nanoconfined MXene-MOF nanolaminate film for molecular removal/collection and multiple sieving. ACS Appl. Mater. Interfaces 2023, 15, 17222–17232. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhang, J.; Huang, J.; Wang, L.; Wang, P.; Cai, C.; Lu, M.; Yao, Z.; Yang, Y. Bimetallic MOF-derived (CuCo)Se nanoparticles embedded in nitrogen-doped carbon framework with boosted electrochemical performance for hybrid supercapacitor. Mater. Res. Bull. 2021, 137, 111196. [Google Scholar] [CrossRef]
- Hu, Q.; Di, J.; Wang, B.; Ji, M.; Chen, Y.; Xia, J.; Li, H.; Zhao, Y. In-situ preparation of NH2-MIL-125(Ti)/BiOCl composite with accelerating charge carriers for boosting visible light photocatalytic activity. Appl. Surf. Sci. 2019, 466, 525–534. [Google Scholar] [CrossRef]
- Omeje, J.S.; Asegbeloyin, J.N.; Ihedioha, J.N.; Ekere, N.R.; Ochonogor, A.E.; Abugu, H.O.; Alum, O.L. Monitoring of pesticide residues in fresh fruits and vegetables available in nigerian markets and assessment of their associated health risks. Environ. Monit. Assess. 2022, 194, 516. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Xiong, X.; He, M.; Tsang, D.C.W.; Gupta, J.; Khan, E.; Harrad, S.; Hou, D.; Ok, Y.S.; Bolan, N.S. Microplastics as pollutants in agricultural soils. Environ. Pollut. 2020, 265, 114980. [Google Scholar] [CrossRef] [PubMed]
- Chandel, M.; Kaur, K.; Sahu, B.K.; Sharma, S.; Panneerselvam, R.; Shanmugam, V. Promise of nano-carbon to the next generation sustainable agriculture. Carbon. 2022, 188, 461–481. [Google Scholar] [CrossRef]
- Ibrahim, A.O.; Adegoke, K.A.; Adegoke, R.O.; AbdulWahab, Y.A.; Oyelami, V.B.; Adesina, M.O. Adsorptive removal of different pollutants using metal-organic framework adsorbents. J. Mol. Liq. 2021, 333, 115593. [Google Scholar] [CrossRef]
- Mahmoud, L.A.M.; Telford, R.; Livesey, T.C.; Katsikogianni, M.; Kelly, A.L.; Terry, L.R.; Ting, V.P.; Nayak, S. Zirconium-based MOFs and their biodegradable polymer composites for controlled and sustainable delivery of herbicides. ACS Appl. Bio Mater. 2022, 5, 3972–3981. [Google Scholar] [CrossRef]
- Yang, J.; Trickett, C.A.; Alahmadi, S.B.; Alshammari, A.S.; Yaghi, O.M. Calcium l-lactate frameworks as naturally degradable carriers for pesticides. J. Am. Chem. Soc. 2017, 139, 8118–8121. [Google Scholar] [CrossRef]
- Reis, R.A.d.; Mahmoud, L.A.M.; Ivanovska, E.H.; Telford, R.; Addicoat, M.A.; Terry, L.R.; Ting, V.P.; Nayak, S. Biodegradable polymer-metal-organic framework (MOF) composites for controlled and sustainable pesticide delivery. Adv. Sustain. Sys. 2023, 7, 2300269. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Taha, M.; Abdel-Gawad, H.; Mahdy, F.; Hegazi, B. Zeolitic imidazolate frameworks: Experimental and molecular simulation studies for efficient capture of pesticides from wastewater. J. Environ. Chem. Eng. 2019, 7, 103499. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Abdel-Gawad, H.; Emam, H.E. Macroporous Cu-MOF@cellulose acetate membrane serviceable in selective removal of dimethoate pesticide from wastewater. J. Environ. Chem. Eng. 2021, 9, 105121. [Google Scholar] [CrossRef]
- Yu, K.; Ahmed, I.; Won, D.I.; Lee, W.I.; Ahn, W.S. Highly efficient adsorptive removal of sulfamethoxazole from aqueous solutions by porphyrinic MOF-525 and MOF-545. Chemosphere 2020, 250, 126133. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.E.; Amira, M.F.; Azab, M.M.H.M.; Abdelfattah, A.M. Effective removal of levofloxacin drug and Cr(VI) from water by a composed nanobiosorbent of vanadium pentoxide@chitosan@MOFs. Int. J. Bio. Macromol. 2021, 188, 879–891. [Google Scholar] [CrossRef]
- Li, Z.; Huang, G.; Liu, K.; Tang, X.; Peng, Q.; Huang, J.; Ao, M.; Zhang, G. Hierarchical BiOX (x = Cl, Br, I) microrods derived from bismuth-MOFs: In situ synthesis, photocatalytic activity and mechanism. J. Clean. Prod. 2020, 272, 122892. [Google Scholar] [CrossRef]
- Xiao, J.; Shi, F.; Zhang, Y.; Peng, M.; Xu, J.; Li, J.; Chen, Z.; Yang, Z. A MOF nanozyme-mediated acetylcholinesterase-free colorimetric strategy for direct detection of organophosphorus pesticides. Chem. Commun. 2024, 60, 996–999. [Google Scholar] [CrossRef]
- Dutta, S.; Mandal, W.; Desai, A.V.; Fajal, S.; Dam, G.K.; Mukherjee, S.; Ghosh, S.K. A luminescent cationic MOF and its polymer composite membrane elicit selective sensing of antibiotics and pesticides in water. Mol. Syst. Des Eng. 2023, 8, 1483–1491. [Google Scholar] [CrossRef]
- Su, B.; Liao, S.; Zhu, H.; Ge, S.; Liu, Y.; Wang, J.; Chen, H.; Wang, L. Fabrication of a 2D metal–organic framework (MOF) nanosheet colloidal system and investigation of its fluorescence response to pesticide molecules. Anal. Methods 2021, 13, 5700–5710. [Google Scholar] [CrossRef]
- Li, Y.X.; Han, Y.C.; Wang, C.C. Fabrication strategies and Cr(VI) elimination activities of the MOF-derivatives and their composites. Chem. Eng. J. 2021, 405, 126648. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Emam, H.E. Modulation of metal organic framework hybrid cotton for efficient sweeping of dyes and pesticides from wastewater. Sustain. Mater. Technol. 2022, 31, e00366. [Google Scholar] [CrossRef]
- Li, T.; Lu, M.; Gao, Y.; Huang, X.; Liu, G.; Xu, D. Double layer MOFs M-ZIF-8@ZIF-67: The adsorption capacity and removal mechanism of fipronil and its metabolites from environmental water and cucumber samples. J. Adv. Res. 2020, 24, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.R.; Sarker, S.; Yu, B.; Kar, A.; Sun, X.; Dey, S.K. TiO2 nanotubes and its composites: Photocatalytic and other photo-driven applications. J. Mater. Res. 2013, 28, 280–293. [Google Scholar] [CrossRef]
- Bhushan, B. Bioinspired oil–water separation approaches for oil spill clean-up and water purification. Philos. Trans. R. Soc. A 2019, 377, 20190120. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Loh, C.H.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog. Polym. Sci. 2018, 77, 69–94. [Google Scholar] [CrossRef]
- Shenvi, S.S.; Isloor, A.M.; Ismail, A.F. A review on RO membrane technology: Developments and challenges. Desalination 2015, 368, 10–26. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Tang, L.; Meng, X.; Deng, D.; Bao, X. Confinement catalysis with 2D materials for energy conversion. Adv. Mater. 2019, 31, 1901996. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Qi, H.; Gong, X.; Zhao, S. Specially wettable membranes for oil–water separation. Adv. Mater. Interfaces 2018, 5, 1800576. [Google Scholar] [CrossRef]
- Wei, C.; Dai, F.; Lin, L.; An, Z.; He, Y.; Chen, X.; Chen, L.; Zhao, Y. Simplified and robust adhesive-free superhydrophobic SiO2-decorated PVDF membranes for efficient oil/water separation. J. Membr. Sci. 2018, 555, 220–228. [Google Scholar] [CrossRef]
- Dai, X.; Cao, Y.; Shi, X.; Wang, X. The PLA/ZIF-8 nanocomposite membranes: The diameter and surface roughness adjustment by ZIF-8 nanoparticles, high wettability, improved mechanical property, and efficient oil/water separation. Adv. Mater. Interfaces 2016, 3, 1600725. [Google Scholar] [CrossRef]
- Yu, Y.; Yue, B.; Wu, J.; Fu, Z.; Cui, Z.; Qu, J.; Hu, J.; Aladejana, J.T.; Lu, Y.; Li, J.; et al. A wood-based MOF membrane with high flux and efficiency for oil-in-water emulsions separation. Colloids Surf. A 2023, 674, 131852. [Google Scholar] [CrossRef]
- Du, J.C.; Zhang, C.Y.; Pu, H.; Li, Y.F.; Jin, S.M.; Tan, L.X.; Zhou, C.L.; Dong, L.C. HKUST-1 MOFs decorated 3D copper foam with superhydrophobicity/superoleophilicity for durable oil/water separation. Colloids Surf. A 2019, 573, 222–229. [Google Scholar] [CrossRef]
- He, W.; Liu, Y.C.; Huang, Z.; Tu, W.W.; Liu, R.; Chen, M.Y. Cubic MOF coated stainless steel mesh with underwater superoleophobicity for highly efficient oil/water separation. Mater. Chem. Phys. 2023, 297, 127346. [Google Scholar] [CrossRef]
- Habibi, N.; Faraji, S.; Pourjavadi, A. Nano graphite platelets/Cu (BDC) MOF coating on polyurethane sponge: A superhydrophobic self-extinguishing adsorbent for static and continuous oil/water separation. Colloids Surf. A 2023, 676, 132186. [Google Scholar] [CrossRef]
- Cao, J.; Su, Y.; Liu, Y.; Guan, J.; He, M.; Zhang, R.; Jiang, Z. Self-assembled MOF membranes with underwater superoleophobicity for oil/water separation. J. Membr. Sci. 2018, 566, 268–277. [Google Scholar] [CrossRef]
- Dalapati, R.; Nandi, S.; Gogoi, C.; Shome, A.; Biswas, S. Metal-organic framework (MOF) derived recyclable, superhydrophobic composite of cotton fabrics for the facile removal of oil spills. ACS Appl. Mater. Interfaces 2021, 13, 8563–8573. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.H.; Fan, H.W.; Li, C.X.; Caro, J.; Meng, H. Robust superhydrophobic/superoleophilic wrinkled microspherical MOF@rGO composites for efficient oil-water separation. Angew. Chem. Int. Ed. 2019, 58, 5297–5301. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Yi, M.; Chai, C.; Li, W.; Qi, P.; Wang, J.; Hao, J. Oxidation stability enhanced MXene-based porous materials derived from water-in-ionic liquid pickering emulsions for wearable piezoresistive sensor and oil/water separation applications. J. Colloid Interface Sci. 2022, 618, 311–321. [Google Scholar] [CrossRef]


| MOF-Based Material | Pollutants Removed | Preparation Method | Advantages | Ref. |
|---|---|---|---|---|
| SM-3 | Iodine and dyes | Solvothermal method | High selectivity | [99] |
| MIL-101 (Cr), MIL-101(Fe) | Methyl orange dye | Infiltration method | Excellent adsorption capacity, simple one-step infiltration, excellent adsorption properties and simple operation | [100] |
| ZIF-L, UiO-66-NH2 | Dye | Spray deposition and post-stabilization | High water permeance and excellent dye rejection | [108] |
| CoSx composite membrane ZIF-67-derived CoSx | Dye Desalination of dyes | Solvothermal method In situ sulfurization | Outstanding permeance and excellent rejection | [109] |
| xOy=Ag2O and ZnO | MB and MO azo dyes | Sol–gel method | Definite durability | [110] |
| iMOF-14C | Antibiotics and pesticides | Solvothermal method | High selectivity and low detection limit | [128] |
| Cu-BTC@ CA | Dimethoate pesticide | In situ synthesis | High adsorbability, large adsorption capacity and good recoverability | [123] |
| MOF-525 and MOF-545 | Sulfamethoxazole | Solvothermal method | Great adsorption capacity and good reusability | [124] |
| V2O5@Ch/Cu-TMA | Levofloxacin drug and Cr(Vl) | Solvothermal method | High efficiency | [125] |
| NH2-UiO-101-Zr@Ox-cotton | Dye | Non-aqueous method | Large adsorption capacity and good recyclability | [131] |
| M-ZIF-8@ZIF-67 | Fipronil and its metabolites | Solvothermal method | High adsorption capacity | [132] |
| PVDF membranes | Oil | Delayed phase inversion | Good stability and excellent recyclability | [140] |
| PLA/ZIF-8 | Dye wastewater | Solvothermal method | Increased oil wettability and significantly improved mechanical property | [141] |
| HKUST-1 MOFs | Oil | Solvothermal method | High separation efficiency and good repeatability | [143] |
| MOF-5/PDA/SSM | Oil | Solvothermal method | Outstanding separation efficiency, excellent recyclability and high water flux | [144] |
| Cu(BDC)MOF | Oil | Solvothermal method | Excellent stability, exhibited good compressibility and reusability | [145] |
| SH-UiO-66 | Heavy and light oils | Solvothermal method | High water resistance, high oil absorption capacity and good reversibility | [147] |
| Ti3C2-Mxene | Oil | Solvothermal method | High hydrophobicity, abundant porosity and sufficient mechanical strength | [149] |
| NH2-MIL-125 (Ti) | Pesticide | Solvothermal method | High removal rate | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; Wang, Y.; He, P.; Wang, Y.; Wei, G. Recent Advances in Metal–Organic Framework (MOF)-Based Composites for Organic Effluent Remediation. Materials 2024, 17, 2660. https://doi.org/10.3390/ma17112660
Tang S, Wang Y, He P, Wang Y, Wei G. Recent Advances in Metal–Organic Framework (MOF)-Based Composites for Organic Effluent Remediation. Materials. 2024; 17(11):2660. https://doi.org/10.3390/ma17112660
Chicago/Turabian StyleTang, Shuxian, Yuxuan Wang, Peng He, Yan Wang, and Gang Wei. 2024. "Recent Advances in Metal–Organic Framework (MOF)-Based Composites for Organic Effluent Remediation" Materials 17, no. 11: 2660. https://doi.org/10.3390/ma17112660






