Adsorption of Phosphate by Two-Step Synthesis of Ceramsite from Electrolytic Manganese Residue/Dredged Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ceramsite
2.3. Preparation of Phosphorus-Containing Wastewater
2.4. Characterization
2.5. Static Adsorption
2.6. Dynamic Adsorption
2.6.1. Dynamic Test Setup
2.6.2. Dynamic Tests
3. Results and Discussion
3.1. Mechanical Behavior of Ceramsite
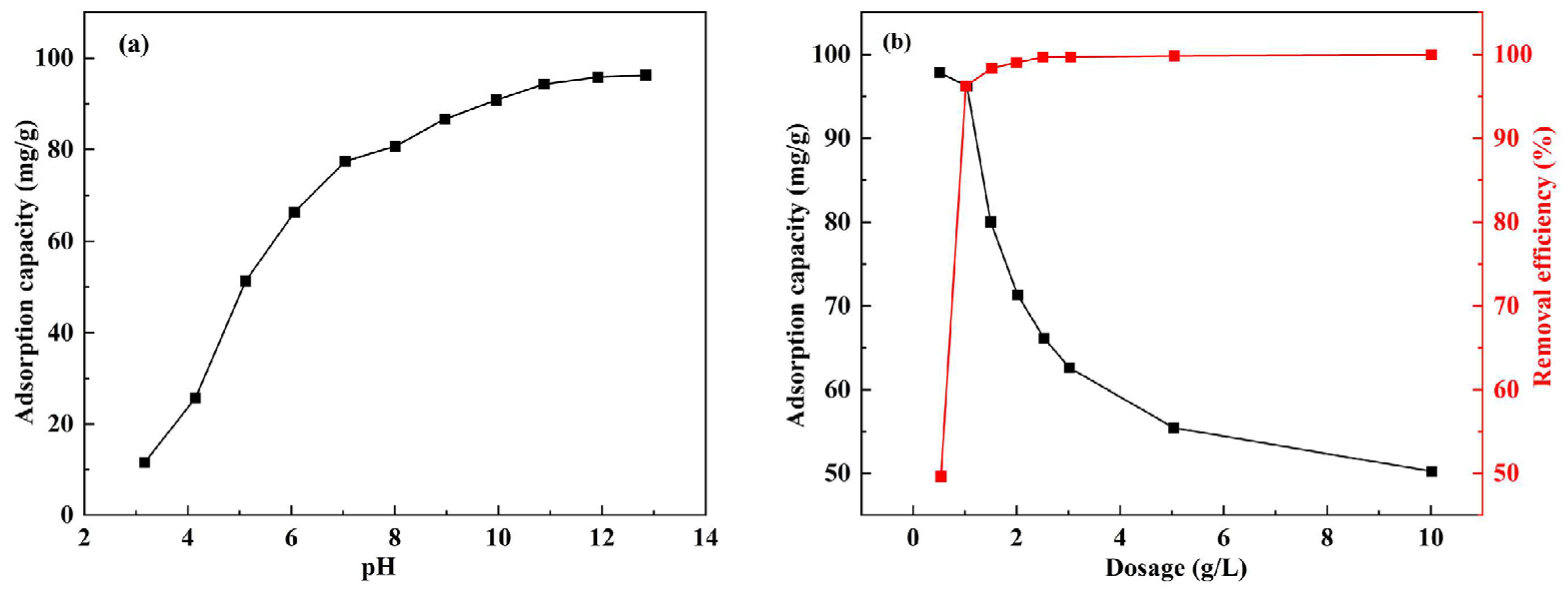
3.2. Effect of pH and Ceramsite Dosage on Adsorption
3.3. Characterization of DS-EMR Ceramsite
3.3.1. Pore Characteristics
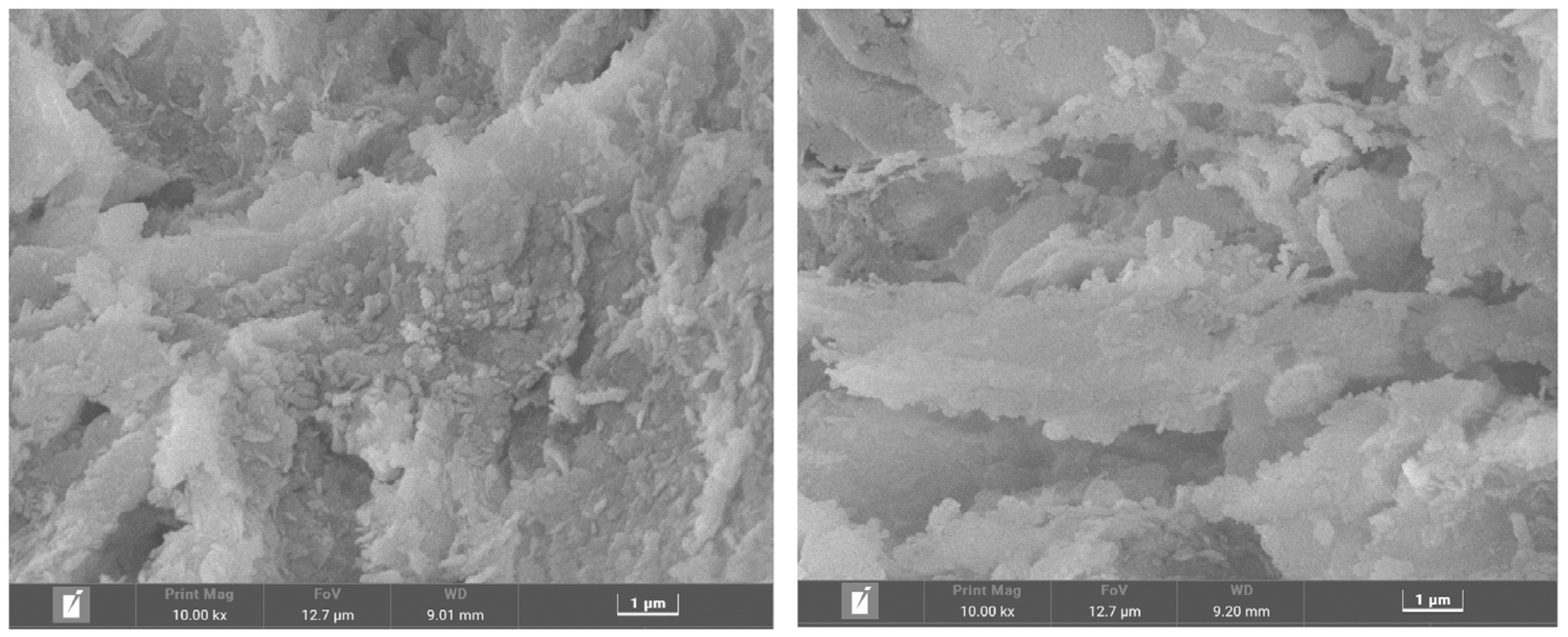
3.3.2. Micromorphology Analysis
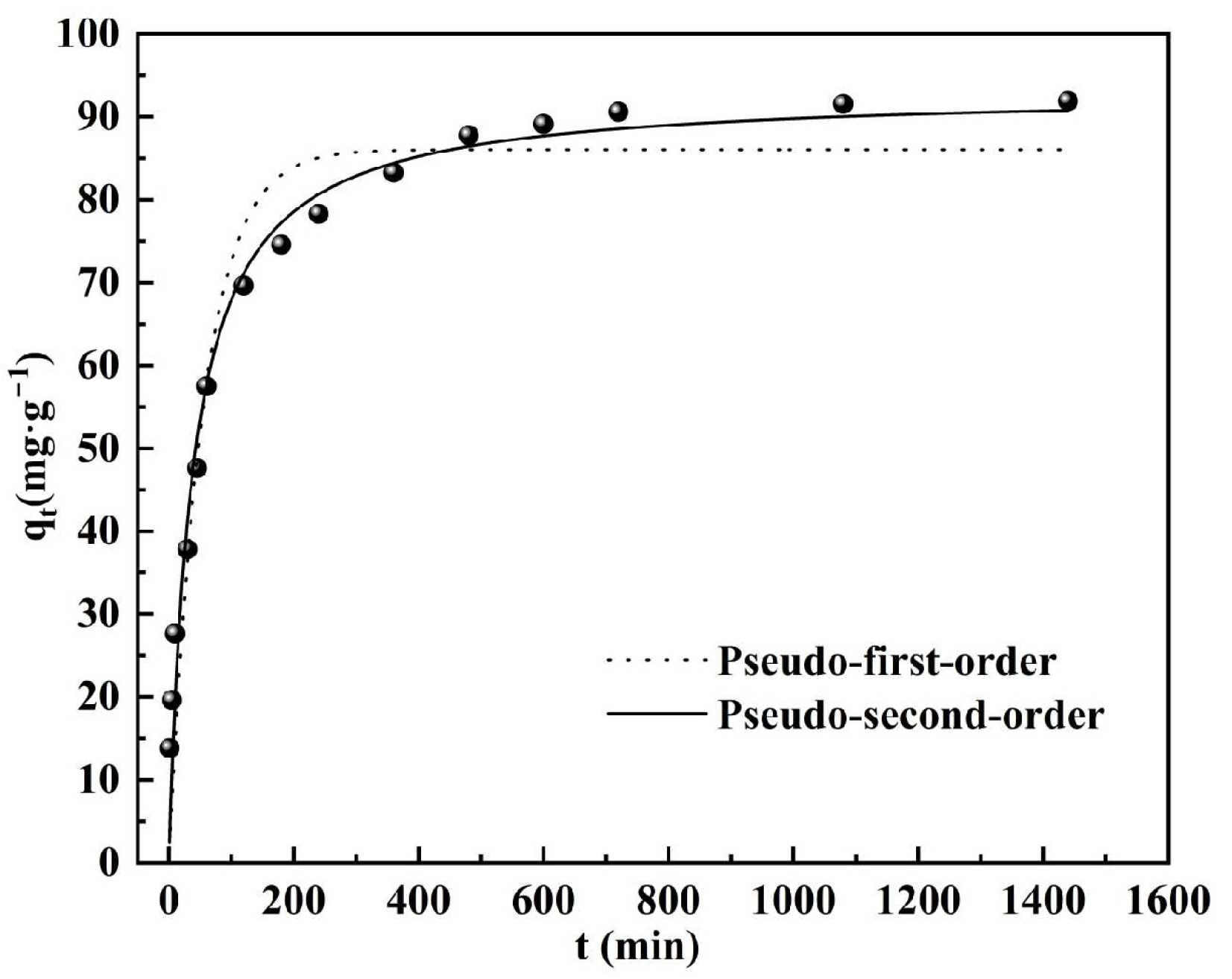
3.4. Adsorption Kinetics
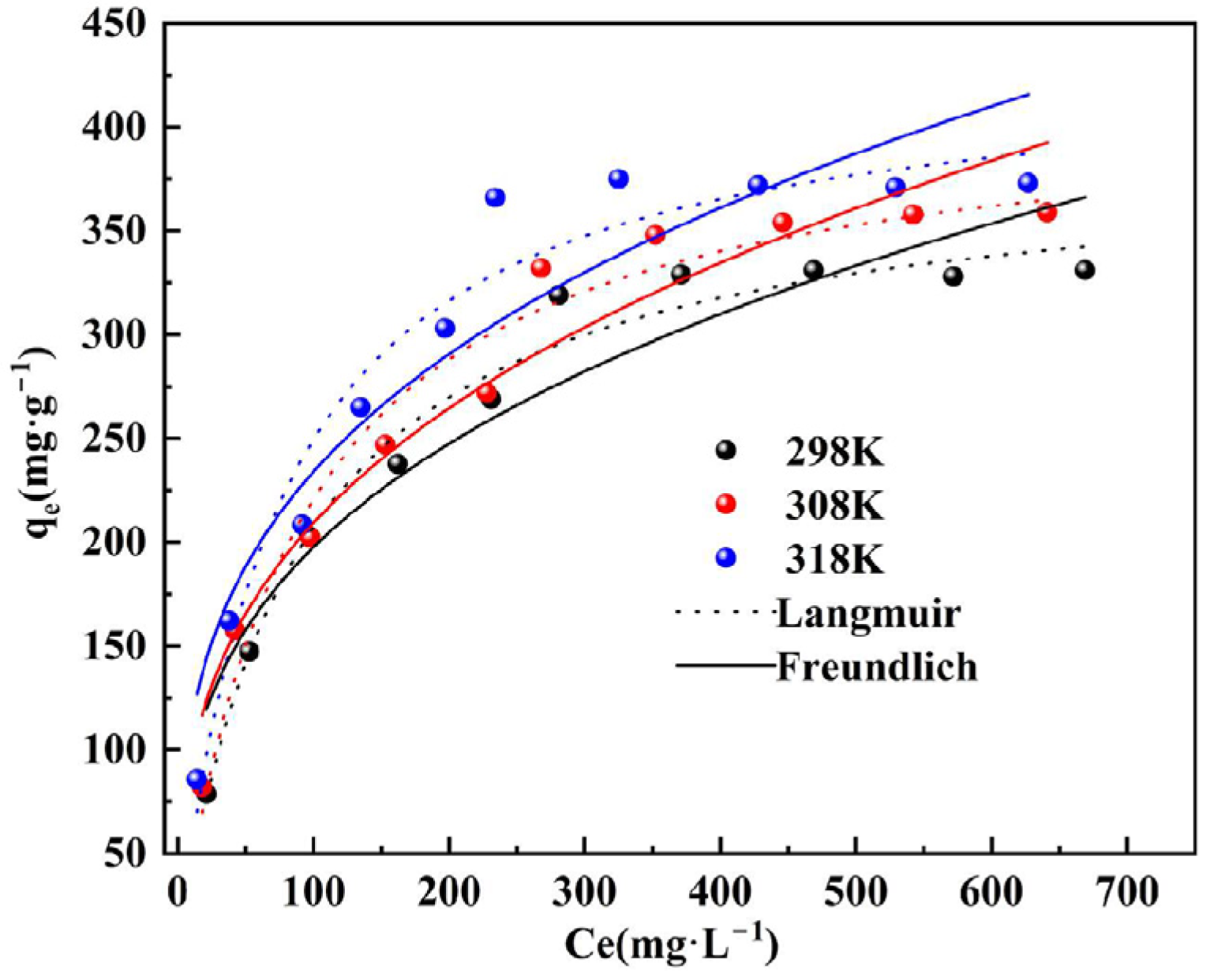
3.5. Adsorption Isotherm
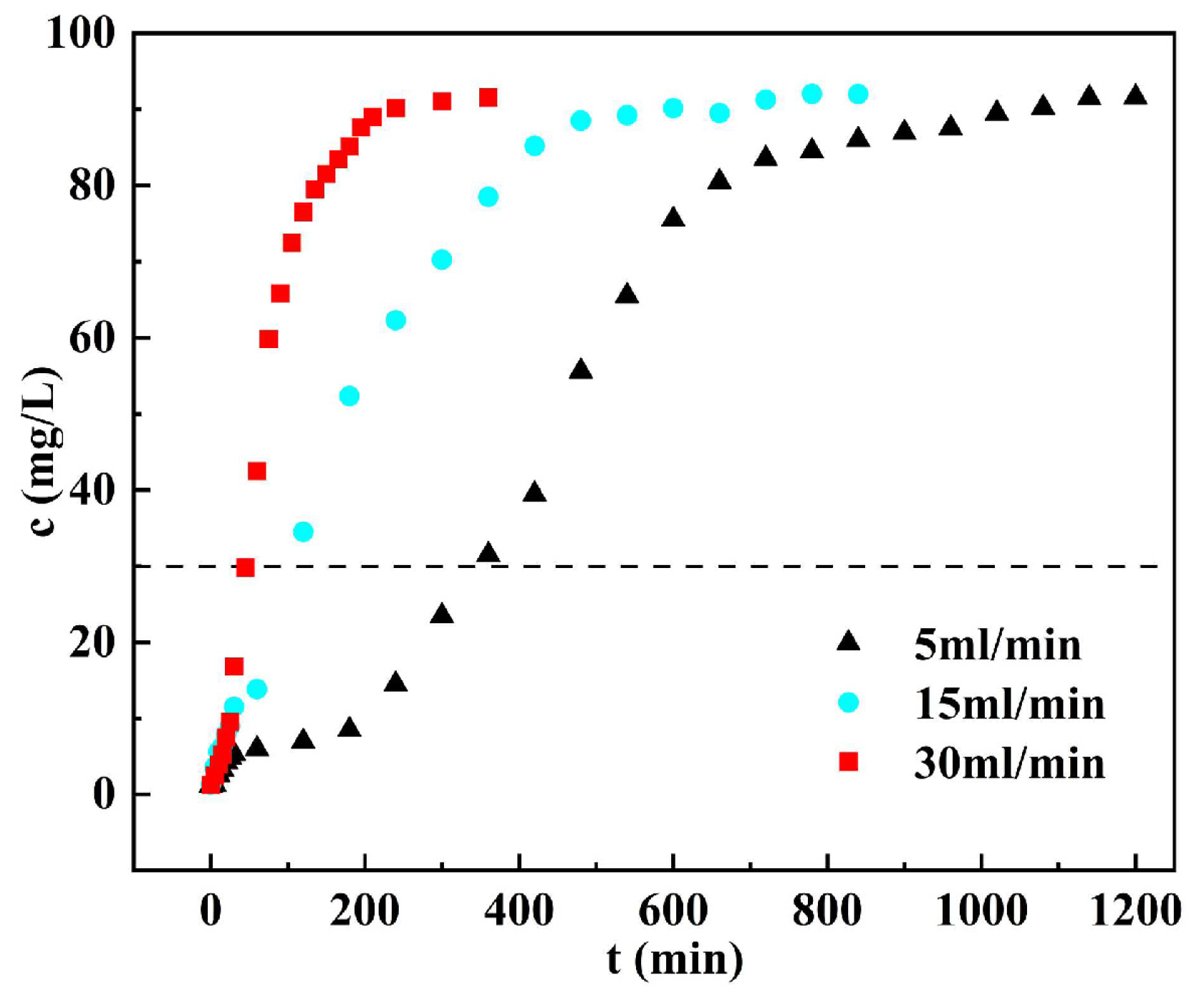
3.6. Dynamic Adsorption
3.6.1. Penetration Curves
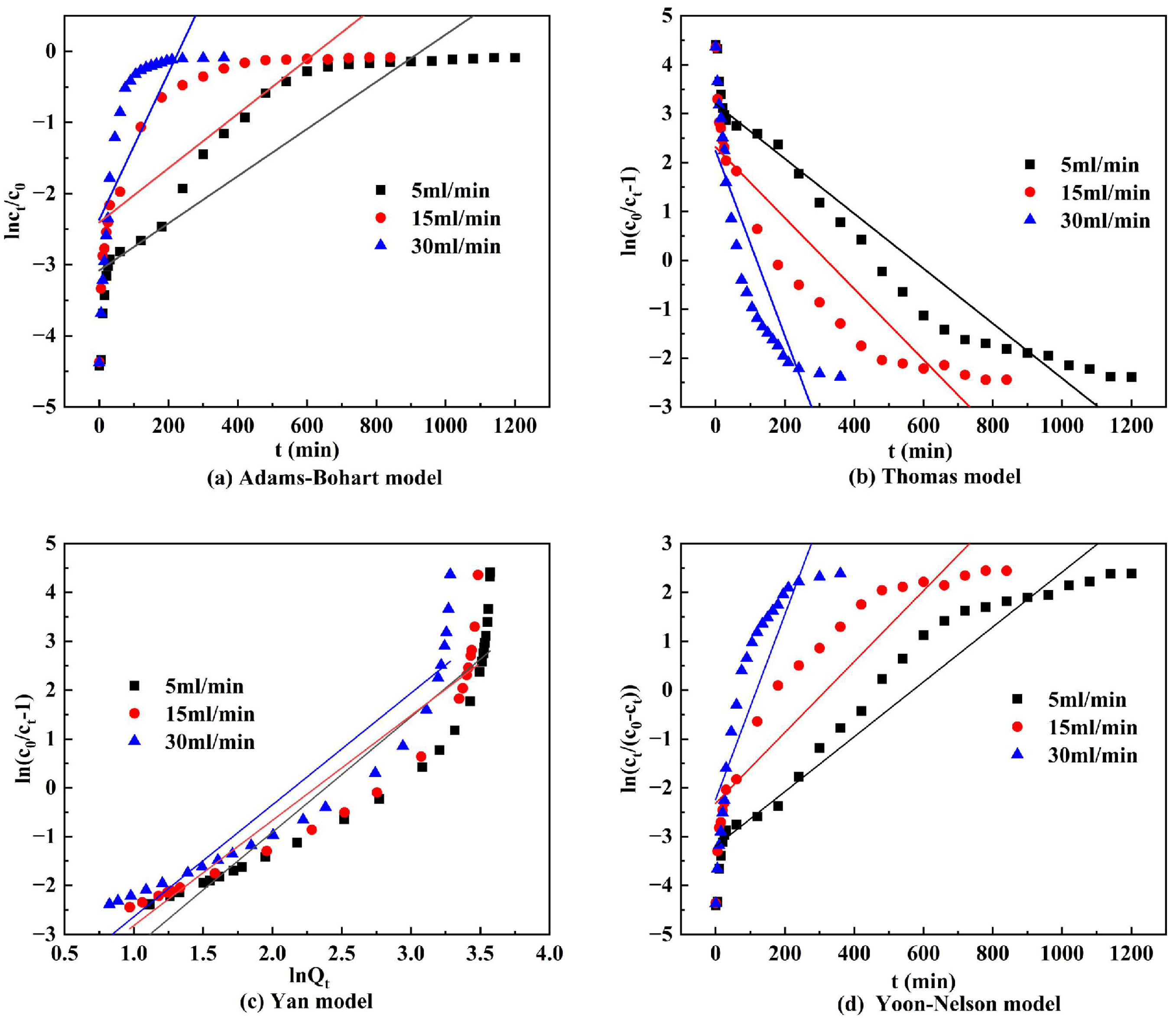
3.6.2. Dynamic Adsorption Models
4. Conclusions
- Through the mechanical and adsorption properties tests, the optimal ceramsite (DS-EMR) was prepared by mixing EMR, DS, and AC at a ratio of 7:2:1 by mass, adding 5 wt% WH as a pore-forming additive, and then sintering at 500 °C.
- Batch adsorption experiments of DS-EMR demonstrated that, at a dosage of 1 g/L, pH = 4 exhibited an adsorption capacity of up to 94.19 mg/g with 96.26% phosphorus removal, indicating that DS-EMR has a significant effect and application prospect for phosphorus removal from wastewater.
- The static adsorption tests showed that the pseudo-second-order kinetic model and Langmuir adsorption isotherm model gave the best fit, indicating that the monolayer chemisorption interactions may play a dominant role in the adsorption of phosphorus by DS-EMR.
- The dynamic adsorption test used four simplified dynamic adsorption models to predict the experimental results, and the DS-EMR ceramic grains fitted well with the Thomas, Yan, and Yoon–Nelson models, indicating that DS-EMR ceramic grains were also able to satisfy the assumptions of the Langmuir adsorption isotherm and pseudo-second-order adsorption kinetics during the dynamic adsorption of phosphorus removal.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biswas, B.K.; Inoue, K.; Ghimire, K.N.; Harada, H.; Ohto, K.; Kawakita, H. Removal and Recovery of Phosphorus from Water by Means of Adsorption onto Orange Waste Gel Loaded with Zirconium. Bioresour. Technol. 2008, 99, 8685–8690. [Google Scholar] [CrossRef]
- Shao, Q.; Zhang, Y.; Liu, Z.; Long, L.; Liu, Z.; Chen, Y.; Hu, X.-M.; Lu, M.; Huang, L.-Z. Phosphorus and Nitrogen Recovery from Wastewater by Ceramsite: Adsorption Mechanism, Plant Cultivation and Sustainability Analysis. Sci. Total Environ. 2022, 805, 150288. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, G.; Li, L.; Xu, Y.; Zhang, Y.; Liu, C.; Zhang, Z. Fabrication of Ceramsite Adsorbent from Industrial Wastes for the Removal of Phosphorus from Aqueous Solutions. J. Chem. 2020, 2020, 8036961. [Google Scholar] [CrossRef]
- Lu, M.; Wang, R.; Xue, Y.; Ren, L.; Chen, S.; Liu, J.; Mei, M.; Wang, T.; Li, J. Eco-Friendly Ceramsite from Dredged Sediment/Biomass for Pb (II) Removal: Process Optimization and Adsorption Mechanistic Insights. J. Environ. Chem. Eng. 2022, 10, 108939. [Google Scholar] [CrossRef]
- Li, T.; Fan, J.; Sun, T. Acid Red G Dye Removal from Aqueous Solutions by Porous Ceramsite Produced from Solid Wastes: Batch and Fixed-Bed Studies. Green Process. Synth. 2020, 9, 770–782. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Zhou, J.; Qian, G. Production of an Effective Catalyst with Increased Oxygen Vacancies from Manganese Slag for Selective Catalytic Reduction of Nitric Oxide. J. Environ. Manag. 2019, 239, 90–95. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Shu, J.; Wang, R.; Chen, M.; Wang, R.; Gao, Y.; Liu, R.; Liu, Z.; Xu, Z.; Tan, D. A Critical Review on Approaches for Electrolytic Manganese Residue Treatment and Disposal Technology: Reduction, Pretreatment, and Reuse. J. Hazard. Mater. 2021, 418, 126235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xiao, D.; Yu, Q.; Chen, S.; Chen, S.; Miao, M. Preparation of Mesoporous Silica from Electrolytic Manganese Slags by Using Amino-Ended Hyperbranched Polyamide as Template. ACS Sustain. Chem. Eng. 2017, 5, 10258–10265. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Gao, G.; Fu, B.; Ye, Z.; Shen, Q. Exploring Responses of Lake Area to River Regulation and Implications for Lake Restoration in Arid Regions. Ecol. Eng. 2019, 128, 18–26. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Peng, Y.; Wang, H.; Wang, X.; Song, J.; Fei, G. Restoration of Aquatic Macrophytes with the Seed Bank Is Difficult in Lakes with Reservoir-like Water-Level Fluctuations: A Case Study of Chaohu Lake in China. Sci. Total Environ. 2022, 813, 151860. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zeng, W.; Ma, B.; Cui, D.; Xie, Y.; Wang, J. Spatiotemporal Variation and Influencing Factors of Nitrogen and Phosphorus in Lake Sediments in China since 1850. J. Clean. Prod. 2022, 368, 133170. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Tao, S. The Challenges and Solutions for Cadmium-Contaminated Rice in China: A Critical Review. Environ. Int. 2016, 92, 515–532. [Google Scholar] [CrossRef]
- Park, J.; Son, Y.; Noh, S.; Bong, T. The Suitability Evaluation of Dredged Soil from Reservoirs as Embankment Material. J. Environ. Manag. 2016, 183, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Xiao, K.; Zhou, W.; Zhu, D.; Zhou, Y.; Yuan, Y.; Xiao, N.; Wan, X.; Hua, Y.; Zhao, J. Analysis of Utilization Technologies for Eichhornia Crassipes Biomass Harvested after Restoration of Wastewater. Bioresour. Technol. 2017, 223, 287–295. [Google Scholar] [CrossRef]
- Kumwimba, M.N.; Dzakpasu, M.; Li, X. Potential of Invasive Watermilfoil (Myriophyllum Spp.) to Remediate Eutrophic Waterbodies with Organic and Inorganic Pollutants. J. Environ. Manag. 2020, 270, 110919. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, J.; Liu, Y.; Sheng, L. Preparation of Ceramsite from Municipal Sludge and Its Application in Water Treatment: A Review. J. Environ. Manag. 2021, 287, 112374. [Google Scholar] [CrossRef]
- Lv, G.; Wu, S. Analytical Pyrolysis Studies of Corn Stalk and Its Three Main Components by TG-MS and Py-GC/MS. J. Anal. Appl. Pyrolysis 2012, 97, 11–18. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and Potential of the Anaerobic Digestion of Waste-Activated Sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Marani, D.; Braguglia, C.M.; Mininni, G.; Maccioni, F. Behaviour of Cd, Cr, Mn, Ni, Pb, and Zn in Sewage Sludge Incineration by Fluidised Bed Furnace. Waste Manag. 2003, 23, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, X.; Wang, W.; Guo, J.; Zhang, L.; Zhang, H. Effect of SiO2 and Al2O3 on Characteristics of Lightweight Aggregate Made from Sewage Sludge and River Sediment. Ceram. Int. 2018, 44, 4313–4319. [Google Scholar] [CrossRef]
- Wang, L.; Shao, Y.; Zhao, Z.; Chen, S.; Shao, X. Optimized Utilization Studies of Dredging Sediment for Making Water Treatment Ceramsite Based on an Extreme Vertex Design. J. Water Process Eng. 2020, 38, 101603. [Google Scholar] [CrossRef]
- Zhang, Y.; Labianca, C.; Chen, L.; De Gisi, S.; Notarnicola, M.; Guo, B.; Sun, J.; Ding, S.; Wang, L. Sustainable Ex-Situ Remediation of Contaminated Sediment: A Review. Environ. Pollut. 2021, 287, 117333. [Google Scholar] [CrossRef]
- Ma, C.; Bao, S.; Zhang, Y.; Luo, Y.; Gui, Y.; Ren, Y. Preparation of Non-Sintered Sewage Sludge Based Ceramsite by Alkali-Thermal Activation and Hydration Mechanism. Ceram. Int. 2022, 48, 31606–31613. [Google Scholar] [CrossRef]
- Qi, Y.; Dai, B.; He, S.; Wu, S.; Huang, J.; Xi, F.; Ma, Y.; Meng, M. Effect of Chemical Constituents of Oxytetracycline Mycelia Residue and Dredged Sediments on Characteristics of Ultra-Lightweight Ceramsite. J. Taiwan Inst. Chem. Eng. 2016, 65, 225–232. [Google Scholar] [CrossRef]
- GB/T 17431.2-2010; Lightweight Aggregates and Its Test Methods—Part 2: Test Methods for Lightweight Aggregates. General Administration of Quality Supervision. Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2010.
- Xue, Y.; Hou, H.; Zhu, S. Adsorption Removal of Reactive Dyes from Aqueous Solution by Modified Basic Oxygen Furnace Slag: Isotherm and Kinetic Study. Chem. Eng. J. 2009, 147, 272–279. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, S.; Zhou, M. Adsorption Characterization of Cu(II) from Aqueous Solution onto Basic Oxygen Furnace Slag. Chem. Eng. J. 2013, 231, 355–364. [Google Scholar] [CrossRef]
- Ren, J.; Leus, K.; Morent, R.; De Geyter, N.; Van Der Voort, P.; Du Laing, G. Fe(III)-Alginate and Layered Aluminum Oxyhydroxide Assisted Hydro Ceramsite Composite for Efficient Removal of Se and as from High-Concentration Sulfate Wastewater. Sep. Purif. Technol. 2024, 334, 126101. [Google Scholar] [CrossRef]
- Łopata, M.; Czerniejewski, P.; Wiśniewski, G.; Czerniawski, R.; Drozdowski, J. The Use of Expanded Clay Aggregate for the Pretreatment of Surface Waters on the Example of a Tributary of Lake Klasztorne Górne in Strzelce Krajeńskie. Limnol. Rev. 2017, 17, 3–9. [Google Scholar] [CrossRef]
- Masłoń, A.A.; Czarnota, J. Efficiency of Brick Dust and Powdered Ceramsite in the Phosphorus Removal from Wastewater. J. Ecol. Eng. 2020, 21, 63–71. [Google Scholar] [CrossRef]
- Zain, Z.M.; Abdulhameed, A.S.; Jawad, A.H.; ALOthman, Z.A.; Yaseen, Z.M. A pH-Sensitive Surface of Chitosan/Sepiolite Clay/Algae Biocomposite for the Removal of Malachite Green and Remazol Brilliant Blue R Dyes: Optimization and Adsorption Mechanism Study. J. Polym. Environ. 2023, 31, 501–518. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, C.; Shen, H.; Du, J.; Li, Q.; Wu, W.; Guo, B.; Liu, G. Green Synthesis of Ceramsite from Industrial Wastes and Its Application in Selective Adsorption: Performance and Mechanism. Environ. Res. 2022, 214, 113786. [Google Scholar] [CrossRef]
- Hao, L.; Song, H.; Zhang, L.; Wan, X.; Tang, Y.; Lv, Y. SiO2/Graphene Composite for Highly Selective Adsorption of Pb(II) Ion. J. Colloid Interface Sci. 2012, 369, 381–387. [Google Scholar] [CrossRef]
- Njaramba, L.K.; Kim, M.; Yea, Y.; Yoon, Y.; Park, C.M. Efficient Adsorption of Naproxen and Ibuprofen by Gelatin/Zirconium-Based Metal–Organic Framework/Sepiolite Aerogels via Synergistic Mechanisms. Chem. Eng. J. 2023, 452, 139426. [Google Scholar] [CrossRef]
- Panda, L.; Rath, S.S.; Rao, D.S.; Nayak, B.B.; Das, B.; Misra, P.K. Thorough Understanding of the Kinetics and Mechanism of Heavy Metal Adsorption onto a Pyrophyllite Mine Waste Based Geopolymer. J. Mol. Liq. 2018, 263, 428–441. [Google Scholar] [CrossRef]
- Isidoro Ribeiro, N.; Barreto Pessanha, O.; Luiza Gomes Soares Pessanha, M.; Guimarães, D. Efficient Phosphate Adsorption by a Composite Composed of Mg6Al2(CO3)(OH)16·4H2O LDH and Chitosan: Kinetic, Thermodynamic, Desorption, and Characterization Studies. Sep. Purif. Technol. 2023, 307, 122717. [Google Scholar] [CrossRef]
- Sellaoui, L.; Gómez-Avilés, A.; Dhaouadi, F.; Bedia, J.; Bonilla-Petriciolet, A.; Rtimi, S.; Belver, C. Adsorption of Emerging Pollutants on Lignin-Based Activated Carbon: Analysis of Adsorption Mechanism via Characterization, Kinetics and Equilibrium Studies. Chem. Eng. J. 2023, 452, 139399. [Google Scholar] [CrossRef]
- Mel’gunov, M.S. Application of the Simple Bayesian Classifier for the N2 (77 K) Adsorption/Desorption Hysteresis Loop Recognition. Adsorption 2023, 29, 199–208. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Peng, S.-Y.; Lee, W.-H.; Lin, Y.-Y.; Hung, M.-J.; Lin, K.-L. Characterization of Cu2+ Adsorption for Eco-Hydroxyapatite Derived from Limestone Sludge via Hydrothermal Synthesis. J. Mater. Cycles Waste Manag. 2023, 25, 1069–1081. [Google Scholar] [CrossRef]
- Van Truong, T.; Kim, Y.-J.; Kim, D.-J. Study of Biochar Impregnated with Al Recovered from Water Sludge for Phosphate Adsorption/Desorption. J. Clean. Prod. 2023, 383, 135507. [Google Scholar] [CrossRef]
- Hamid, U.; Vyawahare, P.; Chen, C.-C. Estimation of Isosteric Heat of Adsorption from Generalized Langmuir Isotherm. Adsorption 2023, 29, 45–64. [Google Scholar] [CrossRef]
- Mussa, Z.H.; Al-Ameer, L.R.; Al-Qaim, F.F.; Deyab, I.F.; Kamyab, H.; Chelliapan, S. A Comprehensive Review on Adsorption of Methylene Blue Dye Using Leaf Waste as a Bio-Sorbent: Isotherm Adsorption, Kinetics, and Thermodynamics Studies. Environ. Monit. Assess. 2023, 195, 940. [Google Scholar] [CrossRef]
- Vilvanathan, S.; Shanthakumar, S. Column Adsorption Studies on Nickel and Cobalt Removal from Aqueous Solution Using Native and Biochar Form of Tectona Grandis. Environ. Prog. Sustain. Energy 2017, 36, 1030–1038. [Google Scholar] [CrossRef]
- Cardenas, C.; Sigot, L.; Vallières, C.; Marsteau, S.; Marchal, M.; Latifi, A.M. Ammonia Capture by Adsorption on Doped and Undoped Activated Carbon: Isotherm and Breakthrough Curve Measurements. Sep. Purif. Technol. 2023, 313, 123454. [Google Scholar] [CrossRef]
- Kebir, M.; Tahraoui, H.; Chabani, M.; Trari, M.; Noureddine, N.; Assadi, A.A.; Amrane, A.; Ben Hamadi, N.; Khezami, L. Water Cleaning by a Continuous Fixed-Bed Column for Cr(VI) Eco-Adsorption with Green Adsorbent-Based Biomass: An Experimental Modeling Study. Processes 2023, 11, 363. [Google Scholar] [CrossRef]
- Phawachalotorn, C.; Wongniramaikul, W.; Taweekarn, T.; Kleangklao, B.; Pisitaro, W.; Limsakul, W.; Sriprom, W.; Towanlong, W.; Choodum, A. Continuous Phosphate Removal and Recovery Using a Calcium Silicate Hydrate Composite Monolithic Cryogel Column. Polymers 2023, 15, 539. [Google Scholar] [CrossRef]
- Hamid, A.; Wilson, A.E.; Torbert, H.A.; Wang, D. Sorptive Removal of Phosphorus by Flue Gas Desulfurization Gypsum in Batch and Column Systems. Chemosphere 2023, 320, 138062. [Google Scholar] [CrossRef]
- Chittoo, B.S.; Sutherland, C. Column Breakthrough Studies for the Removal and Recovery of Phosphate by Lime-Iron Sludge: Modeling and Optimization Using Artificial Neural Network and Adaptive Neuro-Fuzzy Inference System. Chin. J. Chem. Eng. 2020, 28, 1847–1859. [Google Scholar] [CrossRef]


| DS | EMR | WH | AC | |
|---|---|---|---|---|
| SiO2 | 62.19 | 23.32 | 2.67 | 0.57 |
| CaO | 3.04 | 11.36 | 1.50 | 31.33 |
| Fe2O3 | 4.86 | 2.84 | 0.29 | 0.12 |
| MgO | 1.86 | 2.77 | 0.68 | 0.34 |
| Al2O3 | 14.47 | 7.88 | 0.97 | 66.93 |
| Cl | / | / | 2.35 | / |
| SO3 | 0.08 | 26.73 | 0.79 | 0.01 |
| Na2O | 1.10 | 0.39 | 0.18 | 0.35 |
| K2O | 2.52 | 1.64 | 0.47 | 0.02 |
| MnO | / | 5.42 | / | / |
| Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|
| qe/mg·g−1 | k1 | R2 | qe/mg·g−1 | k2 | R2 |
| 7859.51 | 0.00289 | 0.8595 | 94.0734 | 0.00031 | 0.9968 |
| T/°C | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| qm | KL | R2 | KF | n | R2 | |
| 25 | 386.65 | 0.01154 | 0.9787 | 44.28 | 3.08 | 0.9034 |
| 35 | 415.01 | 0.01136 | 0.9650 | 43.96 | 2.95 | 0.9350 |
| 45 | 432.25 | 0.01365 | 0.9545 | 55.29 | 3.19 | 0.8880 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Shi, W.; Liu, S.; Wang, Y.; Song, J.; Long, Y.; Xiang, Y.; Xue, Y. Adsorption of Phosphate by Two-Step Synthesis of Ceramsite from Electrolytic Manganese Residue/Dredged Sludge. Materials 2024, 17, 939. https://doi.org/10.3390/ma17040939
Cheng H, Shi W, Liu S, Wang Y, Song J, Long Y, Xiang Y, Xue Y. Adsorption of Phosphate by Two-Step Synthesis of Ceramsite from Electrolytic Manganese Residue/Dredged Sludge. Materials. 2024; 17(4):939. https://doi.org/10.3390/ma17040939
Chicago/Turabian StyleCheng, Hao, Wei Shi, Song Liu, Yong Wang, Jia Song, Yu Long, Yuan Xiang, and Yongjie Xue. 2024. "Adsorption of Phosphate by Two-Step Synthesis of Ceramsite from Electrolytic Manganese Residue/Dredged Sludge" Materials 17, no. 4: 939. https://doi.org/10.3390/ma17040939
APA StyleCheng, H., Shi, W., Liu, S., Wang, Y., Song, J., Long, Y., Xiang, Y., & Xue, Y. (2024). Adsorption of Phosphate by Two-Step Synthesis of Ceramsite from Electrolytic Manganese Residue/Dredged Sludge. Materials, 17(4), 939. https://doi.org/10.3390/ma17040939






