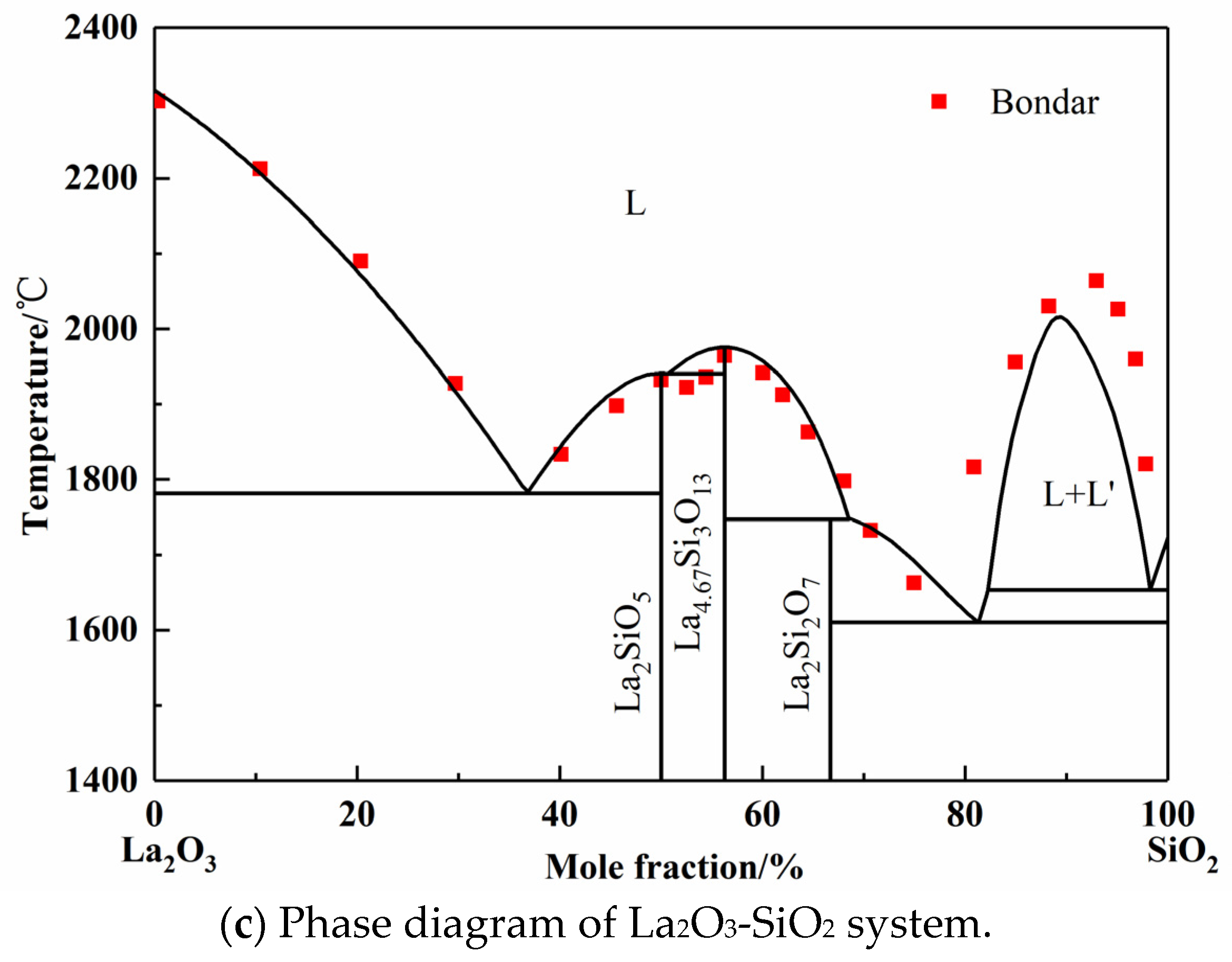
In the original publication [1], there was a mistake in Figure 2c Phase diagram of La2O3-SiO2 system as published. The wrong figure was one progress picture based on the Toropov group’s older literature data, which is inconsistent with the full text description. The corrected Figure 2c for the phase diagram of the La2O3-SiO2 system appears below.
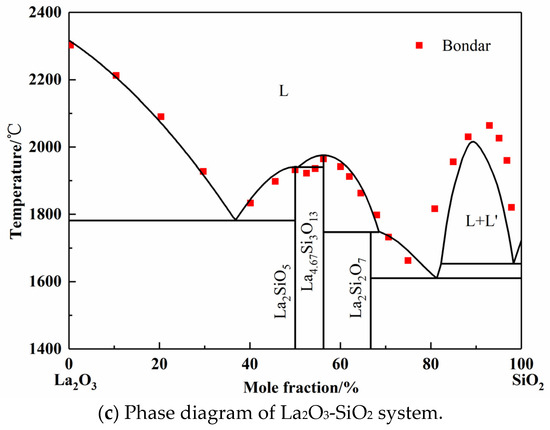
Figure 2.
Calculated phase diagrams of binary systems.
There was an error in the original publication. One chemical formula La4Si3O12 should be La4.67Si3O13 to be consistent with the paper. A correction has been made to 3.1.3. The La2O3-SiO2 System:
The La2O3-SiO2 system is rarely studied by experiment or simulation, and different opinions on the intermediate phases have always been there. In 1961, Toropov released the La2O3-SiO2 phase diagram, and the intermediate compounds are La2Si2O7, La2SiO5, and La4Si3O12. In 1982, Bondar, from the same research group as Toropov, modified the La4Si3O12 phase to La4.67Si3O13. Li finished the calculated La2O3-SiO2 system employing simplified thermodynamic properties in 1999 [11]. However, the adoptive compound was La4Si3O12. Kim only calculated the two-liquid region using the Redlich–Kister expression [44]. However, the number and values of parameters given by Li and Kim are completely different. In this work, La2Si2O7, La2SiO5, and La4.67Si3O13 were chosen as the intermediate compounds, which were found in the equilibrium experimental results, as shown in Figure 5 later. Since the available experimental points are from Toropov and Bondar, the interaction energy of solution phase and the derived thermodynamic parameters of silicates were optimized. The calculated phase diagram is presented in Figure 2c [45,46]. It can be seen that most of the experiment points have good agreement with the calculation results.
The authors state that the scientific conclusions are unaffected. This correction was approved by the Academic Editor. The original publication has also been updated.
Reference
- Li, Y.; Zhang, T.; Feng, Y.; Liu, C.; Jiang, M. Liquid Regions of Lanthanum-Bearing Aluminosilicates. Materials 2020, 13, 450. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).