Effect of Low-Temperature Oxygen Plasma Treatment of Titanium Alloy Surface on Tannic Acid Coating Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Modification
2.2. Sample Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Ahn, T.K.; Lee, D.H.; Kim, T.S.; Jang, C.S.; Oh, J.B.; Ye, G.; Lee, S. Modification of Titanium Implant and Titanium Dioxide for Bone Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1077, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chu, P.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R. Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Variola, F.; Vetrone, F.; Richert, L.; Jedrzejowski, P.; Yi, J.H.; Zalzal, S.; Clair, S.; Sarkissian, A.; Perepichka, D.F.; Wuest, J.D.; et al. Improving biocompatibility of implantable metals by nanoscale modification of surfaces: An overview of strategies, fabrication methods, and challenges. Small 2009, 5, 996–1006. [Google Scholar] [CrossRef]
- Amin Yavari, S.; van der Stok, J.; Chai, Y.C.; Wauthle, R.; Tahmasebi Birgani, Z.; Habibovic, P.; Mulier, M.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Bone regeneration performance of surface-treated porous titanium. Biomaterials 2014, 35, 6172–6181. [Google Scholar] [CrossRef] [PubMed]
- Amin Yavari, S.; Chai, Y.C.; Böttger, A.J.; Wauthle, R.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Effects of anodizing parameters and heat treatment on nanotopographical features, bioactivity, and cell culture response of additively manufactured porous titanium. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 51, 132–138. [Google Scholar] [CrossRef]
- Orapiriyakul, W.; Young, P.S.; Damiati, L.; Tsimbouri, P.M. Antibacterial surface modification of titanium implants in orthopaedics. J. Tissue Eng. 2018, 9, 2041731418789838. [Google Scholar] [CrossRef]
- Cao, Y.; Su, B.; Chinnaraj, S.; Jana, S.; Bowen, L.; Charlton, S.; Duan, P.; Jakubovics, N.S.; Chen, J. Nanostructured titanium surfaces exhibit recalcitrance towards Staphylococcus epidermidis biofilm formation. Sci. Rep. 2018, 8, 1071. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R. Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and Sterilization Using Plasma Technology: Fundamentals and Future Perspectives for Biological Applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef]
- Panariello, B.H.D.; Mody, D.P.; Eckert, G.J.; Witek, L.; Coelho, P.G.; Duarte, S. Low-Temperature Plasma Short Exposure to Decontaminate Peri-Implantitis-Related Multispecies Biofilms on Titanium Surfaces In Vitro. Biomed. Res. Int. 2022, 2022, 1549774. [Google Scholar] [CrossRef] [PubMed]
- Stepczyńska, M. Surface Modification by Low Temperature Plasma: Sterilization of biodegradable material. Plasma Process Polym. 2016, 13, 1080–1088. [Google Scholar] [CrossRef]
- Laschke, M.W.; Augustin, V.A.; Sahin, F.; Anschütz, D.; Metzger, W.; Scheuer, C.; Bischoff, M.; Aktas, C.; Menger, M.D. Surface modification by plasma etching impairs early vascularization and tissue incorporation of porous polyethylene (Medpor®) implants. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Bouaidat, S.; Berendsen, C.; Thomsen, P.; Petersen, S.G.; Wolff, A.; Jonsmann, J. Micro patterning of cell and protein non-adhesive plasma polymerized coatings for biochip applications. Lab. Chip 2004, 4, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Ohl, A.; Schroder, K. Plasma-induced chemical micropatterning for cell culturing applications: A brief review. Surf. Coat. Technol. 1999, 116–119, 820–830. [Google Scholar] [CrossRef]
- Narimisa, M.; Krčma, F.; Onyshchenko, Y.; Kozáková, Z.; Morent, R.; De Geyter, N. Atmospheric Pressure Microwave Plasma Jet for Organic Thin Film Deposition. Polymers 2020, 12, 354. [Google Scholar] [CrossRef] [PubMed]
- Ujino, D.; Nishizaki, H.; Higuchi, S.; Komasa, S.; Okazaki, J. Effect of Plasma Treatment of Titanium Surface on Biocompatibility. Appl. Sci. 2019, 9, 2257. [Google Scholar] [CrossRef]
- Akdoğan, E.; Şirin, H.T. Plasma surface modification strategies for the preparation of antibacterial biomaterials: A review of the recent literature. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112474. [Google Scholar] [CrossRef]
- Nicol, M.J.; Brubaker, T.R.; Honish, B.J., 2nd; Simmons, A.N.; Kazemi, A.; Geissel, M.A.; Whalen, C.T.; Siedlecki, C.A.; Bilén, S.G.; Knecht, S.D.; et al. Antibacterial effects of low-temperature plasma generated by atmospheric-pressure plasma jet are mediated by reactive oxygen species. Sci. Rep. 2020, 10, 3066. [Google Scholar] [CrossRef]
- Teng, F.; Li, J.; Wu, Y.; Chen, H.; Zhang, Q.; Wang, H.; Ou, G. Fabrication and bioactivity evaluation of porous anodised TiO2 films in vitro. Biosci. Trends 2014, 8, 260–265. [Google Scholar] [CrossRef]
- Stepczyńska, M. Research of biocidal effect of corona discharges on poly(lactic acid) packaging films. J. Food Eng. 2014, 126, 56–61. [Google Scholar] [CrossRef]
- Guastaldi, F.P.; Yoo, D.; Marin, C.; Jimbo, R.; Tovar, N.; Zanetta-Barbosa, D.; Coelho, P.G. Plasma treatment maintains surface energy of the implant surface and enhances osseointegration. Int. J. Biomater. 2013, 2013, 354125. [Google Scholar] [CrossRef] [PubMed]
- Naujokat, H.; Harder, S.; Schulz, L.Y.; Wiltfang, J.; Flörke, C.; Açil, Y. Surface conditioning with cold argon plasma and its effect on the osseointegration of dental implants in miniature pigs. J. Craniomaxillofac. Surg. 2019, 47, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.L.; Lim, Y.W.; Kwon, S.Y.; Bahk, J.H.; Kim, J.; Shin, T.; Kim, Y. Non-thermal atmospheric pressure plasma treatment increases hydrophilicity and promotes cell growth on titanium alloys in vitro. Sci. Rep. 2023, 13, 14792. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.; Zhao, H.; Liu, Y.; Liu, J.; Bai, N. Bioactive Effects of Low-Temperature Argon-Oxygen Plasma on a Titanium Implant Surface. ACS Omega 2020, 5, 3996–4003. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Shi, X.; Wu, X. Applications and challenges of low temperature plasma in pharmaceutical field. J. Pharm. Anal. 2021, 11, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.-D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Yan, M.; Hartjen, P.; Gosau, M.; Vollkommer, T.; Grust, A.L.C.; Fuest, S.; Kluwe, L.; Burg, S.; Smeets, R.; Henningsen, A. Effects of a Novel Cold Atmospheric Plasma Treatment of Titanium on the Proliferation and Adhesion Behavior of Fibroblasts. Int. J. Mol. Sci. 2021, 23, 420. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, M.A.; Han, G.J.; Cho, B.H. Plasma in dentistry: A review of basic concepts and applications in dentistry. Acta Odontol. Scand. 2014, 72, 1–12. [Google Scholar] [CrossRef]
- Pan, Y.-H.; Yao, W.-L.; Lin, J.C.Y.; Salamanca, E.; Tsai, P.-Y.; Leu, S.-J.; Yang, K.-C.; Huang, H.-M.; Teng, N.C.; Chang, W.-J. Improvement in the Biological Properties of Titanium Surfaces with Low-Temperature Plasma. Metals 2019, 9, 943. [Google Scholar] [CrossRef]
- Tan, F.; Fang, Y.; Zhu, L.; Al-Rubeai, M. Cold atmospheric plasma as an interface biotechnology for enhancing surgical implants. Crit. Rev. Biotechnol. 2021, 41, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Sileika, T.S.; Barrett, D.G.; Zhang, R.; Lau, K.H.; Messersmith, P.B. Colorless multifunctional coatings inspired by polyphenols found in tea, chocolate, and wine. Angew. Chem. Int. Ed. Engl. 2013, 52, 10766–10770. [Google Scholar] [CrossRef]
- Ge, W.; Cao, S.; Shen, F.; Wang, Y.; Ren, J.; Wang, X. Rapid self-healing, stretchable, moldable, antioxidant and antibacterial tannic acid-cellulose nanofibril composite hydrogels. Carbohydr. Polym. 2019, 224, 115147. [Google Scholar] [CrossRef]
- Sahiner, N.; Butun Sengel, S.; Yildiz, M. A facile preparation of donut-like supramolecular tannic acid-Fe(III) composite as biomaterials with magnetic, conductive, and antioxidant properties. J. Coord. Chem. 2017, 70, 3619–3632. [Google Scholar] [CrossRef]
- Steffi, C.; Shi, Z.; Kong, C.H.; Wang, W. Bioinspired polydopamine and polyphenol tannic acid functionalized titanium suppress osteoclast differentiation: A facile and efficient strategy to regulate osteoclast activity at bone-implant interface. J. R. Soc. Interface 2019, 16, 20180799. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, A.; Satué, M.; Gómez-Florit, M.; Hierro-Oliva, M.; Petzold, C.; Lyngstadaas, S.P.; González-Martín, M.L.; Monjo, M.; Ramis, J.M. Flavonoid-Modified Surfaces: Multifunctional Bioactive Biomaterials with Osteopromotive, Anti-Inflammatory, and Anti-Fibrotic Potential. Adv. Healthc. Mater. 2015, 4, 540–549. [Google Scholar] [CrossRef]
- Geißler, S.; Gomez-Florit, M.; Wiedmer, D.; Barrantes, A.; Petersen, F.C.; Tiainen, H. In Vitro Performance of Bioinspired Phenolic Nanocoatings for Endosseous Implant Applications. ACS Biomater. Sci. Eng. 2019, 5, 3340. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chang, Y.-Y.; Lee, J.; Perikamana, S.K.M.; Kim, E.M.; Jung, Y.-H.; Yun, J.-H.; Shin, H. Surface engineering of titanium alloy using metal-polyphenol network coating with magnesium ions for improved osseointegration. Biomater. Sci. 2020, 8, 3404–3417. [Google Scholar] [CrossRef]
- Steffi, C.; Shi, Z.; Kong, C.H.; Chong, S.W.; Wang, D.; Wang, W. Use of Polyphenol Tannic Acid to Functionalize Titanium with Strontium for Enhancement of Osteoblast Differentiation and Reduction of Osteoclast Activity. Polymers 2019, 11, 1256. [Google Scholar] [CrossRef]
- J.A. Woollam Co., Inc. Guide to Using WVASE32®; Wextech Systems Inc.: New York, NY, USA, 2010. [Google Scholar]
- Fujiwara, H. Spectroscopic Ellipsometry. Principles and Applications, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2007. [Google Scholar]
- Skowronski, L.; Wachowiak, A.A.; Wachowiak, W. Optical and microstructural properties of decorative Al/Ti/TiO2 interference coatings. Appl. Surf. Sci. 2017, 421, 794–801. [Google Scholar] [CrossRef]
- Skowronski, L.; Wachowiak, A.A.; Zdunek, K.; Trzcinski, M.; Naparty, M.K. TiO2-based decorative coatings deposited on the AISI 316l stainless steel and glass using an industrial scale magnetron. Thin Solid. Films 2017, 627, 1–8. [Google Scholar] [CrossRef]
- Skowronski, L.; Wachowiak, A.A.; Grabowski, A. Characterization of optical and microstructural properties of semitransparent TiO2/Ti/glass interference decorative coatings. Appl. Surf. Sci. 2016, 388, 731–740. [Google Scholar] [CrossRef]
- Järrendahl, K.; Arwin, H. Multiple sample analysis of spectroscopic ellipsometry data of semi-transparent films. Thin Solid. Films 1998, 313–314, 114–118. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J.P. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method. Surf. Interface Anal. 1998, 26, 549–564. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, W.M.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics Division, Perkin-Elmer Corp.: Eden Prairie, MI, USA, 1992. [Google Scholar]
- Modic, M.; Kovač, J.; Nicholls, J.R.; Kos, Š.; Serša, G.; Cvelbar, U.; Walsh, J.L. Targeted plasma functionalization of titanium inhibits polymicrobial biofilm recolonization and stimulates cell function. Appl. Surf. Sci. 2019, 487, 1176–1188. [Google Scholar] [CrossRef]
- Biesinger, M.C. Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Skowronski, L.; Chodun, R.; Zdunek, K. TiO2—Based decorative interference coatings produced at industrial conditions. Thin Solid Films 2020, 711, 138294. [Google Scholar] [CrossRef]
- Skowronski, L.; Chorobinski, M. The effect of thickness and optical constants of the dielectric layer on the color behaviour of the glass/Ti/TiO2 decorative coatings. Thin Solid Films 2019, 691, 137595. [Google Scholar] [CrossRef]
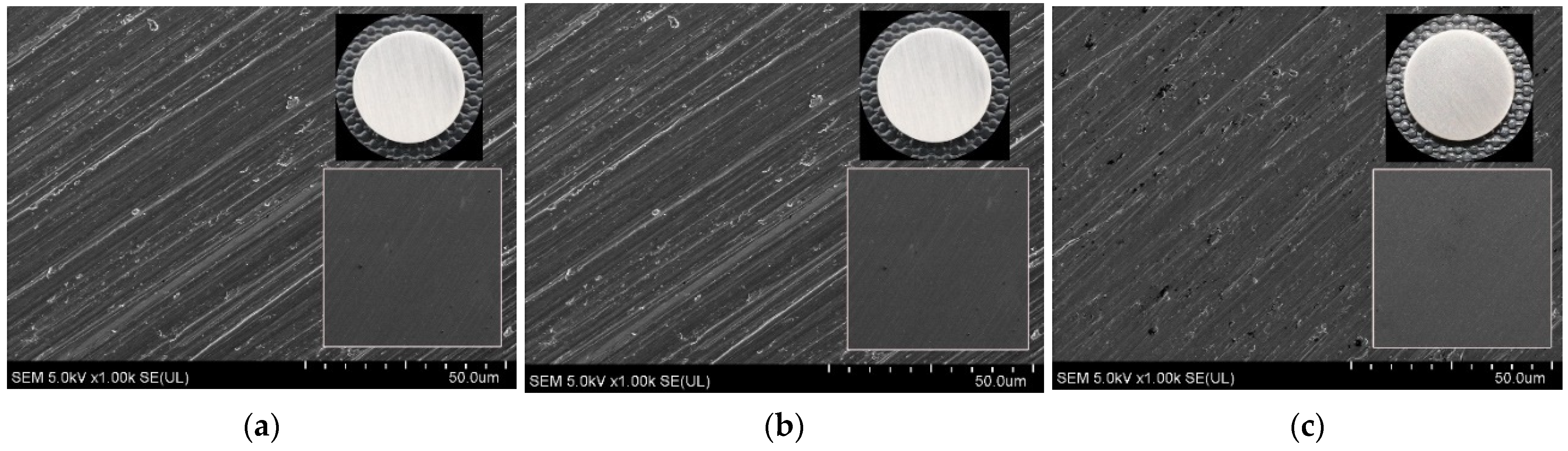
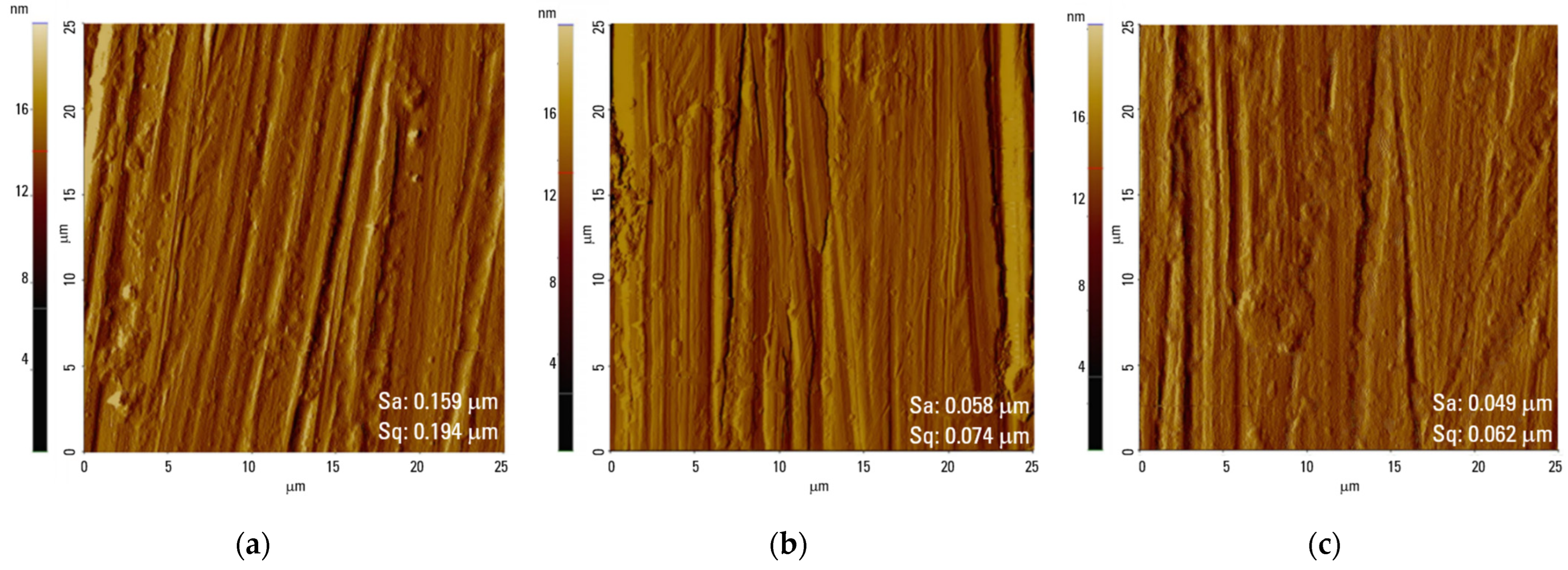
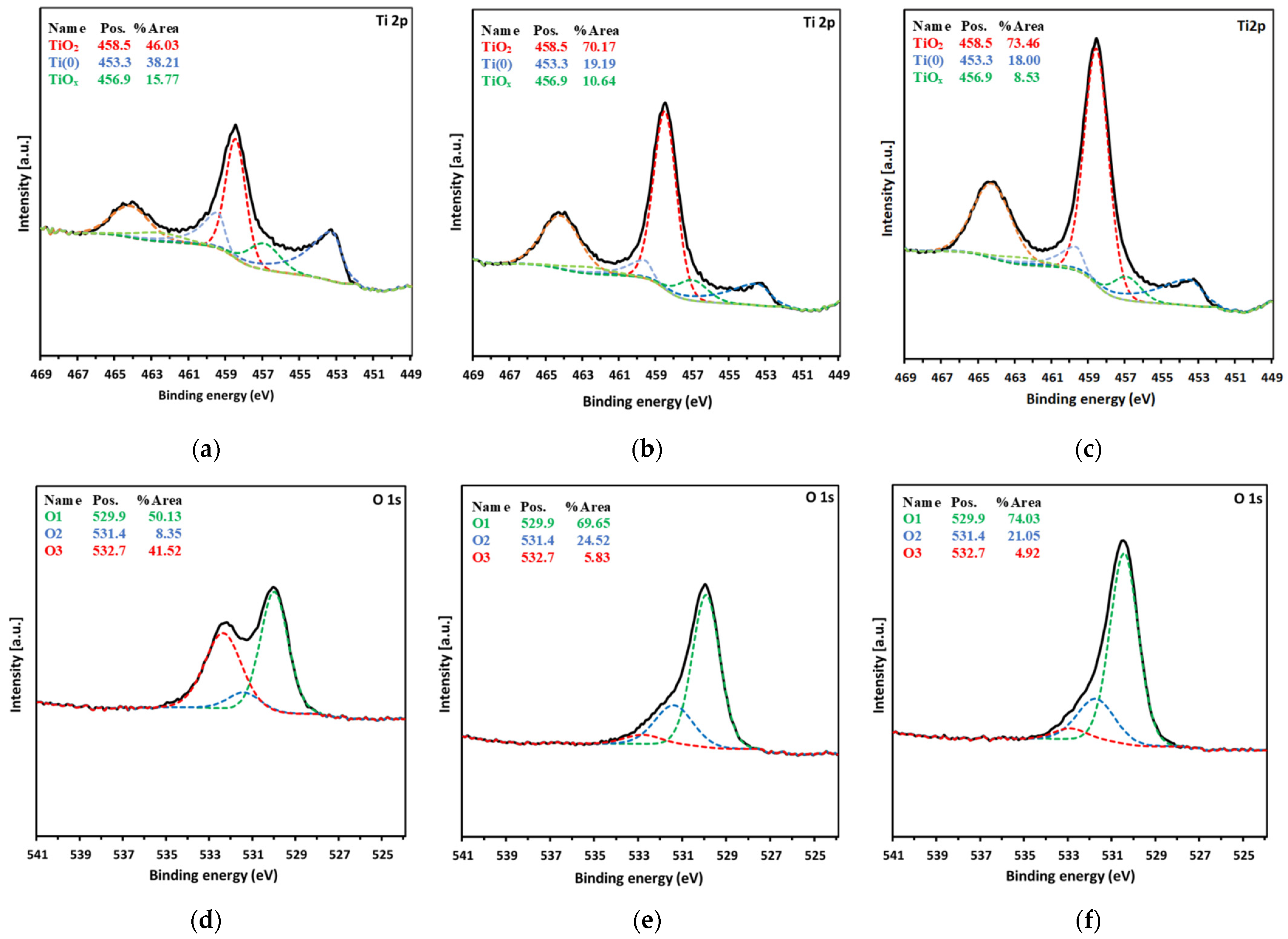
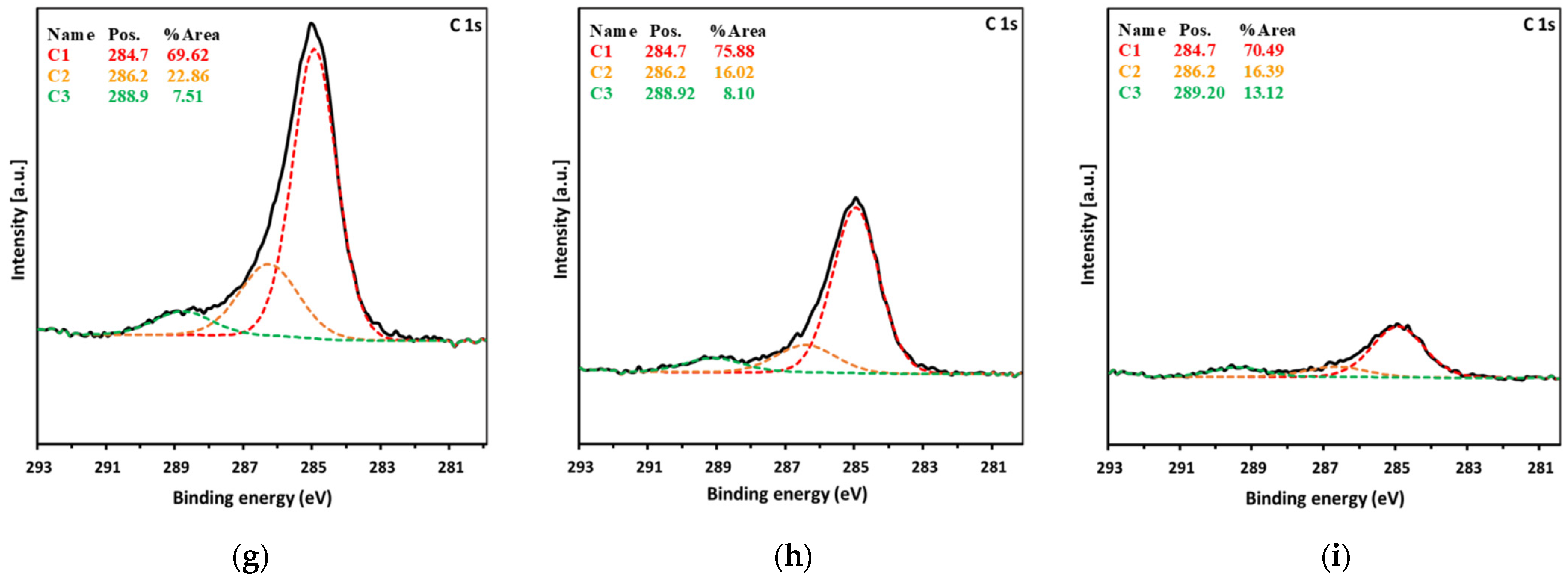
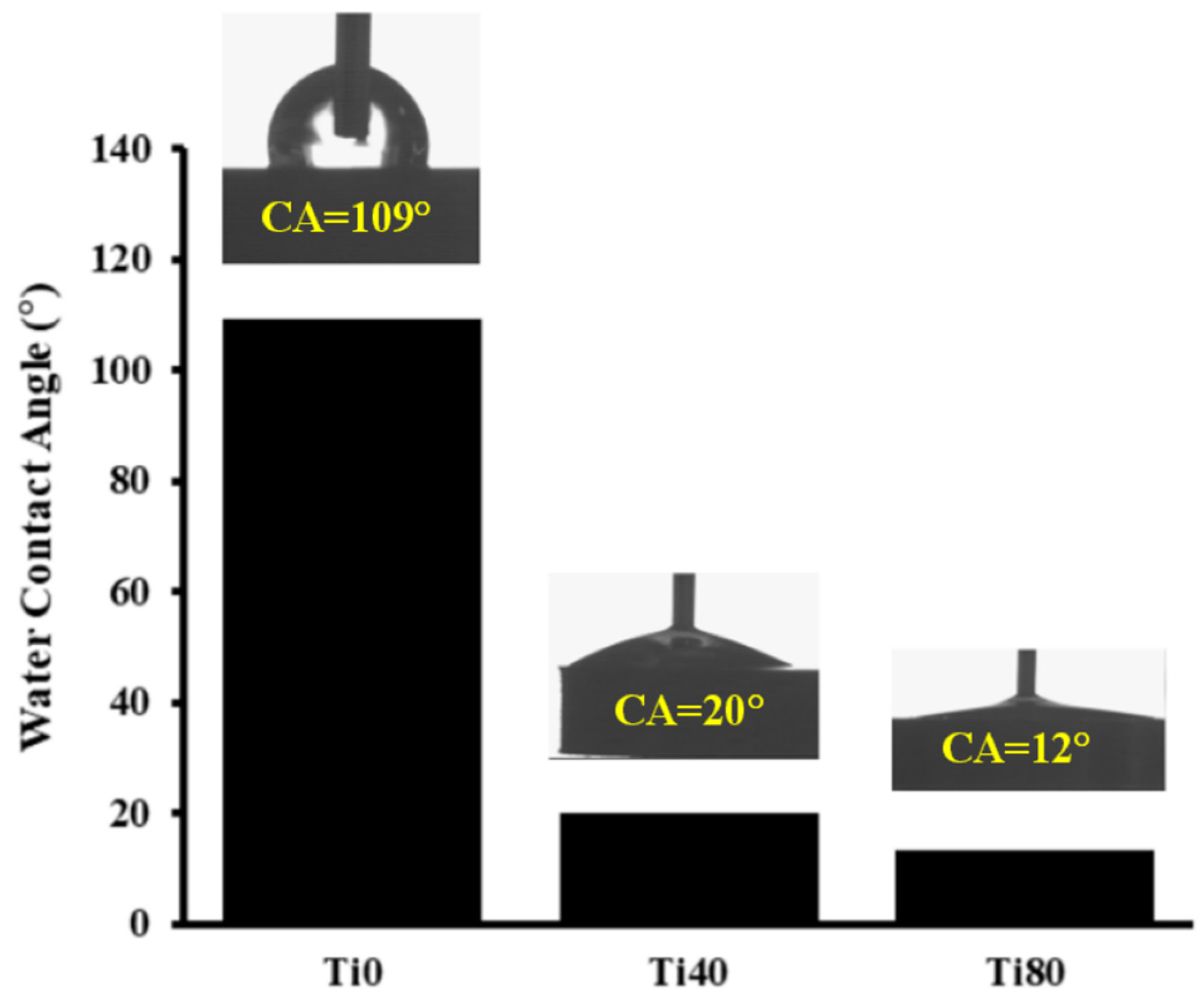
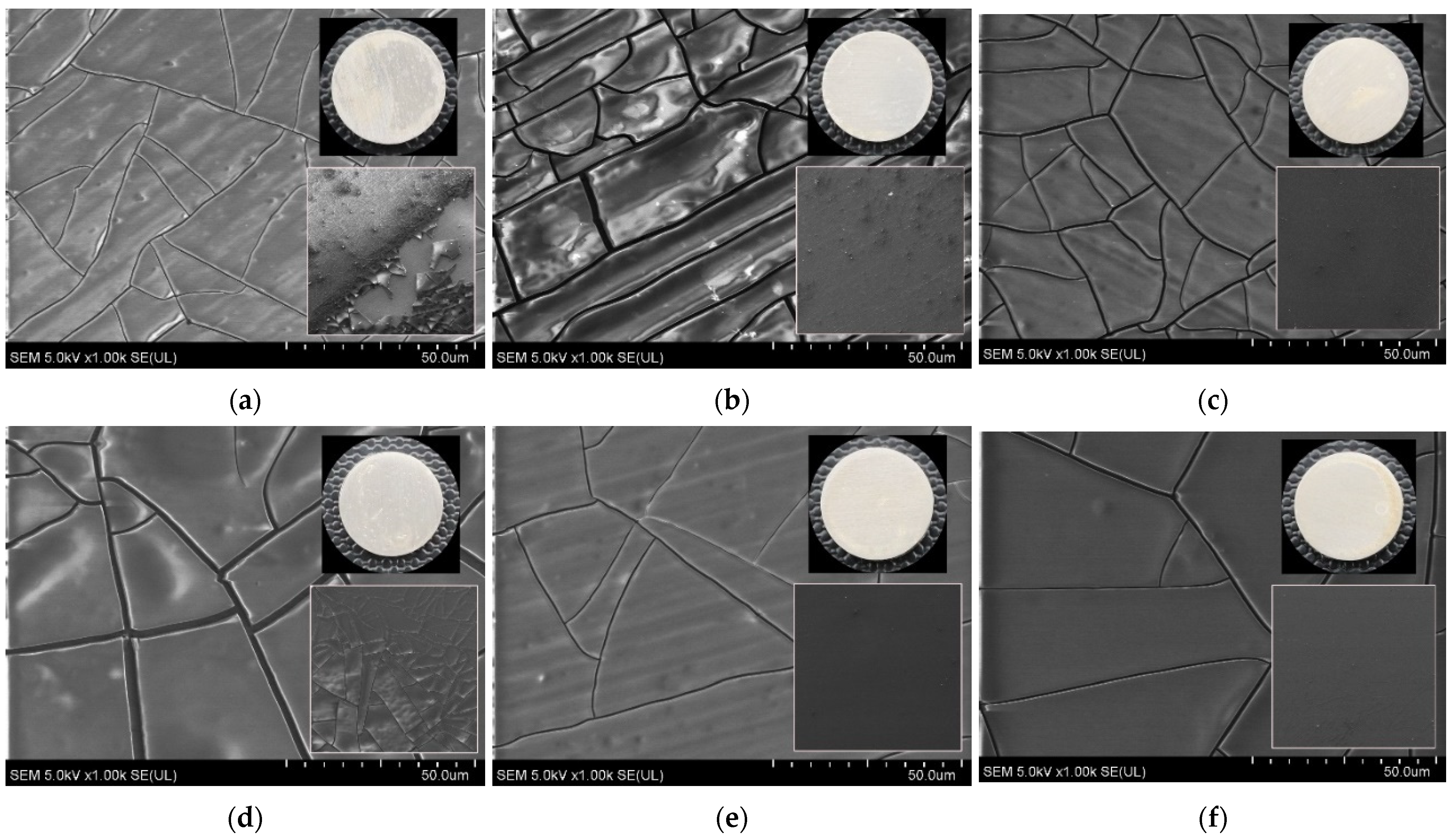


| Spectrum | eV | Assignment of Peak | Ti0 at. % | Total Content | Ti40 at. % | Total Content | Ti80 at. % | Total Content |
|---|---|---|---|---|---|---|---|---|
| Ti2p | 453.3 | Ti(0) | 1.61 | 10.23 | 1.78 | 16.69 | 2.07 | 24.22 |
| 456.9 | Ti2O3 | 3.91 | 3.20 | 4.36 | ||||
| 458.5 | TiO2 | 4.71 | 11.71 | 17.79 | ||||
| O1s | 529.9 | O1 | 15.99 | 31.89 | 28.17 | 40.45 | 40.00 | 54.04 |
| 531.4 | O2 | 2.66 | 9.92 | 11.38 | ||||
| 532.7 | O3 | 13.24 | 2.36 | 2.66 | ||||
| C1s | 284.7 | C–C, C–H | 33.69 | 48.42 | 29.01 | 38.24 | 10.63 | 15.08 |
| 286.2 | C–O | 11.07 | 6.13 | 2.47 | ||||
| 288.9 | O–C=O | 3.64 | 3.10 | 1.98 | ||||
| N | 2.67 | 1.53 | 2.06 | |||||
| Al | 1.58 | 3.09 | 4.60 | |||||
| Si | 5.23 | 0.00 | 0.00 | |||||
| Total | 100.00 | 100.00 | 100.00 |
| Spectrum | eV | Assignment of Peak | Ti0-Ta2 at. % | Total Content | Ti40-Ta2 at. % | Total Content | Ti80-Ta2 at. % | Total Content |
|---|---|---|---|---|---|---|---|---|
| C1s | 284.7 | C–C | 38.66 | 71.47 | 36.84 | 71.09 | 39.52 | 71.60 |
| 286.3 | C–O | 25.49 | 26.98 | 25.50 | ||||
| 288.7 | C=O | 7.32 | 7.27 | 6.58 | ||||
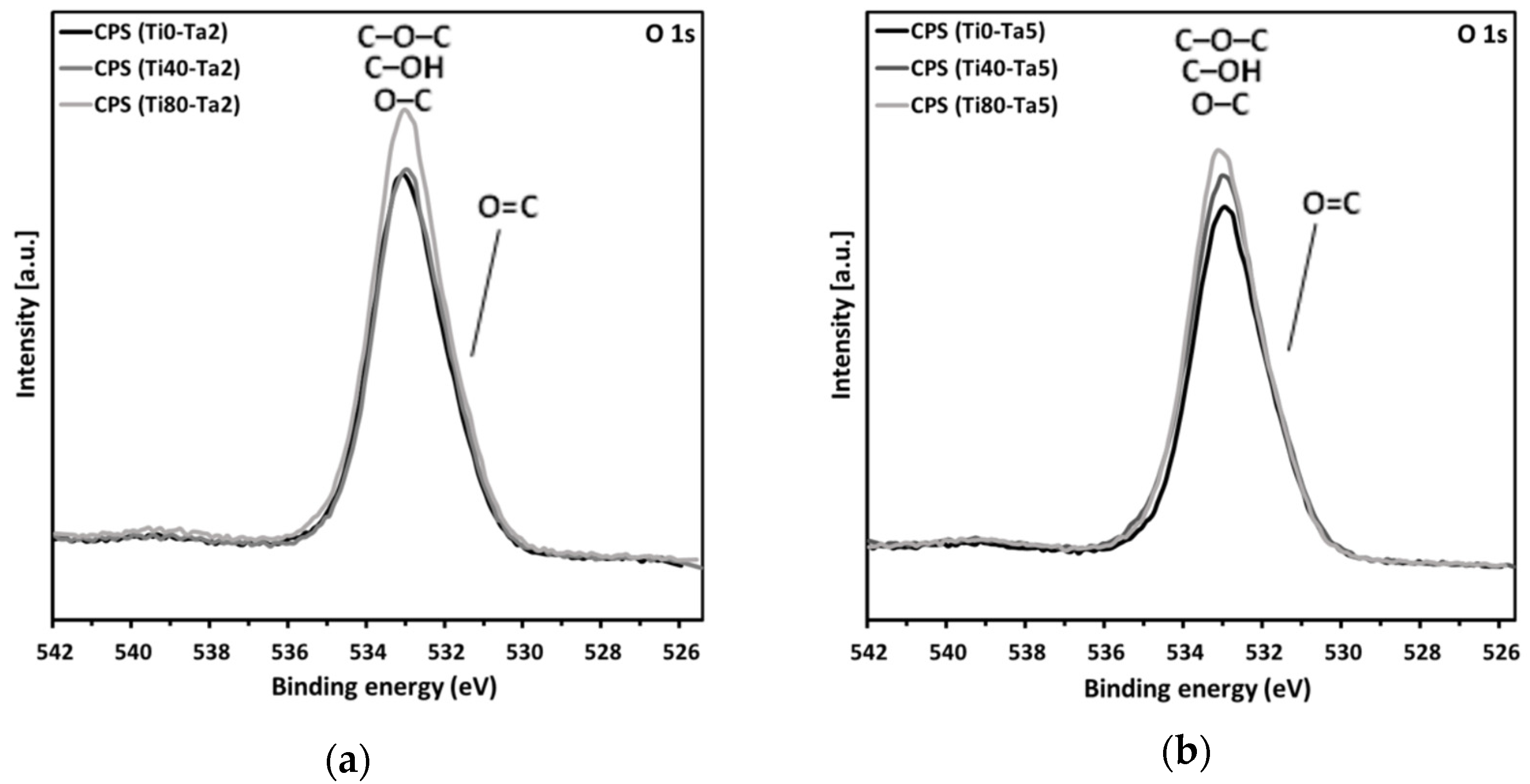
| O1s | 531.8 | O=C | 5.02 | 28.53 | 4.95 | 28.91 | 5.52 | 28.40 |
| 533.3 | C–O–C, C–OH, O–C | 23.51 | 23.96 | 22.88 | ||||
| Total | 100.00 | 100.00 | 100.00 |
| Spectrum | eV | Assignment of Peak | Ti0-Ta5 at. % | Total Content | Ti40-Ta5 at. % | Total Content | Ti80-Ta5 at. % | Total Content |
|---|---|---|---|---|---|---|---|---|
| C1s | 284.7 | C–C | 30.96 | 68.19 | 31.17 | 67.99 | 30.83 | 67.06 |
| 286.3 | C–O | 29.24 | 29.65 | 28.80 | ||||
| 288.7 | C=O | 7.99 | 7.17 | 7.43 | ||||
| O1s | 531.8 | O=C | 3.58 | 31.81 | 3.30 | 32.01 | 4.10 | 32.94 |
| 533.3 | C–O–C, C–OH, O–C | 28.23 | 28.71 | 28.84 | ||||
| Total | 100.00 | 100.00 | 100.00 |
| Sample | di (nm) | d (nm) | Nd (%) |
|---|---|---|---|
| Ti0-Ta2 | 837 ± 23 | 526 ± 4 | 29 ± 2 |
| Ti40-Ta2 | 573 ± 134 | 894 ± 7 | 44 ± 2 |
| Ti80-Ta2 | 546 ± 54 | 2189 ± 20 | 57 ± 2 |
| Ti0-Ta5 | 560 ± 116 | 686 ± 6 | 40 ± 2 |
| Ti40-Ta5 | 672 ± 278 | 1773 ± 20 | 34 ± 2 |
| Ti80-Ta5 | 614 ± 35 | 2708 ± 24 | 27 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winiecki, M.; Stepczyńska, M.; Moraczewski, K.; Skowronski, L.; Trzcinski, M.; Rerek, T.; Malinowski, R. Effect of Low-Temperature Oxygen Plasma Treatment of Titanium Alloy Surface on Tannic Acid Coating Deposition. Materials 2024, 17, 1065. https://doi.org/10.3390/ma17051065
Winiecki M, Stepczyńska M, Moraczewski K, Skowronski L, Trzcinski M, Rerek T, Malinowski R. Effect of Low-Temperature Oxygen Plasma Treatment of Titanium Alloy Surface on Tannic Acid Coating Deposition. Materials. 2024; 17(5):1065. https://doi.org/10.3390/ma17051065
Chicago/Turabian StyleWiniecki, Mariusz, Magdalena Stepczyńska, Krzysztof Moraczewski, Lukasz Skowronski, Marek Trzcinski, Tomasz Rerek, and Rafał Malinowski. 2024. "Effect of Low-Temperature Oxygen Plasma Treatment of Titanium Alloy Surface on Tannic Acid Coating Deposition" Materials 17, no. 5: 1065. https://doi.org/10.3390/ma17051065






