Thermodynamic Study on Hydrogen Reduction of Germanium Tetrachloride to Germanium
Abstract
1. Introduction
2. Analysis of Ge-H-Cl Ternary System
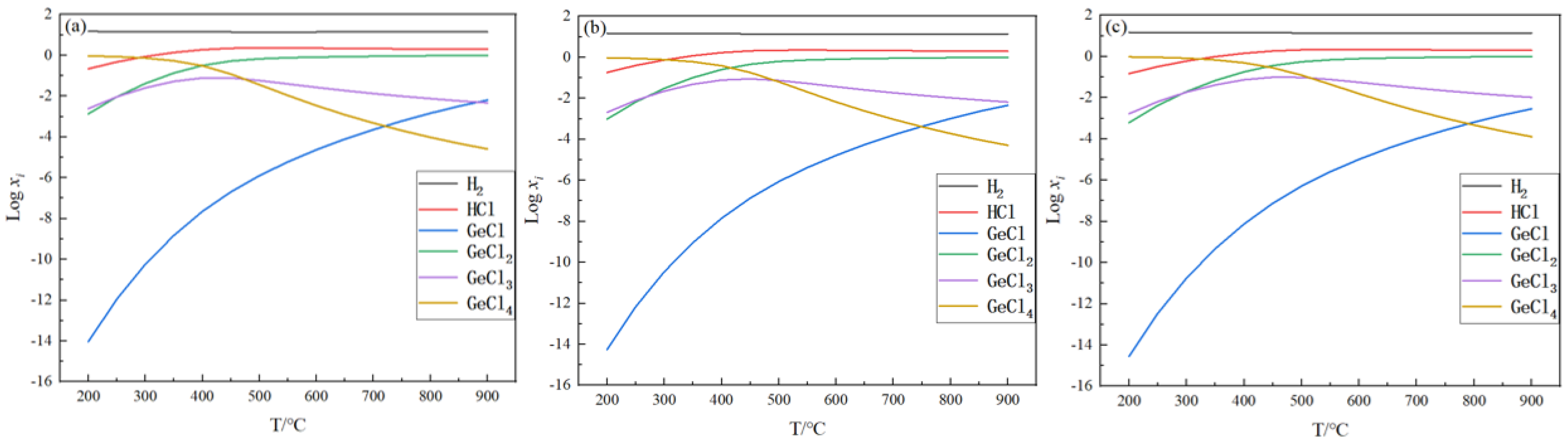
2.1. Equilibrium Component Analysis
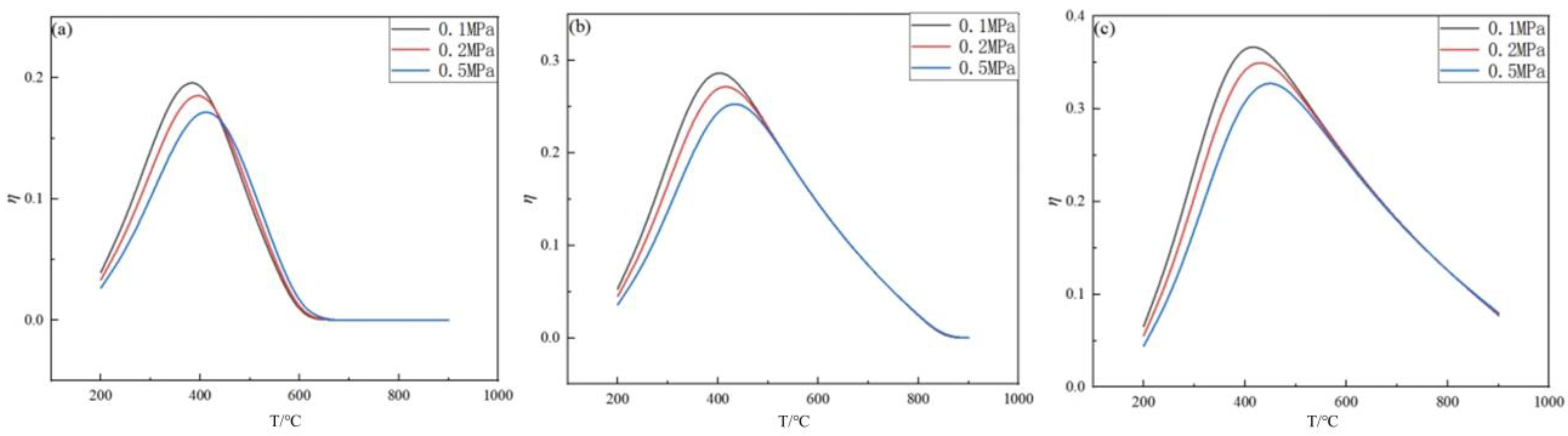
2.2. Effect of Temperature on Germanium Deposition Rate
2.3. Effect of Feed Ratio on Germanium Deposition Rate
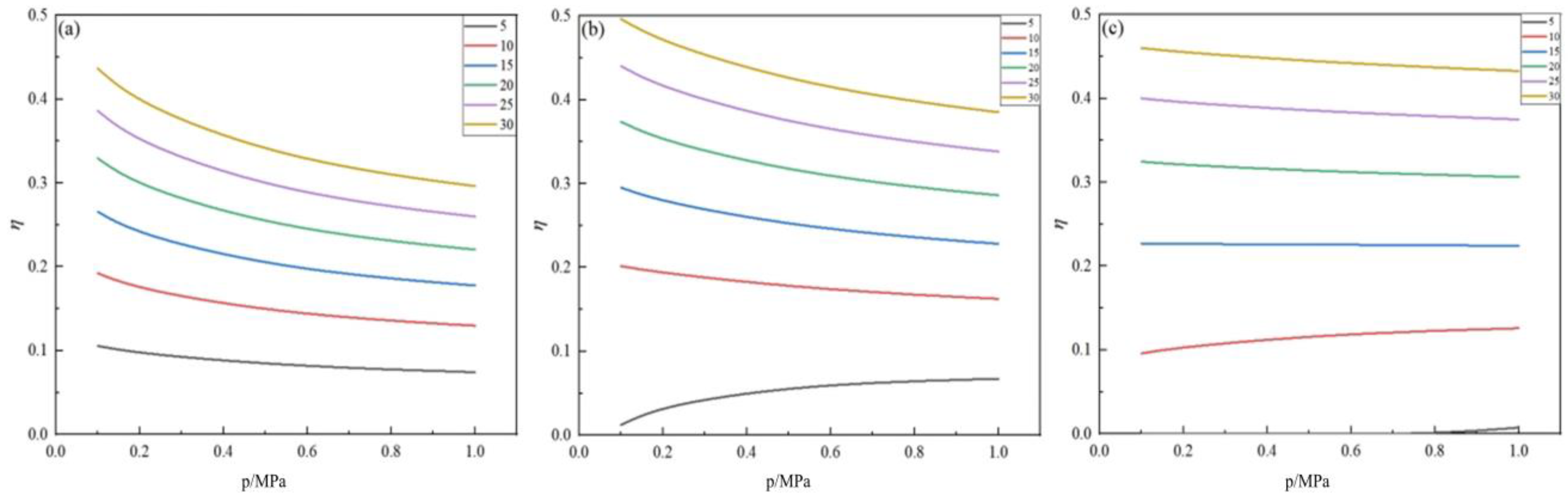
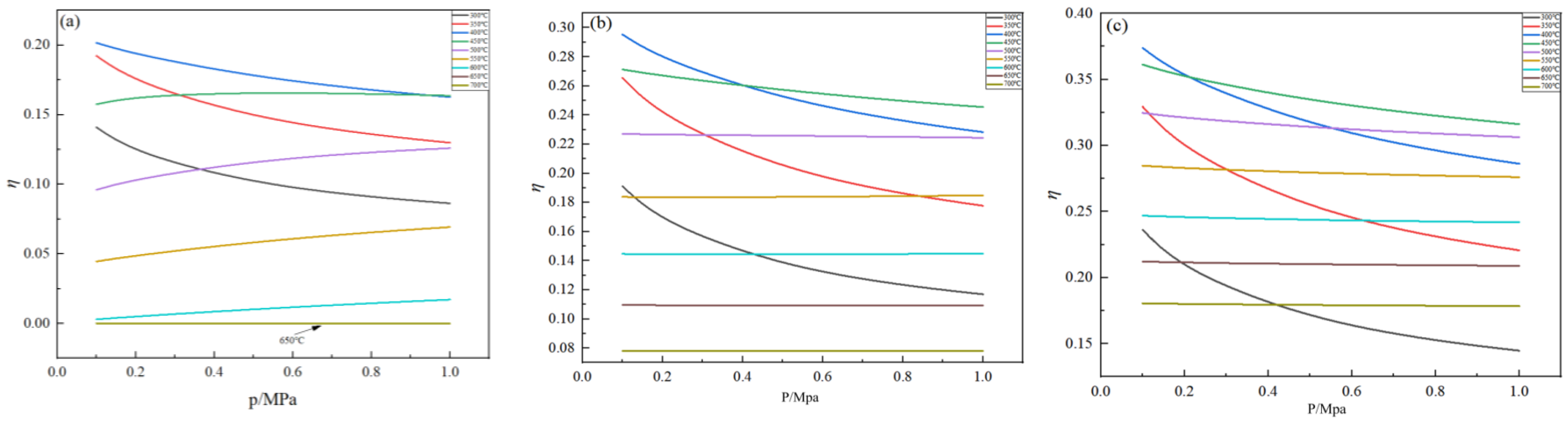
2.4. Effect of Pressure on Germanium Deposition Rate
3. Conclusions
- (1)
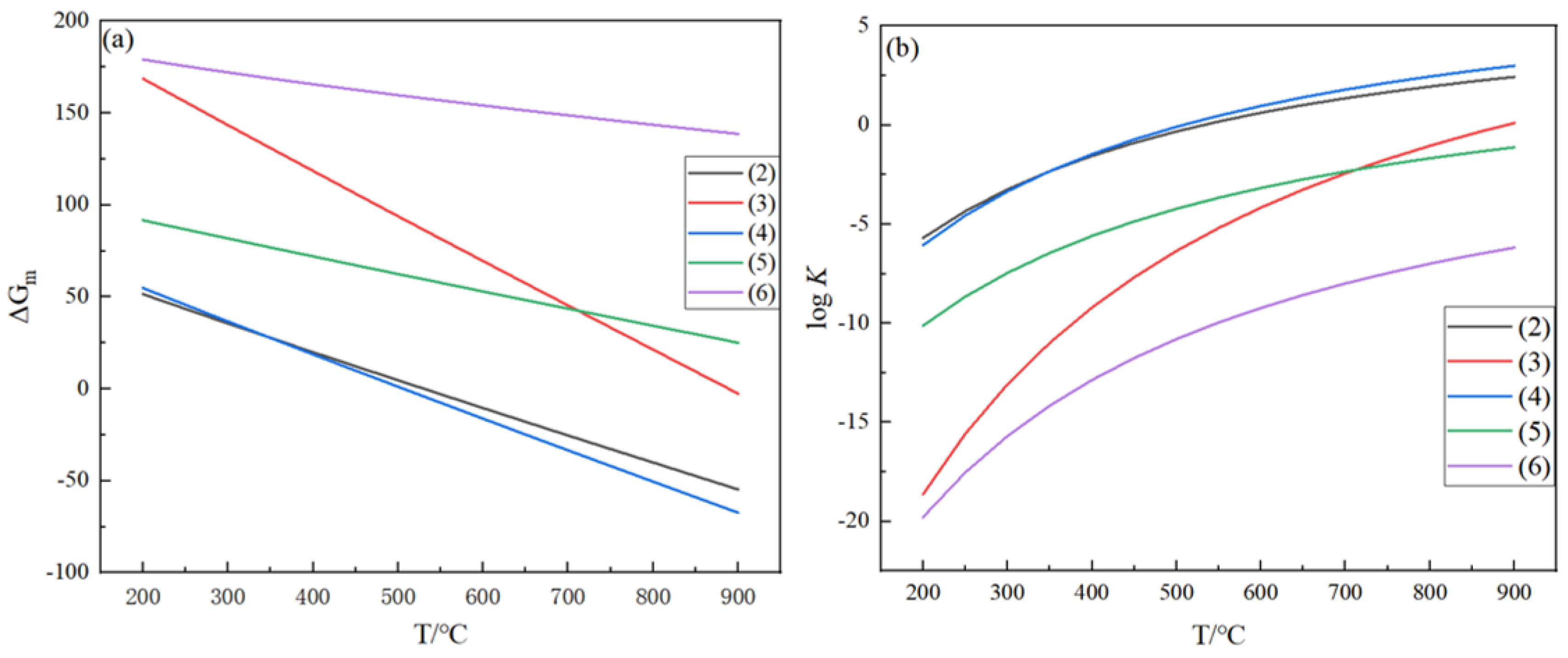
- Relevant thermodynamic data were applied to study the complex chemical reactions of the Ge-H-Cl ternary system in the hydrogen reduction process of germanium tetrachloride, and five independent reactions in the hydrogen reduction process of germanium tetrachloride were identified and plotted with ΔGm-T and logK-T diagrams. In the temperature range from 350 °C to 550 °C, the ΔGm values of the primary reaction (2) and the side reaction (4) are less than 0, and the reaction proceeds efficiently. At high temperatures, the deposition rate of germanium is low because the K value of the side reaction proliferates; at low temperatures, the K value of the primary reaction is minimal, and the reaction proceeds to a low degree. Therefore, in actual production, the low deposition rate of germanium is a normal phenomenon.
- (2)
- The germanium deposition rate increases significantly with increasing temperature, and when the temperature exceeds the optimum temperature, the germanium deposition rate decreases with increasing temperature. When the temperature is lower, the germanium deposition rate is meager, and the change in pressure hardly affects the germanium deposition rate. When the temperature is higher, the germanium deposition rate increases with the increase in the feed ratio. The analysis shows that the optimum operating temperature is 450 °C when the pressure is determined to be 0.1 MPa.
- (3)
- An excess of hydrogen is necessary in the germanium deposition process. As the feed ratio increases, the germanium deposition rate also increases, but the growth rate of the germanium deposition gradually decreases. When the feed ratio increased to a specific value, the effect of further increase on the germanium deposition rate was negligible. Moreover, when the feed ratio was too large, it increased the production of HCl, simultaneously increasing the cost and difficulty of separating hydrogen in the exhaust gas. Consequently, from the above analysis, it can be seen that, in the actual industrial production, germanium tetrachloride should be controlled to the feed ratio of about 20 in the process of germanium preparation by hydrogen reduction.
- (4)
- Within the optimal temperature range, the germanium deposition rate decreases with the increase in pressure. When the temperature was higher, the pressure had almost no effect on the germanium deposition rate, and with the increase in the feed ratio, the temperature range where the pressure did not affect the germanium deposition rate became more considerable. Therefore, in the actual production process, the pressure should be controlled at 0.1 MPa.
- (5)
- The optimum practical production conditions for preparing germanium by the hydrogen reduction of germanium tetrachloride were: temperature T = 450 °C, feed ratio 20, pressure p = 0.1 MPa. The deposition rate of germanium at this time was 36.12%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.; Palit, R.; Kojouharov, I.; Gerl, J.; Gorska-Ott, M.; Schaffner, H.; Habermann, T.; Saha, S.; Das, B.; Dey, P.; et al. Scanning of a Double-Sided Germanium Strip Detector. EPJ Web Conf. 2021, 253, 11009. [Google Scholar] [CrossRef]
- Yenisoy, A.; Yesilyaprak, C.; Ruzgar, K.; Tuzemen, S. Ultra-broad band antireflection coating at mid wave infrared for high efficient germanium optics. Opt. Mater. Express 2019, 9, 3123–3131. [Google Scholar] [CrossRef]
- Brunco, D.P.; De Jaeger, B.; Eneman, G.; Satta, A.; Terzieva, V.; Souriau, L.; Leys, F.E.; Pourtois, G.; Houssa, M.; Opsomer, K.; et al. Germanium: The Past and Possibly a Future Material for Microelectronics. ECS Trans. 2007, 11, 479. [Google Scholar] [CrossRef]
- Lombardero, I.; Ochoa, M.; Miyashita, N.; Okada, Y.; Algora, C. Theoretical and experimental assessment of thinned germanium substrates for III–V multijunction solar cells. Prog. Photovolt. Res. Appl. 2020, 28, 1097–1106. [Google Scholar] [CrossRef]
- Baran, V.; Cat, Y.; Sertel, T.; Ataser, T.; Sonmez, N.A.; Cakmak, M.; Ozcelik, S. A Comprehensive Study on a Stand-Alone Germanium (Ge) Solar Cell. J. Electron. Mater. 2020, 49, 1249–1256. [Google Scholar] [CrossRef]
- Hosni, M.; Norbert, O.; Maximilian, G.; Sergeev, O.; Gehrke, K.; Vehse, M.; Agert, C. Quantum confinement-tunable solar cell based on ultrathin amorphous germanium. Nano Energy 2020, 76, 105048. [Google Scholar]
- Hendrickx, N.W.; Lawrie, W.I.L.; Russ, M.; van Riggelen, F.; de Snoo, S.L.; Schouten, R.N.; Sammak, A.; Scappucci, G.; Veldhorst, M. A four-qubit germanium quantum processor. Nature 2021, 591, 580–585. [Google Scholar] [CrossRef]
- Ohtera, Y.; Miura, K.; Kawashima, T. Ge/SiO2 Photonic Crystal Multichannel Wavelength Filters for Short Wave Infrared Wavelengths. Jpn. J. Appl. Phys. 2007, 46, 1511. [Google Scholar] [CrossRef]
- Kuo, Y.-H.; Huang, Y.-A.; Chen, T.L. A Vertical Germanium Thermooptic Modulator for Optical Interconnects. IEEE Photonics Technol. Lett. 2009, 21, 245–247. [Google Scholar]
- Halbwax, M.; Bouchier, D.; Yam, V.; Debarre, D.; Nguyen, L.H.; Zheng, Y.; Rosner, P.; Benamara, M.; Strunk, H.P.; Clerc, C. Kinetics of Ge growth at low temperature on Si(001) by ultrahigh vacuum chemical vapor deposition. J. Appl. Phys. 2005, 97, 064907. [Google Scholar] [CrossRef]
- Míguez, H.; Chomski, E.; García-Santamaría, F.; Ibisate, M.; John, S.; López, C.; Meseguer, F.; Mondia, J.P.; Ozin, G.A.; Toader, O.; et al. Photonic Bandgap Engineering in Germanium Inverse Opals by Chemical Vapor Deposition. Adv. Mater. 2001, 13, 1634–1637. [Google Scholar] [CrossRef]
- Restrepo, D.T.; Lynch, K.E.; Giesler, K.; Kuebler, S.M.; Blair, R.G. Low-temperature (210 °C) deposition of crystalline germanium via in situ disproportionation of GeI2. Mater. Res. Bull. 2012, 47, 3484–3488. [Google Scholar] [CrossRef]
- Schumb, W.C.; Donald, M.S. The Partial Hydrolysis of Germanium Tetrachloride. J. Am. Chem. Soc. 2002, 77, 2133–2136. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, T.; Liu, Z. Review on resources and recycling of germanium, with special focus on characteristics, mechanism and challenges of solvent extraction. J. Clean. Prod. 2021, 294, 126217. [Google Scholar]
- Moskalyk, R.R. Review of germanium processing worldwide. Miner. Eng. 2004, 17, 393–402. [Google Scholar] [CrossRef]
- Kadomtseva, A.V.; Vorotyntsev, A.V.; Vorotyntsev, V.M.; Petukhov, A.N.; Ob’’Edkov, A.M.; Kremlev, K.V.; Kaverin, B.S. Effect of the catalytic system based on multi-walled carbon nanotubes modified with copper nanoparticles on the kinetics of catalytic reduction of germanium tetrachloride by hydrogen. Russ. J. Appl. Chem. 2015, 88, 595–602. [Google Scholar] [CrossRef]
- Vorotyntsev, A.V.; Zelentsov, S.V.; Vorotyntsev, A.M.; Petukhov, A.; Kadomtseva, A.V. Quantum chemical modeling of dissociative hydrogen chemisorption on metallic nanocluster surfaces. Bull. Acad. Sci. USSR Div. Chem. Sci. 2015, 4, 759–765. [Google Scholar]
- Kadomtseva, A.V.; Ob’’edkov, M.A. Reduction of GeCl4 in the presence of a catalyst based on modified NiCl2. Inorg. Mater. 2017, 53, 1312–1318. [Google Scholar] [CrossRef]
- Garcia-Gil, A.; Biswas, S.; Holmes, J.D. A Review of Self-Seeded Germanium Nanowires: Synthesis, Growth Mechanisms and Potential Applications. Nanomaterials 2021, 11, 2002. [Google Scholar] [CrossRef]
- Kadomtseva, A.V.; Ob’’edkov, A.M.; Zasovskaya, M.A. Effect of Tungsten on the Germanium Tetrachloride Reduction Process. Inorg. Mater. 2020, 56, 229–234. [Google Scholar] [CrossRef]
- Ishii, H.; Takahashi, Y. Growth and Etching of Germanium Films by Chemical Vapor Deposition in a GeCl4-H2 Gas System. J. Electrochem. Soc. 1988, 135, 1539–1543. [Google Scholar] [CrossRef]
- Gresback, R.; Holman, Z.; Kortshagen, U. Nonthermal plasma synthesis of size-controlled, monodisperse, freestanding germanium nanocrystals. Appl. Phys. Lett. 2007, 91, 093119. [Google Scholar] [CrossRef]
- Vaughn, D.D.; Schaak, R.E. Synthesis, properties and applications of colloidal germanium and germanium-based nanomaterials. Chem. Soc. Rev. 2013, 42, 2861–2879. [Google Scholar] [CrossRef]





| T/°C | ΔGm/kJ | ||||
|---|---|---|---|---|---|
| Reaction (2) | Reaction (3) | Reaction (4) | Reaction (5) | Reaction (6) | |
| 200 | 51.524 | 168.76 | 54.852 | 91.678 | 179.172 |
| 250 | 43.509 | 156.087 | 45.716 | 86.719 | 175.581 |
| 300 | 35.585 | 143.502 | 36.662 | 81.801 | 172.16 |
| 350 | 27.744 | 130.998 | 27.683 | 76.92 | 168.885 |
| 400 | 19.979 | 118.57 | 18.774 | 72.074 | 165.739 |
| 450 | 12.285 | 106.212 | 9.931 | 67.261 | 162.705 |
| 500 | 4.654 | 93.918 | 1.148 | 62.477 | 159.771 |
| 550 | −2.919 | 81.685 | −7.578 | 57.722 | 156.923 |
| 600 | −10.44 | 69.507 | −16.253 | 52.992 | 154.153 |
| 650 | −17.915 | 57.382 | −24.88 | 48.287 | 151.45 |
| 700 | −25.348 | 45.304 | −33.462 | 43.604 | 148.806 |
| 750 | −32.745 | 33.271 | −42.002 | 38.941 | 146.215 |
| 800 | −40.109 | 21.279 | −50.502 | 34.299 | 143.669 |
| 850 | −47.444 | 9.327 | −58.967 | 29.674 | 141.163 |
| 900 | −54.754 | −2.59 | −67.397 | 25.067 | 138.693 |
| T/°C | Log K | ||||
|---|---|---|---|---|---|
| Reaction (2) | Reaction (3) | Reaction (4) | Reaction (5) | Reaction (6) | |
| 200 | −5.689 | −18.632 | −6.056 | −10.122 | −19.782 |
| 250 | −4.345 | −15.586 | −4.565 | −8.659 | −17.533 |
| 300 | −3.243 | −13.079 | −3.341 | −7.456 | −15.691 |
| 350 | −2.326 | −10.982 | −2.321 | −6.448 | −14.158 |
| 400 | −1.55 | −9.202 | −1.457 | −5.593 | −12.862 |
| 450 | −0.887 | −7.673 | −0.717 | −4.859 | −11.754 |
| 500 | −0.314 | −6.346 | −0.078 | −4.221 | −10.795 |
| 550 | 0.185 | −5.184 | 0.481 | −3.663 | −9.959 |
| 600 | 0.625 | −4.159 | 0.972 | −3.17 | −9.223 |
| 650 | 1.014 | −3.247 | 1.408 | −2.732 | −8.570 |
| 700 | 1.361 | −2.432 | 1.796 | −2.341 | −7.988 |
| 750 | 1.672 | −1.699 | 2.144 | −1.988 | −7.465 |
| 800 | 1.952 | −1.036 | 2.458 | −1.67 | −6.994 |
| 850 | 2.207 | −0.434 | 2.743 | −1.38 | −6.566 |
| 900 | 2.438 | 0.115 | 3.001 | −1.116 | −6.176 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, D.; Ding, Z.; Wang, T.; Kou, B.; Chen, F.; Hou, Y.; Yang, B.; Xie, G. Thermodynamic Study on Hydrogen Reduction of Germanium Tetrachloride to Germanium. Materials 2024, 17, 1079. https://doi.org/10.3390/ma17051079
Cui D, Ding Z, Wang T, Kou B, Chen F, Hou Y, Yang B, Xie G. Thermodynamic Study on Hydrogen Reduction of Germanium Tetrachloride to Germanium. Materials. 2024; 17(5):1079. https://doi.org/10.3390/ma17051079
Chicago/Turabian StyleCui, Dingfang, Zhiying Ding, Tongbo Wang, Bin Kou, Fengyang Chen, Yanqing Hou, Bin Yang, and Gang Xie. 2024. "Thermodynamic Study on Hydrogen Reduction of Germanium Tetrachloride to Germanium" Materials 17, no. 5: 1079. https://doi.org/10.3390/ma17051079
APA StyleCui, D., Ding, Z., Wang, T., Kou, B., Chen, F., Hou, Y., Yang, B., & Xie, G. (2024). Thermodynamic Study on Hydrogen Reduction of Germanium Tetrachloride to Germanium. Materials, 17(5), 1079. https://doi.org/10.3390/ma17051079






