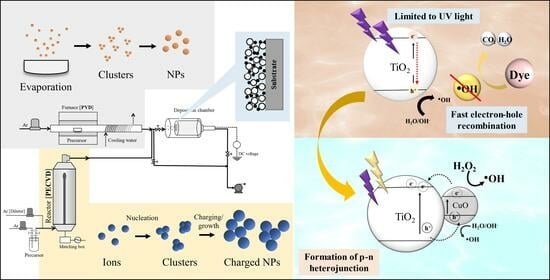
Gas-Phase Fabrication and Photocatalytic Activity of TiO2 and TiO2–CuO Nanoparticulate Thin Films
Abstract
:1. Introduction
- The addition of H2O2, widely known as the Fenton process in AOPs, increases the production of hydroxyl radicals. Cu-based materials possess Fenton-like characteristics and can be used to degrade organic pollutants effectively in wastewater treatment [12].
2. Materials and Methods
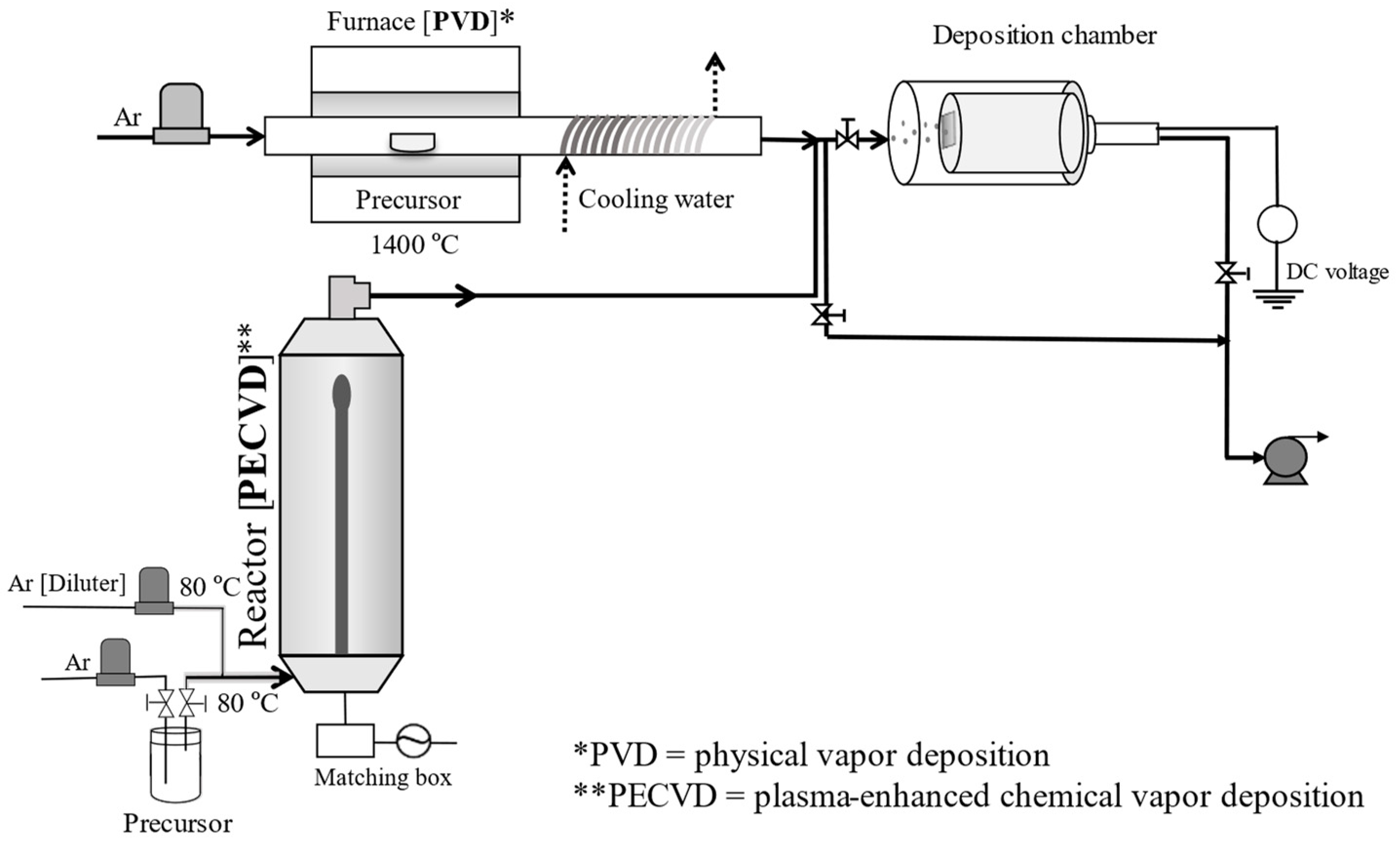
2.1. Preparation of Nanoparticulate Thin Films
2.2. Characterization of Nanoparticulate Thin Films
2.3. Photocatalytic Tests
3. Results and Discussion
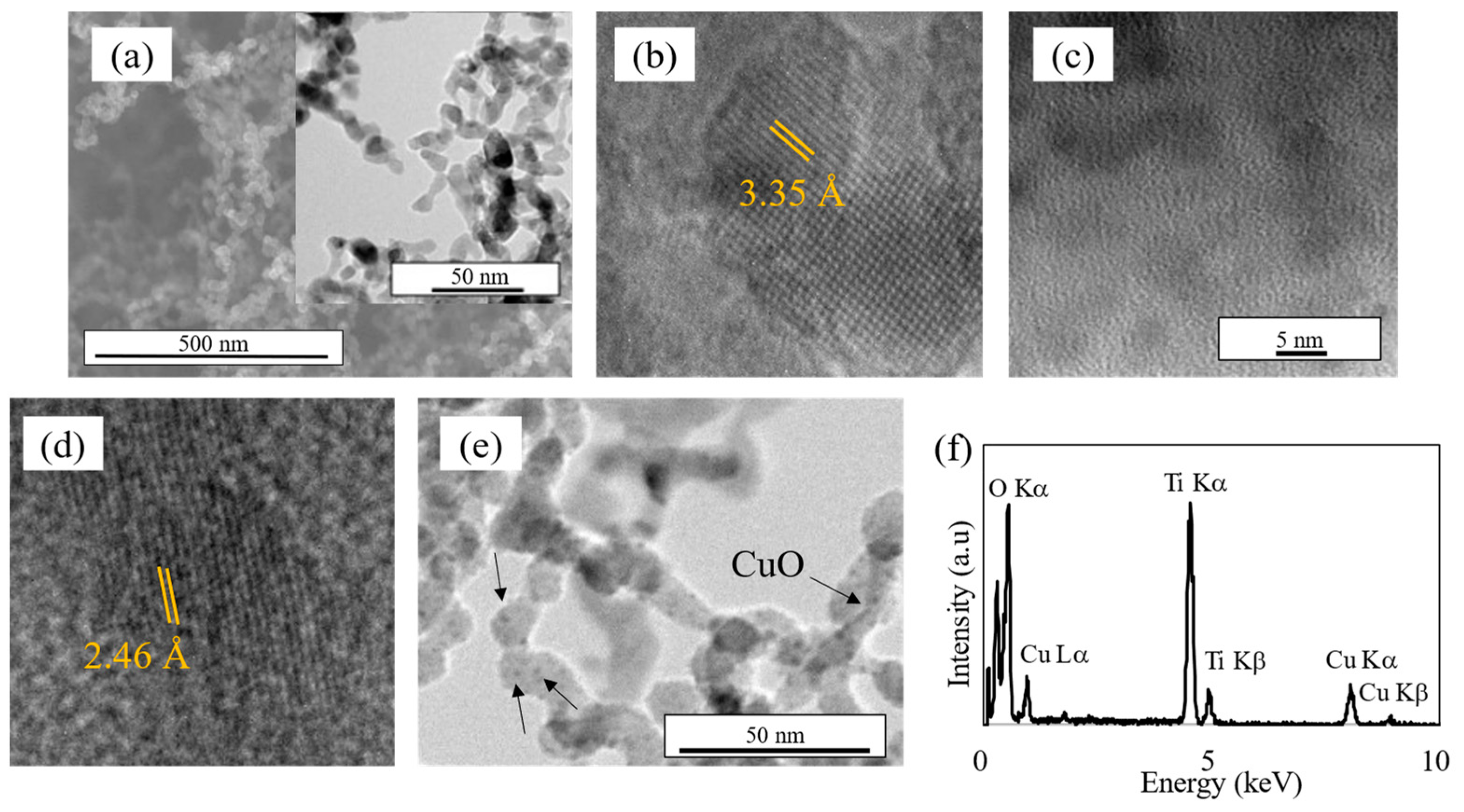
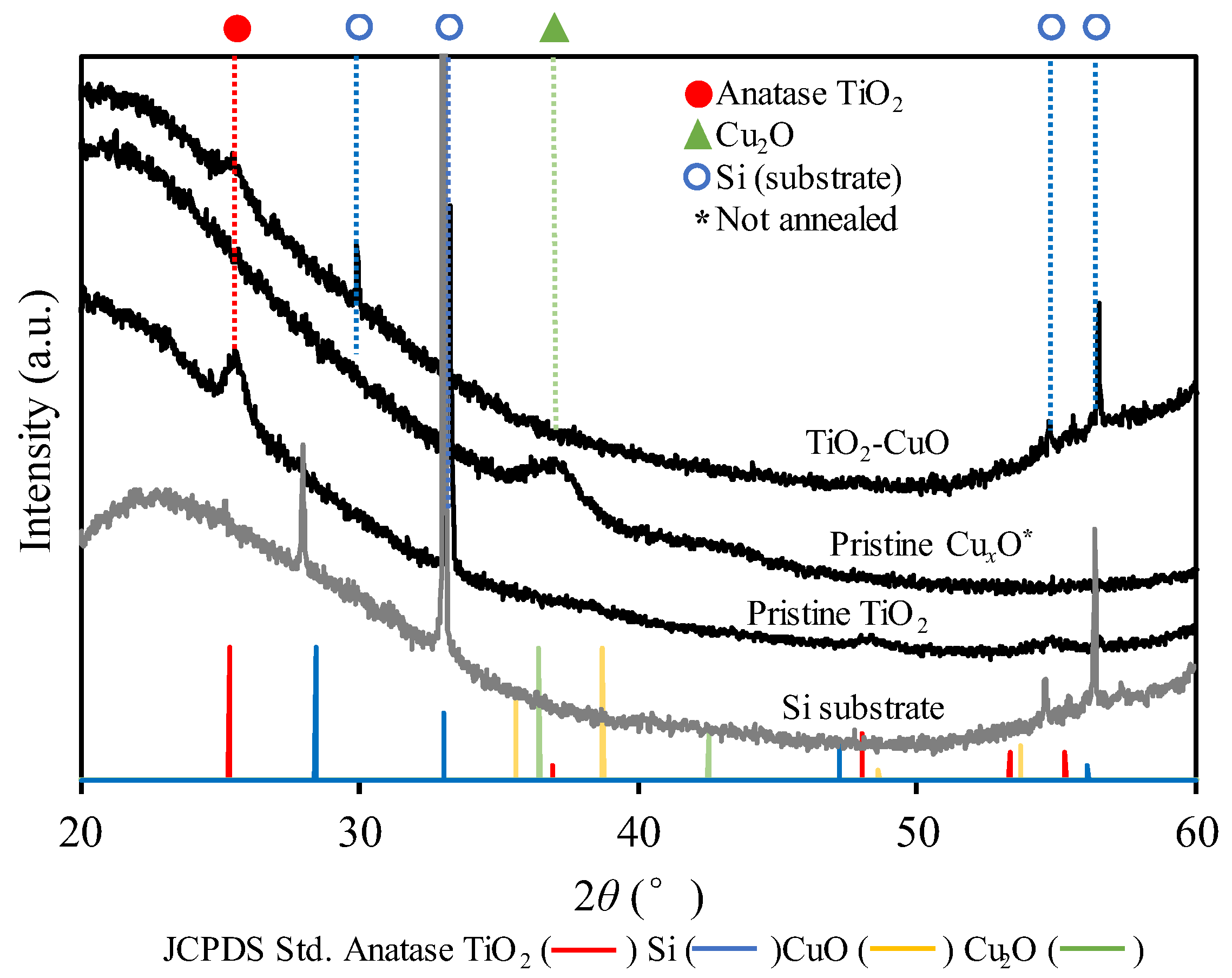
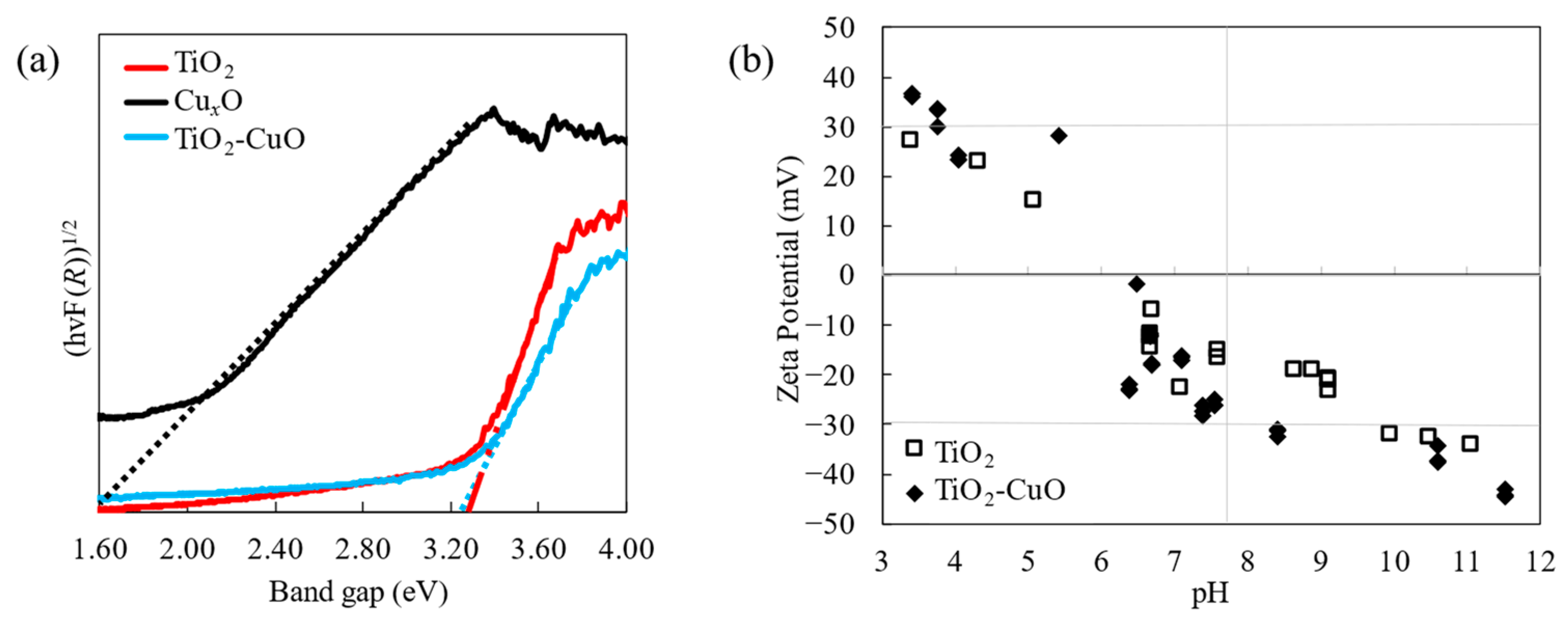
3.1. Characteristics
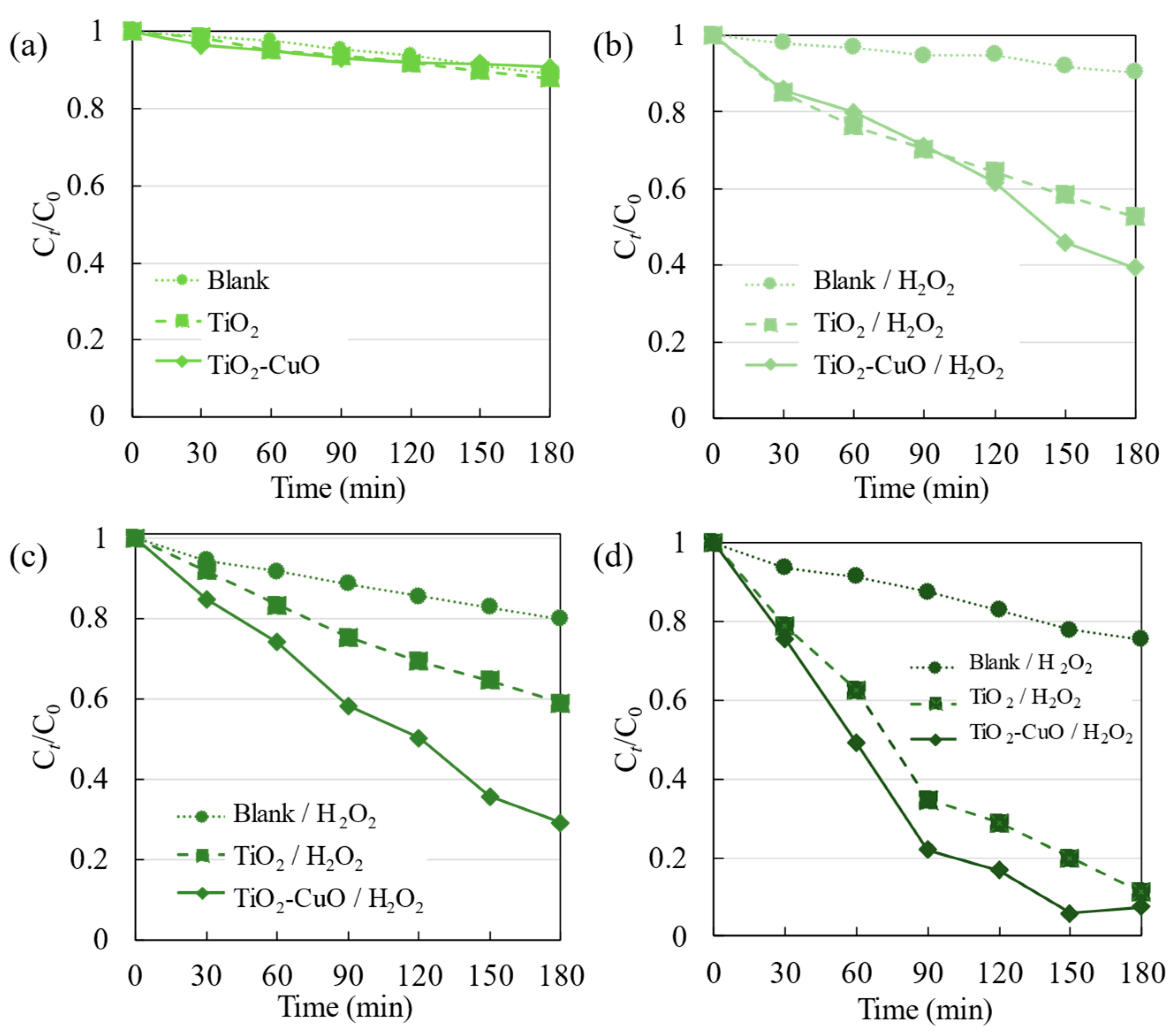
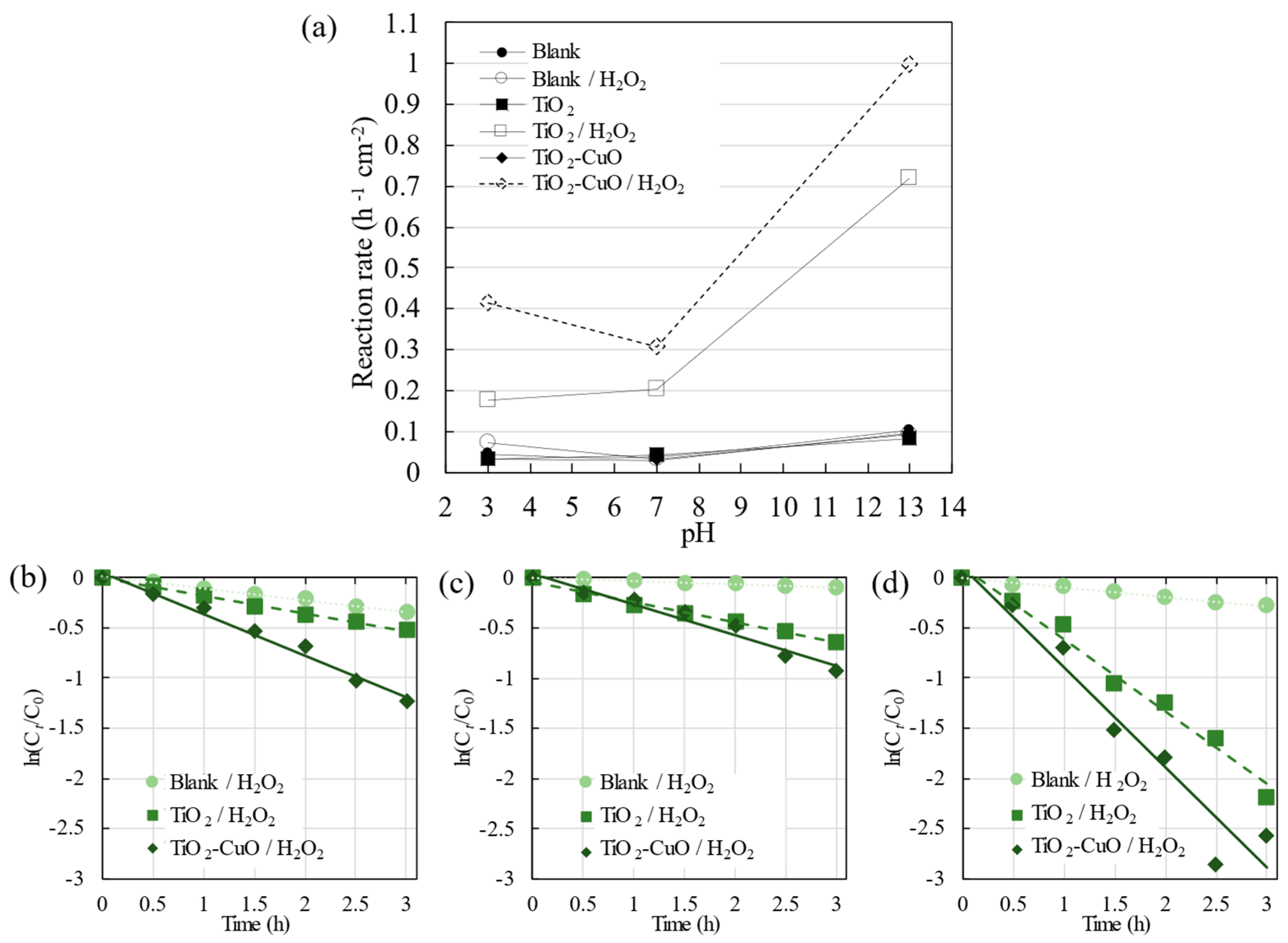
3.2. Photocatalytic Degradation of R6G
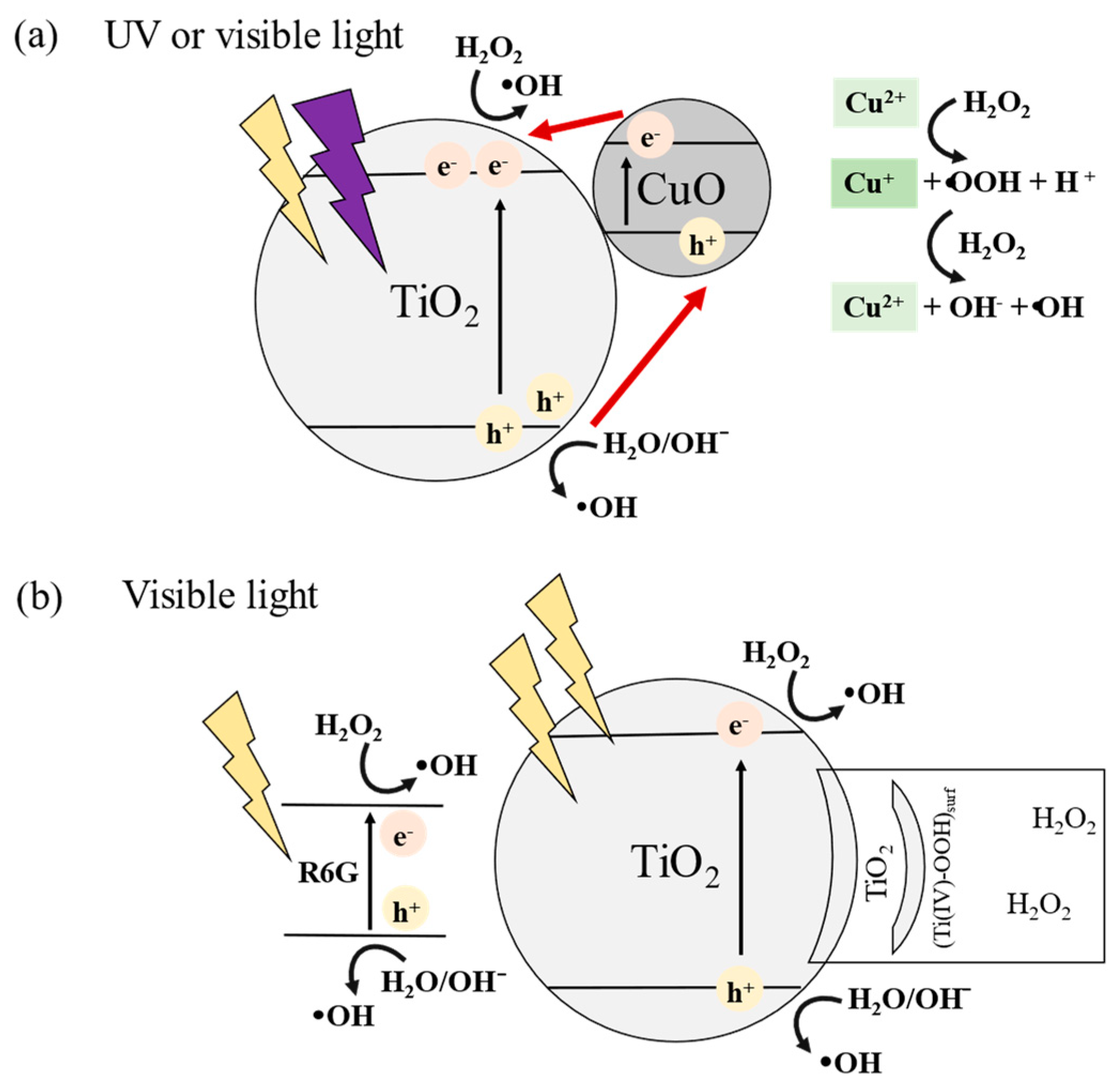
3.2.1. UV Irradiation
3.2.2. Visible Light Irradiation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kubiak, A.; Bielan, Z.; Kubacka, M.; Gabała, E.; Zgoła-Grześkowiak, A.; Janczarek, M.; Zalas, M.; Zielińska-Jurek, A.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Microwave-assisted synthesis of a TiO2-CuO heterojunction with enhanced photocatalytic activity against tetracycline. Appl. Surf. Sci. 2020, 520, 146344. [Google Scholar] [CrossRef]
- Udayabhanu; Lakshmana Reddy, N.; Shankar, M.V.; Sharma, S.C.; Nagaraju, G. One-Pot Synthesis of Cu–TiO2/CuO Nanocomposite: Application to Photocatalysis for Enhanced H2 Production, Dye Degradation & Detoxification of Cr (VI). Int. J. Hydrogen Energy 2020, 45, 7813–7828. [Google Scholar]
- Hardiansyah, A.; Budiman, W.J.; Yudasari, N.; Isnaeni; Kida, T.; Wibowo, A. Facile and Green Fabrication of Microwave-Assisted Reduced Graphene Oxide/Titanium Dioxide Nanocomposites as Photocatalysts for Rhodamine 6G Degradation. ACS Omega 2021, 6, 32166–32177. [Google Scholar] [CrossRef]
- Arshad, R.; Bokhari, T.H.; Javed, T.; Bhatti, I.A.; Rasheed, S.; Iqbal, M.; Nazir, A.; Naz, S.; Khan, M.I.; Khosa, M.K.K.; et al. Degradation Product Distribution of Reactive Red-147 Dye Treated by UV/H2O2/TiO2 Advanced Oxidation Process. J. Mater. Res. Technol. 2020, 9, 3168–3178. [Google Scholar] [CrossRef]
- Ansari, F.; Sheibani, S.; Caudillo-Flores, U.; Fernández-García, M. Effect of TiO2 nanoparticle loading by sol–gel method on the gas-phase photocatalytic activity of CuxO–TiO2 nanocomposite. J. Sol-Gel Sci. Technol. 2020, 96, 464–479. [Google Scholar] [CrossRef]
- Scuderi, V.; Amiard, G.; Sanz, R.; Boninelli, S.; Impellizzeri, G.; Privitera, V. TiO2 coated CuO nanowire array: Ultrathin p–n heterojunction to modulate cationic/anionic dye photo-degradation in water. Appl. Surf. Sci. 2017, 416, 885–890. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, J.; Li, G.; Jiang, Y.; Lan, H.; Guo, W.; Cao, Y. Origin of the improved photocatalytic activity of Cu incorporated TiO2 for hydrogen generation from water. Appl. Surf. Sci. 2016, 382, 170–177. [Google Scholar] [CrossRef]
- Chen, M.; Chen, J.; Chen, C.; Zhang, C.; He, H. Distinct photocatalytic charges separation pathway on CuOx modified rutile and anatase TiO2 under visible light. Appl. Catal. B Environ. 2022, 300, 120735. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.K. Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 2017, 391, 124–148. [Google Scholar] [CrossRef]
- Li, B.; Hao, Y.; Zhang, B.; Shao, X.; Hu, L. A multifunctional noble-metal-free catalyst of CuO/TiO2 hybrid nanofibers. Appl. Catal. A Gen. 2017, 531, 1–12. [Google Scholar] [CrossRef]
- Honda, M.; Ochiai, T.; Listiani, P.; Yamaguchi, Y.; Ichikawa, Y. Low-Temperature Synthesis of Cu-Doped Anatase TiO2 Nanostructures via Liquid Phase Deposition Method for Enhanced Photocatalysis. Materials 2023, 16, 639. [Google Scholar] [CrossRef]
- Date, M.K.; Yang, L.-H.; Yang, T.-Y.; Wang, K.-Y.; Su, T.-Y.; Wu, D.-C.; Cheuh, Y.-L. Three-Dimensional CuO/TiO2 Hybrid Nanorod Arrays Prepared by Electrodeposition in AAO Membranes as an Excellent Fenton-Like Photocatalyst for Dye Degradation. Nanoscale Res. Lett. 2020, 14, 45. [Google Scholar] [CrossRef]
- Chen, W.-T.; Jovic, V.; Sun-Waterhouse, D.; Idriss, H.; Waterhouse, G.I. The role of CuO in promoting photocatalytic hydrogen production over TiO2. Int. J. Hydrogen Energy 2013, 38, 15036–15048. [Google Scholar] [CrossRef]
- Nakate, U.T.; Patil, P.; Na, S.I.; Yu, Y.T.; Suh, E.K.; Hahn, Y.B. Fabrication and Enhanced Carbon Monoxide Gas Sensing Performance of p-CuO/n-TiO2 Heterojunction Device. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125962. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, Q.; Xia, Y.; Wang, J.; Chen, H.; Xu, Q.; Liu, J.; Feng, W.; Chen, S. Preparation and Characterization of Cu-Doped TiO2 Nanomaterials with Anatase / Rutile / Brookite Triphasic Structure and Their Photocatalytic Activity. J. Mater. Sci. Mater. Electron. 2021, 32, 21511–21524. [Google Scholar] [CrossRef]
- Kirk, C.H.; Wang, P.; Chong, C.Y.D.; Zhao, Q.; Sun, J.; Wang, J. TiO2 photocatalytic ceramic membranes for water and wastewater treatment: Technical readiness and pathway ahead. J. Mater. Sci. Technol. 2024, 183, 152–164. [Google Scholar] [CrossRef]
- Khdary, N.H.; Alkhuraiji, W.S.; Sakthivel, T.S.; Khdary, D.N. Nanophotocatalyst Using High Surface Area TiO2. Catalysts 2020, 10, 872. [Google Scholar] [CrossRef]
- Singh, J.; Juneja, S.; Soni, R.K.; Bhattacharya, J. Sunlight Mediated Enhanced Photocatalytic Activity of TiO2 Nanoparticles Functionalized CuO-Cu2O Nanorods for Removal of Methylene Blue and Oxytetracycline Hydrochloride. J. Colloid Interface Sci. 2021, 590, 60–71. [Google Scholar] [CrossRef]
- Méndez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Bahena, D.; Briois, V.; Colbeau-Justin, C.; Rodríguez-López, J.L.; Remita, H. Surface Modification of TiO2 with Ag Nanoparticles and CuO Nanoclusters for Application in Photocatalysis. J. Phys. Chem. C 2016, 120, 5143–5154. [Google Scholar] [CrossRef]
- Kubo, M.; Ishihara, Y.; Mantani, Y.; Shimada, M. Evaluation of the factors that influence the fabrication of porous thin films by deposition of aerosol nanoparticles. Chem. Eng. J. 2013, 232, 221–227. [Google Scholar] [CrossRef]
- Fernández, J.; Kiwi, J.; Lizama, C.; Freer, J.; Baeza, J.; Mansilla, H. Factorial experimental design of Orange II photocatalytic discolouration. J. Photochem. Photobiol. A Chem. 2002, 151, 213–219. [Google Scholar] [CrossRef]
- Rao, Y.; Chu, W. Linuron decomposition in aqueous semiconductor suspension under visible light irradiation with and without H2O2. Chem. Eng. J. 2010, 158, 181–187. [Google Scholar] [CrossRef]
- Li, X.; Chen, C.; Zhao, J. Mechanism of Photodecomposition of H2O2 on TiO2 Surfaces under Visible Light Irradiation. Langmuir 2001, 17, 4118–4122. [Google Scholar] [CrossRef]
- Sathiyan, K.; Bar-Ziv, R.; Mendelson, O.; Zidki, T. Controllable synthesis of TiO2 nanoparticles and their photocatalytic activity in dye degradation. Mater. Res. Bull. 2020, 126, 110842. [Google Scholar] [CrossRef]
- Kusdianto, K.; Jiang, D.; Kubo, M.; Shimada, M. Fabrication of TiO2–Ag nanocomposite thin films via one-step gas-phase deposition. Ceram. Int. 2017, 43, 5351–5355. [Google Scholar] [CrossRef]
- Talla, A.; Suliali, N.J.; Goosen, W.E.; Urgessa, Z.N.; Motloung, S.V.; Botha, J.R. Physica B: Physics of Condensed Matter Effect of Annealing Temperature and Atmosphere on the Structural, Morphological and Luminescent Properties of TiO2 Nanotubes. Phys. B Phys. Condens. Matter 2022, 640, 414026. [Google Scholar] [CrossRef]
- Kumar, P.; Badrinarayanan, S.; Sastry, M. Nanocrystalline TiO2 studied by optical, FTIR and X-ray photoelectron spectroscopy: Correlation to presence of surface states. Thin Solid Films 2000, 358, 122–130. [Google Scholar] [CrossRef]
- Razali, M.H.; Yusoff, M. Highly efficient CuO loaded TiO2 nanotube photocatalyst for CO2 photoconversion. Mater. Lett. 2018, 221, 168–171. [Google Scholar] [CrossRef]
- Li, H.; Liu, B.; Yin, S.; Sato, T.; Wang, Y. Visible Light-Driven Photocatalytic Activity of Oleic Acid-Coated TiO2 Nanoparticles Synthesized from Absolute Ethanol Solution. Nanoscale Res. Lett. 2015, 10, 415. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Pachaiappan, R.; Kumar, P.S.; Hoang, T.K.; Rajendran, S.; Durgalakshmi, D.; Soto-Moscoso, M.; Cornejo-Ponce, L.; Gracia, F. Visible light driven exotic p (CuO)–n (TiO2) heterojunction for the photodegradation of 4-chlorophenol and antibacterial activity. Environ. Pollut. 2021, 287, 117304. [Google Scholar] [CrossRef]
- Sudrajat, H.; Babel, S. Comparison and Mechanism of Photocatalytic Activities of N-ZnO and N-ZrO2 for the Degradation of Rhodamine 6G. Environ. Sci. Pollut. Res. 2016, 23, 10177–10188. [Google Scholar] [CrossRef] [PubMed]
- Berdini, F.; Otalvaro, J.O.; Avena, M.; Brigante, M. Photodegradation of doxycycline in water induced by TiO2-MCM-41. Kinetics, TOC evolution and reusability. Results Eng. 2022, 16, 100765. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hudandini, M.; Kusdianto, K.; Kubo, M.; Shimada, M. Gas-Phase Fabrication and Photocatalytic Activity of TiO2 and TiO2–CuO Nanoparticulate Thin Films. Materials 2024, 17, 1149. https://doi.org/10.3390/ma17051149
Hudandini M, Kusdianto K, Kubo M, Shimada M. Gas-Phase Fabrication and Photocatalytic Activity of TiO2 and TiO2–CuO Nanoparticulate Thin Films. Materials. 2024; 17(5):1149. https://doi.org/10.3390/ma17051149
Chicago/Turabian StyleHudandini, Meditha, Kusdianto Kusdianto, Masaru Kubo, and Manabu Shimada. 2024. "Gas-Phase Fabrication and Photocatalytic Activity of TiO2 and TiO2–CuO Nanoparticulate Thin Films" Materials 17, no. 5: 1149. https://doi.org/10.3390/ma17051149