CO and HCHO Sensing by Single Au Atom-Decorated WS2 Monolayer for Diagnosis of Thermal Aging Faults in the Dry-Type Reactor: A First-Principles Study
Abstract
:1. Introduction
2. Computation Methods
3. Results and Discussion
3.1. Au Decoration Effects on the WS2 Monolayer
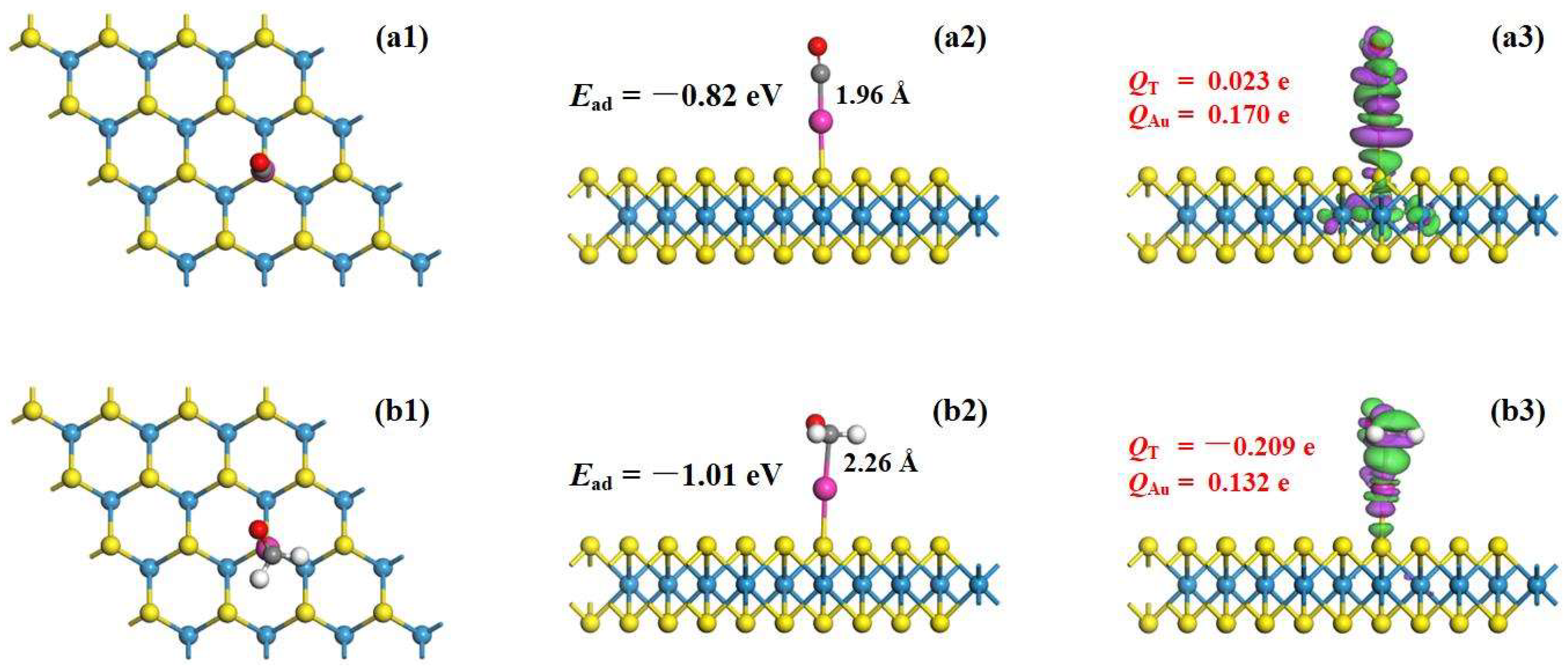
3.2. Adsorption Behavior of Au-WS2 Monolayer
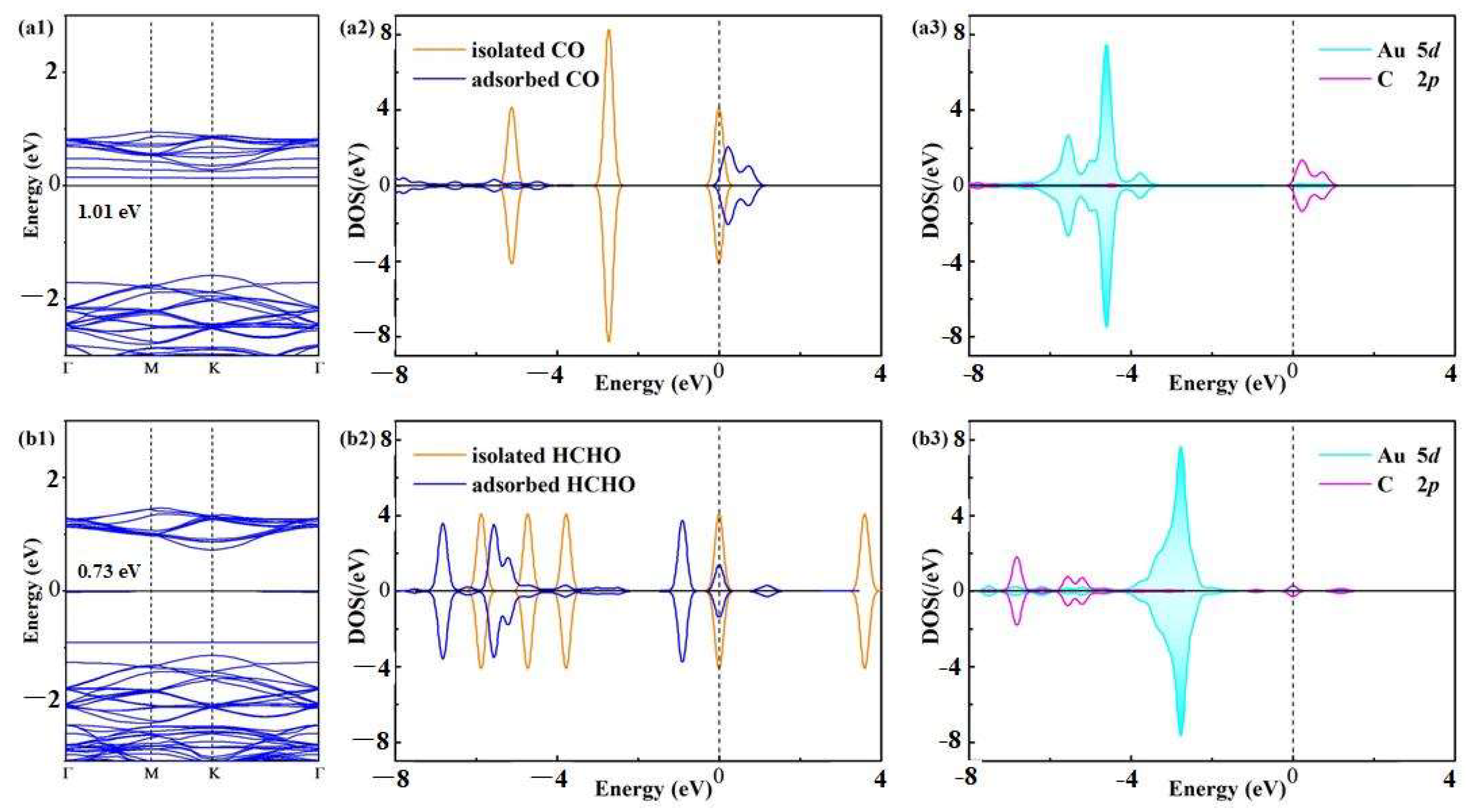
3.3. Electronic Property of Au-WS2 Monolayer in Gas Adsorptions
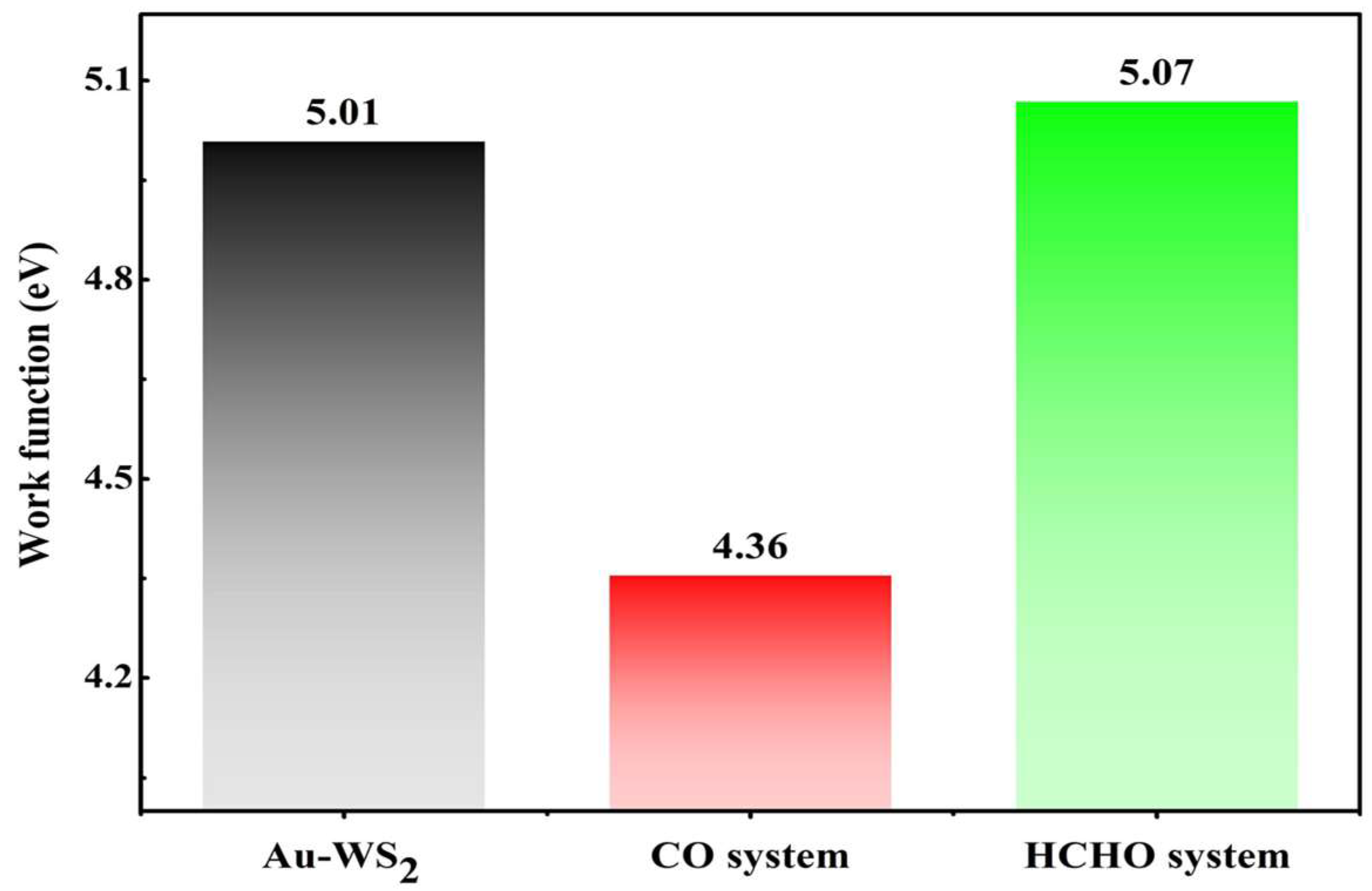
3.4. Gas Sensor Exploration for Au-WS2 Monolayer
4. Conclusions
- i.
- The Au atom can be stably adsorbed on the TS site of the pristine WS2 monolayer with the Ebind of −3.12 eV, making the bandgap 0.80 eV;
- ii.
- The Au-WS2 monolayer performs chemisorption upon CO and HCHO molecules with Ead values of −0.82 and −1.01 eV, respectively, in which the CO molecule behaves as an electron-donator while the HCHO molecule behaves as an electron-acceptor;
- iii.
- The sensing responses of the Au-WS2 monolayer upon CO and HCHO molecules are 58.7% and −74.4%, with recovery times of 0.01 and 11.86 s, respectively, indicating its potential for use as a reusable and room-temperature gas sensor for CO and HCHO detection;
- iv.
- The work function of the Au-WS2 monolayer suffers a 13.0% decrease in the CO system, while it exhibits a 1.2% increase in the HCHO system, suggesting its potential to be used as a WF-based gas sensor for CO detection.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.Z.; Chen, F.; Kang, B.; Ma, X.K. Optimum design of dry-type air-core reactor based on the additional constraints balance and hybrid genetic algorithm. Int. J. Appl. Electromagn. Mech. 2010, 33, 279–284. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, S.Y.; Man, Y.Y.; Li, S.Y.; Li, L.; Li, N.; Ning, Q. Adsorption and sensing performances of Rh-embedded PtSe2 monolayer upon CO and HCHO in dry-type reactors: A first-principles study. Chem. Physics. 2022, 562, 111652. [Google Scholar] [CrossRef]
- Okabe, S.; Ueta, G.; Wada, H. Partial Discharge Signal Propagation Characteristics inside the Winding of Oil-immersed Power Transformer—Using the Three-Winding Transformer Model in Air. IEEE Trans. Dielectr. Electr. Insul. 2012, 18, 2024–2031. [Google Scholar] [CrossRef]
- Iyer, G.; Gorur, R.S.; Krivda, A.; Mahonen, P. Prediction of Electrical Performance of Medium Voltage Epoxy Insulated Equipment. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 334–342. [Google Scholar] [CrossRef]
- Nakagawa, H.; Tsuge, S.; Koyama, T. Studies on thermal-degradation of epoxy-resins by high-resolution pyrolysis-gas chromatography. J. Anal. Appl. Pyrolysis 1987, 12, 97–113. [Google Scholar] [CrossRef]
- Dwyer, D.B.; Isbill, S.B.; Niedziela, J.L.; Kapsimalis, R.J.; Duckworth, D.C. Influence of temperature on accessible pyrolysis pathways of homopolymerized bisphenol A/F epoxies and copolymers. J. Anal. Appl. Pyrolysis 2021, 153, 104978. [Google Scholar] [CrossRef]
- Zhao, Q.; Man, Y.Y.; Li, S.Y.; Li, S.Y.; Li, L.; Li, N.; Ning, Q. Ni-Doped Janus HfSSe monolayer as a promising HCHO and C2H3Cl sensors in Dry-Type Reactor: A First-Principles theory. Comput. Theor. Chem. 2022, 1214, 113744. [Google Scholar] [CrossRef]
- Kalathiripi, H.; Karmakar, S. Analysis of transformer oil degradation due to thermal stress using optical spectroscopic techniques. Int. Trans. Electr. Energy Syst. 2017, 27, e2346. [Google Scholar] [CrossRef]
- Soni, R.; Mehta, B. A review on transformer condition monitoring with critical investigation of mineral oil and alternate dielectric fluids. Electr. Power Syst. Res. 2023, 214, 108954. [Google Scholar] [CrossRef]
- Zhao, Q.; Ran, J.Y.; Qin, C.L.; Yang, L.; Ran, M.C.; Ran, S.Y. An experimental study on the gas and soot formation in ethanol pyrolysis. Int. J. Oil Gas Coal Technol. 2019, 20, 189–211. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, N.; Jarwal, D.K.; Hu, Y.H.; Zhang, J.; Kumar, M. Strategic review on chemical vapor deposition technology-derived 2D material nanostructures for room-temperature gas sensors. J. Mater. Chem. C 2023, 11, 774–801. [Google Scholar] [CrossRef]
- Zhao, Q.; He, J.; Li, S.; Li, S.; Ning, Q.; Cui, H. DFT Calculations of Silver Atom Modified Tungsten Disulfide Monolayer as Promising Sensing Materials for Small Molecular Toxic Gases. Appl. Sci. 2023, 13, 12559. [Google Scholar] [CrossRef]
- Cao, W.; Zhao, Q.; Yang, L.; Cui, H. Enhanced NOx adsorption and sensing properties of MoTe2 monolayer by Ni-doping: A first-principles study. Surf. Interfaces 2021, 26, 101372. [Google Scholar] [CrossRef]
- Li, S.Y.; He, J.; Man, Y.Y.; Li, L.; Li, S.Y.; Li, N.; Zhao, Q. A first-principles study into Pt-embedded NiS2 monolayer as an outstanding gas sensor upon CO and HCHO dry-type reactors. Comput. Theor. Chem. 2023, 1226, 114203. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, Y.X.; He, C.; Wang, F.P.; Li, J. Secondary electron emission behavior of nanostructured fluorocarbon film. Surf. Interfaces 2022, 33, 102195. [Google Scholar] [CrossRef]
- Sharma, A.; Anu; Khan, M.S.; Husain, M.; Khan, M.S.; Srivastava, A. Sensing of CO and NO on Cu-Doped MoS2 Monolayer-Based Single Electron Transistor: A First Principles Study. IEEE Sens. J. 2018, 18, 2853–2860. [Google Scholar] [CrossRef]
- Latzke, D.W.; Zhang, W.T.; Suslu, A.; Chang, T.R.; Lin, H.; Jeng, H.T.; Tongay, S.; Wu, J.Q.; Bansil, A.; Lanzara, A. Electronic structure, spin-orbit coupling, and interlayer interaction in bulk MoS2 and WS2. Phys. Rev. B 2015, 91, 235202. [Google Scholar] [CrossRef]
- Joseph, N.B.; Narayan, A. Topological properties of bulk and bilayer 2M WS2, a first-principles study. J. Phys. Condens. Matter. 2021, 33, 465001. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, F.P.; Wang, K.Z.; Xie, G.B.; Cui, W.Z.; Li, J. Effect of Sputtering Temperature on Fluorocarbon Films: Surface Nanostructure and Fluorine/Carbon Ratio. Nanomaterials 2019, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Roondhe, B.; Dabhi, S.D.; Jha, P.K. A new flatland buddy as toxic gas scavenger: A first principles study. J. Hazard. Mater. 2018, 351, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Mishra, R.K.; Kumar, P.; Gwag, J.S. Electronic and optical properties of Nb/V-doped WS2 monolayer: A first-principles study. Luminescence 2022, 38, 1215–1220. [Google Scholar] [CrossRef]
- Evarestov, R.A.; Kovalenko, A.V.; Bandura, A.V.; Domnin, A.V.; Lukyanov, S.I. Comparison of vibrational and thermodynamic properties of MoS2 and WS2 nanotubes: First principles study. Mater. Res. Express 2018, 5, 115028. [Google Scholar] [CrossRef]
- Gutierrez, H.R.; Perea-Lopez, N.; Elias, A.L.; Berkdemir, A.; Wang, B.; Lv, R.; LopezUrias, F.; Crespi, V.H.; Terrones, H.; Terrones, M. Extraordinary Room-Temperature Photoluminescence in Triangular WS2 Monolayers. Nano Lett. 2013, 13, 3447–3454. [Google Scholar] [CrossRef] [PubMed]
- McCreary, K.M.; Hanbicki, A.T.; Jernigan, G.G.; Culbertson, J.C.; Jonker, B.T. Synthesis of Large-Area WS2 monolayers with Exceptional Photoluminescence. Sci. Rep. 2016, 6, 19159. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Wang, Z.H.; Fu, H.F.; Han, D.M.; Gu, F.B. Insight into the effect of Au particle size on triethylamine sensing properties based on a Au-ZnO nanoflower sensor. J. Mater. Chem. C 2022, 10, 3318–3328. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Tkatchenko, A.; DiStasio, R.A.; Head-Gordon, M.; Scheffler, M. Dispersion-corrected Møller-Plesset second-order perturbation theory. J. Chem. Phys. 2009, 131, 171. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B. 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Li, Q.; Li, P.; Pang, M.S.; Luo, Y.W. Fabrication of Pd-decorated MoSe2 nanoflowers and density functional theory simulation toward ammonia sensing. IEEE Electron Device Lett. 2019, 40, 616–619. [Google Scholar] [CrossRef]
- Duerloo, K.A.N.; Li, Y.; Reed, E.J. Structural phase transitions in two-dimensional Mo- and W-dichalcogenide monolayers. Nat. Commun. 2014, 5, 4214. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, L.M.; Zhao, S.J.; Hu, Z.-Y.; Liu, H. Potential application of metal dichalcogenides double-layered heterostructures as anode materials for li-ion batteries. J. Phys. Chem. C 2016, 120, 4779–4788. [Google Scholar] [CrossRef]
- Gong, C.; Zhang, H.; Wang, W.; Colombo, L.; Wallace, R.M.; Cho, K. Band alignment of two-dimensional transition metal dichalcogenides: Application in tunnel field effect transistors. Appl. Phys. Lett. 2013, 103, 053513. [Google Scholar] [CrossRef]
- Hassan, A.; Guo, Y.G.; Wang, Q.; Kawazoe, Y.; Jena, P. Interfacial properties of penta-graphene-metal contacts. J. Appl. Phys. 2019, 125, 065308. [Google Scholar] [CrossRef]
- Allian, A.D.; Takanabe, K.; Fujdala, K.L.; Hao, X.H.; Truex, T.J.; Cai, J.; Buda, C.; Neurock, M.; Iglesia, E. Chemisorption of CO and Mechanism of CO Oxidation on Supported Platinum Nanoclusters. J. Am. Chem. Soc. 2012, 134, 743. [Google Scholar] [CrossRef]
- Cui, H.; Liu, T.; Zhang, Y.; Zhang, X. Ru-InN Monolayer as a Gas Scavenger to Guard the Operation Status of SF6 Insulation Devices: A First-Principles Theory. IEEE Sens. J. 2019, 19, 5249–5255. [Google Scholar] [CrossRef]
- Cui, H.; Chen, D.; Zhang, Y.; Zhang, X. Dissolved gas analysis in transformer oil using Pd catalyst decorated MoSe2 monolayer: A first principles theory. Sustain. Mater. Technol. 2019, 20, e00094. [Google Scholar] [CrossRef]
- Goutham, S.; Sadasivuni, K.K.; Kumar, D.S.; Rao, K.V. Flexible ultra-sensitive and resistive NO2 gas sensor based on nanostructured Zn(x)Fe(1-x)2O4. RSC Adv. 2018, 8, 3243–3249. [Google Scholar] [CrossRef]
- He, X.; Gui, Y.; Xie, J.; Liu, X.; Wang, Q.; Tang, C. A DFT study of dissolved gas (C2H2, H2, CH4) detection in oil on CuO modified BNNT. Appl. Surf. Sci. 2020, 500, 144030. [Google Scholar] [CrossRef]
- Gui, Y.; Li, W.; He, X.; Ding, Z.; Tang, C.; Xu, L. Adsorption properties of pristine and Co-doped TiO2 (101) toward dissolved gas analysis in transformer oil. Appl. Surf. Sci. 2020, 507, 145163. [Google Scholar] [CrossRef]
- Aasi, A.; Javahersaz, R.; Aghaei, S.M.; Panchapakesan, B. First-principles insight into two-dimensional palladium phosphide tellurium (PdPTe) monolayer as a promising scavenger for detecting SF6 decompositions. J. Mater. Sci. 2022, 57, 5497–5506. [Google Scholar] [CrossRef]
- Vadalkar, S.; Chodvadiya, D.; Som, N.N.; Vyas, K.N.; Jha, P.K.; Chakraborty, B. An Ab-initio Study of the C18 nanocluster for Hazardous Gas Sensor Application. ChemistrySelect 2022, 7, e202103874. [Google Scholar] [CrossRef]
- Aasi, A.; Aghaei, S.M.; Panchapakesan, B. Noble metal (Pt or Pd)-decorated atomically thin MoS2 as a promising material for sensing colorectal cancer biomarkers through exhaled breath. Int. J. Comput. Mater. Sci. Eng. 2024, 13, 2350014. [Google Scholar] [CrossRef]
- Aasi, A.; Bajgani, S.E.; Panchapakesan, B. A first-principles investigation on the adsorption of octanal and nonanal molecules with decorated monolayer WS2 as promising gas sensing platform. AIP Adv. 2023, 13, 025157. [Google Scholar] [CrossRef]
- Zhou, X.; Chu, W.; Zhou, Y.; Sun, W.; Xue, Y. DFT simulation on H2 adsorption over Ni-decorated defective h-BN nanosheets. Appl. Surf. Sci. 2018, 439, 246–253. [Google Scholar] [CrossRef]
- Yu, Y.-J.; Zhao, Y.; Ryu, S.; Brus, L.E.; Kim, K.S.; Kim, P. Tuning the graphene work function by electric field effect. Nano Lett. 2009, 9, 3430–3434. [Google Scholar] [CrossRef]
- Tanvir, N.B.O.; Yurchenko, E.; Laubender, G. Urban. Investigation of low temperature effects on work function based CO2 gas sensing of nanoparticulate CuO films. Sens. Actuators B Chem. 2017, 247, 968–974. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Man, Y.; He, J.; Li, S.; Li, L. CO and HCHO Sensing by Single Au Atom-Decorated WS2 Monolayer for Diagnosis of Thermal Aging Faults in the Dry-Type Reactor: A First-Principles Study. Materials 2024, 17, 1173. https://doi.org/10.3390/ma17051173
Zhao Q, Man Y, He J, Li S, Li L. CO and HCHO Sensing by Single Au Atom-Decorated WS2 Monolayer for Diagnosis of Thermal Aging Faults in the Dry-Type Reactor: A First-Principles Study. Materials. 2024; 17(5):1173. https://doi.org/10.3390/ma17051173
Chicago/Turabian StyleZhao, Qi, Yuyan Man, Jin He, Songyuan Li, and Lin Li. 2024. "CO and HCHO Sensing by Single Au Atom-Decorated WS2 Monolayer for Diagnosis of Thermal Aging Faults in the Dry-Type Reactor: A First-Principles Study" Materials 17, no. 5: 1173. https://doi.org/10.3390/ma17051173





