The Properties of High-Performance Concrete with Manganese Slag under Salt Action
Abstract
:1. Introduction
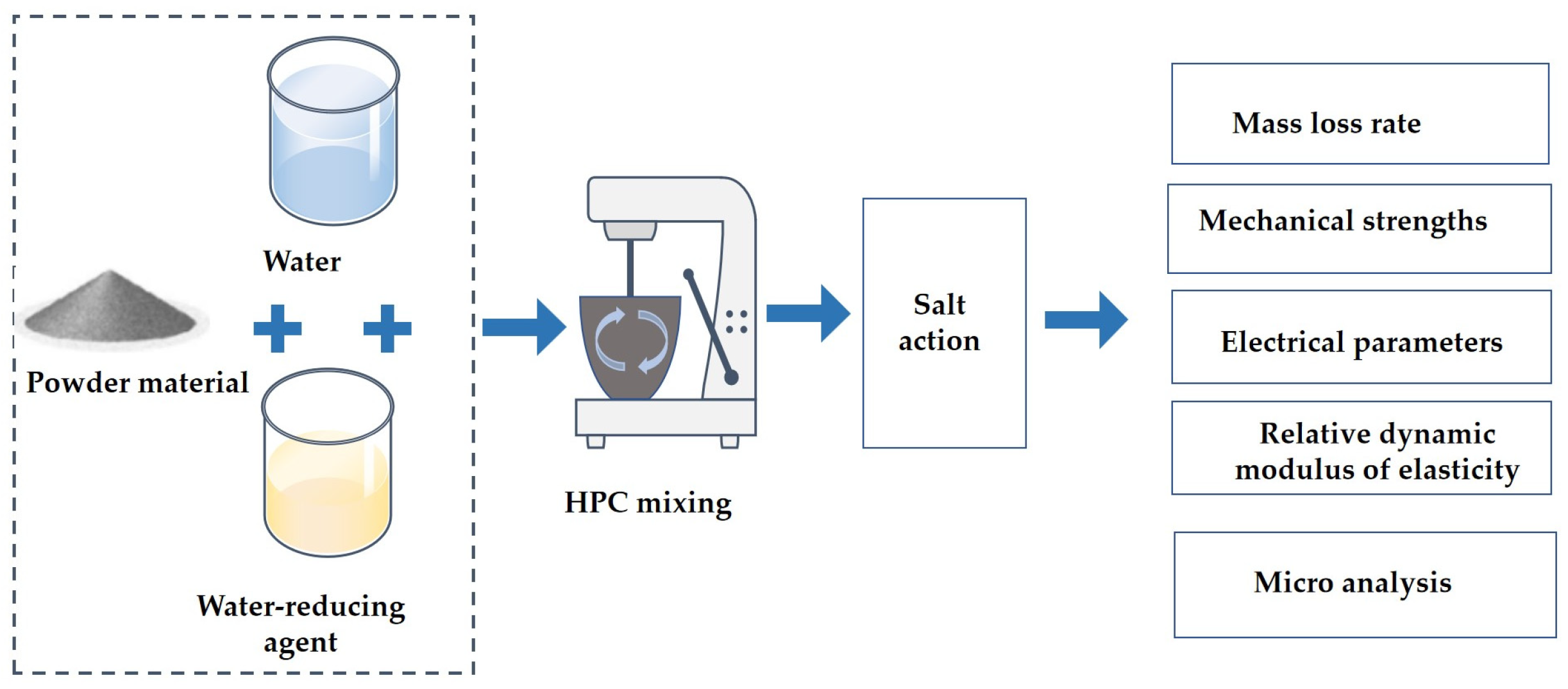
2. Materials and Methods
2.1. Raw Materials
2.2. Specimen Preparations
2.3. Experimental Methodology
2.4. The NaCl F-C Action
2.5. The NaCl and Na2SO4 D-A Actions
2.6. The Measurement of Mass Loss Rate
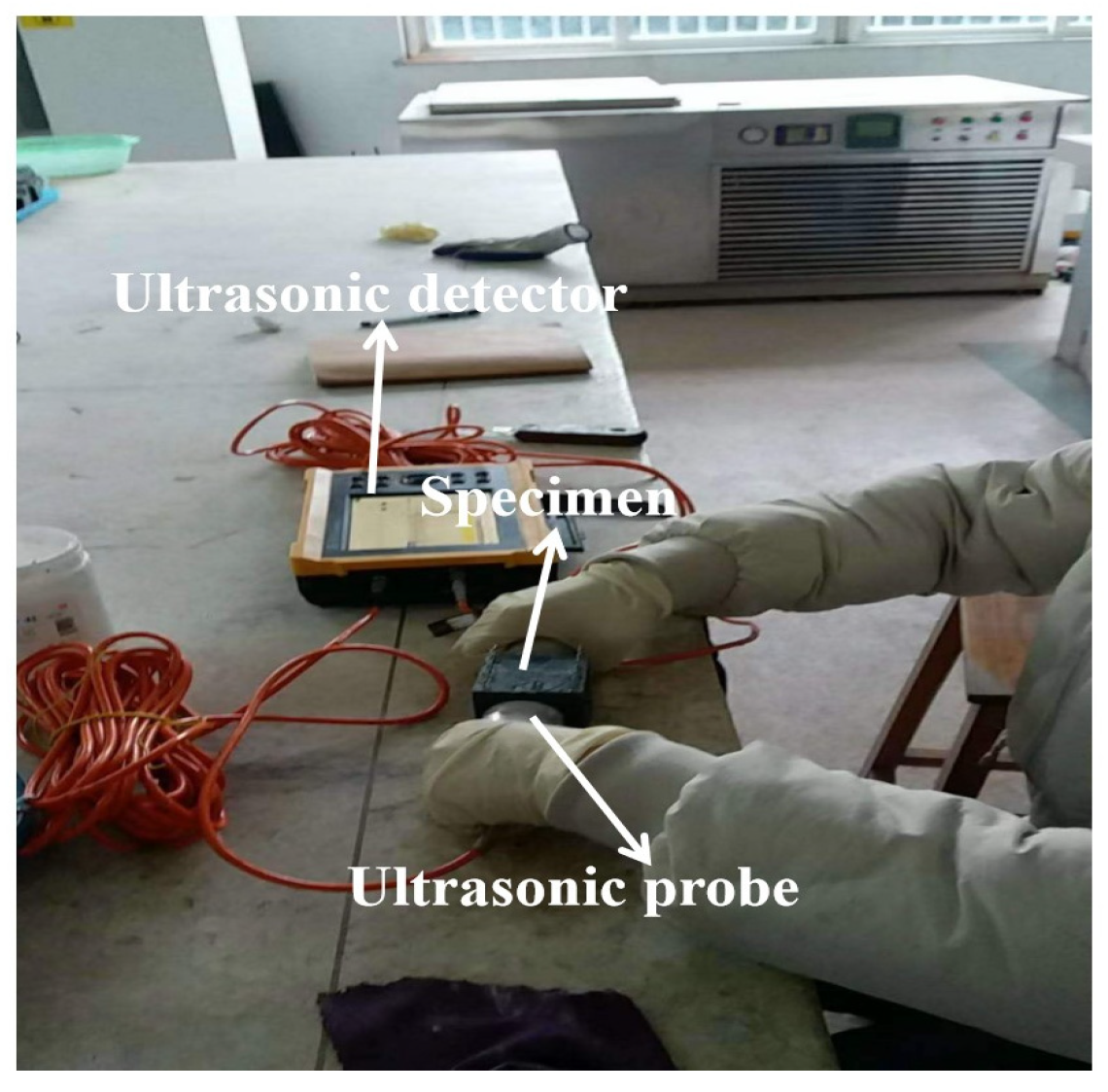
2.7. The Measurement of Relative Dynamic Modulus of Elasticity
2.8. The Measurement of Leached Toxic Heavy Metal Substances
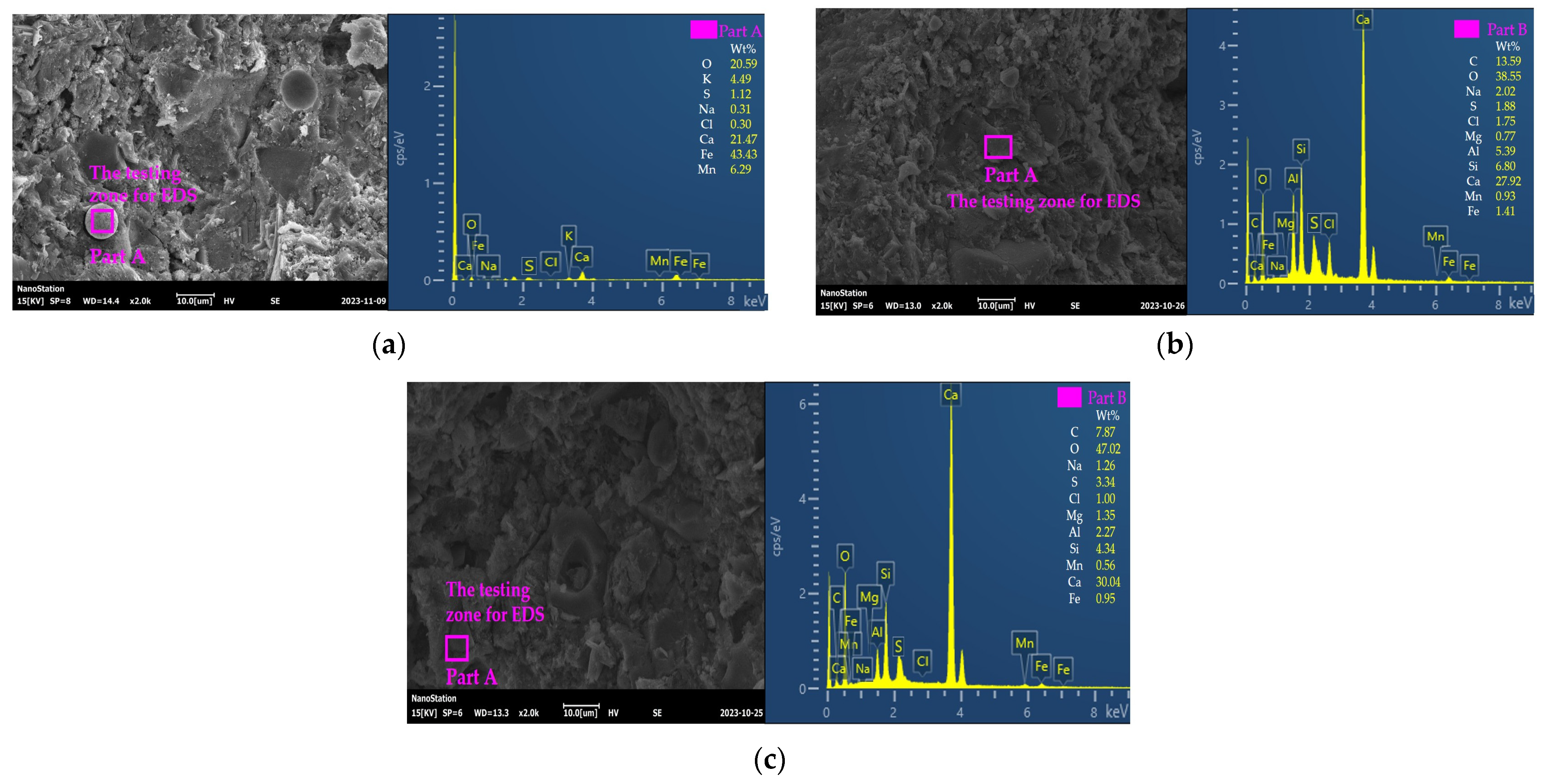
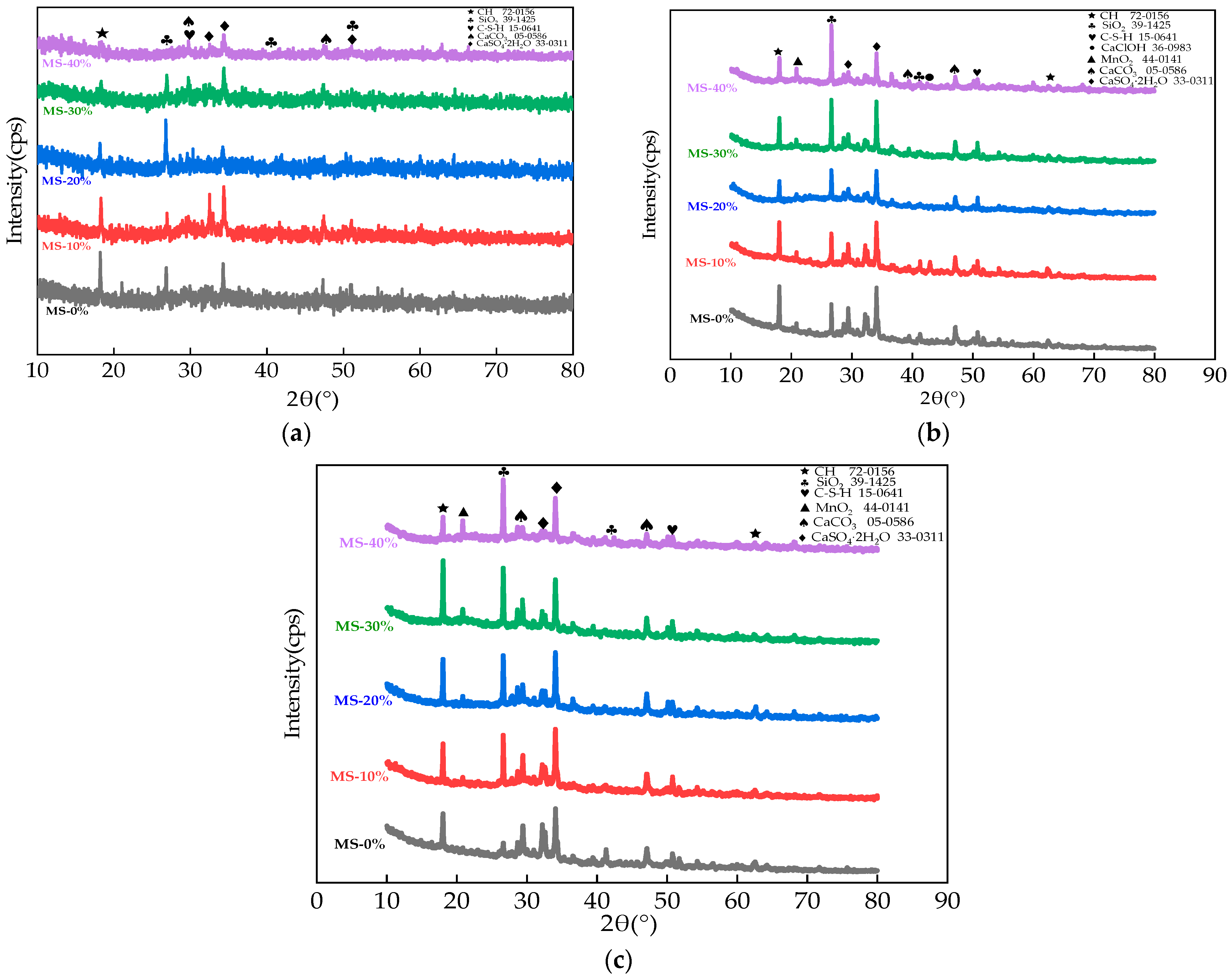
2.9. The Energy-Dispersive X-ray Spectroscopy and XRD Experiment
3. Results and Discussion
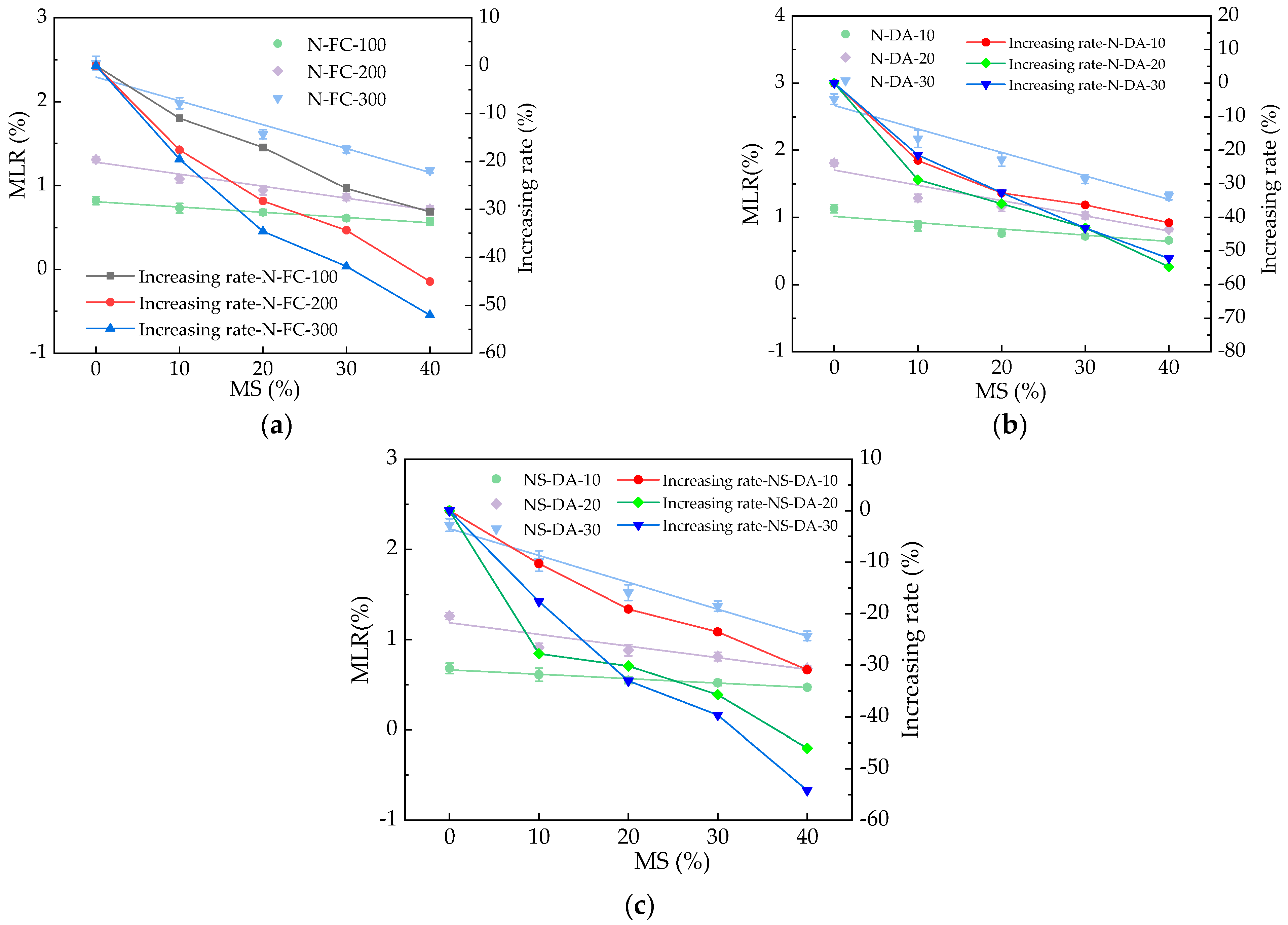
3.1. The MLR of HPC
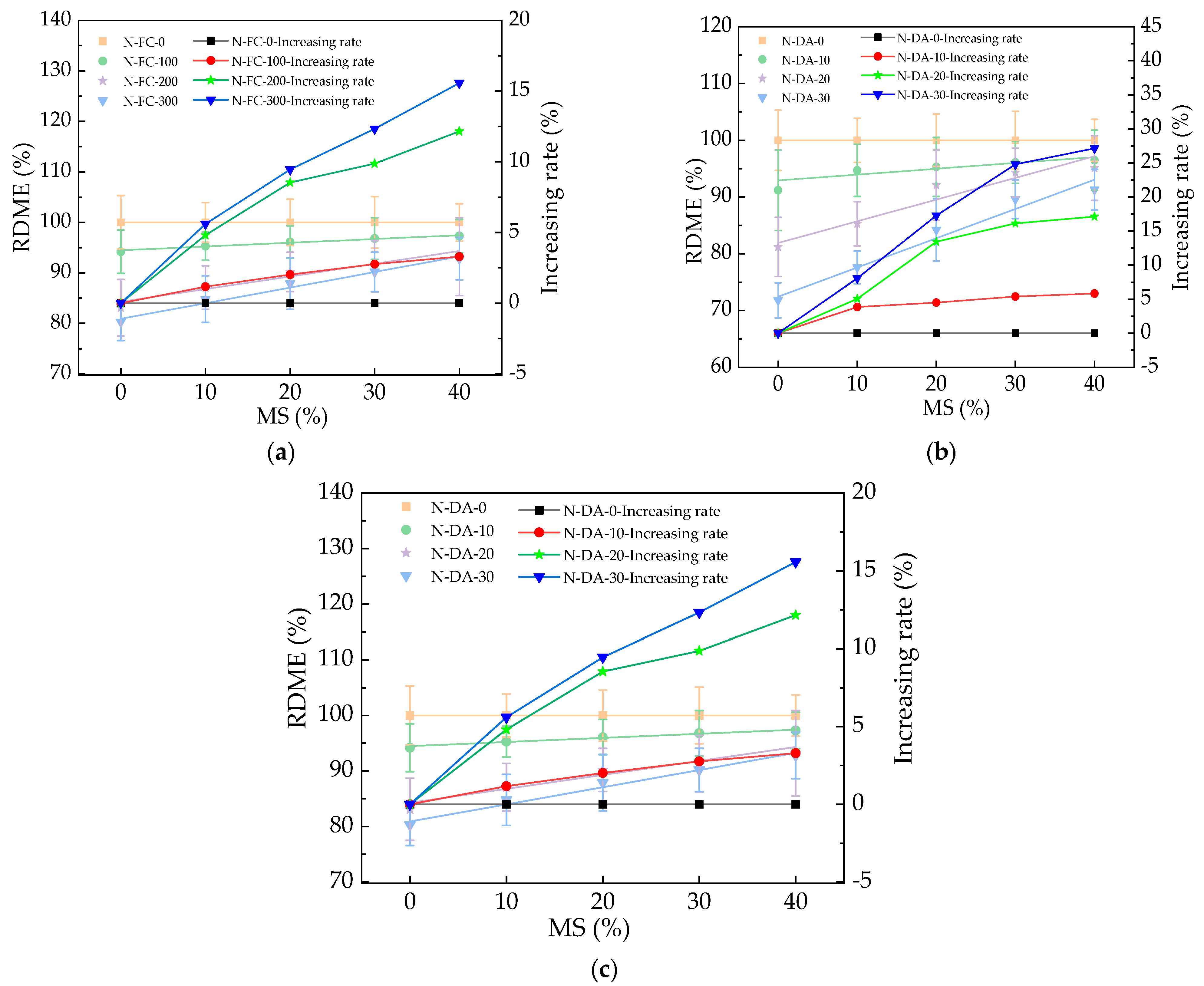
3.2. The RDME of HPC
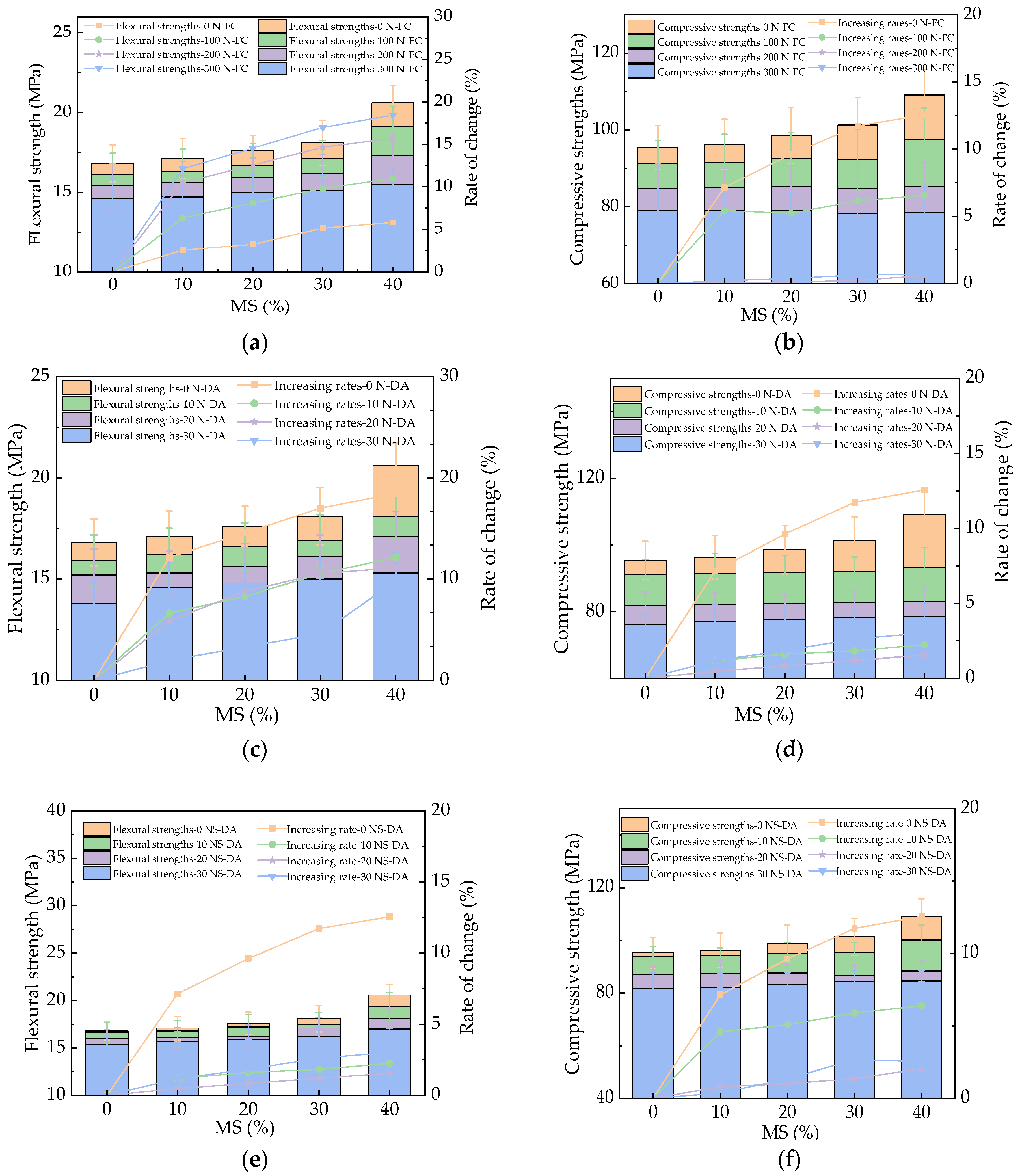
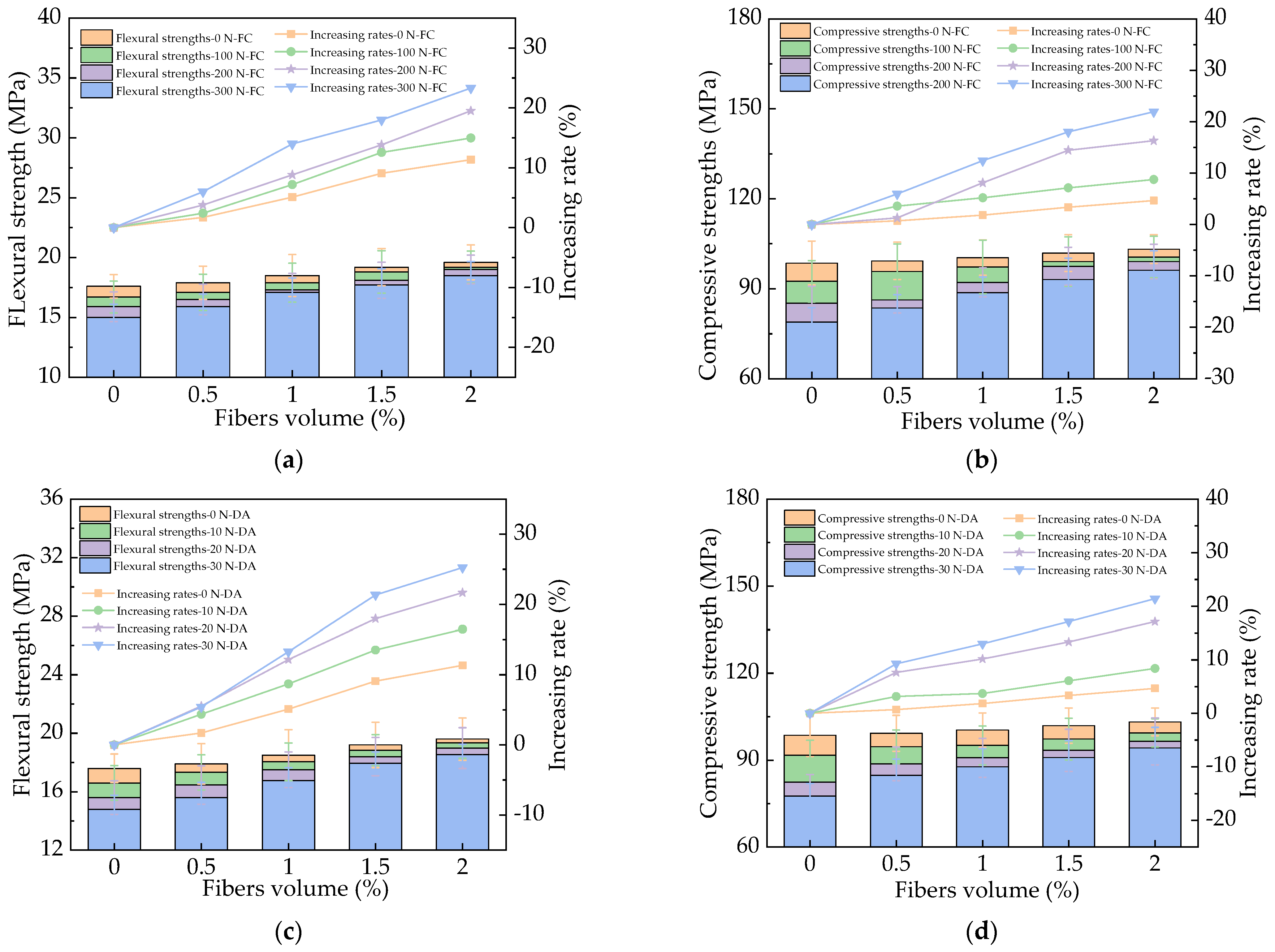
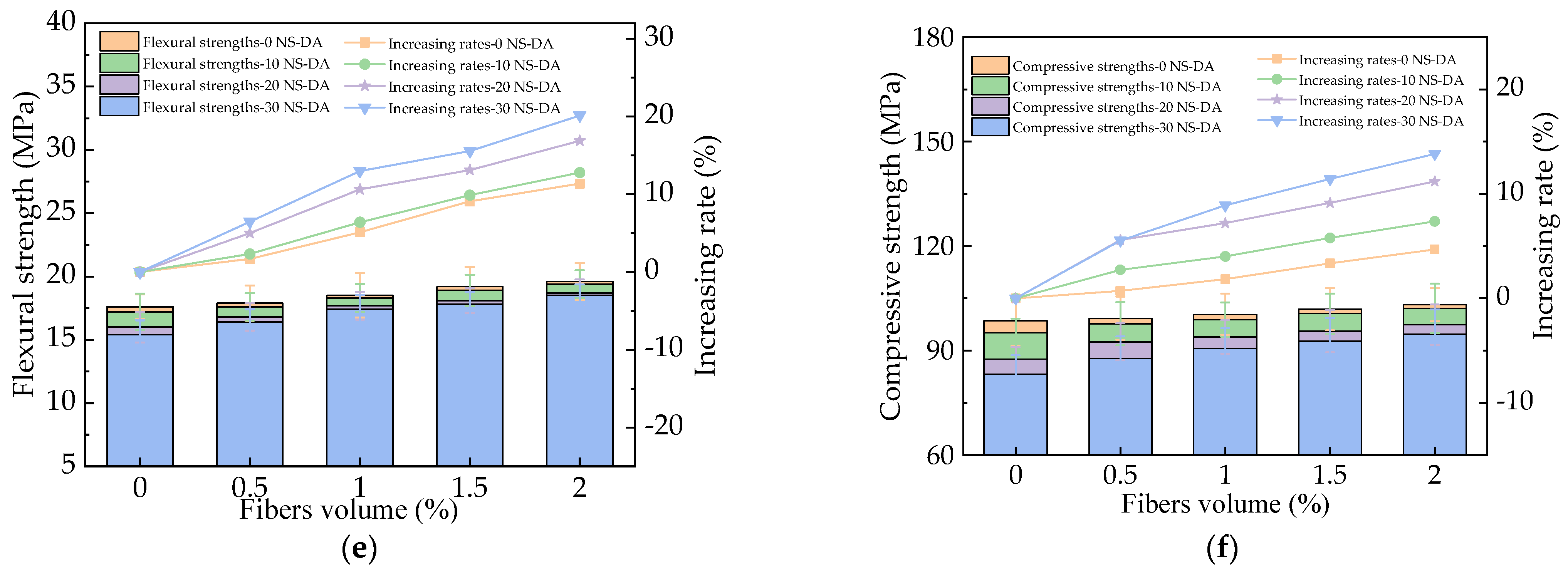
3.3. The Mechanical Strengths of HPC
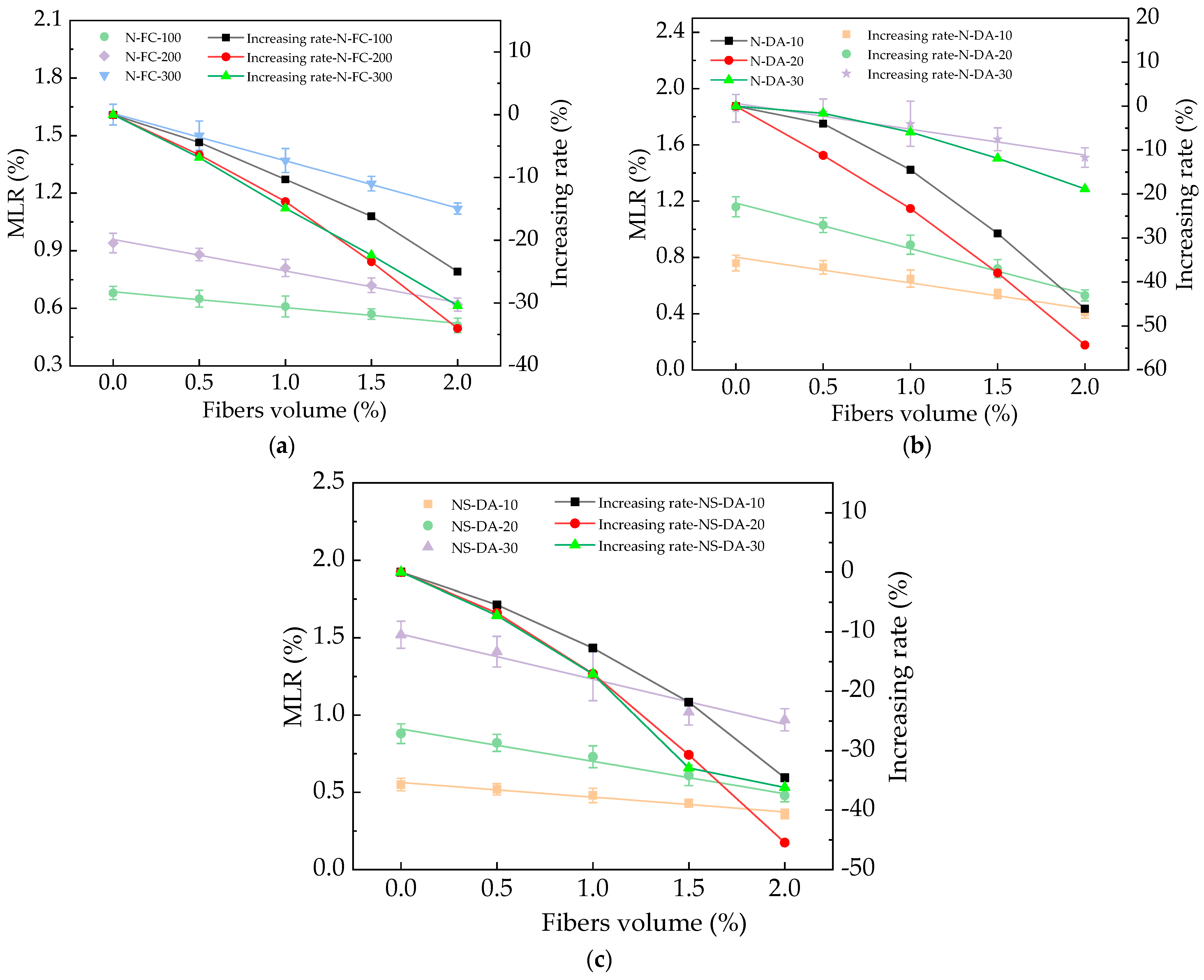
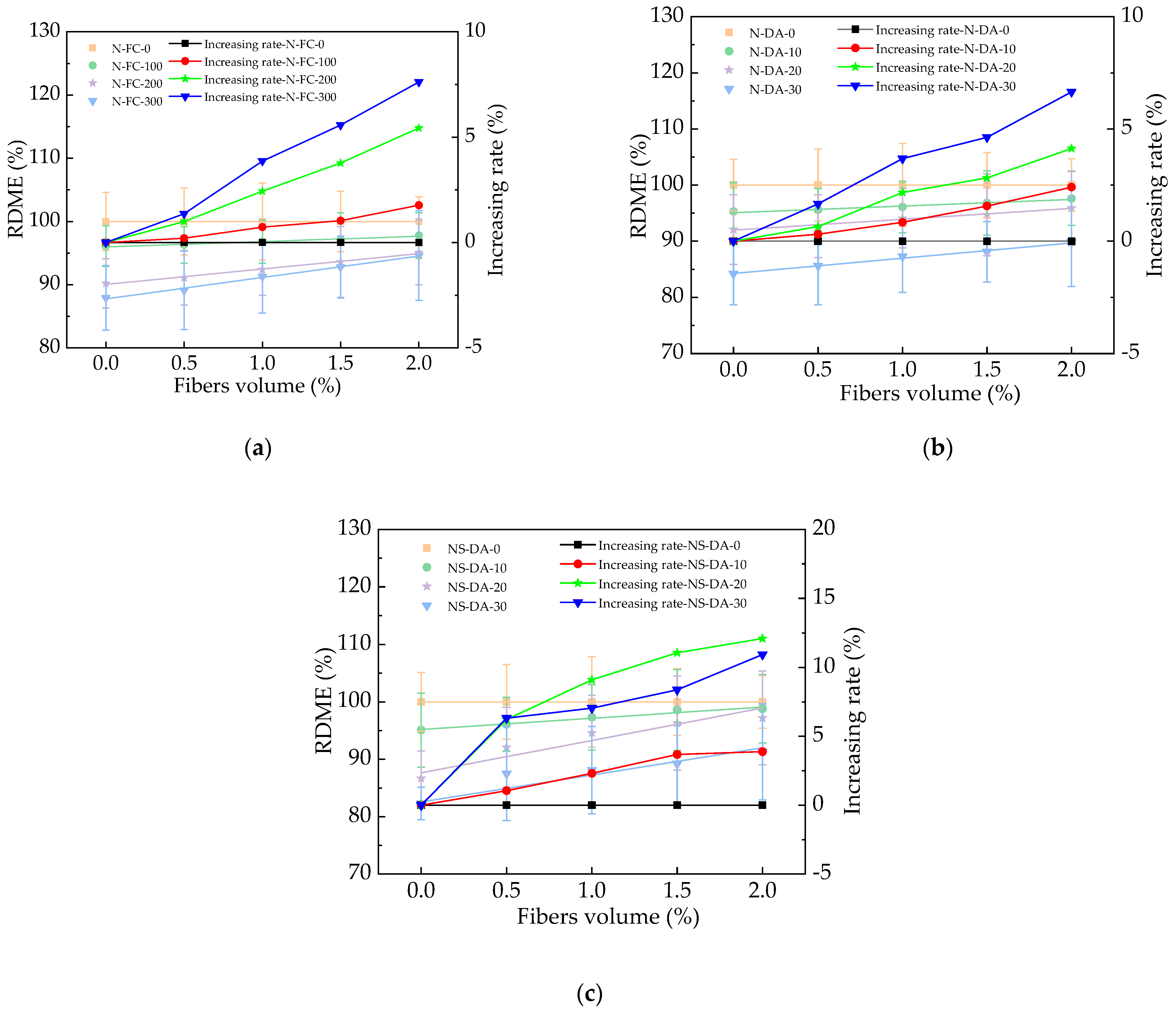
3.4. The Influence of Basalt Fibers
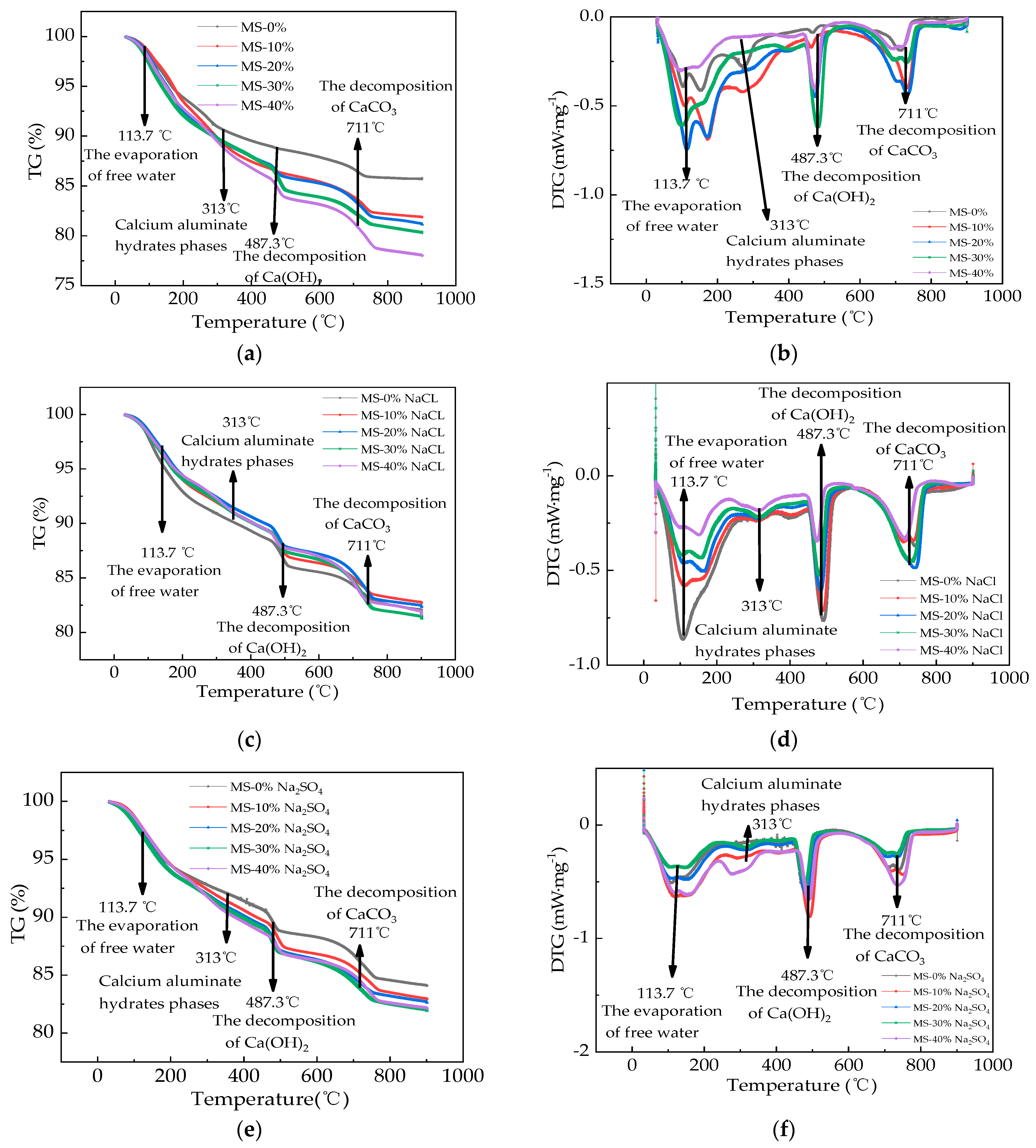
3.5. The Microscopical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MS | manganese slag |
| RDME | relative dynamic modulus of elasticity |
| HPC | high-performance concrete |
| MS-HPC | manganese slag high-performance concrete |
| N-FC | NaCl freeze–thaw cycles |
| N-DA | NaCl dry–wet alternations |
| NS-DA | Na2SO4 dry–wet alternations |
| SEM | scanning electron microscope |
| EDS | energy-dispersive spectrometer |
| TG | thermogravimetric analysis |
| XRD | X-ray diffraction spectroscopy |
| MLR | mass loss rate |
| OPC | Ordinary Portland Cement |
| SF | silica fume |
| QS | quartz sand |
| BFP | blast-furnace slag powder |
| HRWR | high-range water-reducing agent |
| Cr | chromium |
| Zn | zinc |
| C-S-H | calcium silicate hydrate |
References
- Kudyba, A.; Akhtar, S.; Johansen, I.; Safarian, J. Aluminothermic Reduction of Manganese Oxide from Selected MnO-Containing Slags. Materials 2021, 14, 356. [Google Scholar] [CrossRef]
- El-Faramawy, H.; Mattar, T.; Eissa, M.; El-Fawakhry, K.; Ahmed, A.M. Demanganisation of high manganese pig iron to produce high manganese stag. Ironmak. Steelmak. 2004, 31, 23–30. [Google Scholar] [CrossRef]
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Functionalization of graphite oxide with magnetic chitosan for the preparation of a nanocomposite dye adsorbent. Langmuir 2013, 29, 1657–1668. [Google Scholar] [CrossRef]
- Mahapatra, M.; Karmakar, M.; Dutta, A.; Mondal, H.; Roy, J.S.D.; Chattopadhyay, P.K. Microstructural analyses of loaded and/or unloaded semisynthetic porous material for understanding of superadsorption and optimization by response surface methodology. J. Environ. Chem. Eng. 2018, 6, 289–310. [Google Scholar] [CrossRef]
- Nishad, P.A.; Bhaskarapillai, A. Antimony, a pollutant of emerging concern: A review on industrial sources and remediation technologies. Chemosphere 2021, 277, 130252. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Lakouraj, M.M.; Kasirian, N. Development of effective nanobiosorbent based on poly m-phenylenediamine grafted dextrin for removal of Pb (II) and methylene blue from water. Carbohydr. Polym. 2018, 201, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Toro, N.; Rodríguez, F.; Rojas, A.; Robles, P.; Ghorbani, Y. Leaching manganese nodules with iron-reducing agents-a critical review. Miner. Eng. 2021, 163, 106748. [Google Scholar] [CrossRef]
- Witoon, T.; Numpilai, T.; Nijpanich, S.; Chanlek, N.; Kidkhunthod, P.; Cheng, C.K.; Ng, K.; Vo, D.-V.N.; Ittisanronnachai, S.; Wattanakit, C. Enhanced CO2 hydrogenation to higher alcohols over K-Co promoted In2O3 catalysts. Chem. Eng. J. 2022, 431, 133211. [Google Scholar] [CrossRef]
- Sharma, R.; Kim, H.; Pei, J.; Jang, J.G. Dimensional stability of belite-rich cement subject to early carbonation curing. J. Build. Eng. 2023, 63, 105545. [Google Scholar] [CrossRef]
- Khan, R.I.; Ashraf, W.; Olek, J. Amino acids as performance-controlling additives in carbonation-activated cementitious materials. Cem. Concr. Res. 2021, 147, 106501. [Google Scholar] [CrossRef]
- Siddique, S.; Naqi, A.; Jang, J.G. Influence of water to cement ratio on CO2 uptake capacity of belite-rich cement upon exposure to carbonation curing. Cem. Concr. Compos. 2020, 111, 103616. [Google Scholar] [CrossRef]
- Surendran, H.; Akhas, P. Properties of high-performance concrete incorporating toughened glass waste coarse aggregate: An experimental study. Structures 2024, 60, 105897. [Google Scholar] [CrossRef]
- Ferdosian, I.; Camões, A.; Ribeiro, M. High-volume fly ash paste for developing ultra-high performance concrete (UHPC). Ciência Tecnol. Mater. 2017, 29, e157–e161. [Google Scholar] [CrossRef]
- Faleschini, F.; Fernández-Ruíz, M.A.; Zanini, M.; Brunelli, K.; Pellegrino, C.; Hernández-Montes, E. High performance concrete with electric arc furnace slag as aggregate: Mechanical and durability properties. Constr. Build. Mater. 2015, 101, 113–121. [Google Scholar] [CrossRef]
- Rao, K.B.; Nayak, G.; Kamath, M.; Tantri, A. Synergetic effect of binary, ternary and quaternary binders on microstructural, mechanical and durability aspects of EAF aggregate HPC system. Constr. Build. Mater. 2024, 411, 134673. [Google Scholar]
- Abdulkareem, O.M.; Fraj, A.B.; Bouasker, M. Mixture design and early age investigations of more sustainable UHPC. Constr. Build. Mater. 2018, 163, 235–246. [Google Scholar] [CrossRef]
- Norhasri, M.S.M.; Hamidah, M.S.; Fadzil, A.M. Inclusion of nano metaclayed as additive in ultra high performance concrete (UHPC). Constr. Build. Mater. 2019, 201, 590–598. [Google Scholar] [CrossRef]
- Soliman, A.M.; Nehdi, M.L. Effect of partially hydrated cementitious materials and superabsorbent polymer on early-age shrinkage of UHPC. Constr. Build. Mater. 2013, 41, 270–275. [Google Scholar] [CrossRef]
- Yang, H. Influence of Mineral Powder and Fiber on Work and Mechanical Properties of Reactive Powder Concrete. Multipurp. Util. Miner. Resour. 2023, 2, 197–204. [Google Scholar]
- Ge, W.; Zhang, Z.; Ashour, A.; Li, W.; Jiang, H.; Hu, Y.; Shuai, H.; Sun, C.; Li, S.; Liu, Y. Hydration characteristics, hydration products and microstructure of reactive powder concrete. J. Build. Eng. 2023, 69, 106306. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, C.; He, W.; Wu, L. Effects of steel fiber content and shape on mechanical properties of ultra high performance concrete. Constr. Build. Mater. 2016, 103, 8–14. [Google Scholar] [CrossRef]
- Cao, H.; Liang, Z.; Peng, X.; Cai, X.; Wang, K.; Wang, H.; Lyu, Z. Research of Carbon Dioxide Curing on the Properties of Reactive Powder Concrete with Assembly Unit of Sulphoaluminate Cement and Ordinary Portland Cement. Coatings 2022, 12, 209. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, W.; Wang, H. The Corrosion Resistance of Reinforced Reactive Powder Concrete with Secondary Aluminum Ash Exposed to NaCl Action. Materials 2023, 16, 5615. [Google Scholar] [CrossRef]
- Gursel, A.P.; Maryman, H.; Ostertag, C. A life-cycle approach to environmental, mechanical, and durability properties of “green” concrete mixes with rice husk ash. J. Clean. Prod. 2016, 112, 823–836. [Google Scholar] [CrossRef]
- Xu, W.; Yu, J.; Wang, H. The Influence of Manganese Slag on the Properties of Ultra-High-Performance Concrete. Materials 2024, 17, 497. [Google Scholar] [CrossRef]
- Rahat, U.; Jawad, A.; Yuan, Q.; Nikolai, I.V. Ultra-High-Performance Concrete (UHPC): A State-of-the-Art Review. Materials 2022, 15, 4131. [Google Scholar]
- GB/T 2419-2005; Test method for Fluidity of Cements Mortar. The State Bureau of Quality and Technical Supervision Contents: Beijing, China, 2005.
- GB/T 17671-2005; Method of Testing Cements—Determination of Strength. The State Bureau of Quality and Technical Supervision Contents: Beijing, China, 1999.
- UNE-CEN/TS 12390-9:2008 EX; Testing Hardened Concrete-Part 9: Freeze-thaw Resistance-scaling. Aenor: Madrid, Spain, 2008.
- Hong, X.; Wang, H.; Shi, F. Influence of NaCl freeze thaw cycles and cyclic loading on the mechanical performance and peRDMEability of sulphoaluminate cement reactive powder concrete. Coatings 2020, 10, 1227. [Google Scholar] [CrossRef]
- Lei, L.; Wang, Q.; Xu, S.; Wang, N.; Zheng, X. Fabrication of superhydrophobic concrete used in marine environment with anti-corrosion and stable mechanical properties. Constr. Build. Mater. 2020, 251, 118946. [Google Scholar] [CrossRef]
- Huang, G.; Wang, H.; Shi, F. Coupling Effect of Salt Freeze-Thaw Cycles and Carbonation on the Mechanical Performance of Quick Hardening Sulphoaluminate Cement-Based Reactive Powder Concrete with Basalt Fibers. Coatings 2021, 11, 1142. [Google Scholar] [CrossRef]
- Su, Q.; Xu, J.M. Mechanical properties of concrete containing glass sand and rice husk ash. Constr. Build. Mater. 2023, 393, 132053. [Google Scholar] [CrossRef]
- Tarangini, D.; Sravana, P.; Srinivasa Rao, P. Effect of nano silica on frost resistance of pervious concrete. Mater. Today Proc. 2022, 51, 2185–2189. [Google Scholar] [CrossRef]
- Meshram, A.; Gautam, D.; Singh, K. Recycling of white aluminium dross: Production of potash alum. Trans. Indian Inst. Met. 2020, 73, 1239–1248. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Brice, D.G.; Kilcullen, A.R.; Hamdan, S.; Deventer, J.S. Influence of fly ash on the water and chloride peRDMEability of alkali-activated slag mortars and concretes. Constr. Build. Mater. 2013, 48, 1187–1201. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Allahverdi, A. A promising green process for synthesis of high purity activated-alumina nanopowder from secondary aluminum dross. J. Clean. Prod. 2018, 179, 93–102. [Google Scholar] [CrossRef]
- Sarikaya, Y.; Sevinç, I.; Akinç, M. The effect of calcinations temperature on some of the adsorptive properties of fine alumina powders obtained by emulsion evaporation technique. Powder Technol. 2001, 116, 109–114. [Google Scholar] [CrossRef]
- Serelis, E.; Vaitkevicius, V. Effect of waste glass powder and liquid glass on the Physico-Chemistry of Aluminum-Based Ultra-Lightweight concrete. Constr. Build. Mater. 2023, 390, 131615. [Google Scholar] [CrossRef]
- Ng, A.L.; Liew, H.R.; Wong, Y.C. Strength of Concrete with Recycled Manganese Slag. Mater. Sci. Forum 2021, 1030, 88–93. [Google Scholar] [CrossRef]
- Liang, D.Q.; Qin, F.; Li, X.G. Performance of concrete made with manganese slag. Adv. Mat. Res. 2011, 335, 333–338. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Z.; Zhu, Y.; Tian, L. Durability of alkali-activated fly ash concrete: Chloride penetration in pastes and mortars. Constr. Build. Mater. 2014, 65, 51–59. [Google Scholar] [CrossRef]
- Wang, H.; Jin, K.; Zhang, A.; Zhang, L.; Han, Y.; Liu, J.; Shi, F.; Feng, L. External erosion of sodium chloride on the degradation of self-sensing and mechanical properties of aligned stainless steel fiber reinforced reactive powder concrete. Constr. Build. Mater. 2021, 287, 123028. [Google Scholar] [CrossRef]
- Chousidis, N.; Ioannou, I.; Batis, G. Utilization of Electrolytic Manganese Dioxide (E.M.D.) waste in concrete exposed to salt crystallization. Constr. Build. Mater. 2018, 158, 708–718. [Google Scholar] [CrossRef]
- Nath, S.K.; Randhawa, N.S.; Kumar, S. A review on characteristics of silico-manganese slag and its utilization into construction materials. Resour. Conserv. Recycl. 2022, 76, 105946. [Google Scholar] [CrossRef]
- Simao, L.; Fernandes, E.; Hotza, D.; Ribeiro, M.J.; Montedo, O.R.K.; Raupp-Pereira, F. Controlling efflorescence in geopolymers: A new approach. Case Stud. Constr. Mater. 2021, 15, e00740. [Google Scholar] [CrossRef]
- Zhou, Y. Reusing electrolytic manganese residue as an activator: The effect of calcination on its mineralogy and activity. Constr. Build. Mater. 2021, 294, 123533. [Google Scholar] [CrossRef]
- Padilla-Encinas, P.; Palomo, A.; Blanco-Varela, M.T.A. Fernandez-Jimenez, Calcium sulfoaluminate clinker hydration at different alkali concentrations. Cem. Concr. Res. 2020, 138, 106251. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Schmitt, D.; Haha, M.B. Effect of raw mix design and of clinkering process on the formation and mineralogical composition of (ternesite) belite calcium sulphoaluminate ferrite clinker. Cem. Concr. Res. 2014, 59, 87–95. [Google Scholar] [CrossRef]
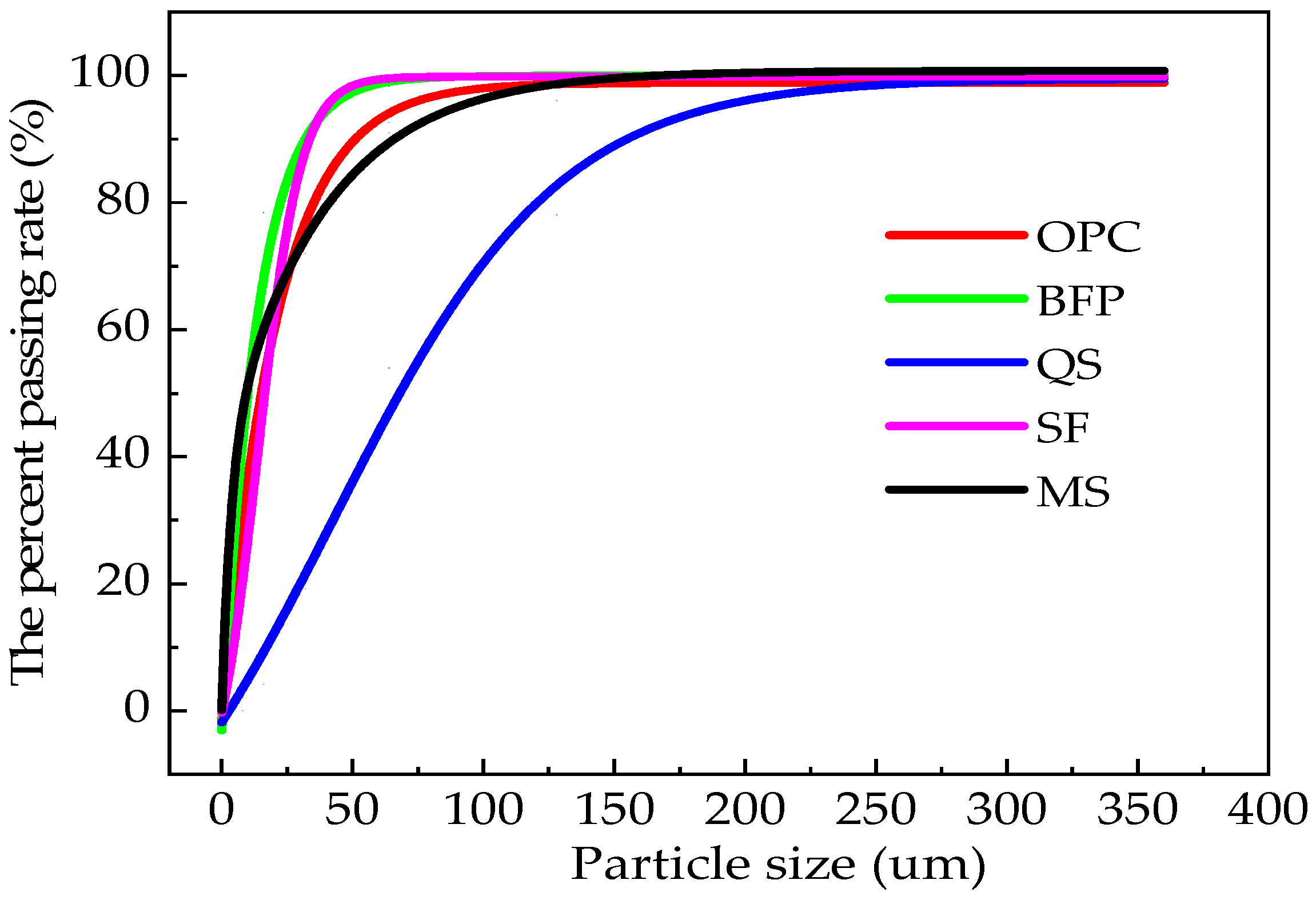



| Types | Particle Size/μm | ||||||
|---|---|---|---|---|---|---|---|
| 0.3 | 0.6 | 1 | 4 | 8 | 64 | 360 | |
| OPC | 0.13 | 0.36 | 3.08 | 14.79 | 29.13 | 92.62 | 100 |
| SF | 32.03 | 59.02 | 82.98 | 99.9 | 99.8 | 100 | 100 |
| QS | 0 | 0 | 0 | 0 | 0.035 | 23.94 | 100 |
| MS | 0.05 | 0.32 | 1.33 | 8.72 | 23.52 | 98.43 | 100 |
| BFP | 0.04 | 0.12 | 3.3 | 19.4 | 35.2 | 98.2 | 100 |
| Types | SiO2 | Al2O3 | FexOy | MgO | CaO | SO3 | K2O | Mn2O | Loss on Ignition |
|---|---|---|---|---|---|---|---|---|---|
| OPC | 20.1 | 5.8 | 3.1 | 1.9 | 62.2 | 3.0 | - | - | 3.0 |
| SF | 90 | 0.4 | 0.5 | 0.6 | 0.35 | 0.3 | 7.4 | - | - |
| QS | 98.3 | - | 1.5 | - | - | - | - | - | - |
| MS | 34.22 | 7.32 | 12.03 | 1.91 | 13.92 | 0.08 | - | 30.52 | - |
| BFP | 34 | 14.9 | 0.5 | 9.8 | 36.9 | 0.3 | 3.6 | - | - |
| Samples | Water | OPC | MS | SF | BFP | QS | BFs | HRWR |
|---|---|---|---|---|---|---|---|---|
| MS-0% | 240 | 658 | 0 | 183 | 111.1 | 962 | 0 | 16 |
| MS-10% | 240 | 567 | 91 | 183 | 111.1 | 962 | 0 | 16 |
| MS-20% | 240 | 477 | 181 | 183 | 111.1 | 962 | 0 | 16 |
| MS-30% | 240 | 383 | 274 | 183 | 111.1 | 962 | 0 | 16 |
| MS-40% | 240 | 293 | 365 | 183 | 111.1 | 962 | 0 | 16 |
| BFs-0% | 240 | 477 | 181 | 183 | 111.1 | 962 | 0 | 16 |
| BFs-0.5% | 240 | 477 | 181 | 183 | 111.1 | 962 | 13 | 16 |
| BFs-1.0% | 240 | 477 | 181 | 183 | 111.1 | 962 | 26 | 16 |
| BFs-1.5% | 240 | 477 | 181 | 183 | 111.1 | 962 | 39 | 16 |
| BFs-2.0% | 240 | 477 | 181 | 183 | 111.1 | 962 | 42 | 16 |
| Equation | Types | a | b | R2 |
|---|---|---|---|---|
| MLR = a + bMS | N-FC-100 | 0.81 | −6.29 × 10−3 | 0.98 |
| N-FC-200 | 1.29 | −1.43 × 10−2 | 0.97 | |
| N-FC-300 | 2.29 | −2.08 × 10−2 | 0.96 | |
| N-DA-10 | 1.01 | −9.34 × 10−3 | 0.92 | |
| N-DA-20 | 1.71 | −2.23 × 10−2 | 0.93 | |
| N-DA-30 | 2.67 | −3.49 × 10−2 | 0.97 | |
| NS-DA-10 | 0.66 | −4.87 × 10−3 | 0.98 | |
| NS-DA-20 | 1.18 | −1.29 × 10−2 | 0.98 | |
| NS-DA-30 | 2.23 | −2.29 × 10−2 | 0.98 |
| Equation | Types | a | b | R2 |
|---|---|---|---|---|
| RDME = a + bMS | RDME(%)-N-FC-0 | 100 | 0 | 1.00 |
| RDME(%)-N-FC-100 | 94.48 | 0.074 | 0.97 | |
| RDME(%)-N-FC-200 | 84.27 | 0.251 | 0.91 | |
| RDME(%)-N-FC-300 | 80.9 | 0.308 | 0.98 | |
| RDME(%)-N-DA-0 | 100 | 0 | 1.00 | |
| RDME(%)-N-DA-10 | 92.92 | 0.103 | 0.90 | |
| RDME(%)-N-DA-20 | 81.89 | 0.382 | 0.92 | |
| RDME(%)-N-DA-30 | 72.4 | 0.52 | 0.97 | |
| RDME(%)-NS-DA-0 | 100 | 0 | 1.00 | |
| RDME(%)-NS-DA-10 | 94.93 | 0.089 | 0.98 | |
| RDME(%)-NS-DA-20 | 88.04 | 0.258 | 0.92 | |
| RDME(%)-NS-DA-30 | 83.73 | 0.27 | 0.91 |
| Equation | Types | a | b | R2 |
|---|---|---|---|---|
| MLR = a + bBF | N-FC-100 | 0.68 | −0.0818 | 0.98 |
| N-FC-200 | 0.96 | −0.165 | 0.99 | |
| N-FC-300 | 1.62 | −0.245 | 0.99 | |
| N-DA-10 | 0.80 | −0.18 | 0.95 | |
| N-DA-20 | 1.19 | −0.32 | 0.99 | |
| N-DA-30 | 1.89 | −0.18 | 0.96 | |
| NS-DA-10 | 0.56 | −0.095 | 0.96 | |
| NS-DA-20 | 0.91 | −0.21 | 0.98 | |
| NS-DA-30 | 1.52 | −0.29 | 0.96 |
| Equation | Types | a | b | R2 |
|---|---|---|---|---|
| RDME = a + bBF | N-FC-0 | 100 | 0 | 1.00 |
| N-FC-100 | 95.98 | 0.85 | 0.96 | |
| N-FC-200 | 90.04 | 2.43 | 0.99 | |
| N-FC-300 | 87.77 | 3.38 | 0.99 | |
| N-DA-0 | 100 | 0 | 1.00 | |
| N-DA-10 | 95.08 | 1.18 | 0.96 | |
| N-DA-20 | 91.97 | 1.93 | 0.98 | |
| N-DA-30 | 84.26 | 2.71 | 0.99 | |
| NS-DA-0 | 100 | 0 | 1.00 | |
| NS-DA-10 | 95.19 | 1.96 | 0.96 | |
| NS-DA-20 | 87.63 | 5.65 | 0.91 | |
| NS-DA-30 | 82.57 | 4.71 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Wang, H.; Peng, L.; Zhao, F. The Properties of High-Performance Concrete with Manganese Slag under Salt Action. Materials 2024, 17, 1483. https://doi.org/10.3390/ma17071483
Yang J, Wang H, Peng L, Zhao F. The Properties of High-Performance Concrete with Manganese Slag under Salt Action. Materials. 2024; 17(7):1483. https://doi.org/10.3390/ma17071483
Chicago/Turabian StyleYang, Junchao, Hui Wang, Ling Peng, and Fei Zhao. 2024. "The Properties of High-Performance Concrete with Manganese Slag under Salt Action" Materials 17, no. 7: 1483. https://doi.org/10.3390/ma17071483




