Effect of Pt on Stress Rupture Properties of Pt-Modified Nickel Aluminide Coatings at 1100 °C
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Different Pt-Content PtAl Coatings
2.2. Stress Rupture Tests and Characterizations
3. Results and Discussion
4. Conclusions
- The increase in Pt content leads to a continuous decrease in the stress rupture life of the PtAl-coated superalloy. Compared to the 0 Pt coating, the stress rupture life decreases by nearly 42% in the 7 Pt coating, while the fracture strain increases from 41.23% to 47.07%.
- Clustered Kirkendall voids and fine columnar crystals at the OZ/IDZ interface of 0 Pt coating can hinder crack propagation.
- The interface of the longitudinal γ′ phase in 3 Pt coating can easily become the path for crack propagation.
- The needle-like TCP phases in the IDZ of 7 Pt coating cause severe stress concentration during creep, promoting the formation of cracks near the substrate and accelerating fracture failure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivakumar, R.; Mordike, B. High temperature coatings for gas turbine blades: A review. Surf. Coat. Technol. 1989, 37, 139–160. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Eggeman, A.S.; Brewster, G.; Xiao, P. Pt effect on early stage oxidation behaviour of Pt-diffused γ-Ni/γ’-Ni3Al coatings. Acta Mater. 2020, 189, 232–247. [Google Scholar] [CrossRef]
- Yang, Y.F.; Jiang, C.Y.; Yao, H.R.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. Cyclic oxidation and rumpling behaviour of single phase β-(Ni,Pt)Al coatings with different thickness of initial Pt plating. Corros. Sci. 2016, 111, 162–174. [Google Scholar] [CrossRef]
- Pauletti, E.; D’Oliveira, A. Influence of Pt concentration on structure of aluminized coatings on a Ni base superalloy. Surf. Coat. Technol. 2017, 332, 57–63. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Y.; Yin, B.; Deng, C.; Liu, M.; Wu, C. Microstructure and Nanoindentation Evolution of (Ni,Pt)Al Coating on IC21 Substrate at 1100 °C. Coatings 2022, 12, 796. [Google Scholar] [CrossRef]
- Hu, T.; Yin, B.; Zhang, X.; Li, W.; Mao, J.; Zhang, X.; Deng, C.; Liu, M. Increasing hot corrosion resistance of β-(Ni, Pt)Al coating by replacing Pt partially with Zr. Corros. Sci. 2023, 220, 111290. [Google Scholar] [CrossRef]
- Zou, H.; Yin, B.; Hu, T.; Deng, P.; Mao, J.; Cai, J.; Hua, Y.; Zhang, X. Impact of Pt/Al ratios on the cyclic oxidation and TCP precipitation of β-(Ni, Pt)Al coated superalloy at 1150 °C. J. Mater. Res. Technol. 2024, 29, 2227–2238. [Google Scholar] [CrossRef]
- Parlikar, C.; Satyanarayana, D.; Chatterjee, D.; Hazari, N.; Das, D.K. Effect of Pt–aluminide bond coat on tensile and creep behavior of a nickel-base single crystal superalloy. Mater. Sci. Eng. A 2015, 639, 575–584. [Google Scholar] [CrossRef]
- Liu, L.; He, J.; Jiang, C.; Fan, D.; Guo, H. Tensile crack inhibition of a PtReAl coated Ni3Al-based single crystal superalloy after long-term oxidation. J. Mater. Res. Technol. 2023, 25, 5110–5121. [Google Scholar] [CrossRef]
- Liu, L.; He, J.; Wu, Y.; Li, J.; Wei, L.; Guo, H. Investigation on the tensile properties of PtAl and PtReAl coated Ni3Al-based single crystal superalloy. Mater. Sci. Eng. A 2023, 867, 144750. [Google Scholar] [CrossRef]
- Alam, Z.; Parlikar, C.; Chatterjee, D.; Das, D.K. Comparative tensile behavior of freestanding γ-γ′ and β-(Ni, Pt)Al bond coats and effect on tensile properties of coated superalloy. Mater. Des. 2017, 114, 505–514. [Google Scholar] [CrossRef]
- Alam, Z.; Satyanarayana, D.; Chatterjee, D.; Sarkar, R.; Das, D. Creep behavior of Pt-aluminide (PtAl) coated directionally solidified Ni-based superalloy CM-247LC after thermal exposure. Mater. Sci. Eng. A 2015, 641, 84–95. [Google Scholar] [CrossRef]
- Tao, X.; Wang, X.; Zhou, Y.; Tan, K.; Liang, J.; Yang, Y.; Liu, J.; Li, J.; Sun, X. Pt–Al bond coat dependence on the creep stress distribution, deformation and fracture behaviour in a second generation Ni-based single crystal superalloy. Mater. Sci. Eng. A 2021, 805, 140575. [Google Scholar] [CrossRef]
- Liu, Y.; Ru, Y.; Zhang, H.; Pei, Y.; Li, S.; Gong, S. Coating-assisted deterioration mechanism of creep resistance at a nickel-based single-crystal superalloy. Surf. Coat. Technol. 2021, 406, 126668. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Wu, M.; Duan, H.; Ru, Y.; Pei, Y.; Li, S.; Gong, S.; Zhang, H. Coating-related deterioration mechanism of creep performance at a thermal exposed single crystal Ni-base superalloy. Mater. Charact. 2022, 187, 111839. [Google Scholar] [CrossRef]
- Tao, X.; Wang, X.; Zhou, Y.; Tan, K.; Meng, J.; Liang, J.; Yang, Y.; Liu, J.; Li, J.; Sun, X. Effect of Pt–Al bond coat on the high-cycle fatigue behaviour of a second-generation single crystal nickel-based superalloy at different temperatures. J. Mater. Res. Technol. 2021, 14, 567–581. [Google Scholar] [CrossRef]
- Tao, X.; Tan, K.; Liang, J.; Wang, X.; Zhou, Y.; Li, J.; Sun, X. Pt-Al bond coat dependence on the high-cycle fatigue rupture and deformation mechanisms of a fourth-generation single crystal superalloy at various temperatures. Mater. Des. 2023, 229, 111880. [Google Scholar] [CrossRef]
- Wang, J.; Guo, T.; Gao, K.; Pang, X. Fatigue behavior of Pt-modified aluminum coated single crystal superalloy at 900 °C. Int. J. Fatigue 2023, 171, 107596. [Google Scholar] [CrossRef]
- Franke, O.; Durst, K.; Göken, M. Microstructure and local mechanical properties of Pt-modified nickel aluminides on nickel-base superalloys after thermo-mechanical fatigue. Mater. Sci. Eng. A 2007, 467, 15–23. [Google Scholar] [CrossRef]
- Taylor, M.; Evans, H.; Busso, E.; Qian, Z. Creep properties of a Pt–aluminide coating. Acta Mater. 2006, 54, 3241–3252. [Google Scholar] [CrossRef]
- Alam, Z.; Kamat, S.; Jayaram, V.; Das, D. Micromechanisms of fracture and strengthening in free-standing Pt-aluminide bond coats under tensile loading. Acta Mater. 2014, 67, 278–296. [Google Scholar] [CrossRef]
- Barjesteh, M.M.; Abbasi, S.M.; Madar, K.Z.; Shirvani, K. Correlation between platinum–aluminide coating features and tensile behavior of nickel-based superalloy Rene®80. Rare Met. 2019, 1–12. [Google Scholar] [CrossRef]
- Srivastava, A.; Gopagoni, S.; Needleman, A.; Seetharaman, V.; Staroselsky, A.; Banerjee, R. Effect of specimen thickness on the creep response of a Ni-based single-crystal superalloy. Acta Mater. 2012, 60, 5697–5711. [Google Scholar] [CrossRef]
- Swadźba, L.; Nawrat, G.; Mendala, B.; Goral, M. The influence of deposition process on structure of platinum-modifed aluminide coatings o Ni-base superalloy. Key Eng. Mater. 2011, 465, 247–250. [Google Scholar] [CrossRef]
- Hayashi, S.; Ford, S.; Young, D.; Sordelet, D.; Besser, M.; Gleeson, B. α-NiPt(Al) and phase equilibria in the Ni–Al–Pt system at 1150 °C. Acta Mater. 2005, 53, 3319–3328. [Google Scholar] [CrossRef]
- Purvis, A.L.; Warnes, B.M. The effects of platinum concentration on the oxidation resistance of superalloys coated with single-phase platinum aluminide. Surf. Coat. Technol. 2001, 146–147, 1–6. [Google Scholar] [CrossRef]
- Jiang, C.; Sordelet, D.; Gleeson, B. Effects of Pt on the elastic properties of B2 NiAl: A combined first-principles and experimental study. Acta Mater. 2006, 54, 2361–2369. [Google Scholar] [CrossRef]
- Mora-García, A.; Mosbacher, M.; Hastreiter, J.; Völkl, R.; Glatzel, U.; Muñoz-Saldaña, J. Creep behavior of polycrystalline and single crystal Ni-based superalloys coated with Ta-containing NiCoCrAlY by high-velocity oxy-fuel spraying. Scr. Mater. 2020, 178, 522–526. [Google Scholar] [CrossRef]
- Bozza, F.; Bolelli, G.; Giolli, C.; Giorgetti, A.; Lusvarghi, L.; Sassatelli, P.; Scrivani, A.; Candeli, A.; Thoma, M. Diffusion mechanisms and microstructure development in pack aluminizing of Ni-based alloys. Surf. Coat. Technol. 2014, 239, 147–159. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, Z.; Yang, Q.; Xie, J. Morphology of the boron-rich phase along columnar grain boundary and its effect on the compression crack of Fe–6.5Si–0.05B alloy. Mater. Sci. Eng. A 2011, 528, 1425–1430. [Google Scholar] [CrossRef]
- Kiruthika, P.; Makineni, S.; Srivastava, C.; Chattopadhyay, K.; Paul, A. Growth mechanism of the interdiffusion zone between platinum modified bond coats and single crystal superalloys. Acta Mater. 2016, 105, 438–448. [Google Scholar] [CrossRef]
- Molins, R.; Hou, P.Y. Characterization of chemical and microstructural evolutions of a NiPtAl bondcoat during high temperature oxidation. Surf. Coat. Technol. 2006, 201, 3841–3845. [Google Scholar] [CrossRef]
- le Graverend, J.-B.; Cormier, J.; Caron, P.; Kruch, S.; Gallerneau, F.; Mendez, J. Numerical simulation of γ/γ′ microstructural evolutions induced by TCP-phase in the MC2 nickel base single crystal superalloy. Mater. Sci. Eng. A 2011, 528, 2620–2634. [Google Scholar] [CrossRef]
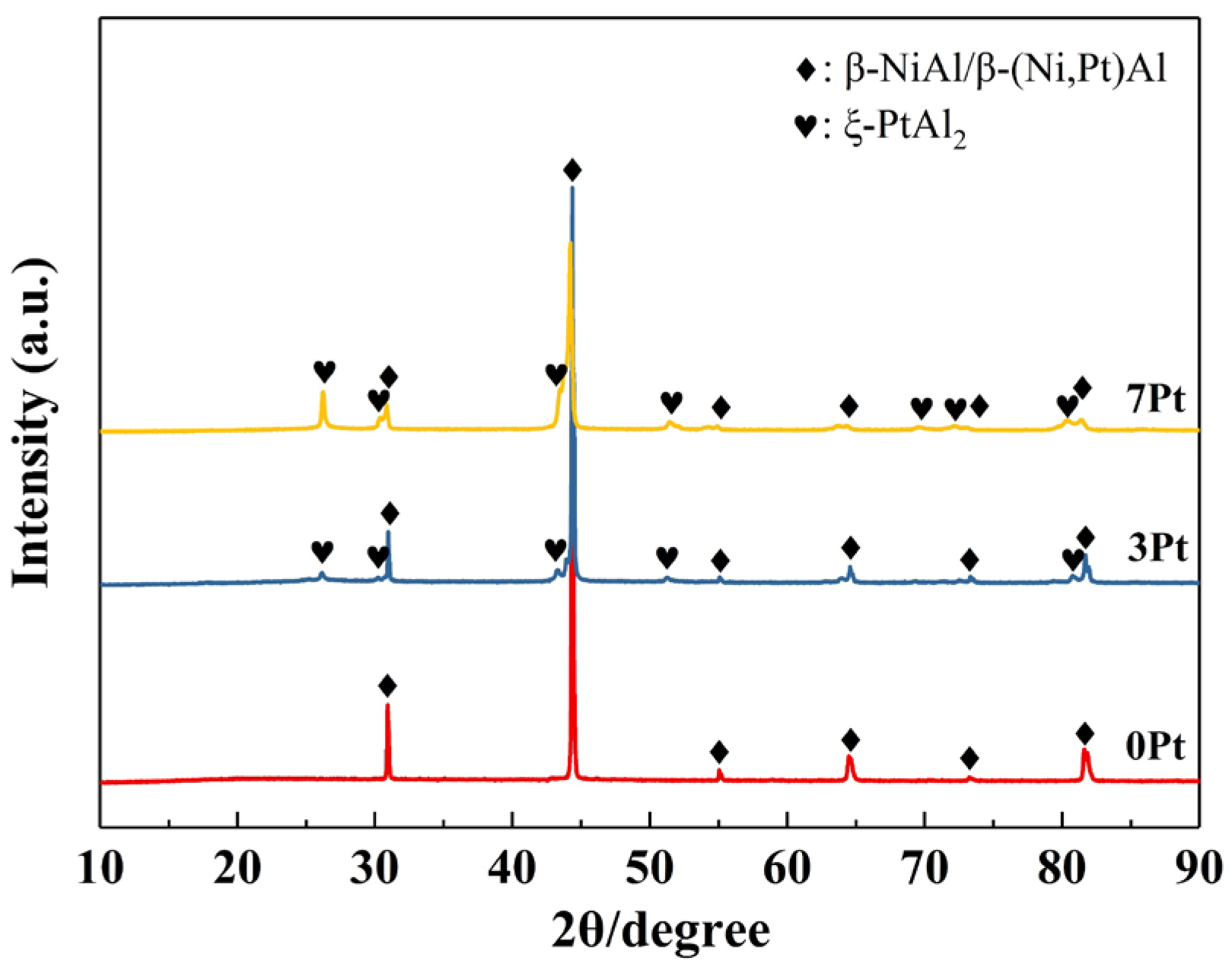


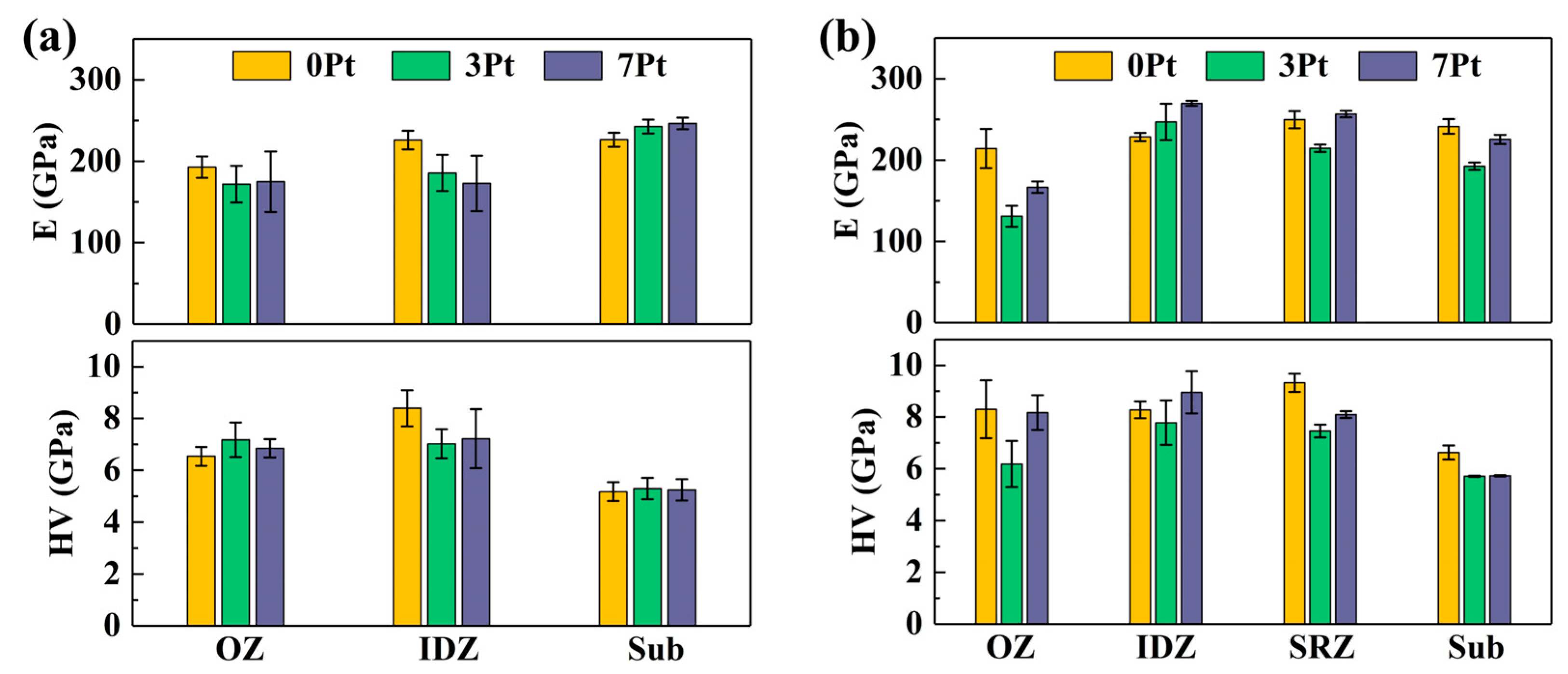


| Specimen | Area | Al | Mo | Cr | Co | Ni | Ta | W | Re | Pt |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 Pt | OZ | 36.11 | 0 | 0.96 | 4.05 | 58.87 | 0 | 0 | 0 | 0 |
| IDZ | 25.87 | 0.75 | 5.58 | 7.24 | 53.13 | 2.36 | 3.21 | 1.85 | 0 | |
| 3 Pt | OZ | 38.62 | 0 | 1.87 | 4.02 | 49.29 | 0 | 0 | 0 | 6.20 |
| IDZ | 25.48 | 0.27 | 5.49 | 6.46 | 50.76 | 2.92 | 2.25 | 1.28 | 5.10 | |
| 7 Pt | OZ | 39.27 | 0 | 2.20 | 3.74 | 44.71 | 0 | 0 | 0 | 10.08 |
| IDZ | 21.45 | 0.16 | 4.90 | 6.40 | 49.90 | 3.70 | 1.94 | 1.39 | 10.16 |
| Area | Al | Mo | Cr | Co | Ni | Ta | W | Pt |
|---|---|---|---|---|---|---|---|---|
| 1 | 24.25 | 0 | 2.28 | 5.46 | 66.80 | 1.22 | 0 | 0 |
| 2 | 23.48 | 0 | 1.82 | 4.14 | 65.63 | 0 | 1.86 | 4.34 |
| 3 | 24.71 | 0 | 1.15 | 3.37 | 62.53 | 0.13 | 0.14 | 7.91 |
| 4 | 22.42 | 0.15 | 1.67 | 4.73 | 60.42 | 2.95 | 0.83 | 6.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Yin, B.; Deng, P.; Deng, C.; Mao, J.; Qiu, Z.; Zeng, D.; Liu, M. Effect of Pt on Stress Rupture Properties of Pt-Modified Nickel Aluminide Coatings at 1100 °C. Materials 2024, 17, 1520. https://doi.org/10.3390/ma17071520
Xue Y, Yin B, Deng P, Deng C, Mao J, Qiu Z, Zeng D, Liu M. Effect of Pt on Stress Rupture Properties of Pt-Modified Nickel Aluminide Coatings at 1100 °C. Materials. 2024; 17(7):1520. https://doi.org/10.3390/ma17071520
Chicago/Turabian StyleXue, Youying, Bin Yin, Peng Deng, Chunming Deng, Jie Mao, Zhaoguo Qiu, Dechang Zeng, and Min Liu. 2024. "Effect of Pt on Stress Rupture Properties of Pt-Modified Nickel Aluminide Coatings at 1100 °C" Materials 17, no. 7: 1520. https://doi.org/10.3390/ma17071520
APA StyleXue, Y., Yin, B., Deng, P., Deng, C., Mao, J., Qiu, Z., Zeng, D., & Liu, M. (2024). Effect of Pt on Stress Rupture Properties of Pt-Modified Nickel Aluminide Coatings at 1100 °C. Materials, 17(7), 1520. https://doi.org/10.3390/ma17071520







