The Influence of Solar Ageing on the Compositions of Epoxy Resin with Natural Polyphenol Quercetin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation Method of Epoxy Resin Composites with Polyphenol (Quercetin)
2.2. Controlled Ageing in a Solar Chamber
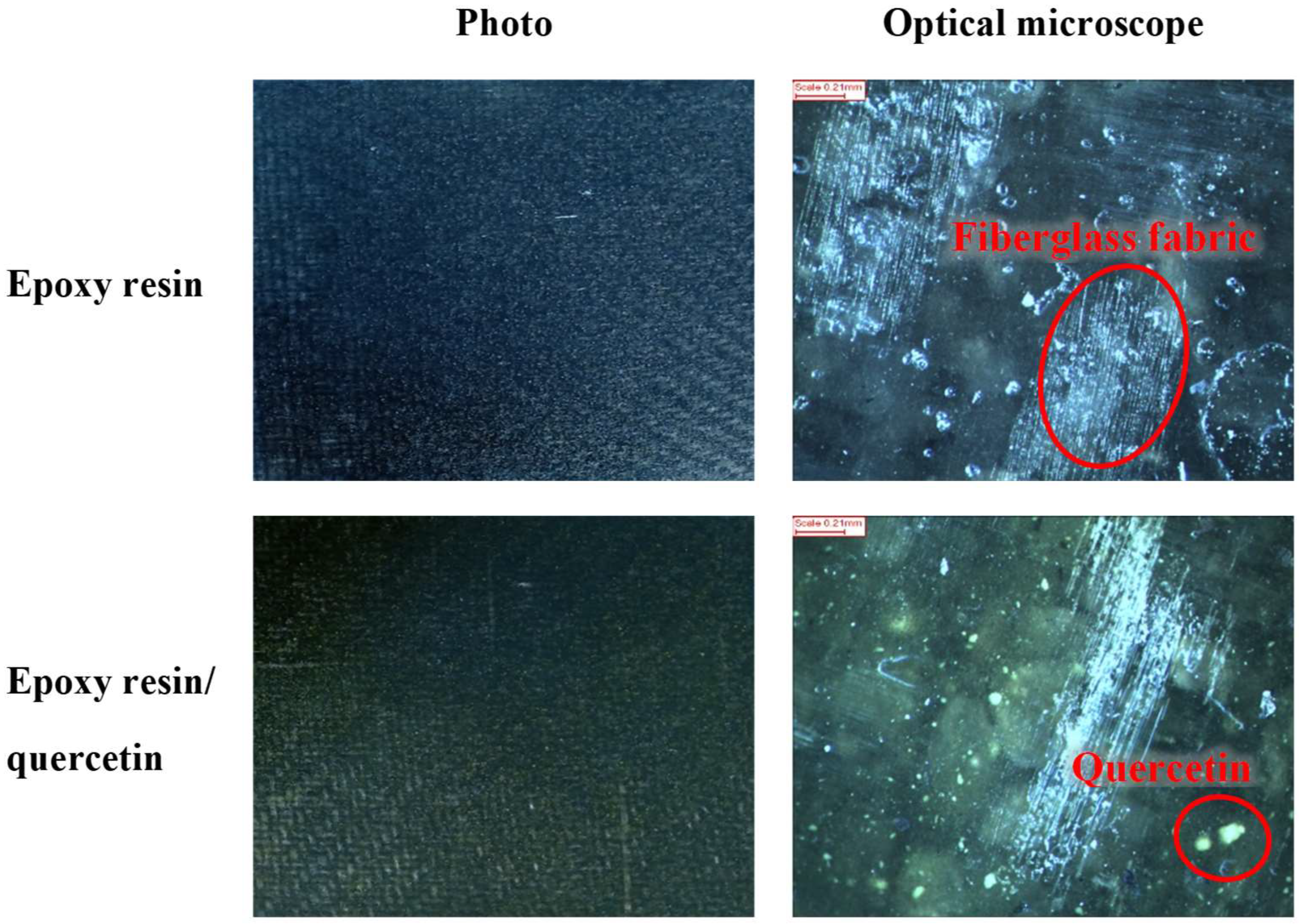
2.3. Optical Microscopy
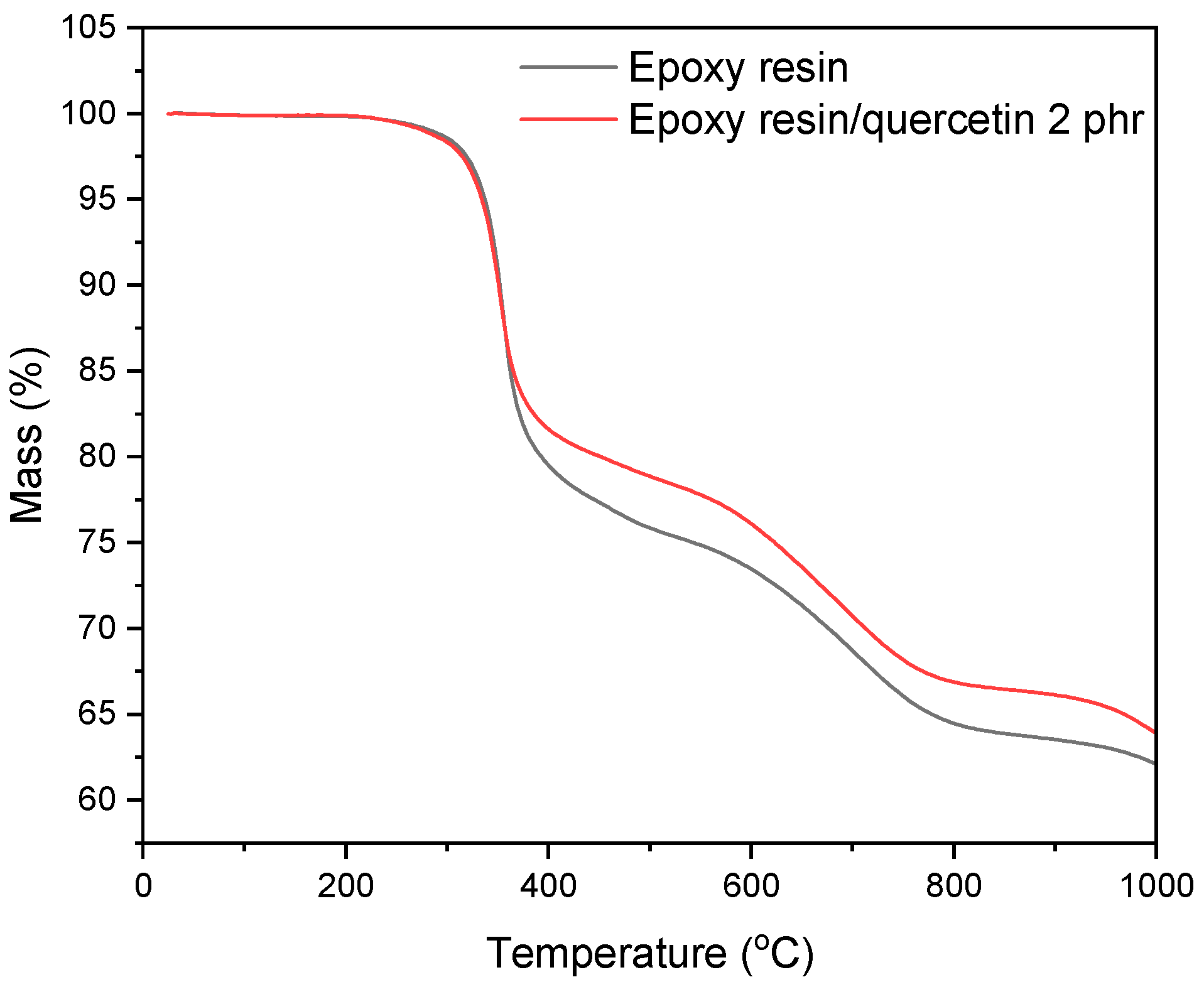
2.4. Thermogravimetric Analysis (TGA) of Epoxy Resin Compositions
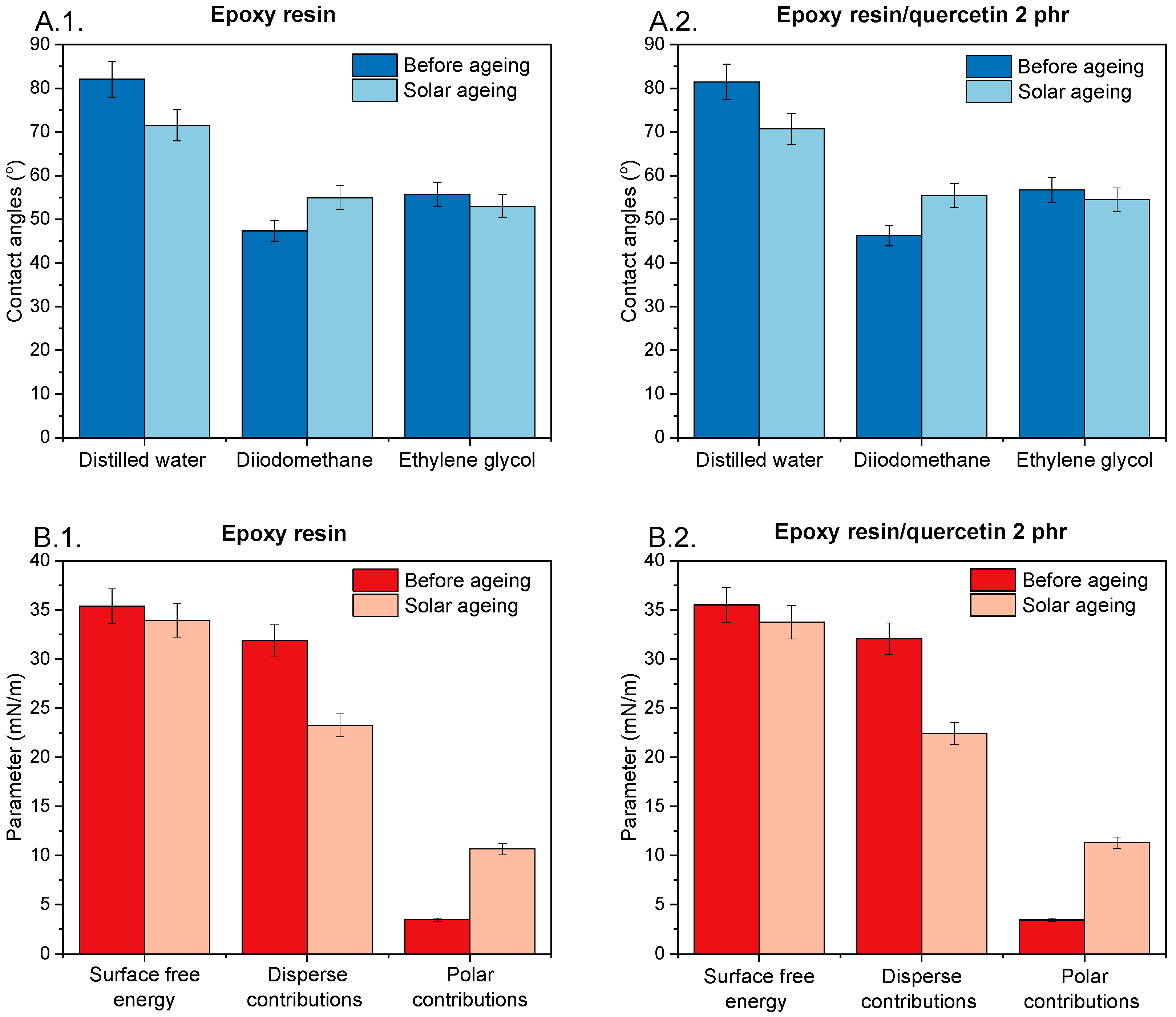
2.5. Investigation of Surface Free Energy of Epoxy Compositions
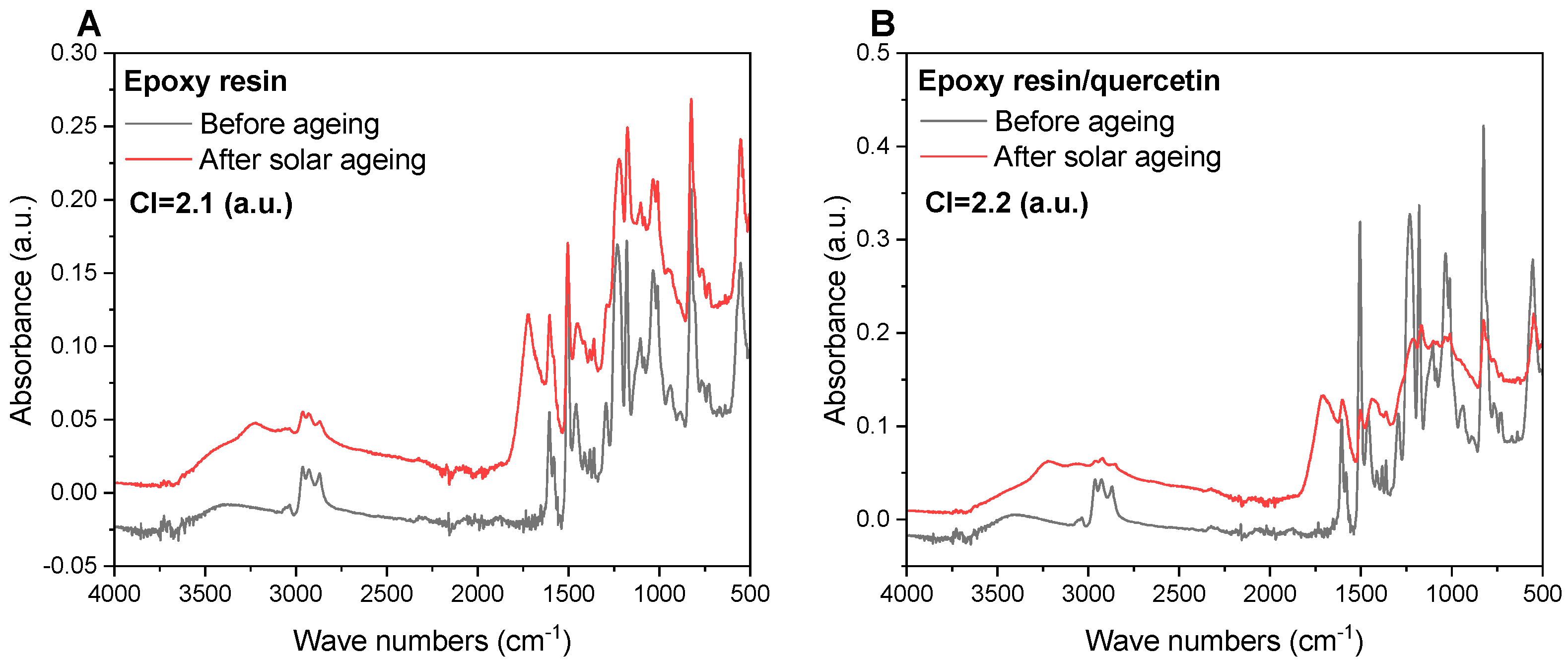
2.6. Determination of Carbonyl Indices (CI) Based on Fourier-Transform Infrared Spectroscopy (FTIR)
- IC=O represents peak intensity characteristic of C=O carbonyl groups (~1700 cm−1) [-], and
- IC-H represents peak intensity characteristic of C-H aliphatic carbon (~2800 cm−1) [-].
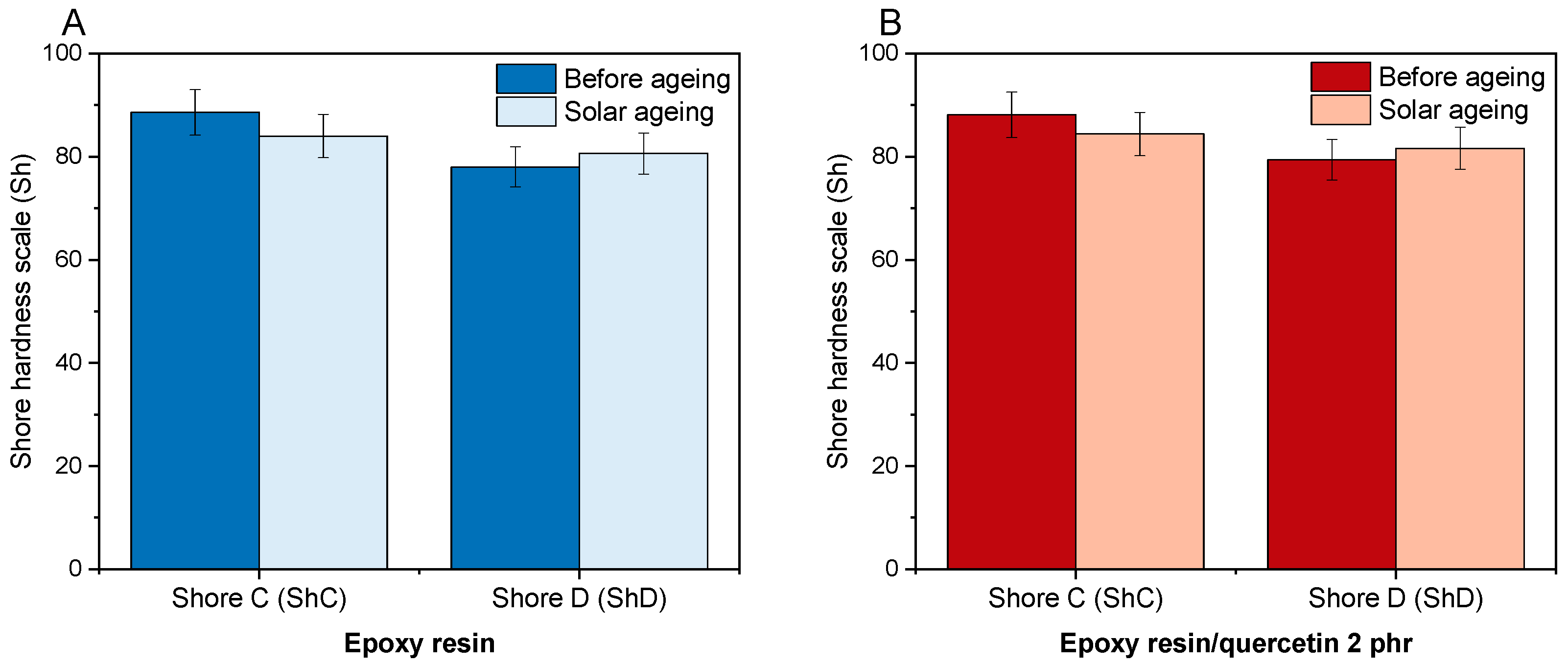
2.7. Hardnesses of Epoxy Compositions on the Shore C and Shore D Scale
2.8. Determination of the Change in Colour Parameters of Epoxy Composites after Solar Ageing
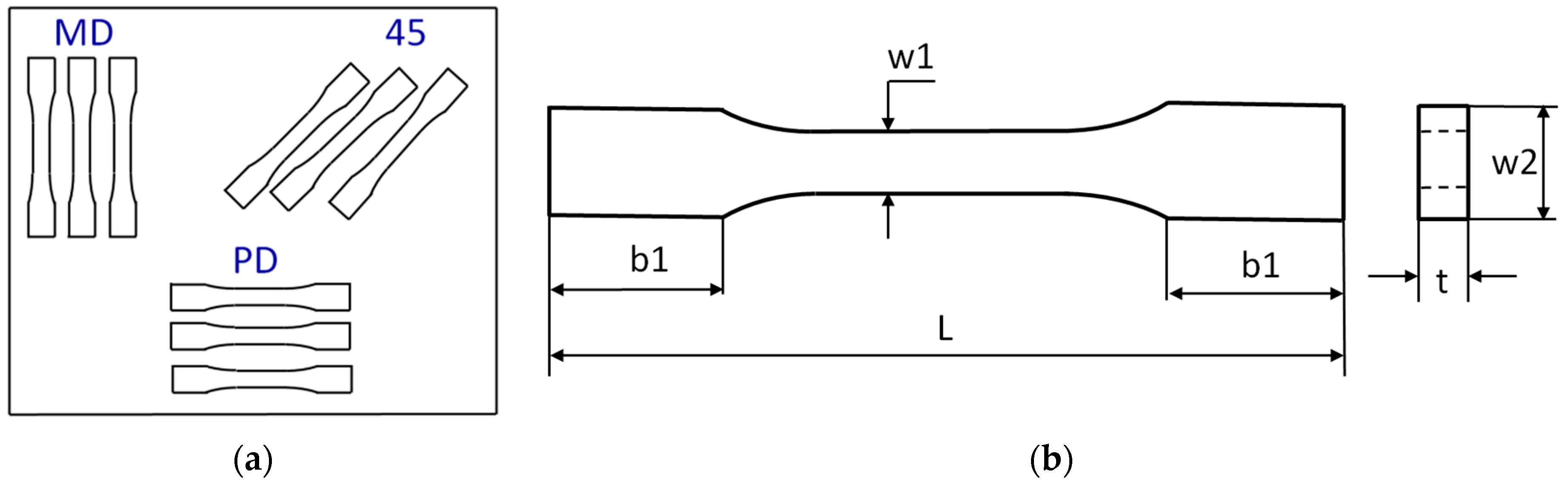
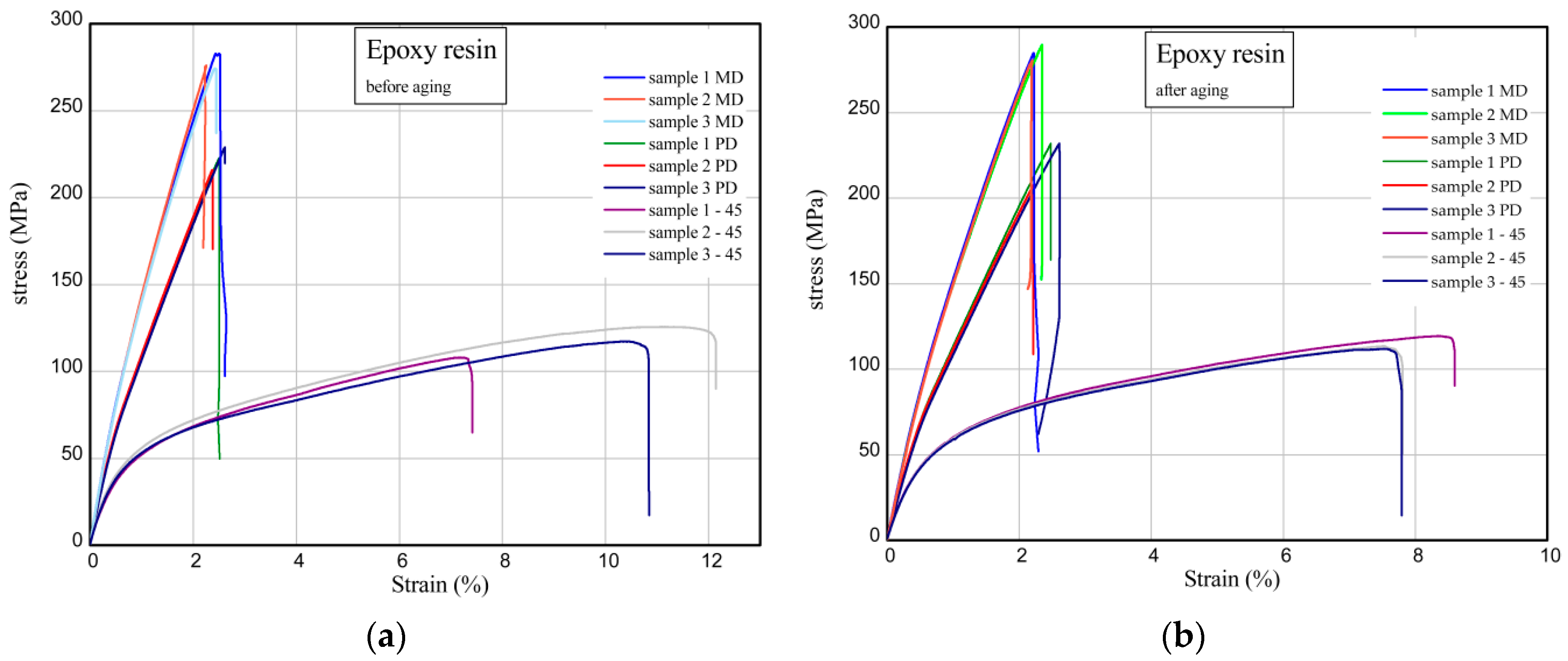
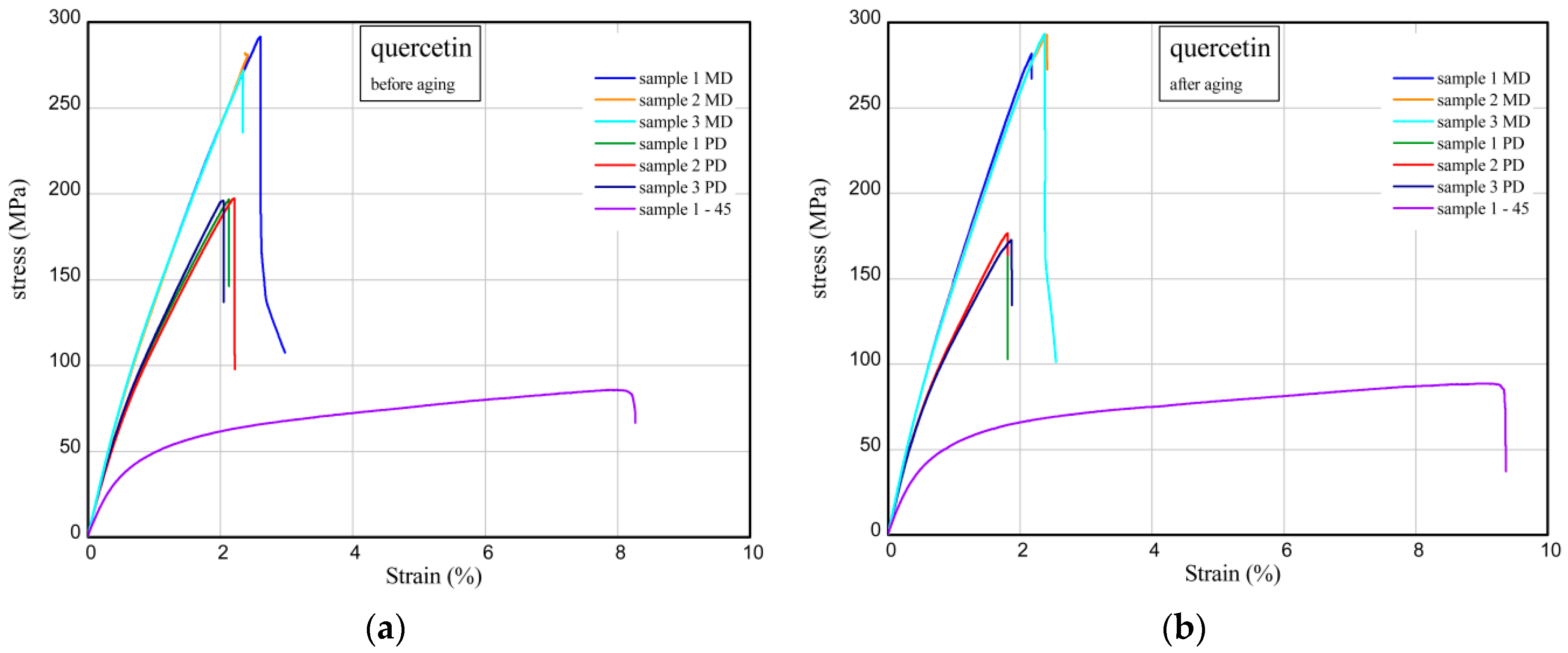
2.9. Uniaxial Tensile Test of Aged and Unaged Epoxy Compositions
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shundo, A.; Yamamoto, S.; Tanaka, K. Network Formation and Physical Properties of Epoxy Resins for Future Practical Applications. JACS Au 2022, 2, 1522–1542. [Google Scholar] [CrossRef] [PubMed]
- Din, I.U.; Ahmed, A.; Tarek, F.; Cantwell, W.; Khan, K.A. Ultra-Thin Composites Membrane for Deployable Structures: XCT Driven Characterization and FE Modeling of Folding Structure. Compos. Sci. Technol. 2024, 245, 110341. [Google Scholar] [CrossRef]
- Ahmed, A.; Ud Din, I.; Ramachandran, R.; Sikandar Bathusha, M.S.; Cantwell, W.; Khan, K.A. Micro-CT Driven Characterization and Validation of Constituents’ Properties Used in Origami-Inspired Foldable Composites Fabricated via Different Manufacturing Processes. Compos. Struct. 2023, 322, 117382. [Google Scholar] [CrossRef]
- Busiak, R.; Masek, A.; Węgier, A.; Rylski, A. Accelerated Aging of Epoxy Biocomposites Filled with Cellulose. Materials 2022, 15, 3256. [Google Scholar] [CrossRef] [PubMed]
- Capricho, J.C.; Fox, B.; Hameed, N. Multifunctionality in Epoxy Resins. Polym. Rev. 2020, 60, 1–41. [Google Scholar] [CrossRef]
- EN 45545-2 R6; Passenger Seat Shell. EN 45545-2: Fire Protection of Railway Vehicles—Part 2: Requirement for Fire Behaviors of Materials and Components (R6). Ecosafene Fire Research Center: Xiamen, China, 2015.
- Du, Y.; Zhao, G.; Shi, G.; Wang, Y.; Li, W.; Ren, S. Effect of Crosslink Structure on Mechanical Properties, Thermal Stability and Flame Retardancy of Natural Flavonoid Based Epoxy Resins. Eur. Polym. J. 2022, 162, 110898. [Google Scholar] [CrossRef]
- Matykiewicz, D. Hybrid Epoxy Composites with Both Powder and Fiber Filler: A Review of Mechanical and Thermomechanical Properties. Materials 2020, 13, 1802. [Google Scholar] [CrossRef] [PubMed]
- Brydson, J.A. Epoxide Resins. In Plastic Materials; Butterworth-Heinemann: Oxford, UK, 1999; pp. 744–777. [Google Scholar]
- Kockott, D. New Method for Accelerated Testing of the Aging Behavior of Polymeric Materials as a Function of Radiation and Temperature. Polym. Test. 2022, 110, 107550. [Google Scholar] [CrossRef]
- Wu, C.; Meng, B.C.; Tam, L.; He, L. Yellowing Mechanisms of Epoxy and Vinyl Ester Resins under Thermal, UV and Natural Aging Conditions and Protection Methods. Polym. Test. 2022, 114, 107708. [Google Scholar] [CrossRef]
- Nikafshar, S.; McCracken, J.; Dunne, K.; Nejad, M. Improving UV-Stability of Epoxy Coating Using Encapsulated Halloysite Nanotubes with Organic UV-Stabilizers and Lignin. Prog. Org. Coat. 2021, 151, 105843. [Google Scholar] [CrossRef]
- Chen, G.; Feng, J.; Qiu, W.; Zhao, Y. Eugenol-Modified Polysiloxanes as Effective Anticorrosion Additives for Epoxy Resin Coatings. RSC Adv. 2017, 7, 55967–55976. [Google Scholar] [CrossRef]
- Kirschweng, B.; Tátraaljai, D.; Földes, E.; Pukánszky, B. Natural Antioxidants as Stabilizers for Polymers. Polym. Degrad. Stab. 2017, 145, 25–40. [Google Scholar] [CrossRef]
- Galano, A.; Álvarez-Diduk, R.; Ramírez-Silva, M.T.; Alarcón-Ángeles, G.; Rojas-Hernández, A. Role of the Reacting Free Radicals on the Antioxidant Mechanism of Curcumin. Chem. Phys. 2009, 363, 13–23. [Google Scholar] [CrossRef]
- Galleano, M.; Verstraeten, S.V.; Oteiza, P.I.; Fraga, C.G. Antioxidant Actions of Flavonoids: Thermodynamic and Kinetic Analysis. Arch. Biochem. Biophys. 2010, 501, 23–30. [Google Scholar] [CrossRef]
- Erlund, I. Review of the Flavonoids Quercetin, Hesperetin, and Naringenin. Dietary Sources, Bioactivities, Bioavailability, and Epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Chen, X.; Zou, L.; Liu, W.; McClements, D.J. Potential of Excipient Emulsions for Improving Quercetin Bioaccessibility and Antioxidant Activity: An in Vitro Study. J. Agric. Food Chem. 2016, 64, 3653–3660. [Google Scholar] [CrossRef]
- Zou, Y.; Qian, Y.; Rong, X.; Cao, K.; McClements, D.J.; Hu, K. Encapsulation of Quercetin in Biopolymer-Coated Zein Nanoparticles: Formation, Stability, Antioxidant Capacity, and Bioaccessibility. Food Hydrocoll. 2021, 120, 106980. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An Insight into Anticancer, Antioxidant, Antimicrobial, Antidiabetic and Anti-Inflammatory Effects of Quercetin: A Review. Polym. Bull. 2022, 80, 241–262. [Google Scholar] [CrossRef]
- Shabir, I.; Kumar Pandey, V.; Shams, R.; Dar, A.H.; Dash, K.K.; Khan, S.A.; Bashir, I.; Jeevarathinam, G.; Rusu, A.V.; Esatbeyoglu, T.; et al. Promising Bioactive Properties of Quercetin for Potential Food Applications and Health Benefits: A Review. Front. Nutr. 2022, 9, 999752. [Google Scholar] [CrossRef]
- Tátraaljai, D.; Földes, E.; Pukánszky, B. Efficient Melt Stabilization of Polyethylene with Quercetin, a Flavonoid Type Natural Antioxidant. Polym. Degrad. Stab. 2014, 102, 41–48. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Richert, A.; Grabska-Zielińska, S.; Rudawska, A.; Bouaziz, M. Antibacterial Films Based on Polylactide with the Addition of Quercetin and Poly(Ethylene Glycol). Materials 2021, 14, 1643. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, G.; Li, J.; Zhang, C.; Ma, Z.; Zhao, M.; Yang, Y.; Han, Y.; Huang, Z.; Weng, Y. Effects of Quercetin and Organically Modified Montmorillonite on the Properties of Poly(Butylene Adipate-Co-Terephthalate)/Thermoplastic Starch Active Packaging Films. ACS Omega 2023, 8, 663–672. [Google Scholar] [CrossRef] [PubMed]
- de Barros Vinhal, G.L.R.R.; Silva-Pereira, M.C.; Teixeira, J.A.; Barcia, M.T.; Pertuzatti, P.B.; Stefani, R. Gelatine/PVA Copolymer Film Incorporated with Quercetin as a Prototype to Active Antioxidant Packaging. J. Food Sci. Technol. 2021, 58, 3924–3932. [Google Scholar] [CrossRef] [PubMed]
- Morici, E.; Arrigo, R.; Dintcheva, N.T. Quercetin as Natural Stabilizing Agent for Bio-Polymer. AIP Conf. Proc. 2014, 1599, 314–317. [Google Scholar]
- Luzi, F.; Pannucci, E.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R.; Puglia, D. Gallic Acid and Quercetin as Intelligent and Active Ingredients in Poly(Vinyl Alcohol) Films for Food Packaging. Polymers 2019, 11, 1999. [Google Scholar] [CrossRef]
- Cichosz, S.; Pęśko, M.; Masek, A. Empirically Distinguished Anti-Aging Behaviour of Quercetin Physically and Chemically Supported on Cellulose Carrier: In-Situ Examination for Ethylene-Norbornene Copolymer. Sustain. Mater. Technol. 2023, 36, e00590. [Google Scholar] [CrossRef]
- Masek, A.; Latos, M.; Piotrowska, M.; Zaborski, M. The Potential of Quercetin as an Effective Natural Antioxidant and Indicator for Packaging Materials. Food Packag. Shelf Life 2018, 16, 51–58. [Google Scholar] [CrossRef]
- Masek, A.; Olejnik, O.; Czechowski, L.; Kaźmierczyk, F.; Miszczak, S.; Węgier, A.; Krauze, S. Epoxy Resin-Based Materials Containing Natural Additives of Plant Origin Dedicated to Rail Transport. Materials 2023, 16, 7080. [Google Scholar] [CrossRef]
- Delor-Jestin, F.; Drouin, D.; Cheval, P.-Y.; Lacoste, J. Thermal and Photochemical Ageing of Epoxy Resin—Influence of Curing Agents. Polym. Degrad. Stab. 2006, 91, 1247–1255. [Google Scholar] [CrossRef]
- UNE-EN ISO 527-1:2020; Plastics—Determination of Mechanical Properties under Static Stretching—Part 1: General Principles. UNE Asociación Española de Normalización: Madrid, Spain, 2020.
- Samper, M.D.; Fages, E.; Fenollar, O.; Boronat, T.; Balart, R. The Potential of Flavonoids as Natural Antioxidants and UV Light Stabilizers for Polypropylene. J. Appl. Polym. Sci. 2013, 129, 1707–1716. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A.; Czechowski, L.; Jastrzębska, A.; Miszczak, S. Effect of the Addition of Naringenin Derived from Citrus on the Properties of Epoxy Resin Compositions. Molecules 2024, 29, 512. [Google Scholar] [CrossRef] [PubMed]
| Parameter | dE × ab (-) | L* (-) | a* (-) | b* (-) |
|---|---|---|---|---|
| Epoxy resin | ||||
| Before ageing | - | 35.05 ± 1.75 | −0.40 ± 0.02 | 3.02 ± 0.15 |
| Solar ageing | 2.25 ± 0.11 | 13.25 ± 0.65 | 1.56 ± 0.07 | 2.37 ± 0.12 |
| Epoxy resin/quercetin 2 phr | ||||
| Before ageing | - | 35.12 ± 1.77 | −4.23 ± 0.21 | 8.40 ± 0.42 |
| Solar ageing | 4.96 ± 0.25 | 33.31 ± 1.67 | −0.33 ± 0.02 | 5.97 ± 0.30 |
| Sample | Unaged | Solar Aged | ||||
|---|---|---|---|---|---|---|
| MD | PD | 45 | MD | PD | 45 | |
| Epoxy resin | 17.30 ± 0.20 | 14.35 ± 0.40 | 9.36 ± 0.65 | 18.41 ± 0.24 | 14.76 ± 0.45 | 10.32 ± 0.18 |
| Epoxy resin/quercetin 2 phr | 16.28 ± 0.17 | 14.83 ± 0.47 | 8.54 | 17.86 ± 0.19 | 15.86 ± 0.19 | 9.41 |
| Sample | Unaged | Solar Aged | ||||
|---|---|---|---|---|---|---|
| MD | PD | 45 | MD | PD | 45 | |
| Epoxy resin | 277.8 ± 4.6 | 223.8 ± 6.7 | 116.7 ± 8.9 | 284.7 ± 4.7 | 223.6 ± 14.2 | 114.8 ± 4.0 |
| Epoxy resin/quercetin 2 phr | 281.9 ± 10.0 | 196.8 ± 0.6 | 85.7 | 289.3 ± 6.6 | 175.2 ± 2.2 | 88.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latos-Brozio, M.; Czechowski, L.; Masek, A. The Influence of Solar Ageing on the Compositions of Epoxy Resin with Natural Polyphenol Quercetin. Materials 2024, 17, 1592. https://doi.org/10.3390/ma17071592
Latos-Brozio M, Czechowski L, Masek A. The Influence of Solar Ageing on the Compositions of Epoxy Resin with Natural Polyphenol Quercetin. Materials. 2024; 17(7):1592. https://doi.org/10.3390/ma17071592
Chicago/Turabian StyleLatos-Brozio, Malgorzata, Leszek Czechowski, and Anna Masek. 2024. "The Influence of Solar Ageing on the Compositions of Epoxy Resin with Natural Polyphenol Quercetin" Materials 17, no. 7: 1592. https://doi.org/10.3390/ma17071592





