Microstructure and Corrosion of Mg-Based Composites Produced from Custom-Made Powders of AZ31 and Ti6Al4V via Pulse Plasma Sintering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
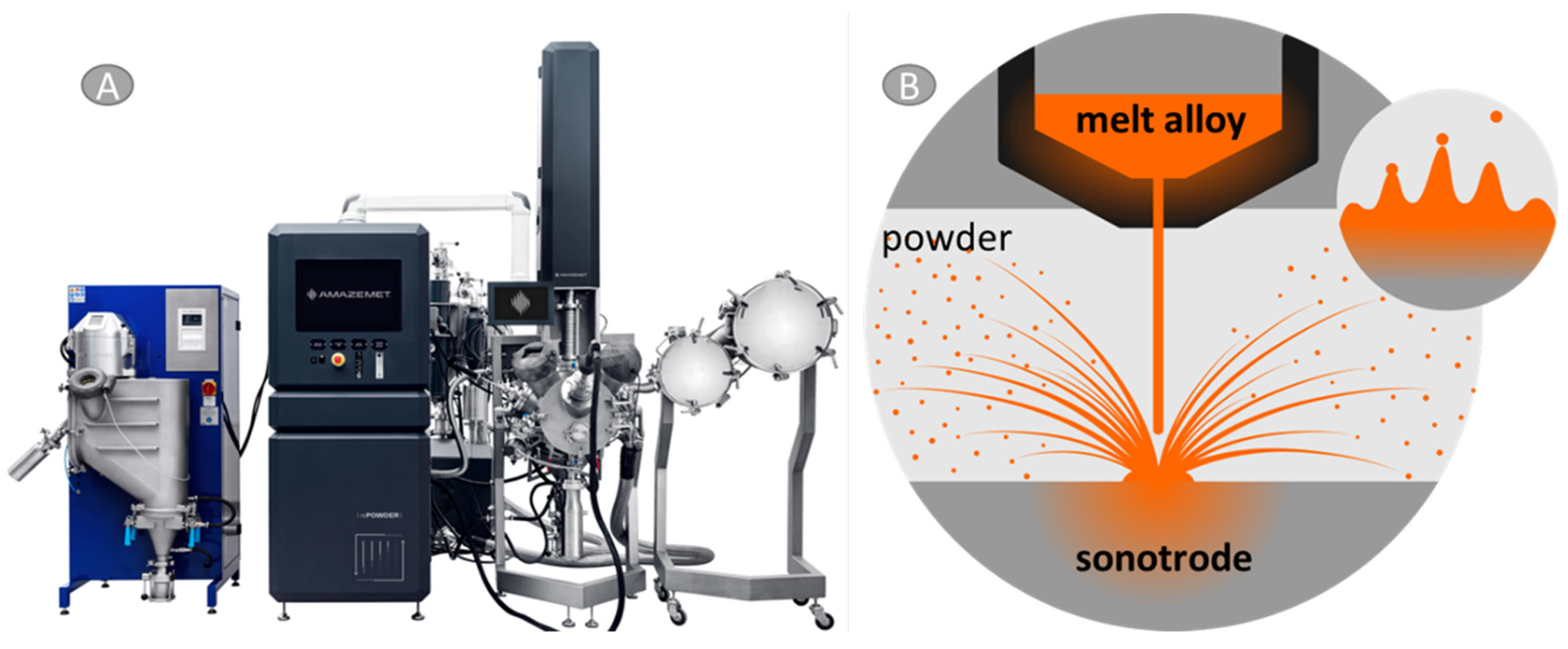
2.2. Methods
3. Results
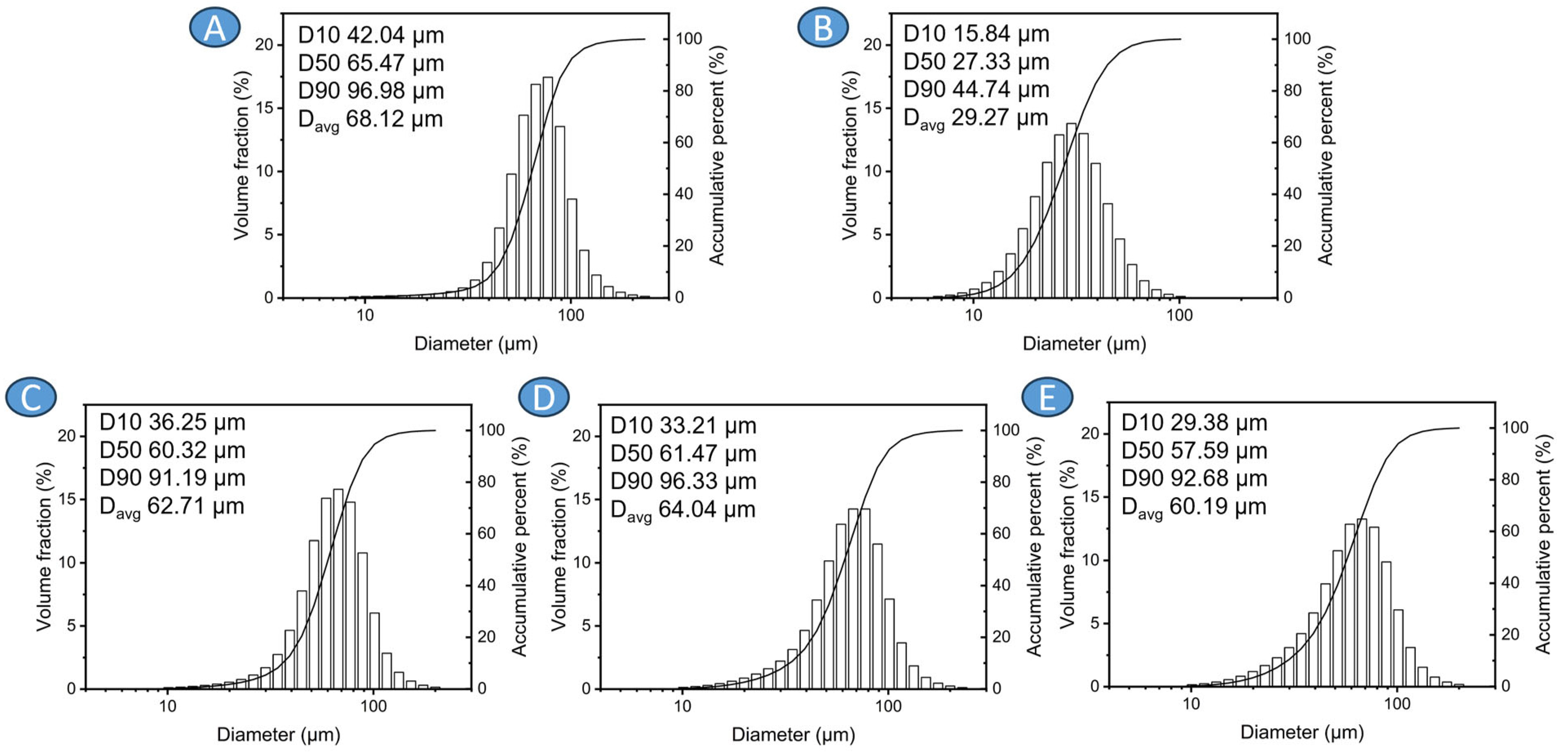
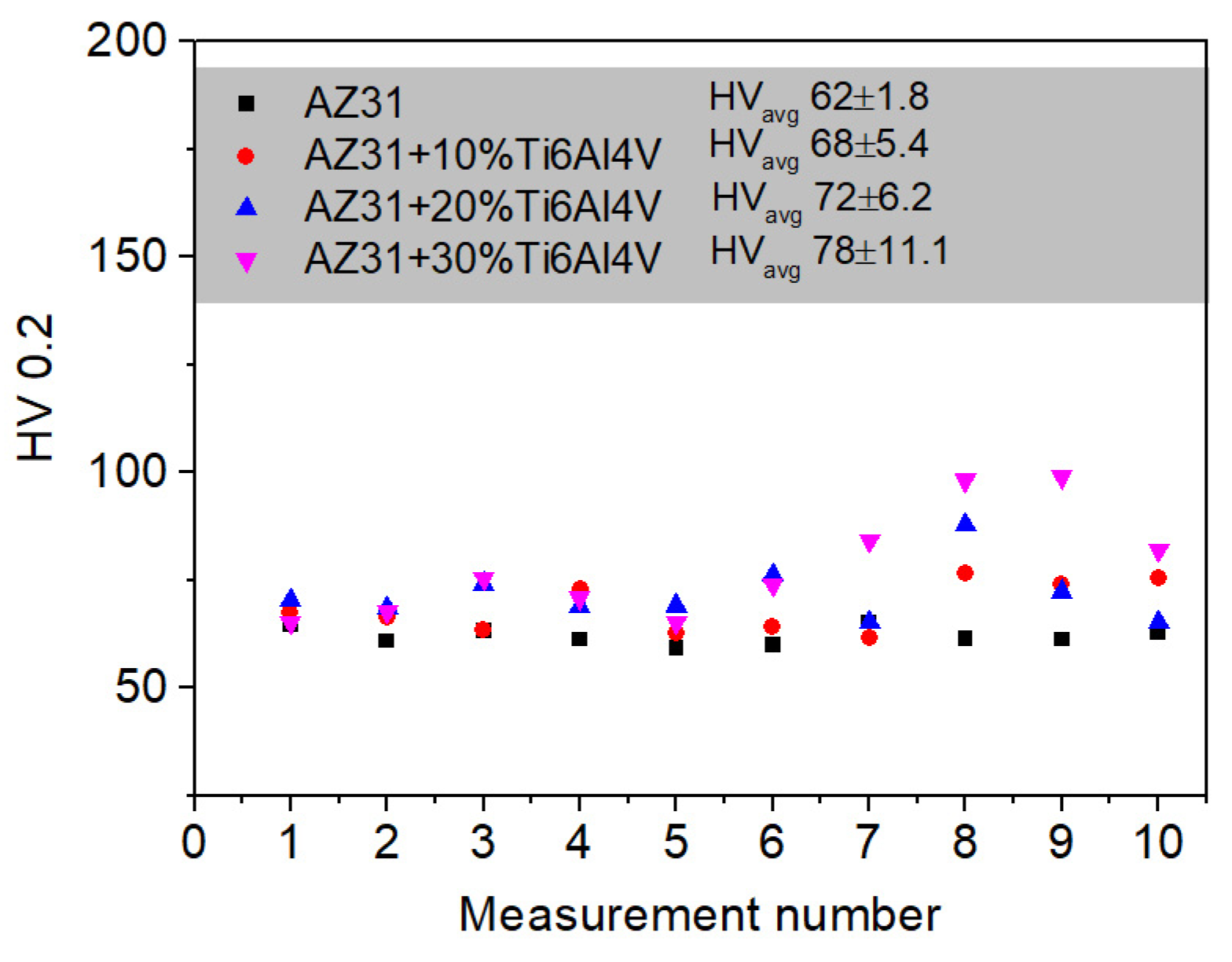
3.1. Precursor Characterization
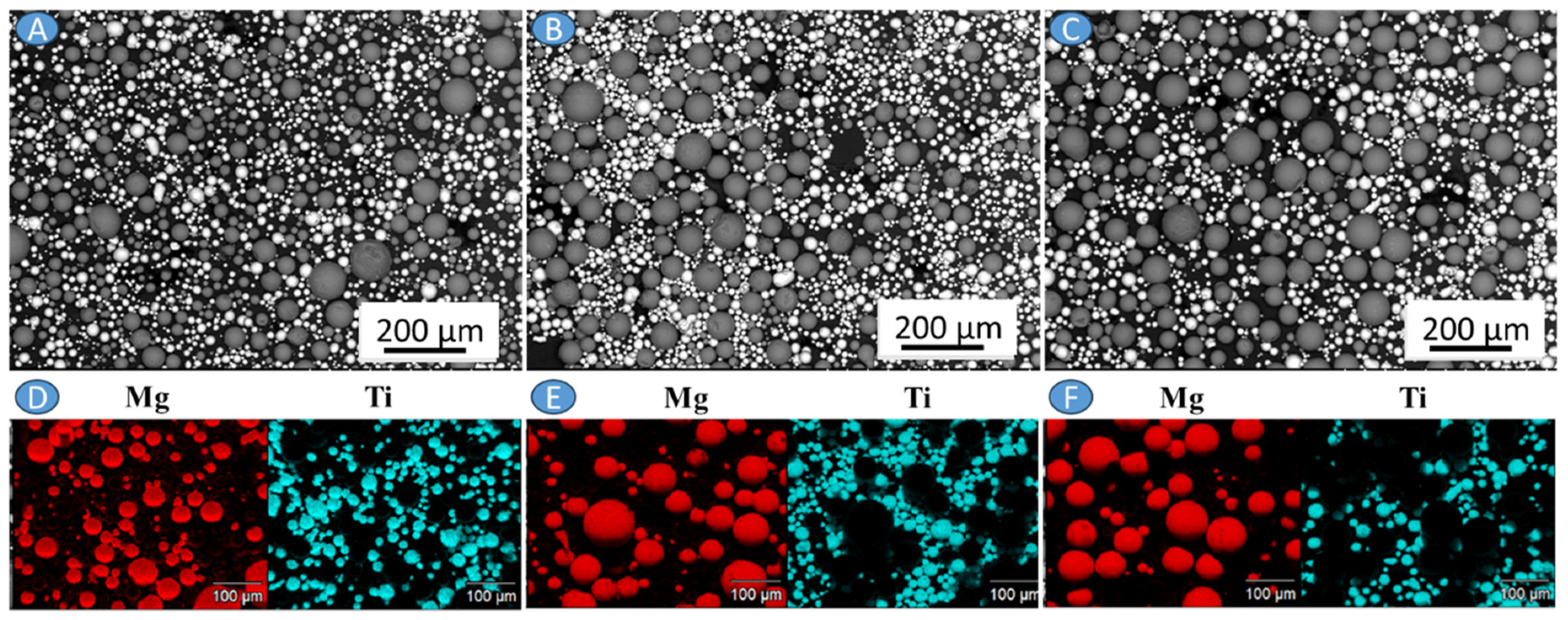
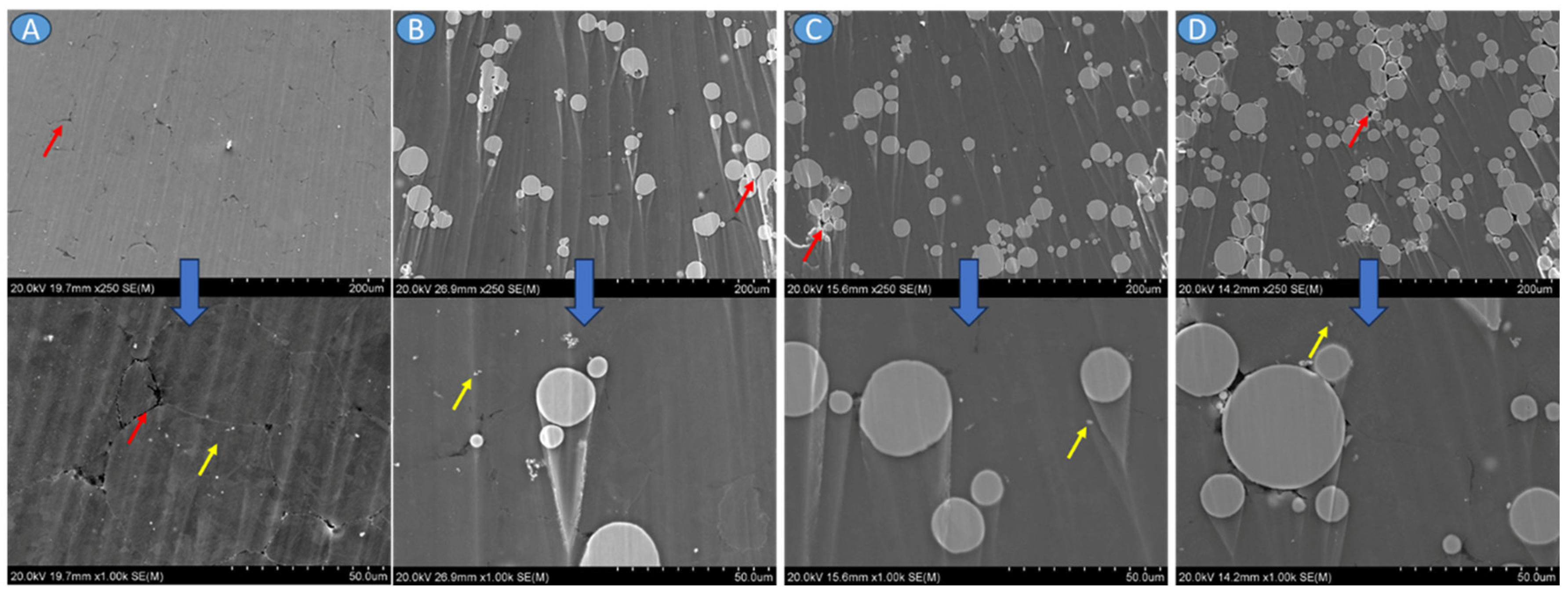
3.2. Materials Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbas, A.T.; Pimenov, D.Y.; Erdakov, I.N.; Taha, M.A.; Soliman, M.S.; El Rayes, M.M. ANN surface roughness optimization of AZ61 magnesium alloy finish turning: Minimum machining times at prime machining costs. Materials 2018, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Liu, X.; Tang, C.P.; Yao, W.; Xiao, Y.; Liu, X.H. Microstructure and texture evolution in LZ91 magnesium alloy during cold rolling. J. Magnes. Alloys 2018, 6, 77–82. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, X.; Ding, W. Effect of Al-4Ti-5B master alloy on the grain refinement of AZ31 magnesium alloy. Scr. Mater. 2006, 54, 269–273. [Google Scholar] [CrossRef]
- Kelen, F.; Gavgali, M.; Aydogmus, T. Microstructure and mechanical properties of a novel TiNi particulate reinforced AZ91 metal matrix composite. Mater. Lett. 2018, 233, 12–15. [Google Scholar] [CrossRef]
- Mordike, B.L.; Ebert, T. Properties—Applications—Potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Chua, B.W.; Lu, L.; Lai, M.O. Influence of SiC particles on mechanical properties of Mg based composite. Compos. Struct. 1999, 47, 595–601. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, L.; Sun, Y.; Zhang, H.; Duan, C.; Yu, H. Synthesis of nanocrystalline AZ31 magnesium alloy with titanium addition by mechanical milling. Mater. Charact. 2016, 113, 108–116. [Google Scholar] [CrossRef]
- Bochenek, A.; Braszczyêska, K.N. Structural analysis of the MgAl5 matrix cast composites containing SiC particles. Mater. Sci. Eng. A 2000, 290, 122–127. [Google Scholar] [CrossRef]
- Dinaharan, I.; Zhang, S.; Chen, G.; Shi, Q. Titanium particulate reinforced AZ31 magnesium matrix composites with improved ductility prepared using friction stir processing, Mater. Sci. Eng. A 2020, 772, 138793. [Google Scholar] [CrossRef]
- Lim, S.C.V.; Gupta, M.; Lu, L. Processing, microstructure, and properties of Mg–SiC composites synthesised using fluxless casting process. Mater. Sci. Technol. 2001, 17, 823–832. [Google Scholar] [CrossRef]
- Wong, W.L.E.; Gupta, M. Development of Mg/Cu nanocomposites using microwave assisted rapid sintering. Compos. Sci. Technol. 2007, 67, 1541–1552. [Google Scholar] [CrossRef]
- Liu, L.; Yu, S.; Liu, E.; Zhao, Y.; Wang, B.; Niu, Y. Effect of Ni addition on the mechanical and degradation properties of hollow glass microsphere/Mg alloy composites. J. Alloys Compd. 2021, 853, 157125. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Lu, S.; Li, W.; Zhao, J.; Johansson, B.; Vitos, L. Elastic properties of vanadium-based alloys from first-principles theory. Phys. Rev. B 2012, 86, 14105. [Google Scholar] [CrossRef]
- Peng, P.; He, X.; She, J.; Tang, A.; Rashad, M.; Zhou, S.; Zhang, G.; Mi, X.; Pan, F. Novel low-cost magnesium alloys with high yield strength and plasticity, Mater. Sci. Eng. A 2019, 766, 138332. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, H.; Sun, Y.; Ren, L.; Wan, Z.; Hu, L. Microstructures and mechanical properties of ultrafine-grained Ti/AZ31 magnesium matrix composite prepared by powder metallurgy. Adv. Powder Technol. 2018, 29, 3241–3249. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Asif, M. Room temperature mechanical properties of Mg-Cu-Al alloys synthesized using powder metallurgy method. Mater. Sci. Eng. A 2015, 644, 129–136. [Google Scholar] [CrossRef]
- Pekguleryuz, M.O.; Kaya, A.A. Creep resistant magnesium alloys for powertrain applications. Adv. Eng. Mater. 2003, 5, 866–878. [Google Scholar] [CrossRef]
- Balasubramani, N.; Srinivasan, A.; Pillai, U.T.S.; Pai, B.C. Effect of Pb and Sb additions on the precipitation kinetics of AZ91 magnesium alloy. Mater. Sci. Eng. A 2007, 457, 275–281. [Google Scholar] [CrossRef]
- Guangyin, Y.; Yangshan, S.; Wenjiang, D. Effects of Sb addition on the microstructure and mechanical properties of AZ91 magnesium alloy. Scr. Mater. 2000, 43, 1009–1013. [Google Scholar] [CrossRef]
- Murad, A.; Hussein, M.A.; Al-Aqeeli, N. Magnesium-based composites and alloys for medical applications: A review of mechanical and corrosion properties. J. Alloys Compd. 2019, 792, 1162–1190. [Google Scholar] [CrossRef]
- Sun, S.; Deng, N.; Zhang, H.; He, L.; Zhou, H.; Han, B.; Gao, K.; Wang, X. Microstructure and mechanical properties of AZ31 magnesium alloy reinforced with novel sub-micron vanadium particles by powder metallurgy. J. Mater. Res. Technol. 2021, 15, 1789–1800. [Google Scholar] [CrossRef]
- Ho, K.F.; Gupta, M.; Srivatsan, T.S. The mechanical behavior of magnesium alloy AZ91 reinforced with fine copper particulates. Mater. Sci. Eng. A 2004, 369, 302–308. [Google Scholar] [CrossRef]
- Pérez, P.; Garcés, G.; Adeva, P. Mechanical properties of a Mg–10 (vol.%)Ti composite. Compos. Sci. Technol. 2004, 64, 145–151. [Google Scholar] [CrossRef]
- Hassan, S.F.; Gupta, M. Development of ductile magnesium composite materials using titanium as reinforcement. J. Alloys Compd. 2002, 345, 246–251. [Google Scholar] [CrossRef]
- Xi, Y.L.; Chai, D.L.; Zhang, W.X.; Zhou, J.E. Titanium alloy reinforced magnesium matrix composite with improved mechanical properties. Scr. Mater. 2006, 54, 19–23. [Google Scholar] [CrossRef]
- Edalati, K.; Emami, H.; Staykov, A.; Smith, D.J.; Akiba, E.; Horita, Z. Formation of metastable phases in magnesium-titanium system by high-pressure torsion and their hydrogen storage performance. Acta Mater. 2015, 99, 150–156. [Google Scholar] [CrossRef]
- Maweja, K.; Phasha, M.; van der Berg, N. Microstructure and crystal structure of an equimolar Mg-Ti alloy processed by Simoloyer high-energy ball mill. Powder Technol. 2010, 199, 256–263. [Google Scholar] [CrossRef]
- Umeda, J.; Kawakami, M.; Kondoh, K.; Ayman, E.S.; Imai, H. Microstructural and mechanical properties of titanium particulate reinforced magnesium composite materials. Mater. Chem. Phys. 2010, 123, 649–657. [Google Scholar] [CrossRef]
- Kitazono, K.; Komatsu, S.; Kataoka, Y. Mechanical properties of titanium particles dispersed magnesium matrix composite produced through accumulative diffusion bonding process. Mater. Trans. 2011, 52, 155–158. [Google Scholar] [CrossRef]
- Braszczyńska-Malik, K.N.; Przełożyńska, E. Analyses of AM50-Tip metal-metal composite microstructure. J. Alloys Compd. 2018, 731, 1181–1187. [Google Scholar] [CrossRef]
- Yu, H.; Sun, Y.; Hu, L.; Wan, Z.; Zhou, H. Microstructure and properties of mechanically milled AZ61 powders dispersed with submicron/nanometer Ti particulates. Mater. Charact. 2017, 127, 272–278. [Google Scholar] [CrossRef]
- Sun, J.; Du, W.; Fu, J.; Liu, K.; Li, S.; Wang, Z.; Liang, H. A review on magnesium alloys for application of degradable fracturing tools. J. Magnes. Alloys 2022, 10, 2649–2672. [Google Scholar] [CrossRef]
- Candan, S.; Unal, M.; Koc, E.; Turen, Y.; Candan, E. Effects of titanium addition on mechanical and corrosion behaviours of AZ91 magnesium alloy. J. Alloys Compd. 2011, 509, 1958–1963. [Google Scholar] [CrossRef]
- Ai, X.; Quan, G. Effect of Ti on the mechanical properties and corrosion of cast AZ91 magnesium alloy. Open Mater. Sci. J. 2012, 6, 6–13. [Google Scholar] [CrossRef]
- Kruszewski, M.J.; Zybała, R.; Ciupiński; Chmielewski, M.; Adamczyk-Cieślak, B.; Michalski, A.; Rajska, M.; Kurzydłowski, K.J. Microstructure and Thermoelectric Properties of Bulk Cobalt Antimonide (CoSb3) Skutterudites Obtained by Pulse Plasma Sintering. J. Electron. Mater. 2016, 45, 1369–1376. [Google Scholar] [CrossRef]
- Pałgan, D.; Dobkowska, A.; Zielińska, A.; Drozdenko, D.; Máthis, K.; Święszkowski, W. The Role of LPSO Structures in Corrosion Resistance of Mg-Y-Zn Alloys. Crystals 2022, 12, 1723. [Google Scholar] [CrossRef]
- Dobkowska, A.; Zrodowski, Ł.; Chlewicka, M.; Koralnik, M.; Adamczyk-cie, B. A comparison of the microstructure-dependent corrosion of dual-structured Mg-Li alloys fabricated by powder consolidation methods: Laser powder bed fusion vs pulse plasma sintering. J. Magnes. Alloys 2022, 10, 3553–3564. [Google Scholar] [CrossRef]
- Paraskevas, D.; Dadbakhsh, S.; Vleugels, J.; Vanmeensel, K.; Dewulf, W.; Duflou, J.R. Solid state recycling of pure Mg and AZ31 Mg machining chips via spark plasma sintering. Mater. Des. 2016, 109, 520–529. [Google Scholar] [CrossRef]
- Yang, L.; Wang, T.; Liu, C.; Ma, Y.; Wu, L.; Yan, H.; Zhao, X.; Liu, W. Microstructures and mechanical properties of AZ31 magnesium alloys fabricated via vacuum hot-press sintering. J. Alloys Compd. 2021, 870, 159473. [Google Scholar] [CrossRef]
- Muhammad, W.N.A.W.; Sajuri, Z.; Mutoh, Y.; Miyashita, Y. Microstructure and mechanical properties of magnesium composites prepared by spark plasma sintering technology. J. Alloys Compd. 2011, 509, 6021–6029. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, J.; Lian, Y.; Han, S.; Zheng, K.; Pan, F. Influence of Micro/Nano-Ti Particles on the Corrosion Behavior of AZ31-Ti Composites. Acta Metall. Sin. Engl. Lett. 2023. [Google Scholar] [CrossRef]
- Zgłobicka, I.B.; Dobkowska, A.; Zielińska, A.; Borucinska, E.; Kruszewski, M.J.; Zybała, R.; Płociński, T.; Idaszek, J.; Jaroszewicz, J.; Paradowski, K.; et al. In-depth analysis of the influence of bio-silica filler (Didymosphenia geminata frustules) on the properties of Mg matrix composites. J. Magnes. Alloys 2023, 11, 2853–2871. [Google Scholar] [CrossRef]

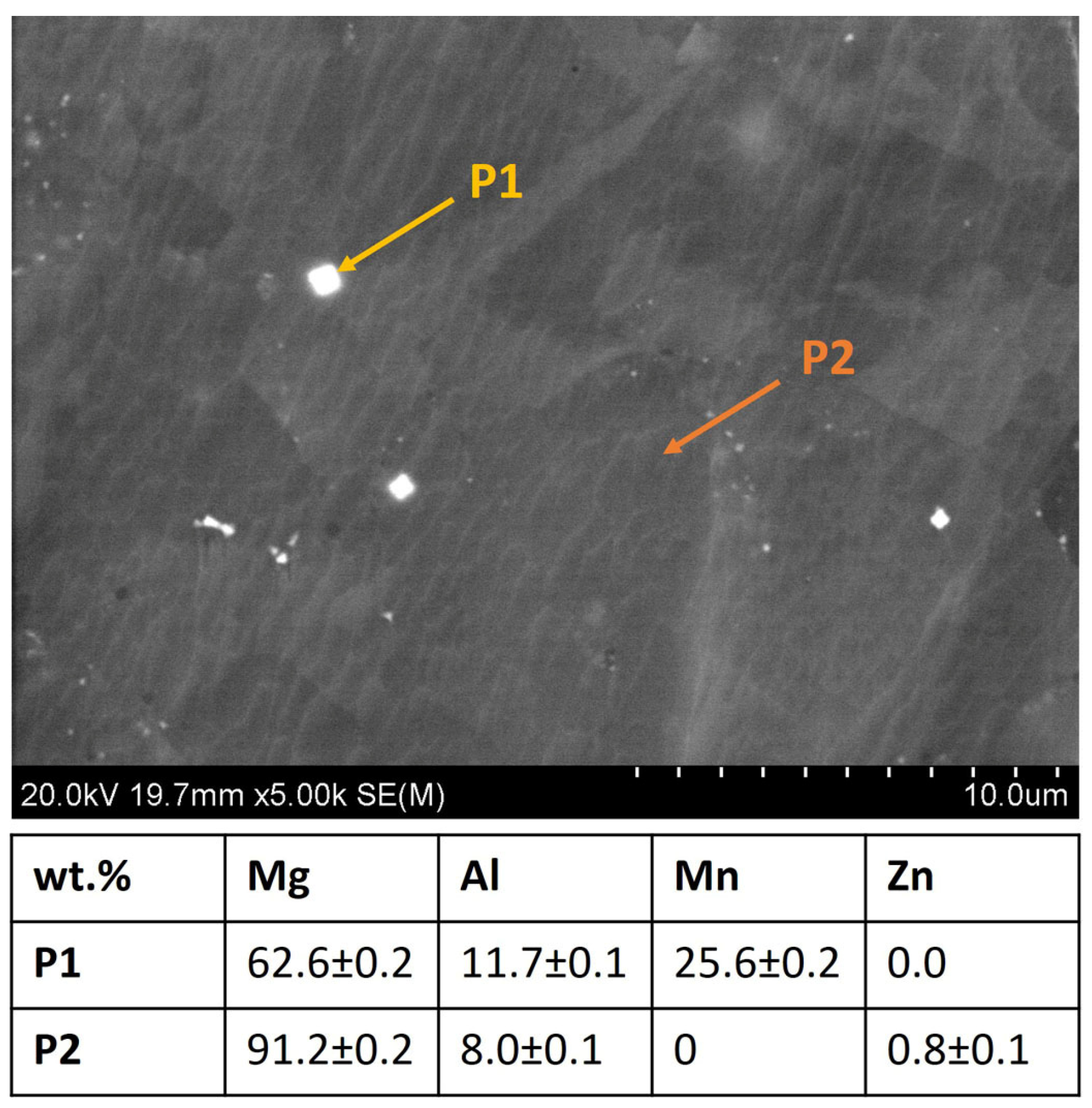
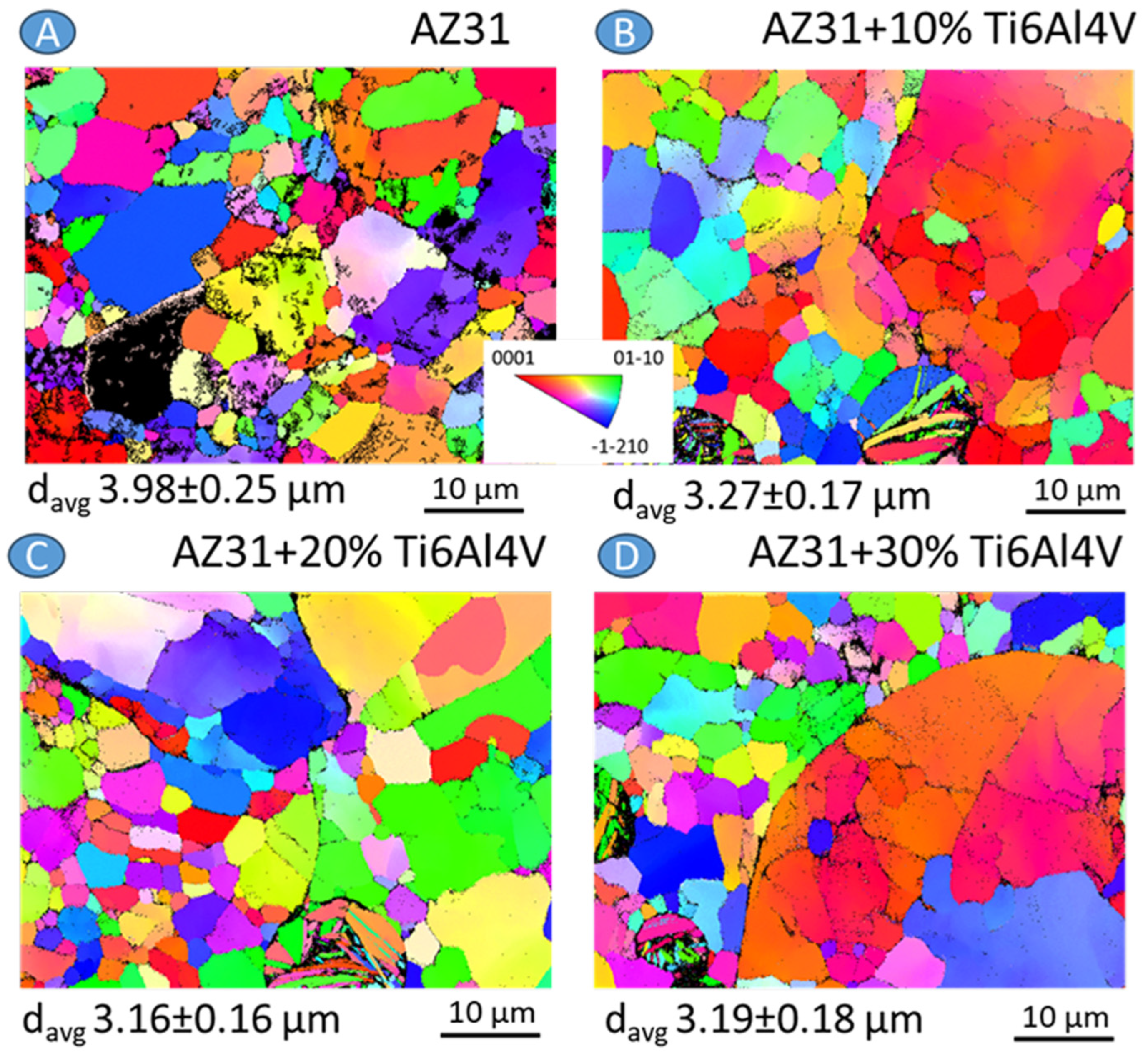
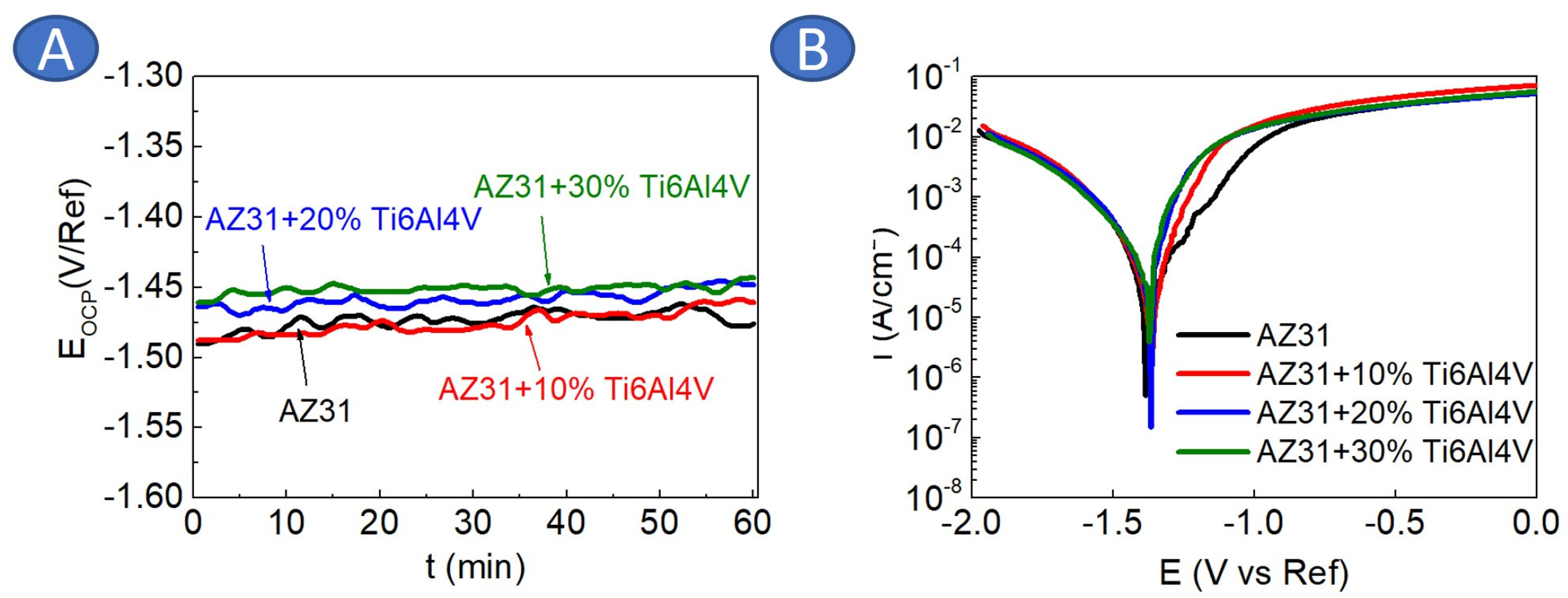
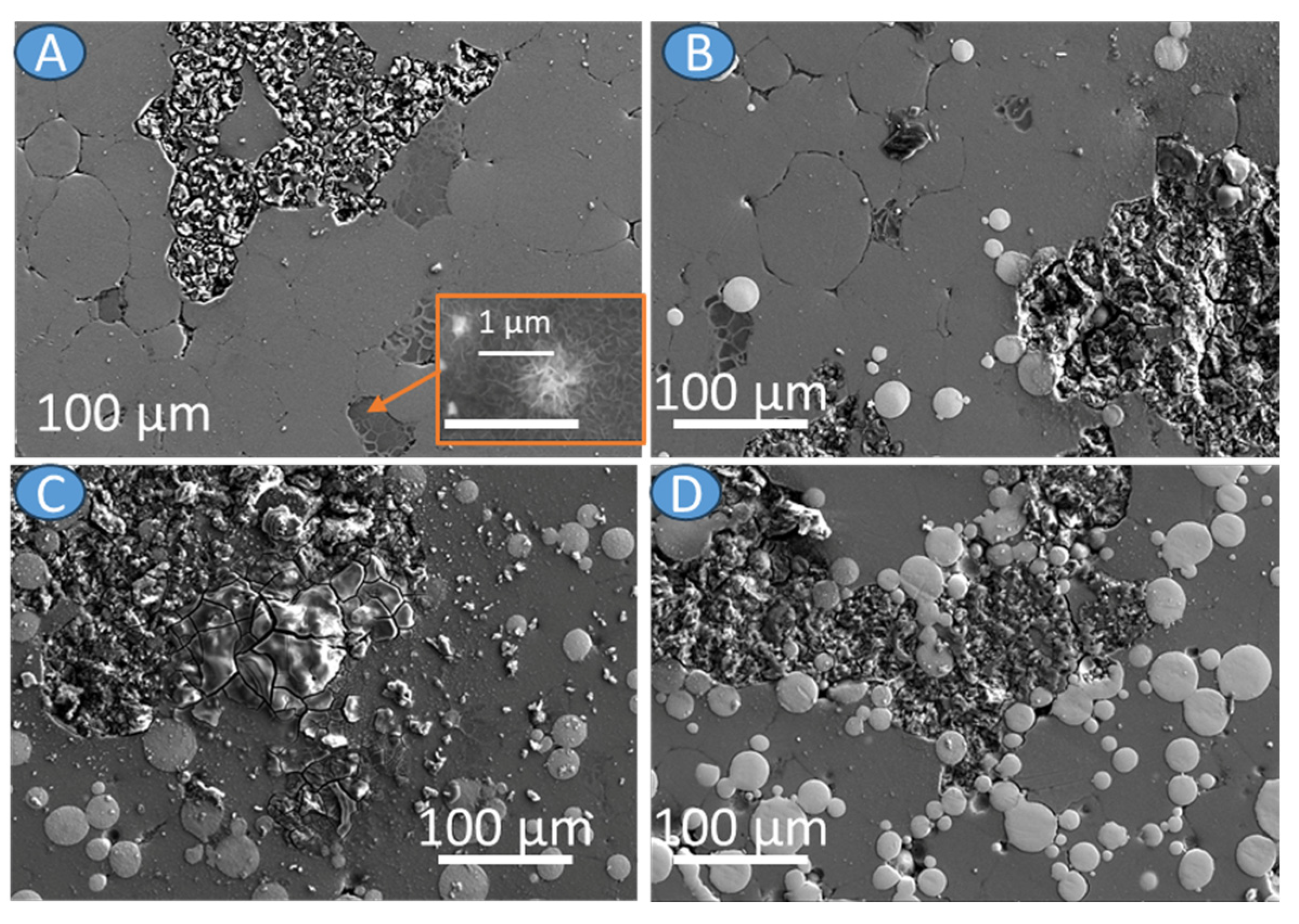

| Material | icorr (µA/cm2) | Ecorr (V vs. Ref) |
|---|---|---|
| AZ31 | 10 ± 2 | −1.38 ± 0.1 |
| AZ31 + 10 vol.%Ti6Al4V | 8 ± 2 | −1.37 ± 0.1 |
| AZ31 + 20 vol.%Ti6Al4V | 33 ± 7 | −1.37 ± 0.1 |
| AZ31 + 30 vol.%Ti6Al4V | 28 ± 11 | −1.37 ± 0.1 |
| Material | CR (mpy) |
|---|---|
| AZ31 | 6.2 ± 0.2 |
| AZ31 + 10 vol.% Ti6Al4V | 6.8 ± 0.1 |
| AZ31 + 20 vol.% Ti6Al4V | 8.7 ± 0.2 |
| AZ31 + 30 vol.% Ti6Al4V | 14.5 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobkowska, A.; Kruszewski, M.J.; Ciftci, J.; Morończyk, B.; Zgłobicka, I.; Zybała, R.; Żrodowski, Ł. Microstructure and Corrosion of Mg-Based Composites Produced from Custom-Made Powders of AZ31 and Ti6Al4V via Pulse Plasma Sintering. Materials 2024, 17, 1602. https://doi.org/10.3390/ma17071602
Dobkowska A, Kruszewski MJ, Ciftci J, Morończyk B, Zgłobicka I, Zybała R, Żrodowski Ł. Microstructure and Corrosion of Mg-Based Composites Produced from Custom-Made Powders of AZ31 and Ti6Al4V via Pulse Plasma Sintering. Materials. 2024; 17(7):1602. https://doi.org/10.3390/ma17071602
Chicago/Turabian StyleDobkowska, Anna, Mirosław Jakub Kruszewski, Jakub Ciftci, Bartosz Morończyk, Izabela Zgłobicka, Rafał Zybała, and Łukasz Żrodowski. 2024. "Microstructure and Corrosion of Mg-Based Composites Produced from Custom-Made Powders of AZ31 and Ti6Al4V via Pulse Plasma Sintering" Materials 17, no. 7: 1602. https://doi.org/10.3390/ma17071602










