The Effect of SiO2 Particle Size on Crystallization Behavior and Space Charge Properties for SiO2/MMT/LDPE Composites
Abstract
1. Introduction
2. Experimental Methods
2.1. Experimental Materials and Samples
2.2. Instrumentation and Equipment
2.3. Crystallization Behavior
2.4. Dielectric Frequency Spectra
2.5. Space Charge
3. Results and Discussion
3.1. Crystalline Morphology of Composites
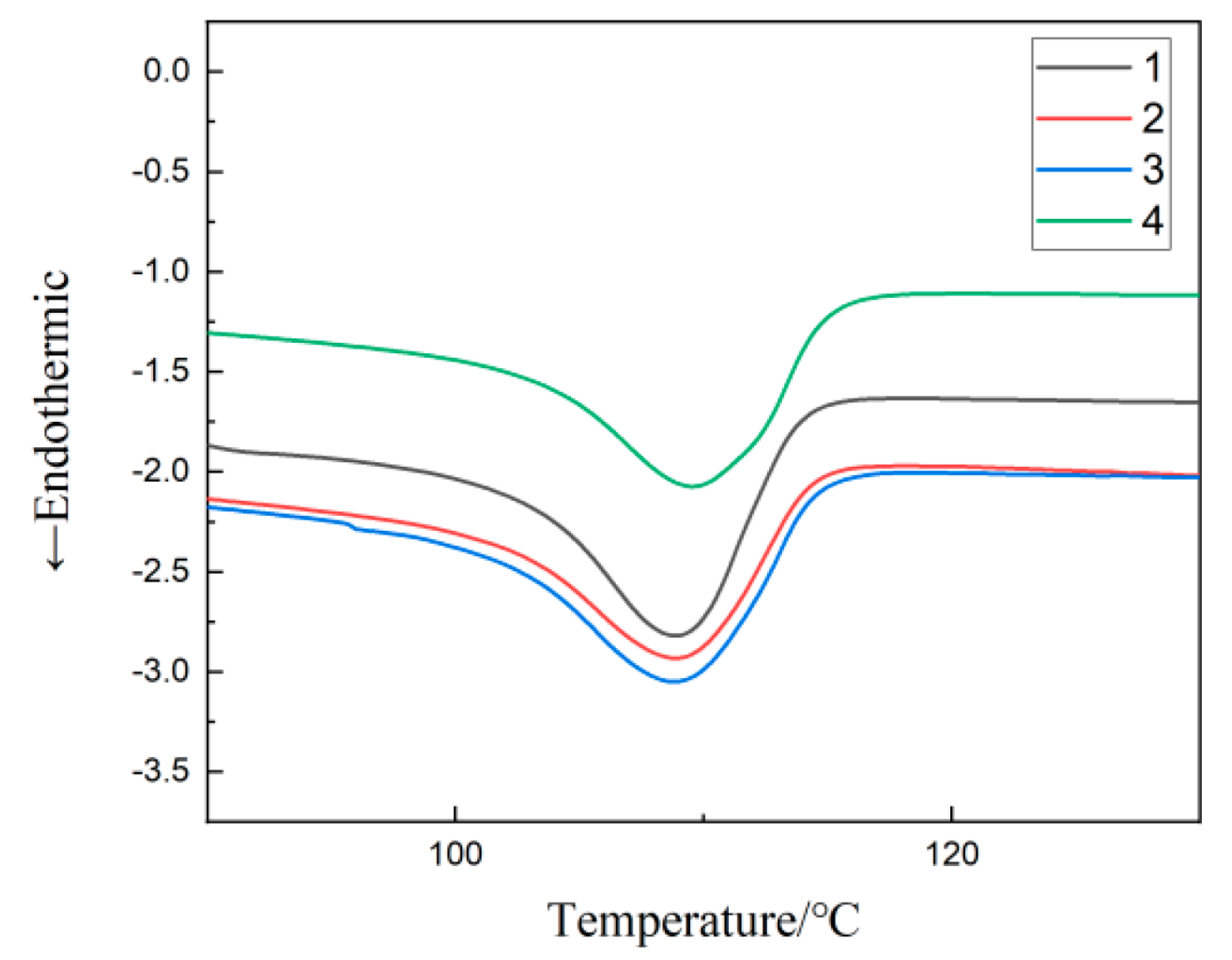
3.2. DSC Testing of Composites
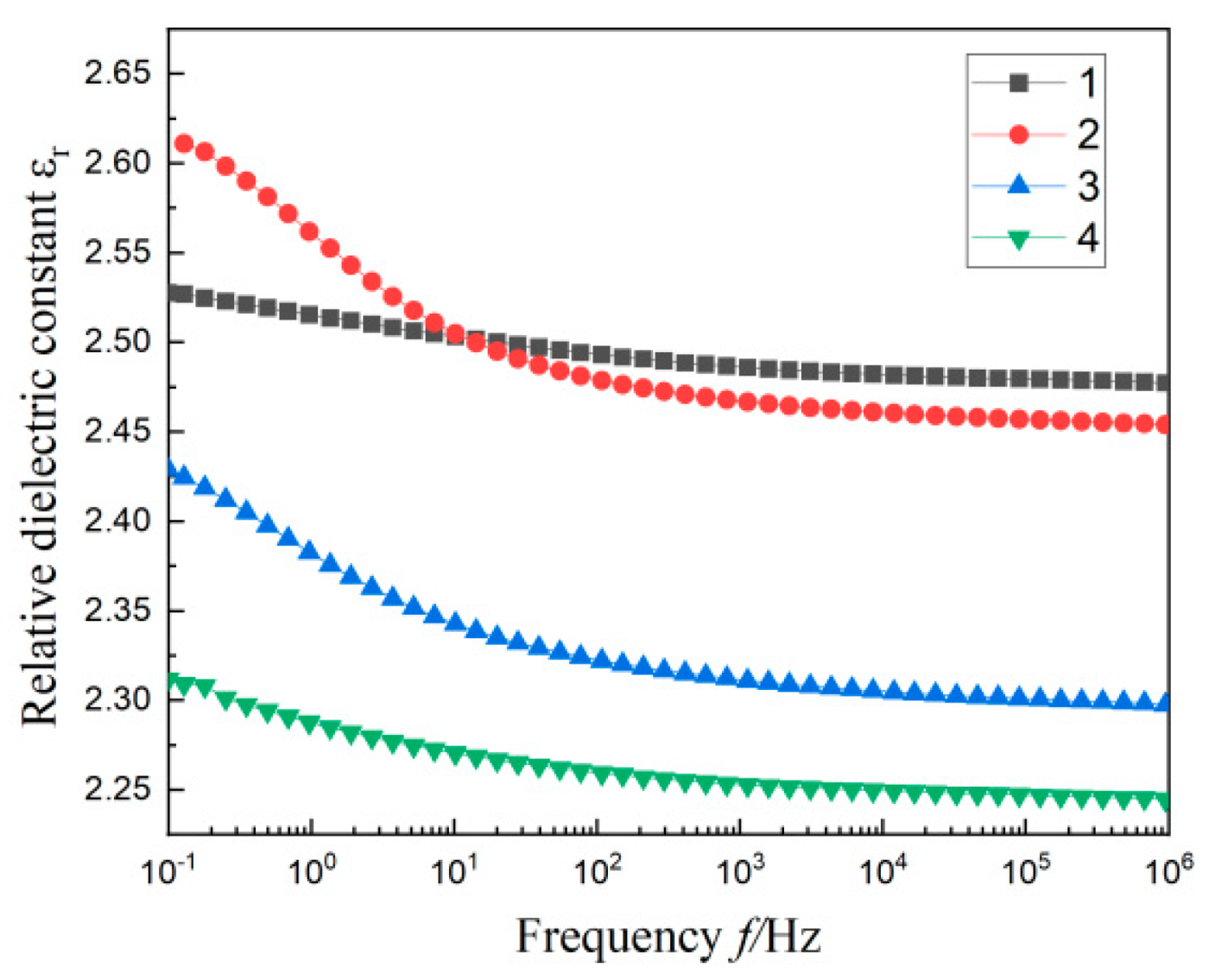
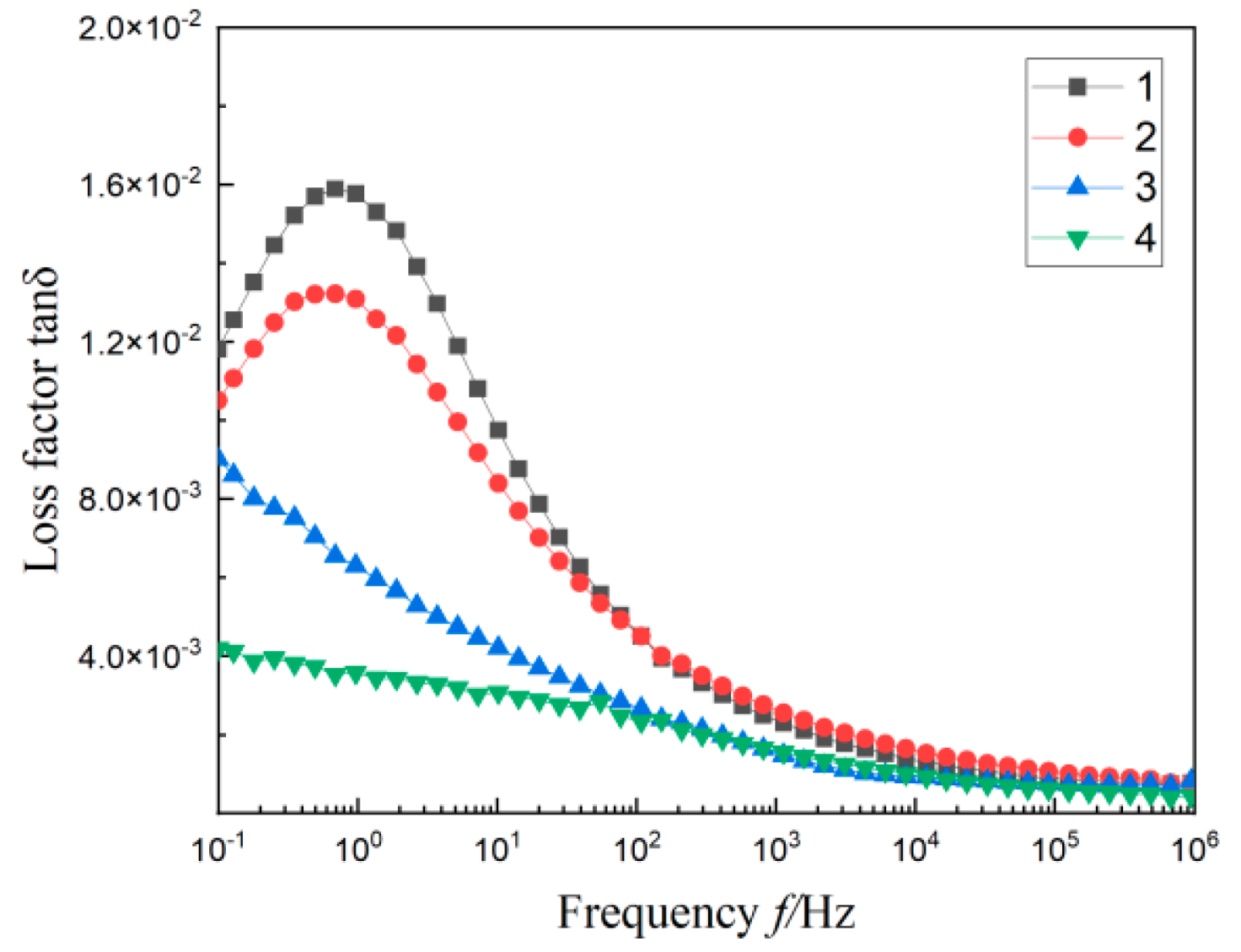
3.3. Dielectric Spectrum of Composites
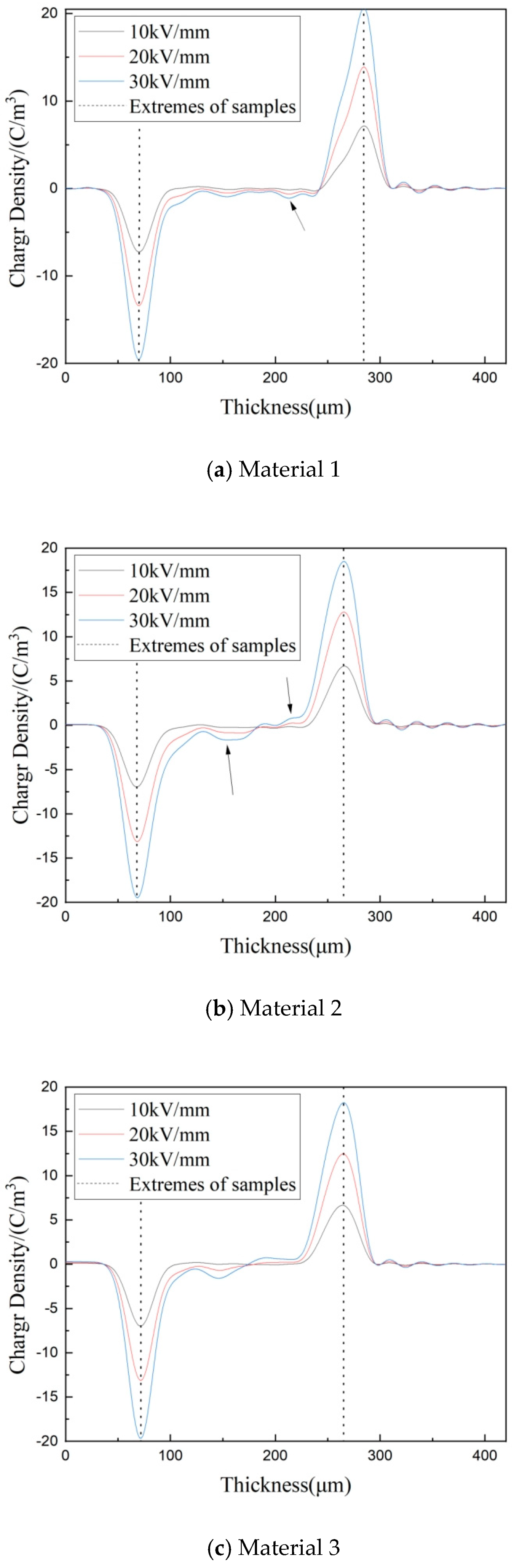
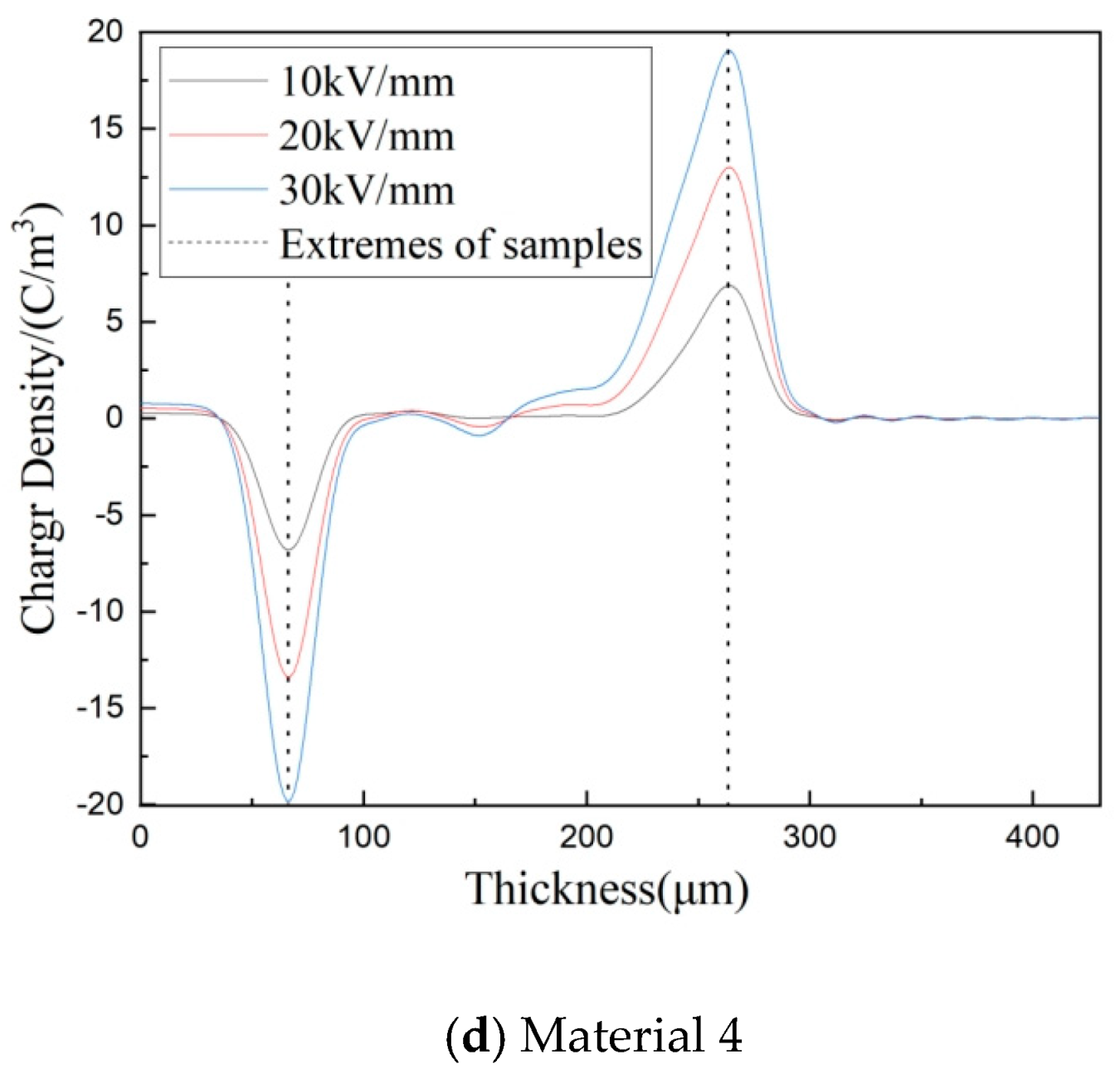
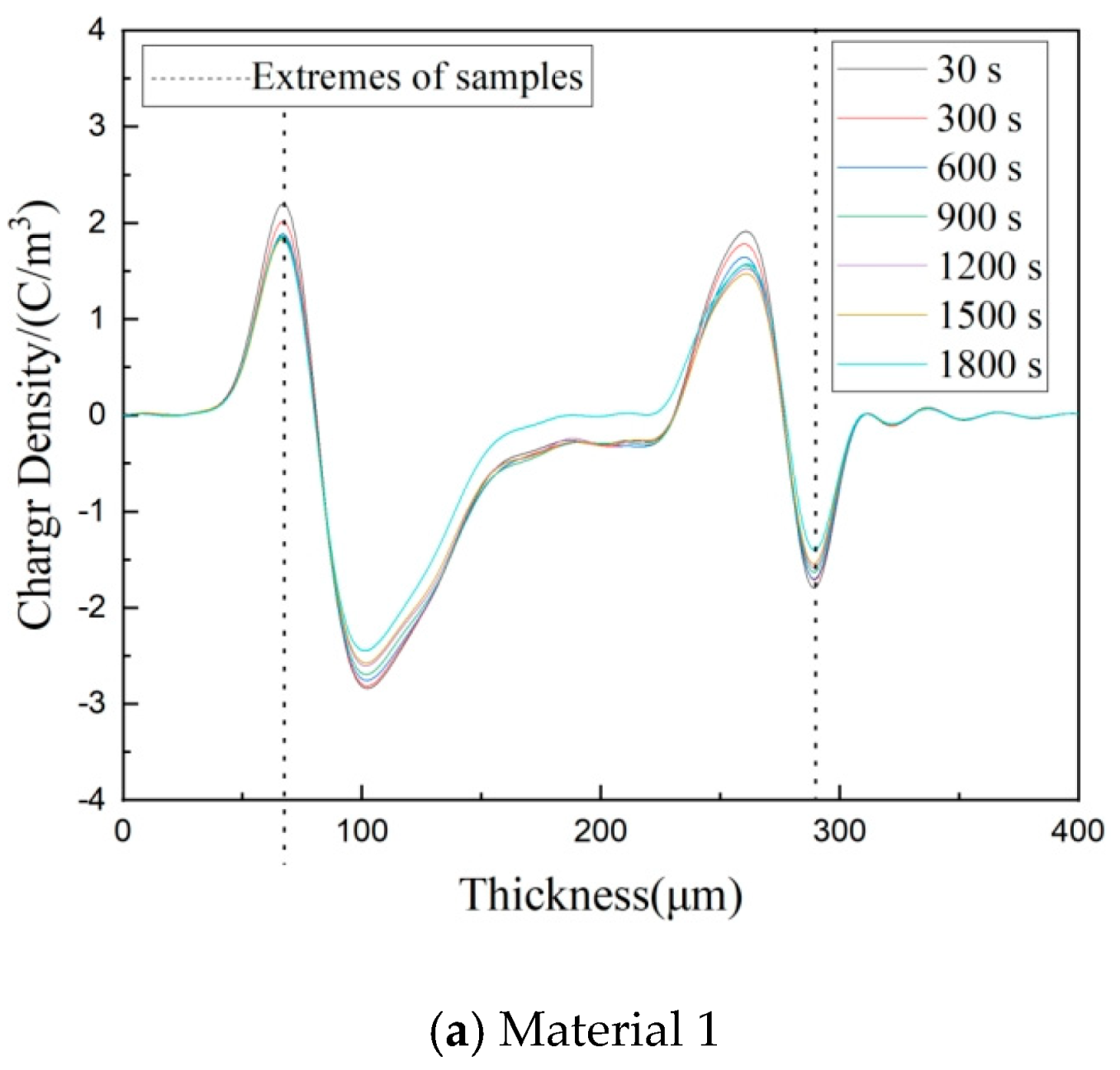
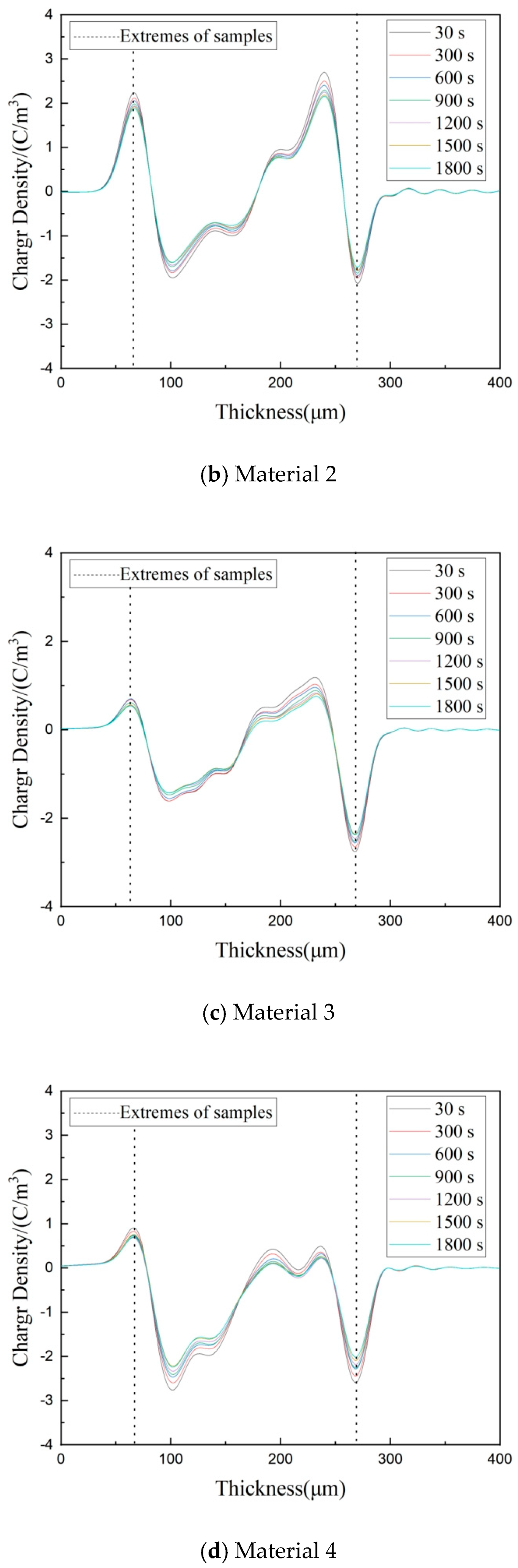
3.4. Space Charge Characteristics of Composites
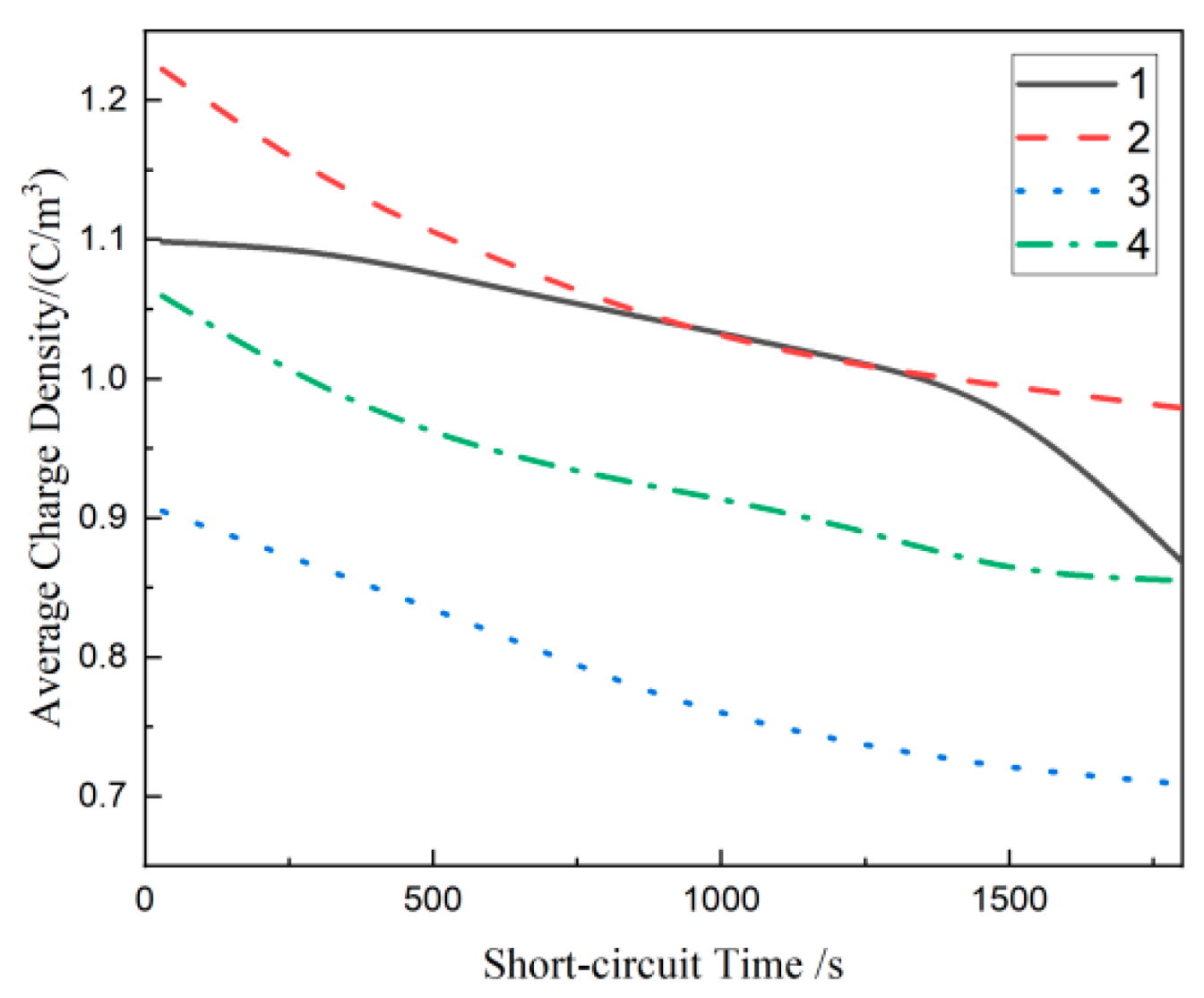
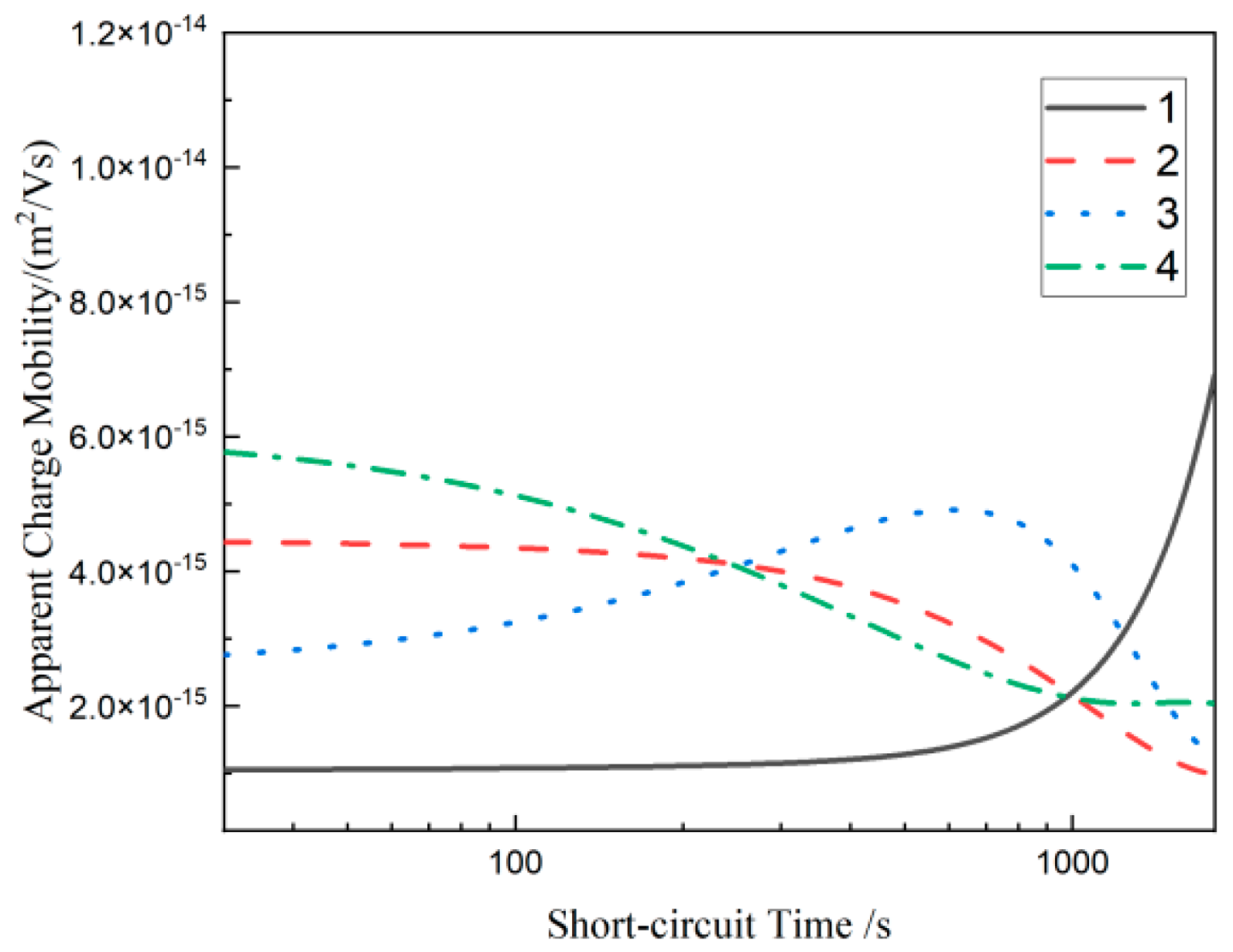
3.5. Depolarization Space Charge Characteristics of Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, S.; Ma, X.; Feng, R. Review of Researches on Power Cables at Home and Abroad. Wire Cable 2021, 3, 1–6. [Google Scholar]
- Amir, M.P.; Claire, P.; Amirhossein, A. XLPE high voltage insulation; A link between DC conductivity and microstructure. Polym. Test. 2024, 131, 108330. [Google Scholar]
- Liu, L.; Liu, T.; Li, X. Performance comparison of semi-conductive shielding layer for high-voltage cable based on polypropylene and low density polyethylene resin. J. Appl. Polym. Sci. 2023, 140, 54510. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, W.; Dinh, M. Comparative Analysis of XLPE and Thermoplastic Insulation-Based HVDC Power Cables. Energies 2022, 16, 167. [Google Scholar] [CrossRef]
- Tanaka, Y.; Chen, G.; Zhao, Y. Effect of additives on morphology and space charge accumulation in low density polyethylene. IEEE Trans. Dielectr. Electr. Insul. 2003, 10, 148–154. [Google Scholar] [CrossRef]
- Maur, S.; Chakraborty, B.; Pradhan, A.K. Relaxation Frequency Distribution Based Approach towards Moisture Estimation of 11kV XLPE Cable Insulation. IEEE Trans. Dielectr. Electr. Insul. 2024, 3, 3355367. [Google Scholar] [CrossRef]
- Mazzanti, G. Updated Review of the Life and Reliability Models for HVDC Cables. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 1371–1390. [Google Scholar] [CrossRef]
- Afeissa, S.; Boukezzi, L.; Bessissa, L. Cross-linking tendency and photo-oxidation degradation in Silane-graftedLDPE insulation under accelerated cyclic weathering aging. Rev. Mex. Física 2024, 70, 021003. [Google Scholar]
- Jiang, Z.; Liu, X.; Wang, W. Preparation and Dielectric Properties of High Temperature Resistant Barium Titanate/Benzoxazole Nano-Composites. J. East China Univ. Sci. Technol. 2023, 49, 55–61. [Google Scholar]
- Ahmed, H.M.; Abd El-Fattah, Z.M.; Anad, N.S. Thermo-mechanical and opto-electrical study of Cr-doped-ZnO-based polyvinyl chloride nanocomposites. J. Mater. Sci. Mater. Electron. 2023, 34, 113. [Google Scholar] [CrossRef]
- Sadek, E.M.; Ahmed, S.M.; El-Nashar, D.E. Effect of modified graphite nanoflakes on curing, mechanical and dielectric properties of nitrile rubber nanocomposites. Polym. Bull. 2023, 80, 847–863. [Google Scholar] [CrossRef]
- Mozaffari, S.; Ghorashi, S.M.B. Effect of Cu@polythiophene yolk–shell nanofiller on the electrical, mechanical and thermal properties of poly(vinyl alcohol) thin films. Polym. Bull. 2022, 80, 895–915. [Google Scholar] [CrossRef]
- He, Z. Simultaneously High Dielectric Constant and Breakdown Strength in CaCu3Ti4O12-Filled Polymer Composites. J. Electron. Mater. 2022, 5, 4521–4528. [Google Scholar] [CrossRef]
- Suresh, M.; Mohan, N.; Gopinathan, A. Montmorillonite-catalysed coupling reactions: A green overview. Org. Biomol. Chem. 2024, 22, 1961–1982. [Google Scholar]
- Ahmed, A.M.; David, M.N.; Ahmed, A.M. Improving the performance of triboelectric nanogenerators using flexible polyurethane nanocomposites foam filled with montmorillonite. Nano Energy 2024, 124, 109426. [Google Scholar]
- Henriques, R.R.; Carelo, J.C.; Soares, B.G. Anti-corrosive and self-healing properties of epoxy coatings loaded with montmorillonite modified with zinc-based ionic liquid. Prog. Org. Coat. 2024, 187, 108185. [Google Scholar]
- Yin, B.Y. Nano Age: Reality and Dream; China Light Industry Press: Beijing, China, 2001; pp. 87–145. [Google Scholar]
- Kaffayatullah, K.; Megat, A.M.J.; Muhammad, N.A. Development and evaluation of basaltic volcanic ash based high performance concrete incorporating metakaolin, micro and nano-silica. Dev. Built Environ. 2024, 17, 100330. [Google Scholar]
- Imai, T.; Sawa, F.; Nakano, T. Effects of nano- and micro-filler mixture on electrical insulation properties of epoxy based composites. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 319–326. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, X.; Gao, J. Conductance Current and Space Charge Characteristics of SiO2/MMT/LDPE Micro-Nano Composites. Materials 2020, 13, 4119. [Google Scholar] [CrossRef]
- Schauer, S.E.; Baskar, R.; Brand, L. Synthesis and characterization of CdSe semiconductor nanoparticles by ultrasonic irradiation. Mater. Sci. Eng. B 2007, 138, 65–68. [Google Scholar]
- Pérez-Maqueda, L.A.; Franco, F.; Avilés, M.A. Effect of sonication on particle size distribution in natural muscovite and biotite. Clays Clay Min. 2003, 51, 701–708. [Google Scholar] [CrossRef]
- Franco, F.; Pérez-Maqueda, L.A.; Pérez-Rodríguez, J.L. The effect of ultra-sound of the particle size and structural disorder of a well-ordered kaolinite. J. Colloid Interface Sci. 2004, 274, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C. Investigation on Dielectric Properties in Polyvinyl Micro/Nano-Composites; Harbin University of Science and Technology: Harbin, China, 2017. [Google Scholar]
- Liang, J.Z. Measurement of the melting enthalpy and crystallizability for HDPES. Guangzhou Chem. Ind. Technol. 1994, 22, 27–30. [Google Scholar]
- Chi, X.; Yu, L.; Zheng, J. Crystallization morphology and electrical tree resistance characteristics of montmorillonite/polypropylene composites. Acta Mater. Compos. Sin. 2015, 32, 76–84. [Google Scholar]
- Roy, M.; Nelson, J.K.; Maccrone, R.K. Polymer Nanocomposite Dielectrics—The Role of the Interface. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 629–643. [Google Scholar] [CrossRef]
- Vinayak, V.J.V.; Deshmukh, K.; Pandey, M. Synergistic effect of nano-BaTiO3 and montmorillonite nanoclay on the AC conductivity and dielectric properties of poly(vinylidenefluoride) nanocomposite films. Ferroelectrics 2024, 618, 561–575. [Google Scholar] [CrossRef]
- Lau, K.Y.; Vaughan, A.S.; Chen, G. On the dielectric response of silica-based polyethylene nanocomposites. J. Phys. D Appl. Phys. 2013, 46, 095303. [Google Scholar] [CrossRef]
- Bruggeman, D.A.G. The calculation of various physical constants of heterogeneous substances. I. The dielectric constants and conductivities of mixtures composed of iso-tropic substances. Ann. Phys. 1935, 24, 636–664. [Google Scholar] [CrossRef]
- Mazzanti, G.; Montanari, G.C.; Palmieri, F. Quantities extracted from space-charge measurements as markers for insulation aging. IEEE Trans. Dielectr. Electr. Insul. 2003, 10, 198–203. [Google Scholar] [CrossRef]
- Li, G.C.; Li, S.T.; Min, D.M. Effect of trap density on space charge formation and accumulation characteristics of low density polyethylene. Sci. Sin. (Technol.) 2013, 43, 375–381. [Google Scholar]
- Yi, S.; Wang, Y.; Peng, Q. Effect of Temperature on Charge Accumulation and Migration in Cross-linked Polyethylene. Proc. Chin. Soc. Electr. Eng. 2017, 37, 5796–5803. [Google Scholar]
- Mazzanti, G.; Montanari, G.C.; Alison, J.M. A space-charge based method for the estimation of apparent mobility and trap depth as markers for insulation degradation-theoretical basis and experimental validation. IEEE Trans. Dielectr. Electr. Insul. 2003, 8, 187–197. [Google Scholar] [CrossRef]
- Lan, L. Effect of temperature on space charge distribution in polymer insulation. Ph.D. Dissertation, Shanghai Jiaotong University, Shanghai, China, 2015. [Google Scholar]
- Kwan, C.K. Dielectric Phenomena in Solids; Elsevier Academic Press: London, UK, 2004; pp. 400–401. [Google Scholar]
- Liu, F.; Ling, Z.; Xie, J. Kinetics of trap generation and electrons capture in solid dielectrics under high electrical strength. J. South China Univ. Technol. (Nat. Sci.) 1993, 1, 100–107. [Google Scholar]

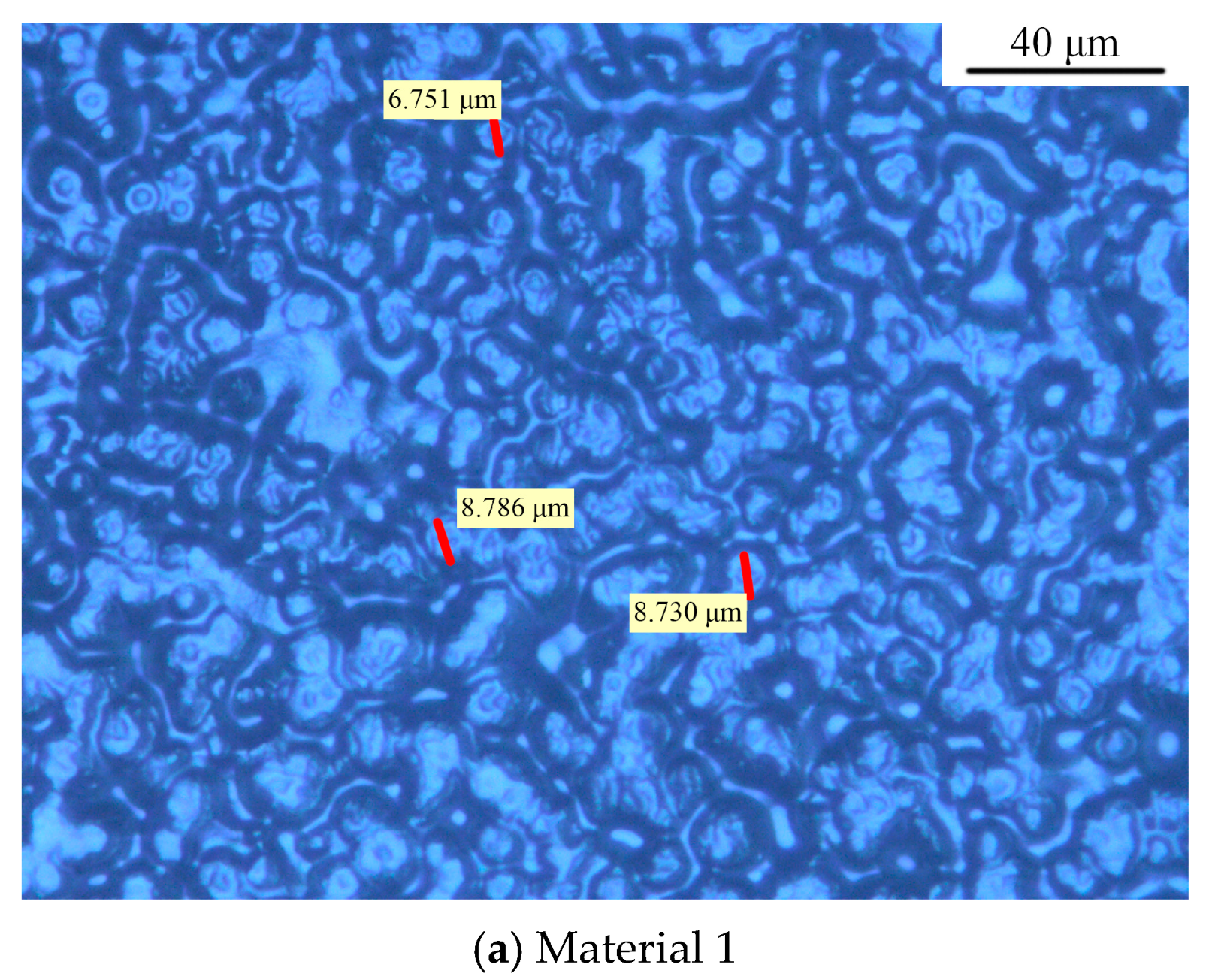
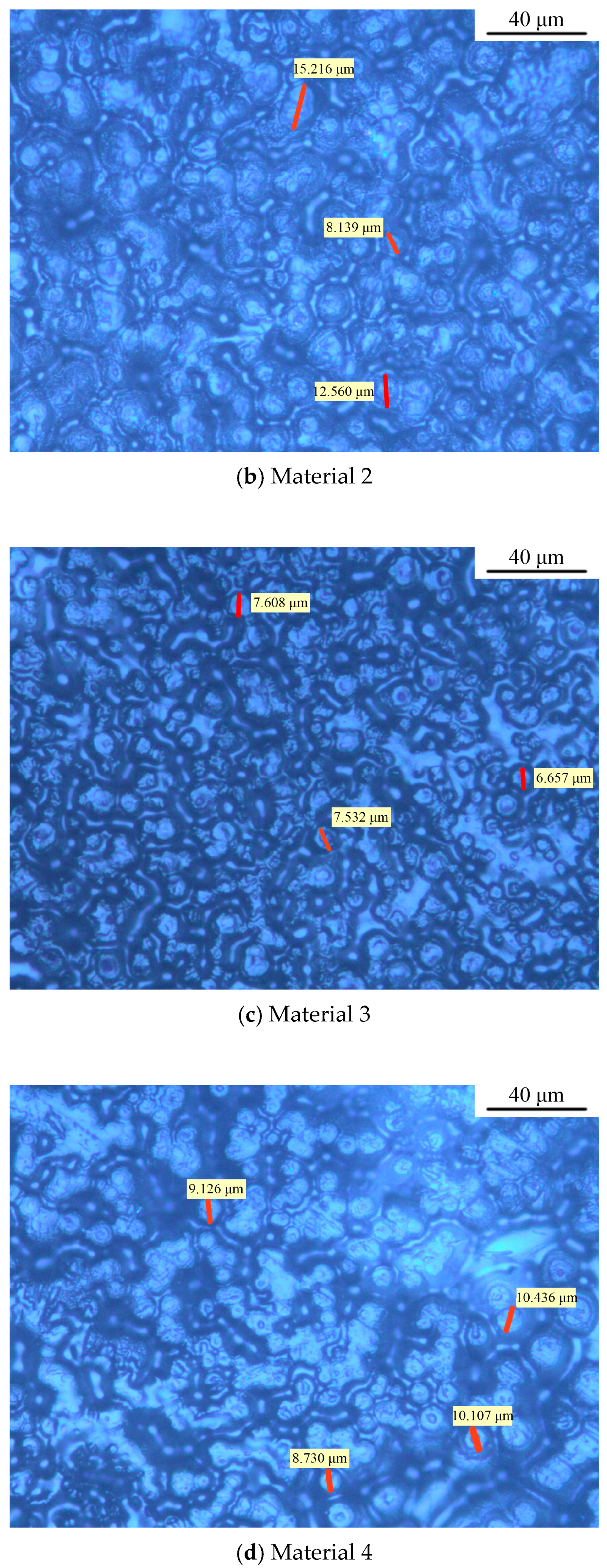
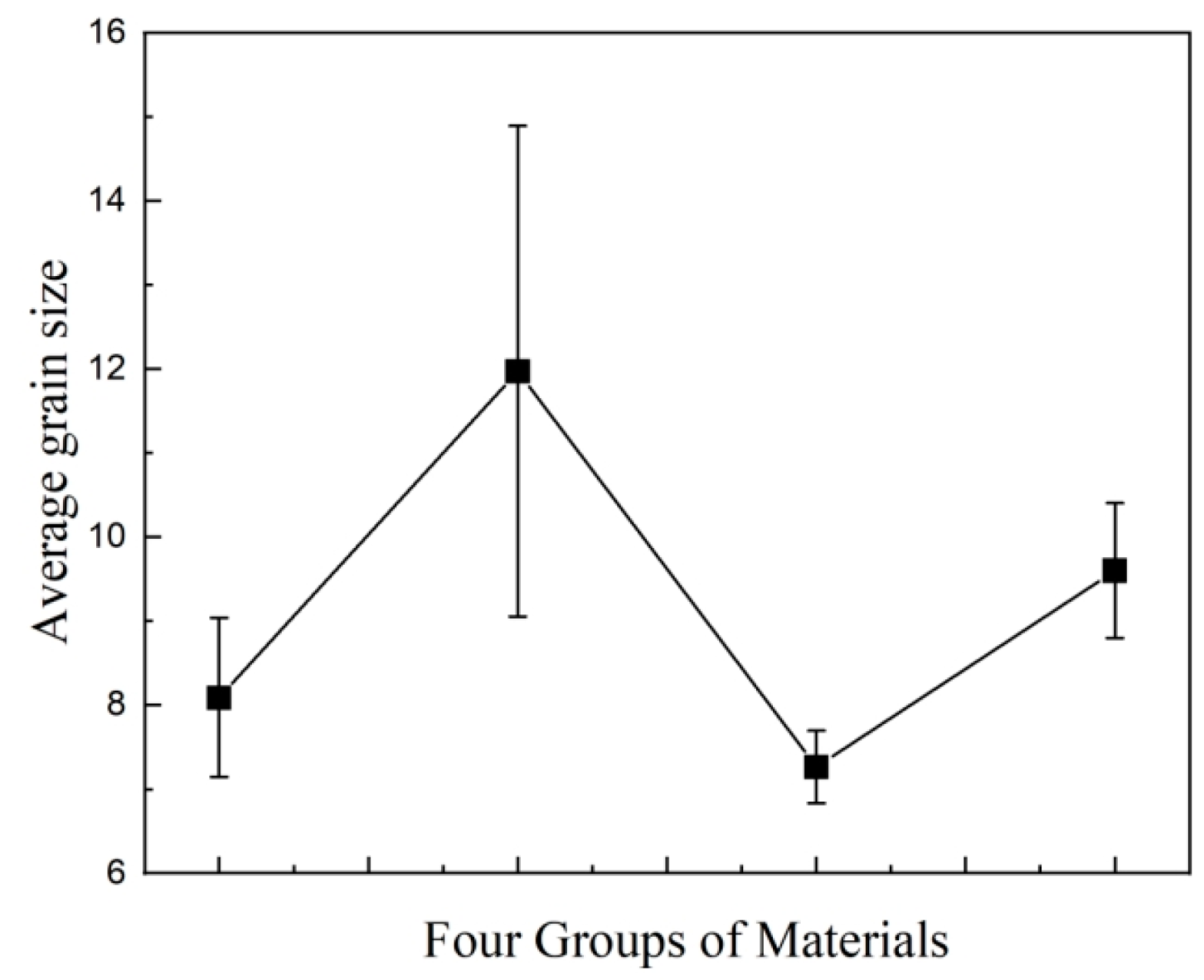
| Materials | Percentage Contents/% | ||||
|---|---|---|---|---|---|
| LDPE | MMT | 1 μmSiO2 | 30 nmSiO2 | 100 nmSiO2 | |
| 1 | 99 | 1 | 0 | 0 | 0 |
| 2 | 98 | 1 | 1 | 0 | 0 |
| 3 | 98 | 1 | 0 | 1 | 0 |
| 4 | 98 | 1 | 0 | 0 | 1 |
| Materials | °C | /% | Melting Heat |
|---|---|---|---|
| 1 | 109.58 | 32.45 | 94.33 |
| 2 | 107.77 | 28.88 | 83.09 |
| 3 | 108.33 | 34.42 | 99.03 |
| 4 | 110.84 | 33.35 | 95.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Yuan, H.; Yu, Q.; Xie, J. The Effect of SiO2 Particle Size on Crystallization Behavior and Space Charge Properties for SiO2/MMT/LDPE Composites. Materials 2024, 17, 1605. https://doi.org/10.3390/ma17071605
Jiang H, Yuan H, Yu Q, Xie J. The Effect of SiO2 Particle Size on Crystallization Behavior and Space Charge Properties for SiO2/MMT/LDPE Composites. Materials. 2024; 17(7):1605. https://doi.org/10.3390/ma17071605
Chicago/Turabian StyleJiang, Hongtao, Hong Yuan, Qunguang Yu, and Jing Xie. 2024. "The Effect of SiO2 Particle Size on Crystallization Behavior and Space Charge Properties for SiO2/MMT/LDPE Composites" Materials 17, no. 7: 1605. https://doi.org/10.3390/ma17071605
APA StyleJiang, H., Yuan, H., Yu, Q., & Xie, J. (2024). The Effect of SiO2 Particle Size on Crystallization Behavior and Space Charge Properties for SiO2/MMT/LDPE Composites. Materials, 17(7), 1605. https://doi.org/10.3390/ma17071605







