Influence of Sulfurization Time on Sb2S3 Synthesis Using a New Graphite Box Design
Abstract
:1. Introduction
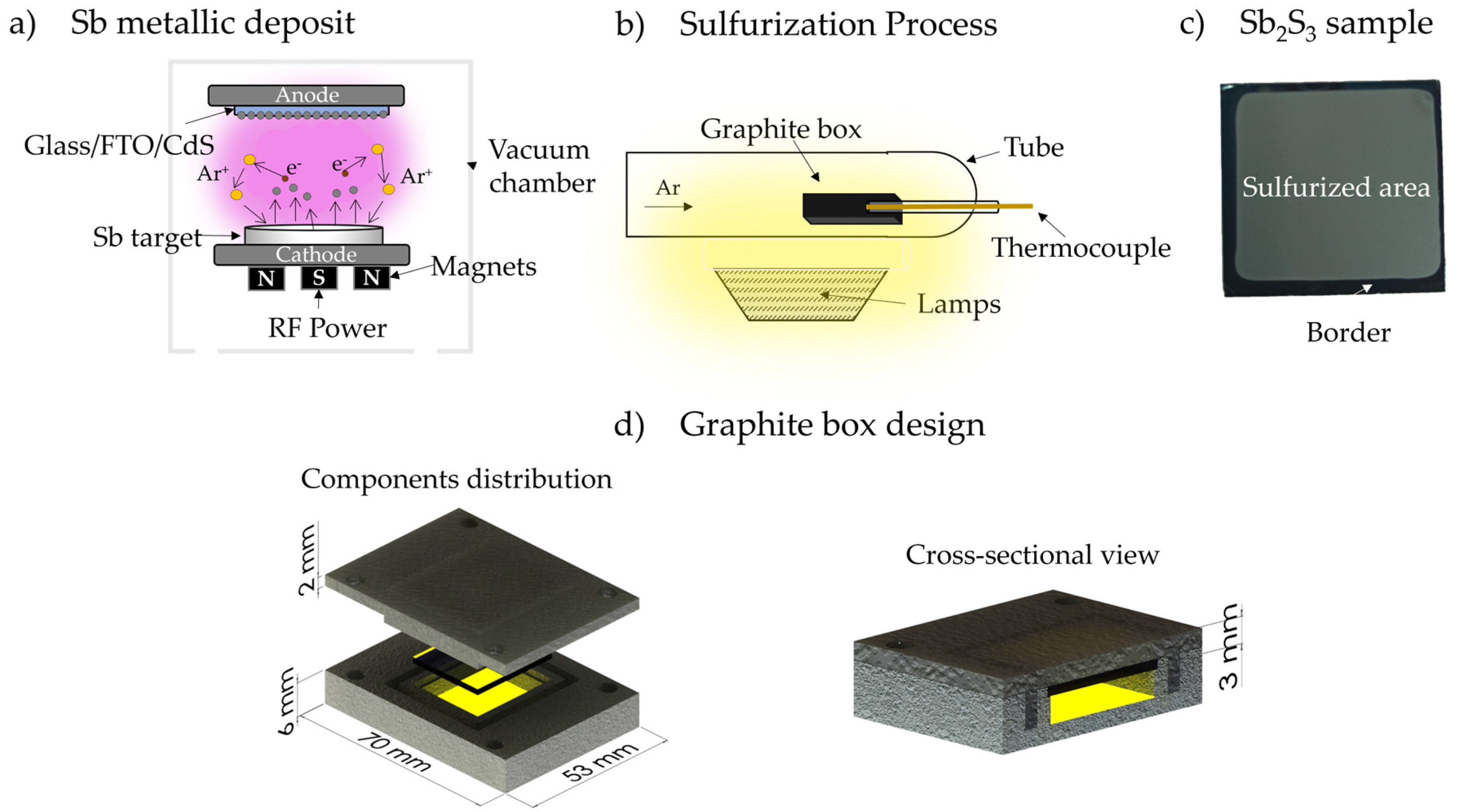
2. Materials and Methods
Measurement and Characterization
3. Results and Discussion
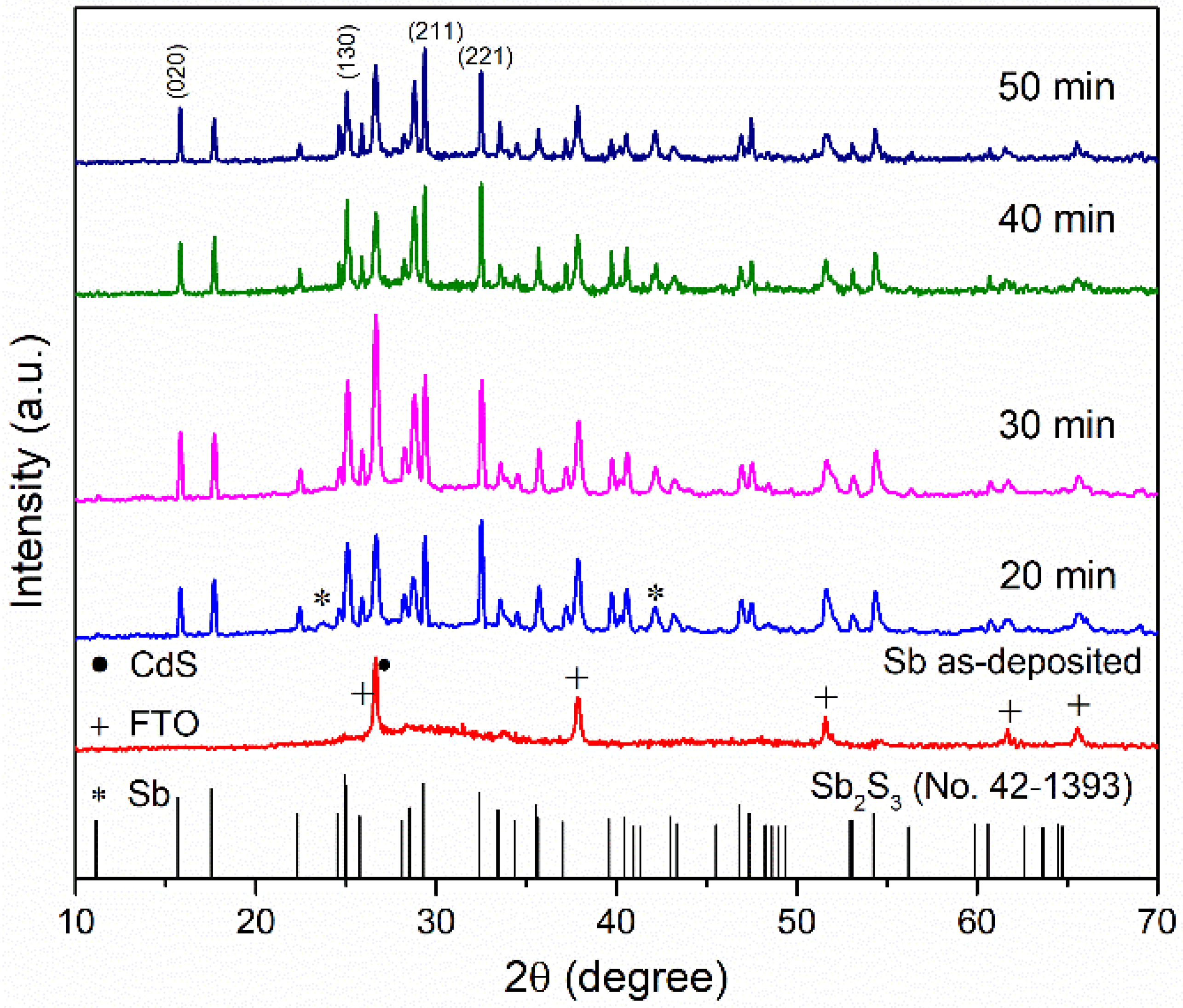
3.1. Structural Analysis
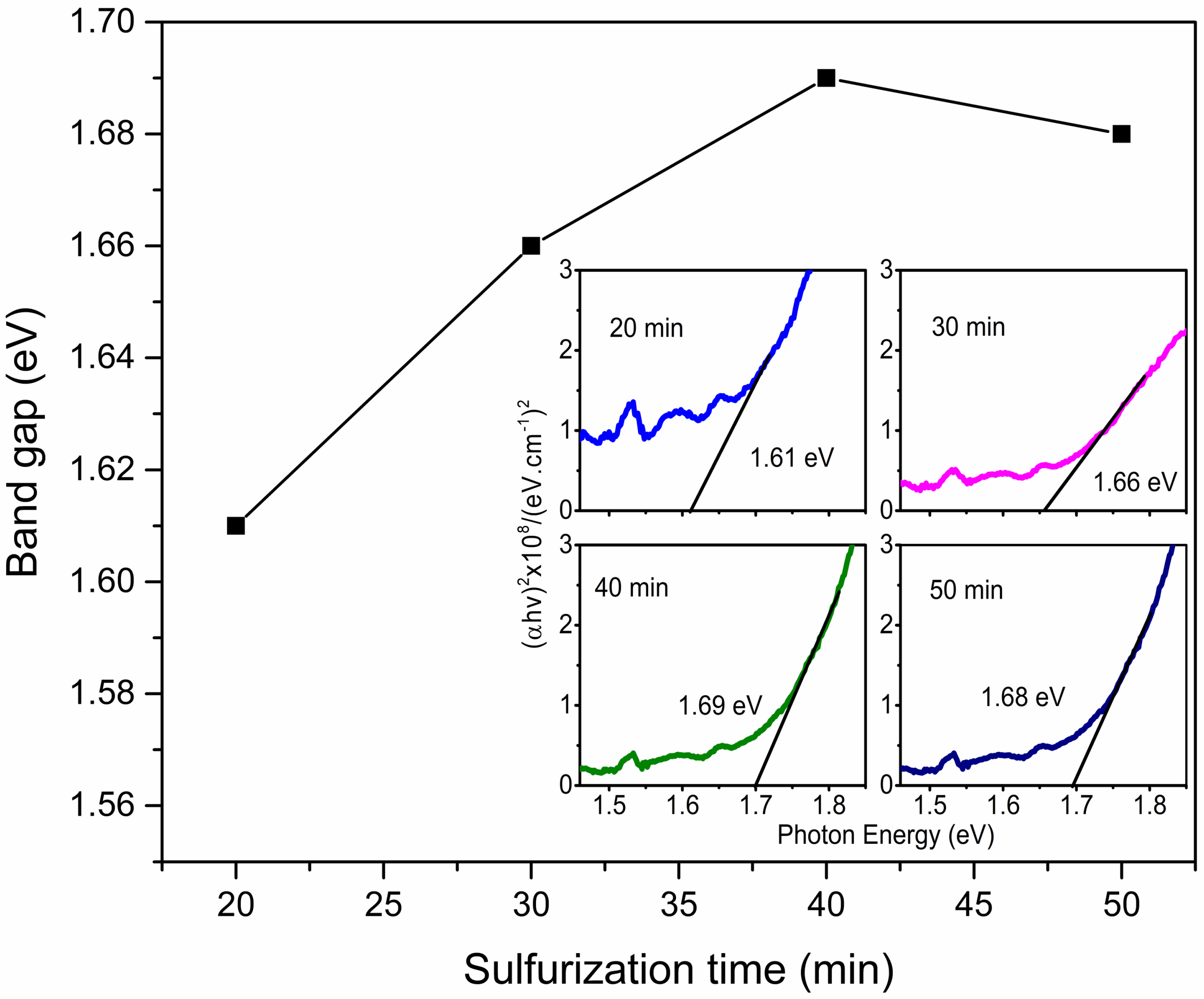
3.2. Optical Analysis
3.3. Morphological Analysis
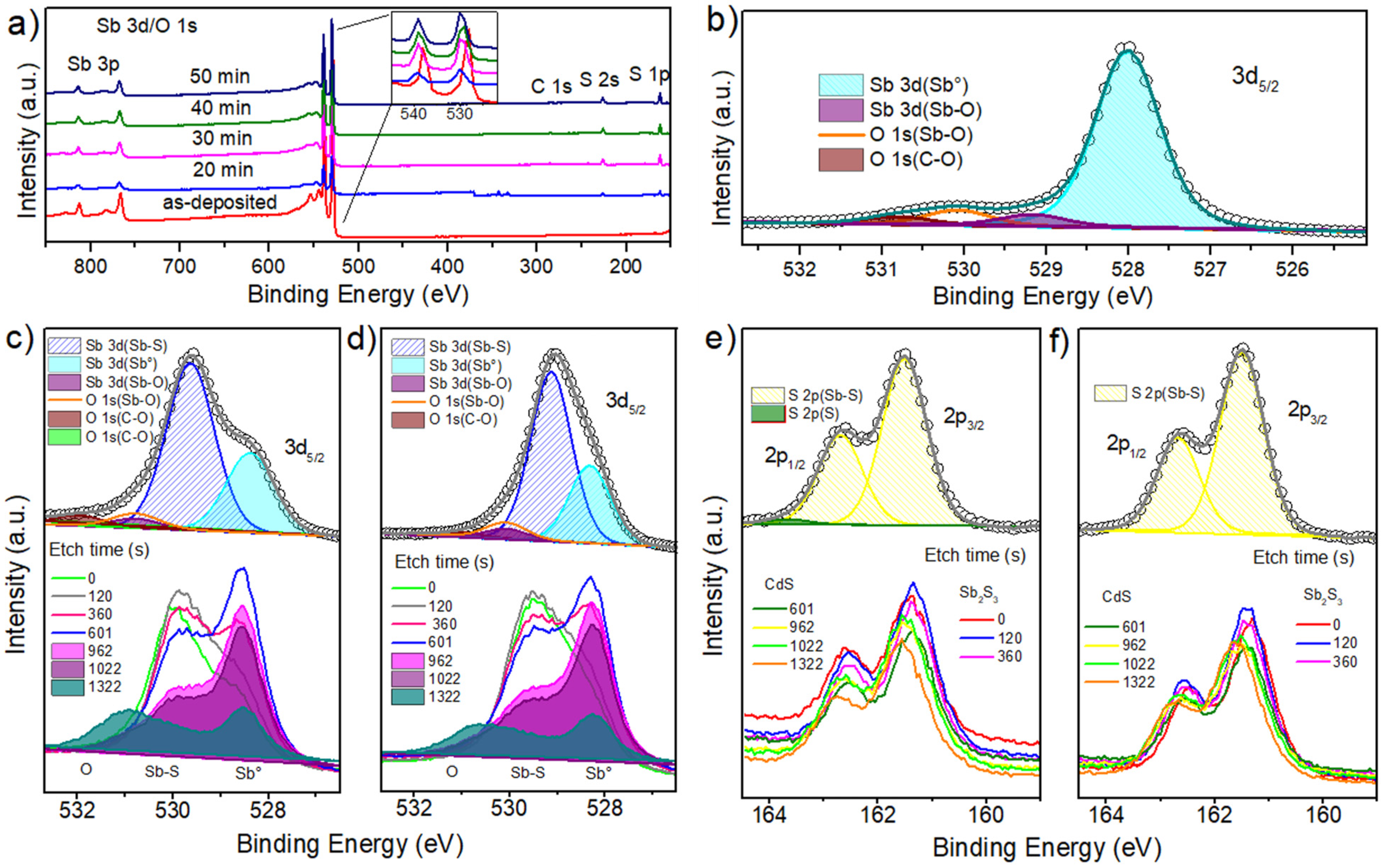
3.4. Chemical Analysis
3.5. Photovoltaic Effect Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Versavel, M.Y.; Haber, J.A. Structural and Optical Properties of Amorphous and Crystalline Antimony Sulfide Thin Films. Thin Solid Films 2007, 515, 7171–7176. [Google Scholar] [CrossRef]
- Moon, D.G.; Rehan, S.; Yeon, S.H.; Lee, S.M.; Park, S.J.; Ahn, S.; Cho, Y.S. A review on binary metal sulfide heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2019, 200, 109963. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, C.; Tang, R.; Jiang, C.; Jiang, G.; Liu, W.; Chen, T.; Zhu, C. Composition Engineering of Sb2S3 Film Enabling High Performance Solar Cells. Sci. Bull. 2019, 64, 136–141. [Google Scholar] [CrossRef]
- Abbas, M.; Zeng, L.; Guo, F.; Rauf, M.; Yuan, X.C.; Cai, B. A Critical Review on Crystal Growth Techniques for Scalable Deposition of Photovoltaic Perovskite Thin Films. Materials 2020, 13, 4851. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.C.; Lee, D.U.; Noh, J.H.; Kim, E.K.; Seok, S.I. Highly Improved Sb2S3 Sensitized-Inorganic–Organic Heterojunction Solar Cells and Quantification of Traps by Deep-Level Transient Spectroscopy. Adv. Funct. Mater. 2014, 24, 3587–3592. [Google Scholar] [CrossRef]
- Chalapathi, U.; Poornaprakash, B.; Ahn, C.H.; Park, S.H. Rapid Growth of Sb2S3 Thin Films by Chemical Bath Deposition with Ethylenediamine Tetraacetic Acid Additive. Appl. Surf. Sci. 2018, 451, 272–279. [Google Scholar] [CrossRef]
- Luo, J.; Xiong, W.; Liang, G.; Liu, Y.; Yang, H.; Zheng, Z.; Zhang, X.; Fan, P.; Chen, S. Fabrication of Sb2S3 Thin Films by Magnetron Sputtering and Postsulfurization/Selenization for Substrate Structured Solar Cells. Alloys Compd. 2022, 826, 154235. [Google Scholar] [CrossRef]
- Yuan, S.; Deng, H.; Yang, X.; Hu, C.; Khan, J.; Ye, W.; Tang, J.; Song, H. Postsurface Selenization for High Performance Sb2S3 Planar Thin FIlms Solar Cells. ACS Photonics 2017, 4, 2862–2870. [Google Scholar] [CrossRef]
- Lian, W.; Jiang, C.; Yin, Y.; Tang, R.; Li, G.; Zhang, L.; Che, B.; Chen, T. Revealing composition and structure dependent deep-Level Defect in Antimony Trisulide Photovoltaics. Nat. Commun. 2021, 12, 3260. [Google Scholar] [CrossRef]
- Gaoa, C.; Huangb, J.; Lia, H.; Sunb, K.; Laia, Y.; Jiaa, M. Fabrication of Sb2S3 Thin Films by Sputtering and Post-annealing for Solar Cells. Ceram. Int. 2019, 45, 3044–3051. [Google Scholar] [CrossRef]
- Kun, W.; Cheng, J.; Yang, X.; Hu, R.; Fu, L.; Huang, J.; Yu, J.; Li, L. Enhanced Photovoltaic Properties in Sb2S3 Planar Heterojunction Solar Cell with a Fast Selenylation Approach. Nano. Res. Lett. 2018, 13, 270. [Google Scholar] [CrossRef]
- Zhang, L.; Zhuang, D.; Zhao, M.; Gong, Q.; Guo, L.; Ouyang, L.; Sun, R.; Wei, Y.; Lyu, X.; Peng, X. “ Sb2S3 Thin Films Prepared by Vulcanizing Evaporated Metallic Precursors. Mater. Lett. 2017, 208, 58–61. [Google Scholar] [CrossRef]
- Lei, H.; Lin, T.; Wang, X.; Cheng, Q.; Chen, X.; Tan, Z.; Chen, V. A Novel in Situ Hydrothermal Preparation Route for Sb2S3 and its Solar Cell Application. Mater. Lett. 2018, 233, 90–93. [Google Scholar] [CrossRef]
- Zhang, J.; Ou, Y.; Yang, J.; Xu, B.; Li, S.L.; Yang, B.; Ma, W.; Cao, C. Fabrication of Sb2S3 Thin Films by Low-Temperature Plasma-Sulfurizing Metallic Sb Layers. Appl. Energy Mater. 2021, 4, 13860–13867. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, H.; Yu, V.; Xu, V.; Tao, V.; Yang, P.; Sun, V.; Chu, J. Effect of Sulfurization Temperature of Solution-Processed Cu2SnS3 Absorber for Low Cost Photovoltaic Cells. Mater. Lett. 2018, 228, 447–449. [Google Scholar] [CrossRef]
- García-Llamas, E.; Merino, J.; Gunder, V.; Neldner, K.; Greiner, D.; Steigert, A.; Giraldo, S.; Izquierdo-Roca, V.; Saucedo, E.; León, M.; et al. Cu2ZnSnS4 Thin Film Solar Cells Grown by Fast Thermal Evaporation and Thermal Treatment. Sol. Energy 2017, 141, 236–241. [Google Scholar] [CrossRef]
- Agawane, G.L.; Shin, S.W.; Vanalakar, S.A.; Suryawanshi, M.P.; Moholkar, A.V.; Yun, J.H.; Gwak, J.; Kim, J.H. Synthesis of Simple, Low Cost and Benign Sol–Gel Cu2ZnSnS4 Thin Films: Influence of Different Annealing Atmospheres. J. Mater. Sci. Mater. Electron. 2015, 26, 1900–1907. [Google Scholar]
- Abusnina, M.; Moutinho, H.; Al-Jassim, M.; DeHart, C.; Martin, M. Fabrication and Characterization of CZTS Thin Films Prepared by the Sulfurization of RF-sputtered Stacked Metal Precursor. J. Electron. Mater. 2014, 43, 3145–3154. [Google Scholar] [CrossRef]
- Henry, J.; Mohanraj, K.; Sivakumar, G. Effect of Annealing Time on the Optical Properties of AZTSe Thin Films. Mater. Lett. 2017, 201, 105–108. [Google Scholar] [CrossRef]
- Almalki, R.; Mkawi, E.; Al-Hadeethi, Y. Fabricating Antimony Sulfde Sb2S3 Microbars Using Solvothermal Synthesis: Efect of the Solvents Used on the Optical, Structural, and Morphological Properties. J. Mater. Sci. Mater. Electron. 2020, 31, 9203–9211. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Olatunde, O.C.; Mathur, S. Structural Studies and Morphological Properties of Antimony Sulphide Nanotowar. Phys. B Condens. Matter 2021, 605, 412691. [Google Scholar] [CrossRef]
- Martínez-Alonso, C.; Soltelo-Lerma, M.; Sato-Berrú, R.Y.; Mayén-Hernández, S.; Hu, H. Purity and Crystallinity of Microwave Synthesized Antimony Sulfide Microrods. Mater. Chem. Phys. 2017, 186, 390–398. [Google Scholar] [CrossRef]
- Luo, Y.D.; Tang, R.; Chen, S.; Hu, J.G.; Liu, Y.K.; Li, Y.F.; Liu, X.S.; Zheng, Z.H.; Su, Z.H.; Ma, X.F.; et al. An Effective Combination Reaction Involved with Sputtered and Selenized Sb Precursor of Efficient Sb2S3 Thin Film Solar Cells. Chem. Eng. J. 2020, 393, 124599. [Google Scholar] [CrossRef]
- Escorcia-García, J.; Becerra, D.; Nair, M.; Nair, P. Heterojunction CdS/Sb2S3 Solar Cells Using Antimony Sulfide Thin Films Prepared by Thermal Evaporation. Thin Solid Films 2014, 569, 28–34. [Google Scholar] [CrossRef]
- Chen, S.; Zhuanghao, Z.; Gathelinaud, M.; Ma, H.; Qiao, Z.; Fan, P.; Liang, G.; Fan, X.; Zhang, X. Magnetron Sputtered Sb2Se3-based Thin Films Towards High Performance Quasi-homojunction Thin Film Solar Cells. Sol. Energy Mater. Sol. Cells 2019, 203, 10154. [Google Scholar] [CrossRef]
- Lan, C.; Lan, H.; Peng, H.; Su, Z.; Zhang, D.; Sun, H.; Luo, J.; Fan, P. Microstructural and Optical Properties of Sb2S3 Film Thermally Evaporated from Antimony Pentasulfide and Efficient Planar Solar Cells. Rapid Res. Lett. 2018, 12, 1800025. [Google Scholar] [CrossRef]
- Sotelo Marquina, R.; Sanchez, T.; Mathews, N.; Mathew, X. Vacuum Coated Sb2S3 Thin Films: Thermal Treatment and the Evolution of its Physical Properties. Mater. Res. Bull. 2017, 90, 285–294. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, B.; Li, S.; Zhang, Q.; Buettner, M.; Li, L.; Qlan, X.; Yan, F. Scalable and Efficient Sb2S3 Thin-Film Solar Cells Fabricated by Close Space Sublimation. APL Mater. 2019, 7, 041105. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bombea, K. Handbook of X-ray Photoelectron Spectroscopy; Pekin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Bodenes, L.; Darwiche, A.; Monconduit, L.; Martínez, H. The Solid Electrolyte Interphase a Key Parameter of the High Performance of Sb in Sodium-ion Batteries: Comparative X-ray Photoelectron Spectroscopy Study of Sb/Na-ion and Sb/Li-ion Batteries. J. Power Sources 2015, 273, 14–24. [Google Scholar] [CrossRef]
- Zakaznova-Herzong, V.; Harmer, S.; Nesbitt, H.; Bancroft, G.; Flemming, R.; Pratt, A. High Resolution XPS Study of the Large Band-gap Semiconductor Stibnite (Sb2S3): Structural Contributions and Surface Reconstruction. Surf. Sci. 2006, 600, 348–356. [Google Scholar] [CrossRef]
- Deng, M.; Li, S.; Hong, W.; Jiang, Y.; Xu, W.; Shuai, H.; Li, H.; Wang, W.; Hou, H.; Ji, X. Natural Stibnite Core (Sb2S3) Embedded in Sulfur-Doped Carbon Sheets: Enhanced Electrochemical Properties as Anode for Sodium Ions Storage. R. Soc. Chem. 2019, 9, 15210–15216. [Google Scholar]
- Wang, C.; Lu, S.; Li, S.; Wang, S.; Lin, X.; Zhang, J.; Kondrotas, R.; Li, K.; Chen, C.; Tang, J. Efficiency improvement of flexible Sb2Se3 solar cells with non-toxic buffer layer via interface engineering. Nano Energy 2020, 71, 104577. [Google Scholar] [CrossRef]
- Muthusamy, T.; Archana, B.; Su, C.-Y.; Tseng, C.-J.; Bhattacharyya, A. Planar Heterojunction Solar Cell Employing a Single-Source Precursor Solution-Processed Sb2S3 Thin Film as the Light Absorber. ACS Omega 2019, 4, 11380–11387. [Google Scholar] [CrossRef]
- Vinayakumar, V.; Obregon Hernandez, C.; Shaji, S.; Avellaneda, D.; Aguilar Martinez, J.; Krishnan, B. Effects of Rapid Thermal Processing on Chemically Deposited Antimony Sulfide Thin Films. Mater. Sci. Semicond. Process. 2018, 80, 9–17. [Google Scholar] [CrossRef]
- Whittles, T.J.; Veal, T.D.; Savory, C.N.; Welch, A.W.; de Souza Lucas, F.W.; Gibbon, J.T.; Birkett, M.; Potter, R.J.; Scanlon, D.O.; Zakutayev, A.; et al. Core Levels, Band Alignments, and Valence-Band States in CuSbS2 for Solar Cell Applications. ACS Appl. Mater. Interfaces 2017, 9, 41916–41926. [Google Scholar] [CrossRef]
- Thompson, M.T. Chapter 3—Review of Diode Physics and the Ideal (and Later, Nonideal) Diode. In Intuitive Analog Circuit Design; Newnes: Boston, MA, USA, 2014; pp. 53–86. [Google Scholar]
- Gibson, P.; Baker, M.; Ozsan, M. Investigation of Sulphur Diffusion at the CdS/CdTe Interface of Thin-film Solar Cells. Surf. Interface Anal. 2002, 33, 825–829. [Google Scholar] [CrossRef]
- Taylor, A.; Major, J.; Kartopu, G.; Lamb, D.; Duenow, J.; Dhere, R.; Maeder, X.; Irvine, S.; Durose, K.; Mendis, B. A Comparative Study of Microstructural Stability and Sulphur Diffusion in CdS/CdTe Photovoltaic Device. Sol. Energy Mater. Sol. Cells 2015, 141, 341–349. [Google Scholar] [CrossRef]
- Yuan, H.; Deng, D.; Dong, X.; Yang, K.; Qiao, C.; Hu, H.; Song, H.; Song, H.; He, Z.; Tang, J. Efficient Planar Antimony Sulfide Thin Film Photovoltaics with Large Grain and Preferential Growth. Sol. Energy Mater. Sol. Cells 2016, 157, 887–893. [Google Scholar] [CrossRef]
- Caballero, R.; Cano-Torres, J.M.; García-Llamas, E.; Fontané, J.M.; Pérez-Rodríguez, A.; Greiner, D.; Kaufman, C.A.; Merino, J.M.; Victorov, I.; Baraldi, G.; et al. Towards the growth of Cu2ZnSn1−xGexS4 thin films by a single-stage process: Effect of substrate temperature and composition. Sol. Energy Mater. Sol. Cells 2015, 139, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Liu, W.; Yang, S.; Zhu, C.; Chen, T. A Fast Chemical Approach Towards Sb2S3 Film with a Large Grain Size for High Performance Planar Heterojunction Solar Cells. Nanoscale 2017, 9, 3386–3390. [Google Scholar] [CrossRef]
- Jiang, C.; Tang, R.; Wang, X.; Ju, H.; Chen, G.; Chen, T. Alkali Metals Doping for High-Performance Planar Heterojunction Sb2S3 Solar Cells. Solar RRL 2018, 3, 180022. [Google Scholar] [CrossRef]
- Zimmermann, E.; Pfadler, T.; Kalb, J.; Dorman, J.A.; Sommer, D.; Hahn, G.; Weickert, J.; Schmidt-Mende, L. Toward High-Efficiency Solution-Processed Planar Heterojunction Sb2S3 Solar Cells. Adv. Sci. 2015, 5, 1500059. [Google Scholar] [CrossRef] [PubMed]


| Sulfurization Time (min) | Crystallite Size (Å) |
|---|---|
| 20 | 7.51 |
| 30 | 7.58 |
| 40 | 15.25 |
| 50 | 14.78 |
| Sulfurization Time (min) | Elements | ||
|---|---|---|---|
| Sb (Atomic %) | S (Atomic %) | S/Sb | |
| 20 | 47.91 | 52.91 | 1.10 |
| 30 | 42.62 | 57.38 | 1.34 |
| 40 | 41.16 | 58.84 | 1.42 |
| 50 | 45.34 | 54.66 | 1.20 |
| Sulfurization Time (min) | Sb2S3 | Sb2O3 | Sb0 | Other Elements | |||||
|---|---|---|---|---|---|---|---|---|---|
| Sb 3d5/2 | S 2p3/2 | S 2p1/2 | Sb 3d5/2 | O1s | Sb 3d5/2 | O1s | O1s | S 2p3/2 | |
| As-deposited | --- | --- | --- | 529.16 | 530.07 | 528.01 | 530.80 | --- | --- |
| 20 | 529.54 | 161.49 | 162.67 | 530.79 | 530.78 | 528.39 | 531.97 | 533.21 | 163.64 |
| 30 | 529.51 | 161.62 | 162.80 | 530.94 | 530.91 | 528.48 | 532.11 | --- | --- |
| 40 | 529.38 | 161.48 | 162.66 | 530.60 | 530.63 | 528.33 | 531.85 | --- | --- |
| 50 | 529.44 | 161.50 | 162.68 | 530.81 | 530.80 | 528.40 | 531.97 | --- | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uc-Canché, S.; Camacho-Espinosa, E.; Mis-Fernández, R.; Loeza-Poot, M.; Ceh-Cih, F.; Peña, J.L. Influence of Sulfurization Time on Sb2S3 Synthesis Using a New Graphite Box Design. Materials 2024, 17, 1656. https://doi.org/10.3390/ma17071656
Uc-Canché S, Camacho-Espinosa E, Mis-Fernández R, Loeza-Poot M, Ceh-Cih F, Peña JL. Influence of Sulfurization Time on Sb2S3 Synthesis Using a New Graphite Box Design. Materials. 2024; 17(7):1656. https://doi.org/10.3390/ma17071656
Chicago/Turabian StyleUc-Canché, Sheyda, Eduardo Camacho-Espinosa, Ricardo Mis-Fernández, Mariely Loeza-Poot, Francisco Ceh-Cih, and Juan Luis Peña. 2024. "Influence of Sulfurization Time on Sb2S3 Synthesis Using a New Graphite Box Design" Materials 17, no. 7: 1656. https://doi.org/10.3390/ma17071656
APA StyleUc-Canché, S., Camacho-Espinosa, E., Mis-Fernández, R., Loeza-Poot, M., Ceh-Cih, F., & Peña, J. L. (2024). Influence of Sulfurization Time on Sb2S3 Synthesis Using a New Graphite Box Design. Materials, 17(7), 1656. https://doi.org/10.3390/ma17071656





