3.1. Friction Reduction after UV Treatment
A macroscopic tribology experiment was conducted on UMT5 and demonstrated the friction reduction achieved by UV treatment. The friction pairs chosen were the Si
3N
4 ball and TiO
2 plate. Si
3N
4, with its high hardness, excellent wear resistance, corrosion resistance, and oxidation resistance, is an excellent material as a friction pair and is well suited for the study of the tribological properties of TiO
2. As shown in
Figure 1b, the friction coefficient stabilized at about 0.035 after 300 s of running the sulfuric acid solution on the TiO
2 surface. After washing off the acid solution and blowing it dry, 30 μL of PAO4 base oil was added, and the experiment was conducted with a load of 3 N and a speed of 56.5 mm/s. The coefficient of friction stabilized at 0.034 and could be maintained for a long time (at least 4 h). If the TiO
2 surface was illuminated by UV, the coefficient of friction stabilized at 0.016 and also maintained for a long time. The friction coefficient of the PAO4 oil-lubricated Si
3N
4-TiO
2 friction system was reduced by 52.9% when treated with UV.
As shown in
Figure 2a, when the experimental speed was set to 56.5 mm/s and the load was changed, on the TiO
2 without UV treatment, the friction coefficient first decreased and then increased and reached the lowest when the load was 3 N. In the UV-treated group, the friction coefficient tended to decrease compared with the one without UV treatment and reached the lowest at 2 N. In
Figure 2b, the load was set to 3 N and the speed during the experiment was changed. On the TiO
2 without UV treatment, the overall friction coefficient was in the range of 0.01–0.015 as the speed became greater than 130 mm/s, while in the UV-treated group, the friction coefficient tended to be lower and even reached a superlubricity state (0.0062 at a speed of 377.0 mm/s). At speeds less than 130 mm/s, although the friction system did not show a transition from non-superlubricity to superlubricity after the UV treatment, a reducing trend could still be observed. At lower speeds of 22 mm/s and 31 mm/s, the coefficient of friction decreased from 0.0545 and 0.0473 to 0.0226 and 0.0196, respectively, with a reduction of 58.6% in both cases.
Since the viscosity of the lubricant is relatively high, it is easy to form a fluid lubrication film during the friction process and reduce the friction coefficient between systems, and for different friction systems, different viscosity lubricants can be used for lubrication. Therefore, it is important to investigate the effect of UV treatment on the regulation of PAO base oil with different viscosities.
Figure 2c–f shows the change in friction coefficient after UV treatment for four PAO base oils with different viscosities when lubricating the Si
3N
4-TiO
2 friction system (all were first run with an acid solution on the friction pairs). The results showed that there was an obvious reduction of friction coefficient after UV treatment in PAO base oil solutions of different viscosities. When PAO2 base oil (10.1 mPa·s) was used, at higher speeds (>150 mm/s), the system went from non-superlubricity to superlubricity after UV treatment and achieved a lower friction coefficient (0.004). But, at lower speed intervals (<70 mm/s) the friction coefficients of the UV-treated and non-UV-treated groups were similar and both were at a high level (0.07–0.08). When lubricated with PAO6 base oil (49.9 mPa·s), in the speed range of 30–250 mm/s, the friction system could achieve the transition from non-superlubricity to superlubricity. The reduction was also obvious at higher speeds, with a range of 30%, and at lower speeds, the friction coefficient reduced more, which was reduced by 61.4% from 0.0335 to 0.0129 at a speed of 22 mm/s. The coefficient of friction was also reduced after UV treatment when lubricated by PAO8 base oil (87.5 mPa·s) and PAO10 base oil (108.6 mPa·s), but the percentage of reduction was relatively low. The friction coefficient on TiO
2 with and without UV treatment both remained in the range of 0.01–0.02 in the tested speed interval.
3.2. Lubrication States When Reducing Friction
The classical Stribeck curve points out that as the bearing characteristic number increases, the friction system transitions from boundary lubrication to mixed lubrication and finally enters the stage of elastohydrodynamic lubrication, and the friction coefficient shows a trend of first decreasing and then slowly increasing. Since the factors and mechanisms affecting the friction system vary under different lubrication states, it is important to determine the lubrication state when lubricated with different base oils to reveal the mechanism of friction coefficient reduction after UV treatment. As shown in
Figure 3a, the film thicknesses of different PAO base oils in the Si
3N
4-TiO
2 friction system at different speeds were calculated according to Hamrock–Dowson (H-D) equations [
37] as follows:
where
,
,
, and
.
hc is the film thickness,
α is the pressure–viscosity coefficient of the oil,
u is the average linear speed of the TiO
2 plate and Si
3N
4 ball,
W is the load,
k is a coefficient, and
E′ is the reduced Young’s modulus of the two contacting solids,
where
E1 and
E2 are Young’s modulus of Si
3N
4 and TiO
2, respectively.
μ1 and
μ2 are Poisson’s ratios of Si
3N
4 and TiO
2, respectively, and
R is the equivalent radius of the ball that could be described by the Hertz contact theory [
38]:
where
D is the diameter of the worn scar of the ball. The percentage reduction of friction coefficient after UV treatment at different speeds when lubricated with different PAO base oils was calculated and is shown in
Figure 3b–f.
The results show that the reduction rate of the friction coefficient after UV treatment is closely related to the lubricant film thickness. For different PAO base oils, the median of the friction coefficient reduction was selected, and the film thickness of the lubricant when the reduction rate of the friction coefficient is larger than the median was calculated. As shown in
Table 3, the median reduction rate of the friction coefficient after UV treatment for different PAO base oil lubricants and the interval of film thickness corresponding to the reduction rate larger than the median are calculated, respectively.
The results show that the high reduction in the friction coefficient mainly corresponds to the same film thickness interval, basically between 30 nm and 100 nm, despite the different viscosities and speeds. The lubrication state could be distinguished by the ratio of the theoretical film thickness to the equivalent surface roughness (Rq), and the ratio
λ could be calculated by the following formula:
where
σ1 and
σ2 are the surface roughness, i.e., the root mean square average of the profile heights over the evaluation length (Rq) of the worn regions of the Si
3N
4 balls and TiO
2 plates, respectively. The system was in the elastohydrodynamic lubrication state if the ratio
λ exceeded three, mixed lubrication state if the ratio
λ was between one and three, and boundary lubrication state if the ratio
λ was less than one. According to the characterization of the upper and lower friction surfaces after the experiment, it was found that the Rq of the upper friction Si
3N
4 was 3.5 nm, the Rq of the lower friction TiO
2 plate was 29.5 nm, and the equivalent roughness
σ* was 29.7 nm; thus, the ratio λ value was 1.01 to 3.37, which was consistent with the
λ value range 1–3 corresponding to the mixed lubrication condition. This shows that in the PAO base oil lubrication with different viscosities, the friction coefficient decreases most significantly when the system is in a mixed lubrication condition.
When the system is in a mixed lubrication state, UV treatment shows the most obvious effect. The mixed lubrication state includes elastohydrodynamic lubrication and boundary lubrication, and according to the research conducted by Han et al. [
35], when using water-based lubricants, such as glycerol solution, UV light modulates the friction and lubrication of the system mainly by interfacial adsorption of molecular films; in other words, the part of boundary lubrication is regulated, while elastohydrodynamic lubrication is not much influenced. However, compared to water-based lubricants, oil-based lubricants have higher viscous pressure coefficients (18 GPa for PAO4 and 6 GPa for propylene glycol), i.e., the state of the lubricant is more influenced by the experimental conditions during oil lubrication. In
Figure 2a, the load affects the friction coefficient significantly greater than UV light does. Therefore, it is necessary to discuss the effect of the elastohydrodynamic lubrication part in the study of UV light on the lubrication of PAO base oil.
Since the surface of Si
3N
4-TiO
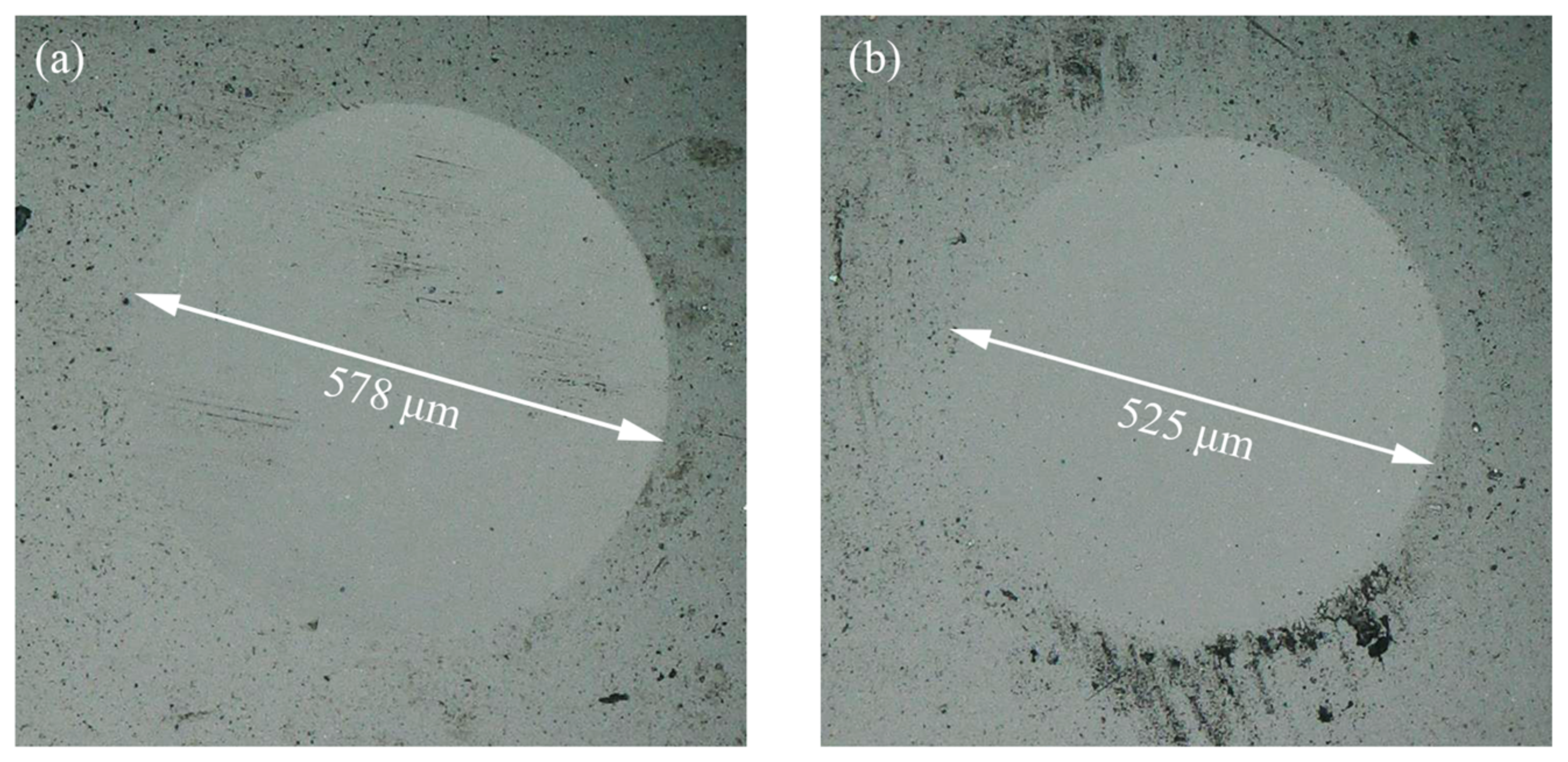
2 friction pairs were treated with an aqueous solution of acid before oil lubrication, the actual contact area was much larger than the calculated value of the point–surface contact model, so the contact pressure of the system could no longer be calculated by using the Hertz contact model. As shown in
Figure 4, the diameter of wear scars on the Si
3N
4 ball surface were therefore used to calculate the actual contact pressure. After the experiments, the diameters of the wear scars were 578.5 μm without UV treatment and 525.2 μm with UV treatment, which corresponded to a calculated actual contact pressure of 11.4 MPa and 13.8 MPa, respectively.
In the case of fluid lubrication, the friction coefficient
μ of the system can be calculated using the following equation
where
A is the contact area between the upper and lower friction pairs during lubrication,
W is the load in the experiment (3 N),
τ is the shear pressure of the lubricating fluid between the friction pairs, and
A and
τ can be calculated by the following equation:
where
η0 is the viscosity of the lubricant,
α is the pressure–viscosity coefficient of the lubricant,
p is the average contact pressure,
u is the average linear velocity (i.e., the velocity in the experiment, which is taken as 56.5 mm/s in this calculation),
hc is the calculated film thickness,
R is the equivalent contact radius, and
E* is the equivalent elastic modulus of the two friction pairs.
According to the above equation, the coefficient of friction μ1 is calculated as 0.0047 for the experiment without UV treatment and 0.0037 for that with UV treatment, while the actual friction coefficients are 0.034 and 0.016. The difference between the two is mainly because it is considered to be an overall elastohydrodynamic lubrication in the calculating process, while the actual experimental conditions had mixed lubrication in the system and there was a contribution of both elastohydrodynamic and boundary lubrication. However, it can be inferred from the calculation of the friction coefficient that from the point of view of the viscous pressure coefficient, the difference in pressure values observed in this experiment is not sufficient to bring about a significant reduction in the friction coefficient.
On the other hand, the study by Jinjin Li et al. [
39] pointed out that the running-in process not only makes the overall contact pressure of the system lower but also causes the upper and lower friction pairs to form a non-parallel surface, as shown in
Figure 5a. There exists a certain inclination angle (
θ) for the upper friction pair, and this inclination angle can help the system to form an elastohydrodynamic film during the elastohydrodynamic lubrication process, thus realizing the elastohydrodynamic effect. As shown in
Figure 5b,c, the wear scars on the Si
3N
4 balls were measured. It was observed that there was indeed a slope with a certain angle, as shown in
Figure 5d,e, and the corresponding inclination angles were 0.017° and 0.024° for θ
1 and θ
2, respectively.
The shear force and friction coefficient can be calculated using the Reynolds equation as follows [
40]:
where
x denotes the velocity direction,
y denotes the vertical paper direction (take
Figure 5a as an example), and
η is the viscosity of the lubricant. Since the role of the viscous pressure effect is negligible in this experiment, as discussed earlier, the value of
η0 is taken. Solving this equation yields the friction coefficient
μ [
39]:
where
L denotes the length of the contact surface in the x-direction and can be considered as the diameter of the wear scar
D.
m is the ratio of the film thickness at the entry
h1 to the film thickness at the exit
h2.
On this basis, it is calculated that the friction coefficient μ1 is 0.0012 for the friction pairs without UV treatment and μ2 is 0.0010 for that after UV treatment, which are also far from the actual friction coefficient values of 0.034 and 0.016. Therefore, the results of this calculation indicate that the slope angle formed during the running-in process cannot explain the significant decrease in the friction coefficient after UV treatment.
From the above analysis, it can be found that the elastohydrodynamic lubrication part of the mixed lubrication has less effect on the system friction coefficient, so the mechanism of UV light on friction lubrication must be further analyzed from the boundary lubrication part.
3.3. Mechanism of Friction Reduction: Photocatalysis
After the macroscopic experiments were completed, the friction coefficient of the wear scar on the surface of the TiO
2 was tested in a dry environment using an AFM and a silica ball probe. The Schematic diagram of the test is shown in
Figure 6a. After the UMT5 friction experiment using PAO4 as a lubricant, the TiO
2 surface was washed with petroleum ether, alcohol, and deionized water, and then the surface was blown dry and finally placed on the AFM bench for microscopic friction experiments.
Figure 6b demonstrates several results of the AFM tribology tests, where the gray and pink areas show the results of TiO
2 without and with UV treatment. The friction coefficient (slope of the data) was 0.223 and 0.405 for experiments, respectively, and this result is consistent with the trend of the macroscopic experiments in
Figure 1.
Figure 6c,d shows that the microstructures of the surface with and without UV treatment are very similar. Since the microscopic experiments were conducted in an air environment, i.e., the friction process involved direct contact between the silica spheres and the wear scar of the TiO
2 plate, the cause of the difference in friction force can only come from the surface of the TiO
2 wear scar. The only possible source of lubrication is the PAO4 oil during the UMT5 macroscopic friction experiment. Therefore, it is presumed that the phenomenon of the reduction in the friction coefficient after UV treatment in both macroscopic and microscopic experiments during oil lubrication comes from the enhanced adsorption of PAO base oil molecules on the surface of inorganic photosensitive materials occurring after UV treatment, forming a lubricating film with a good lubrication effect.
It is well known that PAO base oils mainly consist of hydrocarbons. However, as shown in
Figure 7a, the XPS spectra found that without UV treatment the C signal was dominated by C–C (284.8 eV) with 96.2% of the signal, and about 3.8% of the signal was from C–O (286.1 eV). However, this proportion changed considerably after UV treatment. As shown in
Figure 7b, although the C signal in C–C (284.5 eV) was still the most abundant, occupying 69.5%, the proportion of the C signal in C–O (285.7 eV) increased significantly to 19.3%, in addition to 11.2% of the C signal from C=O. The statistics were deconvoluted using the software OriginPro 2018C, via the Gaussian method.
The results of XPS demonstrated that the residual PAO molecules on the TiO
2 surface were no longer purely C–H and C–C structures, but rather C–O structures or even C=O bonds. The appearance of these structures is attributed to another important application of inorganic photosensitive materials: photocatalysis. When the light energy absorbed by inorganic photosensitive materials (such as TiO
2, ZnO, and WO
3) is greater than their threshold band gap energy, the electrons on their valence bands are excited, generating a large number of electron–hole pairs which are transferred to the surface of the materials and undergo redox reactions with the substances on the surface, playing a photocatalytic role, and this property is often utilized for the degradation of organic substances such as hydrocarbons and phenols [
20,
21,
41]. In the case of photocatalytic degradation of methane on the TiO
2 surface, for example, under the action of light field, electron–hole pairs with high activity are transferred to the TiO
2 surface. Photogenerated electrons reduce oxygen to produce ·O
2− or ·OOH and holes oxidize H
2O and CH
4 to produce ·OH and ·CH
3, and eventually these radicals further generate CH
3OOH and CH
3OH [
24,
25,
42].
In this experiment, the XPS spectra of
Figure 7 show that the PAO base oil molecules on the TiO
2 surface produce C–O and C=O structures internally, and this provides the possibility for the adsorption of PAO base oil molecules on the TiO
2 surface. It forms a hydrogen bond structure with Ti–OH on the TiO
2 surface which then adsorbs, making the friction force in the boundary lubrication part of the mixed lubrication much less than the direct shear resistance between the rough peaks, thus making the friction coefficient of the system significantly lower after the UV treatment.
To prove this conjecture,
Figure 8 tests the adsorption of PAO base oil molecules on the SiO
2 surface and the TiO
2 surface before and after UV treatment using a QCM. Since the viscosity of the liquid has a large effect on the value of the frequency change during the test, a PAO2 base oil with a lower viscosity was chosen for the experiments. A solution of PAO2 with a mass fraction of 20% was prepared using petroleum ether (viscosity of 0.981 mPa·s) as the solvent, with a viscosity value of 2.08 mPa·s.
In the experiment, petroleum ether, petroleum ether solution of PAO2, and petroleum ether flowed sequentially onto the chip of a TiO
2-coated quartz crystal. Triggered by an alternating current, the vibration of the chip and its attenuation was recorded and the resonance frequency was obtained. When different liquid flows over the chip, the substances adsorbed to the surface may affect the frequency [
43,
44]. In
Figure 8a, ∆f
1 in the purple stage represented the total adsorption of PAO2 base oil molecules on the chip surface, and here, ∆f
1 was −2302.9 Hz. On the TiO
2 surface without UV treatment, this value became −2645.6 Hz, while on the UV-treated TiO
2, this value became −4650.6 Hz, shown in ∆f
1 and ∆f
2 in
Figure 8b. In the case of the same test liquid, the difference due to the liquid itself was negligible. Then, the effect of UV treatment on the frequency change in the TiO
2 chip was caused by the change in adsorption of PAO2 molecules. Further evidence was provided in the second gray stage in
Figure 8a: ∆f
2 on the SiO
2 surface represented the interfacial adsorption of PAO2 base oil molecules on the SiO
2 chip, which was greater than the attraction of the petroleum ether solvent and therefore changed the measured frequency. This value reached −321.1 Hz on the SiO
2 surface, which was much smaller in absolute value than Δf
1, proving that the interfacial adsorption of PAO2 base oil molecules accounted for a small fraction on the SiO
2 surface, while on the TiO
2 surface without UV treatment, the Δf
3 value representing the interfacial adsorption was −959.8 Hz; this value was larger than that on the SiO
2 surface, indicating stronger interfacial adsorption on the TiO
2 surface. In the UV group, the corresponding value of Δf
4 was −1885.6 Hz, which was much larger than Δf
3, indicating stronger interfacial adsorption of PAO2 base oil molecules after UV treatment. This is consistent with the previous analysis that the adsorption of PAO base oil molecules on the UV-treated TiO
2 surface was stronger than that on the non-UV-treated surface, which is the essence of the reduced friction coefficient of the system after UV treatment.
To further verify the effect of photocatalysis on the modulation of the friction coefficient of the system, a set of photocatalytic group experiments was added to the original experiments in this study. The experimental procedure was as follows: the same acid solution was run on Si
3N
4 and TiO
2 friction pairs, and the surface was cleaned and blown dry after the running-in, followed by the addition of 30 μL of PAO4 base oil and then a 2 h UV treatment. Compared with the original experiment in
Section 3.1, the order of UV treatment and the addition of oil was switched.
Figure 9a shows the experimental results on TiO
2 with and without UV treatment and the photocatalytic group, where the load is 3 N. The results showed that the friction coefficients of the photocatalytic group and the UV-treated group were close to each other at speeds greater than 200 mm/s, and they both decreased compared to the non-UV-treated group. At speeds between 50 mm/s and 200 mm/s, the friction coefficients of the UV-treated group decreased significantly, and even the transition from non-superlubricity to superlubricity was achieved. This result is consistent with the previous analysis, i.e., the elastohydrodynamic part contributes little to the friction coefficient and the reduction of the friction coefficient in the mixed lubrication stage is greater. There were more hydroxyl, aldehyde, and carboxyl groups in the photocatalytic group; so, the adsorption ability on the surface is stronger and the reduction of the friction coefficient is more obvious.
Figure 9b shows the variation of the friction coefficient and the proportion of the C–O and C=O signal to the carbon XPS energy spectrum signal at the wear scar of the Si
3N
4-TiO
2 friction system under PAO4 lubrication after different UV treatment times. The results showed that the friction coefficient gradually decreased with the increase in the proportion of C in the C–O and C=O to the overall C signal, which was consistent with the previous speculation on the mechanism. With the increase in UV treatment time, more PAO base oil molecules were photocatalyzed, more C–O and C=O bonds were formed, more molecules could be adsorbed on the TiO
2 surface, and a better lubrication effect of the molecular adsorption film formed, which eventually led to the reduction in the friction coefficient.
After the above discussion and analysis, this study proposes the mechanism of friction reduction achieved by UV radiation on a TiO
2 surface, as shown in
Figure 10. In the area where the rough peaks of the Si
3N
4 sphere and TiO
2 plate are in direct contact with each other when no UV treatment is performed, the effective oil film cannot be formed between the rough peaks due to the pressure and the poor adsorption ability of PAO molecules on the surface so the rough peaks directly crash and shear, which makes the friction coefficient of the system relatively high. After the UV treatment, due to the photosensitivity of TiO
2, electron–hole pairs are generated on the surface and redox reactions occur with PAO molecules. Some C–O bonds in PAO molecules are replaced by hydroxyl, aldehyde, or carboxyl groups, which build hydrogen bonds or occupy oxygen vacancies with hydroxyl structures on the upper and lower friction pairs and are finally adsorbed on the surface. In the process of friction, the direct contact of rough peaks becomes the contact of rough peaks and the adsorbed molecular layer. This greatly reduces the friction force and finally reduces the friction coefficient of the system, achieving the purpose of regulating the friction coefficient by light field.