Effect of Zinc and Severe Plastic Deformation on Mechanical Properties of AZ61 Magnesium Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Microstructural Characterization
2.3. Mechanical Characterization
2.4. Corrosion Properties
3. Results
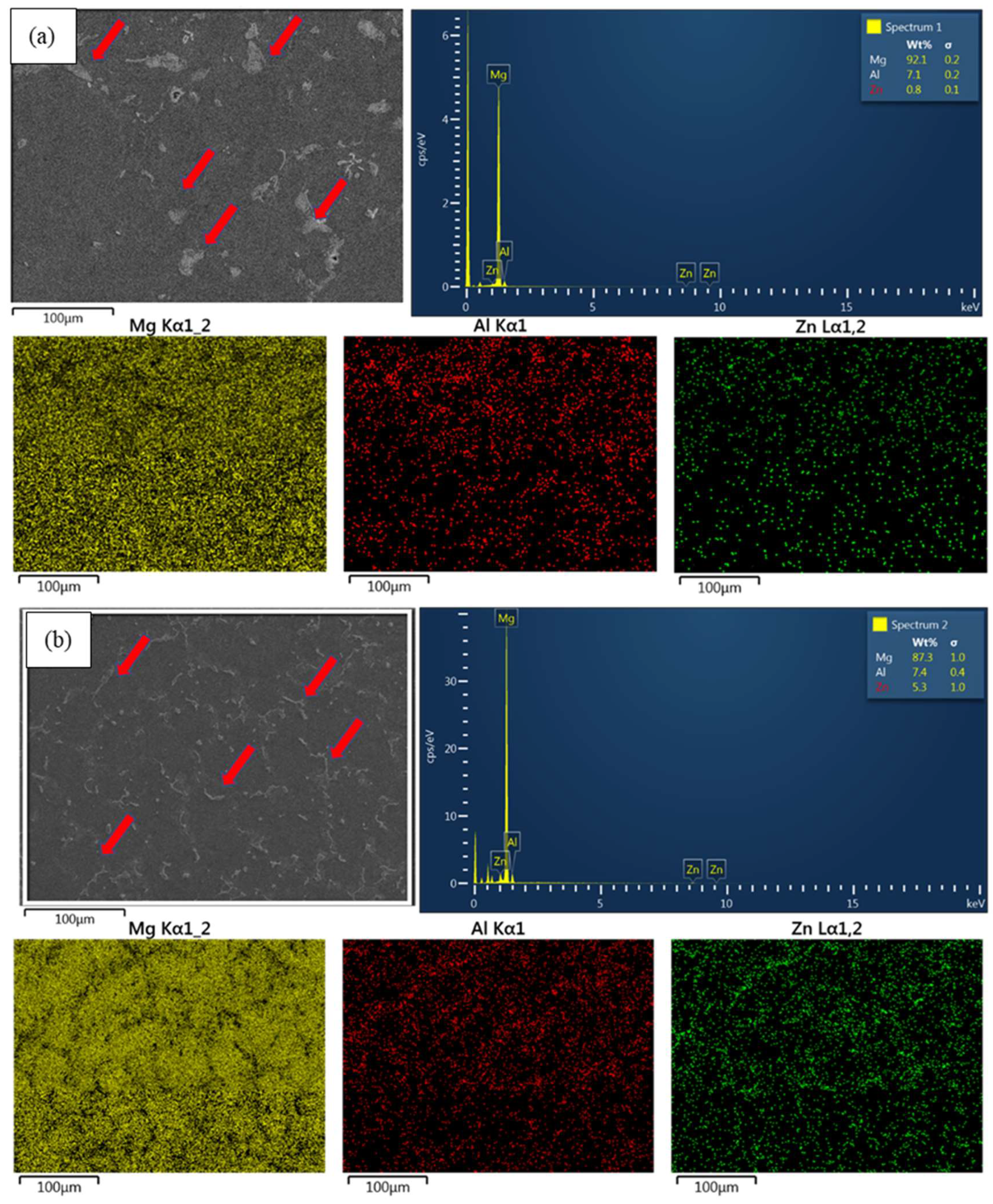
3.1. Phase Characterization
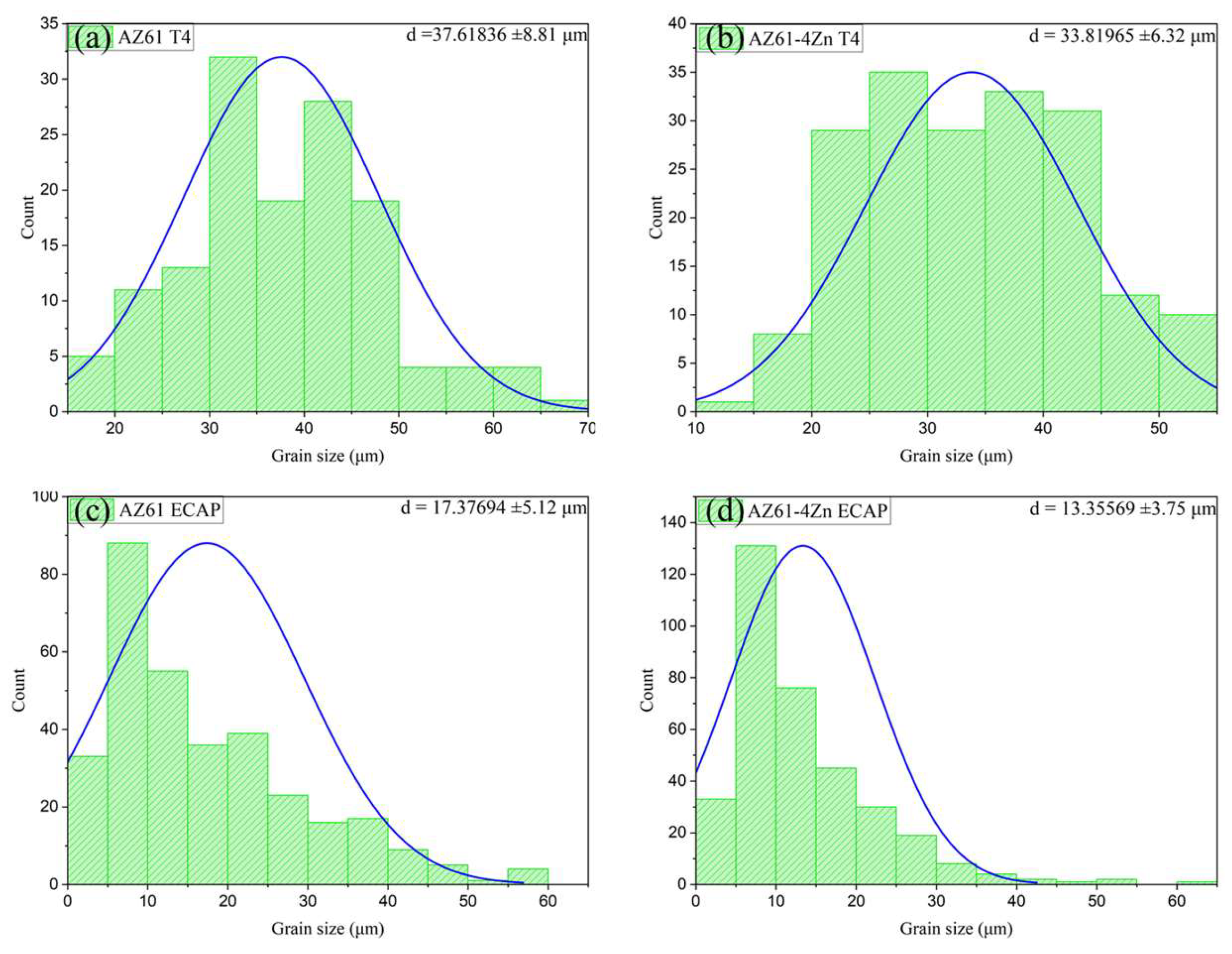
3.2. Microstructure Evaluation
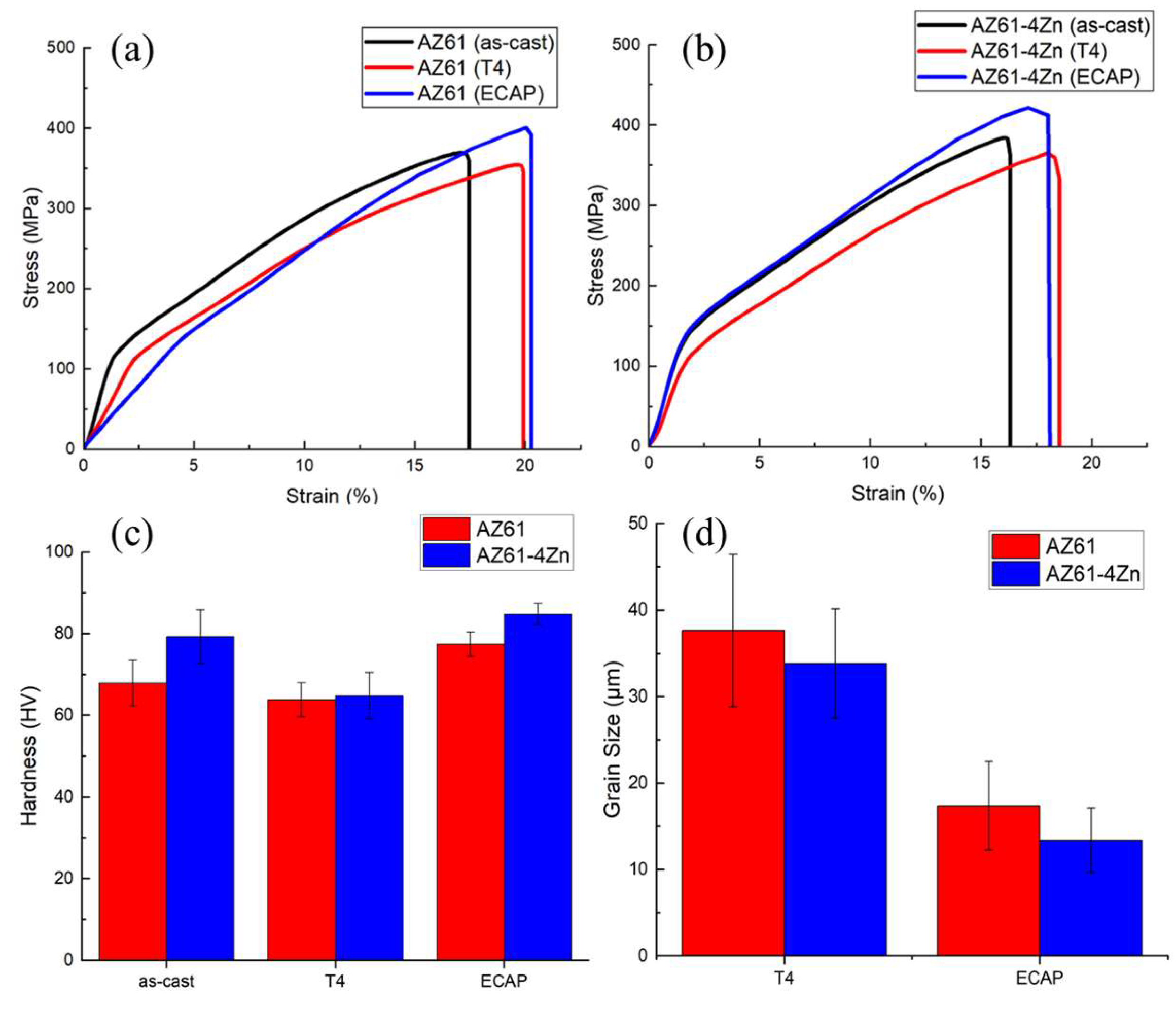
3.3. Mechanical Properties
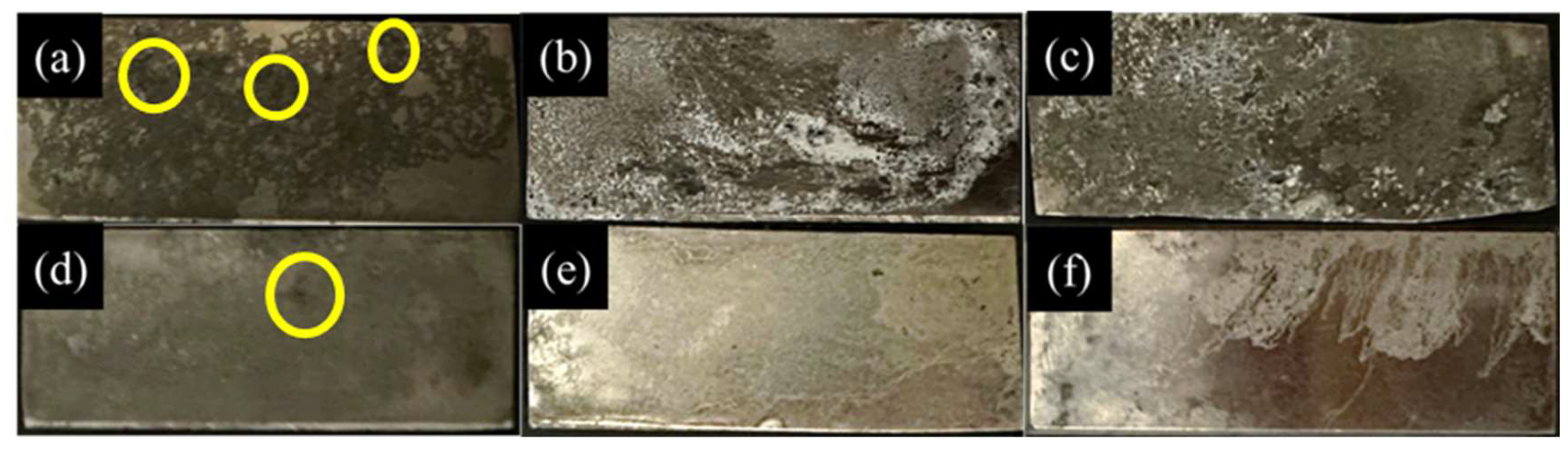
3.4. Corrosion Properties
4. Discussion
5. Conclusions
- (1)
- Microstructural analysis revealed that the addition of 4 wt.% Zn resulted in a significant refinement of grain size and promoted the uniform distribution of Mg-Zn phases and β-Mg17Al12 phases along grain boundaries. This refinement was further enhanced after ECAP processing, leading to a more pronounced grain refinement in AZ61 + 4 wt.% Zn. This refinement played a crucial role in the notable improvement of mechanical and corrosion properties.
- (2)
- The region with the most extensive Dynamic Recrystallization (DRX) grain refinement was observed in AZ61 + 4 wt.% Zn after ECAP processing, showcasing the highest yield strength (145.88 MPa), ultimate compression strength (421.79 MPa), and hardness (84.83 HV).
- (3)
- T4 heat treatment demonstrated a significant impact on the solid solution of Mg-Zn phases and β-Mg17Al12 phases in Mg-Al-Zn alloys. Especially in AZ61 + 4 wt.% Zn, the Mg-Zn phases are completely dissolved in the Mg alloy during the T4 heat treatment.
- (4)
- ECAP has been demonstrated as an effective method for enhancing the mechanical properties and corrosion resistance of magnesium alloys. The results indicate that ECAP has increased the mechanical properties of AZ61 + 4 wt.% Zn, showing improvements of 5.29% in YS and 9.72% in UCS. Furthermore, with regard to corrosion properties, ECAP significantly reduced the corrosion rate and promoted a more uniform corrosion surface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, G.; Lu, Y.; Jia, H.; Ding, Z.; Wu, L.; Shi, Y.; Wang, G.; Luo, Q.; Chen, Y.; Wang, J.; et al. Magnesium-Based Energy Materials: Progress, Challenges, and Perspectives. J. Magnes. Alloys 2023, 11, 3896–3925. [Google Scholar] [CrossRef]
- Balasubramani, N.; Wang, G.; Easton, M.A.; StJohn, D.H.; Dargusch, M.S. A Comparative Study of the Role of Solute, Potent Particles and Ultrasonic Treatment during Solidification of Pure Mg, Mg–Zn and Mg–Zr Alloys. J. Magnes. Alloys 2021, 9, 829–839. [Google Scholar] [CrossRef]
- Osipenko, M.A.; Kharytonau, D.S.; Kasach, A.A.; Ryl, J.; Adamiec, J.; Kurilo, I.I. Inhibitive Effect of Sodium Molybdate on Corrosion of AZ31 Magnesium Alloy in Chloride Solutions. Electrochim. Acta 2022, 414, 140175. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, F.; Wang, S.; Ji, S. The Development of Low-Temperature Heat-Treatable High-Pressure Die-Cast Al–Mg–Fe–Mn Alloys with Zn. J. Mater. Sci. 2021, 56, 11083–11097. [Google Scholar] [CrossRef]
- Kumar, D.; Phanden, R.K.; Thakur, L. A Review on Environment Friendly and Lightweight Magnesium-Based Metal Matrix Composites and Alloys. Mater. Today Proc. 2021, 38, 359–364. [Google Scholar] [CrossRef]
- Gneiger, S.; Papenberg, N.; Mitsche, S.; Fehlbier, M. Manufacturing and Processing of Sheets Using a Mg–Al–Ca–Zn–Y Alloy for Automotive Applications. Results Eng. 2024, 21, 101700. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and Advances in Magnesium Alloy Corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Olugbade, T.O.; Omiyale, B.O.; Ojo, O.T. Corrosion, Corrosion Fatigue, and Protection of Magnesium Alloys: Mechanisms, Measurements, and Mitigation. J. Mater. Eng. Perform. 2022, 31, 1707–1727. [Google Scholar] [CrossRef]
- Nie, K.B.; Deng, K.K.; Wang, X.J.; Wang, T.; Wu, K. Influence of SiC Nanoparticles Addition on the Microstructural Evolution and Mechanical Properties of AZ91 Alloy during Isothermal Multidirectional Forging. Mater. Charact. 2017, 124, 14–24. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Zhang, X.; Yang, Q.; Zhang, J.; Li, X. Development and Application of Magnesium Alloy Parts for Automotive OEMs: A Review. J. Magnes. Alloys 2023, 11, 15–47. [Google Scholar] [CrossRef]
- Wellbrock, W.; Ludin, D.; Röhrle, L.; Gerstlberger, W. Sustainability in the Automotive Industry, Importance of and Impact on Automobile Interior – Insights from an Empirical Survey. Int. J. Corp. Social. Responsib. 2020, 5, 10. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J. Advanced Lightweight Materials for Automobiles: A Review. Mater. Des. 2022, 221, 110994. [Google Scholar] [CrossRef]
- Manjhi, S.K.; Sekar, P.; Bontha, S.; Balan, A.S.S. Additive Manufacturing of Magnesium Alloys: Characterization and Post-Processing. Int. J. Lightweight Mater. Manuf. 2024, 7, 184–213. [Google Scholar] [CrossRef]
- Naseri, M.; Moghadam, A.O.; Anandkumar, M.; Sudarsan, S.; Bodrov, E.; Samodurova, M.; Trofimov, E. Enhancing the Mechanical Properties of High-Entropy Alloys through Severe Plastic Deformation: A Review. J. Alloys Metall. Syst. 2024, 5, 100054. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Attarilar, S.; Gode, C.; Kandavalli, S.R.; Shamsborhan, M.; Wang, Q. Conceptual Analysis on Severe Plastic Deformation Processes of Shape Memory Alloys: Mechanical Properties and Microstructure Characterization. Metals 2023, 13, 447. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Zhilyaev, A.P.; Langdon, T.G. Description of Severe Plastic Deformation (SPD). In Bulk Nanostructured Materials; Wiley: Hoboken, NJ, USA, 2013; pp. 6–21. [Google Scholar]
- Muralidhar, A.; Narendranath, S.; Shivananda Nayaka, H. Effect of Equal Channel Angular Pressing on AZ31 Wrought Magnesium Alloys. J. Magnes. Alloys 2013, 1, 336–340. [Google Scholar] [CrossRef]
- Tan, Y.; Li, W.; Hu, W.; Shi, X.; Tian, L. The Effect of ECAP Temperature on the Microstructure and Properties of a Rolled Rare Earth Magnesium Alloy. Materials 2019, 12, 1554. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.V.S.; Prasad, S.B.; Verma, K.; Mishra, R.K.; Kumar, V.; Singh, S. The Role and Significance of Magnesium in Modern Day Research-A Review. J. Magnes. Alloys 2022, 10, 1–61. [Google Scholar] [CrossRef]
- Bryła, K.; Horky, J. Magnesium Alloys Processed by Severe Plastic Deformation (SPD) for Biomedical Applications: An Overview. Mater. Trans. 2023, 64, 1709–1723. [Google Scholar] [CrossRef]
- Wang, X.; Brünger, E.; Gottstein, G. Microstructure Characterization and Dynamic Recrystallization in an Alloy 800H. Mater. Sci. Eng. A 2000, 290, 180–185. [Google Scholar] [CrossRef]
- Abbas, A.; Huang, S.-J. Investigating the Synergic Effects of WS2 and ECAP on Degradation Behavior of AZ91 Magnesium Alloy. Coatings 2022, 12, 1710. [Google Scholar] [CrossRef]
- Huang, S.-J.; Subramani, M.; Borodianskiy, K.; Immanuel, P.N.; Chiang, C.-C. Effect of Equal Channel Angular Pressing on the Mechanical Properties of Homogenized Hybrid AZ61 Magnesium Composites. Mater. Today Commun. 2023, 34, 104974. [Google Scholar] [CrossRef]
- Guo, C.; Liu, L.; Liu, H.; Qian, F.; Zhou, Y.; Wang, L.; Li, J.; Wang, J. Effect of Indium and Yttrium on the Corrosion Behavior of AZ63 Magnesium Alloy. J. Alloys Compd. 2024, 985, 174068. [Google Scholar] [CrossRef]
- Avvari, M.; Narendranath, S.; Able, M. Microstructure Evolution in AZ61 Alloy Processed by Equal Channel Angular Pressing. Adv. Mech. Eng. 2016, 8, 6. [Google Scholar] [CrossRef]
- Teng, H.; Zhang, X.; Zhang, Z.; Li, T.; Cockcroft, S. Research on Microstructures of Sub-Rapidly Solidified AZ61 Magnesium Alloy. Mater. Charact. 2009, 60, 482–486. [Google Scholar] [CrossRef]
- Zan, Y.N.; Zhou, Y.T.; Liu, Z.Y.; Ma, G.N.; Wang, D.; Wang, Q.Z.; Wang, W.G.; Xiao, B.L.; Ma, Z.Y. Enhancing Strength and Ductility Synergy through Heterogeneous Structure Design in Nanoscale Al2O3 Particulate Reinforced Al Composites. Mater. Des. 2019, 166, 107629. [Google Scholar] [CrossRef]
- Li, Y.; Tan, C.; Yu, X.; Nie, Z.; Zhao, X.; Han, J.; Yuan, S.; Zhao, M.; Guo, C.; Wang, F. Evolution of β Mg17Al12 in Mg Al Zn Ag Alloy over Time. Mater. Sci. Eng. A 2019, 754, 470–478. [Google Scholar] [CrossRef]
- Huang, S.-J.; Subramani, M.; Ali, A.N.; Alemayehu, D.B.; Aoh, J.-N.; Lin, P.-C. The Effect of Micro-SiCp Content on the Tensile and Fatigue Behavior of AZ61 Magnesium Alloy Matrix Composites. Int. J. Met. 2021, 15, 780–793. [Google Scholar] [CrossRef]
- Elambharathi, B.; Kumar, S.D.; Dhanoop, V.U.; Dinakar, S.; Rajumar, S.; Sharma, S.; Kumar, V.; Li, C.; Eldin, E.M.T.; Wojciechowski, S. Novel Insights on Different Treatment of Magnesium Alloys: A Critical Review. Heliyon 2022, 8, e11712. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Wang, J.; Pan, Y.; Ma, K.; Peng, Y.; Ren, J.; Wang, Y.; Wang, D.; Wang, J.; Ma, Y. Tailoring the Microstructural Characteristic and Improving the Corrosion Rate of Mg-Gd-Ni Alloy by Heat Treatment with Different Volume Fraction of LPSO Phase. Corros. Sci. 2023, 210, 110806. [Google Scholar] [CrossRef]
- Sharma, S.K.; Saxena, K.K.; Malik, V.; Mohammed, K.A.; Prakash, C.; Buddhi, D.; Dixit, S. Significance of Alloying Elements on the Mechanical Characteristics of Mg-Based Materials for Biomedical Applications. Crystals 2022, 12, 1138. [Google Scholar] [CrossRef]
- Asadollahi, M.; Gerashi, E.; Alizadeh, R.; Mahmudi, R. Effect of Zn Content and Processing Route on the Microstructure, Mechanical Properties, and Bio-Degradation of Mg–Zn Alloys. J. Mater. Res. Technol. 2022, 21, 4473–4489. [Google Scholar] [CrossRef]
- Ye, X.; Suo, Z.; Heng, Z.; Chen, B.; Wei, Q.; Umeda, J.; Kondoh, K.; Shen, J. An In-Situ Study of Static Recrystallization in Mg Using High Temperature EBSD. J. Magnes. Alloys 2023. [Google Scholar] [CrossRef]
- Liu, F.; Chen, X.; Zhao, Y.; Wang, W.; Zou, D.; Li, R.; Xiong, X.; Su, B. Effects of Severe Plastic Deformation on the Microstructures, Mechanical Properties, and Corrosion Behavior of U-5.5Nb Alloy. J. Nucl. Mater. 2021, 557, 153274. [Google Scholar] [CrossRef]
- Bhat, K.U.; Bhat Panemangalore, D.; Bhat, S. Equal Channel Angular Processing—A Modern Deforming Technique for Quality Products. In Advanced Welding and Deforming; Elsevier: Amsterdam, The Netherlands, 2021; pp. 381–423. [Google Scholar]
- Kapoor, R. Severe Plastic Deformation of Materials. In Materials Under Extreme Conditions; Elsevier: Amsterdam, The Netherlands, 2017; pp. 717–754. [Google Scholar]
- Zhang, Z.; Chen, D.L. Contribution of Orowan Strengthening Effect in Particulate-Reinforced Metal Matrix Nanocomposites. Mater. Sci. Eng. A 2008, 483–484, 148–152. [Google Scholar] [CrossRef]
- Huang, S.-J.; Subramani, M.; Borodianskiy, K. Strength and Ductility Enhancement of AZ61/Al2O3/SiC Hybrid Composite by ECAP Processing. Mater. Today Commun. 2022, 31, 103261. [Google Scholar] [CrossRef]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of Zn on Microstructure, Mechanical Properties and Corrosion Behavior of Mg–Zn Alloys. Mater. Sci. Eng. C 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Hashemi, M.; Alizadeh, R.; Langdon, T.G. Recent Advances Using Equal-Channel Angular Pressing to Improve the Properties of Biodegradable Mg-Zn Alloys. J. Magnes. Alloys 2023, 11, 2260–2284. [Google Scholar] [CrossRef]
- Li, G.; Pan, X.; Jiang, J.; Li, J.; Xie, L.; Liu, H.; Zhang, M. Achieving Ultra-Fine Grains and High Corrosion Resistance of Al–Zn–Mg–Cu Alloy by ECAP and Post Cold Rolling. J. Mater. Res. Technol. 2023, 26, 7354–7368. [Google Scholar] [CrossRef]
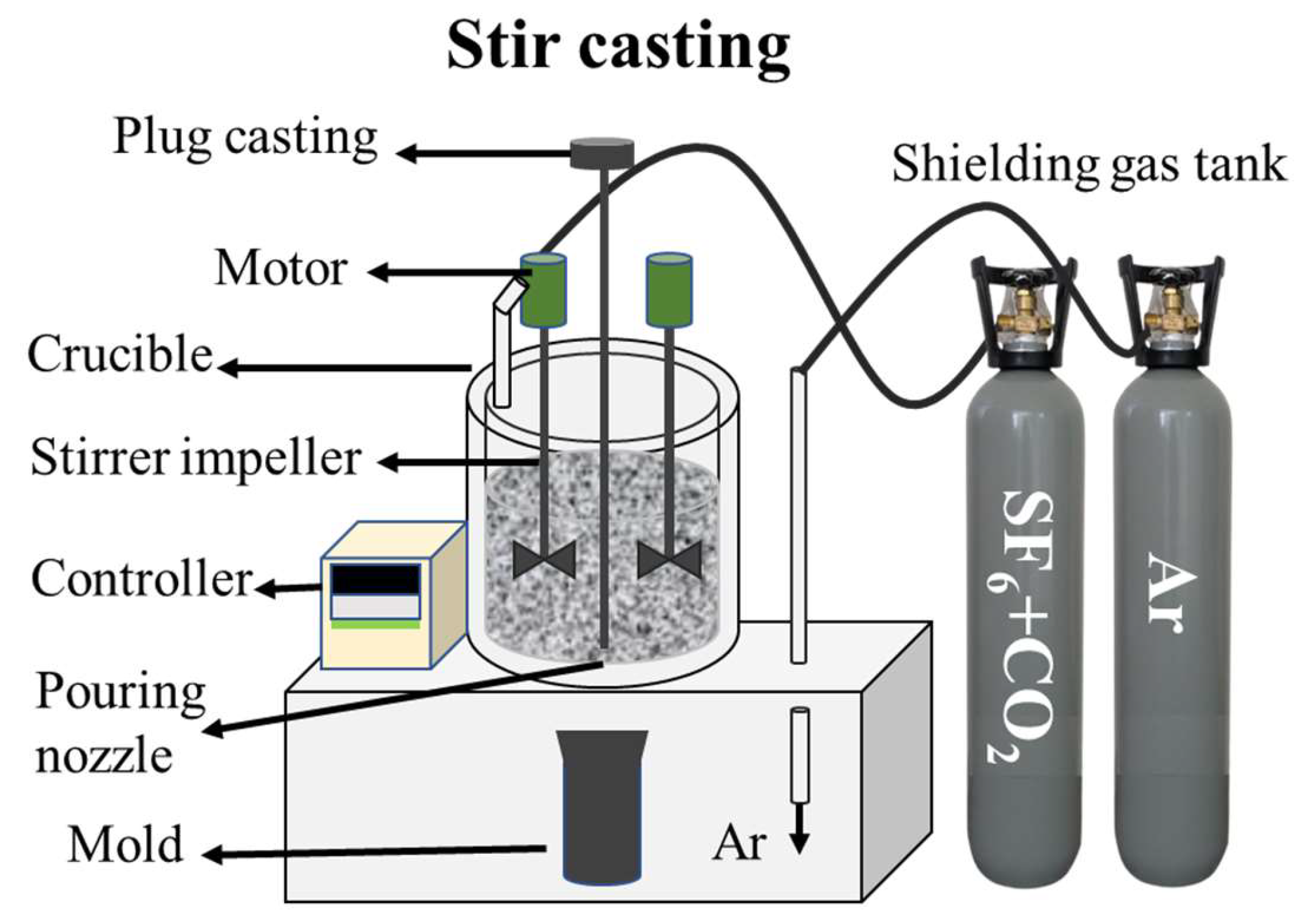
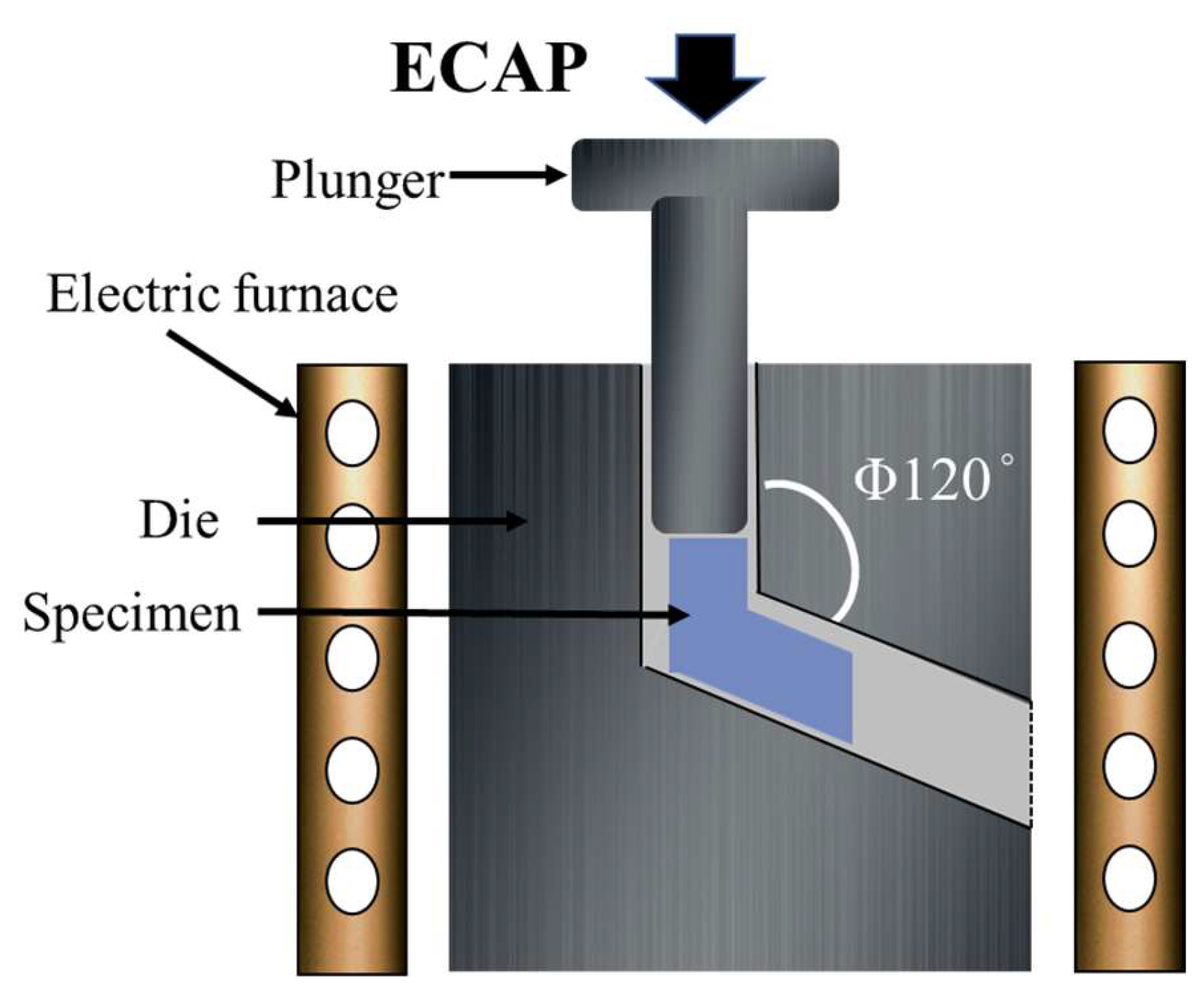
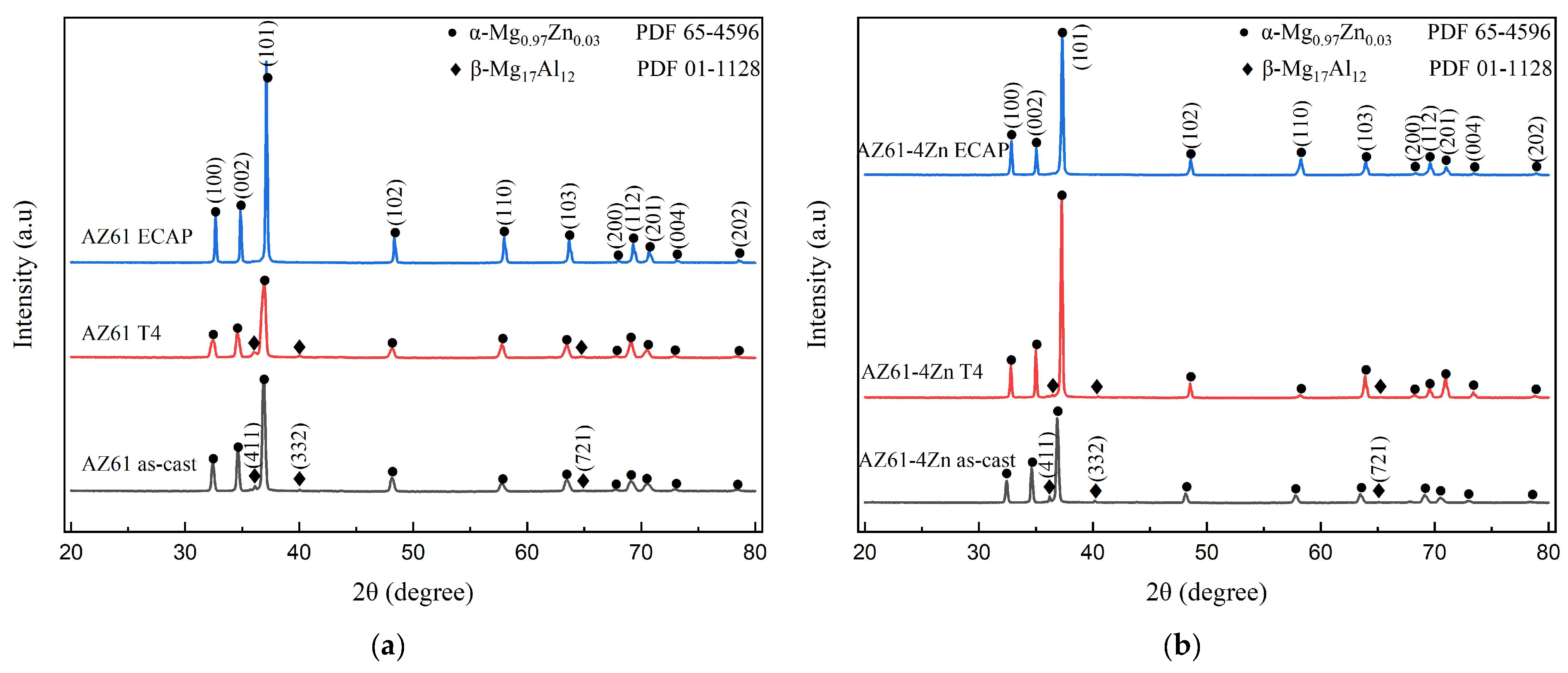
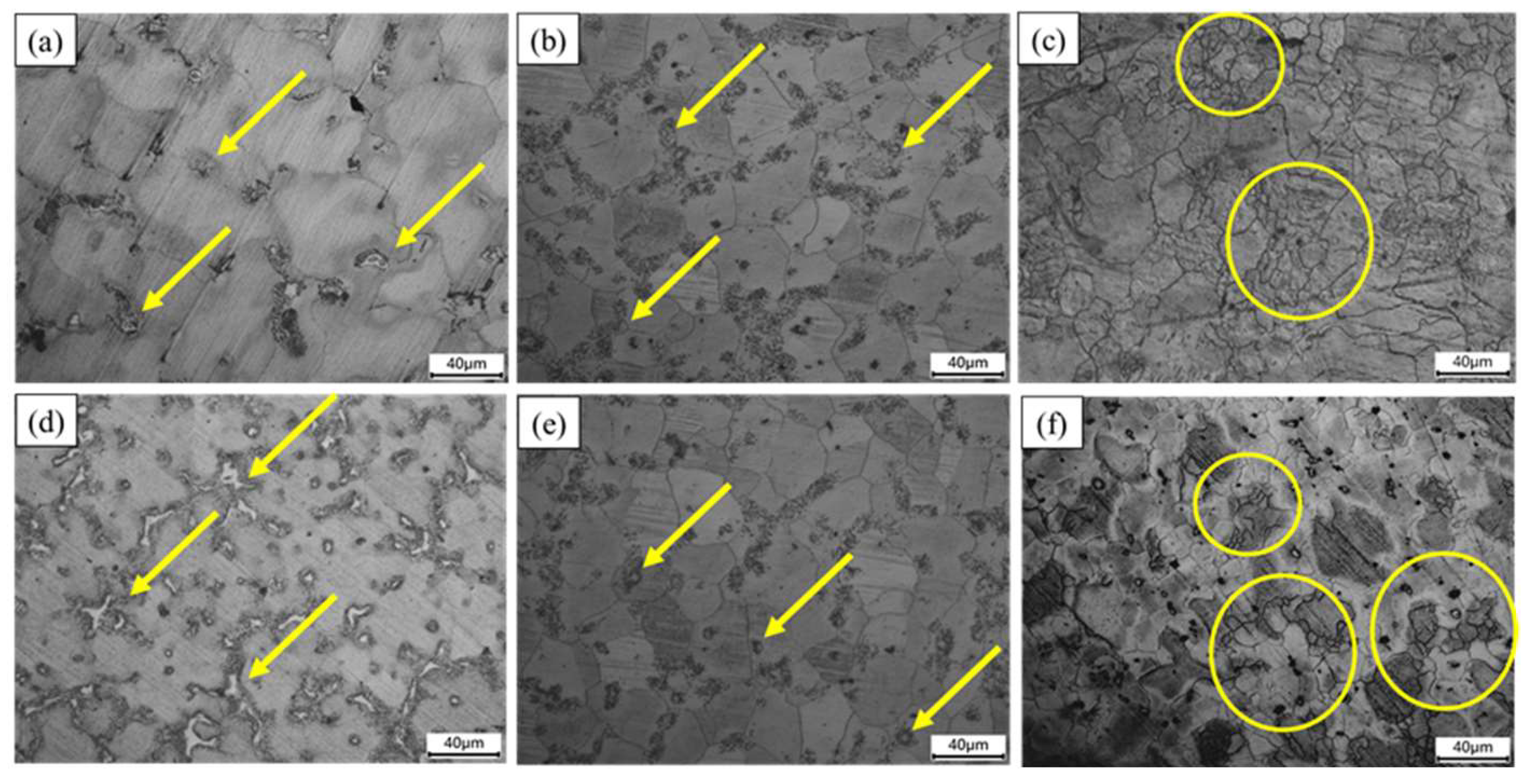
| Elements | Mg | Al | Zn | Mn | Si | Fe | Cu | Ni |
|---|---|---|---|---|---|---|---|---|
| wt.% | balance | 6.25 | 1.24 | 0.27 | 0.06 | 0.03 | 0.01 | 0.01 |
| Ethanol (mL) | DI Water (mL) | Acetic Acid (mL) | Picric Acid (g) | Time (s) |
|---|---|---|---|---|
| 100 | 10 | 5 | 6 | 25 |
| Metallic Alloys | Process | EL (%) | YS (MPa) | UCS (MPa) | Hardness (HV) |
|---|---|---|---|---|---|
| AZ61 | as-cast | 19.26 ± 4.24 | 124.67 ± 3.60 | 369.36 ± 4.24 | 67.88 ± 5.61 |
| T4 | 21.83 ± 2.60 | 115.42 ± 5.05 | 353.97 ± 7.71 | 63.87 ± 4.14 | |
| ECAP | 22.96 ± 2.84 | 141.53 ± 3.80 | 400.48 ± 4.10 | 77.44 ± 3.12 | |
| AZ61 + 4 wt.% Zn | as-cast | 18.49 ± 4.88 | 138.55 ± 4.14 | 384.43 ± 9.55 | 79.33 ± 6.63 |
| T4 | 19.73 ± 2.50 | 117.42 ± 3.40 | 365.43 ± 6.74 | 64.82 ± 5.74 | |
| ECAP | 20.58 ± 3.66 | 145.88 ± 3.14 | 421.79 ± 4.25 | 84.83 ± 2.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-J.; Wu, S.-Y.; Subramani, M. Effect of Zinc and Severe Plastic Deformation on Mechanical Properties of AZ61 Magnesium Alloy. Materials 2024, 17, 1678. https://doi.org/10.3390/ma17071678
Huang S-J, Wu S-Y, Subramani M. Effect of Zinc and Severe Plastic Deformation on Mechanical Properties of AZ61 Magnesium Alloy. Materials. 2024; 17(7):1678. https://doi.org/10.3390/ma17071678
Chicago/Turabian StyleHuang, Song-Jeng, Sheng-Yu Wu, and Murugan Subramani. 2024. "Effect of Zinc and Severe Plastic Deformation on Mechanical Properties of AZ61 Magnesium Alloy" Materials 17, no. 7: 1678. https://doi.org/10.3390/ma17071678
APA StyleHuang, S.-J., Wu, S.-Y., & Subramani, M. (2024). Effect of Zinc and Severe Plastic Deformation on Mechanical Properties of AZ61 Magnesium Alloy. Materials, 17(7), 1678. https://doi.org/10.3390/ma17071678








