Processing of Thin Films Based on Cellulose Nanocrystals and Biodegradable Polymers by Space-Confined Solvent Vapor Annealing and Morphological Characteristics
Abstract
:1. Introduction
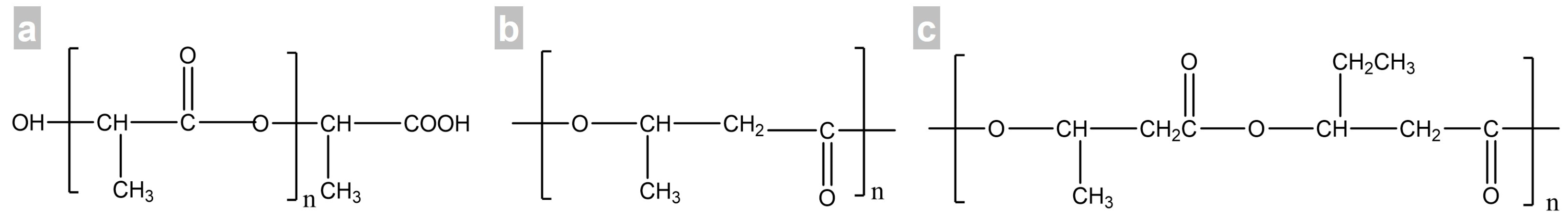
2. Materials and Methods
2.1. Materials
2.2. Production of Cellulose Nanocrystals (CNC)
2.3. Production of Polymer/CNC Composites
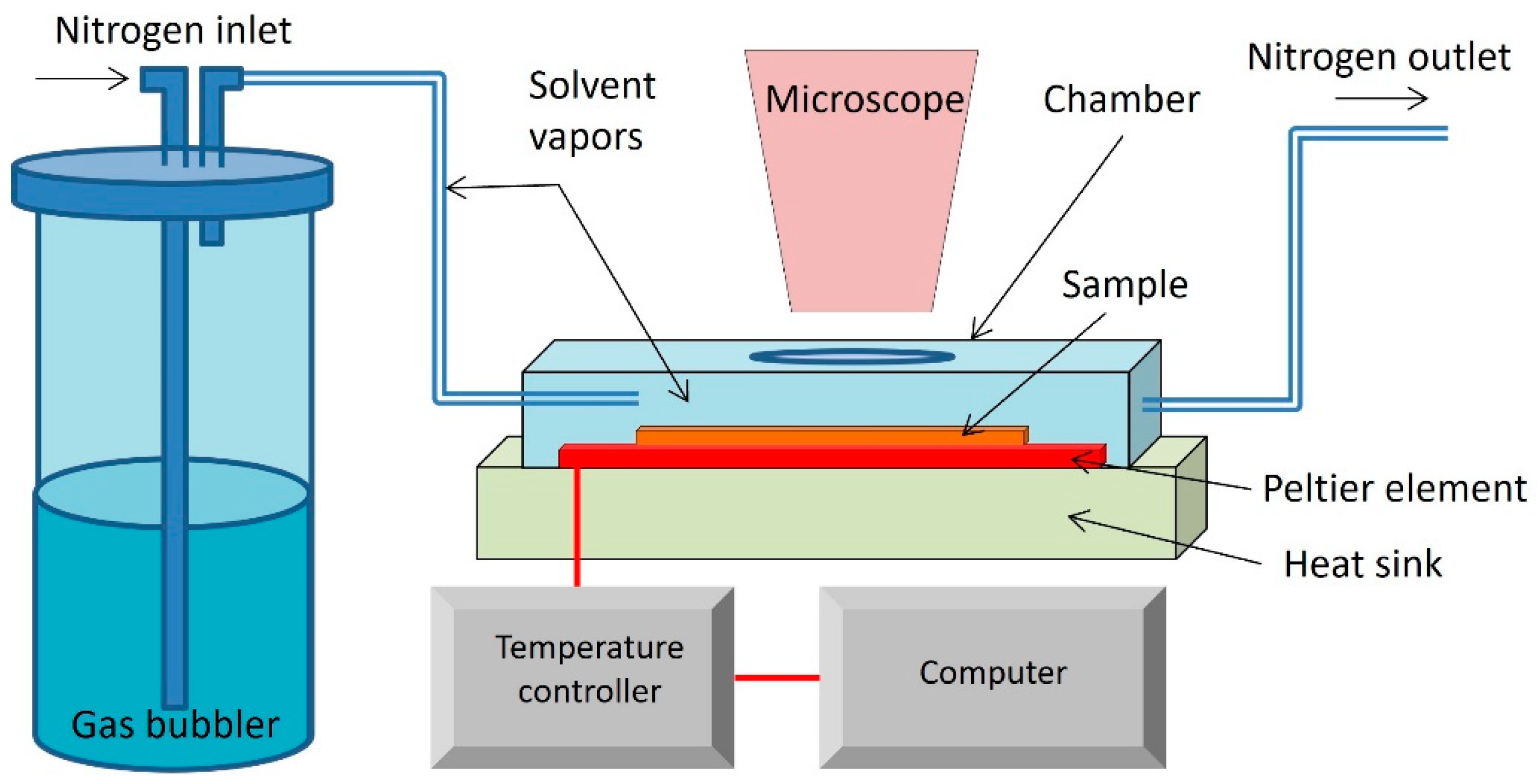
2.4. Processing of Thin Films Based on Cellulose Nanocrystals and Biodegradable Polymers by C-SVA
2.5. Characterization of Biocomposites and Polymers
2.5.1. Atomic Force Microscopy
2.5.2. Thermogravimetric Analysis/Derivative Thermogravimetry (TGA/DTG) Measurements
2.5.3. Raman Spectroscopy
2.5.4. Differential Scanning Calorimetry (DSC) Measurements
3. Results and Discussion
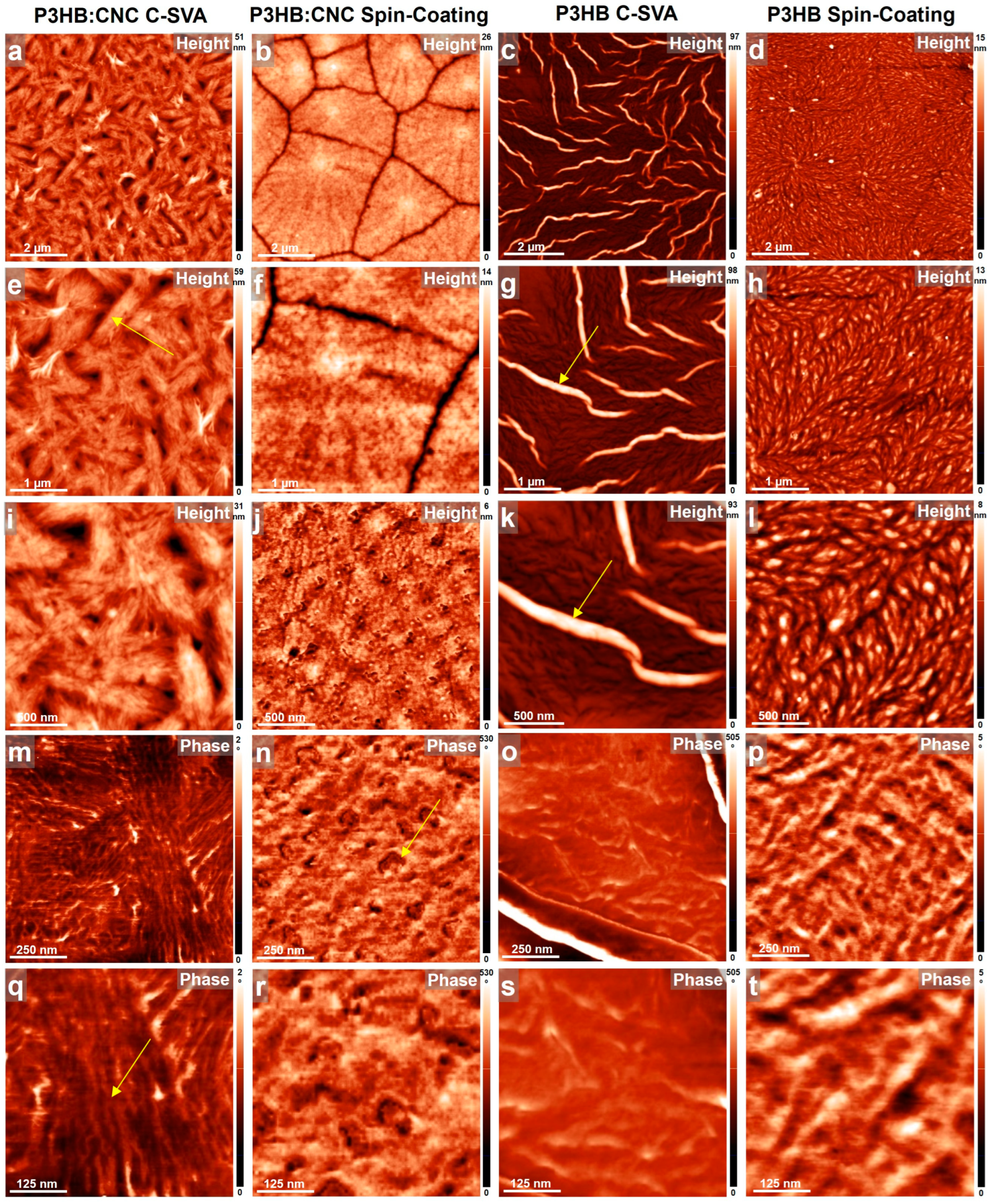
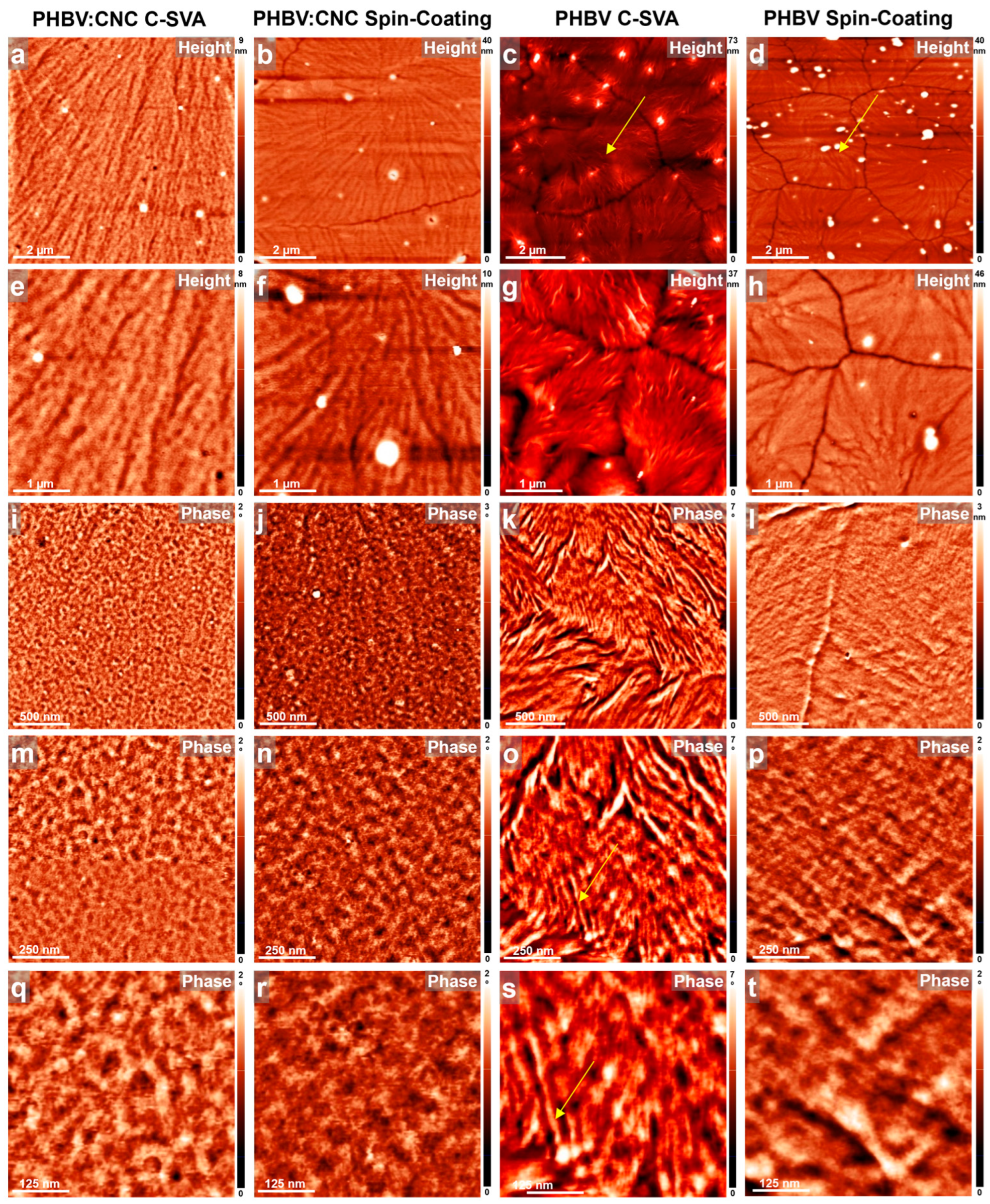
3.1. AFM Studies of Thin Films Produced by C-SVA
3.2. Characterization of the Polymer/CNC Composites
3.2.1. TGA/DTG
3.2.2. Raman Spectroscopy
3.2.3. DSC Characterization of the Polymer/CNC Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhagia, S.; Bornani, K.; Agrawal, R.; Satlewal, A.; Ďurkovič, J.; Lagaňa, R.; Bhagia, M.; Yoo, C.G.; Zhao, X.; Kunc, V.; et al. Critical Review of FDM 3D Printing of PLA Biocomposites Filled with Biomass Resources, Characterization, Biodegradability, Upcycling and Opportunities for Biorefineries. Appl. Mater. Today 2021, 24, 101078. [Google Scholar] [CrossRef]
- Houfani, A.A.; Anders, N.; Spiess, A.C.; Baldrian, P.; Benallaoua, S. Insights from Enzymatic Degradation of Cellulose and Hemicellulose to Fermentable Sugars—A Review. Biomass Bioenergy 2020, 134, 105481. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass Recalcitrance. Part I: The Chemical Compositions and Physical Structures Affecting the Enzymatic Hydrolysis of Lignocellulose. Biofuels Bioprod. Biorefin. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Ciolacu, D.; Rudaz, C.; Vasilescu, M.; Budtova, T. Physically and Chemically Cross-Linked Cellulose Cryogels: Structure, Properties and Application for Controlled Release. Carbohydr. Polym. 2016, 151, 392–400. [Google Scholar] [CrossRef]
- Gauss, C.; Pickering, K.L.; Muthe, L.P. The Use of Cellulose in Bio-Derived Formulations for 3D/4D Printing: A Review. Compos. C Open Access 2021, 4, 100113. [Google Scholar] [CrossRef]
- Landry, V.; Alemdar, A.; Blanchet, P. Nanocrystalline Cellulose: Morphological, Physical, and Mechanical Properties. For. Prod. J. 2011, 61, 104–112. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Q.; Zhang, X.; Ren, S.; Lei, T.; Li, W.; Xu, G.; Zhang, Q. Nanocellulose Films with Combined Cellulose Nanofibers and Nanocrystals: Tailored Thermal, Optical and Mechanical Properties. Cellulose 2018, 25, 1103–1115. [Google Scholar] [CrossRef]
- Revin, V.V.; Liyaskina, E.V.; Parchaykina, M.V.; Kuzmenko, T.P.; Kurgaeva, I.V.; Revin, V.D.; Ullah, M.W. Bacterial Cellulose-Based Polymer Nanocomposites: A Review. Polymers 2022, 14, 4670. [Google Scholar] [CrossRef] [PubMed]
- Qua, E.H.; Hornsby, P.R.; Sharma, H.S.S.; Lyons, G. Preparation and Characterisation of Cellulose Nanofibres. J. Mater. Sci. 2011, 46, 6029–6045. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo De Medeiros, G.; Do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic Biomass from Agricultural Waste to the Circular Economy: A Review with Focus on Biofuels, Biocomposites and Bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- To, A.T.; Chung, P.; Katz, A. Weak-Acid Sites Catalyze the Hydrolysis of Crystalline Cellulose to Glucose in Water: Importance of Post-Synthetic Functionalization of the Carbon Surface. Angew. Chem. Int. Ed. 2015, 54, 11050–11053. [Google Scholar] [CrossRef]
- Pycka, S.; Roman, K. Comparison of Wood-Based Biocomposites with Polylactic Acid (PLA) Density Profiles by Desaturation and X-Ray Spectrum Methods. Materials 2023, 16, 5729. [Google Scholar] [CrossRef]
- Jenkins, A.D.; Kratochvíl, P.; Stepto, R.F.T.; Suter, U.W. Glossary of Basic Terms in Polymer Science (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2287–2311. [Google Scholar] [CrossRef]
- Allara, D.L. Aging of Polymers. Environ. Health Perspect. 1975, 11, 29–33. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R. Microstructure of Polypropylene. Prog. Polym. Sci. 2001, 26, 443–533. [Google Scholar] [CrossRef]
- Babutan, I.; Lucaci, A.-D.; Botiz, I. Antimicrobial Polymeric Structures Assembled on Surfaces. Polymers 2021, 13, 1552. [Google Scholar] [CrossRef]
- Moohan, J.; Stewart, S.A.; Espinosa, E.; Rosal, A.; Rodríguez, A.; Larrañeta, E.; Donnelly, R.F.; Domínguez-Robles, J. Cellulose Nanofibers and Other Biopolymers for Biomedical Applications. A Review. Appl. Sci. 2019, 10, 65. [Google Scholar] [CrossRef]
- Liu, J.; Qi, P.; Chen, F.; Zhang, J.; Li, H.; Sun, J.; Gu, X.; Zhang, S. A Universal Eco-Friendly Flame Retardant Strategy for Polylactic Acid Fabrics and Other Polymer Substrates. Int. J. Biol. Macromol. 2024, 260, 129411. [Google Scholar] [CrossRef]
- Dimov, I.B.; Moser, M.; Malliaras, G.G.; McCulloch, I. Semiconducting Polymers for Neural Applications. Chem. Rev. 2022, 122, 4356–4396. [Google Scholar] [CrossRef]
- He, Y.; Kukhta, N.A.; Marks, A.; Luscombe, C.K. The Effect of Side Chain Engineering on Conjugated Polymers in Organic Electrochemical Transistors for Bioelectronic Applications. J. Mater. Chem. C 2022, 10, 2314–2332. [Google Scholar] [CrossRef]
- Botiz, I.; Durbin, M.M.; Stingelin, N. Providing a Window into the Phase Behavior of Semiconducting Polymers. Macromolecules 2021, 54, 5304–5320. [Google Scholar] [CrossRef]
- Pham, Q.-T.; Chern, C.-S. Applications of Polymers in Lithium-Ion Batteries with Enhanced Safety and Cycle Life. J. Polym. Res. 2022, 29, 124. [Google Scholar] [CrossRef]
- Yarali, E.; Baniasadi, M.; Zolfagharian, A.; Chavoshi, M.; Arefi, F.; Hossain, M.; Bastola, A.; Ansari, M.; Foyouzat, A.; Dabbagh, A.; et al. Magneto-/Electro-responsive Polymers toward Manufacturing, Characterization, and Biomedical/Soft Robotic Applications. Appl. Mater. Today 2022, 26, 101306. [Google Scholar] [CrossRef]
- Angel, N.; Li, S.; Yan, F.; Kong, L. Recent Advances in Electrospinning of Nanofibers from Bio-Based Carbohydrate Polymers and Their Applications. Trends Food Sci. 2022, 120, 308–324. [Google Scholar] [CrossRef]
- Handrea-Dragan, M.; Botiz, I. Multifunctional Structured Platforms: From Patterning of Polymer-Based Films to Their Subsequent Filling with Various Nanomaterials. Polymers 2021, 13, 445. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.A.; Kalra, B. Biodegradable Polymers for the Environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Anwer, A.H.; Ahtesham, A.; Shoeb, M.; Mashkoor, F.; Ansari, M.Z.; Zhu, S.; Jeong, C. State-of-the-Art Advances in Nanocomposite and Bio-Nanocomposite Polymeric Materials: A Comprehensive Review. Adv. Colloid Interface Sci. 2023, 318, 102955. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Lalnundiki, V.; Shelare, S.D.; Abhishek, G.J.; Sharma, S.; Sharma, D.; Kumar, A.; Abbas, M. An Investigation of the Environmental Implications of Bioplastics: Recent Advancements on the Development of Environmentally Friendly Bioplastics Solutions. Environ. Res. 2024, 244, 117707. [Google Scholar] [CrossRef] [PubMed]
- Gavande, V.; Nagappan, S.; Seo, B.; Lee, W.-K. A Systematic Review on Green and Natural Polymeric Nanofibers for Biomedical Applications. Int. J. Biol. Macromol. 2024, 262, 130135. [Google Scholar] [CrossRef]
- Kolybaba, M.; Tabil, L.G.; Panigrahi, S.; Crerar, W.J.; Powell, T.; Wang, B. Biodegradable Polymers: Past, Present, and Future; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2003. [Google Scholar] [CrossRef]
- De, S.; James, B.; Ji, J.; Wasti, S.; Zhang, S.; Kore, S.; Tekinalp, H.; Li, Y.; Ureña-Benavides, E.E.; Vaidya, U.; et al. Biomass-Derived Composites for Various Applications. In Advances in Bioenergy; Elsevier: Amsterdam, The Netherlands, 2023; Volume 8, pp. 145–196. ISBN 978-0-443-18856-5. [Google Scholar]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent Advances in the Development of Biodegradable PHB-Based Toughening Materials: Approaches, Advantages and Applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef]
- Shanmugam, V.; Mensah, R.A.; Försth, M.; Sas, G.; Restás, Á.; Addy, C.; Xu, Q.; Jiang, L.; Neisiany, R.E.; Singha, S.; et al. Circular Economy in Biocomposite Development: State-of-the-Art, Challenges and Emerging Trends. Compos. C Open Access 2021, 5, 100138. [Google Scholar] [CrossRef]
- Bhat, A.R.; Kumar, R.; Mural, P.K.S. Natural Fiber Reinforced Polymer Composites: A Comprehensive Review of Tribo-Mechanical Properties. Tribol. Int. 2023, 189, 108978. [Google Scholar] [CrossRef]
- Bucci, D.Z.; Tavares, L.B.B.; Sell, I. PHB Packaging for the Storage of Food Products. Polym. Test. 2005, 24, 564–571. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Negahi Shirazi, A.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef]
- Li, Z.; Loh, X.J. Recent Advances of Using Polyhydroxyalkanoate-based Nanovehicles as Therapeutic Delivery Carriers. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1429. [Google Scholar] [CrossRef]
- El-Kadi, S.M.; Elbagory, M.; EL-Zawawy, H.A.H.; EL-Shaer, H.F.A.; Shoukry, A.A.; El-Nahrawy, S.; Omara, A.E.-D.; Ali, D.F.I. Biosynthesis of Poly-ß-Hydroxybutyrate (PHB) from Different Bacterial Strains Grown on Alternative Cheap Carbon Sources. Polymers 2021, 13, 3801. [Google Scholar] [CrossRef]
- Holmes, P.A. Applications of PHB—A Microbially Produced Biodegradable Thermoplastic. Phys. Technol. 1985, 16, 32–36. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Khlusov, I.A.; Volova, T.G. A Hybrid PHB–Hydroxyapatite Composite for Biomedical Application: Production, in Vitro and in Vivo Investigation. J. Biomater. Sci. Polym. Ed. 2006, 17, 481–498. [Google Scholar] [CrossRef]
- Poltronieri, P.; Mezzolla, V.; D’Urso, O.F. PHB Production in Biofermentors Assisted through Biosensor Applications. In Proceedings of the 3rd International Electronic Conference on Sensors and Applications, Online, 15–30 November 2016; p. 4. Available online: https://sciforum.net/conference/ecsa-3 (accessed on 29 January 2024).
- Costa, J.A.V.; Moreira, J.B.; Lucas, B.F.; Braga, V.D.S.; Cassuriaga, A.P.A.; Morais, M.G.D. Recent Advances and Future Perspectives of PHB Production by Cyanobacteria. Ind. Biotechnol. 2018, 14, 249–256. [Google Scholar] [CrossRef]
- Anjana; Raturi, G.; Shree, S.; Sharma, A.; Panesar, P.S.; Goswami, S. Recent Approaches for Enhanced Production of Microbial Polyhydroxybutyrate: Preparation of Biocomposites and Applications. Int. J. Biol. Macromol. 2021, 182, 1650–1669. [Google Scholar] [CrossRef]
- Song, W.; Yang, Z.; Zhang, S.; Fei, B.; Zhao, R. Properties Enhancement of Poly(β-Hydroxybutyrate) Biocomposites by Incorporating Surface-Modified Wheat Straw Flour: Effect of Pretreatment Methods. Int. J. Biol. Macromol. 2023, 232, 123456. [Google Scholar] [CrossRef]
- Kostag, M.; El Seoud, O.A. Sustainable Biomaterials Based on Cellulose, Chitin and Chitosan Composites—A Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100079. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Nicolae, C.A.; Gabor, A.R.; Trusca, R. Thermal and Mechanical Properties of Poly(3-Hydroxybutyrate) Reinforced with Cellulose Fibers from Wood Waste. Ind. Crops Prod. 2020, 145, 112071. [Google Scholar] [CrossRef]
- Diyana, Z.N.; Jumaidin, R.; Selamat, M.Z.; Ghazali, I.; Julmohammad, N.; Huda, N.; Ilyas, R.A. Physical Properties of Thermoplastic Starch Derived from Natural Resources and Its Blends: A Review. Polymers 2021, 13, 1396. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Ismail, H. Thermoplastic/Natural Filler Composites: A Short Review. JPS 2019, 30, 81–99. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Chong, E.W.N.; Owolabi, F.A.T.; Asniza, M.; Tye, Y.Y.; Rizal, S.; Nurul Fazita, M.R.; Mohamad Haafiz, M.K.; Nurmiati, Z.; Paridah, M.T. Enhancement of Basic Properties of Polysaccharide-based Composites with Organic and Inorganic Fillers: A Review. J. Appl. Polym. Sci. 2019, 136, 47251. [Google Scholar] [CrossRef]
- Ling, S.; Chen, W.; Fan, Y.; Zheng, K.; Jin, K.; Yu, H.; Buehler, M.J.; Kaplan, D.L. Biopolymer Nanofibrils: Structure, Modeling, Preparation, and Applications. Prog. Polym. Sci. 2018, 85, 1–56. [Google Scholar] [CrossRef]
- Senila, L.; Cadar, O.; Kovacs, E.; Gal, E.; Dan, M.; Stupar, Z.; Simedru, D.; Senila, M.; Roman, C. L-Poly(Lactic Acid) Production by Microwave Irradiation of Lactic Acid Obtained from Lignocellulosic Wastes. Int. J. Mol. Sci. 2023, 24, 9817. [Google Scholar] [CrossRef]
- Todor-Boer, O.; Petrovai, I.; Tarcan, R.; Vulpoi, A.; David, L.; Astilean, S.; Botiz, I. Enhancing Photoluminescence Quenching in Donor–Acceptor PCE11:PPCBMB Films through the Optimization of Film Microstructure. Nanomaterials 2019, 9, 1757. [Google Scholar] [CrossRef]
- Botiz, I. Prominent Processing Techniques to Manipulate Semiconducting Polymer Microstructures. J. Mater. Chem. C 2023, 11, 364–405. [Google Scholar] [CrossRef]
- Todor-Boer, O.; Petrovai, I.; Tarcan, R.; David, L.; Astilean, S.; Botiz, I. Control of Microstructure in Polymer: Fullerene Active Films by Convective Self-Assembly. Thin Solid Films 2020, 697, 137780. [Google Scholar] [CrossRef]
- Babutan, I.; Todor-Boer, O.; Atanase, L.I.; Vulpoi, A.; Botiz, I. Crystallization of Poly(Ethylene Oxide)-Based Triblock Copolymers in Films Swollen-Rich in Solvent Vapors. Coatings 2023, 13, 918. [Google Scholar] [CrossRef]
- Babutan, I.; Todor-Boer, O.; Atanase, L.I.; Vulpoi, A.; Simon, S.; Botiz, I. Self-Assembly of Block Copolymers on Surfaces Exposed to Space-Confined Solvent Vapor Annealing. Polymer 2023, 273, 125881. [Google Scholar] [CrossRef]
- Babutan, I.; Todor-Boer, O.; Atanase, L.I.; Vulpoi, A.; Botiz, I. Self-Assembly of Block Copolymers in Thin Films Swollen-Rich in Solvent Vapors. Polymers 2023, 15, 1900. [Google Scholar] [CrossRef]
- Posselt, D.; Zhang, J.; Smilgies, D.-M.; Berezkin, A.V.; Potemkin, I.I.; Papadakis, C.M. Restructuring in Block Copolymer Thin Films: In Situ GISAXS Investigations during Solvent Vapor Annealing. Prog. Polym. Sci. 2017, 66, 80–115. [Google Scholar] [CrossRef]
- Senila, L.; Gál, E.; Kovacs, E.; Cadar, O.; Dan, M.; Senila, M.; Roman, C. Poly(3-Hydroxybutyrate) Production from Lignocellulosic Wastes Using Bacillus Megaterium ATCC 14581. Polymers 2023, 15, 4488. [Google Scholar] [CrossRef] [PubMed]
- Senila, L.; Kovacs, E.; Scurtu, D.A.; Cadar, O.; Becze, A.; Senila, M.; Levei, E.A.; Dumitras, D.E.; Tenu, I.; Roman, C. Bioethanol Production from Vineyard Waste by Autohydrolysis Pretreatment and Chlorite Delignification via Simultaneous Saccharification and Fermentation. Molecules 2020, 25, 2606. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yao, Z.; Zhou, J.; He, M.; Jiang, Q.; Li, A.; Li, S.; Liu, M.; Luo, S.; Zhang, D. Improvement of Polylactic Acid Film Properties through the Addition of Cellulose Nanocrystals Isolated from Waste Cotton Cloth. Int. J. Biol. Macromol. 2019, 129, 878–886. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, J.; Liang, X.; Huang, Z.; Li, J.; Peng, S. Crystallization Behaviors Regulations and Mechanical Performances Enhancement Approaches of Polylactic Acid (PLA) Biodegradable Materials Modified by Organic Nucleating Agents. Int. J. Biol. Macromol. 2023, 233, 123581. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; López, J.; Kenny, J.M. Bionanocomposite Films Based on Plasticized PLA–PHB/Cellulose Nanocrystal Blends. Carbohydr. Polym. 2015, 121, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Murakami, R.; Sato, H.; Dybal, J.; Iwata, T.; Ozaki, Y. Formation and Stability of β-Structure in Biodegradable Ultra-High-Molecular-Weight Poly(3-Hydroxybutyrate) by Infrared, Raman, and Quantum Chemical Calculation Studies. Polymer 2007, 48, 2672–2680. [Google Scholar] [CrossRef]
- Briassoulis, D.; Tserotas, P.; Athanasoulia, I.-G. Alternative Optimization Routes for Improving the Performance of Poly(3-Hydroxybutyrate) (PHB) Based Plastics. J. Clean. Prod. 2021, 318, 128555. [Google Scholar] [CrossRef]
- Schulz, G.L.; Ludwigs, S. Controlled Crystallization of Conjugated Polymer Films from Solution and Solvent Vapor for Polymer Electronics. Adv. Funct. Mater. 2017, 27, 1603083. [Google Scholar] [CrossRef]
- Mukaffa, H.; Asrofi, M.; Sujito; Asnawi; Hermawan, Y.; Sumarji; Qoryah, R.D.H.; Sapuan, S.M.; Ilyas, R.A.; Atiqah, A. Effect of Alkali Treatment of Piper Betle Fiber on Tensile Properties as Biocomposite Based Polylactic Acid: Solvent Cast-Film Method. Mater. Today Proc. 2022, 48, 761–765. [Google Scholar] [CrossRef]
- Samrudhi, B.M.; Devadiga, D.; Ahipa, T.N. Flame-Retardant Polymer Nanocomposite Films and Coatings. In Polymer Nanocomposite Films and Coatings; Elsevier: Amsterdam, The Netherlands, 2024; pp. 259–291. ISBN 978-0-443-19139-8. [Google Scholar]
- Mokhena, T.; Sefadi, J.; Sadiku, E.; John, M.; Mochane, M.; Mtibe, A. Thermoplastic Processing of PLA/Cellulose Nanomaterials Composites. Polymers 2018, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Abdalkarim, S.Y.H.; Yu, H.-Y.; Wang, D.; Yao, J. Electrospun Poly(3-Hydroxybutyrate-Co-3-Hydroxy-Valerate)/Cellulose Reinforced Nanofibrous Membranes with ZnO Nanocrystals for Antibacterial Wound Dressings. Cellulose 2017, 24, 2925–2938. [Google Scholar] [CrossRef]
- Sessini, V.; Navarro-Baena, I.; Arrieta, M.P.; Dominici, F.; López, D.; Torre, L.; Kenny, J.M.; Dubois, P.; Raquez, J.-M.; Peponi, L. Effect of the Addition of Polyester-Grafted-Cellulose Nanocrystals on the Shape Memory Properties of Biodegradable PLA/PCL Nanocomposites. Polym. Degrad. Stab. 2018, 152, 126–138. [Google Scholar] [CrossRef]
- Morgan, J.J.; Craciun, M.F.; Eichhorn, S.J. Quantification of Stress Transfer in a Model Cellulose Nanocrystal/Graphene Bilayer Using Raman Spectroscopy. Compos. Sci. Technol. 2019, 177, 34–40. [Google Scholar] [CrossRef]
- Lizundia, E.; Fortunati, E.; Dominici, F.; Vilas, J.L.; León, L.M.; Armentano, I.; Torre, L.; Kenny, J.M. PLLA-Grafted Cellulose Nanocrystals: Role of the CNC Content and Grafting on the PLA Bionanocomposite Film Properties. Carbohydr. Polym. 2016, 142, 105–113. [Google Scholar] [CrossRef]
- Rincon-Granados, K.L.; Vázquez-Olmos, A.R.; Rodríguez-Hernández, A.-P.; Prado-Prone, G.; Garibay-Febles, V.; Almanza-Arjona, Y.C.; Sato-Berrú, R.Y.; Mata-Zamora, E.; Silva-Bermúdez, P.S.; Vega-Jiménez, A. Bactericidal and Cytotoxic Study of Hybrid Films Based on NiO and NiFe2O4 Nanoparticles in Poly-3-Hydroxybutyrate. J. Clust. Sci. 2024, 35, 167–178. [Google Scholar] [CrossRef]
- Cao, X.; Mohamed, A.; Gordon, S.H.; Willett, J.L.; Sessa, D.J. DSC Study of Biodegradable Poly(Lactic Acid) and Poly(Hydroxy Ester Ether) Blends. Thermochim. Acta 2003, 406, 115–127. [Google Scholar] [CrossRef]

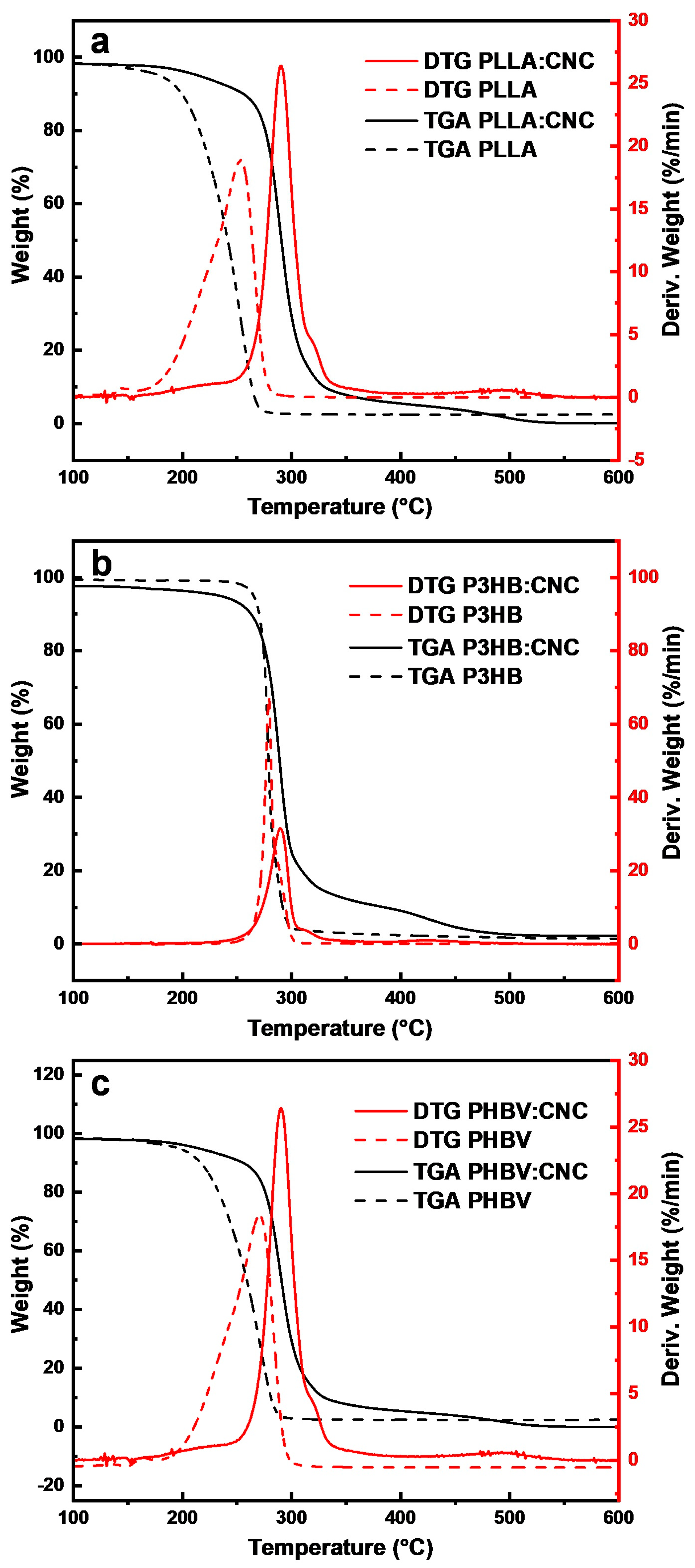
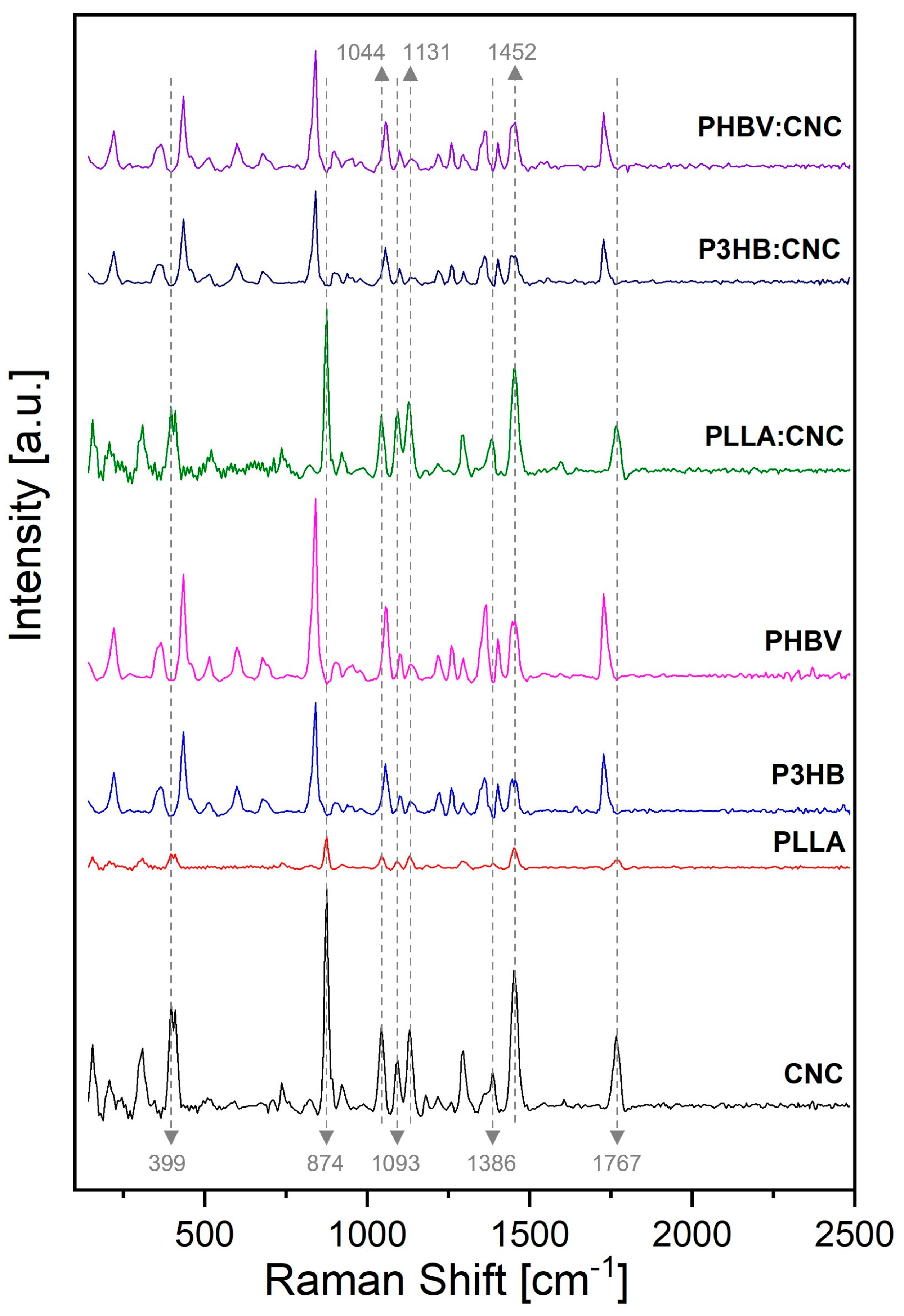
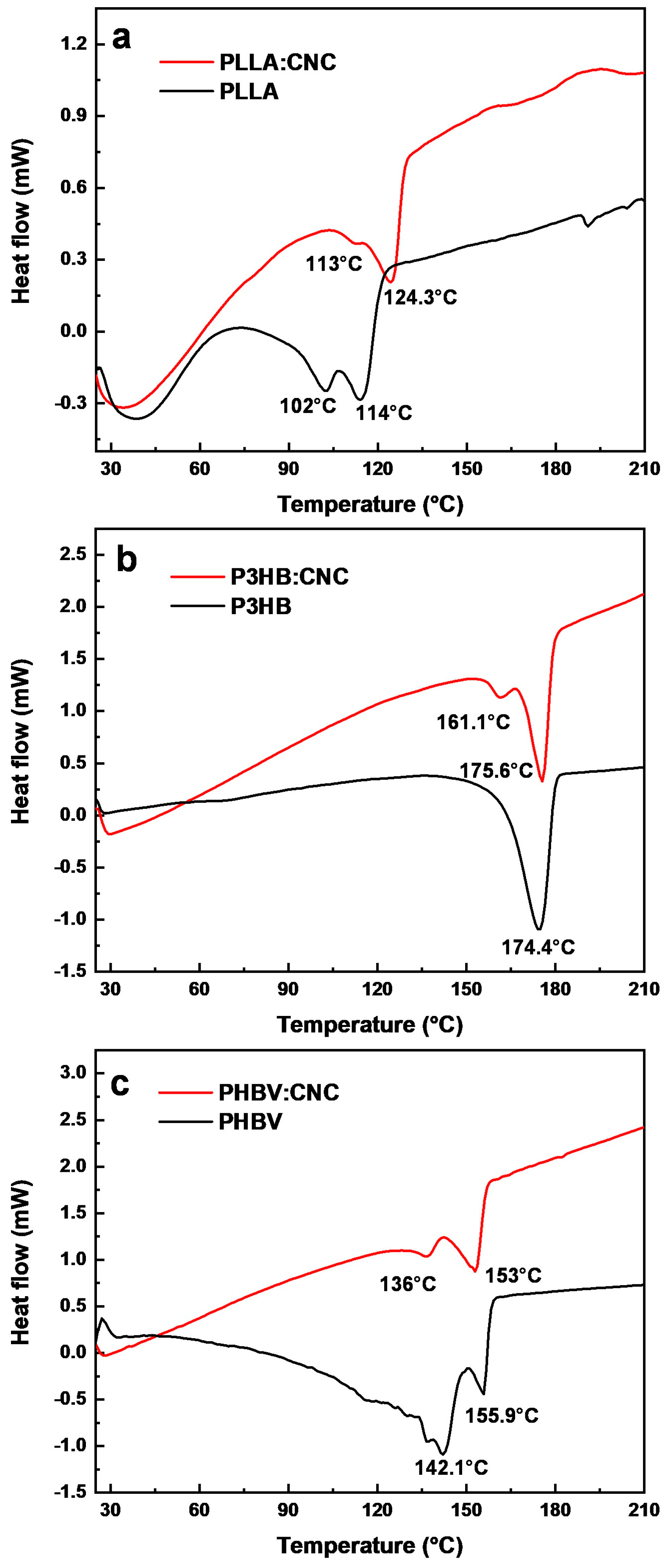
| Material | Tc (°C) | Tm (°C) | ΔHc (J/g) | ΔHm (J/g) |
|---|---|---|---|---|
| PLLA | 102.92 | 114.15 | −2.69 | −8.44 |
| PLLA:CNC | 113.07 | 124.28 | −0.49 | −10.7 |
| P3HB | - | 174.89 | - | −58.89 |
| P3HB:CNC | 161.12 | 175.64 | −3.03 | −27.44 |
| PHBV | 142.15 | 155.89 | −4.57 | −6.75 |
| PHBV:CNC | 136 | 153 | −3.40 | −20.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senila, L.; Botiz, I.; Roman, C.; Simedru, D.; Dan, M.; Kacso, I.; Senila, M.; Todor-Boer, O. Processing of Thin Films Based on Cellulose Nanocrystals and Biodegradable Polymers by Space-Confined Solvent Vapor Annealing and Morphological Characteristics. Materials 2024, 17, 1685. https://doi.org/10.3390/ma17071685
Senila L, Botiz I, Roman C, Simedru D, Dan M, Kacso I, Senila M, Todor-Boer O. Processing of Thin Films Based on Cellulose Nanocrystals and Biodegradable Polymers by Space-Confined Solvent Vapor Annealing and Morphological Characteristics. Materials. 2024; 17(7):1685. https://doi.org/10.3390/ma17071685
Chicago/Turabian StyleSenila, Lacrimioara, Ioan Botiz, Cecilia Roman, Dorina Simedru, Monica Dan, Irina Kacso, Marin Senila, and Otto Todor-Boer. 2024. "Processing of Thin Films Based on Cellulose Nanocrystals and Biodegradable Polymers by Space-Confined Solvent Vapor Annealing and Morphological Characteristics" Materials 17, no. 7: 1685. https://doi.org/10.3390/ma17071685









